Abstract
Background
This retrospective study was performed to verify the advantages and disadvantages of the free lateral arm flap for defect reconstruction of the forearm and hand.
Patients and Methods
Between 2001 and 2010, 21 patients underwent defect coverage of the forearm and hand with the free lateral arm flap. The mean patient age was 48 years (17–78). The results concerning defect origin, flap size, pedicle length, operative time, revisions of the anastomoses or other complications, donor site morbidity, and length of hospital stay were evaluated.
Results
The majority of defects were caused by infections or chronic wounds. The defects were localized at the forearm in 6 cases and at the hand in 15 cases. The flap width ranged from 3 to 8 cm, and the length was from 5 to 20 cm. All flaps survived. Only in one case, a revision of the anastomosis was necessary. Primary closure of the donor site was possible in all patients. No complications occurred during the healing procedure. The majority of the patients were satisfied with the aesthetic result at the recipient site as well as at the donor site.
Conclusion
The free lateral arm flap is a very reliable option for defect coverage at the forearm and hand for small and medium size defects. A satisfactory aesthetic appearance, an excellent tissue quality, and frequent primary donor site closure are great advantages for selecting this flap.
Keywords: Free lateral arm flap, Reconstruction of the forearm and hand, Defect coverage, Donor site morbidity
Introduction
Complex injuries in the forearm and hand with exposition of functional important structures such as bone, tendon, blood vessels, and nerves often require free tissue transplantation for coverage. For choosing an adequate flap, the tissue pliability and quality are as important as the patients' personal profile.
Further aspects for an adequate indication are a relatively short operation time, a constant vessel anatomy, and a low donor site morbidity. The general condition of the patient and his personal profile have to be included in the planning. The goal is an optimal reconstruction of form, function, and aesthetics.
The free lateral arm flap was initially described by Song et al. in 1982 as a septocutaneous flap. It replaced the pedicled lateral “cross-arm-flap” and was established as a routine procedure in reconstructive surgery by the work of Katsaros et al. [18]. Later, numerous variations of the flap were described, partly using a combination of different tissue components as the osteofasciocutaneous flap (with distal humerus corticalis [10, 13, 23]), sensitive flap (posterior brachial cutaneous nerve [12, 13, 17]), musculotendofasciocutaneous flap [13] using triceps muscle components, or just as a fascial flap [3, 5, 8]. The lateral arm flap can also be extended distally to the lateral epicondyle of the elbow [12]. With its variability in applications, the lateral arm flap is an excellent option for defect coverage of small and medium sizes on the forearm and hand.
The maximum flap size is 8 cm of width and 20 cm of length. The pedicle length can be up to 11 cm with a caliber of 2–2.5 mm [23]. If the indication for the lateral arm flap is correct, the donor defect can be primarily closed. The skin island supported by the septocutaneous vessels contains the whole lateral arm.
In this article, the variability and the reliability of the lateral arm flap for small and medium size defects of the forearm and hand are compared with alternative free flaps (Table 1). The current literature is discussed as well.
Table 1.
Comparison of alternative fasciocutaneous flaps
| Lateral arm flap | Radial forearm flap | Anterolateral thigh flap | Scapular/parascapular flap | |
|---|---|---|---|---|
| Maximum size skin island (cm) | 8 × 25 | 10 × 20 | 7 × 25 | 15 × 28 |
| Maximum length vascular pedicle (cm) | 11 | 15 | 15 | 10 |
| Only fascia | Yes | Yes | – | – |
| Tendon component | Lateral triceps tendon | Palmaris longus tendon, half of flexor carpi radialis tendon | Fascia lata | – |
| Muscle component | – | – | – | Latissimus dorsi muscle, anterior serratus muscle |
| Bone component | Distal humerus | Distal radius | – | Lateral margin of scapular |
| Nerve component | Posterior antebrachial cutaneous nerve | Lateral antebrachial cutaneous nerve | Lateral femoral cutaneous nerve | – |
| Patient position | Supine position or lateral position | Supine position or lateral position | Supine position | Lateral position |
Patients and Methods
From 2002 to 2010 in the two senior authors' (M.S. and G.G.) institutions, 21 defects of the hand or forearm with small or medium size defects where covered using the free lateral arm flap (Table 2).
Table 2.
Collective of patients
| No. | Patient | Gender | Age | Etiology of defect | Localization | Size of defect/flap | Type of arterial anastomosis |
|---|---|---|---|---|---|---|---|
| 1 | L. A. | Male | 44 | Infection of the back of hand after soft tissue injury | Hand | 6 × 9 cm | EEA A. radialis |
| 2 | G. B. | Female | 46 | Rheumatoid arthritis, chronic wound healing disorder after wrist arthrodesis | Dorsum of hand | 3.5 × 8 cm | EEA A. radialis |
| 3 | A.-E. E. | Male | 57 | Third degree open comminuted fracture of radius | Forearm | 6 × 14 cm | ESA A. ulnaris |
| 4 | Th. G. | Male | 20 | Bruise with decollement | Hand | 5.5 × 13 cm | ESA A. radialis |
| 5 | L. G. | Male | 48 | Polytrauma, exposed wrist arthrodesis | Hand and wrist | 6 × 14 cm | ESA A. radialis |
| 6 | M. Ge. | Male | 69 | Soft tissue defect on the forearm after infection | Forearm | 6 × 15 cm | EEA A. radialis |
| 7 | M. Go. | Male | 73 | Infection with necrosis of thumb | Hand | 5.5 × 20 cm | EEA A. radialis |
| 8 | K. H. | Male | 66 | Soft tissue defect caused by osteitis non-union of the radius | Forearm | 8 × 14 cm | ESA A. radialis |
| 9 | S. I. | Female | 53 | Synovia cell sarcoma of the wrist | Hand and wrist | 8 × 15 cm | ESA A. radialis |
| 10 | I. K. | Female | 49 | Heavy bruise by hydraulic machine | Hand | 7 × 12 cm | EEA A. ulnaris |
| 11 | T. K. | Male | 21 | Loss of ALT flap after flap thinning | (Back of) hand | 4 × 8 cm | ESA A. ulnaris |
| 12 | D. K. | Male | 33 | Infection of the thumb | Thumb | 3 × 7 cm | EEA A. radialis |
| 13 | H. L. | Female | 70 | Suicide attempt with numerous cutting wounds | Hand/forearm | 4 × 7 cm | EEA A. radialis |
| 14 | Ch. M. | Male | 78 | Extravasation during chemotherapy | Forearm | 7 × 14 cm | ESA A. radialis |
| 15 | Th. M. | Male | 45 | Necrotizing fasciitis | Forearm | 6 × 13 cm | ESA A. radialis |
| 16 | Ch. Mu. | Male | 17 | Soft tissue infection after cooking injury with contaminated fowl knife | Forearm | 5.5 × 15 cm | ESA A. radialis |
| 17 | St. Re. | Male | 20 | Crush in floating screed machine | Hand | 7 × 12 cm | ESA A. radialis |
| 18 | P. R.-T. | Female | 50 | Relapse of phlegmon on the wrist | Hand | 6 × 10 cm | ESA A. radialis |
| 19 | K. S. | Male | 37 | Heavy bruise injury by a cylinder | Hand | 5 × 17 cm | ESA A. radialis |
| 20 | S.S. | Male | 45 | Soft tissue defect after open fracture of the basal phalanx of index | Hand | 4 × 10 cm | ESA A. radialis |
| 21 | K.-H. Sch. | Male | 63 | Heavy bruise injury | Hand | 6 × 10 cm | EEA A. radialis |
Patient data were analyzed retrospectively. Patient's age, gender, profession, the size and localization of the defect, time of pre-clinical treatment, operation time, intra-operative complications, revisions, and general postoperative complications were recorded. Also, the overall treatment time, complications in the donor site, and interventions for secondary correction were analyzed. The study design was obtained in an internal IRB approval.
Results
Our patient collectively consisted of 5 women (23, 8%) and 16 men (76, 2%) between 17 and 78 years (median 48 years) treated from 2002 to 2010 (Table 2).
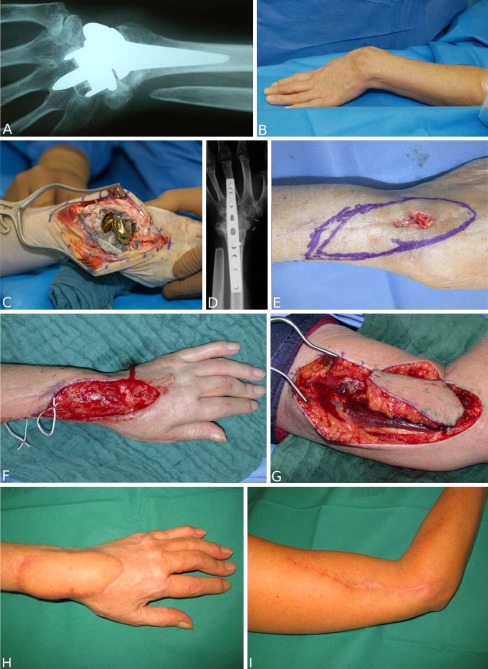
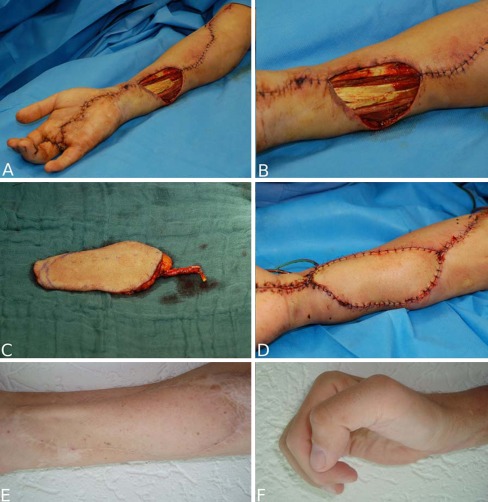
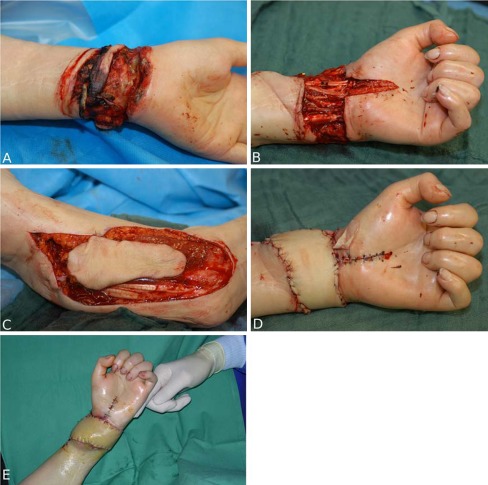
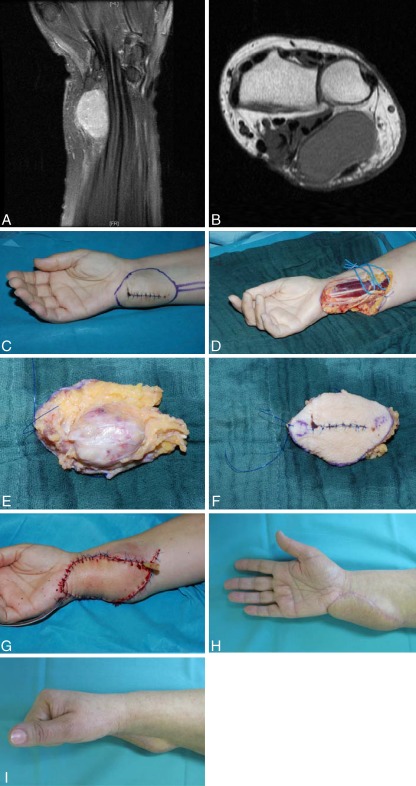
In 15 cases, the defect was localized at the hand, in 6 at the forearm. The defect was caused by chronic infected wounds in nine cases (Figs. 1a–i and 2a–f). In seven patients, suicide attempts (Fig. 3a–e), automutilation, or traffic accidents/other accidents caused injuries like contusions, amputations, slash wounds, or decollement injuries. There were also two treated cases of open forearm/finger fractures and one case of a non-healing pseudarthrosis in the forearm after bony fixation with a plate. In one patient, the defect was caused by a tumor resection (Fig. 4a–i), in one case by a paravasate on the dorsum of the hand.
Fig. 1.
a–b 46-year-old patient with an elsewhere implanted wrist prosthesis and joint malalignment with functional impairment. The patient is suffering from rheumatoid arthritis. c–d Explantation of the wrist implant and total wrist fusion with a plate. e Postoperative wound healing disorder over the extensor tendons and arthrodesis implant. f Debridement dorsally at the wrist with exposed important structures. g Harvested free lateral arm flap. h–i Postoperative result 2 years after flap transplantation (end-to-side anastomosis to radial artery, end-to-end anastomosis to concomitant veins of radial artery). The patient is contented with the scar in the donor site
Fig. 2.
a–b 45-year-old physician with soft tissue defect after necrotizing fasciitis in the right forearm. The infection started on the fourth finger, which had been amputated before in another hospital. c Harvested free lateral arm flap. d Situation immediately postoperative after flap transplantation. e Result 4 years postoperatively. f The patient works as an orthopedic physician again; he is able to use his hand and fingers in treating patients in his office
Fig. 3.
a 70-year-old woman with complete sectioning of median nerve, ulnar nerve, all flexor tendons, and radial/ulnar artery in the distal forearm/wrist after suicide attempt with multiple slash injuries. The patient had been lying in her apartment for 24 h with this injury. b Reconstruction of all structures at the wrist. Median nerve reconstruction with sural nerve interposition grafts. c Harvesting of a free lateral arm flap. d Immediate postoperative result in the operation room. e Result 3 days postoperative; the flap is perfused. The patient has been moved to a psychiatric facility. A postoperative tendon rehabilitation program was planned
Fig. 4.
a–b 53-year-old patient with a suspicious mass at the wrist level. The tumor displaces the ulnar palmar wrist structures (flexor tendons, ulnar nerve, ulnar artery, median nerve). c After incisional biopsy (synovial cell sarcoma G2), there was indication for radical resection and defect coverage. d–f In the radical tumor resection, all tendons, median nerve, and ulnar nerve could be preserved after epineural stripping. The tumor was resected with clear margins. g The defect was covered with a free lateral arm flap from the contralateral side (end-to-end-anastomosis of the flap artery to the ulnar artery), which had been resected for the purpose of oncological radicality. h–i Two years after the operation with adjuvant radiation therapy (60 Gray). No tumor recurrence and excellent function of the hand and wrist
In one of the 21 cases, a fascial flap was carried out, in all other cases a fasciocutaneous lateral arm flap (95, 2%). The flap size was dependent on the defects that had to be covered and ranged from 3 to 8 cm in width and 7–20 cm in length. The pedicles in our cases had a maximum length of 8 cm.
In 14 cases, end-to-end-anastomoses were performed with concomitant veins (in 7 cases, only one concomitant vein was used). In five patients, the cephalic vein was used as venous drainage vessel; in two different cases, another subcutaneous vein of the upper extremity was used. In 18 cases, the arterial recipient vessel was the radial artery; in 3 patients, the ulnar artery was used. In 13 cases, the arterial anastomosis was performed in an end-to-side-technique; in 8 cases, an end-to-end-anastomosis was carried out. The venous anastomosis was implemented in an end-to-end-technique in 19 cases; in 2 cases, an end-to-side-anastomosis was performed.
The mean operation time was 5 h, ranging from 209 to 540 min. The operation which lasted 540 min was done for coverage of a defect after a complex amputation injury of the right hand by a hydraulic machine, so that most of the operation time was needed for reconstruction and preparation of the recipient site.
The mean hospitalization time was 34 days, being prolonged if the complexity of the wounds/injuries was higher. The length of stay in hospital ranged from 15 to 60 days. Of the patients, 23% (5/21) had been treated up to 150 days in other facilities before the first admittance in our hospitals. Therefore, the time until the definitive microsurgical reconstruction was delayed for a median of 22 days. If the wound or injury was primarily treated by our hospital, the delay was 17.5 days after the occurrence of the defect. In our data, there was no significant correlation concerning postoperative complications with the delay between occurrence of the defect and operative treatment.
Within the first 24 h, there were no revisions of the anastomosis in our patients. In one case, a venous drainage problem of the flap occurred 72 h after the operation. The revision was successful. As a postoperative complication in one case, a diffuse postoperative bleeding was treated because of a coagulation disorder. In another case, a flap demonstrated an unclear swelling. In two cases, there were minor postoperative wound healing disorders which could be managed by conservative treatment. All lateral arm flaps could be transplanted successfully.
A primary closure of the donor site defect was possible in all cases. Wound healing disorders at the donor site did not occur. In one patient, a secondary operation for flap thinning was performed 1 month after the flap transplantation. None of the patients complained about donor site problems.
Discussion
Reconstruction of defects of the forearm and hand is technically demanding because very important functional structures are near to each other and sometimes have to be reconstructed as well. Chronic infections, wound healing disorders, or trauma often lead to complex defects, particularly when there are accompanying injuries. An adequate soft tissue coverage at the forearm and hand requires careful planning. The planned tissue transplantation has to provide a definitive closure of the defect, together with mechanical stability and pliability for tendon gliding and coverage of other important structures. In more complex situations with loss of different structures, the aim is to replace or reconstruct all structures in one step.
Subsequently, an ideal flap should be variable regarding its tissue complexity [16, 21, 25, 27, 29] and provide the option of being planned either as a fascia-only flap [3, 5, 8, 11, 26, 28] and osteocutaneous flap [7, 13, 15, 20, 23] including a tendon component as a tendon replacement or with the possibility of sensory nerval connection. The flap should be variable in its size and provide a good substitute tissue quality, e.g., equal skin thickness, hairiness, texture, and pigmentation, and an adequate overall thickness of the different components of the flap. For the practicability a constant vessel anatomy and a long pedicle are favorable. This may allow an easy, secure, and variable anastomosis at the recipient site. Finally, the functional and aesthetical impairment in the donor site should be minimized.
Anatomy of the Lateral Arm Flap and Surgical Technique
The blood supply of the lateral arm flap is provided by 3–5 septocutaneous perforator arteries in the lateral intermuscular septum of the upper arm, which are derived from the collateral radial posterior artery. This artery is a branch of the deep brachial artery and runs between triceps brachii muscle and brachial muscle distally along the humerus. Because of anastomoses with recurrent arteries of the periarticular plexus of the elbow joint, the lateral arm flap can be distally extended with blood supply far over the lateral epicondyle of the humerus [12, 21].
The operation is done in supine position with a sterile applied arm blood tourniquet filled with 300 mmHg. First, the skin and subcutaneous tissue are prepared on a virtual axis between the deltoid muscle apex and the lateral epicondyle of the humerus down to the level of muscle fascia. Starting from posterior, the fascia is dissected step by step from the muscle and prepared towards the intermuscular septum until the blood vessels are in sight. The same procedure is done from the ventral side.
When the flap is already mobile and connected to the underground only by interseptal vessels, the necessary length of the flap pedicle and the cutaneous nerve is prepared from proximally starting distally of the humeral insertion of the deltoid muscle. In some sections, the preparation area is along the way of the radial nerve. When the pedicle mobilization is adequate and the blood supply of the flap is satisfactory after opening the blood tourniquet, the flap can be harvested by ligation. If an osteocutaneous composite lateral arm flap is dissected, the periosteum is not stripped off the bone, but only incised, and an oscillating saw is used for corticotomy. The release of the bone segment is carefully finished with a sharp osteotome [23].
Donor Site Defect
Because the width of the lateral arm flap has a maximum of 8 cm, the donor site usually can be closed primarily. In our patients split skin grafting was not necessary in any case—irrespective of the donor defect width. The fact that no patient felt impaired by the donor site morbidity reflects the complexity of the surgical procedure in the patients mind and the primary closure of the donor site as well as the satisfaction with the overall result. Because of the donor site scar, Graham et al. [9] prefer the flap in male patients, but describe an excessive hair growth in the recipient site. In our patients, we could not endorse this finding.
The posterior antebrachial cutaneous nerve often cannot be preserved during preparation of the lateral arm flap. Therefore, in more than 50% of the patients, a hypaesthesia on the forearm skin remains [9]. Only a few patients consider this as an impairment [14]. There are also described transient move impairments after raising a composite flap with a vascularized humerus block. In the work of Graham et al. [9], in 19.4% of the cases, postoperative elbow pain is described. The radial nerve should be handled with care during flap dissection. Proximally, it runs together with the posterior collateral radial artery along the humerus and then passes through the space between the brachial muscle and the brachioradial muscle in the cubital fossa. Therefore, in few cases, a transient irritation of the radial nerve can occur, at worst leading to a transient radial nerve palsy. However, we did not see this in our series.
Advantages of the Lateral Arm Flap
The tissue quality of the lateral arm flap provides some clear advantages. The fascia serves as an ideal material to provide tendon gliding. Furthermore, the tissue is easier to enforce so that three-dimensional defects can be covered excellently. Chen and El-Gamm [3], Moser et al [21], and Atzei et al. [1] mentioned these advantages of the lateral arm flap especially for the reconstruction of defects on the hand and fingers. Another advantage is the possibility of performing the whole procedure without the need for general anesthesia, but in axillary plexus anesthesia. By ipsilateral raise of the free lateral arm flap, the donor defect is minimized because no other extremity is involved aesthetically and functionally. Also advantageous is the practicability concerning the operation management, which is often noted in literature. Because of the lateral position of the flap on the arm, there is no need for intra-operative repositioning of the patient. The mean operation time of 315 min with two teams described in our retrospective analysis is derived from the complexity of injuries. In some cases, there was a huge wound defect with the need for meticulous debridement and time-consuming preparation of the recipient vessels as well as reconstruction of different damaged anatomical structures.
Yousif et al. [32] carried out detailed studies of the blood supply of lateral arm flap on 25 cadavers. In all cases, the supplying artery was the posterior collateral radial artery. The mean diameter was 2 mm. On its constant course from the radial margin of the humerus through the lateral intermuscular septum between the brachial muscle and the brachioradial@@ muscle, it runs to the lateral condylus of humerus and provides several periostal, muscular, fascial, and cutaneous branches. In our series, we can approve that there is a reliable vessel anatomy of the lateral arm flap, which is often mentioned in literature [4, 12, 31].
The average length of the pedicle is 7.8 cm, as described by Yousif et al. The pedicle length in our patients had a maximum of 8 cm, complying with these findings. The pedicle length can be varied by the positioning of the skin island of the flap. By choosing a line of surgical dissection which is relatively distal, a longer pedicle length of up to 11 cm can be obtained. However, in general, the pedicle length of the lateral arm flap is shorter in comparison to the radial forearm or the anterolateral thigh flap.
Fasciocutaneous transplants often lead to a voluminous and aesthetically annoying tissue excess, particularly at the hand as a highly exposed body area. This excess of subcutaneous tissue has to be thinned in secondary operations, either by open surgery or by liposuction. Moser et al. [21] describe the necessity for flap thinning by a secondary procedure after transplantation of free lateral arm flap to the hand in half of the cases. Nevertheless, by this correction, a good functional result at a low complication rate could be obtained.
In our 21 series, only in one case, a secondary flap thinning was necessary.
The free lateral arm flap provides a good option for reconstruction of simple and complex multistructural defects of small to medium size at the hand and forearm. Besides the great variety of tissue components that are included, this flap provides a constant anatomy with a relatively long vascular pedicle. An intra-operative change of position mostly is not necessary, and the operation time can be lowered by working simultaneously with two teams. The rate of revision, complication, or flap loss is low. Secondary debulking procedures are usually not necessary on a frequent basis. A primary closure of the donor defect is worth aspiring to for an aesthetically satisfactory result.
As an alternative to the lateral arm flap, the anterior lateral thigh flap (ALT flap) is one of the most frequently used flaps in modern reconstructive surgery. Depending on patient habitus, the flap can be raised up to 7 × 30 cm. For closure of the donor site, additional split skin grafting is often necessary. The ALT flap allows an integration of tendon components by including vascularized parts of the fascia lata. Furthermore, the implementation of sensory function of the skin island is possible by microsurgical coaptation of the lateral cutaneous femoral nerve. The maximum pedicle length of up to 15 cm is one of the longest of all established free tissue flaps. The ALT flap—like the lateral arm flap—can be prepared in supine position. In young women, the donor defect scar can be hidden better than a scar on the lateral arm, but it is also aesthetically unpleasant.
Unlike the ALT flap, the radial forearm flap can include not only tendon transplants (e.g., long palmaris muscle or flexor carpi radialis muscle) or a sensory skin nerve (lateral antebrachial cutaneous nerve), but also a vascularized distal radial bone block in a composite flap. Like the lateral arm flap, it can be dissected also as a fascial flap. An advantage in comparison to the lateral arm flap is the vascular pedicle, which is much longer (up to 15 cm), and the caliber of the vessel is larger. It can be used as well as a flow-through flap. The disadvantages are the limited flap size (maximum 10 × 18 cm), the requirement of sacrificing a main artery for the hand, and the higher donor site morbidity. In particular, the high aesthetic impairment has to be noted because, in most cases, skin grafting is necessary.
The scapular flap can be risen with a size of up to 15 × 35 cm. The donor site morbidity is relatively low because it can be closed primarily under most circumstances. Combinations with other flaps from the subscapular graft system (dorsal latissimus muscle or anterior serratus muscle) or a vascularized bone graft from the scapula are reliable [19, 22]. The pedicle length is comparable with the ALT flap. A great disadvantage is the necessity of having the patient in a lateral position for flap dissection.
Fascial flaps are interesting alternatives to the lateral arm flap. The free serratus fascia flap is a favorable flap especially in reconstruction of soft tissue defects of the hand with exposure of tendons [6, 24]. A simultaneous split skin graft allows a defect coverage with a very thin flap in a one-step procedure. The temporo-parietal fascia flap also provides an optimal gliding tissue for tendons [2]. However, fascia flaps tend to secondary contraction, both when the flap harvest is preliminated and when defect coverage is done in a one-step operation with split skin grafting. A disadvantage of the serratus fascia flap in comparison to the lateral arm flap and the other flaps mentioned is the difficult postoperative monitoring. Because of misjudgment of the perfusion situation, the adequate time for revision may often be missed.
Raising the temporo-parietal fascia flap contains the risk of injuring the frontal branch of the facial nerve. Another disadvantage—especially in female patients—is the risk of a secondary alopecia areata.
Muscle flaps equally show a great variation in use for defect coverage in the hand and forearm. Especially, the free gracilis flaps can lead to good results [30]. The additional vascularization implied by the muscle tissue provides a better oxygenation of the wound ground and better immunological conditions and, respectively, better effect of a systemic antibiotical therapy [11].
On exposed tendons, the higher tendency to develop adherences can be a disadvantage of muscle flaps. Secondary tenolyses because of postoperative impairment of movement are sometimes necessary, especially if the wrist joint area is covered with the muscle flap. In this situation, contractures of the wrist joint may occur.
Because of the satisfying results in our series as well as in the literature, the free lateral arm flap has become a first choice option for coverage at the forearm and hand in small or medium size defect when a free flap may be necessary.
Conflict of interest
The authors declare that they have no conflicts of interest, commercial associations, or intent of financial gain regarding this research.
References
- 1.Atzei A, Pignatti M, Udali G, et al. The distal lateral arm flap for resurfacing of extensive defects of the digits. Microsurg. 2007;27:8–16. doi: 10.1002/micr.20308. [DOI] [PubMed] [Google Scholar]
- 2.Biswas G, Lohani I, Chari PS. The sandwich temporoparietal free fascial flap for tendon gliding. Plast Reconstr Surg. 2001;108:1639–1645. doi: 10.1097/00006534-200111000-00031. [DOI] [PubMed] [Google Scholar]
- 3.Chen HC, El-Gammal TA. The lateral arm fascial free flap for resurfacing of the hand and fingers. Plast Reconstr Surg. 1997;99:454–459. doi: 10.1097/00006534-199702000-00021. [DOI] [PubMed] [Google Scholar]
- 4.Chowdary RP, Murphy RX. Delayed debulking of free muscle flaps. Br J Plast Surg. 1992;45:38–41. doi: 10.1016/0007-1226(92)90113-C. [DOI] [PubMed] [Google Scholar]
- 5.Flügel A, Heitmann C, Kehrer A, et al. Coverage of soft-tissue defects of the hand with free fascial flaps. Microsurg. 2005;25:47–53. doi: 10.1002/micr.20070. [DOI] [PubMed] [Google Scholar]
- 6.Flügel A, Kehrer A, Heitmann C, et al. Defect coverage of the hand with the free serratus fascial flap. Handchir Mikrochir Plast Chir. 2005;37:186–192. doi: 10.1055/s-2005-837700. [DOI] [PubMed] [Google Scholar]
- 7.Gedebou TM, Wei FC, Lin CH. Clinical experience of 1284 free anterolateral thigh flaps. Handchir Mikrochir Plast Chir. 2002;34:239–244. doi: 10.1055/s-2002-36290. [DOI] [PubMed] [Google Scholar]
- 8.Gohla T, Kehrer A, Holle G, et al. Functional and aesthetic refinements of free flap coverage at the dorsum of the hand and distal forearm. Unfallchirurg. 2007;110:5–13. doi: 10.1007/s00113-006-1203-5. [DOI] [PubMed] [Google Scholar]
- 9.Graham B, Adkins P, Scheker LR. Complications and morbidity of the donor and recipient sites in 123 lateral arm flaps. J Hand Surg (Br.) 1992;17:189–192. doi: 10.1016/0266-7681(92)90086-h. [DOI] [PubMed] [Google Scholar]
- 10.Haas F, Rappl T, Koch H, et al. Free osteocutaneous lateral arm flap: anatomy and clinical applications. Microsurgery. 1992;23:87–95. doi: 10.1002/micr.10112. [DOI] [PubMed] [Google Scholar]
- 11.Hallock GG. The utility of both muscle and fascia flaps in severe upper extremity trauma. J Trauma. 2002;53:61–65. doi: 10.1097/00005373-200207000-00013. [DOI] [PubMed] [Google Scholar]
- 12.Harpf C, Papp C, Ninkovic M, et al. The lateral arm flap: review of 72 cases and technical refinements. J Reconstr Microsurg. 1998;14:39–48. doi: 10.1055/s-2007-1006900. [DOI] [PubMed] [Google Scholar]
- 13.Hennerbichler A, Etzer C, Gruber S, et al. Lateral arm flap: analysis of its anatomy and modification using a vascularized fragment of the distal humerus. Clin Anat. 2003;16:204–214. doi: 10.1002/ca.10140. [DOI] [PubMed] [Google Scholar]
- 14.Hoefert S, Hoffmann J, Zerfowski N et al. Funktionelle und ästhetische Entnahmemorbidität lateraler Oberarmlappen. Symposium der Arbeitsgemeinschaft für Kieferchirurgie 2005. www.ag-kiefer.de/symposium_Mai_2005/abstracts.htm
- 15.Hou SM, Lou TK. Vascularized tendon graft using lateral arm flap. 5 microsurgery cases. Acta Orthop Scand. 1993;64:373–376. doi: 10.3109/17453679308993648. [DOI] [PubMed] [Google Scholar]
- 16.Ito O, Igawa HH, Suzuki S, et al. Evaluation of the donor site in patients who underwent reconstruction with a free radial forearm flap. J Reconstr Microsurg. 2005;21:113–117. doi: 10.1055/s-2005-864844. [DOI] [PubMed] [Google Scholar]
- 17.Karamursel S, Bagdaty D, Markal N, et al. Versatility of the lateral arm free flap in various anatomic defect reconstructions. J Reconstr Microsurg. 2005;21:107–112. doi: 10.1055/s-2005-864843. [DOI] [PubMed] [Google Scholar]
- 18.Katsaros J, Schustermann M, Beppu M, et al. The lateral upper arm flap: anatomy and clinical applications. Ann Plast Surg. 1984;12:489–500. doi: 10.1097/00000637-198406000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Kremer T, Bickert B, Germann G, et al. Outcome assessment after reconstruction of complex defects of the forearm and hand with osteocutaneous free flaps. Plast Reconstr Surg. 2006;118:443–454. doi: 10.1097/01.prs.0000227742.66799.74. [DOI] [PubMed] [Google Scholar]
- 20.Kreutzer C, Sauerbier M. Plastic reconstruction on the lower limb. Z Orthop Unfall Up2date. 2010;5:97–124. doi: 10.1055/s-0029-1244016. [DOI] [Google Scholar]
- 21.Moser VL, Gohritz A, Schoonhoven J, et al. The free lateral arm flap in reconstructive hand surgery. Eur J Plast Surg. 2004;27:81–85. doi: 10.1007/s00238-004-0608-z. [DOI] [Google Scholar]
- 22.Sauerbier M, Erdmann D, Bickert B, et al. Defect coverage of the hand and forearm with a free scapula–parascapula flap. Handchir Mikrochir Plast Chir. 2001;33:20–25. doi: 10.1055/s-2001-12076. [DOI] [PubMed] [Google Scholar]
- 23.Sauerbier M, Giessler GA. The lateral arm flap for defect closure at the hand and wrist. Master techniques in orthopedic surgery series—soft tissue. 1st ed.. In: Cooney WP, Moran SL, editors. Lippincott Williams & Wilkins; 2009. p. 179–89
- 24.Sauerbier M, Ofer N, Germann G, et al. Microvascular reconstruction in burn and electrical burn injuries of the severely traumatized upper extremity. Plast Reconstr Surg. 2007;119:605–615. doi: 10.1097/01.prs.0000246512.47204.da. [DOI] [PubMed] [Google Scholar]
- 25.Song R, Song Y, Yu Y, et al. The upper arm free flap. Clin Plast Surg. 1982;9:27–35. [PubMed] [Google Scholar]
- 26.Sullivan MJ, Carrol WR, Kuriloff DB. Lateral arm free flap in head and neck reconstruction. Arch Otolaryngol Head Neck Surg. 1992;118:1095–1101. doi: 10.1001/archotol.1992.01880100087017. [DOI] [PubMed] [Google Scholar]
- 27.Summers AN, Matloub HS, Sanger JR. Salvage of ischemic digits using a lateral arm fascial flap. Plast Reconstr Surg. 2001;107:398–407. doi: 10.1097/00006534-200102000-00015. [DOI] [PubMed] [Google Scholar]
- 28.Ulusal BG, Lin YT, Ulusal AE, et al. Free lateral arm flap for 1-stage reconstruction of soft tissue and composite defects of the hand: a retrospective analysis of 118 cases. Ann Plast Surg. 2007;58:173–178. doi: 10.1097/01.sap.0000232832.18894.2b. [DOI] [PubMed] [Google Scholar]
- 29.Waterhouse N, Healy C. The versatility of the lateral arm flap. Br J Plast Surg. 1990;43:398–402. doi: 10.1016/0007-1226(90)90002-H. [DOI] [PubMed] [Google Scholar]
- 30.Wechselberger G, Schoeller T, Pülzl P, et al. Free tissue transplantation for defect coverage of the dorsum of the hand: aesthetic and functional aspects. Handchir Mikrochir Plast Chir. 2003;35:245–250. doi: 10.1055/s-2003-42135. [DOI] [PubMed] [Google Scholar]
- 31.Yildirim S, Taylan G, Eker G, et al. Free flap choice for soft tissue reconstruction of the severely damaged upper extremity. J Reconstr Microsurg. 2006;22:599–609. doi: 10.1055/s-2006-956234. [DOI] [PubMed] [Google Scholar]
- 32.Yousif NJ, Warren R, Matloub HS, et al. The lateral arm fascial free flap: its anatomy and use in reconstruction. Plast Reconstr Surg. 1990;86:1138–1145. doi: 10.1097/00006534-199012000-00016. [DOI] [PubMed] [Google Scholar]