Abstract
Intravenous (i.v.) administration of autoantigen effectively induces Ag-specific tolerance against experimental autoimmune encephalomyelitis (EAE). We and others have shown enhanced EAE severity in mice lacking IL-12 or its receptor, strongly suggesting an immunoregulatory effect of IL-12 signaling. To examine the role of IL-12 responsiveness in autoantigen-induced tolerance in EAE, we administered autoantigen i.v. in two distinct treatment regimes to wildtype and IL-12Rβ2−/− mice, immunized to develop EAE. Administration at the induction phase suppressed EAE in wildtype and IL-12Rβ2−/− mice however the effect was somewhat less potent in the absence of IL-12Rβ2. Expression of pro-inflammatory cytokines such as IFN-γ, IL-17 and IL-2, was inhibited in wild-type tolerized mice but less so in IL-12Rβ2−/− mice. I.v. antigen was also effective in suppressing disease in both genotypes when given during the clinical phase of disease with similar CNS inflammation, demyelination and peripheral inflammatory cytokine profiles observed in both genotypes. There was however a mild impact of a lack of IL-12 signaling on Treg induction during tolerance induction compared to WT mice in this treatment regime. These findings show that the enhanced severity of EAE that occurs in the absence of IL-12 signaling can be effectively overcome by i.v. autoantigen, indicating that this therapeutic effect is not primarily mediated by IL-12 and that i.v. tolerance could be a powerful approach to suppressing severe and aggressive MS.
Keywords: tolerance, experimental autoimmune encephalomyelitis, IL-12 receptor, autoimmunity
1. Introduction
Induction of intravenous (i.v.) tolerance with specific autoantigens has been accomplished by administration of antigen in a variety of tolerogenic forms, including soluble protein/peptide, and antigen-coupled splenocytes (Ferguson et al., 2003; Kennedy et al., 1988; Li et al., 2008; Santambrogio et al., 1995). A range of mechanisms are involved in the induction of i.v. tolerance, including selective suppression of antigen-specific Th1 cytokines and induction of regulatory cytokines (Benson et al., 2000; Ilarregui et al., 2009; Smith and Miller, 2006). We have shown that when soluble antigen is administered after clinical disease onset, clonal deletion of antigen-reactive T cells by apoptosis is a major mechanism for the reversal of EAE (Zhang et al., 1999). In contrast, when soluble antigen is administered before or immediately after immunization for EAE induction, an antigen-specific regulatory mechanism, but not deletion, prevails and appears to be the main mechanism for tolerance induction (Hilliard et al., 2000).
IL-12 is a heterodimeric cytokine composed of a heavy chain (p40) and a light chain (p35). Heterodimeric IL-12 is produced mainly by activated APCs and has been shown to play an important role in the differentiation and expansion of Th1 cells (Mullen et al., 2001; Trinchieri, 1998). IL-12 exerts its effects through a receptor (IL-12R) composed of two chains, IL-12Rβ1 and IL-12Rβ2 (Gately, 1998). In the mouse, IL-12Rβ1 mediates both low and high affinity binding to IL-12, whereas IL-12Rβ2 is thought to mediate transmembrane signaling, with negligible effects on binding (Gately, 1998). Gene targeting studies suggest that the absence of either IL-12Rβ1 or IL-12Rβ2 impairs functional responses to IL-12 (Wu et al., 1997; Wu et al., 2000). The p40 subunit of IL-12 is also shared by IL-23, also a heterodimeric cytokine (p40 and p19 subunits) which has a strong effect in promoting the function and survival of Th17 cells (Langrish et al., 2005). The receptor for IL-23 is a heterodimer composed of IL-12Rβ1 and IL-23R (Parham et al., 2002). IL-12Rβ2 is required for biological response of IL-12 but not IL-23. Thus, the IL-12Rβ2-deficient model is a valuable tool to differentiate the role of these closely related cytokines and to specifically investigate a role for IL-12 signaling in the induction of tolerance. The enhanced clinical and pathological disease in this EAE model also provides an opportunity to test the effect of i.v. tolerance on highly severe EAE, which is of significant importance for clinical therapy of aggressive MS.
2. METHODS
Mice, EAE induction, and i.v. tolerance induction
Female, 8–12 week old, IL-12Rβ2−/− mice and wild type C57BL/6 mice were purchased from the Jackson Laboratories (Bar Harbor, ME). To induce EAE, mice were injected subcutaneously with 200 μg MOG35-55 in CFA containing 4 mg/ml Mycobacterium tuberculosis H37Ra (Difco, Detroit, MI) over 2 sites on the back. Two hundred ng pertussis toxin was given i.p. on days 0 and 2 post immunization (p.i.). To induce i.v. tolerance, MOG35-55 at a dose of 200 μg/mouse was i.v. injected either on days −3, 0, +6 p.i or at days 14 and 17 p.i. Identical volumes (100 μl) of PBS were i.v. injected in parallel to serve as controls. EAE was scored according to a 0–5 scale as follows (Benson et al., 2000): 1, limp tail or waddling gait with tail tonicity; 2, waddling gait with limp tail (ataxia); 2.5, ataxia with partial limb paralysis; 3, full paralysis of one limb; 3.5, full paralysis of one limb with partial paralysis of second limb; 4, full paralysis of 2 limbs; 4.5, moribund; and 5, death. All work was performed in accordance with the Thomas Jefferson University guidelines for animal use and care.
Histopathology
Mice were extensively perfused transcardially with PBS and spinal cords were harvested. Five-μm sections were stained with H&E or Luxol fast blue (myelin stain) to examine inflammation and demyelination respectively.
Flow cytometric analysis of mononuclear cells (MNCs) in the CNS
MNCs from pooled spinal cords were isolated as previously described (Zhang et al., 1999). Cells were washed in staining buffer (1% FCS + 0.1% NaN3 in PBS). After blocking with anti-CD16/CD32 antibodies, cells were incubated with fluorescent antibodies to murine CD4 and CD11b (BD PharMingen, San Jose, CA) for 20 mins in the dark at 4° C. Cells were fixed and data were acquired on a FACSAria (Becton-Dickinson, Mountain View, CA). MNCs were gated and fluorescence was analyzed using CellQuest (Beckson-Dickson) software.
Cytokine production and proliferation assay
Suspensions of MNCs from spleen were prepared as previously described (Zhang et al., 2002). Cells were cultured at a density of 2.5 × 106/ml in complete RPMI with 10% FCS and activated with MOG35-55 (25 μg/ml), Con A (5 μg/ml), or cultured without antigen/mitogen. Supernatants were collected after 48 h. Quantitative ELISAs for IFN-γ IL-2, TNF-α, and IL-17 were performed using paired mAbs according to the manufacturer's recommendation (BD PharMingen). To assess proliferation, cells were cultured in triplicate with of MOG35-55 (25 μg/ml), Con A (5 μg/ml), or without antigen/mitogen. After 60 h, cells were pulsed for 12 h with 1 μCi of 3H-methylthymidine. Cells were harvested and analyzed using a β-counter. Results were expressed as cpm or stimulation index (SI).
Statistics
Datasets were analyzed for statistical significance using unpaired Student's t tests or Mann-Whitney tests for non-parametric data and tests were performed on values of area under the curve for each mouse in EAE clinical score datasets.
3. RESULTS
Intravenous MOG35-55 given at disease induction inhibits severe EAE in IL-12Rβ2−/− mice
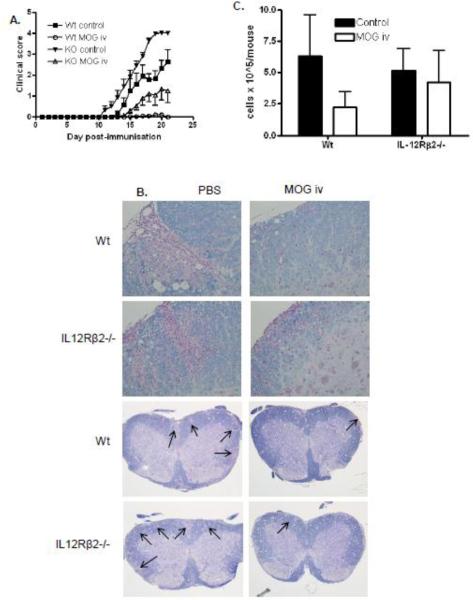
Previously we have shown that IL-12Rβ2−/− mice developed earlier and more severe EAE than wildtype animals, with significantly enhanced antigen-specific IL-17 responses (Zhang et al., 2003). We have shown that complete tolerance to EAE can be induced with MOG35-55 given i.v. before and shortly after EAE immunization of wildtype mice (Zhang et al., 2005). To investigate if i.v. tolerance can suppress severe EAE and if IL-12R signaling is required for i.v. tolerance, we immunized IL-12Rβ2−/− mice, and wildtype control mice, to develop EAE and induced tolerance with i.v. autoantigen at the induction phase of disease. To this end, wildtype and IL-12Rβ2−/− mice were given either MOG35-55 (200 μg/mouse/day) i.v. or an equivalent volume of PBS i.v. on days −3, 0 and +6 of EAE immunization. With this treatment regime, i.v. MOG35-55 completely suppressed the development of EAE in wildtype mice (Fig. 1A; data pooled from 3 independent experiments). Interestingly however, the induction of tolerance was significant, but incomplete in IL-12Rβ2−/− mice (Fig. 1A) suggesting, that IL-12R signaling may play a partial, albeit minor role in the induction of tolerance by i.v. autoantigen during the priming phase of disease, before clinical signs have developed. It is also possible that the extent of tolerance was similar in both genotypes but incomplete in IL-12Rβ2−/− mice due to enhanced disease severity. However neither interpretation supports a major role for IL-12 signaling in the early induction of tolerance.
Figure 1. Intravenous MOG35-55 given at disease induction inhibits severe EAE in IL-12Rβ2−/− mice.
Wildtype and IL-12Rβ2−/− mice immunized to develop EAE were given MOG35-55 i.v. on days −3, 0 and +6 days p.i. (200 μg/mouse/day). (A) Mice were scored daily for clinical signs (n=7–9). (B) Spinal cord inflammation and demyelination were characterized histologically. (C) Mononuclear cells recovered from pooled spinal cords of each group were quantified between days 14–21 p.i. (n=3). Results shown are pooled from three independent experiments with a total n=7–9 mice. WT control vs. WT MOG ***p=0.0003, KO control vs. KO MOG **p=0.0038, both statistically significant.
We examined the pathological and immunological profile of the CNS at day 14–21 post immunization (p.i.). Spinal cord histopathology demonstrated extensive inflammation and demyelination in the spinal cords of PBS-treated mice of both genotypes with the most severe pathology clearly evident in IL-12Rβ2−/− mice (Fig. 1B). However, little pathology was detectable in wildtype mice treated with MOG35-55 i.v. (Fig. 1B). Moderate inflammation and demyelination was detectable in the spinal cords of IL-12Rβ2−/− mice treated with MOG35-55 i.v. demonstrating CNS immunopathology as a result of incomplete induction of tolerance (Fig 1B). Analysis of mononuclear cells recovered from pooled spinal cords showed considerable variability, however, there was a clear trend of reduced CNS infiltration in tolerized WT mice compared to PBS treated controls, which was not as striking in IL-12Rβ2−/− mice (Fig. 1C).
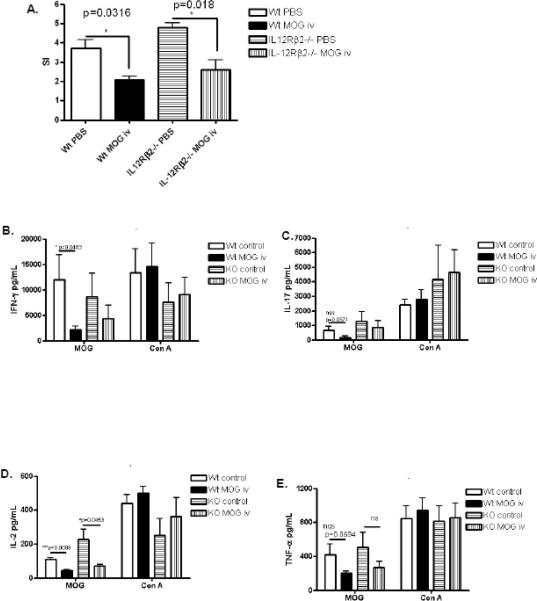
We also examined the profile of the peripheral immune system at day 14–121 post-immunization in this model of early tolerance induction. We found significant suppression of proliferative responses to MOG35-55 in wildtype and IL-12Rβ2−/− mice that received MOG35-55 i.v. suggesting that IL-12 signaling does not play a major role in suppression of T cell proliferation induced by tolerance (Fig. 2A). Splenocytes from these mice were cultured with MOG35-55 or Con A and pro-inflammatory cytokine expression was measured by ELISA. Administration of i.v. autoantigen did not affect mitogen-stimulated expression of IL-2, IL-17, IFN-γ or TNF-α (Fig. 2 B–E). However, activation of splenocytes with MOG35-55 demonstrated that in WT animals, i.v. autoantigen significantly inhibited IFN-γ and IL-2 expression with a trend towards suppression of IL-17 and TNF-α. While there was a trend towards suppression of these cytokines in tolerized IL-12Rβ2−/− mice, this only reached statistical significance in the case of IL-2 (Fig. 2D) suggesting that IL-12 signaling may play a partial role in the suppression of pro-inflammatory cytokines such as IFN-γ, IL-17 and TNF-α during early tolerance induction.
Figure 2. Peripheral immune response during EAE and early tolerance induction.
Splenocytes were harvested between days 14–21 p.i. and stimulated with MOG35-55 or concanavalin A. (A) Proliferative responses were measured in MOG-stimulated cells by [3H]thymidine incorporation after 60 hours (n=3–4). (B–E) Cytokine production was measured by ELISA after 48 hours, (n=4). Results shown are pooled from four independent experiments.
Intravenous tolerance effectively overcomes enhanced ongoing EAE in IL-12Rβ2−/− mice
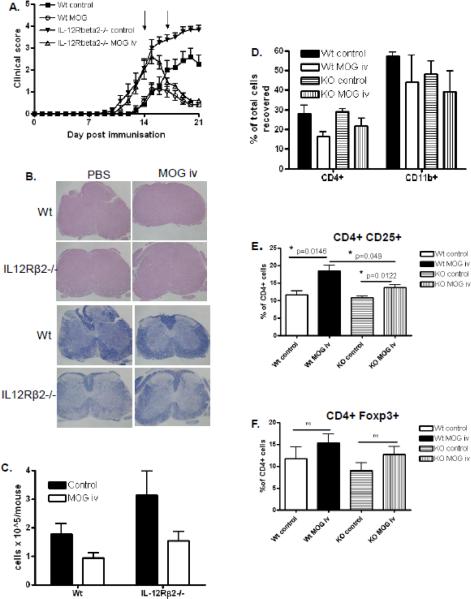
To investigate if i.v. tolerance can also suppress severe EAE during the clinical phase of disease which is relevant to treating human disease, we immunized IL-12Rβ2−/− mice and wildtype controls to develop EAE and i.v. injected either MOG35-55 (200 μg/mouse/day) or an equivalent volume of PBS as a control on days 14 and 17 p.i. via the tail vein. In agreement with our previous findings (Zhang et al., 2003), IL-12Rβ2−/− mice developed earlier and more severe EAE than wildtype animals (Fig. 3A; data pooled from 3 independent experiments). Intravenous administration of MOG35-55 during the clinical phase (day 14 and 17) effectively suppressed disease in wildtype mice and also in IL-12Rβ2−/− mice. Interestingly, and somewhat in contrast to early tolerance induction (Fig 1A), administration of i.v. autoantigen during clinical disease suppressed EAE in both genotypes to a comparable degree, despite enhanced severity of EAE in IL-12Rβ2−/− mice (Fig 3A; data pooled from 3 independent experiments) demonstrating the potency of this therapeutic approach. Histopathology of spinal cords of mice in each group correlated with clinical findings. More extensive inflammation (H+E) and demyelination (Luxol Fast Blue; LFB) was observed in IL-12Rβ2−/− mice compared to wildtype mice and i.v. antigen largely inhibited the development of pathology in both genotypes (Fig 3B). Analysis of mononuclear cells recovered from pooled spinal cords also correlated with clinical disease as the greatest yield was from spinal cords of the IL-12Rβ2−/− group and i.v. tolerance resulted in less infiltration of mononuclear cells in both genotypes (Fig 3C; data pooled from 3 independent experiments). Qualitative immunophenotyping of mononuclear cells isolated from spinal cords showed that i.v. tolerance in both genotypes resulted in a mild reduction of CD4+ cells as well as CD11b+ cells (Fig 3D; data pooled from 3 independent experiments), however, as spinal cords were pooled in each of 3 experiments, statistical analysis was not possible. The findings from this study demonstrate that i.v. tolerance is an effective approach for suppressing ongoing EAE, even in a highly enhanced EAE model, and the mechanism of i.v. tolerance during clinical disease is independent of IL-12R signaling.
Figure 3. Intravenous MOG35-55 given during clinical disease inhibits severe EAE.
Wildtype and IL-12Rβ2−/− mice immunized to develop EAE were given MOG35-55 i.v. on days 14 and 17 p.i. (200μg/mouse/day). (A) Mice were scored daily for clinical signs. (B) Spinal cord inflammation and demyelination were characterized histologically. (C) Mononuclear cells recovered from pooled spinal cords of each group were quantified between days 18–21 p.i. (n=3). (D) Mononuclear cells from CNS tissue were immunophenotyped by flow cytometry. Splenocytes were harvested between days 18–21 p.i., stained with fluorescently labeled antibodies and analyzed by flow cytometry. (E) Proportions of CD4+CD25+ Treg in splenocyte CD4+ populations (n=4–5). (F) Proportions of CD4+Foxp3+ Treg in splenocyte CD4+ populations (n=4–5). Results shown are pooled from three independent experiments with a total n=8–10 mice. WT control vs. WT MOG *p=0.0311, KO control vs. KO MOG **p=0.0015, both statistically significant.
To investigate if similar tolerogenic mechanisms were at play both wildtype and IL-12Rβ2−/− mice, we analysed the recall immune response of each group. Proliferative responses to MOG antigen and Con A did not differ significantly in control groups of each genotype compared to MOG-treated mice (data not shown). We examined pro-inflammatory cytokine production by antigen (MOG35-55) and mitogen (Con A) stimulated splenocytes from control and tolerized mice and while there were trends of suppression of IFN-γ, IL-17 and IL-2 in wildtype mice, these did not reach statistical significance (data not shown). Similar trends were observed in IL-12Rβ2−/− groups suggesting that mechanistically, the regulation of pro-inflammatory cytokines during tolerance is not dependent on IL-12 signaling in the effector phase of disease. These data also differ somewhat from pro-inflammatory cytokine profiles that were suppressed significantly when tolerance was induced at the induction phase of disease (Figure 2) reinforcing the concept of distinct tolerogenic mechanisms at different phases of disease.
We next examined the proportion of regulatory T cells in the peripheral immune system of tolerized and control EAE mice. The frequency of naturally occurring CD4+CD25+ regulatory T cells was comparable in both wildtype and IL-12Rβ2−/− mice (Fig. 3E) and this proportion significantly increased in both genotypes after i.v. autoantigen. However, the increase in the WT group was more robust than that observed in the IL-12Rβ2−/− group which resulted in a significantly higher proportion of CD4+CD25+ Tregs in tolerized WT mice than tolerized IL-12Rβ2−/− mice (Fig. 3E). This suggests that IL-12 signaling may contribute to autoantigen-induced expansion of Tregs. There was also a trend towards increased Foxp3+ cells also which did not quite reach statistical significance (Fig. 3F). These data demonstrate robust increases in Tregs as a consequence of i.v. autoantigen and while this is not dependent on IL-12 signaling, there does appear to be an influence of IL-12 signaling in Treg expansion during tolerance.
4. DISCUSSION
Here we report that i.v. autoantigen efficiently induced tolerance to EAE even in the clinically severe model of EAE that develops in IL-12Rβ2−/− mice. This finding also demonstrates that IL-12 signaling does not play a major role in tolerance induction. Tolerance resulted in reduced infiltration of inflammatory cells into the CNS and concomitantly less inflammatory lesions and demyelination. Expansion of Tregs in the periphery was a feature of tolerance induction in both genotypes and is likely a major cellular mechanism limiting EAE in this model.
Interestingly however, IL-12 signaling appeared to be somewhat more influential in tolerance induced during the priming phase compared to during the effector phase of EAE. While treatment of WT mice with MOG35-55 i.v. at day −3, 0 and +6 of immunization completely suppressed the development of disease, parallel treatment of IL-12Rβ2−/− mice resulted in only partial tolerance. It is notable however that, as we have previously reported (Zhang et al., 2003), IL-12Rβ2−/− mice develop more severe EAE than WT and thus it is possible that incomplete tolerance is due to the magnitude of disease. In this early treatment regime, in contrast to treatment during clinical disease, significant suppression of pro-inflammatory cytokine responses to antigen stimulation were observed in WT mice, however while IL-12Rβ2−/− mice showed similar trends, of the cytokines examined, only IL-2 expression was significantly suppressed in the absence of IL-12 signaling. This would suggest that IL-12 signaling contributes to constraint of the expression of pro-inflammatory cytokines such as IL-17, IFN-γ and TNF-α by antigen-specific cells. Interestingly, there was no suppression of pro-inflammatory cytokine responses to mitogen stimulation in tolerized mice of either genotype demonstrating that immunoregulatory pathways induced by i.v. autoantigen targeted antigen specific responses.
However, the data presented here demonstrate that IL-12 signaling plays a complex role during the induction of tolerance as the presence of exogenous IL-12 and the lack of IL-12 signaling both prevent complete induction of tolerance (Zhang et al., 2002). These seemingly contradictory findings may be explained by recent studies showing that IFN-γ and other Th1-associated factors promote the development of induced Tregs from CD4+CD25− cells both in vivo and in vitro (Hong et al., 2005; Ouaked et al., 2009; Wang et al., 2006). IL-12 potently induces IFN-γ and a lack of IL-12 signaling may result in changes to IFN-γ production in vivo that affects the downstream production of Tregs. Indeed, our data here show significantly less Treg induction following i.v. autoantigen during clinical disease in the absence of IL-12Rβ2. However, it is important to note that our analysis of changes in CD4+ CD25+ Treg may include predominantly naturally occurring Treg but also a population of effector T cells that upregulated CD25 expression following activation. Indeed, given the complete induction of tolerance in IL12Rβ2−/− mice in the face of severe EAE and our cautious interpretation of impaired Treg generation in IL-12Rβ2−/− mice, these findings would argue against Treg as principal mediators of tolerance in this model The role of exogenous IL-12 in abrogating the induction of tolerance is also understandable given the potent role of IL-12 in inducing pro-inflammatory Th1 cells (Gately, 1998; Trinchieri, 1998).
While Ouaked et al. did not find an effect of exogenous IL-12 on Treg development in vitro (Ouaked et al., 2009), the data presented above suggest that IL-12 signaling plays a role in the development of CD4+CD25+ Tregs in vivo. In support of this, we have previously shown that induction of Treg by TGF-β is significantly impaired in IL-12Rβ2−/− mice (Zhao et al., 2008). Interestingly, Ilarregui et al. observed increased IL-12 production from T cells incubated with tolerogenic dendritic cells and also observed that incubation of CD4+ T cells with the same tolerogenic dendritic cells led to increased proportions of CD4+CD25+ Tregs (Ilarregui et al., 2009). The moderate difference in efficacy of tolerance induction in IL-12Rβ2−/− mice at induction versus effector phases of EAE reported here aligns with our previously published findings. These studies showed that apoptosis of effector cells primarily underlies tolerance induced at the effector stage while tolerance induction at the time of immunization is dependent on an antigen specific immunoregulatory mechanism that may include CD4+CD25+ Treg cells (Zhang et al., 1999; Zhang et al., 2002).
Taken together, our studies demonstrate that enhanced severity of clinical EAE and pro-inflammatory responses can be effectively overcome by i.v. autoantigen, indicating that i.v. tolerance may be a powerful approach to suppress severe and aggressive MS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests: The authors declare no competing interests
Authors' contributions: GXZ designed the study and co-wrote the manuscript. DF analyzed and interpreted the data and co-wrote the manuscript. SY, ZZ and MC performed experiments. AR oversaw the study and the writing of the manuscript.
6. REFERENCES
- Benson JM, Campbell KA, Guan Z, Gienapp IE, Stuckman SS, Forsthuber T, Whitacre CC. T-cell activation and receptor downmodulation precede deletion induced by mucosally administered antigen. J Clin Invest. 2000;106:1031–1038. doi: 10.1172/JCI10738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson TA, Stuart PM, Herndon JM, Griffith TS. Apoptosis, tolerance, and regulatory T cells--old wine, new wineskins. Immunol Rev. 2003;193:111–123. doi: 10.1034/j.1600-065x.2003.00042.x. [DOI] [PubMed] [Google Scholar]
- Gately MK, Renzetti LM, Magram J, Stern AS, Adorini L, Gubler U, Presky DH. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu Rev Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- Hilliard BA, Kamoun M, Ventura E, Rostami A. Mechanisms of suppression of experimental autoimmune encephalomyelitis by intravenous administration of myelin basic protein: role of regulatory spleen cells. Exp Mol Pathol. 2000;68:29–37. doi: 10.1006/exmp.1999.2290. [DOI] [PubMed] [Google Scholar]
- Hong J, Li N, Zhang X, Zheng B, Zhang JZ. Induction of CD4+CD25+ regulatory T cells by copolymer-I through activation of transcription factor Foxp3. Proc Natl Acad Sci U S A. 2005;102:6449–6454. doi: 10.1073/pnas.0502187102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilarregui JM, Croci DO, Bianco GA, Toscano MA, Salatino M, Vermeulen ME, Geffner JR, Rabinovich GA. Tolerogenic signals delivered by dendritic cells to T cells through a galectin-1-driven immunoregulatory circuit involving interleukin 27 and interleukin 10. Nat Immunol. 2009;10:981–991. doi: 10.1038/ni.1772. [DOI] [PubMed] [Google Scholar]
- Kennedy MK, Dal Canto MC, Trotter JL, Miller SD. Specific immune regulation of chronic-relapsing experimental allergic encephalomyelitis in mice. J Immunol. 1988;141:2986–2993. [PubMed] [Google Scholar]
- Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Zhang GX, Chen Y, Xu H, Fitzgerald DC, Zhao Z, Rostami A. CD11c+CD11b+ dendritic cells play an important role in intravenous tolerance and the suppression of experimental autoimmune encephalomyelitis. J Immunol. 2008;181:2483–2493. doi: 10.4049/jimmunol.181.4.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen AC, High FA, Hutchins AS, Lee HW, Villarino AV, Livingston DM, Kung AL, Cereb N, Yao TP, Yang SY, Reiner SL. Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science. 2001;292:1907–1910. doi: 10.1126/science.1059835. [DOI] [PubMed] [Google Scholar]
- Ouaked N, Mantel PY, Bassin C, Burgler S, Siegmund K, Akdis CA, Schmidt-Weber CB. Regulation of the foxp3 gene by the Th1 cytokines: the role of IL-27-induced STAT1. J Immunol. 2009;182:1041–1049. doi: 10.4049/jimmunol.182.2.1041. [DOI] [PubMed] [Google Scholar]
- Parham C, Chirica M, Timans J, Vaisberg E, Travis M, Cheung J, Pflanz S, Zhang R, Singh KP, Vega F, To W, Wagner J, O'Farrell AM, McClanahan T, Zurawski S, Hannum C, Gorman D, Rennick DM, Kastelein RA, De Waal Malefyt R, Moore KW. A Receptor for the Heterodimeric Cytokine IL-23 Is Composed of IL-12Rbeta1 and a Novel Cytokine Receptor Subunit, IL-23R. J Immunol. 2002;168:5699–5708. doi: 10.4049/jimmunol.168.11.5699. [DOI] [PubMed] [Google Scholar]
- Santambrogio L, Crisi GM, Leu J, Hochwald GM, Ryan T, Thorbecke GJ. Tolerogenic forms of auto-antigens and cytokines in the induction of resistance to experimental allergic encephalomyelitis. J Neuroimmunol. 1995;58:211–222. doi: 10.1016/0165-5728(95)00022-t. [DOI] [PubMed] [Google Scholar]
- Smith CE, Miller SD. Multi-peptide coupled-cell tolerance ameliorates ongoing relapsing EAE associated with multiple pathogenic autoreactivities. J Autoimmun. 2006;27:218–231. doi: 10.1016/j.jaut.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G. Interleukin-12: a cytokine at the interface of inflammation and immunity. Adv Immunol. 1998;70:83–243. doi: 10.1016/s0065-2776(08)60387-9. [DOI] [PubMed] [Google Scholar]
- Wang Z, Hong J, Sun W, Xu G, Li N, Chen X, Liu A, Xu L, Sun B, Zhang JZ. Role of IFN-gamma in induction of Foxp3 and conversion of CD4+ CD25− T cells to CD4+ Tregs. J Clin Invest. 2006;116:2434–2441. doi: 10.1172/JCI25826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Ferrante J, Gately MK, Magram J. Characterization of IL-12 receptor beta1 chain (IL-12Rbeta1)-deficient mice: IL-12Rbeta1 is an essential component of the functional mouse IL-12 receptor. J Immunol. 1997;159:1658–1665. [PubMed] [Google Scholar]
- Wu C, Wang X, Gadina M, O'Shea JJ, Presky DH, Magram J. IL-12 receptor beta 2 (IL-12R beta 2)-deficient mice are defective in IL-12-mediated signaling despite the presence of high affinity IL-12 binding sites. J Immunol. 2000;165:6221–6228. doi: 10.4049/jimmunol.165.11.6221. [DOI] [PubMed] [Google Scholar]
- Zhang GX, Gran B, Yu S, Li J, Siglienti I, Chen X, Kamoun M, Rostami A. Induction of experimental autoimmune encephalomyelitis in IL-12 receptor-beta 2-deficient mice: IL-12 responsiveness is not required in the pathogenesis of inflammatory demyelination in the central nervous system. J Immunol. 2003;170:2153–2160. doi: 10.4049/jimmunol.170.4.2153. [DOI] [PubMed] [Google Scholar]
- Zhang GX, Liu TT, Ventura ES, Chen Y, Rostami A. Reversal of spontaneous progressive autoimmune encephalomyelitis by myelin basic protein-induced clonal deletion. Autoimmunity. 1999;31:219–227. doi: 10.3109/08916939908994067. [DOI] [PubMed] [Google Scholar]
- Zhang GX, Xu H, Kishi M, Calida D, Rostami A. The role of IL-12 in the induction of intravenous tolerance in experimental autoimmune encephalomyelitis. J Immunol. 2002;168:2501–2507. doi: 10.4049/jimmunol.168.5.2501. [DOI] [PubMed] [Google Scholar]
- Zhang GX, Yu S, Li Y, Ventura ES, Gran B, Rostami A. A paradoxical role of APCs in the induction of intravenous tolerance in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2005;161:101–112. doi: 10.1016/j.jneuroim.2004.12.017. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Yu S, Fitzgerald DC, Elbehi M, Ciric B, Rostami AM, Zhang GX. IL-12R beta 2 promotes the development of CD4+CD25+ regulatory T cells. J Immunol. 2008;181:3870–3876. doi: 10.4049/jimmunol.181.6.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]