Abstract
Natalizumab inhibits the influx of leukocytes into the central nervous system (CNS) via blockade of alpha-4 subunit of very late activation antigen (VLA)-4. The association of natalizumab therapy with progressive multifocal leukoencephalopathy (PML) suggests a disturbance of CNS immune surveillance in a small percentage of Multiple Sclerosis (MS) patients exposed to the medication. Natural killer (NK) cells are known to play an important role in modulating the evolution of different phases of this lymphocyte mediated disease, and we investigated the effects of natalizumab on the NK cell phenotype and infiltration in the CNS in experimental autoimmune encephalomyelitis (EAE), a murine model of MS. Our data show that both resting (from naïve mice) and activated (from EAE mice) NK cells express high levels of VLA-4, and anti-VLA-4 antibody treatment significantly decreases NK cells frequency in the CNS of EAE mice. Moreover, we find that anti-VLA-4 possibly impairs NK cells migratory potential, since unblocked VLA-4 expression levels were downregulated on those NK cells that penetrate the CNS. These data suggest that treatment with antibody to VLA-4 may alter immune surveillance of the CNS by impacting NK cell functions and might contribute to the understanding of the mechanisms leading to the development of PML in some MS patients.
Keywords: Natalizumab, VLA-4, Multiple Sclerosis, EAE, PML, Flow cytometry
1. Introduction
Designed on the basis of progenitor antibodies capable of preventing leukocyte accumulation in the central nervous system (CNS) of experimental autoimmune encephalomyelitis (EAE, the mouse model of multiple sclerosis) (Kent et al., 1995; Yednock et al., 1992), natalizumab (Tysabri®) is the first humanized monoclonal antibody approved for treatment of the relapsing-remitting form of multiple sclerosis (MS) (Miller et al, 2003; Biogen Idec, 2011). It selectively binds to the alpha-4 integrin subunit of very late activation antigen-4 (VLA-4, alpha-4/beta-1 integrin) on immune cells, and inhibits transmigration of autoreactive cells across the blood-brain barrier into CNS by blocking the interaction of lymphocyte VLA-4 with its endothelial ligand cell adhesion molecule 1 (VCAM-1) (von Andrian and Engelhardt, 2003). Unfortunately, the impressive efficacy in reducing relapse rates and disease progression obtained in MS patients with this medication (Polman et al., 2006), has been counterbalanced by the association of the use of natalizumab with the occurrence of progressive multifocal leukoencephalopathy (PML), a rare and potentially fatal demyelinating disease caused by JC virus (JCV) (Kleinschmidt-DeMasters and Tyler, 2005; Langer-Gould et al., 2005; Biogen Idec, 2011).
A relationship between duration of therapy with natalizumab and prior exposures to other chemotherapies to treat their MS, have been established as increased risk factors for the development of PML (Kappos et al., 2011; Biogen Idec, 2011). Thus, it is likely that treatment with natalizumab results in impaired immune surveillance of the CNS in an overall small percentage of patients, and the potential for an immune-suppression specific to the CNS could be related to the effects of the drug on particular immune cells subsets. Evidence from studies in patients has shown that natalizumab induces a sustained decrease in immune cell (T and B cells) numbers in the cerebrospinal fluid (CSF) (Stüve et al., 2006) as well as depletion of dendritic cells in cerebral perivascular spaces (del Pilar Martin et al., 2008). Inhibition of T-cell trafficking is thought to underlie both drug efficacy and its potential toxicity. However, the impact on other cell subsets may be relevant and we were interested in evaluating natural killer (NK) cells because of their described immunosuppressive activity, the reduced levels detected in MS subjects (Segal, 2007), and the accelerated onset and increased severity of MOG35–55 peptide induced EAE in NK-depleted mice (Zhang et al., 1997). CD56+ NK cells were reported to increase in peripheral blood of patients after natalizumab treatment (Skarica et al., 2011) that might be part of the mechanism of action of natalizumab. Despite considerable efforts and in part due to limitations from methodologies, the biological activity of NK cells in the CNS during exposure to natalizumab is poorly understood.
Considering that VLA-4 was demonstrated to be expressed in human NK cells (Gismondi et al., 1991; Macías et al., 2000), we wondered 1) whether their homing to CNS would be affected by natalizumab, and 2) whether natalizumab-restricted CNS-infiltrating NK cells would show an altered phenotype. In the present study, we used a murine version of natalizumab, anti-mouse alpha-4 integrin subunit of VLA-4 (CD49d) mAb, to investigate whether natalizumab interferes with NK cells homing to CNS during inflammatory and autoimmune responses within the CNS in an EAE model in C57BL/6 (B6) mice.
2. Materials and methods
2.1. Mice
Female C57BL/6 mice and SCID mice, 7 to 8 weeks of age, were purchased from Taconic (Germantown, NY) and The Jackson Laboratory (Bar Harbor, ME), respectively. All mice were maintained in specific pathogen-free condition in accordance with the guidelines prescribed by the Institutional Animal Care and Use Committee (IACUC) at the animal facilities of Barrow Neurological Institute, St. Joseph’s Hospital and Medical Center.
2.2. Antigens and antibodies
The murine MOG35–55 peptide (M-E-V-G-W-Y-R-S-P-F-SR-V-V-H-L-Y-R-N-G-K) was synthesized (purity > 95%) by BioSynthesis Inc (Lewisville, TX). Purified NA/LE Rat anti-Mouse CD49d mAb (R1–2) used for in vivo blocking VLA-4 was obtained from BD Pharmingen (San Diego, CA). Purified NA/LE Rat IgG2b, κ (BD Pharmingen) was used as the isotype control antibody for anti-CD49d mAb.
2.3. EAE induction
EAE was induced in mice by subcutaneous (s.c.) injections into the flank and tail base with 100 μg of MOG35–55 peptide emulsified in complete Freund’s adjuvant (CFA) containing 500 μg of heat inactivated Mycobacterium tuberculosis (Difco, Detroit, MI). Supplemental injections of 200 ng pertussis toxin were given intravenously (i.v.) on the same day and two days later (ListBiologic, Campbell, CA) (Mendel et al., 1995).
2.4. Treatment regimes
For the blocking experiment, animals were injected with 62.5 μg of anti-Mouse CD49d mAb (R1–2, in 100μl sterile PBS), 62.5 μg of isotype control Ab (in 100μl sterile PBS) or an equivalent volume of sterile PBS by i.v. injection under anesthesia (1 ml/kg 10:1 ketamine: xylazine) on day 0 and day 2 post-immunization (p.i.). The mice were sacrificed on day 5 and day 7 p.i. for the observation of NK cells infiltration in the CNS and the VLA-4 expression level during the initiation stage of EAE.
2.5. Mononuclear cells isolation
Cells from the CNS, spleens and draining lymph nodes were isolated and prepared in single cell suspensions as described previously (Liu et al., 2007; Vollmer et al., 2005). Briefly, splenocytes and lymphocytes were isolated mechanically by gentle scraping of the fresh spleen tissues and lymph nodes through a 70 μM strainer into RPMI 1640 with 1% FBS. After red blood cell lysis with BD Pharm Lyse™ buffer (BD Bioscience, San Jose, CA), cells were washed in culture medium and filtered through a 40 μM strainer. The CNS-infiltrating mononuclear cells were isolated from the fresh brain and spinal cords. The tissues were cut into small pieces, and digested in 10 mM Hepes/NaOH buffer containing 1 mg/ml of collagenase IV (Sigma, St. Louis, MO) at 37°C for 1h. Tissues were then homogenized with a syringe, filtered through a 70 μM cell strainer to obtain single cell suspension, and centrifuged. Cell pellets were resuspended in 30% Percoll and centrifuged against 70% Percoll. The cell monolayer between the 30%–70% Percoll interface was collected. Single cell suspensions were washed in 1% FBS buffer.
2.6. Flow cytometry
106 cells were stained for surface markers with fluorochrome-conjugated mAbs: anti-CD3- Alexa Fluor 647 (17A2), anti-CD4-PeCy7 (RM4–5), anti-CD8α-ApcCy7 (53–6.7), anti-CD19-FITC (1D3), anti-NK 1.1-PerCP-Cy5.5 (PK136), and anti-CD49d-PE (9C10) (BD Pharmingen, San Diego, CA) for 30 min at 4°C. Flow cytometric data were collected on a FACSAria™ flow cytometer (Becton Dickinson, Mountain View, MD) and analyzed with Diva™ software.
2.7. Statistical analyses
A Student’s t-test was used for the comparison of the frequencies of NK, CD4+, CD8+, CD19+ subpopulations of leukocytes in the target organs and the α4 integrin expression levels on NK/T/B cells. A P value <0.05 was considered significant.
3. Results
3.1. Expression of VLA-4 on NK cells
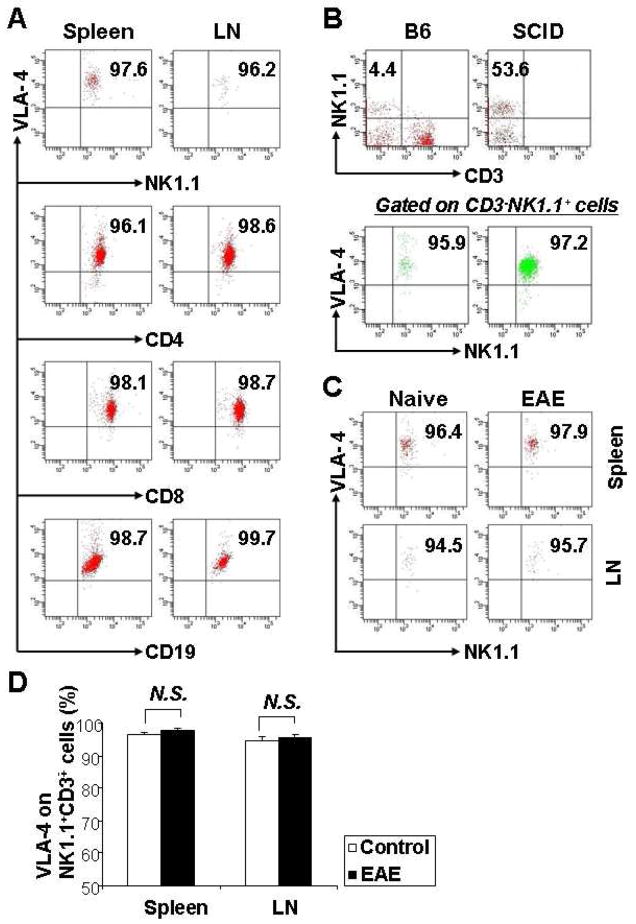
To determine expression of VLA-4 on NK cells, we isolated mononuclear cells from the spleen and lymph nodes of naïve B6 mice, then the cells were stained for surface marker NK1.1 with fluorochrome-conjugated mAb. FACS analysis showed that VLA-4 was expressed on almost all lymphocyte subsets (Table 1). Dot plots of fluorescence for VLA-4 on peripheral lymphocytes are shown in Fig. 1. The expression of VLA-4 on each subpopulation of lymphocytes NK1.1+, CD4+, CD8+, and CD19+ was similar in spleen and lymph nodes (Fig. 1A), which is consistent with the data from the human peripheral blood cells (Ogino et al., 1998).
Table 1. Expression of VLA-4 antigen on lymphocytes.
Expression of VLA-4 antigen were assessed in mononuclear cells isolated from the spleens of naïve B6 mice (n=15) from multiple experiments. Cells were stained for surface markers with fluorochrome-conjugated mAbs as designed and analyzed by FACS. Values are mean ± SD.
| VLA-4 antigen | Spleen (% expression) | Lymph nodes (% expression) |
|---|---|---|
| NK cell | 96.2 ± 3.5 | 96.1 ± 4.1 |
| CD4+ cell | 95.3 ± 5.0 | 96.9 ± 5.9 |
| CD8+ cell | 98.7 ± 7.9 | 98.1 ± 4.2 |
| CD19+ cell | 98.9 ± 6.0 | 99.1 ± 7.5 |
Figure 1.
VLA-4 Expression on NK cells. Mononuclear cells of spleen and/or lymph nodes from naïve B6 mice and SCID mice, MOG35–55-induced EAE mice were harvested, stained with the indicated mAbs, and analyzed by flow cytometry. Dot plots were gated on lymphocytes. (A) VLA-4 expression on NK, CD4+ and CD8+ T cells, CD19+ B cells are shown in representative plots from 15 individual naïve B6 mice. (B) The portion of NK cells in the splenocytes of naïve B6 mice and naïve SCID mice, and their VLA-4 expression are shown as representative of 3 independent experiments (n=2 to 3 mice/group). (C) The VLA-4 expression levels on NK cells from spleen and LN during the peak phase (day 14 post-immunization) of EAE were compared with naïve mice. The plots are representative of 6 separate experiments (n=2 mice/group). (D) There were no significant differences between these two groups (P>0.5). N.S., not significant.
Abbreviation: LN, lymph nodes.
The majority of NK cells (NK1.1+CD3−) expressed high levels of VLA-4 (Fig. 1A upper panel). There were no differences in the positive rate and mean fluorescence intensity of VLA-4 between spleen and lymph nodes (data not shown). Further, we verified the expression pattern of VLA-4 on NK cells from the spleen of Severe Combined Immunodeficiency (SCID) mice, a transgenic strain lacking T and B lymphocytes due to a chromosomal mutation (Dorshkind et al., 1985). We found that SCID mice have a large population of NK cells (mean ~53.6%) with the same levels of expressions of VLA-4 as in NK cells from naïve B6 mice (Fig. 1B).
Some in vitro experiments indicated that cytokines such as IL-2 could upregulate, while IL-4 downregulate VLA-4 expression on human lymphocytes (Macías et al., 2000; Sasaki et al., 2009), and to ascertain whether the microenvironment constituted by chemokines/cytokines (mimicking a disease state) impacted the VLA-4 expression on NK cells, we measured it at the peak phase of the EAE model on B6 mice. There were no differences in naïve B6 mice compared to EAE mice (Fig. 1C); the bar chart shows results from statistic analysis (Fig. 1D)
3.2. Transmigration of NK cells to CNS is impaired by anti-VLA-4
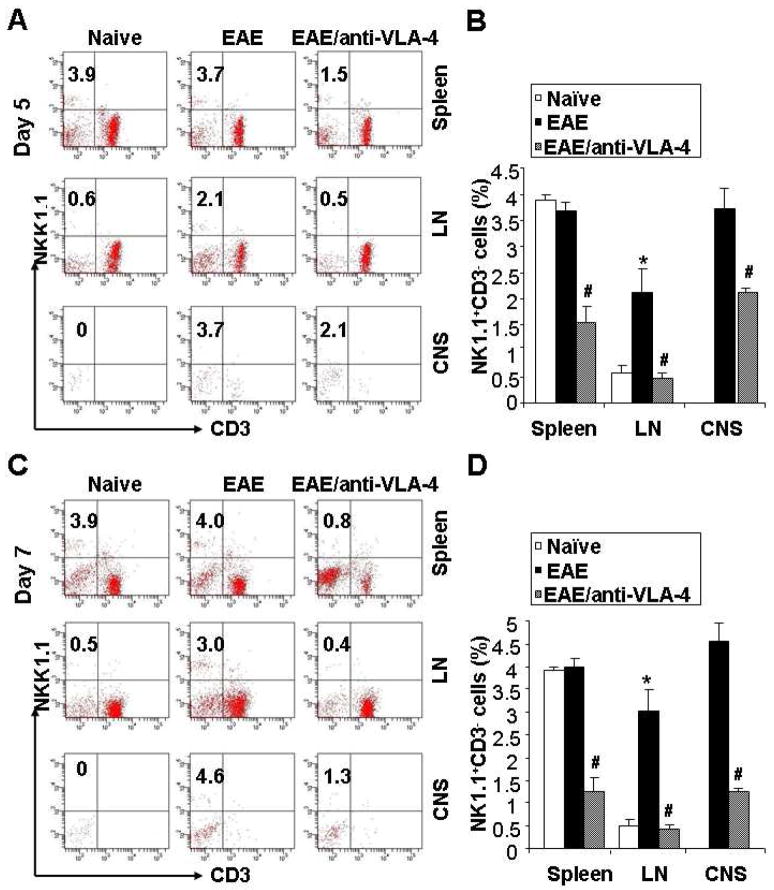
To evaluate the possible functional effects of anti-VLA-4 mAb on the blockade of NK cells transmigration to the CNS, the EAE mice were administrated this antibody on day 0 and day 2 after MOG35–55 immunization. Control animals received the same amount of isotype control Ab or an equivalent volume of sterile PBS. The expected onset of neurological deficits in this EAE model induced with MOG peptide is between day 9 and 12, and to capture the early stages of the disease process when the role of NK cells is at its peak, we sacrificed the mice on day 5 and day 7 p.i.. More NK cells infiltrated the lymph nodes and CNS (including brain and spinal cord, no difference between these two locations, data not shown) of EAE mice compared to naïve mice, while no significant difference was observed in the spleen between these two groups. Anti-VLA-4 administration in vivo demonstrates a dramatic inhibition of NK cells infiltration in the CNS, spleen and lymph nodes (Fig. 2A, 2B). The blockade effects range from 46.5% to 76.2%, among which the inhibition rate in the CNS is 43.2~71.7%. This inhibition persisted throughout the 5 days of observation following the last antibody injection (day 2) (Fig. 2C, 2D). The same blocking effects of anti-VLA-4 were observed in the infiltration of CD4+ and CD8+ T cells, CD19+ B cells into the CNS (data not shown), which is supportive of this being the mechanism of action of natalizumab in human.
Figure 2.
Anti-VLA-4 inhibits infiltration of NK cells into the CNS and peripheral lymph organs during EAE. EAE was induced in mice as described in the Materials and methods. On day 0 (initial of immunization) and day 2 post-immunization, animals received 62.5 μg of anti-Mouse CD49d mAb (R1–2, in 100μl sterile PBS), 62.5 μg of isotype control Ab (in 100μl sterile PBS) or an equivalent volume of sterile PBS by i.v. injection. The mice were sacrificed to observe the frequencies of NK cells in the spleen, lymph nodes (LN) and the CNS on day 5 (A) and day 7 (C). EAE mice showed higher infiltration of NK cells in the LN and CNS (middle column); treatment of anti-VLA-4 significantly blocked the infiltration (right column). The statistical analysis is expressed as the mean percentage of NK1.1+CD3− cells among the total lymphocytes. Values represent the mean ± SEM of four replicates obtained from two different experiments (B and D). *, P<0.01 vs Naïve; #, P<0.01 vs EAE. Abbreviation: LN, lymph nodes.
3.3. Unblocked alpha-4 integrin expression on NK cells is downregulated by anti-VLA-4
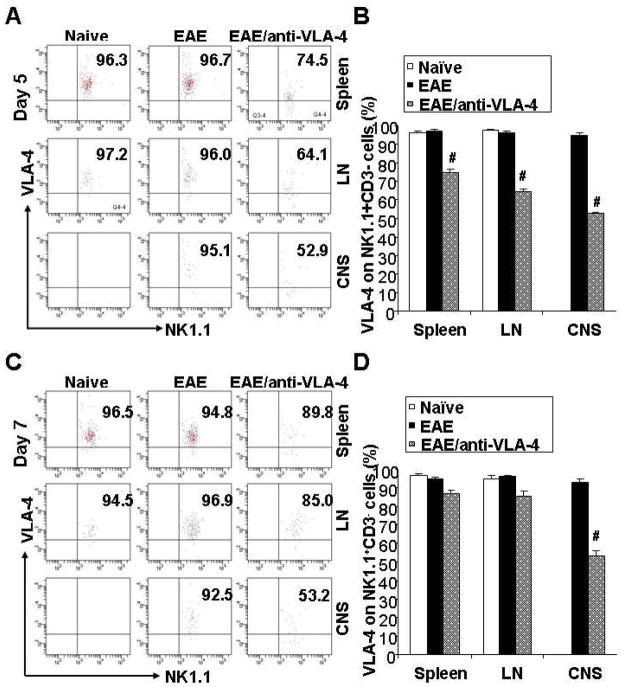
To ascertain whether the binding of anti-VLA-4 to alpha-4 integrin on NK cells surface impact the biological function of these cells, we compared the VLA-4 expression with or without anti-VLA-4 treatment by flow cytometry. Interestingly, we found that the anti-VLA-4 treated mice showed a significant, albeit partial decreased alpha-4 integrin expression on NK cells extracted on day 5 from all three organs, spleen, lymph nodes and the CNS (Fig. 3A, 3B). The decrease in unblocked alpha-4 levels on NK cells in CNS approximated 50%, whereas in spleen and lymph nodes the reduction was 25% and 35%, respectively. On day 7, unblocked α4-integrin expression levels on NK cells in spleen and lymph nodes seemed to recover, while remained low in cells from the CNS (Fig. 3C, 3D). These data suggest that VLA-4 is downregulated as long as antibody titers in the circulation are sufficiently high, and this is consistent with observations that effective antibody concentrations are maintained for several days following the antibody administration (Kent et al., 1995; Khatri et al., 2009).
Figure 3.
Anti-VLA-4 suppresses VLA-4 expression on NK cells in the CNS and peripheral lymph organs during EAE. The induction of EAE model and anti-VLA-4 administration was described previously. The VLA-4 expression levels on NK cells from the Spleen, LN and the CNS on day 5 and day 7 were showed in (A) and (C), respectively. There were no differences between the naive and EAE mice (left two columns of panel A and C). VLA-4 expression was downregulated on NK cells from the anti-VLA-4 treated EAE mice on day 5, compared to non-treated EAE mice (B). The suppression by anti-VLA-4 was released on day 7 in the spleen and LN, but no in the CNS (D). *, P<0.01 vs Naïve; #, P<0.01 vs EAE. Abbreviation: LN, lymph nodes.
4. Discussion
Earlier studies, aimed at clarifying the immunological events associated with the treatment with natalizumab, focused on the most abundant cells of the immune system, namely T and B lymphocytes. Here, we report on the modifications occurring on NK cells, a subset of immune cells whose function is drawing increasing attention from the researchers (Shi and Ransohoff, 2010). Based on the notion that human NK cells express high levels of VLA-4 on the cell surface, we sought to investigate the effects on CNS-infiltrating NK cells and the impact on the CNS immune surveillance of an anti-murine VLA-4 antibody. In accordance with previous data discussed above, our flow cytometric analyses show that both resting (isolated from naïve mice) and activated NK cells (isolated from EAE mice) express high levels of VLA-4 molecules, granting the rationale to study the blockade effects of anti-VLA-4 antibody on NK cells in EAE. In the present study, we extended the existing data on transmigration of T and B lymphocytes, to NK cells. We find that in vivo treatment with anti-VLA-4 can heavily inhibit the NK cells transmigration into the CNS of EAE mice via the blockade of the interaction between VLA-4 on the cell surface and its ligand VCAM-1 on the endothelial cells.
Since the early 1980s, many studies have documented decreased NK cell numbers and impairment of NK cell function in the peripheral blood of patients with MS, while other investigations have suggested that peripheral-blood NK cell functions are defective in systemic autoimmune syndromes secondary to sequestration of NK cells in target tissues, such as the CNS (Flodström-Tullberg et al., 2009). In an assay reproducing pathological conditions, NK cells appear to readily home to the CNS (Shi and Ransohoff, 2010) where they can interact with microglia significantly altering myelin-reactive T cell expansion (Hao et al., 2010; Huang et al., 2006).
Growing evidence from investigations in human (Fearon and Locksley, 1996; Horwitz et al., 1997) and from murine models (Shi et al., 2000; Takeda and Dennert, 1993; Zhang et al., 1997) suggests that NK cells may be involved in immune surveillance of CNS. In this regard, even though direct proof is lacking, we would speculate that a reduction of CNS-resident NK cells might play of contributory role to the risk of PML associated with the use of natalizumab. This opportunistic disease caused by the JCV is typically seen in immuno-compromised individuals, and NK cells, considered part of the innate immune system, are key elements in the initial defense against pathogens. They are particularly important in responding to viral infections (Andoniou et al., 2006; Biron et al., 1999; French and Yokoyama, 2003; Paya et al., 1989) and studies with murine cytomegalovirus (MCMV) proved their function in limiting viral replication in vivo (Sun et al., 2009), while other studies in humans have implicated NK cells in controlling the severity of herpesvirus, viral hepatitis, human immunodeficiency virus (HIV), dengue virus and yellow fever infections (Andoniou et al., 2006). Hence, the relevance of NK cells in limiting viral infections is undisputed and they might furnish a critical contribution to restrict JCV infection as well.
In addition to CNS, we observed that the anti-VLA-4 treated EAE mice had smaller numbers of NK cells in the peripheral lymphatic system, including spleen and lymph nodes, compared to non-treated EAE mice, suggesting that the VLA-4 blockades have a broad impact on the trafficking of NK cells both systemically and to specific organs. We did not measure the frequency of peripheral blood NK cells in the mice, but data from MS patients treated with natalizumab provide evidence of an increase of circulating CD56+ NK cells (Skarica et al., 2011) and allow us to hypothesize that this increase is due to redistribution in lymphoid organs and CNS. Whether such altered distribution of NK cells has an impact on the therapeutic effects of natalizumab remains unknown. The most significant finding of this study is the observation that after anti-VLA-4 exposure there is a decrease in apha-4 integrin surface expression on NK cells, CD4+ T cells, CD8+ T cells, and CD19+ B cells. In line with our results, recent clinical investigations have demonstrated that the levels of surface-bound natalizumab correlate with diminished alpha-4 expression on T, B, NK, and NKT subsets of peripheral blood mononuclear cells from patients on natalizumab therapy (Harrer et al., 2011; Skarica et al., 2011; Stüve et al., 2006). Moreover, we found that while the expression levels on NK cells recovered in the spleen and lymph nodes 5 days after the last antibody injection, they continued to be suppressed throughout the observation period in NK cells extracted from the CNS. We cannot conclude whether anti-VLA-4 exposure causes a genuine downregulation of the molecule or this is an artifact secondary to steric hindrance of antibody binding, as we used a different clone of VLA-4 detection antibody (9C10) compared to the blocking antibody (R1–2). For this reason we applied the term “unblocked” for the detectable VLA-4 molecule by flow cytometry in the context of anti-VLA-4 blocked NK cells.
A tempting interpretation of this fact is that the exposure to the target antibody directly drives the expression of the integrin molecule on the cell surface. We speculate that with the rising titers of the blocking antibody coinciding with the external administrations, the expression of the integrin molecule is downregulated, whereas clearance of the antibody allows recover of the adhesion molecule expression. In support of this view, are an in vitro binding assay on CD3+ T cells that showed an inverse correlation between increased amounts of cell-bound natalizumab and surface expression of VLA subunits (Harrer et al., 2011) and a clinical study that demonstrated how after the typical initial decrease, unblocked alpha-4 integrin expression levels tended to recover and were similar to baseline 14 weeks after the last natalizumab infusion (Wipfler et al., 2011). It is then fair to conclude that natalizumab diminishes the functional surface expression of the alpha-4 integrin hence the migratory capacity of the circulating immune cells and a threshold of unbound VLA-4 may be required for the capacity to migrate across the endothelial barrier thus explaining the impaired circulation of NK cells and other lymphocytes into the CNS.
The reason why NK cells extracted from the CNS as opposed to peripheral lymphoid organs, maintained low levels of integrin expression is unclear, and to clarify the dynamics of NK recirculation further studies that include multiple time-point sampling are needed. At this juncture, we can only speculate that the NK cells confined in the CNS, no longer exposed to the changes of the antibody titers, may not reset their integrin surface molecules.
Finally, in vitro studies showed that the cytokine IL-2 could upregulate while IL-4 downregulate the VLA-4 expression on human lymphocytes (Macías et al., 2000; Sasaki et al., 2009). We attempted to validate these findings but our data collected from the naïve and EAE mice did not yield differences on VLA-4 expression pattern, and argue against a major impact of the cytokine/chemokine milieu on the VLA-4 expression in this EAE model.
In conclusion, our study demonstrates that treatment with a murine anti-VLA-4 alters NK cell distribution and transmigration to the CNS via modification of the VLA-4 expression in an EAE model, and we provide evidence that VLA-4 blockade may interfere with the immune surveillance functions of these cells. The dynamic qualities of the changes induced by the artificial stimulation of the integrin receptor that we observed in the experiments reported here, allow important speculations relevant to the use of natalizumab in human. For instance, manipulation of factors, such as alternative dosing strategies, could further reduce the potential for complications of this highly effective medication and should be further explored.
Acknowledgments
This work was supported in part by grants from Biogen-Idec Corporation (F.-D.S.), Muscular Dystrophy Association (F.-D.S.), Arizona Biomedical Research Commission (F.-D. S.), Barrow Neurological Foundation (F.-D.S.) and NIH R01AI083294 (F.-D.S.).
The authors thank Dr. Junwei Hao, and Ms. Pallavi Dhadvai for the exceptional expert technical assistance.
Footnotes
Disclosures
R. Bomprezzi has functioned as principal investigator in clinical trials sponsored by Genzyme Corporation, Novartis, Merck Serono, Biogen Idec, and UCB and has served as consultant in scientific advisory boards for Teva Pharmaceutical Industries Ltd., EMD Serono, Biogen Idec. and Genzyme Corporation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andoniou CE, Andrews DM, Degli-Esposti MA. Natural killer cells in viral infection: more than just killers. Immunol Rev. 2006;214:239–250. doi: 10.1111/j.1600-065X.2006.00465.x. [DOI] [PubMed] [Google Scholar]
- Biogen Idec. Medical information webpage. 2011 https://medinfo.biogenidec.com/
- Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- del Pilar Martin M, Cravens PD, Winger R, Frohman EM, Racke MK, Eagar TN, Zamvil SS, Weber MS, Hemmer B, Karandikar NJ, Kleinschmidt-DeMasters BK, Stuve O. Decrease in the numbers of dendritic cells and CD4+ T cells in cerebral perivascular spaces due to natalizumab. Arch Neurol. 2008;65:1596–1603. doi: 10.1001/archneur.65.12.noc80051. [DOI] [PubMed] [Google Scholar]
- Dorshkind K, Pollack SB, Bosma MJ, pHILLIPS RA. Natural killer (NK) cells are present in mice with severe combined immunodeficiency (scid) J Immunol. 1985;134:3798–3801. [PubMed] [Google Scholar]
- Fearon DT, Locksley RM. The instructive role of innate immunity in the acquired immune response. Science. 1996;272:50–54. doi: 10.1126/science.272.5258.50. [DOI] [PubMed] [Google Scholar]
- Flodström-Tullberg M, Bryceson YT, Shi FD, Höglund P, Ljunggren HG. Natural killer cells in human autoimmunity. Curr Opin Immunol. 2009;21:634–640. doi: 10.1016/j.coi.2009.09.012. [DOI] [PubMed] [Google Scholar]
- French AR, Yokoyama WM. Natural killer cells and viral infections. Curr Opin Immunol. 2003;15:45–51. doi: 10.1016/s095279150200002x. [DOI] [PubMed] [Google Scholar]
- Gismondi A, Morrone S, Humphries MJ, Piccoli M, Frati L, Santoni A. Human natural killer cells express VLA-4 and VLA-5, which mediate their adhesion to fibronectin. J Immunol. 1991;146:384–392. [PubMed] [Google Scholar]
- Hao J, Liu R, Piao W, Zhou Q, Vollmer TL, Campagnolo DI, Xiang R, La Cava A, Van Kaer L, Shi FD. Central nervous system (CNS)-resident natural killer cells suppress Th17 responses and CNS autoimmune pathology. J Exp Med. 2010;207:1907–1921. doi: 10.1084/jem.20092749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrer A, Wipfler P, Einhaeupl M, Pilz G, Oppermann K, Hitzl W, Afazel S, Haschke-Becher E, Strasser P, Trinka E, Kraus J. Natalizumab therapy decreases surface expression of both VLA-heterodimer subunits on peripheral blood mononuclear cells. J Neuroimmunol. 2011;234:148–154. doi: 10.1016/j.jneuroim.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Horwitz DA, Gray JD, Ohtsuka K, Hirokawa M, Takahashi T. The immunoregulatory effects of NK cells – the role of TGF-β and implications for autoimmunity. Immunol Today. 1997;18:538–542. doi: 10.1016/s0167-5699(97)01149-3. [DOI] [PubMed] [Google Scholar]
- Huang D, Shi F-D, Jung S, Pien GC, Wang J, Salazar-Mather TP, He TT, Weaver JT, Ljunggren H-G, Biron CA, Littman DR, Ransohoff RM. The neuronal chemokine CX3CL1/fractalkine selectively recruits NK cells that modify experimental autoimmune encephalomyelitis within the central nervous system. FASEB J. 2006;20:896–905. doi: 10.1096/fj.05-5465com. [DOI] [PubMed] [Google Scholar]
- Kappos L, Bates D, Edan G, Eraksoy M, Garcia-Merino A, Grigoriadis N, Hartung HP, Havrdová E, Hillert J, Hohlfeld R, Kremenchutzky M, Lyon-Caen O, Miller A, Pozzilli C, Ravnborg M, Saida T, Sindic C, Vass K, Clifford DB, Hauser S, Major EO, O'Connor PW, Weiner HL, Clanet M, Gold R, Hirsch HH, Radü EW, Sørensen PS, King J. Natalizumab treatment for multiple sclerosis: updated recommendations for patient selection and monitoring. Lancet Neurol. 2011;10:745–758. doi: 10.1016/S1474-4422(11)70149-1. [DOI] [PubMed] [Google Scholar]
- Khatri BO, Man S, Giovannoni G, Koo AP, Lee JC, Tucky B, et al. Effect of plasma exchange in accelerating natalizumab clearance and restoring leukocyte function. Neurology. 2009;72:402–409. doi: 10.1212/01.wnl.0000341766.59028.9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent SJ, Karlik SJ, Cannon C, Hines DK, Yednock TA, Fritz LC, Horner HC. A monoclonal antibody to alpha 4 integrin suppresses and reverses active experimental allergic encephalomyelitis. J Neuroimmunol. 1995;58:1–10. doi: 10.1016/0165-5728(94)00165-k. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt-DeMasters BK, Tyler KL. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N Engl J Med. 2005;353:369–374. doi: 10.1056/NEJMoa051782. [DOI] [PubMed] [Google Scholar]
- Langer-Gould A, Atlas SW, Green AJ, Bollen AW, Pelletier D. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N Engl J Med. 2005;353:375–381. doi: 10.1056/NEJMoa051847. [DOI] [PubMed] [Google Scholar]
- Liu JQ, Carl JW, Jr, Joshi PS, RayChaudhury A, Pu XA, Shi FD, Bai XF. CD24 on the resident cells of the central nervous system enhances experimental autoimmune encephalomyelitis. J Immunol. 2007;178:6227–6235. doi: 10.4049/jimmunol.178.10.6227. [DOI] [PubMed] [Google Scholar]
- Macías C, Ballester JM, Hernándeza P. Expression and functional activity of the very late activation antigen-4 molecule on human natural killer cells in different states of activation. Immunology. 2000;100:77–83. doi: 10.1046/j.1365-2567.2000.00994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendel I, Kerlero de Rosbo N, Ben-Nun A. A myelin oligodendrocyte glycoprotein peptide induces typical chronic experimental autoimmune encephalomyelitis in H-2b mice: fine specificity and T cell receptor V beta expression of encephalitogenic T cells. Eur J Immunol. 1995;25:1951–1959. doi: 10.1002/eji.1830250723. [DOI] [PubMed] [Google Scholar]
- Miller DH, Khan OA, Sheremata WA, Blumhardt LD, Rice GPA, Libonati MA, Willmer-Hulme AJ, Dalton CM, Miszkiel KA, O'Connor PW. A controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2003;348:15–23. doi: 10.1056/NEJMoa020696. [DOI] [PubMed] [Google Scholar]
- Ogino M, Namie S, Ozono Y, Miyazaki M, Harada T, Kohno S. Expression of VLA-4 on peripheral mononuclear cells in patients on chronic haemodialysis with carpal tunnel syndrome. Nephrol Dial Transplant. 1998;13:3126–3131. doi: 10.1093/ndt/13.12.3126. [DOI] [PubMed] [Google Scholar]
- Polman CH, O’Connor PW, Havrdova EA, et al. A Randomized , Placebo-Controlled Trial of Natalizumab for Relapsing Multiple Sclerosis. NEJM. 2006;354:899–910. doi: 10.1056/NEJMoa044397. [DOI] [PubMed] [Google Scholar]
- Paya CV, Patick AK, Leibson PJ, Rodriguez M. Role of natural killer cells as immune effectors in encephalitis and demyelination induced by Theiler’s virus. J Immunol. 1989;143:95–102. [PubMed] [Google Scholar]
- Sasaki K, Pardee AD, Qu Y, Zhao X, Ueda R, Kohanbash G, Bailey LM, Okada H, Muthuswamy R, Kalinski P, Basse PH, Falo LD, Storkus WJ. IL-4 suppresses very late antigen-4 expression which is required for therapeutic Th1 T-cell trafficking into tumors. J Immunother. 2009;32:793–802. doi: 10.1097/CJI.0b013e3181acec1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal BM. The role of natural killer cells in curbing neuroinflammation. J Neuroimmunol. 2007;191:2–7. doi: 10.1016/j.jneuroim.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F-D, Ransohoff RM. Nature killer cells in the central nervous system. In: Lotze MT, Thomson AW, editors. Nature Killer Cells. Academic Press; Maryland Heights: 2010. pp. 373–383. [Google Scholar]
- Shi FD, Takeda K, Akira S, Sarvetnick N, Ljunggren HG. IL-18 directs autoreactive T cells and promotes autodestruction in the central nervous system via induction of IFN-gamma by NK cells. J Immunol. 2000;165:3099–3104. doi: 10.4049/jimmunol.165.6.3099. [DOI] [PubMed] [Google Scholar]
- Skarica M, Eckstein C, Whartenby KA, Calabresi PA. Novel mechanisms of immune modulation of natalizumab in multiple sclerosis patients. J Neuroimmunol. 2011;235:70–76. doi: 10.1016/j.jneuroim.2011.02.010. [DOI] [PubMed] [Google Scholar]
- Stüve O, Marra CM, Jerome KR, Cook L, Cravens PD, Cepok S, Frohman EM, Phillips JT, Arendt G, Hemmer B, Monson NL, Racke MK. Immune surveillance in multiple sclerosis patients treated with natalizumab. Ann Neurol. 2006;59:743–747. doi: 10.1002/ana.20858. [DOI] [PubMed] [Google Scholar]
- Sun JC, Beike JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:511–628. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Dennert G. The development of autoimmunity in C57BL/6 lpr mice correlates with the disappearance of natural killer type 1-positive cells: evidence for their suppressive action on bone marrow stem cell proliferation, B cell immunoglobulin secretion, and autoimmune symptoms. J Exp Med. 1993;177:155–164. doi: 10.1084/jem.177.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer TL, Liu R, Price M, Rhodes S, La Cava A, Shi FD. Differential effects of IL-21 during initiation and progression of autoimmunity against neuroantigen. J Immunol. 2005;174:2696–2701. doi: 10.4049/jimmunol.174.5.2696. [DOI] [PubMed] [Google Scholar]
- von Andrian UH, Engelhardt B. Alpha4 integrins as therapeutic targets in autoimmune disease. N Engl J Med. 2003;348:68–72. doi: 10.1056/NEJMe020157. [DOI] [PubMed] [Google Scholar]
- Wipfler P, Oppermann K, Pilz G, Afazel S, Haschke-Becher E, Harrer A, Huemer M, Kunz A, Golaszewski S, Staffen W, Ladurner G, Kraus J. Adhesion molecules are promising candidates to establish surrogate markers for natalizumab treatment. Mult Scler. 2011;17:16–23. doi: 10.1177/1352458510383075. [DOI] [PubMed] [Google Scholar]
- Yednock TA, Cannon C, Fritz LC, Sanchez-Madrid F, Steinman L, Karin N. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature. 1992;356:63–66. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]
- Zhang BN, Yamamura T, Kondo T, Fujiwara M, Tabira T. Regulation of experimental autoimmune encephalomyelitis by natural killer (NK) cells. J Exp Med. 1997;186:1677–1687. doi: 10.1084/jem.186.10.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]