Abstract
Objective
This study investigated the role of L-arginine supplementation to undernourished and Cryptosporidium parvum-infected suckling mice.
Methods
The following regimens were initiated on the 4th day of life and given subcutaneously daily: either 200mM of L-arginine or PBS for the C. parvum-infected controls. L-arginine-treated mice were grouped to receive either 20mM of NG-nitroarginine-methyl-ester (L-NAME) or PBS. Infected mice received orally 106 excysted-C. parvum oocysts on day 6 and were euthanized on day 14th at the infection peak.
Results
L-arginine improved weight gain compared to the untreated infected controls. L-NAME profoundly impaired body weight gain as compared to all other groups. Cryptosporidiosis was associated with ileal crypt hyperplasia, villus blunting, and inflammation. L-arginine improved mucosal histology following infection. L-NAME abrogated these arginine-induced improvements. Infected control mice showed an intense arginase expression, which was even greater with L-NAME. L-arginine reduced parasite burden, an effect that was reversed by L-NAME. C. parvum infection increased urine NO3-/NO2- concentration when compared to uninfected controls, which was increased by L-arginine supplementation, an effect that was also reversed by L-NAME.
Conclusion
These findings show a protective role of L-arginine during C. parvum infection in undernourished mice with involvement of arginase I and nitric oxide synthase enzymatic actions.
Keywords: Cryptosporidium parvum, diarrhea, malnutrition, arginine, growing mice
INTRODUCTION
Cryptosporidium parvum is an enteric protozoan spread by fecal-to-oral transmission that causes debilitating diarrhea in its host. C. parvum begins infection after intracellular oocytes release sporozoites that bind to intestinal epithelial cells and produce superficial parasitophorous vacuoles [1,2]. C. parvum is found worldwide, but is particularly common in developing areas where inadequate sanitation and water contamination are prevalent. In immune-compromised hosts such as those with HIV, this infection is potentially life-threatening. Mortality and risk of protracted C. parvum infection is worse in patients with uncontrolled AIDS [3,4]. In addition, undernourished children are at greater risk for acquiring and spreading this chlorine-resistant protozoan, which has been shown to endure and spread in the environment [5].
L-arginine is considered an essential amino acid in growing children whose anabolic protein requirement is continuously high [6]. In addition, arginine deficiency has been associated with severe undernutrition and with other catabolic states, like trauma and sepsis, and during heavy infection burdens [7]. L-arginine has a myriad of biological roles, since it is a substrate to two enzymatic systems, nitric oxide synthase (NOS) and arginase isoforms, which lead to either nitric oxide or to ornithine and polyamine synthesis, respectively. L-arginine is therefore a key constituent of complex immunological, inflammatory, and wound healing responses [8,9].
Recently, we have demonstrated that early post-natal malnutrition worsens C. parvum infection in C57BL6J mice in a model of maternal-offspring separation [10], somewhat analogous to premature weaning seen in Brazilian shantytown areas [11].
During suckling time, breast-milk-derived arginine is not sufficient to overcome the higher demand of this nutrient during bursts of growth [12,13]. In this current study, we investigate whether L-arginine supplementation improves intestinal adaptation following C. parvum challenge in undernourished suckling mice. We also explore the involvement of arginase and inducible nitric oxide synthase (iNOS; NOS2) in controlling the infection. Our findings support an arginine-based therapy to prevent the devastating and lasting effects of the vicious cycle of diarrhea and malnutrition in the developing world.
Materials and methods
Malnutrition protocol
The protocol described herein is in accordance with the Institutional Animal Care and Use Committee (IACUC) policies of the University of Virginia. Pregnant inbred mice (C57BL6J) were purchased from Charles River Lab. (Wilmington, MA) and monitored daily before delivery with free access to standard chow diet and water (12 h cycle/light and dark). After birth, litter size was adjusted to 6–8 pups. Their first day of life was recorded as post-natal day (D)1. Half of the offspring was separated from their lactating dam for increasing intervals starting on D4 of life, as described previously [14,15]; four hours at D4, eight hours at D5 and then twelve hours from D6 until D13. Separated pups were kept in an incubator box under controlled temperature (28 ± 2C). Severely underweight pups (< 1.8 g) at D4 were not included. Nourished controls stayed with their mothers. Litters were kept undisturbed during D1 to D4 to reduce stress and to assure full colostrum intake. Body weight was recorded daily until mice were euthanized. Care was taken to keep the same level of animal handling for all groups. The maternal-offspring separation model described here is analogous to the early weaning often seen in Brazilian shantytown children [11].
Experimental Groups
Data from nourished and undernourished mice are presented; the latter had shown approximately 10-fold heavier infections than their nourished sibilings given the same ammount [10]. We therefore expected that the effect of intervention with L-arginine in the undernourished groups would be greater and more quantifiable with more severe infections with malnutrition.
In this study, we have used the following six groups: uninfected nourished controls (N=11); uninfected undernourished controls (N=16); infected nourished controls (N=7); infected undernourished controls (N=11), infected undernourished treated with 200 mM of L-arginine (N=14), and infected undernourished treated with 200 mM of L-arginine and 20mM of NG-nitroarginine-methyl-ester (L-NAME) (N=7).
Treatment regimens were initiated on the 4th day of life. Based on a previous dose-response curve (data not shown), a dose of 200 mM of L-arginine was chosen for this study. L-NAME treatment was given immediately prior to L-arginine injection. Treatments were given subcutaneously in different dorsal areas of the animals daily starting D4 of life until D13, at volumes of 40 µl during D4–D10 and 80µl during D11 until D13. L-NAME treatment was given twice daily, each dose given at half of the volume administered for the single L-arginine treatment dose.
Cryptosporidium parvum infection
At D6, 1 hour prior to parasite inoculation, pups were separated from their lactating dams to empty their stomachs. Each pup received an oral inoculum of 106 excysted C. parvum oocysts in 10µl of PBS (pH 7.2). Oocysts were obtained from experimentally infected calves (Iowa isolate; Waterborne Inc., New Orleans, LA). Littermate controls were kept and handled separately and received PBS orally at the same time that the infected mice received their inoculation. Body weight was measured daily.
Tissue collection and histology
Animals were euthanized at D14, 8 days post-inoculum, at the peak of the infection, after being anesthetized with sodium pentobarbital (8 mg/100g i.p.), followed by cervical dislocation. After opening the abdominal cavity, approximately 1 cm of ileum (proximal to the ileocecal valve) was removed and prepared for histology (24 hr in 10% zinc formalin). Another 2 cm-segment, proximal to the first removed segment, was removed and milked free of stool to perform quantitative real-time PCR for cryptosporidial DNA (frozen and stored in −20°C). A final 1-cm ileal segment was harvested and prepared for immunoblotting, as described below (frozen immediately in liquid nitrogen and stored in −80°C).
Digital micrographs of intestinal histology were taken using a high resolution microscope and Photoshop CS software (BH-2, Olympus, Tokyo, Japan). In order to address mucosal villus surface and crypt hyperplasic response, villus height and crypt depth and area were measured using Image J software, at low magnification (100X), from ileum samples at day 14 (8 days post-inoculation), following calibration, as described elsewhere [16]. Recognizable longitudinal sections of crypts and villi were measured per ileum. In order to perform direct visualization of oocysts, and to confirm infectivity, higher magnifications were used (400X).
Immunoblotting
In brief, ileal segments (0.5 cm to the ileocecal valve) were harvested and immediately frozen in liquid nitrogen. Thawed specimens were pulverized in glass homogenizers, containing lysis buffer and then transferred to test tubes with protease inhibitor and centrifuged at 14 000 rpm. Supernatants were assayed using the bicinchoninic acid method, BCA Protein Assay Kit (Pierce, Rockford, IL) to standardize 50 µg of protein product. Samples were loaded into 15% denaturating polyacryamide gels (Biorad, Hercules, CA), and gels were transferred overnight and then blotted onto nitrocellulose membranes. Membranes were incubated with rabbit arginase I IgG antibody (at dilution of 1:500) for 2 hours and then rinsed 3 times in rinsing buffer then incubated in a horseradish peroxidase-conjugated secondary antibody (1:1000) and rinsed as described above. Each membrane was washed and exposed to Kodak X-Omat AR film (Kodak, Rochester, NY). Following stripping, blots were further incubated with GAPDH for densitometry normalization.
Immunohistochemistry
Four-micrometer sections were cut from each paraffin tissue block (n = 4 for each group). Immunohistochemical staining was performed using the avidin-biotin complex technique (VectaStain Kit, Vector Laboratories, Burlingame, CA), using the automated DakoCytomation System (Carpinteria, CA). The rabbit polyclonal antibody Arginase I (Santa Cruz,Biotechnology, Santa Cruz, CA) was used at 1:100 dilution after antigen retrieval. Briefly, sections of specimens and controls were incubated in a 0.01 mMol/L sodium citrate buffer (pH 6.0) in a pressure cooker at full steam pressure for 10 minutes. Positive control included normal human liver. Negative controls for each experiment omitted the primary antibody. Negative controls, which consisted of omission of the primary antiserum, were uniformly negative. Positive controls were used to confirm immunostaining (brown). Slides were counterstained with Harris’ hematoxylin (blue).
Quantitative real-time PCR
DNA from intestinal samples was extracted using a Qiagen Tissue kit (Valencia, CA). Quantification of the infection burden was done by comparing the curves with a known amount of DNA derived from a standard curve. Master mix was prepared by mixing 12.5µl of SYBR green mastermix (Biorad, Hercules, CA), 5.5 µl of nuclease free water (Fisher Scientific, Pittsburgh, PA) and 1.0 µl of both forward and reverse primers. The primers are specific for the 18s rRNA gene of the parasite (forward: 5’- TAG AGA TTG GAG GTT GTT CCT – 3’; reverse: 5’- CTC CAC CAA CTA AGA ACG GCC – 3’) (GenBank no. X64341). The total reaction mixture for each sample was 20µl, to which 5µl of the DNA sample was added. DNA amplification was previously described in detail elsewhere [17].
Nitrate and Nitrite Colorimetric Assay
A reliable measurement of total NO is the sum of both its oxidative metabolites, the nitrate and nitrite concentrations in urine since NO is scavenged rapidly (t1/2 = 4 seconds). The first step is the conversion of nitrate to nitrite utilizing nitrate reductase followed by the addition of the Griess reagent that converts nitrite into a deep purple azo compound. Photometric measurement of the absorbance due to this azo chromophore accurately determines NO2- concentration. Urine samples from anesthetized animals, prior to euthanasia, were collected by urinary bladder puncture using a 1-ml syringe and stored at −80°C until analysis. Samples were thawed and diluted ten times with the Assay Buffer solution for standard curve reading. Following dilution, 80µL of each sample were plated in triplicate, and 20µL total of an enzyme cofactor and nitrate reductase were added into each well. The plate was covered and incubated at room temperature for 1 hour. After incubation, Griess Reagent R1 was added and immediately followed by Griess Reagent R2. The plate was incubated for 10 minutes at room temperature and the absorbance was read at 550nm using an ELISA plate reader. All materials were purchased from Cayman Chemical (Ann Arbor, Michigan).
Statistical analyses
The data analysis and graphs were performed with GraphPad 4.01 for Windows. All statistical analyses were done from raw data using either 1-way analyses of variance with post-hoc Bonferroni’s comparison test or Student’s unpaired t-tests, as appropriate. A P-value < 0.05 was considered significant. Data are represented as either mean ± standard error for graphs or mean ± standard deviation in table 1.
Table 1.
Morphometry of the ileal villus and crypt from experimental mice at fourteen days old, eight days post-inoculum.
| Groups Variables |
Nourished control | N | Nourished infected |
N | Uninfected Control |
N | Infected control |
N | L-Arg (200 mM) |
N | L-NAME (20 mM) |
N |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Villus height (µm) | 193.69 ± 23.58# | 30 | 102.88 ± 16.38** | 30 | 147.5 ± 34.4# | 43 | 83.18 ± 13.21 | 23 | 92.0 ± 38.97** | 39 | 67.81 ± 15 | 19 |
| Crypt length (µm) | 29.70 ± 3.22Ψ | 30 | 55.296 ± 5.85δ | 30 | 25.27 ± 5.04Ψ | 43 | 74.72 ± 15.71 | 59 | 59.27 ± 12.58*** | 46 | 67.5 ± 20.57 | 42 |
p<0.05 vs all groups
p<0.05 vs infected control.
p<0.05 vs L-NAME (20mM)
p<0.05 vs nourished infected, infected control, L-Arg 200 and L-NAME groups
p<0.05 vs nourished control, uninfected control, infected control and L-NAME groups
Comparisons were done by Student Unpaired T test. Data are presented as Mean ± SD. N= number of measured ileal villi and crypts from H&E stained sections in at least four animals per group. Villi and crypts were only measured when their full longitudinal axis was found. Groups: Nourished uninfected control; nourished infected control; undernourished uninfected control; undernourished infected mice treated with L-arginine 200 mM; undernourished infected mice treated with L-arginine 200 mM and the NOS inhibitor NG-nitroarginine methyl ester (L-NAME), at the dose of 20 mM. Infection inoculum: 106 C. parvum oocysts.
RESULTS
Physical Growth
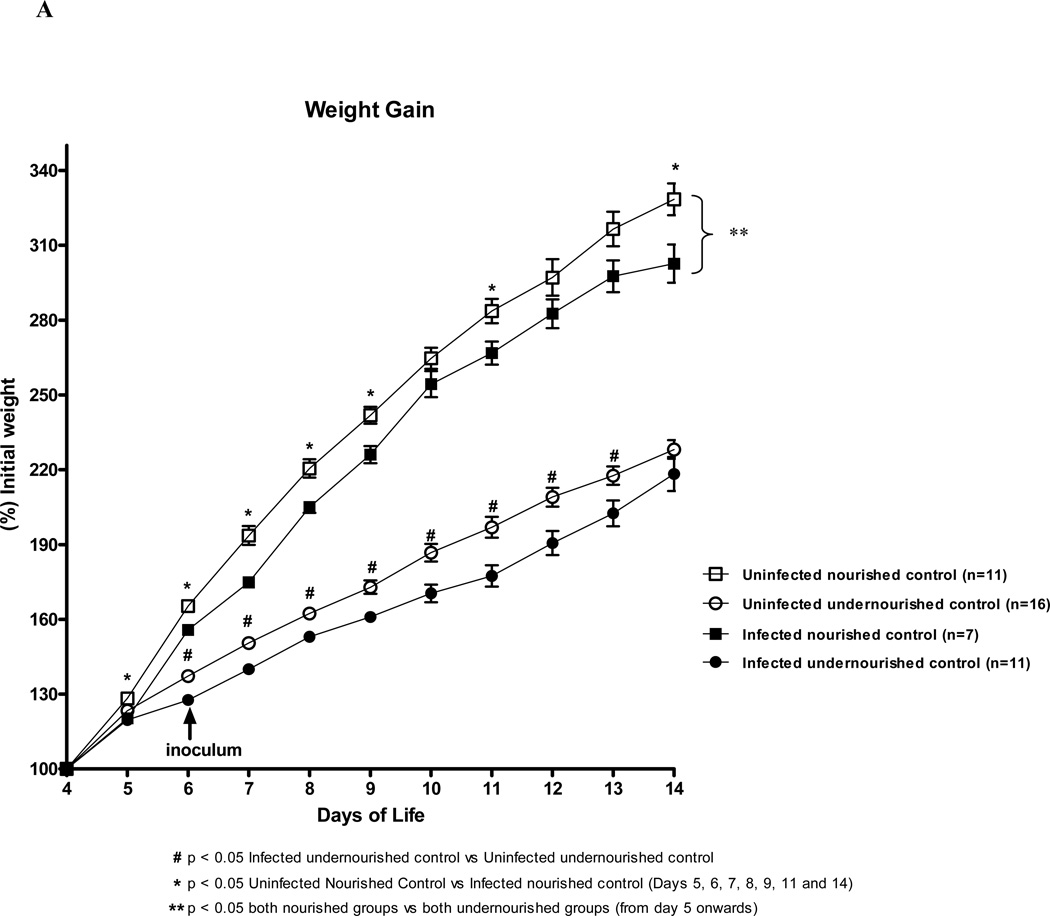
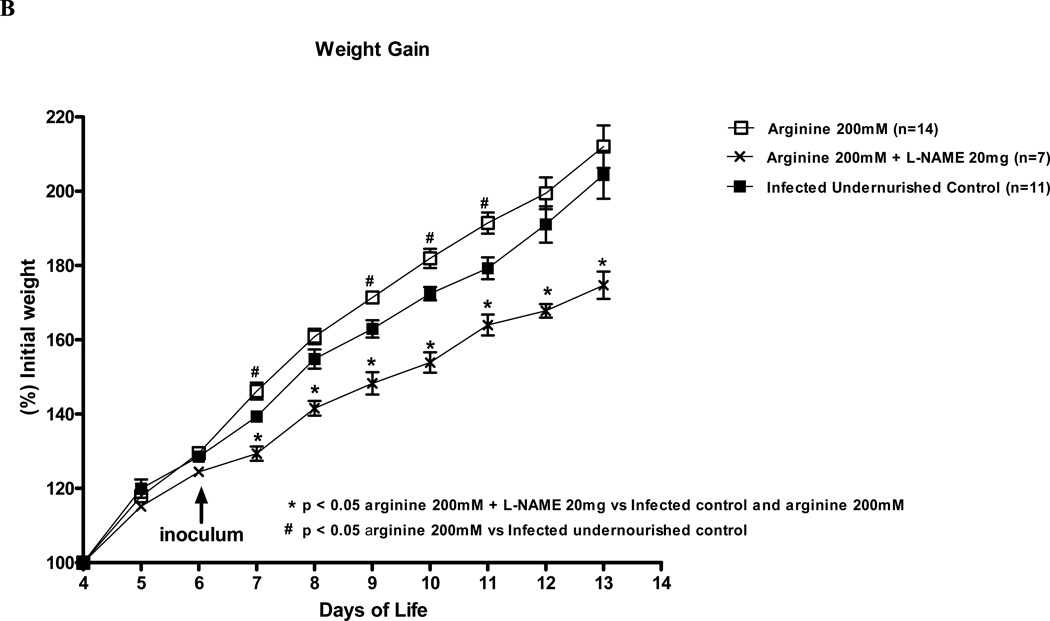
We analyzed the daily weight gain of the experimental mice over a 9 day-period. As expected, undernourished and C. parvum infected mice had significant reductions in the body weight gain during most of the experimental time course as compared with the uninfected undernourished control (Fig. 1A). In undernourished mice, treatment with 200mM of L-arginine significantly improved weight gain as soon as the first days post-inoculum, as compared to the untreated infected control, with growth advantages (Fig. 1B). L-NAME treatment (20mM) profoundly impaired the relative body weight gain over the same period, reducing weight gains by 30% at the end point, as compared to the infected PBS control, thus reversing the L-arginine protective effect against C. parvum. Most notably, L-NAME treatment (20mM) reduced weight gains from the first day of treatment and worsened during the post-infection period - decrements that were maintained until the end of the experimental course (Fig. 1).
FIGURE 1.
Weight gain curves of the experimental mice challenged by malnutrition from post-natal day 4 until day 13 (% initial weight). (A) Infected mice were inoculated with 106 C.parvum oocysts at post-natal day 6 by gavage with or without L-arginine treatment (200 mM) given subcutaneously. (B) A subset of 200 mM L-arginine treated and infected mice received the NOS inhibitor NG-nitroarginine methyl ester (L-NAME), 20 mM. Curves are presented as percentage of initial weight at day 4.
Histology
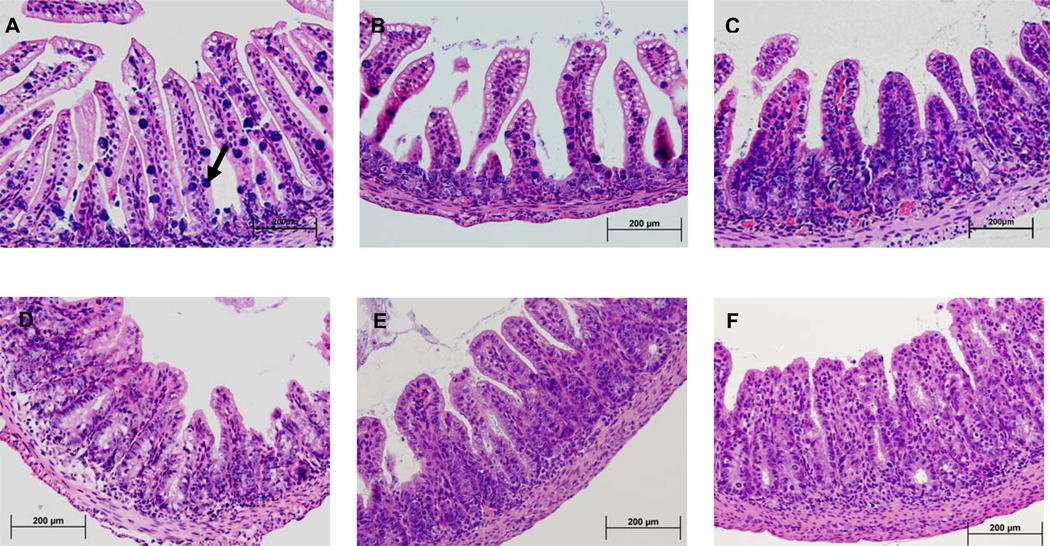
As previously described [15], the undernourished non-infected mice showed profound crypt derangement and shortening, with almost complete mitotic blunting. Rarely Paneth and goblet cells were found within the crypt. In addition, villi were thin and scattered (Fig. 2B) as compared to the nourished control (Fig 2A). Cryptosporidial infections were associated with crypt hyperplasia, villus blunting and inflammation in the lamina propria and submucosa (Fig. 2C–F). Oral arginine treatment (200mM) resulted in improved mucosal histology to the level of the uninfected undernourished control group (Fig 2E). We found significant improvements in villus height in L-arginine treated groups in the ileal mucosa (Table 1). L-NAME abrogated the histological improvements seen with L-arginine treatment. In addition, the L-NAME-treated group showed marked inflammatory infiltrates in the submucosa. The ileal histology from 200 mM L-arginine + L-NAME showed profound villus flattening, accompanied by surrounding crypt enlargement (Fig. 2F).
FIGURE 2.
Representative ileal histology of the experimental groups, H&E, 100X. (a) Nourished uninfected control; (b) undernourished uninfected control; (c) nourished infected control; (d) undernourished infected control; (e) undernourished infected mice treated with L-arginine 200 mM; (f) undernourished infected mice treated with L-arginine 200 mM and the NOS inhibitor NG-nitroarginine methyl ester (L-NAME), at the dose of 20 mM. Blue spots represent goblet cells scattered within villi. Note abundance of goblet cells in uninfected nourished controls (arrow) and cell number reductions in the ileum from C. parvum-infected mice. Marked villus atrophy, crypt hyperplasia, and inflammatory infiltrates are seen in C. parvum infected and undernourished mice. Note improvements with arginine treatment.
Immunoblotting and immunohistochemistry
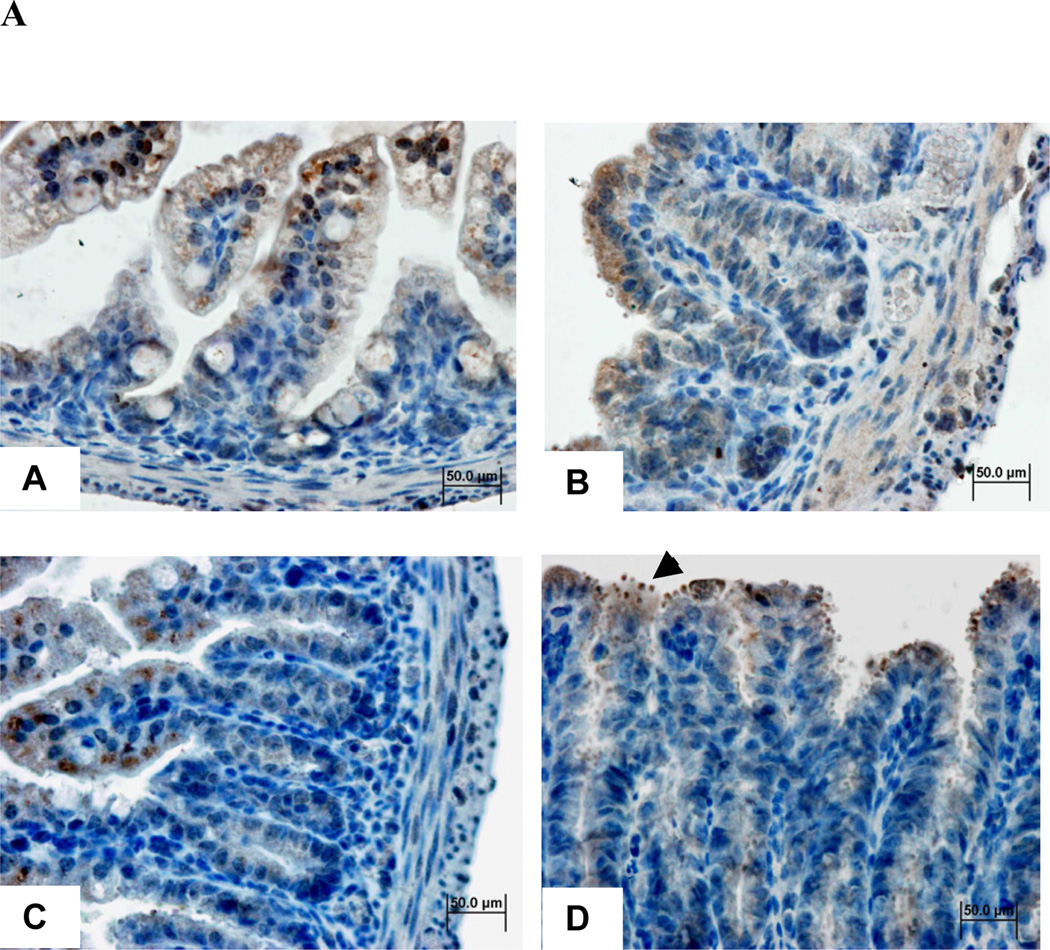
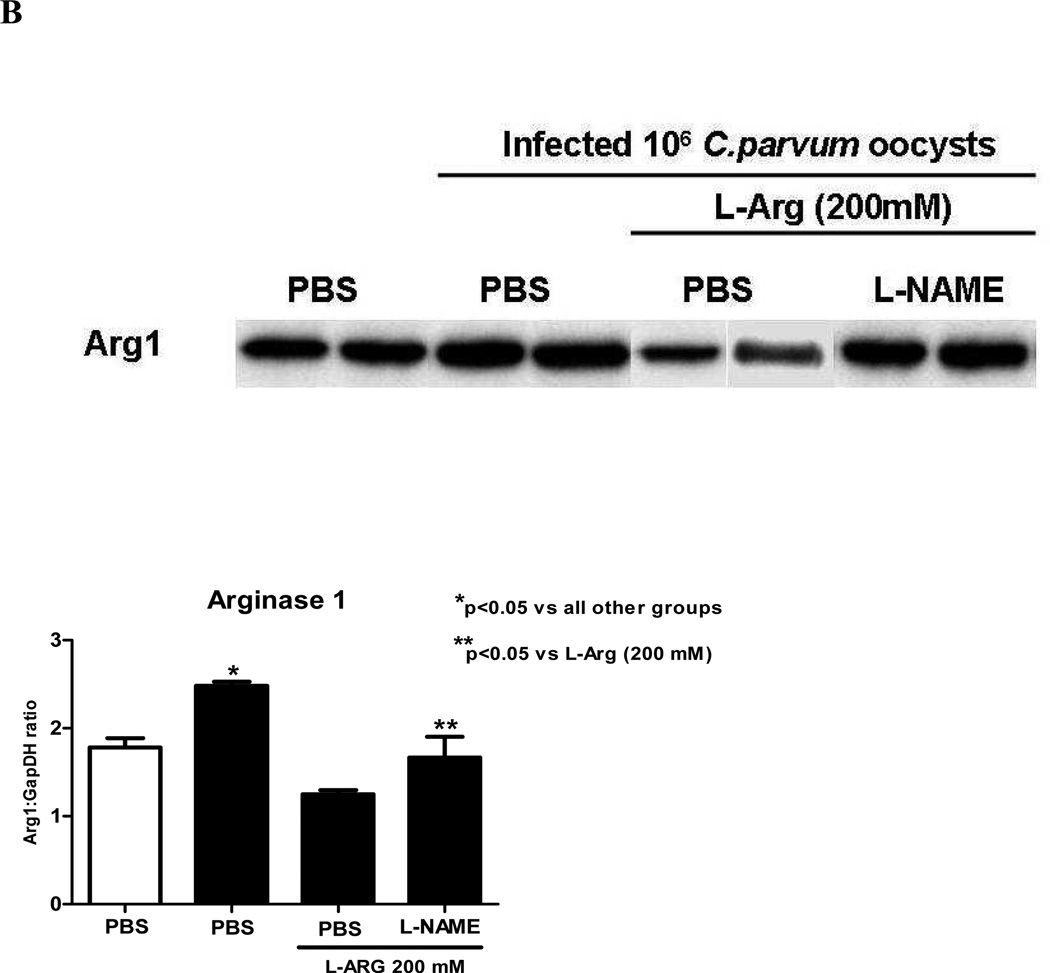
To assess the activity of arginine enzymatic pathway, we performed immuohistochemistry and western blots to determine ileal arginase I (ARG I) expression. Interestingly ARG1 immunostaining was found in the ileum of undernourished uninfected mice and among the ileum of infected mice, in the vicinity where C. parvum oocysts clustered, namely in the apical surface of infected enterocytes (Fig 3A). Additionally, the C. parvum parasites were seen in large numbers in the ileum of L-NAME-treated animals. ARG1 immunolabeling was markedly seen in lining epithelia, in the apical region of the enterocytes nearby C. parvum oocysts but the immunostaing was found weaker within crypts.
FIGURE 3.
(A) Ileal arginase I immunohistochemistry from experimental groups, 400X (a) Undernourished uninfected control, (b) undernourished infected control; (c) undernourished infected mice treated with L-arginine 200 mM; (d) undernourished infected mice treated with L-arginine 200 mM and the NOS inhibitor NG-nitroarginine methyl ester (L-NAME), at the dose of 20 mM; arrowhead indicates C.parvum oocysts. Brown color represents arginase 1 immunostaining. Counterstaining with Harris’ hematoxylin.
(B) Ileal immunoblots of the experimental groups for arginase I (ARG1). Band densitometry was plotted after normalizing for the housekeeping protein GADPH. Statistical analyses were done using Student T test. The results are shown mean ± SEM. *p< 0.05, significant increase versus uninfected control and **p<0.05, significant decrease versus infected control. N=4 for each group.
Unexpectedly, expression of ARG I protein was seen in the ileum of undernourished uninfected mice, as detected by western blotting. A marked increase of ileal ARG I expression was seen when C. parvum infection was present (Fig 3B). Undernourished mice treated with L-arginine showed reduced expression of ARG1, as compared to infected undernourished controls (p<0.05). This effect was reversed by L-NAME treatment (Fig 3B).
Intensity of cryptosporidial infection
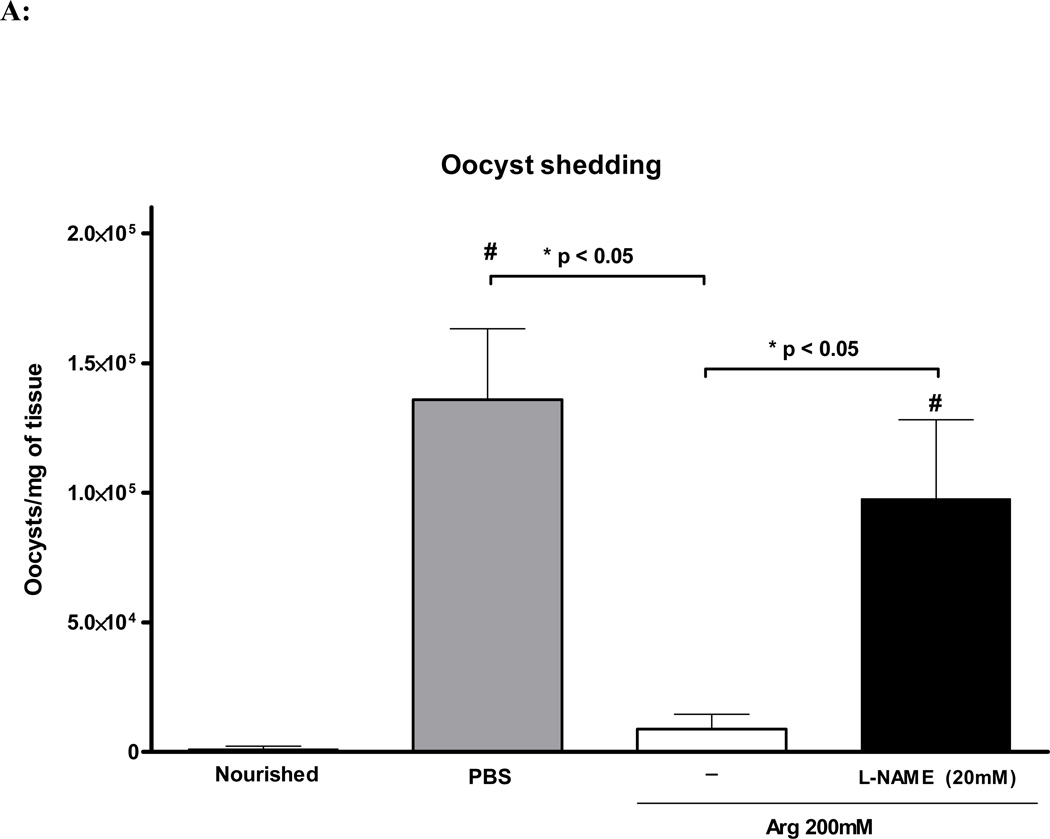
In order to better evaluate the anti-parasitic effect of L-arginine supplementation in C. parvum-infected undernourished mice, we determined the intensity of cryptosporidial infection in the ileum using Real Time-PCR. L-arginine treatment at dose of 200mM significantly reduced intensity of cryptosporidial infection per mg of ileal tissue (p<0.001), as opposed to the infected PBS control mice. The non-selective nitric oxide synthase inhibitor L-NAME (20mM) significantly reversed the effects of L-arginine (p<0.05), as depicted in Fig. 4A. Nourished infected mice showed much lower ileal oocyst shedding as opposed to the undernourished controls.
FIGURE 4.
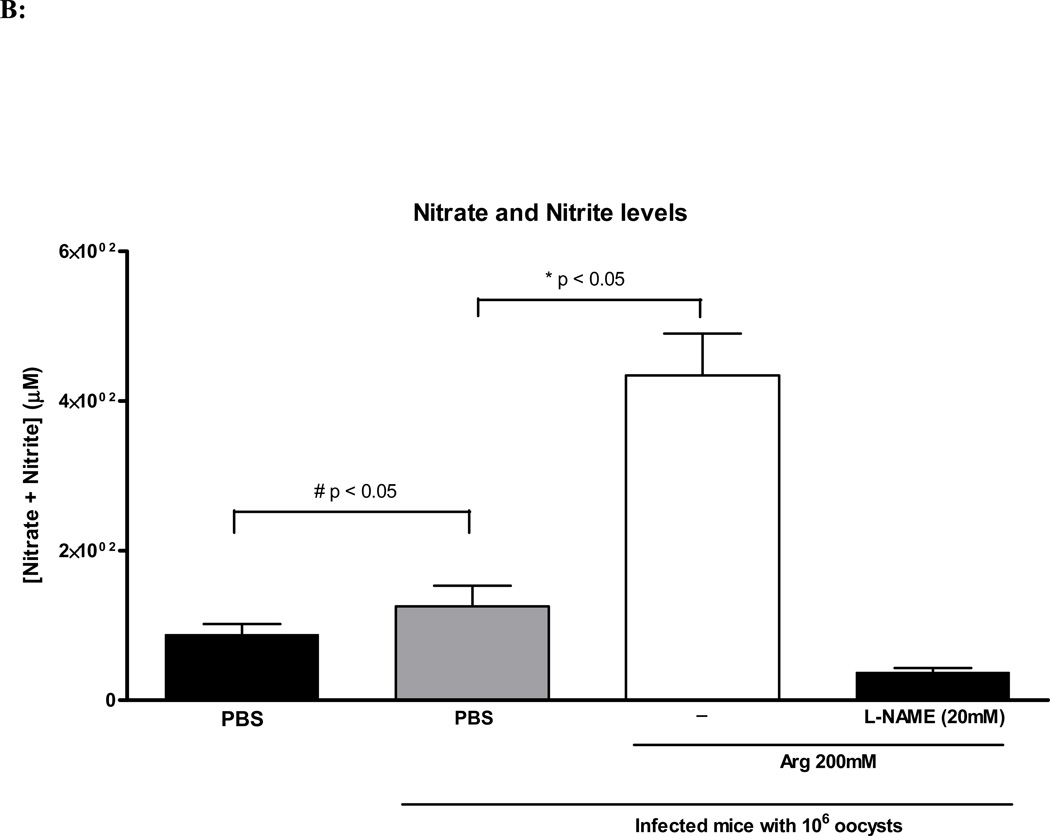
(A) Quantification of fecal shedding from the harvested ileal tissue by real-time PCR at day 14 of age (day 8 of infection). Nourished infected controls (N=5); undernourished infected controls (N=7); undernourished infected mice treated with L-arginine 200 mM (N=6); undernourished infected mice treated with L-arginine 200 mM and the NOS inhibitor NG-nitroarginine methyl ester (L-NAME), at the dose of 20 mM (N=5). Statistical analyses were done using Student T test. The results are shown mean ± SEM and represent the average oocyst shedding from each mg of ileal tissue. (B) Nitrate and Nitrite colorimetric assay for ELISA absorbance at 550nm using an ELISA plate reader. Undernourished uninfected controls (N=4); undernourished infected controls (N=6); undernourished infected mice treated with L-arginine 200 mM (N=6); undernourished infected mice treated with L-arginine 200 mM and the NOS inhibitor NG-nitroarginine methyl ester (L-NAME), at the dose of 20 mM (N=5). Statistical analyses were done using Student T test. The results are shown mean ± SEM. Meaningful statistical difference was set at P < 0.05.
Nitrate and Nitrite Quantification
In order to assess the production of nitric oxide synthase-derived nitric oxide, we measured its oxidative metabolites in the urine, nitrate (NO3−) and nitrite (NO2−). C. parvum infection significantly increased urine NO3- and NO2- concentration when compared to non-infected controls. 200mM L-arginine treatment significantly increased nitric oxide metabolites when compared to infected and non-infected controls. The combined treatment of 200mM of L-arginine and L-NAME (20 mM) reversed this observed effect (Fig. 4B).
DISCUSSION
Cryptosporidiosis is one of the most important etiologies of persistent and debilitating watery-diarrhea in children in the developing world and profoundly jeopardizes physical growth and cognitive performance [18,19,20]. Undernourished children living in crowded households without adequate sanitation are at particular risk of acquiring and spreading this enteric protozoan [5]. The vicious cycle between malnutrition and diarrhea creates a self-amplifying loop which may have a lasting impact on physical and cognitive development that may extend over an entire life-span and even through generations [2,21].
In an earlier study, we demonstrated that undernutrition caused by breastfeeding restriction during the suckling time in rodents could lead to severe stunting, poor weight gain [15] and much heavier cryptosporidial infections [10]. In the current study, we have evaluated the potential benefit of the conditional amino acid L-arginine during intestinal adaptations against C. parvum infection in suckling mice afflicted with early post-natal malnutrition. This malnutrition model is particularly valuable to dissect the components of the vicious cycle of infection during early post-natal development since L-arginine is not present in sufficient amounts in rodent (or human) breast-milk to provide full support to the rapid growth during the suckling time [22], a demand in which might be increased even more during a catabolic state like heavy cryptosporidiosis. Suckling mice have been shown to be susceptible to C. parvum infection in the ileum tissue [23,24] and malnutrition induced by maternal-offspring separation was found to determine profound and lasting effects on the intestinal barrier function [25]. Supplementation of L-arginine has been shown to enhance growth in suckling piglets [13] and growing arginase I overexpressing transgenic mice, which develop severe and selective arginine deficiency, have impaired immunity and lymphoid tissue formation [26].
Additionally, experimental cryptosporidiosis causes a significant reduction in amino acid absorption even after clearance of the parasite with concomitant up-regulation of intestinal peptide transporters [27].
Since intestinal Th1-directed pro-inflammatory cytokines are critical to eradicate C. parvum in the small intestine [28,29], the rationale for L-arginine supplementation (NOS substrate to generate nitric oxide, a potential inducer of IFN-γ and TNF-α) to control C. parvum infection includes its greater need at a time of its increased demand, as seen during catabolic states.
Our findings clearly demonstrate the beneficial role of L-arginine supplementation against C. parvum infection, as depicted by reduced ileal- C. parvum oocyst shedding as compared to controls and as shown by elsewhere studies in athymic nude mice [30] and neonatal piglets [31]. Gookin et al, showed the role of NO-mediated prostaglandin synthesis in maintaining intestinal barrier function (14). We found similar levels of NO metabolites in C. parvum-challenged mice at day 14, as shown by Leitch and He [32] emphasizing the importance of NO production in controlling infection. Further evidence comes from iNOS knock-out murine models and prolonged treatment with NO donor compounds, in which the rate of infection and intestinal epithelial integrity is significantly impacted and protected, respectively [32].
L-arginine is a substrate to two enzymatic pathways, arginase and NOS isoforms. Interestingly, arginase (ARG) is poorly expressed in the murine neonatal small intestine until day 18, when thereafter there is a peak of enzymatic activity in growing mice, corresponding to the intestinal adaptation to the weaning [33,34]. In fact, unlike adults, the neonatal small intestine is a main source of arginine synthesis for which demand is heavy during the anabolic state of the growth burst [35]. Thus, poor arginase expression in the neonatal small intestine further emphasizes the need of the amino acid arginine for protein synthesis during intestinal development. In our study, we demonstrated the expression of arginase I (ARG I) in the ileum of uninfected undernourished mice, suggesting that higher ARG I activity is required for the compensatory mechanisms accompanying the intestinal adaptation to malnutrition. Additionally, increased ileal ARG I expression was directly correlated with the inflammatory and infection states associated with ileal cryptosporidiosis in mice, especially following treatment with L-NAME. Although, clearly documented with a Th-2 anti-inflammatory response, ARG I was found to be up-regulated together with iNOS in the colon of Citrobacter rodentium [36].
Arginine is predominantly transported across the intestinal membrane via a Na+-independent CAT-1 (cationic transporter) system induced by mitogen-activated protein kinase [8,37]. Following the C. parvum-induced inflammatory response, the increased ARG I expression we observed may associate with ongoing mucosal healing since ARG I is co-localized with ornithine decarboxylase (ODC) in the cytosol. This is significant because ARG I may regulate the conversion of ornithine to polyamines [38,39], to enhance cell proliferation and mucosal repair during C. parvum infection. In addition, arginine-derived polyamines have been shown to support migration by a focal adhesion kinase mechanism in intestinal cell lines during monolayer wound healing, which may aid in repair of the intestinal epithelial barrier following its disruption by the infection [40].
Arginine supplementation in our model may provide sufficient amino acid substrate for both enzymes (iNOS and ARG 1) and thus efficiently enhancing the innate immune system to fight against the infection or alternatively improving ARG-I dependent RhoA activity, which has been associated in human inflammatory bowel disease (IBD) with submucosal tissues near microvessels [41].
Recently, it has been shown that apoptotic cells through TGF-β might induce anti-inflammatory responses and reduce NO synthesis via increased arginase (ARG) breakdown of arginine in these cells [42]. ARG has also been found to protect against mucosal damage in a murine model of colitis [36]. Therefore, L-arginine treatment might help to control the severe pro-inflammatory responses due to C. parvum infection and initiate earlier mucosal healing. This effect appears to be mediated in part by increased intestinal ARG activity.
Our findings further support the importance of NO-mediated responses against cryptosporidial infections and document for the first time the benefit of L-arginine treatment when malnutrition is present with C. parvum infection, and an arginase-dependent role in mucosal healing. Furthermore, we show that Cryptosporidium infection induces increased expression of arginase I in the ileum during suckling, and that this increase is localized around the apical membrane of the enterocytes near the site of parasite attachment, suggesting that C. parvum may induce higher enterocyte’s expression of ARG1 to limit the availability of arginine to the iNOS pathway as a survival mechanism. This feature was found in heavily infected ilea, as the enterocyte’s apical membrane infected with C. parvum oocysts is highly immunostained by arginase I within the ileal epithelia, suggesting that in addition to the scarcity of iNOS driven pro-inflammatory cytokines to kill the parasite due to inhibitory action of L-NAME, the C. parvum parasite may indirectly induce L-arginine breakdown, as noted above. Such survival advantage of increased C.parvum driven-arginine uptake by the ileal epithelium and by shifting arginine from iNOS toward the arginase pathway was recently described by Gookin et al. for arginase II [43], findings which warrant further studies to dissect their relative importance. Interestingly, we also found some immunolabeling within the parasite itself, which may represent antigenic cross-reactivity, since Cryptosporidium species do not express arginase I but rather arginine decarboxylase [44].
In addition, a marked elevation in arginase activity found with C. parvum infection in untreated undernourished mice may reduce NO synthesis in the ileal tissue by diverging arginine away from iNOS, thereby contributing to parasite survival. Similar findings were described by Gaur and colleagues in a model of murine cutaneous Leishmaniasis [45]. Furthermore, plasma arginase levels may increase after being released from challenged tissues driving systemic arginine deficiency [46], which may even be further compromised by undernutrition.
In summary, our findings support the concept of testing arginine-based therapy against cryptosporidiosis in growing children afflicted by infection and malnutrition, and suggest that arginine may provide a double benefit of both antiparasitic and mucosal restitution effects.
ACKNOWLEDGMENTS
This research was supported by NIH Cooperative Agreement U54 AI57168 for the Mid-Atlantic Regional Center for Excellence (MARCE) funded by the National Institute of Allergy and Infectious Diseases and by the Fogarty International Center Global Infectious Diseases Research Training (GIDRT) program at NIH, grant number D43 TW006578 and in part by NIH Research Grant #5R01HD053131, funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the NIH Office of Dietary Supplements (ODS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors’ contribution:
IBC, BBO, JWSS and BPC conducted most of the crypto infection handling of the experimental mice. JES and RLG contributed with the oocyst shedding PCR analyses. AAML, RLG and RBO contributed with the study design, study analysis and manuscript preparation.
References
- 1.Sasahara T, Maruyama H, Aoki M, Kikuno R, Sekiguchi T, Takahashi A, Satoh Y, Kitasato H, Takayama Y, Inoue M. Apoptosis of intestinal crypt epithelium after Cryptosporidium parvum infection. J Infect Chemother. 2003;9:278–281. doi: 10.1007/s10156-003-0259-1. [DOI] [PubMed] [Google Scholar]
- 2.Dillingham RA, Lima AA, Guerrant RL. Cryptosporidiosis: epidemiology and impact. Microbes Infect. 2002;4:1059–1066. doi: 10.1016/s1286-4579(02)01630-1. [DOI] [PubMed] [Google Scholar]
- 3.Eisenberg JN, Priest JW, Lammie PJ, Colford JM., Jr The Serologic response to Cryptosporidium in HIV-infected persons: implications for epidemiologic research. Emerg Infect Dis. 2001;7:1004–1009. doi: 10.3201/eid0706.010614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pozio E, Rezza G, Boschini A, Pezzotti P, Tamburrini A, Rossi P, Di FM, Smacchia C, Schiesari A, Gattei E, Zucconi R, Ballarini P. Clinical cryptosporidiosis and human immunodeficiency virus (HIV)-induced immunosuppression: findings from a longitudinal study of HIV-positive and HIV-negative former injection drug users. J Infect Dis. 1997;176:969–975. doi: 10.1086/516498. [DOI] [PubMed] [Google Scholar]
- 5.Newman RD, Sears CL, Moore SR, Nataro JP, Wuhib T, Agnew DA, Guerrant RL, Lima AA. Longitudinal study of Cryptosporidium infection in children in northeastern Brazil. J Infect Dis. 1999;180:167–175. doi: 10.1086/314820. [DOI] [PubMed] [Google Scholar]
- 6.Li P, Yin YL, Li D, Kim SW, Wu G. Amino acids and immune function. Br J Nutr. 2007;98:237–252. doi: 10.1017/S000711450769936X. [DOI] [PubMed] [Google Scholar]
- 7.Jahoor F, Badaloo A, Villalpando S, Reid M, Forrester T. Arginine flux and intravascular nitric oxide synthesis in severe childhood undernutrition. Am J Clin Nutr. 2007;86:1024–1031. doi: 10.1093/ajcn/86.4.1024. [DOI] [PubMed] [Google Scholar]
- 8.Pan M, Choudry HA, Epler MJ, Meng Q, Karinch A, Lin C, Souba W. Arginine transport in catabolic disease states. J Nutr. 2004;134:2826S–2829S. doi: 10.1093/jn/134.10.2826S. [DOI] [PubMed] [Google Scholar]
- 9.Ziegler TR, Evans ME, Fernandez-Estivariz C, Jones DP. Trophic and cytoprotective nutrition for intestinal adaptation, mucosal repair, and barrier function. Annu Rev Nutr. 2003;23:229–261. doi: 10.1146/annurev.nutr.23.011702.073036. [DOI] [PubMed] [Google Scholar]
- 10.Coutinho BP, Oria RB, Vieira CM, Sevilleja JE, Warren CA, Maciel JG, Thompson MR, Pinkerton RC, Lima AA, Guerrant RL. Cryptosporidium infection causes undernutrition and, conversely, weanling undernutrition intensifies infection. J Parasitol. 2008;94:1225–1232. doi: 10.1645/GE-1411.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lima AA, Guerrant RL. Persistent diarrhea in children: epidemiology, risk factors, pathophysiology, nutritional impact, and management. Epidemiol Rev. 1992;14:222–242. doi: 10.1093/oxfordjournals.epirev.a036088. [DOI] [PubMed] [Google Scholar]
- 12.Davis TA, Fiorotto ML, Reeds PJ. Amino acid compositions of body and milk protein change during the suckling period in rats. J Nutr. 1993;123:947–956. doi: 10.1093/jn/123.5.947. [DOI] [PubMed] [Google Scholar]
- 13.Kim SW, Wu G. Dietary arginine supplementation enhances the growth of milk-fed young pigs. J Nutr. 2004;134:625–630. doi: 10.1093/jn/134.3.625. [DOI] [PubMed] [Google Scholar]
- 14.Calikoglu A, Karayal A, D'Ercole A. Nutritional regulation of IGF-I expression during brain development in mice. Pediatr Res. 2001;49:197–202. doi: 10.1203/00006450-200102000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Oria RB, Vieira CMG, Pinkerton RC, De Castro-Costa CM, Lopes MB, Hussaini I, Shi W, Brito GAC, Lima AAM, Guerrant RL. Apolipoprotein E knockout mice have accentuated malnutrition with mucosal disruption and blunted insulin-like growth factor responses to refeeding. Nutrition Research. 2007;26:427–435. doi: 10.1016/j.nutres.2006.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carneiro-Filho BA, Oria RB, Wood RK, Brito GA, Fujii J, Obrig T, Lima AA, Guerrant RL. Alanyl-glutamine hastens morphologic recovery from 5-fluorouracil-induced mucositis in mice. Nutrition. 2004;20:934–941. doi: 10.1016/j.nut.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 17.Parr JB, Sevilleja JE, Samie A, Alcantara C, Stroup SE, Kohli A, Fayer R, Lima AA, Houpt ER, Guerrant RL. Detection and quantification of Cryptosporidium in HCT-8 cells and human fecal specimens using real-time polymerase chain reaction. Am J Trop Med Hyg. 2007;76:938–942. [PMC free article] [PubMed] [Google Scholar]
- 18.Tzipori S, Ward H. Cryptosporidiosis: biology, pathogenesis and disease. Microbes Infect. 2002;4:1047–1058. doi: 10.1016/s1286-4579(02)01629-5. [DOI] [PubMed] [Google Scholar]
- 19.Lorntz B, Soares AM, Moore SR, Pinkerton R, Gansneder B, Bovbjerg VE, Guyatt H, Lima AM, Guerrant RL. Early childhood diarrhea predicts impaired school performance. Pediatr Infect Dis J. 2006;25:513–520. doi: 10.1097/01.inf.0000219524.64448.90. [DOI] [PubMed] [Google Scholar]
- 20.Moore SR, Lorntz B, Lima AA, Guerrant RL. Risk factors for adverse outcomes in developing countries. Lancet. 2007;369:824–825. doi: 10.1016/S0140-6736(07)60405-X. [DOI] [PubMed] [Google Scholar]
- 21.Guerrant RL, Oria RB, Moore SR, Oria MO, Lima AA. Malnutrition as an enteric infectious disease with long-term effects on child development. Nutr Rev. 2008;66:487–505. doi: 10.1111/j.1753-4887.2008.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Jonge WJ, Kwikkers KL, te Velde AA, van Deventer SJ, Nolte MA, Mebius RE, Ruijter JM, Lamers MC, Lamers WH. Arginine deficiency affects early B cell maturation and lymphoid organ development in transgenic mice. J Clin Invest. 2002;110:1539–1548. doi: 10.1172/JCI16143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ernest JA, Blagburn BL, Lindsay DS, Current WL. Infection dynamics of Cryptosporidium parvum (Apicomplexa: Cryptosporiidae) in neonatal mice (Mus musculus) J Parasitol. 1986;72:796–798. [PubMed] [Google Scholar]
- 24.Armson A, Sargent K, MacDonald LM, Finn MP, Thompson RC, Reynoldson JA. A comparison of the effects of two dinitroanilines against Cryptosporidium parvum in vitro and in vivo in neonatal mice and rats. FEMS Immunol Med Microbiol. 1999;26:109–113. doi: 10.1111/j.1574-695X.1999.tb01377.x. [DOI] [PubMed] [Google Scholar]
- 25.Soderholm JD, Yates DA, Gareau MG, Yang PC, MacQueen G, Perdue MH. Neonatal maternal separation predisposes adult rats to colonic barrier dysfunction in response to mild stress. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1257–G1263. doi: 10.1152/ajpgi.00314.2002. [DOI] [PubMed] [Google Scholar]
- 26.de Jonge WJ, Hallemeesch MM, Kwikkers KL, Ruijter JM, de Gier-de VC, van Roon MA, Meijer AJ, Marescau B, de Deyn PP, Deutz NE, Lamers WH. Overexpression of arginase I in enterocytes of transgenic mice elicits a selective arginine deficiency and affects skin, muscle, and lymphoid development. Am J Clin Nutr. 2002;76:128–140. doi: 10.1093/ajcn/76.1.128. [DOI] [PubMed] [Google Scholar]
- 27.Barbot L, Windsor E, Rome S, Tricottet V, Reynes M, Topouchian A, Huneau JF, Gobert JG, Tome D, Kapel N. Intestinal peptide transporter PepT1 is over-expressed during acute cryptosporidiosis in suckling rats as a result of both malnutrition and experimental parasite infection. Parasitol Res. 2003;89:364–370. doi: 10.1007/s00436-002-0776-3. [DOI] [PubMed] [Google Scholar]
- 28.McDonald SA, O'Grady JE, Bajaj-Elliott M, Notley CA, Alexander J, Brombacher F, McDonald V. Protection against the early acute phase of Cryptosporidium parvum infection conferred by interleukin-4-induced expression of T helper 1 cytokines. J Infect Dis. 2004;190:1019–1025. doi: 10.1086/422761. [DOI] [PubMed] [Google Scholar]
- 29.Lacroix S, Mancassola R, Naciri M, Laurent F. Cryptosporidium parvum-specific mucosal immune response in C57BL/6 neonatal and gamma interferon-deficient mice: role of tumor necrosis factor alpha in protection. Infect Immun. 2001;69:1635–1642. doi: 10.1128/IAI.69.3.1635-1642.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leitch GJ, He Q. Arginine-derived nitric oxide reduces fecal oocyst shedding in nude mice infected with Cryptosporidium parvum. Infect Immun. 1994;62:5173–5176. doi: 10.1128/iai.62.11.5173-5176.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gookin JL, Duckett LL, Armstrong MU, Stauffer SH, Finnegan CP, Murtaugh MP, Argenzio RA. Nitric oxide synthase stimulates prostaglandin synthesis and barrier function in C. parvum-infected porcine ileum. Am J Physiol Gastrointest Liver Physiol. 2004;287:G571–G581. doi: 10.1152/ajpgi.00413.2003. [DOI] [PubMed] [Google Scholar]
- 32.Leitch GJ, He Q. Reactive nitrogen and oxygen species ameliorate experimental cryptosporidiosis in the neonatal BALB/c mouse model. Infect Immun. 1999;67:5885–5891. doi: 10.1128/iai.67.11.5885-5891.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Jonge WJ, Dingemanse MA, de Boer PA, Lamers WH, Moorman AF. Arginine-metabolizing enzymes in the developing rat small intestine. Pediatr Res. 1998;43:442–451. doi: 10.1203/00006450-199804000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Hurwitz R, Kretchmer N. Development of arginine-synthesizing enzymes in mouse intestine. Am J Physiol. 1986;251:G103–G110. doi: 10.1152/ajpgi.1986.251.1.G103. [DOI] [PubMed] [Google Scholar]
- 35.Bertolo RF, Brunton JA, Pencharz PB, Ball RO. Arginine, ornithine, and proline interconversion is dependent on small intestinal metabolism in neonatal pigs. Am J Physiol Endocrinol Metab. 2003;284:E915–E922. doi: 10.1152/ajpendo.00269.2002. [DOI] [PubMed] [Google Scholar]
- 36.Gobert AP, Cheng Y, Akhtar M, Mersey BD, Blumberg DR, Cross RK, Chaturvedi R, Drachenberg CB, Boucher JL, Hacker A, Casero RA, Jr, Wilson KT. Protective role of arginase in a mouse model of colitis. J Immunol. 2004;173:2109–2117. doi: 10.4049/jimmunol.173.3.2109. [DOI] [PubMed] [Google Scholar]
- 37.Pan M, Meng QH, Wolfgang CL, Lin CM, Karinch AM, Vary TC, Souba WW. Activation of intestinal arginine transport by protein kinase C is mediated by mitogen-activated protein kinases. J Gastrointest Surg. 2002;6:876–882. doi: 10.1016/s1091-255x(02)00052-5. [DOI] [PubMed] [Google Scholar]
- 38.Li H, Meininger CJ, Hawker JR, Jr, Haynes TE, Kepka-Lenhart D, Mistry SK, Morris SM, Jr, Wu G. Regulatory role of arginase I and II in nitric oxide, polyamine, and proline syntheses in endothelial cells. Am J Physiol Endocrinol Metab. 2001;280:E75–E82. doi: 10.1152/ajpendo.2001.280.1.E75. [DOI] [PubMed] [Google Scholar]
- 39.Popovic PJ, Zeh HJ, III, Ochoa JB. Arginine and immunity. J Nutr. 2007;137:1681S–1686S. doi: 10.1093/jn/137.6.1681S. [DOI] [PubMed] [Google Scholar]
- 40.Rhoads JM, Chen W, Gookin J, Wu GY, Fu Q, Blikslager AT, Rippe RA, Argenzio RA, Cance WG, Weaver EM, Romer LH. Arginine stimulates intestinal cell migration through a focal adhesion kinase dependent mechanism. Gut. 2004;53:514–522. doi: 10.1136/gut.2003.027540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horowitz S, Binion DG, Nelson VM, Kanaa Y, Javadi P, Lazarova Z, Andrekopoulos C, Kalyanaraman B, Otterson MF, Rafiee P. Increased arginase activity and endothelial dysfunction in human inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1323–G1336. doi: 10.1152/ajpgi.00499.2006. [DOI] [PubMed] [Google Scholar]
- 42.Freire-de-Lima CG, Xiao YQ, Gardai SJ, Bratton DL, Schiemann WP, Henson PM. Apoptotic cells, through transforming growth factor-beta, coordinately induce anti-inflammatory and suppress pro-inflammatory eicosanoid and NO synthesis in murine macrophages. J Biol Chem. 2006;281:38376–38384. doi: 10.1074/jbc.M605146200. [DOI] [PubMed] [Google Scholar]
- 43.Gookin JL, Stauffer SH, Stone MR. Induction of arginase II by intestinal epithelium promotes the uptake of L-arginine from the lumen of Cryptosporidium parvum-infected porcine ileum. J Pediatr Gastroenterol Nutr. 2008;47:417–427. doi: 10.1097/MPG.0b013e31816f6c02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keithly JS, Zhu G, Upton SJ, Woods KM, Martinez MP, Yarlett N. Polyamine biosynthesis in Cryptosporidium parvum and its implications for chemotherapy. Mol Biochem Parasitol. 1997;88:35–42. doi: 10.1016/s0166-6851(97)00063-7. [DOI] [PubMed] [Google Scholar]
- 45.Gaur U, Roberts SC, Dalvi RP, Corraliza I, Ullman B, Wilson ME. An effect of parasite-encoded arginase on the outcome of murine cutaneous leishmaniasis. J Immunol. 2007;179:8446–8453. doi: 10.4049/jimmunol.179.12.8446. [DOI] [PubMed] [Google Scholar]
- 46.Wu G, Bazer FW, Davis TA, Kim SW, Li P, Marc RJ, Carey SM, Smith SB, Spencer TE, Yin Y. Arginine metabolism and nutrition in growth, health and disease. Amino Acids. 2009;37:153–168. doi: 10.1007/s00726-008-0210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]