I. What is a neural crest cell?
The neural crest is an embryonic cell type that is unique to vertebrates and forms numerous, diverse derivatives. For example, these cells give rise to critical components of the craniofacial skeleton, such as the jaws and skull, as well as melanocytes and ganglia of the peripheral nervous system. Interestingly, non-vertebrate chordates and invertebrates have many differentiated cell types that, in vertebrates, are derived from the neural crest (e.g. melanocytes, sensory neurons, and even cartilage). Whereas these derivatives arise from endomesoderm in invertebrates, they arise in vertebrates either solely from the neural crest, as is the case for melanocytes, or from both neural crest and other cell types. The latter is true for cranial sensory neurons, which arise from both neural crest and ectodermal placodes, as well as for cartilage/bone, which comes from neural crest plus mesoderm at cranial levels and from mesoderm alone in the trunk. As a consequence, the neural crest is sometimes referred to as ectomesenchyme, comprising the “fourth germ layer” (Hall, 2000).
The neural crest arises from a region at the border of the neural plate, between the neural plate and the adjacent non-neural ectoderm. Neural crest cells are specified at this border region by a combination of inducing signals that initiate during gastrulation. They remain at the neural plate border during neurulation, as the neural plate transforms into the rising neural folds and, after neural tube closure, come to reside in a domain of the dorsal neural tube. The neural crest precursor population expresses a characteristic suite of transcription factors, including Snail2 (Slug), Sox10, FoxD3 and Sox9, termed neural crest specifier genes.
Neural crest cells first become morphologically identifiable as individual or groups of migratory cells when they lose their connections to other neuroepithelial cells during the delamination phase. This involves either a complete or partial epithelial – to-mesenchymal transition (EMT). At this time, clonal analysis in vivo (Bronner-Fraser and Fraser, 1988) and in vitro (Sieber-Blum and Cohen, 1980; Baroffio et al., 1988; Stemple and Anderson, 1992; LeDouarin et al., 2008; Dupin et al., 2010) has revealed that many early migrating neural crest cells are multipotent. Combined with a limited capacity for self-renewal, neural crest cells are regarded as stem-cell like. They then migrate along defined pathways, and, following the migratory phase, settle in diverse and sometimes distant destinations in the periphery where they differentiate into a vast array of derivatives.
Taking these traits together, a neural crest cell can be defined by its initial location and molecular signature, coupled with its ability to undergo EMT, migrate and form multiple characteristic derivatives. Therefore, the neural crest can be defined operationally as a cell population that: 1) arises at the neural plate border; 2) expresses a combination of neural crest markers; and 3) migrates away from the neural tube to form multiple derivatives.
II. Historical perspective
The neural crest was first identified in the developing chick embryo by His in 1868. Described as a strip of cells lying between the neural tube and presumptive epidermis, it received its name based on its position at the “crest” of the closing neural tube. Initial experiments to identify derivatives of the neural crest involved extirpation experiments performed in amphibians and later in birds. By ablating the neural folds or neural tube followed by analysis of missing derivatives, it was determined that neural crest cells contribute to much of the peripheral nervous system and some facial skeleton pieces (rev. Horstadius, 1950). Such ablation experiments were used to infer neural crest derivatives by their absence but could not distinguish between a direct contribution of the neural crest from a requirement for an interaction with the neural crest in order to form that derivative. Subsequently, this was followed by transplantation experiments between the neural folds of closely related urodele species that labeled populations of cells at different levels of the neural axis (Horstadius,1950). These experiments confirmed an important contribution of the neural crest to diverse structures in vertebrate embryos.
Such interspecific transplantation experiments revealed the basic patterns of neural crest migration at different axial levels. Interestingly, in the pigment cell lineage, migration pathways appear to be intrinsically programmed to the neural crest in some species. For example, neural fold transplantations performed between different species of urodeles with either striped or spotted expression gave rise to a pigmentation pattern close but not exactly similar to that of the donor neural folds (Twitty and Niu, 1948).
A major advance in our understanding of the neural crest came with the advent of techniques that made it possible to follow the neural crest in higher vertebrates (Weston, 1963; Chibon, 1967; LeDouarin, 1969, 1973; LeDouarin and Teillet, 1974). Using tritiated thymidine as label, Weston transplanted labeled chick neural tubes into unlabeled hosts and was able to show that neuronal derivatives arose from ventrally migrating trunk neural crest cells whereas presumptive pigment cells migrated dorsolaterally (Weston, 1963). Moreover, ganglionic precursors populate their derivatives in a ventral to dorsal order, with sympathetic ganglia being populated by the most ventrally migrating cells and dorsal root ganglia by those cells that coalesced close to the neural tube. However, this approach diluted rapidly, obviating long term examination of neural crest derivatives.
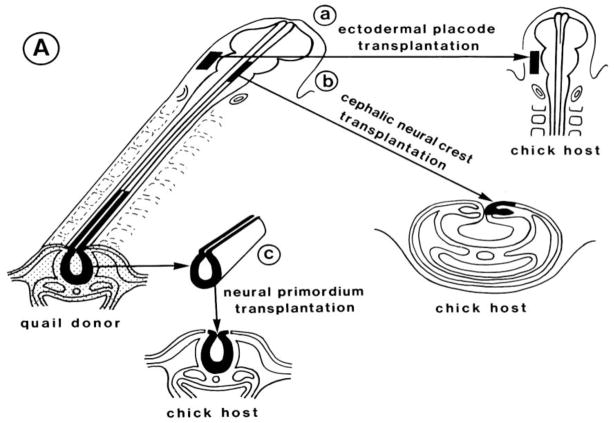
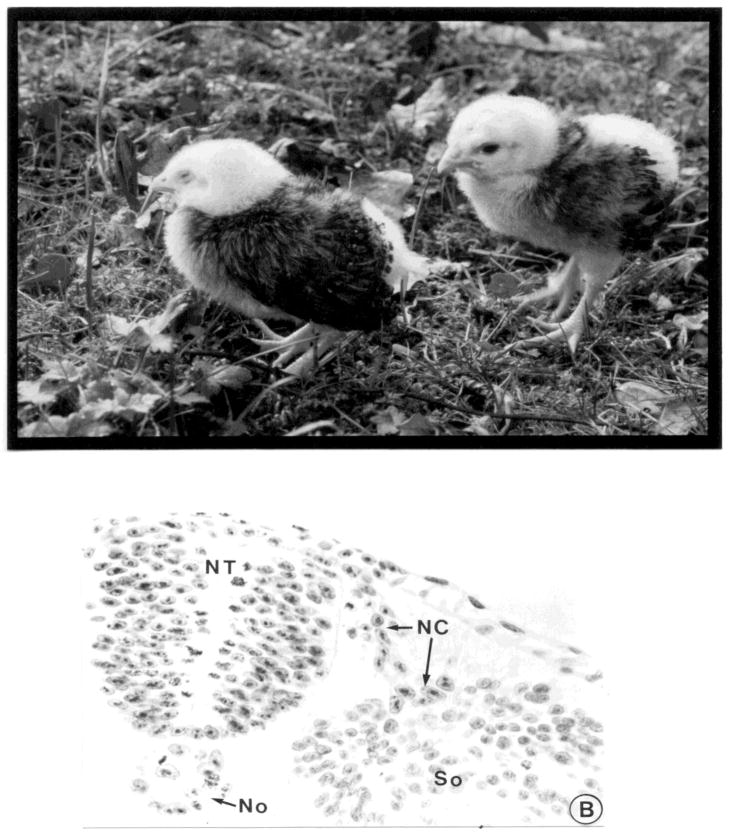
The advent of the quail/chick chimera propelled studies of the neural crest into the modern age with the ability to characterize in detail the derivatives of the neural crest along the body axis at single cell resolution (LeDouarin, 1973; 1982; Figure 1; Figure 2A). By replacing chick neural folds at different axial levels with quail tissue of similar age and location, a detailed contribution of the neural crest was determined at all levels of the neural axis (LeDouarin, 1982; Noden, 1983). Initial identification of quail cells was made by staining for DNA, since quail cells have condensed heterochromatin in their nucleolus, and can be distinguished from chick cells that have uniformly distributed heterochromatin during interphase (Figure 2B). The availability of quail specific antibodies has made it possible to detect transplanted quail cells at still higher resolution. More recently, the production of transgenic GFP chickens has made it possible to do intraspecific transplants in which every donor cell can be recognized with ease.
Figure 1.
Schematic diagram illustrating the grafting technique whereby donor quail tissue is transplanted in place of a similar region of host chick tissue. This can be done at different axial levels and for different tissue; e.g. to transplant ectodermal placodes (a), cranial neural folds (b), and trunk neural tubes (c). (reprinted with permission).
Figure 2.
Quail tissue can be recognized after transplantation into chick hosts. a) examples of hatchlings in which trunk quail neural tubes were grafted into white leghorn chick hosts, which lack melanocytes. Since neural crest cells give rise to melanocytes, the quail-derived donor cells have populated the feathers of the wings, which have quail pigmentation. b) a transverse section of an embryo into which a quail neural tube (NT) plus notochord (No) was transplanted. After staining for DNA, quail cells (arrows) can be recognized by the condensed heterochromatin and can be seen migrating away from the donor neural tube. (reprinted with permission).
III. Differences in neural crest development along the rostrocaudal axis and between species
Neural crest cells initiate migration in a spatiotemporally controlled sequence that, in most vertebrates, occurs first in the head shortly after neural tube closure, and then proceeds tailward. However, in some mammals and amphibians, neural crest emigration starts prior to tube closure; as a consequence, cranial neural crest cells emigrate from the open neural folds that close after neural crest emigration is complete at that axial level (for review see Baker and Bronner-Fraser, 1997). In fish and jawless vertebrates, the neural tube form via secondary neurulation, which involves ectoderm thickening, followed by cavitation (Damas, 1944; Papan and Campos-Ortega, 1999; Kelsh et al., 2009). Common to all vertebrate embryos, premigratory neural crest cells can be identified at the neural plate border and subsequently the dorsal aspect of the neural tube by their expression of neural crest specifier genes (Meulemans and Bronner-Fraser, 2004).
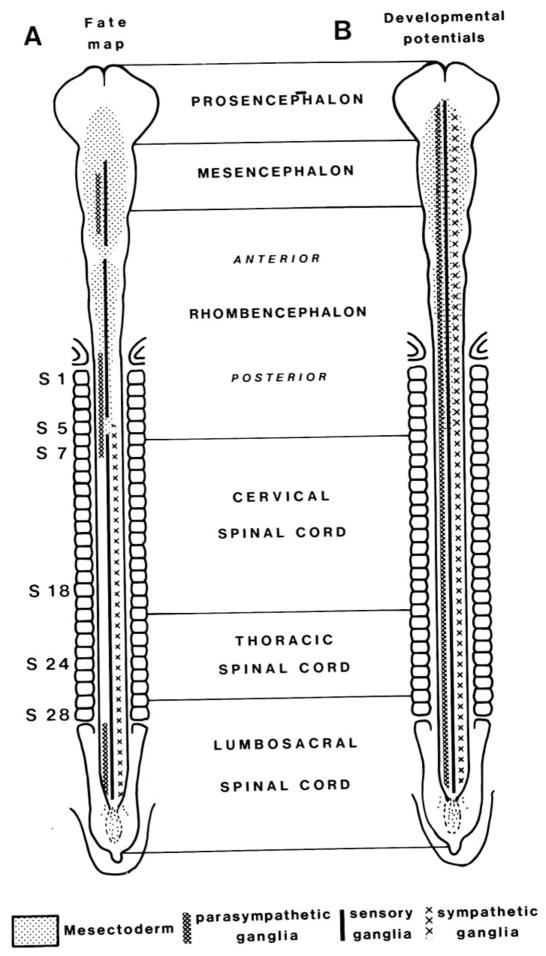
Interspecific grafts such as quail-chick chimeras have revealed regionalization in the fates of neural crest cells arising at different axial levels. The neural crest cells follow distinct migratory pathways, and also give rise to a stereotyped and sometimes divergent set of derivatives, that vary depending upon their axial level of origin. The levels from which different neural crest populations emerge along the body axis are often designated as cranial, vagal, trunk and lumbosacral (LeDouarin, 1982). The cranial neural crest encompasses the prosencephalic, mesencephalic and anterior rhomencephalic regions; vagal crest includes the posterior rhombencephalic crest at the levels of somites 1–7; trunk crest comprises cervical and thoracic levels adjacent to somites 8–28; and lumbosacral corresponds to the regions caudal the the 28th somite (Figure 3). Cranial neural crest cells contribute to facial skeleton and connective tissue, glia and Schwann cells, and ciliary and cranial sensory ganglia (LeDouarin, 1982; D'Amico-Martel and Noden, 1983). Vagal neural crest cells populate the enteric nervous system, first in the rostral and then in progressively more caudal regions of the gut. A subpopulation, termed the cardiac neural crest, contributes to the outflow tract and cardiac septum. In the trunk region, neural crest cells form sensory and sympathetic ganglia, Schwann cells and adrenomedullary cells. Sacral neural crest cells, like vagal neural crest cells, contribute to the enteric nervous system. Thus neural crest cells from different axial levels contribute to some distinct and some overlapping differentiated cell types. Melanocytes arise from all of these regions.
Figure 3.
Schematic diagram illustrating different levels of the body axis and the types of derivatives arising from neural crest at those levels. (reprinted with permission).
Normally, only cranial neural crest cells contribute to facial bone and cartilage. In fact, heterotopic grafts of trunk neural crest cells to the head fail to form these derivatives, although they can contribute to neurons, glia and melanocytes of the head (LeDouarin, 1982; Lwigale et al., 2004). This led to the idea that different regions of the neuraxis have differential potential to contribute to neural crest derivatives. However, recent experiments suggest that appropriate culture conditions can divert trunk neural crest cells into cartilaginous and bone lineages (McGonnell and Graham, 2002; Calloni et al., 2007). This is particularly striking in clonal cultures of neural crest cells, which have chondrogenic and osteogenic potential (LeDouarin et al., 2008; see Dupin & LeDouarin Chapter), particularly in the presence of growth factors like Shh.
Across gnathostome vertebrates, migratory pathways of cranial neural crest cells are largely conserved, as are neural crest derivatives (see Theveneau & Mayor Chapter). Agnathans also have similar migratory pathways for cranial neural crest cells, though they lack an important neural crest derivative – the jaw. In contrast to cranial neural crest, migratory pathways followed by trunk neural crest cells are highly divergent between different species. In amniotes, trunk neural crest cells migrate in a segmental pattern through the anterior half of each somite, but fail to migrate through its posterior half (Rickmann et al., 1985; Bronner-Fraser, 1986; Kalcheim and Teillet, 1989), due to inhibitory cues in the latter (Gammill et al., 2006). In contrast, trunk neural crest cells in fish and amphibians migrate either between the neural tube and adjacent somite, or intersomitically (Krotoski et al., 1988). In lamprey, trunk neural crest cells only migrate a short distance to form dorsal root ganglia or mesenchymal cells of the fin, but fail to form sympathetic ganglia (Häming et al, 2011), that appear to be a novelty of gnathostomes.
IV. Multipotent versus restricted
Morphologically detectable neural crest cells usually are first observed as the cells individualize and delaminate upon emigration from the neural tube, following their epithelial to mesenchymal transition (EMT), in which they convert from a tightly adherent sheet of cells to a disperse and more individual mesenchymal population. Prior to EMT, however, it is difficult or impossible at most axial levels and in most species to distinguish presumptive neural crest cells from cells that will form dorsal neural tube derivatives. However, there are exceptions, such as a subpopulation of cranial neural crest cells in chick and mouse that appears to be set aside and morphologically distinct at midbrain levels. In addition, some species such as axolotl have a clearly segregated population of neural crest cells that exist as a ridge on the dorsal neural tube.
A long-standing debate in the neural crest literature has been whether neural crest cells are multipotent and/or restricted in their developmental potential. In other words, can a single neural crest precursor form only one type of derivative or are the cells multipotent and able to produce multiple derivatives. Single cell lineage experiments (Bronner-Fraser and Fraser, 1988), in which individual cells within the chick dorsal neural tube are labeled with vital dye, show that some of the labeled clones contribute to multiple differentiated cell types in the periphery, including melanocytes, sympathetic and sensory ganglion cells. Thus, the original precursor was “multipotent” in its developmental potential to form neural crest derivatives. In addition to neural crest derivatives, single dorsal neuroepithelial cell also give rise both to migrating neural crest cells and cells that remain in the dorsal neural tube, such as roof plate cells and dorsal sensory neurons and/or interneurons. This suggests a shared lineage between the neural tube and neural crest, at least at this stage. However, labeling migrating neural crest cells in vivo also produced clones that could contribute to more than one neural crest lineage (Fraser and Bronner-Fraser, 1991), again supporting the idea that some of the migrating population retained multipotency.
Clonogenic culture of neural crest cells cultured shortly after their emigration from the neural tube definitely show that many early migrating neural crest cells are multipotent in vitro as well (Sieber-Blum and Cohen, 1980; Baroffio et al., 1988; Stemple and Anderson, 1992; LeDouarin et al., 2008, Calloni et al., 2007, 2009). Exposure to different growth factors can profoundly influence their lineage decisions (e.g. Lahav et al., 1998; see Dupin & LeDouarin Chapter). Furthermore, they have a capacity for self-renewal, at least for a few cell divisions (Stemple and Anderson, 1992; Trentin et al., 2004).
The fact that individual neural crest cells can form multiple derivatives has led to the idea that they have stem cell properties. Stem cells are defined as individual progenitor cells that can generate one or more specialized cell types. A cardinal feature of stem cells is their ability to self-renew, that is, to divide so as to give rise to at least one daughter cell that maintains the multipotent character of its parent. The fact that cloned neural crest cells have a limited ability to self-renew has led to the idea that they are stem-like (progenitors) cells rather than true stem cells. Interestingly, however, neural crest stem cells can be derived from adult tissues (Fernandes et al., 2008; Shakhova and Sommer, 2010 ; see Chapter Dupin and Sommer), suggesting that they can remain quiescent for long periods of time or maintain long term self-renewal ability when left in situ.
The presence of some multipotent neural crest precursors cannot, however, rule out the possibility that other precursors may be more restricted in their developmental potential. In fact, experiments in zebrafish suggested that neural crest cells contribute to different sets of derivatives accordingly to their migration order (Raible and Eisen, 1994). However, if the leader cell was ablated, the next cell in line took up the fate that would have been filled by the ablated cell (Raible and Eisen, 1996). Similarly, early migrating neural crest cells normally exhibit a broader range of derivatives than later migrating cells; however, when the early population is ablated and replaced by late migrating cells, the late migrating cells assume a broader developmental potential than that prescribed by their normal fate (Baker et al, 1997). This raises a very important issue: that developmental potential is greater than or equal to a cell’s normal fate. Only by challenging the cell by putting it into a new environment can one test for restriction of cell fate. This is best exemplified by experiments in which the potential of neural crest populations was challenged by performing heterotopic transplants between different axial levels (LeDouarin and Teillet, 1974), such as exchanging cranial and trunk, or vagal and trunk populations. The results demonstrate a combination of flexibility in cell fate and some axial level-autonomous characteristics. For example, cranial neural crest cells normally make cartilage and bone of the face whereas trunk neural crest do not. Transplantation of cranial neural folds to the trunk results in production of many normal trunk derivatives, as well as the formation of ectopic cartilage nodules (LeDouarin and Teillet, 1974; Le Lièvre et al., 1980). Conversely, transplantation of trunk neural folds to the head results in contributions to cranial neurons and glia of cranial ganglia, but not to cartilage, although some connective tissues and pericytes derive from this graft (Nakamura and Le Lièvre, 1982). This reveals some flexibility in fate, but a more limited ability to form skeletal derivatives (LeDouarin et al., 2004). However, trunk neural crest cells can form cartilage in vitro under appropriate culture conditions (McGonnell and Graham, 2002; Calloni et al., 2007). Because challenging prospective neural crest fate is a difficult experiment, it is much easier to prove multipotency than restricted cell fate, leaving the question of whether or not there are lineage-restricted neural crest precursors still open to debate (see Krispin et al., 2010).
V. A cranial neural crest gene regulatory network
Comparative analysis of conserved molecular mechanisms can help understand the fundamental principles underlying neural crest formation--from the origin of these cells at the neural plate border to their differentiation into diverse cell types. The neural crest has been studied in a number of different vertebrate models, ranging from jawless vertebrates (Sauka-Spengler et al., 2007; Ota et al., 2007) to mice, and even human embryos (Betters et al., 2010). Assembling information obtained from diverse vertebrate models into a hypothetical gene regulatory network (NC-GRN) may help explain and generalize the complex events underlying formation of the cranial neural crest (Meulemans and Bronner-Fraser, 2004; Sauka-Spengler and Bronner-Fraser, 2008; Betancur et al., 2010).
Although it is always tempting to generalize, it is critical to keep in mind that there are several populations of neural crest cells along the neural axis, such that one NC-GRN cannot account for the process of neural crest formation at all rostrocaudal levels. To date, the NC-GRN has been compiled from data in a number of species and has focused on the cranial neural crest, since it is the easiest population to visualize and the one that appears to be most conserved with respect to cell migration patterns across vertebrates. Given the marked differences in developmental potential, migratory pathways, and derivatives along the neural axis, it is important to emphasize that the GRN responsible for formation of cranial neural crest cells is sure to be different than that regulating the trunk neural crest.
From a temporal perspective, the process of neural crest formation can be subdivided into a series of steps, in the form of distinct regulatory modules. This multistep process is initiated by several environmental signals, exerting their effects on cells at the neural plate border. These extracellular signals include molecules like Wnts, BMPs and FGFs, that function at gastrula stages to activate a transcriptional program that imbues the neural plate border with the competence to give rise to the neural crest and dorsal neural tube.
This is accomplished by upregulation of a “neural plate border specifier” module comprised of transcription factors like Msx, Pax3/7, and Zic1. These are expressed in the neural plate border as well as in neighboring domains. Interestingly, it is the region of overlap of these genes that defines the broad territory of the neural plate border. These border specifiers are expressed broadly, have functions in not only the neural crest but also other ‘border” populations like cranial ectodermal placodes, and Rohon-Beard cells and, later, are down-regulated in the migrating neural crest cells.
Further refinement of the border region results from cooperation between the extracellular signaling module and the neural plate border specifier module to activate “neural crest specifiers”. Genes in this module include transcription factors like Snail/Slug, FoxD3, Id, cMyc, and Sox9/10, all of which are expressed in pre- and migratory neural crest cells. These genes confer the ability to undergo an epithelial to mesenchymal transition, allowing neural crest cells to leave the neural tube, migrate and subsequently differentiate into diverse derivatives. In addition, these genes are activated by Wnt and other signals, together with neural plate border markers, Zic, Msx1/2 and Pax3/7 . In addition, they repress the neural tube marker Sox2.
The neural crest specifiers control expression of numerous effector genes that mediate cell adhesion and motility, such as cadherins and Rho GTPases. They also function in specifying various neural crest lineages. Often the same transcription factors that function early in neural crest specification are later deployed to control differentiation of one or more neural crest program. The prime example is that of Sox10, which is upstream of the differentiation program controlling melanocyte, sensory, autonomic and glial cell lineages. Its paralogue, Sox9, in turn is responsible for cartilage cell differentiation.
This version of the NC-GRN is, by definition preliminary and likely to be missing numerous important players. Moreover, direct connections, feedback loops, and cross-regulation within the network is only now beginning to be dissected. It is also becoming increasingly clear that, in addition to a hard-wired NC-GRN, post-transcriptional, post-translational and epigenetic modfications also play large roles in neural crest development. Despite these caveats, formulation of a NC-GRN provides a useful hypothetical scaffold for testing and further analysis.
One tool for establishing additional components and direct connections within the network is via the identification and dissection of neural crest enhancers (Betancur, et. al, 2010). For example, identification of a cranial enhancer for Sox10 has shown that c-Myb, Ets1 and Sox9 are direct inputs that mediate expression of Sox10 in the cranial neural crest. This adds new transcription factors (c-Myb and Ets1) to the neural crest specifier module and establishes direct inputs.
VI. Evolution of the neural crest
During vertebrate evolution, many already existing cell types came under the umbrella of the neural crest lineage, making these a population of cells with combined ectodermal and mesenchymal properties, comprising what has been referred to as a “fourth germ layer” (Hall, 2000). Thus, the addition of this novel cell population essentially transformed the triploblastic chordate body plan of ecto-, meso- and endoderm, into a more sophisticated quadroblastic, body plan. This transformation in turn led to a huge expansion of cell diversity.
In vertebrates, the invention of the neural crest cells together with ectodermal placodes allowed for the formation of a new set of sensory organs in the “New Head”, as formulated by Gans and Northcutt (1983). Indeed, neural crest cells contribute to many novel features specific to vertebrates, including craniofacial bone and sensory ganglia. Further modifications of the first branchial arch into the upper and lower jaws of gnathostomes (jawed vertebrates) are thought to have facilitated predatory behavior. By imbuing vertebrates with enhanced predation, evolution of neural crest may have endowed vertebrates with enhanced growth of the head, skull and brain. In fact, new data provides definitive evidence that the neural crest acts as a brain organizing center important for growth of the skull and that the modern brain requires interactions with neural crest (Creuzet et al., 2006; see LeDouarin & Creuzet Chapter).
The existence of a conserved cranial NC-GRN that is surprisingly similar between animals as different as amphibians and mice, raises the intriguing question of when was the NC-GRN invented? From a regulatory perspective, one possibility is that this network or parts thereof already existed in non-vertebrate chordates and was added to step-wise during vertebrate evolution.
The first bona fide neural crest cells and derivatives are apparent in jawless (agnathan) basal vertebrates, lamprey and hagfish (see Maisey, 1986; Northcutt, 1996; Ota et al., 2007). These extant animals are morphologically similar to early fossil vertebrates (see Forey and Janvier, 1993; Smith and Hall, 1993; Tucker et al., 2006). Therefore, agnathans occupy a critical position to provide important insights into our understanding of evolution of the neural crest. From an embryological point of view, lamprey embryos are more accessible than hagfish and have provided more information regarding the neural crest and their derivatives. Early experiments by Languille and Hall (1986) showed that ablation of the lamprey dorsal neural tube at neurula stages resulted in defects of the cranial and visceral skeleton. Subsequent DiI labeling experiments (McCauley and Bronner-Fraser, 2003) confirmed that the cranial neural crest of lamprey followed relatively similar migratory pathways to those of jawed vertebrates. However, hagfish and lamprey lack some critical neural crest derivatives such as jaws and sympathetic ganglia.
Molecular analysis of the cranial neural crest gene regulatory network in the basal lamprey reveals a high degree of conservation to the base of vertebrates (Sauka-Spengler et al., 2007). By identifying and examining the expression patterns of nearly a hundred lamprey homologues of NC-GRN components, it was found that patterning signals, comprised of BMPs, Wnts, FGFs and Notch, are similar in lamprey to those observed in jawed vertebrates. Similarly, the same suite of genes (Zic, Msx, Pax3/7) is expressed at the neural plate border of lamprey as in other vertebrates. Similarly, there is high conservation at the level of neural crest specifier module, with genes like Snail, SoxE, FoxD3, AP-2, expressed in neural folds and dorsal neural tube. In fact, only two transcription factors, Ets1 and Twist, appear to be differentially regulated in lamprey compared with other vertebrates. Rather than being expressed early in the forming neural crest as neural crest specifier genes, these were first deployed later at the level of effector genes. Thus, these may confer some species specific traits. However, the overwhelming majority of genes appear to be conserved in their deployment at the neural plate border and in the nascent neural crest. Thus, analysis of the lamprey NC-GRN strongly suggests that it is largely conserved to the base of vertebrates, for over 550 million years.
Functional analysis of selected neural crest GRN components also suggests a high level of conservation across vertebrates. For example, knock-down of over eight transcription factors operating either at the neural plate border or in the neural crest specifier module suggests conserved functions of these genes in lamprey compared with those operating in jawed vertebrates (Sauka-Spengler et al., 2007). Indeed, fine tuned analysis of interconnections in the neural plate border module of lamprey (Nikitina et al, 2008) reveals remarkably similar connections to those observed in Xenopus (deCroze et al., 2011; see Monsoro-Burq Chapter).
Based on identifying amphioxus homologues of genes involved in the putative vertebrate NC-GRN, there is good evidence for the existence of components of the NC-GRN even in non-vertebrate chordates. For example, the extracellular signaling module, comprised of BMPs, Wnts, and FGFs, as well as the neural plate border module (Zic, Msx, Pax3/7) already exist in basal chordates, such as amphioxus, and urochordates like Ciona (Shoguchi et al., 2008). These genes are involved in patterning the neural plate border in urochordates (Shoguchi et al., 2008) and cephalochordates (Holland, 2009) as are in vertebrates). In contrast, the neural crest specifier module appears to be largely missing. Only amphioxus and urochordate homologues of Snail are expressed at the border of the neural and non-neural ectoderm. For example, although other homologues of neural crest specifier genes are present in other tissues in amphioxus (e.g. FoxD3, SoxE, AP-2), only the Snail homologue is expressed in the neural plate border (Yu et al, 2008). One intriguing possibility is that evolution of the vertebrate neural crest may have involved elaboration of the ‘neural crest specifier’ module. This could occur by intercalation of genetic sub-networks that promoted an epithelial to mesenchymal transition and cell migratory properties to precursor cells within the dorsal neural tube. One possibility is that this may have occurred via elaboration or modification of existing regulatory programs involved in formation of differentiated cell types and structures that were already present in invertebrates. Such co-option may have been enabled by a shift in signalling field.
This idea is supported by the discovery of migrating neural crest-like pigment cell precursors in urochordates (Jeffery et al. 2004). This was discovered by DiI labeling in the vicinity of the neural tube, which resulted in labeling a population of migratory cells that later differentiated into pigment cells. Since these cells display a subset of the molecular properties of vertebrate neural crest cells, possibly reflecting a transitional state.
Interestingly, another vertebrate innovation, ectodermal placodes may have co-evolved with neural crest. In the vertebrate head, the peripheral sensory nervous system has a dualorigin from both neural crest and cranial ectodermal placodes (Ayer-Le Lièvre at al., 1982; D’Amico-Martel and Noden, 1983; Baker and Bronner-Fraser, 2001). Although derived from ectoderm, like neural crest cells, placode cells leave the ectoderm either by invaginating or delaminating. Ultimately, they condense to form cranial sensory ganglia as well as the paired sense organs, the lens, nose and ears. Whereas many of the cranial sensory ganglia are comprised of neurons entirely derived from placodes, others such as the trigeminal, and vestibuloacoustic ganglia contain both neural crest- and placode-derived neurons. In contrast, neural crest cells contribute all of the supporting cells of these ganglia.
VII. Where is the field going?
The neural crest field is relatively young and has made great strides since its discovery by His in 1868. In the past 150 years, the field has moved from describing morphology, to experimental embryology, to detailed molecular analysis. Importantly, comparative analysis of multiple species has provided unique insights into which features of the neural crest are vertebrate-wide versus species specific. There are numerous positive aspects of having multiple animal models, ranging from basal vertebrates like lamprey and hagfish, to fish and amphibianslike zebrafish and Xenopus, to amniotes like chick and mouse. These make it possible to determine broadly applicable concepts and universal rules.
The combined data give a picture of the neural crest that is defined by the nature of its regulatory state, position at particular developmental times and ability to differentiate into broad derivatives. From a regulatory perspective, future work must continue to define and refine the cranial neural crest regulatory network at the transcriptional and post-transcriptional level. Direct interactions must be established within the network and additional regulatory elements need to be identified in all modules. Another important step will be characterization of the sub-networks that bestow stem-cell characteristics to the neural crest, that maintain the progenitor state, and regulate proliferation and cell death. Importantly, these should be compared with sub-networks and modules that exist in chordates in order to better understand neural crest evolution via regulatory changes.
An important continuation of this effort will be to expand knowledge of the GRN to other axial levels, like the vagal and trunk. This will require in depth analysis at different times, axial levels and across species. Further analysis of neural crest enhancer elements holds the promise of identifying yet more neural crest genes and establishing more direct connections in the NC-GRN. Furthermore, it is increasingly clear that in addition to a “hard-wired” neural crest GRN, epigenetic modification is extremely important for controlling the timing of important events in the formation and differentiation of these cells.
At a cellular level, future work must be directed toward understanding the molecular and cell biological mechanisms responsible for cell motility and cell fate decisions. Important questions include understanding what confers migratory and stem cell properties to neural crest progenitors, how dynamic changes in cell morphology and cell cycle progression occur, and how their fate decisions are made.
The technological explosion in molecular biology and genomics will play an increasingly important role in studies of the neural crest. By applying multiplexed analysis of perturbations of neural crest genes and next generation technologies to perform genome-wide profiling, investigators will be able to gain a systems-level understanding of what makes a neural crest cell. Transcriptome analysis of discrete neural crest populations will help define not only the changes that occur in a neural crest cell during the process of maturation and differentiation but also what might account for differences in developmental potential along the neural axis, making it possible to expand the cranial GRN to other axial levels.
The advent of new technologies makes this a very exciting time in all areas of biology, including developmental biology. Although the questions sometimes seem very daunting in their complexity, the great strides made in the past few years make it clear that there will be exponential growth of knowledge and technology, allowing greater understanding of this fascinating cell population – the Neural Crest.
Acknowledgments
We would like to acknowledge Les Treilles Foundation, whose generous support and wonderful atmosphere provided the participants of our Neural Crest study group with an environment that stimulated discussion and promoted fruitful interactions. We are particularly grateful to Mme. Catherine Bachy, whose organizational assistance greatly contributed to the success of our endeavor.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ayer-Le Lievre CS, LeDouarin NM. The early development of cranial sensory ganglia and the potentialities of their component cells studied in quail-chick chimeras. Dev Biol. 1982;94:291–310. doi: 10.1016/0012-1606(82)90349-9. [DOI] [PubMed] [Google Scholar]
- Baker CVH, Bronner-Fraser M, LeDouarin NM, Teillet MA. Early- and late-migrating cranial neural crest populations have equivalent developmental potential in vivo. Development. 1997;124:3077–3087. doi: 10.1242/dev.124.16.3077. [DOI] [PubMed] [Google Scholar]
- Baker CVH, Bronner-Fraser M. The origins of the neural crest. Part I: Embryonic induction. Mechanisms of Development. 1997;69:3–11. doi: 10.1016/s0925-4773(97)00132-9. [DOI] [PubMed] [Google Scholar]
- Baker C, Bronner-Fraser M. Vertebrate cranial placodes: I. Embryonic induction Dev Biol. 2001;232:1–61. doi: 10.1006/dbio.2001.0156. [DOI] [PubMed] [Google Scholar]
- Baroffio A, Dupin E, LeDouarin NM. Clone-forming ability and differentiation potential of migratory neural crest cells. Proc Natl Acad Sci U S A. 1988;85(14):5325–9. doi: 10.1073/pnas.85.14.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancur P, Bronner-Fraser M, Sauka-Spengler T. Genomic code for Sox10, a key regulatory enhancer for cranial neural crest. Proc Natl Acad Sci USA. 2010;107:3570–5. doi: 10.1073/pnas.0906596107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancur P, Bronner-Fraser M, Sauka-Spengler T. Assembling neural crest regulatory circuits into a gene regulatory network. Annu Rev Cell Dev Biol. 2010;26:581–603. doi: 10.1146/annurev.cellbio.042308.113245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betters E, Liu Y, Kjaeldgaard A, Sundström E, García-Castro MI. Analysis of early human neural crest development. Dev Biol. 2010;344:578–92. doi: 10.1016/j.ydbio.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronner-Fraser M. Analysis of the early stages of trunk neural crest migration in avian embryos using the monoclonal antibody HNK-1. Devl Biol. 1986;115:44–55. doi: 10.1016/0012-1606(86)90226-5. [DOI] [PubMed] [Google Scholar]
- Bronner-Fraser M, Fraser S. Cell lineage analysis shows multipotentiality of some avian neural crest cells. Nature. 1988;335(8):161–164. doi: 10.1038/335161a0. [DOI] [PubMed] [Google Scholar]
- Calloni GW, Glavieux-Pardanaud C, LeDouarin NM, Dupin E. Sonic Hedgehog promotes the development of multipotent neural crest progenitors endowed with both mesenchymal and neural potentials. Proc Natl Acad Sci U S A. 2007;104:19879–884. doi: 10.1073/pnas.0708806104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calloni GW, LeDouarin NM, Dupin E. High frequency of cephalic neural crest cells shows coexistence of neurogenic, melanogenic, and osteogenic differentiation capacities. Proc Natl Acad Sci U S A. 2009;106:8947–52. doi: 10.1073/pnas.0903780106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chibon P. Marquage nuclé aire par la thymidine tritiée des dérivés de la cre te neurale chez l’Amphibien Urodè le Pleurodeles waltlii. Michah J Embryol Exp Morphol. 1967;18:343–358. [PubMed] [Google Scholar]
- Creuzet SE, Martinez S, LeDouarin NM. The cephalic neural crest exerts a critical effect on forebrain and midbrain development. Proc Natl Acad Sci U S A. 2006;103:14033–8. doi: 10.1073/pnas.0605899103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Crozé N, Maczkowiak F, Monsoro-Burq AH. Reiterative AP2a activity controls sequential steps in the neural crest gene regulatory network. Proc Natl Acad Sci U S A. 2011;108:155–60. doi: 10.1073/pnas.1010740107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupin E, Calloni GW, LeDouarin NM. The cephalic neural crest of amniote vertebrates is composed of a large majority of precursors endowed with neural, melanocytic, chondrogenic and osteogenic potentialities. Cell Cycle. 2010;9(2):238–49. doi: 10.4161/cc.9.2.10491. [DOI] [PubMed] [Google Scholar]
- Fernandes KJ, Toma JG, Miller FD. Multipotent skin-derived precursors: adult neural crest-related precursors with therapeutic potential. Philos Trans R Soc Lond B Biol Sci. 2008;363:185–98. doi: 10.1098/rstb.2006.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser SE, Bronner-Fraser M. Migrating neural crest cells in the trunk of the avian embryo are multipotent. Development. 1991;112:913– 920. doi: 10.1242/dev.112.4.913. [DOI] [PubMed] [Google Scholar]
- D'Amico-Martel A, Noden DM. Contributions of placodal and neural crest cells to avian cranial peripheral ganglia. Am J Anat. 1983;166:445–68. doi: 10.1002/aja.1001660406. [DOI] [PubMed] [Google Scholar]
- Damas H. Recherches sur le dévelopment de Lampetra fluviatilis L. Contribution à l' étude de la céphalogenèse des Vertébrés. Arch Biol. 1944;55:1–285. [Google Scholar]
- Forey P, Janvier P. Agnathans and the origin of jawed vertebrates. Nature. 1993;361:129–134. [Google Scholar]
- Gammill L, Gonzalez C, Gu C, Bronner-Fraser M. Guidance of trunk neural crest migration requires Neuropilin-2/Semaphorin3F signaling. Development. 2006;133:99–106. doi: 10.1242/dev.02187. [DOI] [PubMed] [Google Scholar]
- Gans C, Northcutt RG. Neural Crest and the Origin of Vertebrates: A New Head. Science. 1983;220:268–273. doi: 10.1126/science.220.4594.268. [DOI] [PubMed] [Google Scholar]
- Hall BK. The neural crest as a fourth germ layer and vertebrates as quadroblastic not triploblastic. Evol Dev. 2000;2:3–5. doi: 10.1046/j.1525-142x.2000.00032.x. [DOI] [PubMed] [Google Scholar]
- Häming* D, Simoes-Costa M, Uy B, Valencia J, Sauka-Spengler T, Bronner-Fraser M. Expression of sympathetic nervous system marker genes in lamprey embryos suggests cooption from notochord. 2011 doi: 10.1371/journal.pone.0026543. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- His W. Die erste entwickelung des huhnchens im Ei. Leipzig: FCW Vogel; 1868. Untersuchungen uber die erste Anlage des Wirbeltierleibes. [Google Scholar]
- Holland LZ. Chordate roots of the vertebrate nervous system: expanding the molecular toolkit. Nat Rev Neurosci. 2009;10:736–46. doi: 10.1038/nrn2703. [DOI] [PubMed] [Google Scholar]
- Hörstadius S. The neural crest: Its properties and derivatives in the light of experimental research. London: Oxford University Press; 1950. [Google Scholar]
- Jeffery WR, Strickler AG, Yamamoto Y. Migratory neural crest-like cells form body pigmentation in a urochordate embryo. Nature. 2004;431:696–9. doi: 10.1038/nature02975. [DOI] [PubMed] [Google Scholar]
- Kalcheim C, Teillet MA. Consequences of somite manipulation on the pattern of dorsal root ganglion development. Development. 1989;106:85–93. doi: 10.1242/dev.106.1.85. [DOI] [PubMed] [Google Scholar]
- Krispin S, Nitzan E, Kalcheim C. The dorsal neural tube: a dynamic setting for cell fate decisions. Dev Neurobiol. 2010;70:796–812. doi: 10.1002/dneu.20826. [DOI] [PubMed] [Google Scholar]
- Kelsh RN, Harris ML, Colanesi S, Erickson CA. Stripes and belly-spots– A review of pigment cell morphogenesis in vertebrates. Seminars in Cell and Developmental Biology. 2009;20:90–104. doi: 10.1016/j.semcdb.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krotoski D, Fraser S, Bronner-Fraser M. Mapping of neural crest migration in Xenopus laevis embryos using inter- and intra-specific cell markers. Devl Biol. 1988;127:119–132. doi: 10.1016/0012-1606(88)90194-7. [DOI] [PubMed] [Google Scholar]
- Lahav R, Ziller C, Dupin E, LeDouarin NM. Endothelin 3 promotes neural crest cell proliferation and mediates a vast increase in melanocyte number in culture. Proc Natl Acad Sci U S A. 1996;93:3892–7. doi: 10.1073/pnas.93.9.3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahav R, Dupin E, Lecoin L, Glavieux C, Champeval D, Ziller C, LeDouarin NM. Endothelin 3 selectively promotes survival and proliferation of neural crest-derived glial and melanocytic precursors in vitro. Proc Natl Acad Sci U S A. 1998;95:14214–9. doi: 10.1073/pnas.95.24.14214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langille RM, Hall BK. Evidence of cranial neural crest contribution to the skeleton of the sea lamprey, Petromyzon marinus. Prog Clin Biol Res. 1986;217B:263–6. [PubMed] [Google Scholar]
- LeDouarin N. The neural crest. Cambridge University Press; Cambridge: 1982. [Google Scholar]
- LeDouarin NM, Calloni GW, Dupin E. The stem cells of the neural crest. Cell Cycle. 2008;7:1013–9. doi: 10.4161/cc.7.8.5641. [DOI] [PubMed] [Google Scholar]
- LeDouarin NM, Creuzet S, Couly G, Dupin E. Neural crest cell plasticity and its limits. Development. 2004;131:4637–50. doi: 10.1242/dev.01350. [DOI] [PubMed] [Google Scholar]
- LeDouarin NM, Teillet MA. The migration of neural crest cells to the wall of the digestive tract in avian embryo. J Embryol Exp Morphol. 1973;30:31–48. [PubMed] [Google Scholar]
- LeDouarin NM, Teillet MA. Experimental analysis of the migration and differentiation of neuroblasts of the autonomic nervous system and of neurectodermal mesenchymal derivatives, using a biological cell marking technique. Dev Biol. 1974;41:162–84. doi: 10.1016/0012-1606(74)90291-7. [DOI] [PubMed] [Google Scholar]
- LeDouarin NM, Teillet MA, Le Lievre C. Influence of the tissue environment on the differentiation of neural crest cells. Soc Gen Physiol Ser. 1977;32:11–27. [PubMed] [Google Scholar]
- Le Lievre CS, Schweizer GG, Ziller CM, LeDouarin NM. Restrictions of developmental capabilities in neural crest cell derivatives as tested by in vivo transplantation experiments. Dev Biol. 1980;77:362–78. doi: 10.1016/0012-1606(80)90481-9. [DOI] [PubMed] [Google Scholar]
- LeDouarin N. A biological cell labeling technique and its use in experimental embryology. Dev Biol. 1973;30:217–22. doi: 10.1016/0012-1606(73)90061-4. [DOI] [PubMed] [Google Scholar]
- LeDouarin NM, Teillet MA. Experimental analysis of the migration and differentiation of neuroblasts of the autonomic nervous system and of neurectodermal mesenchymal derivatives, using a biological cell marking technique. Dev Biol. 1974;1:162–84. doi: 10.1016/0012-1606(74)90291-7. [DOI] [PubMed] [Google Scholar]
- LeDouarin NM, Calloni GW, Dupin E. The stem cells of the neural crest. Cell Cycle. 2008;7:1013–9. doi: 10.4161/cc.7.8.5641. [DOI] [PubMed] [Google Scholar]
- Lwigale PY, Conrad G, Bronner-Fraser M. Graded potential of neural crest to form cornea, sensory neurons and cartilage along the rostrocaudal axis. Development. 2004;131:1979–1991. doi: 10.1242/dev.01106. [DOI] [PubMed] [Google Scholar]
- Ota KG, Kuraku S, Kuratani S. Hagfish embryology with reference to the evolution of the neural crest. Nature. 2007;446:672–5. doi: 10.1038/nature05633. [DOI] [PubMed] [Google Scholar]
- Maisey JG. Heads and tails: a chordate phylogeny. Cladistics. 1986;2:201–256. doi: 10.1111/j.1096-0031.1986.tb00462.x. [DOI] [PubMed] [Google Scholar]
- McCauley D, Bronner-Fraser M. Neural crest contributions to the lamprey head. Development. 2003;130:2317–2327. doi: 10.1242/dev.00451. [DOI] [PubMed] [Google Scholar]
- McGonnell IM, Graham A. Trunk neural crest has skeletogenic potential. Curr Biol. 2002;12:767–71. doi: 10.1016/s0960-9822(02)00818-7. [DOI] [PubMed] [Google Scholar]
- Meulemans D, Bronner-Fraser M. Gene-regulatory interactions in neural crest evolution and development. Dev Cell. 2004;7:291–299. doi: 10.1016/j.devcel.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Ayer-le Lievre CS. Mesectodermal capabilities of the trunk neural crest of birds. J Embryol Exp Morphol. 1982;70:1–18. [PubMed] [Google Scholar]
- Nikitina N, Sauka-Spengler T, Bronner-Fraser M. Dissecting early regulatory relationships in the lamprey neural crest gene regulatory network. Proc Natl Acad Sci USA. 2008;105:20083–8. doi: 10.1073/pnas.0806009105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noden DM. The role of the neural crest in patterning of avian cranial skeletal, connective, and muscle tissues. Dev Biol. 1983;96:144–65. doi: 10.1016/0012-1606(83)90318-4. [DOI] [PubMed] [Google Scholar]
- Northcutt RG. The agnathan ark: the origin of craniate brains. Brain Behav Evol. 1996;48:237–247. doi: 10.1159/000113203. [DOI] [PubMed] [Google Scholar]
- Papan C, Campos-Ortega JA. Region-specific cell clones in the developing spinal cord of the zebrafish. Dev Genes Evol. 1999;209:135–44. doi: 10.1007/s004270050237. [DOI] [PubMed] [Google Scholar]
- Raible DW, Eisen JS. Restriction of neural crest cell fate in the trunk of the embryonic zebrafish. Development. 1994;120:495–503. doi: 10.1242/dev.120.3.495. [DOI] [PubMed] [Google Scholar]
- Raible DW, Eisen JS. Regulative interactions in zebrafish neural crest. Development. 1996;122:501–7. doi: 10.1242/dev.122.2.501. [DOI] [PubMed] [Google Scholar]
- Rickmann M, Fawcett JW, Keynes RJ. The migration of neural crest cells and growthcones of motor axons through the rostral half of the chick somite. J Embryo Exp Morphol. 1985;90:437–455. [PubMed] [Google Scholar]
- Sieber-Blum M, Cohen AM. Clonal analysis of quail neural crest cells: they are pluripotent and differentiate in vitro in the absence of noncrest cells. Dev Biol. 1980;80:96–106. doi: 10.1016/0012-1606(80)90501-1. [DOI] [PubMed] [Google Scholar]
- Sauka-Spengler T, Meulemans D, Bronner-Fraser M. Ancient evolutionary origin of the neural crest gene regulatory network. Dev Cell. 2007;13:405–420. doi: 10.1016/j.devcel.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Sauka-Spengler T, Bronner-Fraser M. A gene regulatory network orchestrates neural crest formation. Nat Rev Mol Cell Biol. 2008;9:557–568. doi: 10.1038/nrm2428. [DOI] [PubMed] [Google Scholar]
- Shakhova O, Sommer L. StemBook 2008–2010. Harvard Stem Cell Institute; Cambridge (MA): 2010. Neural crest-derived stem cells. [PubMed] [Google Scholar]
- Shoguchi E, Hamaguchi M, Satoh N. Genome-wide network of regulatory genes for construction of a chordate embryo. Dev Biol. 2008 Apr 15;316(2):498–509. doi: 10.1016/j.ydbio.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Stemple DL, Anderson DJ. Lineage diversification of the neural crest: in vitro investigations. Dev Biol. 1993;159:12–23. doi: 10.1006/dbio.1993.1218. [DOI] [PubMed] [Google Scholar]
- Trentin A, Glavieux-Pardanaud C, LeDouarin NM, Dupin E. Self-renewal capacity is a widespread property of various types of neural crest precursor cells. Proc Natl Acad Sci U S A. 2004;101:4495–500. doi: 10.1073/pnas.0400629101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker RP, Drabikowski K, Hess JF, Ferralli J, Chiquet-Ehrismann R, Adams JC. Phylogenetic analysis of the tenascin gene family: evidence of origin early in the chordate lineage. BMC Evol Biol. 2006;6:60. doi: 10.1186/1471-2148-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twitty VC, Niu MC. Causal analysis of chromatophore migration. J Exp Zool. 1948;108(3):405–37. doi: 10.1002/jez.1401080305. [DOI] [PubMed] [Google Scholar]
- Yu JK, Meulemans D, Bronner-Fraser M. Insights from the amphioxus genome on origin of neural crest in the “new” vertebrate head. Genome Biology. 2008 doi: 10.1101/gr.076208.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston JA. A radioautographic analysis of the migration and localization of trunk neural crest cells in the chick. Dev Biol. 1963;6:279–310. doi: 10.1016/0012-1606(63)90016-2. [DOI] [PubMed] [Google Scholar]