Abstract
Background
Periodic Leg Movements in Sleep (PLMS) are non-epileptiform, repetitive movements of the lower limbs that have been associated with apparent dopamine deficiency. We hypothesized that elderly patients with a disease characterized primarily by dopamine depletion (Parkinsonism) would have higher rates of PLMS than aged matched controls or a different neurodegenerative condition not primarily involving a hypodopaminergic state, Alzheimer’s Disease (AD).
Methods
We compared rates of PLMS derived from in-lab overnight polysomnography in patients with Parkinsonism (n = 79), AD (n = 28), and non-neurologically impaired, community-based controls (n = 187).
Results
Parkinsonian patients not receiving levo-dopa had significantly higher rates of PLMS than did Parkinsonian patients receiving levo-dopa, as well as higher rates than seen in AD and controls. Other medications did not appear to exert the pronounced effect of levo-dopa on PLMS in this Parkinsonian patient population. The symptom of leg kicking was reported more frequently in Parkinsonism, and it was associated with higher rates of PLMS. Caregiver reported leg kicking was unrelated to PLMS in AD.
Conclusions
Results are broadly compatible with a dopaminergic hypothesis for PLMS in Parkinsonism. The clinical significance of the negative findings in AD patient requires further investigation.
Keywords: Parkinsonism, Alzheimer’s Disease, Periodic Leg Movements in Sleep, Restless Legs Syndrome, Willis-Ekbom Disease
INTRODUCTION
Periodic Leg Movements in Sleep (PLMS) are repetitive, non-epileptiform, movements of the lower limbs that show marked, age-dependent prevalence [1] and appear to be at least partially under dopaminergic control. Evidence for the latter includes pharmacologic trials successfully decreasing the presence of PLMS with levo-dopa [2] or D2/D3 receptor agonists [3,4], as well as neuroimaging studies of such patients suggesting down regulation of flurodopa (18F-DOPA) binding in the putamen and/or caudate [5] and decreased single photon emission computed tomography (SPECT) D2 receptor binding throughout the striatum [6,7]. Not all data, however, suggest a primary deficiency of dopamine as the pathophysiologic basis for PLMS and its overlapping, but not identical, clinical condition, restless legs syndrome (RLS). For example, SPECT neuroimaging of the dopamine transporter (DAT) has not shown changes in patients with PLMS [8], and cerebrospinal fluid analyses for dopamine and metabolites in patients with PLMS/RLS have not shown consistent differences from controls [9, 10]. In this study we further examined the hypo-dopaminergic hypothesis by studying the prevalence of PLMS of a broad range of elderly Parkinsonian patients, as well as age-matched controls. We also included, as a positive control group, unmedicated patients with Alzheimer’s Disease, a patient population on which few data exist on PLMS prevalence.
METHODS
Patients
We performed overnight, in-laboratory polysomnography (PSG) from 79 (63 men, 16 women) patients with Parkinsonian syndromes (including 70 with idiopathic Parkinson’s Disease, 5 with possible multiple system atrophy (P type, N = 3; C-type N = 2) [11], 3 post-encephalitic Parkinsonism and 1 with drug-induced Parkinsonism), 28 patients (18 men, 10 women) with Alzheimer’s Disease (AD) and 187 (53 men, 134 women) elderly community dwelling controls without known neurodegenerative disease. These studies were conducted by grants from the United States National Institutes of Health (see Acknowledgements).
Parkinsonian patients met cardinal criteria for the condition, having at least 2 of the following: bradykinesia, rigidity, resting tremor and postural instability. They had been diagnosed 8.1 (SD = 6.9) years prior to study. Hoehn-Yahr ratings (1–5) [12] indicated a mean rating of 2.9 (SD = 1.1); 73 % of patients had at least moderate bilateral involvement (i.e., Hoehn-Yahr rating > 2). Medications used by the Parkinsonian patients receiving levo-dopa (n = 56) included: various dopamine agonists (n = 30), monoamine oxidase uptake inhibitors (n = 22), amantadine (n = 17), trihexyphenidyl (n = 9), anti-depressants including tricylic antidepressants and serotoninergic/noradrenergic reuptake inhibitors (n = 26), benzodiazepines (n = 11), and anti-psychotics with partial dopaminergic blockage (n = 5). Among the 56 patients receiving levo-dopa, the mean daily dose was 779.5 (SD = 506.0) mg (range 50–2650 mg). Patients receiving levo-dopa were more likely to have a Hoehn-Yahr rating > 2 (89.1% vs 31.8% chi-square = 25.99, p < .0001), but were not significantly older (t = 0.88, p = 0.38) than those not receiving levo-dopa. Of the 23 Parkinsonian patients not receiving levo-dopa, usage of various medications included small numbers of patients receiving dopamine agonists (n = 2), trihexyphenidyl (n = 1), monoamine oxidase uptake inhibitors (n = 2), benzodiazepines (n = 3), and anti-depressants (n = 6). Of the 30 patients receiving levo-dopa and a dopamine agonist, the combined mean levo-dopa dose equivalence [13] was 1203.0 (SD = 628.9) mg (range 375–2750 mg).
All AD patients met NINDS-consensus criteria for probable AD [14]. AD patients (mean age = 67.8 [SD = 8.7]) were all community dwelling and resided with caregivers at the time of study. They were moderately demented (mean [SD] Mini-Mental State Exam = 17.8 [6.8]) [15]; cognitive decline was estimated by caregivers as beginning 5.3 (SD =3.5) years before undergoing polysomnography. None had Parkinsonism, which together with fluctuations and hallucinations, would be suggestive of Lewy Body Dementia. AD patients were unmedicated with any psychotropic medications at the time of polysomnographic evaluation; none were receiving cholinesterase inhibitors.
Controls (mean age = 65.3 [SD = 8.2]) served as a community-based population for the study of sleep disordered breathing [16–18]; 17% used psychotropic medications on an intermittent basis, including 14 (7.5%) on anti-depressant medications, but, whenever possible, they were asked to suspend these prior to the lab night.
Table 1 indicates comparable age composition across groups. Patients and controls provided Informed Consent under IRB-approved protocols at Emory University and Stanford University. For AD patients, caregivers provided Informed Consent.
Table 1.
Polysomnography Across Groups
| Parkinsonism (on Levo- dopa) (n=56) |
Parkinsonism (not on Levo-dopa) (n=23) |
Alzheimer’s Disease (n=28) |
Controls (n=187) |
F |
p |
Scheffe Comparisons |
|||
|---|---|---|---|---|---|---|---|---|---|
| Agea | 63.9 (10.7) | 66.4 (12.1) | 67.6 (8.7) | 65.3 (8.2) | 1.12 | .34 | ---- | ||
| Total Sleep Timeb | 280.3 (97.2) | 287.2 (85.7) | 368.1 (106.7) | 367.2 (81.1) | 18.12 | .0001 | PL-DOPA < AD, CON PNO L-DOPA < AD, CON |
||
| Sleep Efficiencyc | 64.4 (19.8) | 64.5 (18.3) | 68.3 (15.7) | 74.5 (14.5) | 7.63 | .0001 | PL-DOPA, PNO L-DOPA < CON | ||
| REMd | 15.3 (12.1) | 14.6 (10.0) | 13.6 (5.9) | 18.0 (6.8) | 3.84 | .01 | none | ||
| Leg Kickinge | 3.0 (1.0) | 3.6 (1.0) | 2.1 (1.2) | 1.8 (0.9) | 24.34 | .0001 | AD, CON < PL-DOPA, PNO L-DOPA | ||
Footnotes: All values represent mean (SD).
in years.
in minutes
percentage of Time in Bed
percentage of Total Sleep Time
expressed on a 1 – 5 scale with 1 indicating “never” and 5 indicating “always;” (See text for further description of this variable)
Procedures
Conventional PSG, including electroencephalography, electroocculography, surface mentalis electromyography (EMG), single lead (II) electrocardiography, respiratory effort and airflow, pulse oximetry and two channels of bilateral anterior tibialis (AT) EMG were recorded. To maintain high reliability for scoring sleep stages in patients with neurodegenerative disease we scored only waking, NREM and REM sleep in the Parkinsonian and AD patients, as we have previously established high reliability for only those stages in such patients [19]. AT EMG recordings were scored for the presence of PLMS using conventional criteria for both movements and accompanying arousals [20, 21]. The total numbers of PLMS and PLMS with arousal were adjusted for a rate per hour to yield a PLMS Index (PLMSI) and a PLMS with Arousal Index (PLMSAI). We scored a movement as associated with an arousal only when the arousal occurred concurrently with a leg movement or within 2 seconds subsequent to that movement. Arousals occurring before the initiation of a leg movement were assumed to be related to other factors or represented spontaneous events and were not included in the tally of the PLMSAI.
PLMS are often associated with symptoms such as leg kicking during sleep. They also are often associated with restless legs syndrome (RLS). In this study, we did not select participants on the basis of the presence or absence of RLS, however, we employed a single questionnaire item inquiring how often (over the preceding 6 months) the individual experienced leg twitches or kicks during the night during sleep. This could be answered never (1), rarely (2), sometimes (3), most of the time (4), always (5) or left blank. Controls and Parkinsonian patients completed this question individually. Caregivers completed the question for the AD patients. For group comparisons on this item, we used the aforementioned scaled numeric responses for those individuals who responded. We also examined the association between responses on this item and PLMS by combining categories (never/rarely versus sometimes/most of the time/always) and relating these to a PLMSI value of 10 events/hr or higher.
RESULTS
Table 1 shows PSG results from the 4 groups and indicates that both Parkinsonian groups demonstrated lower Total Sleep Times (TSTs) and Sleep Efficiencies (SEs) relative to Controls. REM (expressed as a percentage of TST) was higher in Controls relative to all 3 patient groups, although specific comparisons did not exceed the Scheffe threshold. Comparisons of REM% between individuals using and not using anti-depressant medications among the Parkinsonian patients (32 of 79) and among the controls (14 of 187) were not statistically significant (t = .40, p = .69; t = .73, p = .47, respectively).
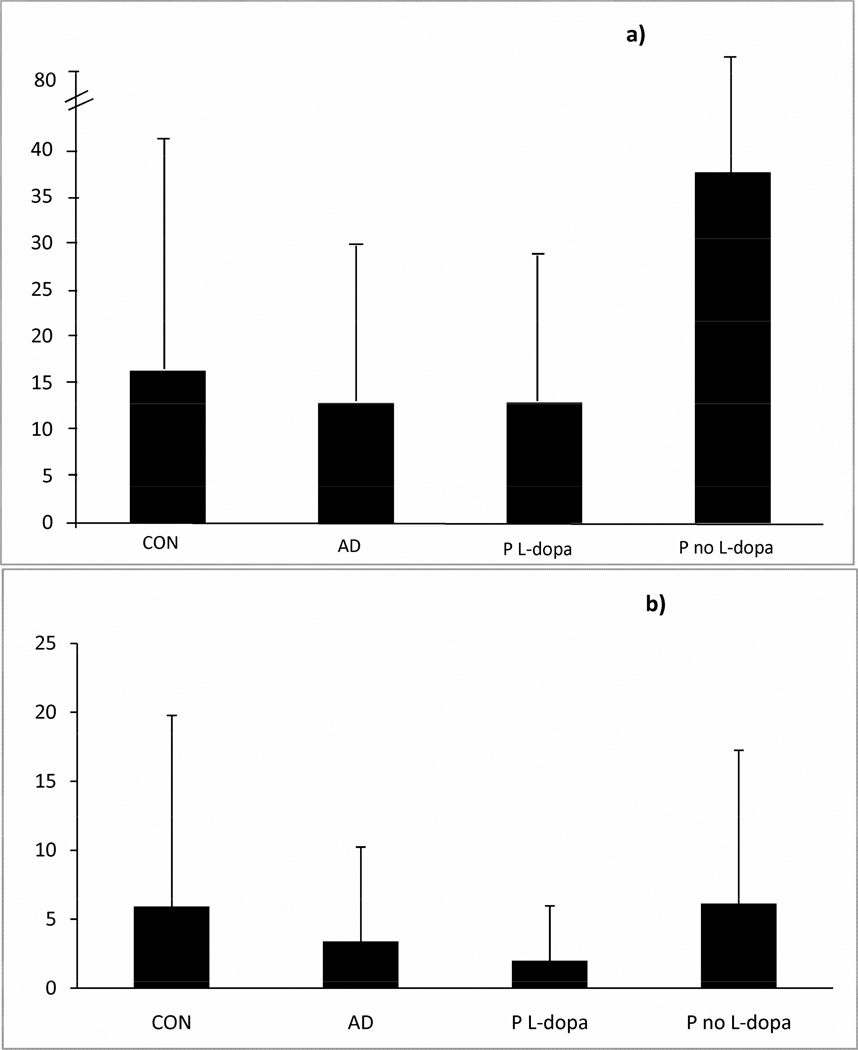
Figures 1a and 1b illustrate the PLMSI and PLMSAI (events/hr) across the 4 groups. There were no sex differences in PLMSI or PLMSAI in any patient group, although among Controls, men had higher PLMSI than women (25.4 [36.0] versus 12.7 [20.1], t = 2.43, p = < .02). Oneway Analyses of Variance indicated a significant main effect for PLMSI (F = 5.62, p = .0009) but not for PLMSAI (F = 1.83, p = .14) (for both: df 3, 290). Scheffe adjusted contrasts indicated that Parkinsonian patients unmedicated with levo-dopa showed significantly higher PLMSI levels than either of the three other groups. Among the 56 patients receiving L-dopa, dosage was related only marginally to PLMSI (rho = −.216, p = .109) and PLMSAI (rho = −.217 p = .108). Younger patients and patients with longer disease duration were more likely to be receiving higher doses (rho = −.414, p < .002 and rho = .539, p < .0001, respectively).
Figure 1.
Periodic Leg Movements in Sleep Across Four Groups. Figure 1a shows mean (SD) PLMS Index (movements per hour of sleep) on the y-axis for each group. Figure 1b shows mean (SD) PLMS Arousal Index (movements with arousal per hour of sleep) on the y-axis for each group. Oneway Analyses of Variance indicated a significant main effect for PLMSI but not PLMSAI (see text).
There were no significant differences in PLMSI or PLMSAI when comparing those using and not using any of the foregoing medication classes (i.e., agonists, monoamine oxidase B inhibitors, amantadine, trihexyphenidyl, benzodiazepines, atypical anti-psychotics), either within the 56 levo-dopa medicated Parkinsonian patients, or among those 23 individuals not receiving levo-dopa. There were also no differences in PLMSI or PLMSAI when comparing those individuals taking versus not taking anti-depressants. Patients not receiving levo-dopa had significantly shorter disease duration (2.9 [4.1] vs 10.3 [6.7] years, t = 5.92, p < .0001) than those receiving levo-dopa.
We examined further the possible effects of dopamine agonists on PLMS among those 30 Parkinsonian patients receiving both levo-dopa and dopamine agonists. In these analyses, we converted each patient’s dopamine agonist dose to its levo-dopa equivalent and then summed the total daily dose of each patient as a cumulative levo-dopa dose equivalent. The correlations between summed levo-dopa daily dose equivalent and PLMSI/PLMSAI and between levo-dopa dose alone and PLMSI/PLMSAI were all non-significant and did not differ in magnitude, suggesting that dopamine agonist use among these patients did not contribute a pharmacologic effect on the absence or presence of PLMS.
The symptom of leg kicking significantly differentiated the 4 groups (Table 1) with Scheffe comparisons indicating that both groups of Parkinsonian patients more likely to report this symptom than either Controls or the AD patients (via caregiver report). The 2 Parkinsonian groups did not differ on this symptom and therefore were combined to examine associations with PLMS. Parkinsonian patients reporting that they never or rarely experienced leg kicking during sleep were significantly less likely to demonstrate a PLMSI of ≥ 10 movements/hour than those reporting that they sometimes, often or always experienced this symptom (12.5 % vs 55.6 %, p = .047, Fisher’s exact test, 2-tailed). A similar pattern was seen in controls (33.8 % vs 54.6 %, p =.044, Fisher’s exact test, 2-tailed) but not in AD (Fisher’s exact test, 2-tailed, p = 1.00).
DISCUSSION
Our data indicate that Parkinsonian patients not receiving levo-dopa have higher rates of PLMS when compared aged Parkinsonian patients receiving levo-dopa, the latter not differing from AD patients or elderly control subjects. The Parkinsonian patients also showed a greater likelihood of symptomatic leg kicking than did AD patients or controls, however, we did not collect systematically any data from bedpartners or spouses of Parkinsonian patients. Such data would have provided an additional valuable source of data on behavioral abnormalities among those patients. Additionally, asking patients themselves questions regarding the sensory components of restless legs syndrome would have provided valuable adjunctive information. At least one previous study [22] suggested higher rates of PLMS in unmedicated Parkinson’s Disease patients relative to controls, though that study did not report severity of symptoms associated with PLMS. The mean PLMSI in our non-levo-dopa treated patients approached, but was somewhat lower, than that reported by Wetter et al [22] in unmedicated patients (37.6 vs 68.3 events per hour), although our controls were slightly higher (16.3 vs 10.6 events per hour) than theirs. These data thus are confirmatory of high rates of PLMS in Parkinsonism and are broadly compatible with either pre- and/or post-synaptic dopamine dysfunction underlying their occurrence [23]. We did not detect any evidence that usage of anti-depressant medication was associated with higher rates of occurrence PLMS (or lower REM%) in either the controls or the Parkinsonian patients, a finding well-documented in other studies of non-Parkinsonian patients, however this effect may not be uniform across various types of anti-depressants [24, 25]. Our study did not have sufficient power to examine the effects of different types of medications on PLMS within this drug class. We also did not see any effect of other medication classes on PLMS for Parkinsonian patients using levo-dopa. In addition to insufficient power to detect such effects, this could reflect the relatively advanced state of disease of these patients.
The absence of serotonergic influences on PLMS in this study was somewhat surprising given the known effects reported elsewhere [24, 25], although the mechanisms underlying the worsening of PLMS with serotonergic agonism are uncertain [26]. Impairments in iron metabolism have also been thought to represent a substrate for the presence of PLMS, based on diverse evidence such as circadian fluctuation in serum iron in early evening (coinciding with peak restless legs symptoms), lower brain iron in basal ganglia structures on brain MRI in RLS patients, and post-mortem findings of decreased staining for iron and ferritin (see [27] for review). Unfortunately, we did not have measurements of iron metabolism in this study with which to test this hypothesis.
We did not find that PLMS accompanied by arousal (PLMSAI) differentiated these groups, possibly reflecting the somewhat lower inter-rater reliability for scoring leg movements with arousal [28]. In fact, the lower levels of reliability in assessing brief arousals during sleep [29] have led some individuals to focus more specifically on autonomic variability accompanying PLMS as an indicator for arousal [30]. We did not have such data to explore this possibility here.
Interestingly, although some selective monoaminergic depletion in AD has been recognized for many years (e.g., loss of D2 receptors in caudate, putamen, amyglada and hippocampus) [31–33], even in the absence of extrapyramidal signs [34], and this depletion has been implicated in behavioral syndromes in AD [35–37], our AD patients did not demonstrate elevated rates of PLMS. It is possible that these particular AD patients, unlike our patients with Parkinsonism, simply did not incur as widespread and pervasive a dopaminergic deficit that predisposes for the presence of PLMS. Absence of a detectable association between PLMS and reported leg kicking in the AD patients also suggested reduced caregiver awareness of this symptom.
The summary statement from the 2002 National Institutes of Health Workshop on Restless Legs Syndrome [38] raised the possibility that RLS in dementia patients could be manifested as wandering and pacing, but remained ambiguous as to whether this was considered to be relevant to all forms of dementia or was limited to a particular form of dementia (e.g., AD, Vascular Dementia, Dementia with Lewy Bodies, Frontotemporal Dementia). Perhaps the most likely forms of dementia expected to demonstrate RLS (and perhaps, by inference, PLMS as well) would be the synucleinopathies [39], rather than the amyloidopathies, although empirical studies in support of this remain scant [40]. No studies to date have reported on prevalence of PLMS in well-characterized AD patients. Caregiver reports of RLS symptoms suggested greater likelihood in Dementia with Lewy Bodies relative to AD [41], but an earlier study suggested that the caregiver reported prevalence of leg kicking in AD patients over twice that of controls [42]. Evidence supporting an association between wandering in dementia and RLS and/or PLMS has been reviewed elsewhere and is largely speculative [1]. As one example of such evidence, neuroimaging studies have suggested reduced dopamine reuptake in the caudate and putamen among AD patients who wander relative to those who did not [43, 44]. Attempts to have mild-to-moderate AD patients respond to detailed questions regarding RLS symptoms proved unproductive [45], although behaviorally observed signs of RLS in AD [46] in the early evening hours were predictive of agitation on a standard rating scale [47]. Our data, using both PSG-recorded PLMS as well as a simple caregiver-based question about leg kicking in sleep, were unable to demonstrate that AD patients demonstrated increased risk for PLMS. Future studies, examining PLMS in patients with both amyloidopathies and synucleinopathies and relying on caregiver observations of wandering and pacing will be necessary to more fully understand PLMS, both with and without concomitant RLS, in dementia.
ACKNOWLEDGEMENTS
This work was supported by National Institutes of Health grants NS-050595 and NS-35345 to DLB
Footnotes
CONFLICTS OF INTEREST
The authors disclose the following Conflicts of Interest:
Bliwise (Consultant: Ferring)
Trotti (Consultant: UCB)
Yesavage (No disclosures)
Rye (Consultant: UCB, Merck)
REFERENCES
- 1.Bliwise DL. Restless legs syndrome and periodic limb movements in the elderly with and without dementia. In: Hening WA, Allen RP, Chokroverty S, Earley CJ, editors. Restless Legs Syndrome. Philadelphia: Elsevier/Saunders; 2009. pp. 178–184. [Google Scholar]
- 2.Benes H, Kurella B, Kummer J, Kazenwadel J, Selzer R, Kohnen R. Rapid onset of action of levodopa in restless legs syndrome: a double-blind, randomized, multicenter, crossover trial. Sleep. 1999;22:1073–1081. doi: 10.1093/sleep/22.8.1073. [DOI] [PubMed] [Google Scholar]
- 3.Allen R, Becker PM, Bogan R, Schmidt M, Kushida CA, Fry JM, et al. Ropinirole decreases periodic leg movements and improves sleep parameters in patients with restless legs syndrome. Sleep. 2004;27:907–914. doi: 10.1093/sleep/27.5.907. [DOI] [PubMed] [Google Scholar]
- 4.Partinen M, Hirvonen K, Jama L, Alakuijala A, Hublin C, Tamminen I, et al. Efficacy and safety of pramipexole in idiopathic restless legs syndrome: a polysomnographic dose-finding study—the PRELUDE study. Sleep Med. 2006;7:407–417. doi: 10.1016/j.sleep.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Routtinen HM, Partinen M, Hublin C, Bergman J, Haaparanta M, Solin O, et al. An FDOPA PET study in patients with periodic limb movement disorder and restless legs syndrome. Neurology. 2000;54:502–504. doi: 10.1212/wnl.54.2.502. [DOI] [PubMed] [Google Scholar]
- 6.Staedt J, Stoppe G, Kogler A, Munz D, Riemann H, Emrich D, et al. Dopamine D2 receptor alteration in patients with periodic leg movements in sleep (nocturnal myoclonus) J Neural Trans [Gen Sect] 1993;93:71–74. doi: 10.1007/BF01244940. [DOI] [PubMed] [Google Scholar]
- 7.Michaud M, Soucy JP, Chabli A, Lavigne G, Montplaisir J. SPECT imaging of striatal pre- and postsynaptic dopaminergic status in restless legs syndrome with periodic leg movements in sleep. J Neurol. 2002;249:164–170. doi: 10.1007/pl00007859. [DOI] [PubMed] [Google Scholar]
- 8.Eisensehr I, Wetter TC, Linke R, Noachtar S, von Lindeiner H, Gildehaus FJ, et al. Normal IPT and IBZM SPECT in drug-naïve and levodopa-treated idiopathic restless legs syndrome. Neurology. 2001;57:1307–1309. doi: 10.1212/wnl.57.7.1307. [DOI] [PubMed] [Google Scholar]
- 9.Earley CJ, Hyland K, Allen RP. CSF dopamine, serotonin, and biopterin metabolites in patients with restless legs syndrome. Mov Disord. 2001;16:144–149. doi: 10.1002/1531-8257(200101)16:1<144::aid-mds1009>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 10.Stiasny-Kolster K, Moller JC, Zschocke J, Bandmann O, Cassel W, Oertel WH, et al. Normal dopaminergic and serotonergic metabolites in cerebrospinal fluid and blood of restless legs syndrome patients. Mov Disord. 2004;19:192–196. doi: 10.1002/mds.10631. [DOI] [PubMed] [Google Scholar]
- 11.Gilman S, Low P, Quinn N, Albanese A, Ben-Shlomo Y, Fowler C, et al. Consensus statement on the diagnosis of multiple system atrophy. Clin Auton Res. 1998;8:359–362. doi: 10.1007/BF02309628. [DOI] [PubMed] [Google Scholar]
- 12.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 13.Hobson DE, Lang AE, Martin WRW, Razmy A, Rivest J, Fleming J. Excessive daytime sleepiness and sudden onset sleep in Parkinson’s disease: a survey by the Canadian Movement Disorders Group. JAMA. 2002;287:455–463. doi: 10.1001/jama.287.4.455. [DOI] [PubMed] [Google Scholar]
- 14.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadian EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 15.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State: A practical method for grading the cognitive state of patients for the clinician. J Psychiatry Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 16.Bliwise DL, Bliwise NG, Partinen M, Pursley AM, Dement WC. Sleep apnea and mortality in an aged cohort. Am J Public Health. 1988;78:544–547. doi: 10.2105/ajph.78.5.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bliwise DL. Sleep and aging. In: Pressman MR, Orr WC, editors. Understanding Sleep: The Evaluation and Treatment of Sleep Disorders. Washington, DC: American Psychological Association; 1997. pp. 441–464. [Google Scholar]
- 18.Bliwise DL. Epidemiology of age-dependence of sleep disordered breathing in old age. Sleep Medicine Clinics. 2009;4:57–64. doi: 10.1016/j.jsmc.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bliwise DL, Williams ML, Irbe D, Ansari FP, Rye DB. Inter-rater reliability for identification of REM sleep in Parkinson’s Disease. Sleep. 2000;23:671–676. [PubMed] [Google Scholar]
- 20.American Sleep Disorders Association. EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–184. [PubMed] [Google Scholar]
- 21.American Sleep Disorders Association. Atlas and scoring rules. Sleep. 1993;16:748–759. [Google Scholar]
- 22.Wetter T, Collado-Seidel V, Pollmacher T, Yassouridis A, Trenkwalder C. Sleep and periodic leg movement patterns in drug-free patients with Parkinson’s disease and multiple system atrophy. Sleep. 2000;23:361–367. [PubMed] [Google Scholar]
- 23.Freeman AAH, Rye DB. The brain’s dopamine systems and their relevance to restless legs syndrome. In: Hening WA, Allen RP, Chokroverty S, Earley CJ, editors. Restless Legs Syndrome. Philadelphia: Elsevier/Saunders; 2009. pp. 69–77. [Google Scholar]
- 24.Rottach KG, Schaner BM, Kirch MH, Zivotofsky AZ, Teufel LM, Gallwitz T, et al. Restless legs syndrome as side effect of second generation antidepressants. J Psychiatr Res. 2008;43:70–75. doi: 10.1016/j.jpsychires.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 25.Yang C, White DP, Winkelman JW. Anti-depressants and periodic leg movements of sleep. Biol Psychiatry. 2005;58:510–514. doi: 10.1016/j.biopsych.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 26.Buchbuhrer MJ, Gunzel J. Nonpharmacologic considerations and treatment of restless legs syndrome. In: Hening WA, Allen RP, Chokroverty S, Earley CJ, editors. Restless Legs Syndrome. Philadelphia: Elsevier/Saunders; 2009. pp. 228–237. [Google Scholar]
- 27.Trotti LM, Rye DB. Restless legs syndrome. Handb Clin Neurol. 2011;100:661–673. doi: 10.1016/B978-0-444-52014-2.00047-1. [DOI] [PubMed] [Google Scholar]
- 28.Bliwise DL, Keenan S, Burnburg D, Mattice C, Minkley P, Pursley A, et al. Inter-rater reliability for scoring periodic leg movements in sleep. Sleep. 1991;14:249–251. doi: 10.1093/sleep/14.3.249. [DOI] [PubMed] [Google Scholar]
- 29.Whitney CW, Gottlieb DJ, Redline S, Norman RG, Didge RR, Shahar E, et al. Reliability of scoring respiratory disturbance indices and sleep staging. Sleep. 1998;21:749–757. doi: 10.1093/sleep/21.7.749. [DOI] [PubMed] [Google Scholar]
- 30.Winkelman JW. The evoked heart rate response to periodic leg movements of sleep. Sleep. 1999;22:575–580. doi: 10.1093/sleep/22.5.575. [DOI] [PubMed] [Google Scholar]
- 31.Joyce JN, Myers AJ, Gurevich E. Dopamine D2 receptor bands in normal human temporal cortex are absent in Alzheimer’s disease. Brain Res. 1998;784:7–17. doi: 10.1016/s0006-8993(97)01005-6. [DOI] [PubMed] [Google Scholar]
- 32.Ryoo HL, Joyce JN. Loss of dopamine D2 receptors varies along the rostrocaudal axis of the hippocampal complex in Alzheimer’s disease. J Comp Neurol. 1994;348:94–110. doi: 10.1002/cne.903480105. [DOI] [PubMed] [Google Scholar]
- 33.Kemppainen N, Ruottinen H, Nagren K, Rinne JO. PET shows that striatal dopamine D1 and D2 receptors are differentially affected in AD. Neurology. 2000;55:205–209. doi: 10.1212/wnl.55.2.205. [DOI] [PubMed] [Google Scholar]
- 34.Pizzolato G, Chierichetti F, Fabbri M, Cagnin A, Dam M, Ferlin G, et al. Reduced striatal dopamine receptors in Alzheimer’s disease: single photon emission tomography study with the D2 tracer [123I]-IBZM. Neurology. 1996;47:1065–1068. doi: 10.1212/wnl.47.4.1065. [DOI] [PubMed] [Google Scholar]
- 35.Reeves S, Brown R, Howard R, Grasby P. Increased striatal dopamine (D2/D3) receptor availability and delusions in Alzheimer’s disease. Neurology. 2009;72:528–534. doi: 10.1212/01.wnl.0000341932.21961.f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pritchard AL, Pritchard CW, Bentham P, Lendon CL. Investigation of the role of the dopamine transporter in susceptibility to behavioural and psychological symptoms of patients with probable Alzheimer’s disease. Dement Geriatr Cogn Disord. 2008;26:257–260. doi: 10.1159/000160958. [DOI] [PubMed] [Google Scholar]
- 37.Sweet RA, Hamilton RL, Healy MT, Wisniewski SR, Henteleff R, Pollock BG, et al. Alterations of striatal dopamine receptor binding in Alzheimer disease are associated with Lewy body pathology and antemortem psychosis. Arch Neurol. 2001;58:466–472. doi: 10.1001/archneur.58.3.466. [DOI] [PubMed] [Google Scholar]
- 38.Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisir J, et al. Restless legs syndrome: diagnostic criteria, special considerations and epidemiology. A report from the Restless Legs Syndrome Diagnosis and Epidemiology Workshop at the National Institutes of Health. Sleep Med. 2003;4:101–119. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 39.Boeve BF, Silber MH, Saper CB, Ferman TJ, Dickson DW, Parisi JE, et al. Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease. Brain. 2007;130:2770–2788. doi: 10.1093/brain/awm056. [DOI] [PubMed] [Google Scholar]
- 40.Iranzo A, Comella CL, Santamaria J, Oertel W. Restless legs syndrome in Parkinson’s disease and other neurodegenerative diseases of the central nervous system. Mov Disord. 2007;22(Suppl):S424–S430. doi: 10.1002/mds.21600. [DOI] [PubMed] [Google Scholar]
- 41.Rongve A, Boeve BF, Aarsland D. Frequency and correlates of caregiver-reported sleep disturbances in a sample of persons with early dementia. J Am Geriatr Soc. 2010;58:480–486. doi: 10.1111/j.1532-5415.2010.02733.x. [DOI] [PubMed] [Google Scholar]
- 42.Tractenberg RE, Singer CM, Kaye JA. Symptoms of sleep disturbance in persons with Alzheimer’s disease and normal elderly. J Sleep Res. 2005;14:177–185. doi: 10.1111/j.1365-2869.2005.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanaka Y, Meguro K, Yamaguchi S, Ishii H, Watanuki S, Funaki Y, et al. Decreased striatal D2 receptor density associated with severe behavioral abnormality in Alzheimer’s disease. Ann Nucl Med. 2003;17:567–573. doi: 10.1007/BF03006670. [DOI] [PubMed] [Google Scholar]
- 44.Meguro K, Yamaguchi S, Itoh M, Fujiwara T, Yamadori A. Striatal dopamine metabolism correlated with frontotemporal glucose utilization in Alzheimer’ disease: a double-tracer PET study. Neurology. 1997;49:941–945. doi: 10.1212/wnl.49.4.941. [DOI] [PubMed] [Google Scholar]
- 45.Richards K, Shue VM, Beck CK, Lambert CW, Bliwise DL. Restless legs syndrome risk factors, behaviors and diagnoses in persons with elderly to moderate dementia and sleep disturbance. Beh Sleep Med. 2010;8:48–61. doi: 10.1080/15402000903425769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rose KM, Beck C, Tsai P-F, Liem PH, Davila DG, Kleban M, et al. Sleep disturbances and nocturnal agitation behaviors in older adults with dementia. Sleep. 2011;34:779–786. doi: 10.5665/SLEEP.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cohen-Mansfield J. Agitated behaviors in the elderly. II Preliminary results in the cognitively deteriorated. J Am Geriatr Soc. 1986;34:722–727. doi: 10.1111/j.1532-5415.1986.tb04303.x. [DOI] [PubMed] [Google Scholar]