Abstract
The interaction between the endocannabinoid system and catecholaminergic circuits has gained increasing attention as it is recognized that the development of synthetic cannabinoid receptor agonists/antagonists or compounds targeting endocannabinoid synthesis/metabolism may hold some therapeutic potential for the treatment of psychiatric disorders. The noradrenergic system plays a critical role in the modulation of emotional state, primarily related to anxiety, arousal, and stress. Recent evidence suggests that the endocannabinoid system mediates stress responses and emotional homeostasis, in part, by targeting noradrenergic circuits. This review summarizes our current knowledge regarding the anatomical substrates underlying regulation of noradrenergic circuitry by the endocannabinoid system. It then presents biochemical evidence showing an important effect of cannabinoid modulation on adrenergic receptor signaling. Finally, new evidence from behavioral pharmacology studies is provided demonstrating that norepinephrine is a critical determinant of cannabinoid-induced aversion, adding another dimension to how central noradrenergic circuitry is regulated by the cannabinoid system.
Keywords: Cannabinoid receptor type1, adrenergic receptor, nucleus accumbens, nucleus of the solitary tract, Sprague-Dawley
Introduction
For centuries, cannabis preparations have been used for their medicinal properties. However, psychotropic and mood altering properties are common and cannabis users have described “visions of devils” and “communication with spirits” (Zuardi, 2006). In the Western world, the use of cannabis for therapeutic purposes did not reach prominence primarily due to difficulties in obtaining reproducible effects in clinical studies, and because of the development of more effective medications. However, cannabis has been, and still is, used for recreational purposes and is exploited for its euphoric and sedative properties. Nevertheless, adverse effects, such as anxiety, panic and depression, are also commonly reported (Johns, 2001).
A link between cannabis use and the development of serious mental illnesses, including schizophrenia, bipolar disease and major depression, has been debated for several decades (Johns, 2001; Degenhardt et al., 2003; Strakowski et al., 2007; van Rossum et al., 2009). It is still not clear whether cannabis use can trigger or facilitate the onset of a psychiatric disorder or whether the genetic predisposition for mental illness leads to consumption of cannabis to compensate for any disturbance in the endocannabinoid system. In summary, there is a significant amount of evidence implicating the endocannabinoid system in psychiatric disorders (Degenhardt et al., 2003; Viveros et al., 2005; Fernandez-Espejo et al., 2009; Parolaro et al., 2010). Considering that the monoamine system is critically involved in the pathophysiology of depression, anxiety and post-traumatic stress disorder (PTSD), the goal of the present review is to explore the association between the endocannabinoid and noradrenergic systems with a particular emphasis on the pathophysiology of psychiatric disorders.
1. Cannabinoids, norepinephrine and mood regulation
There are a number of contradictory reports in the literature regarding the effects of cannabinoids on mood. For example, both cannabinoid type 1 receptor (CB1r) agonists (Gobbi et al., 2005; Hill & Gorzalka, 2005; Morrish et al., 2009) and antagonists (Shearman et al., 2003; Tzavara et al., 2003; Griebel et al., 2005) have been shown to exert an antidepressant-like effect in pre-clinical animal studies. Furthermore, cannabinoid receptor agonists/antagonists have been shown to exert anxiolytic effects in some studies but anxiogenic effects in others (Haller et al., 2004b; Degroot, 2008; Moreira & Lutz, 2008; Carvalho et al., 2010b). In human studies, dual effects have been reported. Occasional users often report that cannabis increases well-being, euphoria and contentment (Velez et al., 1989). However, increased anxiety, dysphoria and depressive mood have been reported following moderate cannabis use (Reilly et al., 1998). The use of cannabis seems to exacerbate psychotic symptoms, such as delusions and hallucinations (Negrete et al., 1986; Cleghorn et al., 1991; Baigent et al., 1995), as well as increase anxiety and symptoms of psychosis (Morrison et al., 2009). Adverse effects of cannabis have been linked to potential toxic effects induced by the consumption of high doses of the drug as, unlike other drugs of abuse, cannabis rarely induces life-threatening events and, thus, users may consume extremely high doses.
Dysregulation of the noradrenergic system has been implicated in several mood disorders, including hyperarousal, anxiety, depression and PTSD (Friedman et al., 1999; Southwick et al., 1999; Nutt, 2002; Nutt, 2006; Itoi & Sugimoto, 2010). The noradrenergic system, together with the serotonergic, cholinergic and dopaminergic systems, is typically viewed as a neuromodulatory system (Sara, 2009). The noradrenergic system, in particular, has its cell bodies grouped in nuclei in the brainstem, namely the locus coeruleus (LC) and the nucleus of the solitary tract (NTS) (Foote et al., 1983; Weinshenker & Schroeder, 2007; Itoi & Sugimoto, 2010). While the LC is a homogeneous nucleus in which most cells are noradrenergic (Foote et al., 1983), the NTS contains several other neurotransmitters (Barraco et al., 1992). The noradrenergic neurons of the NTS are distributed throughout the caudal NTS (subpostremal and commissural NTS) (Barraco et al., 1992). The LC, located within the dorsal wall of the rostral pons, in the lateral floor of the fourth ventricle, is the largest noradrenergic nucleus in the brain (Foote et al., 1983) and is the sole source of norepinephrine (NE) in the forebrain (Sara, 2009). The LC is seen as the “arousal” center, important for regulation of sleep and vigilance, and activation of the LC is important for selective attention (Southwick et al., 1999; Sara, 2009). On the other hand, the NTS works as relay station for sensory signals arising from the viscera, integrating visceral information with other regulatory information coming from the brainstem, diencephalon and forebrain (Barraco et al., 1992; Itoi & Sugimoto, 2010). The NTS is known to send efferents to autonomic centers in the brainstem but also to send ascending efferents to higher levels of the neuroaxis (Barraco et al., 1992).
NE can interact with three families of adrenergic receptors (ARs): α1, α2 and β(1–3) receptors that exhibit different signal transduction. For example, α1 receptors are coupled to Gq proteins, activating phospholipase C and the phosphotidyl inositol intracellular pathway, resulting in activation of protein kinase C and release of intracellular calcium (Duman & Nestler, 1995). In contrast, α2-ARs, found pre- and postsynaptically (MacDonald et al., 1997), are coupled to Gi proteins, which can lead to a decrease in intracellular cAMP (Duman & Nestler, 1995). Presynaptically distributed α2-ARs are considered autoreceptors, since activation of these receptors will decrease intracellular cAMP and Ca2+, thereby inhibiting neurotransmitter release. Finally, β-ARs are coupled to Gs proteins, activating adenylyl cyclase and increasing intracellular cAMP (Duman & Nestler, 1995). Several studies have revealed alterations in the levels of adrenergic receptor expression in depressed suicide victims. The density of α2-ARs is increased in brains of depressed suicide victims (Meana et al., 1992; De Paermentier et al., 1997; Callado et al., 1998), while β1-AR density is decreased (De Paermentier et al., 1990). These changes are not widespread suggesting that specific areas of the brain may contribute to the pathophysiology of mood disorders. Moreover, pharmacological depletion of monoamines, using reserpine, for example, produces depressive-like behaviors in animal models, suggesting a role for monoamines (including NE) in the pathophysiology of depression (Nutt, 2006). Additionally, most antidepressants drugs act by increasing the levels of synaptic monoamines suggesting that low levels of NE account for the expression of depressive-like symptoms. Interestingly, higher levels of plasma NE were correlated with longer periods of remission to a new depressive episode in patients that had suffered their first major depression episode, suggesting a protective effect of NE (Johnston et al., 1999). However, it has also been described that patients with melancholic depression show dysregulation of the hypothalamic-pituitary-adrenal axis, with high levels of plasma cortisol and cerebrospinal fluid NE being reported (Wong et al., 2000). Thus, although the molecular mechanisms underlying depression are still largely unclear, abnormalities in noradrenergic transmission certainly play an important part in its pathophysiology.
2. The interplay between the endocannabinoid and noradrenergic systems
Manipulation of the endocannabinoid system results in effects on mood and cognition that share similarities with the noradrenergic system. Briefly, increasing endocannabinoid tone has been shown to improve mood similar to increasing noradrenergic tone with antidepressants. This has been shown in preclinical studies, where the antidepressant effects of chronic CB1r agonist administration implicate a role for NE (Morrish et al., 2009). Moreover, over-activation of the endocannabinoid system can cause mania (Henquet et al., 2006), a side effect that has been reported by patients using antidepressants (Peet, 1994; Bond et al., 2008; Tondo et al., 2010). Taken together, the effects of manipulating the endocannabinoid system and modulating noradrenergic transmission suggest that the two systems may interact or share some common signaling pathways. Consistent with this, a study performed in human subjects revealed that administration of the β-AR blocker, propranolol, before consumption of marijuana prevented cannabinoid-induced cardiovascular effects and prevented cannabinoid-induced learning impairment (Sulkowski et al., 1977). In agreement with this, early anatomical studies using autoradiography have identified moderate CB1r binding and CB1r mRNA in the principal noradrenergic nuclei, the LC and NTS (Herkenham et al., 1991; Mailleux & Vanderhaeghen, 1992; Matsuda et al., 1993; Derbenev et al., 2004; Jelsing et al., 2008). Characterization of CB1r distribution in the LC showed that CB1r is localized to somato-dendritic profiles as well as within axon terminals and neurochemical characterization of LC neurons showed that some of the CB1r-positive neurons are noradrenergic (Scavone et al., 2010). The existence of CB1r in the LC and NTS suggests that cannabinoids may modulate noradrenergic activity. In fact, administration of cannabinoid-like agents has been shown to increase Fos expression in LC noradrenergic neurons (Patel & Hillard, 2003; Oropeza et al., 2005) and in NTS neurons (Jelsing et al., 2009). Moreover, cannabinoid-like agents are also able to modulate LC and NTS firing (Himmi et al., 1996; Himmi et al., 1998; Mendiguren & Pineda, 2004; Mendiguren & Pineda, 2006; Muntoni et al., 2006) suggesting that CB1r in the LC and NTS are functionally active. These anatomical and physiological studies reveal a potential mechanism by which cannabinoids exert their effects on mood, cognition and arousal. Moreover, cannabinoids have been shown to increase NE release in the prefrontal cortex (PFC, Oropeza et al., 2005). Interestingly, activation of α2-AR in the hypothalamus leads to the production of endocannabinoids (Kuzmiski et al., 2009) and CB1r and β2-AR have been shown to physically interact in vitro (Hudson et al., 2010), contributing to the notion that the two systems interact.
2.1 Anatomical localization of CB1r in noradrenergic circuits
With respect to the noradrenergic system, autoradiographic binding studies have shown the existence of a moderate density of CB1r protein and mRNA in the LC and NTS (Herkenham et al., 1991; Mailleux & Vanderhaeghen, 1992; Matsuda et al., 1993; Derbenev et al., 2004; Jelsing et al., 2008). Some studies using dual immunohistochemical detection of dopamine-β-hydroxylase (or tyrosine hydroxylase, TH) and CB1r have shown that some of the CB1r-positive neurons in the LC (Scavone et al., 2006; Scavone et al., 2010) and NTS (Carvalho et al., 2010a) are noradrenergic. Moreover, electron microscopic analysis revealed that most of CB1r found in the LC are distributed post-synaptically. The role of post-synaptic CB1r is not yet fully understood although reports of post-synaptic CB1r inhibiting cortical interneurons in an autocrine manner have been described (Bacci et al., 2004). In Scavone’s study (2010), most of post-synaptic CB1r were found in the cytoplasm, which may reflect newly synthesized receptor on its way to dendritic processes or axon terminals in target regions. It was also shown that CB1r localized to post-synaptic profiles received mostly asymmetric (excitatory) type synapses. One can speculate that upon activation by excitatory (glutamatergic) terminals, endocannabinoids are produced and released to act on post-synaptic CB1r, thus directly inhibiting transmission without altering glutamate transmission. CB1r was also detected within pre-synaptic profiles in the LC, where the synaptic specializations were more commonly of the symmetric (inhibitory) type. Symmetric (inhibitory) synapses are thought to be GABAergic, thus suggesting that cannabinoids can have a greater impact on GABAergic transmission as compared to glutamatergic transmission. It appears that cannabinoids in the LC may mediate different signal transduction pathways depending on the pre vs post-synaptic localization of CB1r.
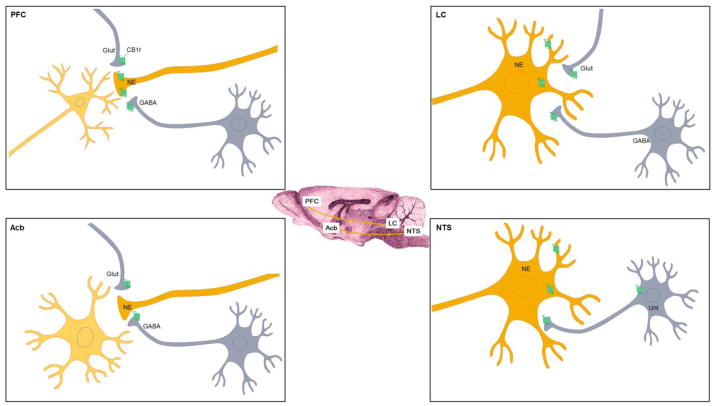
Interestingly, the PFC and the Acb, two brain regions implicated in mood disorders and that receive noradrenergic afferents from the LC and NTS respectively, show a very different pattern of CB1r distribution with respect to noradrenergic terminals (Figure 1). In the PFC, CB1r can be found in noradrenergic terminals (approximately 30% of CB1r-positive fibers were noradrenergic) (Oropeza et al., 2007) while in the Acb the percentage of co-localization of CB1r and DβH is very low (Carvalho et al., 2010a). This may reflect a different consequence to the modulation of NE by endocannabinoids in these two brain regions. In line with this, the impact of systemic WIN 55,212-2 administration on AR expression differs between the PFC and Acb (Carvalho et al., 2010a) (see below). CB1r shows an interesting topographical distribution in the Acb, with higher expression of CB1r being found in the shell of the Acb at mid-rostral levels and higher CB1r expression in the core of the Acb at caudal levels (Carvalho et al., 2010a). The heterogeneous distribution of CB1r throughout the Acb may reflect different effects of the endocannabinoid system on the modulation of behavioral output in the Acb. It is proposed that the subregions of the Acb (shell and core) can be further subdivided with respect to function (Zahm, 1999). For instance, anatomical and behavioral studies support a rostro-caudal gradient for appetitive versus aversive behaviors (Reynolds & Berridge, 2001; Reynolds & Berridge, 2002; Reynolds & Berridge, 2003). In line with this, the possibility exists that the influence of cannabinoids on Acb function are greater with respect to certain behaviors as compared to others, due to the heterogeneous distribution of CB1r in this limbic-motor region.
Figure 1.
The distribution of CB1r (indicated by green receptor) in noradrenergic circuitry. The LC is the sole source of NE to the PFC while the Acb primarily receives NE from the NTS (depicted in saggital section schematic at center of figure). In the LC, CB1r has been found both pre and post-synaptically. When post-synaptic, CB1r are localized to somatodendritic processes receiving both symmetric (putative GABAergic) and asymmetric (putative glutamatergic) synapses. In addition, CB1r are localized to axon terminals that are either inhibitory (GABA-containing terminals) or excitatory (glutamate-containing terminals) in the LC. In the PFC, NE terminals exhibit immunolabeling for CB1r, while in the Acb NE terminals are seldom immunoreactive for CB1r. Localization of CB1r on glutamatergic and GABAergic axon terminals in both the PFC and Acb have been well described. In the Acb, CB1r is found in terminals forming both symmetric and asymmetric type synapses. In the NTS, CB1r has been localized to NE neurons as well as to neurons whose phenotype has yet to be defined (defined as unlabeled, unl).
2.2 Effects of cannabinoids on noradrenergic transmission
Effects on LC activity
Several studies have reported cannabinoid-induced effects on LC neuronal activity. Namely, cannabinoid receptor agonists have been shown to increase LC spontaneous firing (Mendiguren & Pineda, 2004; Mendiguren & Pineda, 2006; Muntoni et al., 2006). Patel and Hillard showed increased Fos labeling in noradrenergic neurons in the LC following systemic injection of CP55940 and WIN 55,212-2 (2003). In this study, it was also shown that both CB1r agonists increase Fos expression in dopaminergic neurons. However, the activation of dopaminergic neurons by cannabinoid receptor agonists is blocked by an α1-AR antagonist and by an α2-AR agonist, suggesting that CP55940 and WIN 55,212-2 may be activating dopaminergic neurons by first activating LC-NE neurons. In another study, Oropeza and colleagues (2005) showed that systemic WIN 55,212-2 induces Fos expression in noradrenergic neurons of the LC. This effect was blocked by the CB1r antagonist SR 141716A, suggesting a role for CB1r. Recordings from LC-NE neurons in anaesthetized rats have shown that systemic and central administration of cannabinoids, dose-dependently, increased the firing rate of the LC (Mendiguren & Pineda, 2006; Muntoni et al., 2006). This effect was blocked by administration of the CB1r antagonist SR141716A. Interestingly, administration of SR141716A alone caused a significant reduction of LC spontaneous firing, suggesting that LC is under the control of an endogenous cannabinoid tone. This hypothesis is further supported by evidence showing that URB597, a selective inhibitor of fatty acid amide hydrolase (FAAH), the enzyme responsible for degradation of the endocannabinoid, anandamide, is able to enhance the spontaneous firing rate of LC-NE neurons (Gobbi et al., 2005).
Cannabinoids have also been shown to inhibit KCL-evoked excitation of the LC (Mendiguren & Pineda, 2007), indicating that cannabinoids may have a protective role in the LC by preventing over-activation of neuronal activity. Hyper-activity of the LC has been proposed to alter behavioral flexibility and disable focused or selective attention (Aston-Jones et al., 1999a; Aston-Jones et al., 1999b; Aston-Jones et al., 1999b; Usher et al., 1999; Aston-Jones, 2002). On the other hand, the phasic firing of the LC is important for optimal performance on tasks that require focused attention. Thus, excess inhibitory actions of cannabinoids may lead to a decrease in the phasic activation of the LC, which could result in an overall disruption of attention in both animals and humans (Jentsch et al., 1997; Solowij et al., 2002; Arguello & Jentsch, 2004).
Effects on NTS activity
There is compelling evidence for complex actions of cannabinoids in the NTS. In the NTS not all neurons are sensitive to Δ9-THC or other cannabinoid-based analogs (Himmi et al., 1996; Himmi et al., 1998). About 50% of NTS neurons are responsive to cannabinoid-based analogs, a response apparently mediated by CB1r. Interestingly, a subset of NTS neurons exhibit increased activity following cannabinoid exposure, while others exhibit decreased neuronal activity. Moreover, both WIN 55,212-2 and the antagonist rimonabant were able to increase Fos expression in the NTS, albeit in different subsets of neurons (Jelsing et al., 2009). In a study focusing on cardiovascular function, a subset of NTS neurons with baroreceptive properties was found to increase discharge after application of endocannabinoid anandamide and the endocannabinoid uptake inhibitor AM404 (Seagard et al., 2005), similarly to conditions in which there is an increase in blood pressure. The different responses to cannabinoid analogs observed in the NTS may be due to the fact that the NTS is a heterogeneous nucleus containing a large variety of neurotransmitters and neuropeptides. Catecholaminergic, serotonergic, dopaminergic, GABAergic and cholinergic neurons can be found within similar subregions of the NTS (Barraco et al., 1992). Since most studies fail to identify the neurochemical properties of the neuronal population analyzed, it is hard to speculate regarding the functional implications of these findings. In any case, the different studies reveal that cannabinoids can strongly influence activity of NTS neurons. With respect to NTS noradrenergic neurons, it has been shown that noradrenergic neurons in the NTS are positive for CB1r (Carvalho et al., 2010a), providing anatomical evidence for a potential action of cannabinoids on noradrenergic neurons. In addition, some Δ9-THC-sensitive neurons were depressed when clonidine, a α2-AR agonist, was co-administered, suggesting that these neurons are likely noradrenergic (Himmi et al., 1996).
The effects of cannabinoids on NE release in target regions
Several studies have reported that systemic and local administration of cannabinoid analogs alters the release of NE in specific areas of the brain. Systemic administration of WIN 55,212-2 or Δ9-THC has been shown to increase the release of NE in the PFC and in the Acb (Jentsch et al., 1997; Oropeza et al., 2005; Page et al., 2007). Jentsch and colleagues (1997) showed an increase in NE turnover in the PFC and Acb of rats after systemic injection of Δ9-THC. They also show that Δ9-THC increased dopamine turnover but only in the PFC; no effects were observed in serotonin turnover. Oropeza and colleagues (2005) report an increase of NE release in the PFC with concomitant Fos activation in noradrenergic neurons of the LC; importantly, this effect was blocked by SR 141716A, a CB1r antagonist. In another study, repeated administration of WIN 55,212-2 increased the release of NE in PFC with increased expression of TH in the LC (Page et al., 2007). Consistent with this, rats administered Δ9-THC or WIN 55,212-2 exhibited an increased activity rate of TH and increased levels of NE turnover in the LC, hippocampus, cortex, hypothalamus and cerebellum (Moranta et al., 2004). In addition, decreased synthesis of serotonin and dopamine were observed upon Δ9-THC or WIN 55,212-2 administration. Interestingly, an in vitro study, has shown that cannabinoids have the ability to inhibit the activity of monoamine oxidase (MAO), the enzyme responsible for the metabolism of monoamine neurotransmitters, such as NE and dopamine (Fisar, 2010), which could be another mechanism that results in increases in NE levels. In line with increased release of NE in the PFC and in the Acb, another study has reported alterations in the expression of ARs, as well as in the NE transporter (NET) (Reyes et al., 2009). Reyes and colleagues have shown that acute administration of WIN 55,212-2 decreases NET expression in the PFC, which in addition to LC activation (Oropeza et al., 2005), increased TH activity in the LC (Moranta et al., 2004; Page et al., 2007) and inhibition of MAO (Fisar, 2010) may account for the increased release of NE. Furthermore, repeated systemic administration of WIN 55,212-2 was shown to decrease the levels of β1-AR in the PFC (Reyes et al., 2009). In contrast, abstinence from WIN 55,212-2 induced an upregulation of β1-AR, which could be interpreted as a rebound effect attributed to a return to basal levels following a period of abstinence. No changes were observed in α2A-AR levels. In the Acb, it has been shown that β1-AR expression was decreased with acute or repeated administration of WIN 55,212-2 (Carvalho et al., 2010a). Additionally, α2A-AR was decreased but only after repeated administration; this effect persisted with abstinence from WIN 55,212-2 (Carvalho et al., 2010a). The lower levels of β1-AR may represent an adaptive mechanism following increases in extracellular NE in the Acb after WIN 55,212-2 treatment. The decreased in α2A-AR expression only after repeated exposure to WIN 55,212-2 may reflect a secondary mechanism to increase NE release. Activation of α2A-AR is known to decrease cAMP production in the axon terminal, decreasing the release of vesicular NE (Wozniak et al., 2000).
Interestingly, some reports have also shown that the CB1r antagonist, SR141716A, is capable of increasing NE release in the PFC (Tzavara et al., 2003) and in the hypothalamus (Tzavara et al., 2001), and the administration of SR141716A is accompanied by antidepressant effects in the forced swim test. However in another study, SR141716A alone did not trigger an effect in the levels of NE compared to vehicle treated animals; however, in this study, it was observed that SR141716A blocked the effects of WIN 55,212-2-induced NE release (Oropeza et al., 2005). These contradictory effects can be explained in part by the different doses used in these studies. In the latter, SR141716A was used at 0.2mg/kg while in the former study the doses applied ranged from 1mg/kg to 10mg/kg. The findings from studies involving CB1r antagonism can also reflect the existence of a basal tone of endocannabinoids in these regions.
Based on the reported effects of cannabinoids on NE transmission, it is of great interest to understand the functional consequences of NE on cannabinoid-induced behaviors, namely aversion and anxiety.
3. Contribution of norepinephrine to cannabinoid-induced behaviors
Emerging studies have revealed an important role for NE in cognitive and limbic function. While, for many decades, the LC-NE system was seen as the main source of forebrain NE and was intensely investigated for its role in attention, memory and behavior, increased interest in the NTS has contributed to increasing the complexity of how this neuromodulator regulates forebrain targets. Several studies have reported the existence of direct ascending projections from the NTS to limbic areas such as the bed nucleus of the stria terminalis (BNST), central nucleus of the amygdala (Ricardo & Koh, 1978; Reyes & Van Bockstaele, 2006) or Acb (Delfs et al., 1998) and these ascending projections have been shown to significantly impact motivated behaviors (Aston-Jones et al., 1999a; Delfs et al., 2000). Blockade of β-ARs is known to impair memory, decrease anxiety and increase depressive symptoms (Gottschalk et al., 1974; Sternberg et al., 1986; Patten, 1990) by targeting structures such as the hippocampus, PFC, amygdala or BNST (Delfs et al., 2000; Aston-Jones, 2002; Tully & Bolshakov, 2010). Thus, the effects of NE are region specific and rely on highly intricate neurocircuitries within cortical and limbic systems. The next section details the impact of cannabinoids on selected NE circuits.
3.1 Cannabinoid-induced aversion
Cannabinoid agents have been shown to produce both preference and aversion in the place conditioning paradigm. Murray and Bevins (Murray & Bevins, 2010) recently considered the variability in behaviors associated with cannabinoid receptor agonist exposure and found that the most consistent factor impacting behavioral outcome was the dose of the cannabinoid receptor agent used. Low doses have a tendency to induce preference while high doses have a tendency to induce aversion. Place conditioning is a classical conditioning paradigm in which animals learn to associate the effect of a drug (or other discrete treatment) with particular environmental (contextual) cues. Place conditioning can identify both conditioned place preference and conditioned place aversion, and thus it can be used to study both rewarding and aversive effects of drugs (Bardo & Bevins, 2000; Carlezon, 2003). Place conditioning is useful in probing neural circuits involved in reward and aversion. For example, microinjection of amphetamine into the Acb produces conditioned place preference, whereas microinjection of amphetamine into the area postrema produces a conditioned taste aversion (Carr & White, 1983; Carr & White, 1986). Other studies have shown that microinjection of μ opioid receptor agonists into the ventral tegmental area produces conditioned place preference, whereas microinjection of kappa opioid receptor into the ventral tegmental area, Acb, medial PFC or lateral hypothalamus produces conditioned place aversion (Shippenberg & Elmer, 1998). Hence, place conditioning studies enable parsing out the neural circuits involved in drug reward and aversion and identifying which drugs induce reward or aversion depending on the region and receptor subtypes being activated. Accordingly, monoaminergic transmission in several limbic structures (e.g. amygdala, PFC, bed nucleus of the stria terminalis and Acb) has been reported to be important for the expression of aversive behaviors (Aston-Jones et al., 1999a; Delfs et al., 2000; Ventura et al., 2007; Kerfoot et al., 2008).
The neural circuitry involved in mediating cannabinoid-induced aversion was recently elucidated (Figure 2) (Carvalho et al., 2010b; Carvalho & Van Bockstaele, 2011). Both the Acb and bed nucleus of the stria terminalis receive direct noradrenergic projections from the NTS (Delfs et al., 1998; Forray et al., 2000; Forray & Gysling, 2004). Activation of the NTS has been shown to occur when conditioned taste aversion acquisition and expression occur (Sakai & Yamamoto, 1997; Swank, 2000). Although these studies did not provide any neurochemical characterization of the activated neurons, the possibility exists that some of the activated neurons are noradrenergic considering that the highest neuronal activation was seen in the caudal and intermediate NTS. The localization of CB1r to noradrenergic neurons in the NTS (Carvalho et al., 2010a) and the ability of WIN 55,212-2 to induce NTS activation (Jelsing et al., 2009) underlie the hypothesis that WIN 55,212-2 induces aversion by increasing NE release in target regions. Our results show that NE in the Acb, but not the BNST, is critical for WIN 55,212-2-induced aversion, as decreasing NE signaling in the Acb, either by immunotoxin depletion of noradrenergic fibers (Carvalho et al., 2010b) or by blockade of β1-ARs (Carvalho & Van Bockstaele, 2011), impaired its expression. In addition, it is known that blockade of β1-AR reduces the excitability of accumbal neurons which may trigger aversion (Kombian et al., 2006; Carlezon & Thomas, 2009). Interestingly, blockade of β1-AR did not impair lithium chloride-induced aversion (Carvalho & Van Bockstaele, 2011), suggesting that noradrenergic transmission may be specific to aversion to cannabinoid-based agents. Moreover, the lack of effect of in lithium chloride-induced aversion suggests that the β1-AR blocker did not impact learning.
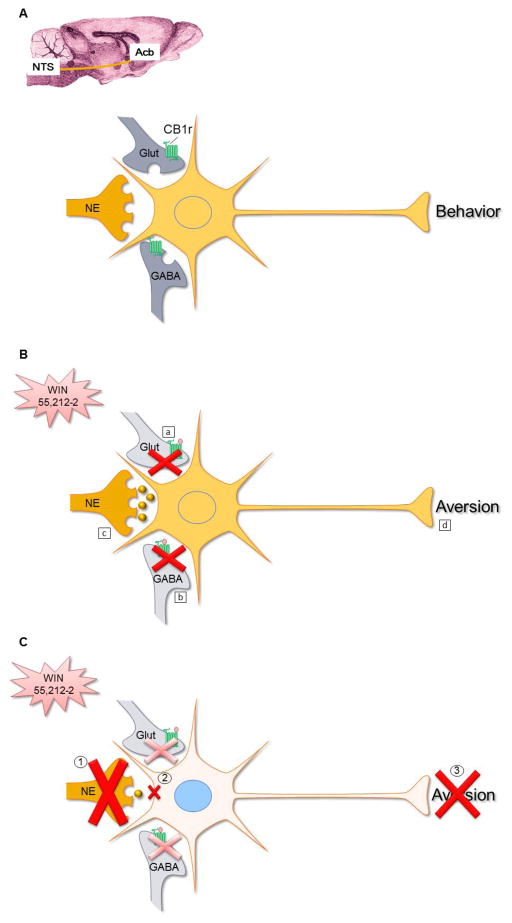
Figure 2. Schematic diagram summarizing proposed involvement of NE in neural circuitry underlying cannabinoid-induced aversion.
A. Schematic diagram depicting glutamatergic (Glut), GABAergic (GABA) and noradrenergic (NE) innervation of Acb neurons. These neuromodulators are well known to regulate Acb activity and consequently behavior. CB1r (depicted in green) is primarily associated with GABA and Glut axon terminals in this region, and few NE terminals express CB1r.
B. In the presence of a cannabinoid receptor agonist (e.g. WIN 55,212-2), glutamate release is reduced (a, Robbe et al, 2001) together with a reduction in GABA (b, Manzoni and Bockaert, 2001). WIN 55,212-2 causes a concomitant increase in NE (c, Jentsch et al, 1997) that, in combination with a decrease in glutamate and GABA, induces activation of Acb neurons triggering aversion (d, Carlezon and Thomas, 2009).
C. Blocking NE transmission either by depleting NE (1) input or by blocking β1-adrenergic receptors (2), prevents the expression of WIN 55,212-2-induced aversion (3) (Carvalho et al, 2010; Carvalho and Van Bockstaele, 2011).
Noradrenergic transmission in the bed nucleus of the stria terminalis has been implicated in the signaling of aversion in opiate withdrawal (Delfs et al., 2000; Cecchi et al., 2007) and visceral pain (Deyama et al., 2009; Minami, 2009). However, our results seem to suggest that NE in the bed nucleus of the stria terminalis is not critical for WIN 55,212-2-induced aversion (Carvalho et al., 2010b). While technical limitations should be taken into consideration, as the noradrenergic depletion achieved may have not been sufficient to remove all noradrenergic inputs, the possibility that NE in bed nucleus of the stria terminalis is not required for the expression of WIN 55,212-2 aversion is also plausible.
3.2 Cannabinoid-induced anxiety
Cannabinoids have been shown to induce anxiolytic and anxiogenic effects using the elevated plus maze (EPM) or the elevated zero maze (EZM). The EZM is a modification of the well-established EPM. Both EPM and EZM are based on the natural conflict of rodents to explore a novel environment and their innate aversion to open, elevated and brightly lit spaces. As a consequence of the aversive properties of the open arms, subjects spend a greater amount of time on the closed arms and the proportion of total exploration in the open arms provides a measure of anxiety, such that increases in percent time spent on the open arms is considered to be indicative of anxiolytic drug action (Handley & Mithani, 1984; Pellow & File, 1986). Conversely, decreases in percent time spent on open arms reflect an anxiogenic effect of the drug.
The differential results on anxiety following exposure to cannabinoid agents may be due to some of the following variables: prior drug use, dose used, basal anxiety levels and regional endocannabinoid basal tone (Degroot, 2008). Generally, the anxiogenic properties of cannabinoid agents occur more frequently in drug-naïve subjects and in novel/stressful environments (Haller et al., 2004a; Viveros et al., 2005; Degroot, 2008). This suggests that basal endocannabinoid tone is important in the response to exogenous cannabinoids. It has been shown that increases in endocannabinoid levels in specific brain areas are important for coping with anxiety-provoking stimuli (Marsicano et al., 2002). In this scenario, endocannabinoids are thought to work to restore homeostasis. While under particular physiological situations, this increase in endocannabinoids may be restricted to specific brain regions, such as the amygdala (Marsicano et al., 2002), in cases where exogenous/systemic cannabinoids are administered, the diverse nature of cannabinoid receptor activation may trigger an anxiogenic effect. Although, decreased NE tone in the Acb was able to reverse WIN 55,212-2-induced aversion, it was not sufficient to block WIN 55,212-2-induced anxiety (Carvalho et al., 2010b). Decreasing NE tone in the BNST also failed to prevent WIN 55,212-2-induced aversion. These results suggest that WIN 55,212-2-induced anxiety is not mediated by NE input to the Acb or the BNST. These findings are not surprising as the Acb has not been implicated in the development of anxiety-like behaviors. On the other hand, the results obtained from NE depletion from the BNST are quite fascinating. The BNST is seen as an important nucleus for the expression of anxiety (Davis, 1998; Walker et al., 2003; Davis, 2006) and is one of the richest areas in NE in the CNS (Forray & Gysling, 2004). Although NE in the BNST has been shown to mediate anxiety to certain stressors, it does not mediate anxiety in response to all types of stressors (Cecchi et al., 2002). Considering this, it has been proposed that NE effects on anxiety are stimuli-specific. Moreover, other neurotransmitters have also been implicated in signaling anxiety in the bed nucleus of the stria terminalis, such as corticotropin releasing factor (Smith & Aston-Jones, 2008). It has been suggested that anxiogenic effects of endocannabinoids can be mediated by transient receptor potential vanilloid type-1 (TRPV1) activation (Campos & Guimaraes, 2009; Micale et al., 2009) as anandamide but not 2- arachidonyl glycerol (2-AG) is a TRPV1 agonist (Zygmunt et al., 1999). It is not clear whether WIN 55,212-2 has the ability to direct modulate TRPV1. WIN 55,212-2 has been shown to inhibit TRPV1 in trigeminal ganglion neurons (Patwardhan et al., 2006; Wang et al., 2011) but the role of TRPV1 in WIN 55,212-2-induced anxiety is not yet clear. Taken all together, the results suggest that WIN 55,212-2-induced anxiety is independent of noradrenergic transmission in the Acb and the bed nucleus of the stria terminalis.
Conclusion
Growing evidence suggests an interaction between the cannabinoid and noradrenergic systems that has significant functional and behavioral implications. Importantly, cannabinoids can modulate noradrenergic transmission in both noradrenergic nuclei and target regions. This modulation seems to be circuit specific and may depend on the basal status of cannabinoid and NE levels. In addition, NE seems to be important for particular cannabinoid-induced behaviors. However, many questions remain regarding cannabinoid-adrenergic interactions in disease. It is clear that the noradrenergic system plays a role in certain psychiatric disorders. It is tempting to speculate that, under certain conditions, drugs targeting the endocannabinoid system may provide an effective tool to modulate and reverse impairments in noradrenergic transmission. However, numerous safety issues persist with cannabinoid-based agents that may preclude their widespread utility. The question also arises as to whether prevention of side effects induced by cannabinoid-based agents may involve a combination of cannabinoid-based agents and modulators of the noradrenergic system.
Highlights.
Growing evidence suggests an interaction between the cannabinoid and noradrenergic systems that has significant functional and behavioral implications.
The functional consequences of cannabinoid-based ligands on noradrenergic transmission impact conditioned place aversion and anxiety.
Cannabinoid-based ligands can modulate noradrenergic transmission in both noradrenergic nuclei and target regions.
Modulation of noradrenergic pathways is circuit specific and may depend on the basal status of brain norepinephrine levels.
Acknowledgments
NIDA DA020129
List of Abbreviations (in alphabetical order)
- AR
adrenergic receptor
- CB1r
cannabinoid type 1 receptor
- Δ9-THC
Δ9-tetrahydrohydrocannabinol
- FAAH
fatty acid amide hydrolase
- LC
locus coeruleus
- MAO
monoamine oxidase
- NE
norepinephrine
- NET
norepinephrine transporter
- Acb
nucleus accumbens
- NTS
nucleus of the solitary tract
- PTSD
post-traumatic stress disorder
- PFC
prefrontal cortex
- TH
tyrosine hydroxylase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anand A, Charney DS. Norepinephrine dysfunction in depression. J Clin Psychiatry. 2000;61(Suppl 10):16–24. [PubMed] [Google Scholar]
- Arguello PA, Jentsch JD. Cannabinoid CB1 receptor-mediated impairment of visuospatial attention in the rat. Psychopharmacology (Berl) 2004;177:141–150. doi: 10.1007/s00213-004-1953-0. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G. Norepinephrine. In: Davis KL, Charney D, Coyle JT, Nemeroff C, editors. Neuropsychopharmacology: The Fifth Generation of Progress. Lippincott, Williams & Wilkins; Philadelphia, USA: 2002. pp. 47–57. [Google Scholar]
- Aston-Jones G, Delfs JM, Druhan J, Zhu Y. The bed nucleus of the stria terminalis. A target site for noradrenergic actions in opiate withdrawal. Ann N Y Acad Sci. 1999a;877:486–498. doi: 10.1111/j.1749-6632.1999.tb09284.x. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Cohen J. Role of locus coeruleus in attention and behavioral flexibility. Biol Psychiatry. 1999b;46:1309–1320. doi: 10.1016/s0006-3223(99)00140-7. [DOI] [PubMed] [Google Scholar]
- Bacci A, Huguenard JR, Prince DA. Long-lasting self-inhibition of neocortical interneurons mediated by endocannabinoids. Nature. 2004;431:312–316. doi: 10.1038/nature02913. [DOI] [PubMed] [Google Scholar]
- Baigent M, Holme G, Hafner RJ. Self reports of the interaction between substance abuse and schizophrenia. Aust N Z J Psychiatry. 1995;29:69–74. doi: 10.3109/00048679509075894. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: What does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Barraco R, el-Ridi M, Ergene E, Parizon M, Bradley D. An atlas of the rat subpostremal nucleus tractus solitarius. Brain Res Bull. 1992;29:703–765. doi: 10.1016/0361-9230(92)90143-l. [DOI] [PubMed] [Google Scholar]
- Bond DJ, Noronha MM, Kauer-Sant’Anna M, Lam RW, Yatham LN. Antidepressant-associated mood elevations in bipolar II disorder compared with bipolar I disorder and major depressive disorder: A systematic review and meta-analysis. J Clin Psychiatry. 2008;69:1589–1601. doi: 10.4088/jcp.v69n1009. [DOI] [PubMed] [Google Scholar]
- Callado LF, Meana JJ, Grijalba B, Pazos A, Sastre M, Garcia-Sevilla JA. Selective increase of alpha2A-adrenoceptor agonist binding sites in brains of depressed suicide victims. J Neurochem. 1998;70:1114–1123. doi: 10.1046/j.1471-4159.1998.70031114.x. [DOI] [PubMed] [Google Scholar]
- Campos AC, Guimaraes FS. Evidence for a potential role for TRPV1 receptors in the dorsolateral periaqueductal gray in the attenuation of the anxiolytic effects of cannabinoids. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1517–1521. doi: 10.1016/j.pnpbp.2009.08.017. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Thomas MJ. Biological substrates of reward and aversion: A nucleus accumbens activity hypothesis. Neuropharmacology. 2009;56(Suppl 1):122–132. doi: 10.1016/j.neuropharm.2008.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA., Jr Place conditioning to study drug reward and aversion. Methods Mol Med. 2003;84:243–249. doi: 10.1385/1-59259-379-8:243. [DOI] [PubMed] [Google Scholar]
- Carr GD, White NM. Anatomical disassociation of amphetamine’s rewarding and aversive effects: An intracranial microinjection study. Psychopharmacology (Berl) 1986;89:340–346. doi: 10.1007/BF00174372. [DOI] [PubMed] [Google Scholar]
- Carr GD, White NM. Conditioned place preference from intra-accumbens but not intra-caudate amphetamine injections. Life Sci. 1983;33:2551–2557. doi: 10.1016/0024-3205(83)90165-0. [DOI] [PubMed] [Google Scholar]
- Carvalho AF, Van Bockstaele EJ. Direct intra-accumbal infusion of a beta-adrenergic receptor antagonist abolishes WIN 55,212-2-induced aversion. Neurosci Lett. 2011;500:82–85. doi: 10.1016/j.neulet.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho AF, Mackie K, Van Bockstaele EJ. Cannabinoid modulation of limbic forebrain noradrenergic circuitry. Eur J Neurosci. 2010a;31:286–301. doi: 10.1111/j.1460-9568.2009.07054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho AF, Reyes AR, Sterling RC, Unterwald E, Van Bockstaele EJ. Contribution of limbic norepinephrine to cannabinoid-induced aversion. Psychopharmacology (Berl) 2010b;211:479–491. doi: 10.1007/s00213-010-1923-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchi M, Capriles N, Watson SJ, Akil H. Beta1 adrenergic receptors in the bed nucleus of stria terminalis mediate differential responses to opiate withdrawal. Neuropsychopharmacology. 2007;32:589–599. doi: 10.1038/sj.npp.1301140. [DOI] [PubMed] [Google Scholar]
- Cecchi M, Khoshbouei H, Javors M, Morilak DA. Modulatory effects of norepinephrine in the lateral bed nucleus of the stria terminalis on behavioral and neuroendocrine responses to acute stress. Neuroscience. 2002;112:13–21. doi: 10.1016/s0306-4522(02)00062-3. [DOI] [PubMed] [Google Scholar]
- Cleghorn JM, Kaplan RD, Szechtman B, Szechtman H, Brown GM, Franco S. Substance abuse and schizophrenia: Effect on symptoms but not on neurocognitive function. J Clin Psychiatry. 1991;52:26–30. [PubMed] [Google Scholar]
- Davis M. Neural systems involved in fear and anxiety measured with fear-potentiated startle. Am Psychol. 2006;61:741–756. doi: 10.1037/0003-066X.61.8.741. [DOI] [PubMed] [Google Scholar]
- Davis M. Are different parts of the extended amygdala involved in fear versus anxiety? Biol Psychiatry. 1998;44:1239–1247. doi: 10.1016/s0006-3223(98)00288-1. [DOI] [PubMed] [Google Scholar]
- De Paermentier F, Mauger JM, Lowther S, Crompton MR, Katona CL, Horton RW. Brain alpha-adrenoceptors in depressed suicides. Brain Res. 1997;757:60–68. doi: 10.1016/s0006-8993(97)00138-8. [DOI] [PubMed] [Google Scholar]
- De Paermentier F, Cheetham SC, Crompton MR, Katona CL, Horton RW. Brain beta-adrenoceptor binding sites in antidepressant-free depressed suicide victims. Brain Res. 1990;525:71–77. doi: 10.1016/0006-8993(90)91321-7. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Hall W, Lynskey M. Exploring the association between cannabis use and depression. Addiction. 2003;98:1493–1504. doi: 10.1046/j.1360-0443.2003.00437.x. [DOI] [PubMed] [Google Scholar]
- Degroot A. Role of cannabinoid receptors in anxiety disorders. In: Köfalvi A, editor. Cannabinoids and the Brain. Springer; USA: 2008. pp. 559–572. [Google Scholar]
- Delfs JM, Zhu Y, Druhan JP, Aston-Jones G. Noradrenaline in the ventral forebrain is critical for opiate withdrawal-induced aversion. Nature. 2000;403:430–434. doi: 10.1038/35000212. [DOI] [PubMed] [Google Scholar]
- Delfs JM, Zhu Y, Druhan JP, Aston-Jones GS. Origin of noradrenergic afferents to the shell subregion of the nucleus accumbens: Anterograde and retrograde tract-tracing studies in the rat. Brain Res. 1998;806:127–140. doi: 10.1016/s0006-8993(98)00672-6. [DOI] [PubMed] [Google Scholar]
- Derbenev AV, Stuart TC, Smith BN. Cannabinoids suppress synaptic input to neurones of the rat dorsal motor nucleus of the vagus nerve. J Physiol. 2004;559:923–938. doi: 10.1113/jphysiol.2004.067470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyama S, Katayama T, Kondoh N, Nakagawa T, Kaneko S, Yamaguchi T, Yoshioka M, Minami M. Role of enhanced noradrenergic transmission within the ventral bed nucleus of the stria terminalis in visceral pain-induced aversion in rats. Behav Brain Res. 2009;197:279–283. doi: 10.1016/j.bbr.2008.08.024. [DOI] [PubMed] [Google Scholar]
- Duman RS, Nestler EJ. Signal transduction pathways for catecholamine receptors. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The Fourth Generation of Progress. Raven Press; New York: 1995. p. 303. [Google Scholar]
- Fernandez-Espejo E, Viveros MP, Nunez L, Ellenbroek BA, Rodriguez de Fonseca F. Role of cannabis and endocannabinoids in the genesis of schizophrenia. Psychopharmacology (Berl) 2009;206:531–549. doi: 10.1007/s00213-009-1612-6. [DOI] [PubMed] [Google Scholar]
- Fisar Z. Inhibition of monoamine oxidase activity by cannabinoids. Naunyn Schmiedebergs Arch Pharmacol. 2010;381:563–572. doi: 10.1007/s00210-010-0517-6. [DOI] [PubMed] [Google Scholar]
- Foote SL, Bloom FE, Aston-Jones G. Nucleus locus ceruleus: New evidence of anatomical and physiological specificity. Physiol Rev. 1983;63:844–914. doi: 10.1152/physrev.1983.63.3.844. [DOI] [PubMed] [Google Scholar]
- Forray MI, Gysling K. Role of noradrenergic projections to the bed nucleus of the stria terminalis in the regulation of the hypothalamic-pituitary-adrenal axis. Brain Res Brain Res Rev. 2004;47:145–160. doi: 10.1016/j.brainresrev.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Forray MI, Gysling K, Andres ME, Bustos G, Araneda S. Medullary noradrenergic neurons projecting to the bed nucleus of the stria terminalis express mRNA for the NMDA-NR1 receptor. Brain Res Bull. 2000;52:163–169. doi: 10.1016/s0361-9230(00)00229-x. [DOI] [PubMed] [Google Scholar]
- Friedman JI, Adler DN, Davis KL. The role of norepinephrine in the pathophysiology of cognitive disorders: Potential applications to the treatment of cognitive dysfunction in schizophrenia and alzheimer’s disease. Biol Psychiatry. 1999;46:1243–1252. doi: 10.1016/s0006-3223(99)00232-2. [DOI] [PubMed] [Google Scholar]
- Gobbi G, Bambico FR, Mangieri R, Bortolato M, Campolongo P, Solinas M, Cassano T, Morgese MG, Debonnel G, Duranti A, Tontini A, Tarzia G, Mor M, Trezza V, Goldberg SR, Cuomo V, Piomelli D. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc Natl Acad Sci U S A. 2005;102:18620–18625. doi: 10.1073/pnas.0509591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschalk LA, Stone WN, Gleser GC. Peripheral versus central mechanisms accounting for antianxiety effects of propranolol. Psychosom Med. 1974;36:47–56. doi: 10.1097/00006842-197401000-00004. [DOI] [PubMed] [Google Scholar]
- Griebel G, Stemmelin J, Scatton B. Effects of the cannabinoid CB1 receptor antagonist rimonabant in models of emotional reactivity in rodents. Biol Psychiatry. 2005;57:261–267. doi: 10.1016/j.biopsych.2004.10.032. [DOI] [PubMed] [Google Scholar]
- Haller J, Varga B, Ledent C, Barna I, Freund TF. Context-dependent effects of CB1 cannabinoid gene disruption on anxiety-like and social behaviour in mice. Eur J Neurosci. 2004a;19:1906–1912. doi: 10.1111/j.1460-9568.2004.03293.x. [DOI] [PubMed] [Google Scholar]
- Haller J, Varga B, Ledent C, Freund TF. CB1 cannabinoid receptors mediate anxiolytic effects: Convergent genetic and pharmacological evidence with CB1-specific agents. Behav Pharmacol. 2004b;15:299–304. doi: 10.1097/01.fbp.0000135704.56422.40. [DOI] [PubMed] [Google Scholar]
- Handley SL, Mithani S. Effects of alpha-adrenoceptor agonists and antagonists in a maze-exploration model of ‘fear’-motivated behaviour. Naunyn Schmiedebergs Arch Pharmacol. 1984;327:1–5. doi: 10.1007/BF00504983. [DOI] [PubMed] [Google Scholar]
- Heninger GR, Delgado PL, Charney DS. The revised monoamine theory of depression: A modulatory role for monoamines, based on new findings from monoamine depletion experiments in humans. Pharmacopsychiatry. 1996;29:2–11. doi: 10.1055/s-2007-979535. [DOI] [PubMed] [Google Scholar]
- Henquet C, Krabbendam L, de Graaf R, ten Have M, van Os J. Cannabis use and expression of mania in the general population. J Affect Disord. 2006;95:103–110. doi: 10.1016/j.jad.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: A quantitative in vitro autoradiographic study. J Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Gorzalka BB. Pharmacological enhancement of cannabinoid CB1 receptor activity elicits an antidepressant-like response in the rat forced swim test. Eur Neuropsychopharmacol. 2005;15:593–599. doi: 10.1016/j.euroneuro.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Himmi T, Perrin J, El Ouazzani T, Orsini JC. Neuronal responses to cannabinoid receptor ligands in the solitary tract nucleus. Eur J Pharmacol. 1998;359:49–54. doi: 10.1016/s0014-2999(98)00630-x. [DOI] [PubMed] [Google Scholar]
- Himmi T, Dallaporta M, Perrin J, Orsini JC. Neuronal responses to delta 9-tetrahydrocannabinol in the solitary tract nucleus. Eur J Pharmacol. 1996;312:273–279. doi: 10.1016/0014-2999(96)00490-6. [DOI] [PubMed] [Google Scholar]
- Hudson BD, Hebert TE, Kelly ME. Physical and functional interaction between CB1 cannabinoid receptors and beta2-adrenoceptors. Br J Pharmacol. 2010;160:627–642. doi: 10.1111/j.1476-5381.2010.00681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoi K, Sugimoto N. The brainstem noradrenergic systems in stress, anxiety, and depression. J Neuroendocrinol. 2010 doi: 10.1111/j.1365-2826.2010.01988.x. [DOI] [PubMed] [Google Scholar]
- Jelsing J, Galzin AM, Guillot E, Pruniaux MP, Larsen PJ, Vrang N. Localization and phenotypic characterization of brainstem neurons activated by rimonabant and WIN55,212-2. Brain Res Bull. 2009;78:202–210. doi: 10.1016/j.brainresbull.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Jelsing J, Larsen PJ, Vrang N. Identification of cannabinoid type 1 receptor expressing cocaine amphetamine-regulated transcript neurons in the rat hypothalamus and brainstem using in situ hybridization and immunohistochemistry. Neuroscience. 2008;154:641–652. doi: 10.1016/j.neuroscience.2008.03.051. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Andrusiak E, Tran A, Bowers MB, Jr, Roth RH. Delta 9-tetrahydrocannabinol increases prefrontal cortical catecholaminergic utilization and impairs spatial working memory in the rat: Blockade of dopaminergic effects with HA966. Neuropsychopharmacology. 1997;16:426–432. doi: 10.1016/S0893-133X(97)00018-3. [DOI] [PubMed] [Google Scholar]
- Johns A. Psychiatric effects of cannabis. Br J Psychiatry. 2001;178:116–122. doi: 10.1192/bjp.178.2.116. [DOI] [PubMed] [Google Scholar]
- Johnston TG, Kelly CB, Stevenson MR, Cooper SJ. Plasma norepinephrine and prediction of outcome in major depressive disorder. Biol Psychiatry. 1999;46:1253–1258. doi: 10.1016/s0006-3223(99)00134-1. [DOI] [PubMed] [Google Scholar]
- Kerfoot EC, Chattillion EA, Williams CL. Functional interactions between the nucleus tractus solitarius (NTS) and nucleus accumbens shell in modulating memory for arousing experiences. Neurobiol Learn Mem. 2008;89:47–60. doi: 10.1016/j.nlm.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kombian SB, Ananthalakshmi KV, Edafiogho IO. Enaminones and norepinephrine employ convergent mechanisms to depress excitatory synaptic transmission in the rat nucleus accumbens in vitro. Eur J Neurosci. 2006;24:2781–2788. doi: 10.1111/j.1460-9568.2006.05152.x. [DOI] [PubMed] [Google Scholar]
- Kuzmiski JB, Pittman QJ, Bains JS. Metaplasticity of hypothalamic synapses following in vivo challenge. Neuron. 2009;62:839–849. doi: 10.1016/j.neuron.2009.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald E, Kobilka BK, Scheinin M. Gene targeting--homing in on alpha 2-adrenoceptor-subtype function. Trends Pharmacol Sci. 1997;18:211–219. doi: 10.1016/s0165-6147(97)01063-8. [DOI] [PubMed] [Google Scholar]
- Mailleux P, Vanderhaeghen JJ. Distribution of neuronal cannabinoid receptor in the adult rat brain: A comparative receptor binding radioautography and in situ hybridization histochemistry. Neuroscience. 1992;48:655–668. doi: 10.1016/0306-4522(92)90409-u. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, Di Marzo V, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Bonner TI, Lolait SJ. Localization of cannabinoid receptor mRNA in rat brain. J Comp Neurol. 1993;327:535–550. doi: 10.1002/cne.903270406. [DOI] [PubMed] [Google Scholar]
- Meana JJ, Barturen F, Garcia-Sevilla JA. Alpha 2-adrenoceptors in the brain of suicide victims: Increased receptor density associated with major depression. Biol Psychiatry. 1992;31:471–490. doi: 10.1016/0006-3223(92)90259-3. [DOI] [PubMed] [Google Scholar]
- Mendiguren A, Pineda J. CB(1) cannabinoid receptors inhibit the glutamatergic component of KCl-evoked excitation of locus coeruleus neurons in rat brain slices. Neuropharmacology. 2007;52:617–625. doi: 10.1016/j.neuropharm.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Mendiguren A, Pineda J. Systemic effect of cannabinoids on the spontaneous firing rate of locus coeruleus neurons in rats. Eur J Pharmacol. 2006;534:83–88. doi: 10.1016/j.ejphar.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Mendiguren A, Pineda J. Cannabinoids enhance N-methyl-D-aspartate-induced excitation of locus coeruleus neurons by CB1 receptors in rat brain slices. Neurosci Lett. 2004;363:1–5. doi: 10.1016/j.neulet.2004.02.073. [DOI] [PubMed] [Google Scholar]
- Micale V, Cristino L, Tamburella A, Petrosino S, Leggio GM, Drago F, Di Marzo V. Anxiolytic effects in mice of a dual blocker of fatty acid amide hydrolase and transient receptor potential vanilloid type-1 channels. Neuropsychopharmacology. 2009;34:593–606. doi: 10.1038/npp.2008.98. [DOI] [PubMed] [Google Scholar]
- Minami M. Neuronal mechanisms for pain-induced aversion behavioral studies using a conditioned place aversion test. Int Rev Neurobiol. 2009;85:135–144. doi: 10.1016/S0074-7742(09)85010-1. [DOI] [PubMed] [Google Scholar]
- Moranta D, Esteban S, Garcia-Sevilla JA. Differential effects of acute cannabinoid drug treatment, mediated by CB1 receptors, on the in vivo activity of tyrosine and tryptophan hydroxylase in the rat brain. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:516–524. doi: 10.1007/s00210-004-0921-x. [DOI] [PubMed] [Google Scholar]
- Moreira FA, Lutz B. The endocannabinoid system: Emotion, learning and addiction. Addict Biol. 2008;13:196–212. doi: 10.1111/j.1369-1600.2008.00104.x. [DOI] [PubMed] [Google Scholar]
- Morrish AC, Hill MN, Riebe CJ, Gorzalka BB. Protracted cannabinoid administration elicits antidepressant behavioral responses in rats: Role of gender and noradrenergic transmission. Physiol Behav. 2009;98:118–124. doi: 10.1016/j.physbeh.2009.04.023. [DOI] [PubMed] [Google Scholar]
- Morrison PD, Zois V, McKeown DA, Lee TD, Holt DW, Powell JF, Kapur S, Murray RM. The acute effects of synthetic intravenous Delta9-tetrahydrocannabinol on psychosis, mood and cognitive functioning. Psychol Med. 2009;39:1607–1616. doi: 10.1017/S0033291709005522. [DOI] [PubMed] [Google Scholar]
- Muntoni AL, Pillolla G, Melis M, Perra S, Gessa GL, Pistis M. Cannabinoids modulate spontaneous neuronal activity and evoked inhibition of locus coeruleus noradrenergic neurons. Eur J Neurosci. 2006;23:2385–2394. doi: 10.1111/j.1460-9568.2006.04759.x. [DOI] [PubMed] [Google Scholar]
- Murray JE, Bevins RA. Cannabinoid conditioned reward and aversion: Behavioral and neural processes. ACS Chem Neurosci. 2010;1:265–278. doi: 10.1021/cn100005p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrete JC, Knapp WP, Douglas DE, Smith WB. Cannabis affects the severity of schizophrenic symptoms: Results of a clinical survey. Psychol Med. 1986;16:515–520. doi: 10.1017/s0033291700010278. [DOI] [PubMed] [Google Scholar]
- Nutt DJ. The role of dopamine and norepinephrine in depression and antidepressant treatment. J Clin Psychiatry. 2006;67(Suppl 6):3–8. [PubMed] [Google Scholar]
- Nutt DJ. The neuropharmacology of serotonin and noradrenaline in depression. Int Clin Psychopharmacol. 2002;17(Suppl 1):S1–12. doi: 10.1097/00004850-200206001-00002. [DOI] [PubMed] [Google Scholar]
- Oropeza VC, Mackie K, Van Bockstaele EJ. Cannabinoid receptors are localized to noradrenergic axon terminals in the rat frontal cortex. Brain Res. 2007;1127:36–44. doi: 10.1016/j.brainres.2006.09.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oropeza VC, Page ME, Van Bockstaele EJ. Systemic administration of WIN 55,212-2 increases norepinephrine release in the rat frontal cortex. Brain Res. 2005;1046:45–54. doi: 10.1016/j.brainres.2005.03.036. [DOI] [PubMed] [Google Scholar]
- Page ME, Oropeza VC, Sparks SE, Qian Y, Menko AS, Van Bockstaele EJ. Repeated cannabinoid administration increases indices of noradrenergic activity in rats. Pharmacol Biochem Behav. 2007;86:162–168. doi: 10.1016/j.pbb.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parolaro D, Realini N, Vigano D, Guidali C, Rubino T. The endocannabinoid system and psychiatric disorders. Exp Neurol. 2010;224:3–14. doi: 10.1016/j.expneurol.2010.03.018. [DOI] [PubMed] [Google Scholar]
- Patel S, Hillard CJ. Cannabinoid-induced fos expression within A10 dopaminergic neurons. Brain Res. 2003;963:15–25. doi: 10.1016/s0006-8993(02)03797-6. [DOI] [PubMed] [Google Scholar]
- Patten SB. Propranolol and depression: Evidence from the antihypertensive trials. Can J Psychiatry. 1990;35:257–259. doi: 10.1177/070674379003500312. [DOI] [PubMed] [Google Scholar]
- Patwardhan AM, Jeske NA, Price TJ, Gamper N, Akopian AN, Hargreaves KM. The cannabinoid WIN 55,212-2 inhibits transient receptor potential vanilloid 1 (TRPV1) and evokes peripheral antihyperalgesia via calcineurin. Proc Natl Acad Sci U S A. 2006;103:11393–11398. doi: 10.1073/pnas.0603861103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peet M. Induction of mania with selective serotonin re-uptake inhibitors and tricyclic antidepressants. Br J Psychiatry. 1994;164:549–550. doi: 10.1192/bjp.164.4.549. [DOI] [PubMed] [Google Scholar]
- Pellow S, File SE. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: A novel test of anxiety in the rat. Pharmacol Biochem Behav. 1986;24:525–529. doi: 10.1016/0091-3057(86)90552-6. [DOI] [PubMed] [Google Scholar]
- Reilly D, Didcott P, Swift W, Hall W. Long-term cannabis use: Characteristics of users in an australian rural area. Addiction. 1998;93:837–846. doi: 10.1046/j.1360-0443.1998.9368375.x. [DOI] [PubMed] [Google Scholar]
- Reyes BAS, Rosario JC, Piana PMT, Van Bockstaele EJ. Cannabinoid Modulation of cortical adrenergic receptors and transporters. J Neurosci Res. 2009 doi: 10.1002/jnr.22158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes BA, Van Bockstaele EJ. Divergent projections of catecholaminergic neurons in the nucleus of the solitary tract to limbic forebrain and medullary autonomic brain regions. Brain Res. 2006;1117:69–79. doi: 10.1016/j.brainres.2006.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. Glutamate motivational ensembles in nucleus accumbens: Rostrocaudal shell gradients of fear and feeding. Eur J Neurosci. 2003;17:2187–2200. doi: 10.1046/j.1460-9568.2003.02642.x. [DOI] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. Positive and negative motivation in nucleus accumbens shell: Bivalent rostrocaudal gradients for GABA-elicited eating, taste “liking”/”disliking” reactions, place preference/avoidance, and fear. J Neurosci. 2002;22:7308–7320. doi: 10.1523/JNEUROSCI.22-16-07308.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. Fear and feeding in the nucleus accumbens shell: Rostrocaudal segregation of GABA-elicited defensive behavior versus eating behavior. J Neurosci. 2001;21:3261–3270. doi: 10.1523/JNEUROSCI.21-09-03261.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricardo JA, Koh ET. Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain Res. 1978;153:1–26. doi: 10.1016/0006-8993(78)91125-3. [DOI] [PubMed] [Google Scholar]
- Sakai N, Yamamoto T. Conditioned taste aversion and c-fos expression in the rat brainstem after administration of various USs. Neuroreport. 1997;8:2215–2220. doi: 10.1097/00001756-199707070-00025. [DOI] [PubMed] [Google Scholar]
- Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci. 2009;10:211–223. doi: 10.1038/nrn2573. [DOI] [PubMed] [Google Scholar]
- Scavone JL, Mackie K, Van Bockstaele EJ. Distribution and trafficking of the cannabinoid receptor CB1 in the rat noradrenergic locus coeruleus. Society for Neuroscience Abstracts 2006 [Google Scholar]
- Scavone JL, Mackie K, Van Bockstaele EJ. Characterization of cannabinoid-1 receptors in the locus coeruleus: Relationship with mu-opioid receptors. Brain Res. 2010;1312:18–31. doi: 10.1016/j.brainres.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seagard JL, Hopp FA, Hillard CJ, Dean C. Effects of endocannabinoids on discharge of baroreceptive NTS neurons. Neurosci Lett. 2005;381:334–339. doi: 10.1016/j.neulet.2005.02.044. [DOI] [PubMed] [Google Scholar]
- Shearman LP, Rosko KM, Fleischer R, Wang J, Xu S, Tong XS, Rocha BA. Antidepressant-like and anorectic effects of the cannabinoid CB1 receptor inverse agonist AM251 in mice. Behav Pharmacol. 2003;14:573–582. doi: 10.1097/00008877-200312000-00001. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Elmer GI. The neurobiology of opiate reinforcement. Crit Rev Neurobiol. 1998;12:267–303. doi: 10.1615/critrevneurobiol.v12.i4.10. [DOI] [PubMed] [Google Scholar]
- Smith RJ, Aston-Jones G. Noradrenergic transmission in the extended amygdala: Role in increased drug-seeking and relapse during protracted drug abstinence. Brain Struct Funct. 2008;213:43–61. doi: 10.1007/s00429-008-0191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solowij N, Stephens RS, Roffman RA, Babor T, Kadden R, Miller M, Christiansen K, McRee B, Vendetti J Marijuana Treatment Project Research Group. Cognitive functioning of long-term heavy cannabis users seeking treatment. JAMA. 2002;287:1123–1131. doi: 10.1001/jama.287.9.1123. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Bremner JD, Rasmusson A, Morgan CA, 3rd, Arnsten A, Charney DS. Role of norepinephrine in the pathophysiology and treatment of posttraumatic stress disorder. Biol Psychiatry. 1999;46:1192–1204. doi: 10.1016/s0006-3223(99)00219-x. [DOI] [PubMed] [Google Scholar]
- Sternberg DB, Korol D, Novack GD, McGaugh JL. Epinephrine-induced memory facilitation: Attenuation by adrenoceptor antagonists. Eur J Pharmacol. 1986;129:189–193. doi: 10.1016/0014-2999(86)90353-5. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, DelBello MP, Fleck DE, Adler CM, Anthenelli RM, Keck PE, Jr, Arnold LM, Amicone J. Effects of co-occurring cannabis use disorders on the course of bipolar disorder after a first hospitalization for mania. Arch Gen Psychiatry. 2007;64:57–64. doi: 10.1001/archpsyc.64.1.57. [DOI] [PubMed] [Google Scholar]
- Sulkowski A, Vachon L, Rich ES., Jr Propranolol effects on acute marihuana intoxication in man. Psychopharmacology (Berl) 1977;52:47–53. doi: 10.1007/BF00426599. [DOI] [PubMed] [Google Scholar]
- Swank MW. Conditioned c-fos in mouse NTS during expression of a learned taste aversion depends on contextual cues. Brain Res. 2000;862:138–144. doi: 10.1016/s0006-8993(00)02101-6. [DOI] [PubMed] [Google Scholar]
- Tondo L, Vazquez G, Baldessarini RJ. Mania associated with antidepressant treatment: Comprehensive meta-analytic review. Acta Psychiatr Scand. 2010;121:404–414. doi: 10.1111/j.1600-0447.2009.01514.x. [DOI] [PubMed] [Google Scholar]
- Tully K, Bolshakov VY. Emotional enhancement of memory: How norepinephrine enables synaptic plasticity. Mol Brain. 2010;3:15. doi: 10.1186/1756-6606-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzavara ET, Davis RJ, Perry KW, Li X, Salhoff C, Bymaster FP, Witkin JM, Nomikos GG. The CB1 receptor antagonist SR141716A selectively increases monoaminergic neurotransmission in the medial prefrontal cortex: Implications for therapeutic actions. Br J Pharmacol. 2003;138:544–553. doi: 10.1038/sj.bjp.0705100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzavara ET, Perry KW, Rodriguez DE, Bymaster FP, Nomikos GG. The cannabinoid CB(1) receptor antagonist SR141716A increases norepinephrine outflow in the rat anterior hypothalamus. Eur J Pharmacol. 2001;426:R3–4. doi: 10.1016/s0014-2999(01)01228-6. [DOI] [PubMed] [Google Scholar]
- Usher M, Cohen JD, Servan-Schreiber D, Rajkowski J, Aston-Jones G. The role of locus coeruleus in the regulation of cognitive performance. Science. 1999;283:549–554. doi: 10.1126/science.283.5401.549. [DOI] [PubMed] [Google Scholar]
- van Rossum I, Boomsma M, Tenback D, Reed C, van Os J EMBLEM Advisory Board. Does cannabis use affect treatment outcome in bipolar disorder? A longitudinal analysis J Nerv Ment Dis. 2009;197:35–40. doi: 10.1097/NMD.0b013e31819292a6. [DOI] [PubMed] [Google Scholar]
- Velez CN, Johnson J, Cohen P. A longitudinal analysis of selected risk factors for childhood psychopathology. J Am Acad Child Adolesc Psychiatry. 1989;28:861–864. doi: 10.1097/00004583-198911000-00009. [DOI] [PubMed] [Google Scholar]
- Ventura R, Morrone C, Puglisi-Allegra S. Prefrontal/accumbal catecholamine system determines motivational salience attribution to both reward- and aversion-related stimuli. Proc Natl Acad Sci U S A. 2007;104:5181–5186. doi: 10.1073/pnas.0610178104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viveros MP, Marco EM, File SE. Endocannabinoid system and stress and anxiety responses. Pharmacol Biochem Behav. 2005;81:331–342. doi: 10.1016/j.pbb.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Wang W, Cao X, Liu C, Liu L. Cannabinoid WIN 55,212–2 inhibits TRPV1 in trigeminal ganglion neurons via PKA and PKC pathways. Neurol Sci. 2011 doi: 10.1007/s10072-011-0620-6. [DOI] [PubMed] [Google Scholar]
- Weinshenker D, Schroeder JP. There and back again: A tale of norepinephrine and drug addiction. Neuropsychopharmacology. 2007;32:1433–1451. doi: 10.1038/sj.npp.1301263. [DOI] [PubMed] [Google Scholar]
- Wong ML, Kling MA, Munson PJ, Listwak S, Licinio J, Prolo P, Karp B, McCutcheon IE, Geracioti TD, Jr, DeBellis MD, Rice KC, Goldstein DS, Veldhuis JD, Chrousos GP, Oldfield EH, McCann SM, Gold PW. Pronounced and sustained central hypernoradrenergic function in major depression with melancholic features: Relation to hypercortisolism and corticotropin-releasing hormone. Proc Natl Acad Sci U S A. 2000;97:325–330. doi: 10.1073/pnas.97.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak M, Schramm N, Limbird L. The noradrenergic receptor subtypes. In: Bloom F, Kupfer D, editors. Psychopharmacology: The Fourth Generation of Progress. Raven Press; New York: 2000. [Google Scholar]
- Zahm DS. Functional-anatomical implications of the nucleus accumbens core and shell subterritories. Ann N Y Acad Sci. 1999;877:113–128. doi: 10.1111/j.1749-6632.1999.tb09264.x. [DOI] [PubMed] [Google Scholar]
- Zuardi AW. History of cannabis as a medicine: A review. Rev Bras Psiquiatr. 2006;28:153–157. doi: 10.1590/s1516-44462006000200015. [DOI] [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, Julius D, Hogestatt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]