Abstract
Heterotrimeric G proteins transduce signals sensed by transmembrane G protein coupled receptors (GPCRs). A subfamily of G protein βγ subunit types has been shown to selectively translocate from the plasma membrane to internal membranes on receptor activation. Using 4D imaging we show here that Gβγ translocation is not restricted to some subunit types but rather all twelve members of the family of mammalian γ subunits are capable of supporting βγ translocation. Translocation kinetics varies widely depending on the specific γ subunit type, with t1/2 ranging from 10 seconds to many minutes. Using fluorescence complementation, we show that the β and γ subunits translocate as βγ dimers with kinetics determined by the γ subunit type. The expression patterns of endogenous γ subunit types in HeLa cells, hippocampal neurons and cardiomyocytes are distinctly different. Consistent with these differences, the βγ translocation rates vary widely. βγ translocation rates exhibit the same γ subunit dependent trends regardless of the specific receptor type or cell type showing that the translocation rates are intrinsic to the γ subunit types. βγ complexes with widely different rates of translocation had differential effects on muscarinic stimulation of GIRK channel activity. These results show that G protein βγ translocation is a general response to activation of GPCRs and may play a role in regulating signaling activity.
Keywords: GPCR, G protein, live cell imaging, translocation, GIRK channel
1. Introduction
G protein subunits (α and βγ) are associated with the plasma membrane and are central to the regulation of cell physiology [1]. Early evidence suggested that the subunits are activated by transmembrane GPCRs and regulate the levels of second messengers by regulating effector activity at the plasma membrane. This lead to a long standing model that G proteins are constrained to the plasma membrane where they function as transducers of signals from outside the cell. However recent evidence suggests that G protein subunits are present inside the cell in the Golgi, ER, nucleus, endosomes and mitochondria [2,3,4,5,6,7]. The mechanism that allows G proteins to reside at both the plasma membrane and intracellular membranes was not clear. Evidence for constitutive shuttling of G protein subunits between the plasma membrane and intracellular membranes [8,9] as well as translocation of some βγ subunit types on receptor activation to the Golgi and ER [10,11] identified mechanisms that allow G proteins to reach various intracellular sites from the plasma membrane. Gβγ translocation was previously observed by using wide field microscopy for relatively short periods of time [10,11]. Here we imaged the properties of all members of the G protein γ subunit family in live cells using more sensitive 4D imaging methods for longer periods of time to ensure that even limited translocation of subunits would be detected. To ascertain that the properties identified were not particular to certain cell types or receptors we examined G protein subunit movement in different cells types on activation of different endogenous receptors. The results showed that the entire family of G protein βγ subunit types demonstrates receptor stimulated translocation, albeit at vastly different rates.
To examine if differential translocation rates of G protein βγ subunit types modulate downstream signaling activity differentially, we examined the effect of two βγ subunit type combinations with widely different translocation rates on muscarinic receptor activation of GIRK (G protein-coupled inwardly-rectifying potassium channel) activity. Gi coupled muscarinic receptors are known to activate GIRK channels through direct interaction of the βγ complex with the channel [12]. We examined the kinetics of channel activation in the presence of a rapidly or slowly translocating γ subunit. These experiments suggested a functional consequence for cells possessing G protein subunit types with differential translocation kinetics.
2. Materials and methods
2.1. Constructs and cell lines
G protein constructs used in this study have been previously described [8,11,13,14]. Receptors and G protein subunits were cloned into pcDNA3.1 (Invitrogen), GalT-DsRed was from Clontech, USA. Venus155–239-β1 and Venus1–155-γ2 have been described before [15] and were from N. Lambert, (Georgia Health Sciences University). Venus1–155-γ2 in pcDNA3.1 was cut with BamH1 and XbaI to release the γ2 fragment. γ5 and γ9 PCR fragments respectively were then cut with BamH1 and XbaI and cloned into these sites to make Venus1–155-γ5 and Venus1–155-γ9. GFP-GPI was from V.R. Caiolfa, (San Raffaele Institute of Research). The HeLa cell line was obtained from ATCC and cultured in the recommended medium. The HL-1 cardiomyocyte cell line was from W. Claycomb, (Louisiana State University Medical Center) and was grown in complete supplemented Claycomb medium (Sigma) [16]. Hippocampal cultures were prepared by coating the glass bottom of 30 mm plastic culture dishes with 0.15% agarose. Cell suspensions were prepared from postnatal day 1–3 rat hippocampus using papain and mechanical dispersion and cultured as described [17]. A-68930, norepinephrine, yohimbine, carbachol, atropine, SDF-1α, AMD3100, isoproterenol, N6-Cyclopentyladenosine were from Sigma. Quantitative PCR was performed as previously described [13].
2.2 Live cell imaging
All the imaging was performed using an Andor-Leica spinning disk laser confocal imaging system. It consisted of a Leica DMI 6000B microscope with adaptive corrective focus (AFC) that prevents drift in long term experiments, a Yokogawa CSU X1 spinning disk unit and an Andor iXon EM CCD camera. Excitation was controlled with 4 solid state lasers: 445, 488, 515 and 595 nm. Excitation and emission wavelength filters (Semrock) were as follows: CFP fluorescence - 445 excitation and 478/30 emission; GFP fluorescence - 488 excitation and 515/20 emission; YFP fluorescence - 515 excitation and 528/20 emission, Red fluorescence - 595 excitation and 628/20 emission. Cells were cultured and transiently transfected either in 29 mm glass bottom culture dishes (In vitro Scientific) or on 40 mm glass coverslips. Images were acquired with a 63× objective (1.4 NA). Confocal planes of cells for Z stacks were imaged at 0.4 µm intervals and the topographic Z projection images of maximum intensity were created using Andor iQ 2.5 software. Mean pixel fluorescence intensity changes in the entire cell or in selected areas of the cell were determined using Andor iQ 2.5 software. Z stacked images were acquired at 15–40 sec intervals before and after addition of agonist to activate an endogenous receptor. When measuring fluorescence in internal membranes, the top few planes were removed in order to avoid interference from the plasma membrane.
2.3. Electrophysiology
Whole-cell patch-clamp current recordings were performed with an EPC 9 amplifier driven by the Pulse program (Heka-Electronik) using pipettes with a resistance of 2–3 MΩ, pulled from filamented borosilicated glass capillaries (WPI, 1B150F-4) using a micropipette laser puller (P-2000 Sutter Instruments, Novato, CA). Cells were clamped at −50 mV and bathed in an extracellular solution containing (millimolar): NaCl 120, KCl 20, CaCl2 2, MgCl2 1, HEPES 10, pH 7.4, while the intracellular solution was (millimolar): K gluconate 110, KCl 20, NaCl 10, MgCl2 1, Mg ATP 2, EGTA 2 GTP 0.3, pH 7.4. Approximately 2 min after the whole cell configuration was established, the cell membrane capacitance was measured in voltage-clamp by using the automatic compensation circuitry of the EPC-9 amplifier at a holding potential of −50 mV. Series resistance was electronically compensated (80%). Drugs were applied by a gravity driven perfusion system allowing switching between different test solutions. Solution exchange time with this system is typically < 0.5 s. All experiments were performed at room temperature (25°C).
Transiently transfected HL-1 cells with GFP tagged γ subunits or GPI membrane marker were visualized with a Nikon Eclipse TE2000-U epifluorescence microscope using a 40X oil immersion objective (0.6 N.A.). GFP fluorescence was detected with D492/18 excitation and D535/30 emission filters (Chroma) Images were recorded at 1 sec exposures with 4 binning using a Hamamatsu CCD Orca-ER camera (12 bit). Images were acquired using MetaMorph software and cells with approximately equal intensities of GFP were selected for patch clamping. During agonist application, cells were clamped at −80 mV (nominal EK=−49 mV). Agonist application elicited an inward current reaching a steady-state plateau. After removal of the agonist, the current decays to the initial baseline. Current activation and deactivation kinetics were fitted to a single exponential function Aexp(−t/τ)+C, where A is the current amplitude at the start of the fit, t is time, τ is the activation or the deactivation time constant, and C is the steady-state asymptote. Currents were analyzed using IgorPro (WaveMetrics). The time course of the current was fitted to the indicated equations by using Origin v. 7.5. Results are expressed as mean ± S.E.M. Statistical differences between means were analyzed using the Kolmogorov-Smirnov test (*p<0.05 and **p<0.01).
3. 3. Results and Discussion
3.1. Translocation of all members of the G protein γ subunit family can be detected
Earlier results showed that six members of the γ subunit family support receptor mediated βγ translocation from the plasma membrane [11]. Based on wide field imaging, it was concluded that γ2, γ3, γ4, γ7, γ8 and γ12 were not capable of translocation. Here we examined Gβγ translocation using confocal microscopy and 4D imaging. Translocation of all members of the γ subunit family was examined in HeLa cells by activating two endogenous receptors, CXCR4 and α2 adrenergic (α2AR). Cells were imaged using a spinning disk confocal microscope for >10 min after receptor activation. To obtain a 3D view of the translocation, confocal images along the Z axis were captured and stacked as described in the Materials and Methods section.
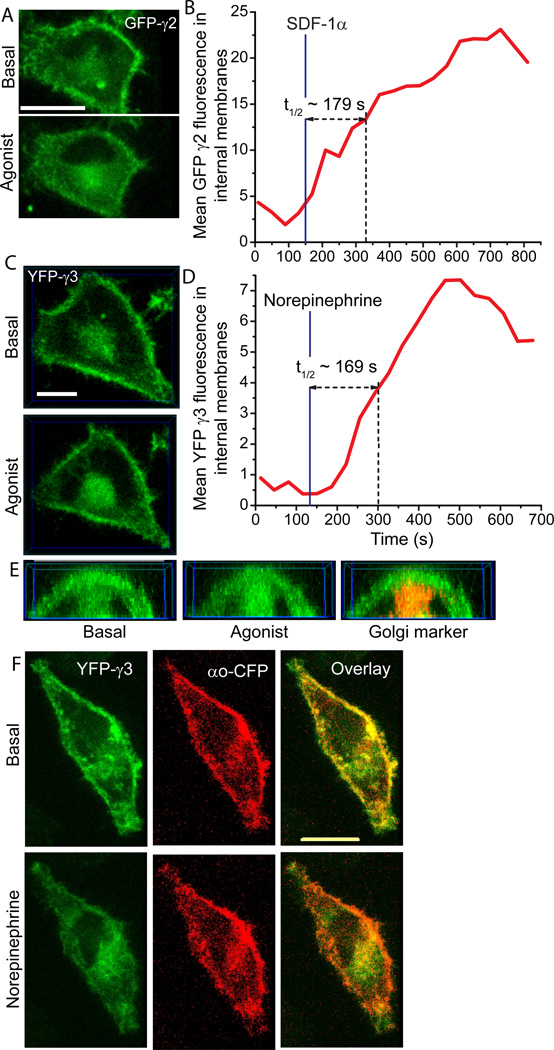
Fig. 1A shows the translocation of FP (Fluorescence Protein)-γ2 to intracellular membranes when CXCR4 receptors in HeLa cells were activated with SDF-1α. Translocation occurred over a relatively long period of time (Fig. 1A, B) compared to subunit types that we previously categorized as those capable of translocation. The t1/2 for translocation as determined by time lapse 3D imaging was about 180 sec. The closely related γ3 subunit also translocated on a similar time scale (t1/2 ~170 sec) to the Golgi when HeLa endogenous α2 adrenergic receptors in HeLa cells were activated with norepinephrine (Fig 1C, D). This result suggested that the receptor stimulated translocation of γ2 is not a peculiarity specific to that subunit type. 3D images showed that on translocation γ3 colocalized with a Golgi marker - galactosyltransferase (GalT-dsRed (Fig. 1E). The slow translocating γ subunit types thus translocate predominantly to the Golgi similar to the rapidly translocating γ subunit types examined earlier [10,11].
Fig.1. Receptor activated slow translocation of the βγ2 and βγ3 subunits to intracellular membranes.
(A) Representative 3D images viewed from the top of the cell (n=6). HeLa cells transiently transfected with GFP-γ2 before and after activation of endogenous CXCR4 receptor with 100 ng/ml SDF-1 α. (B) GFP intensity changes in the internal membranes. (C) Representative 3D images of transiently transfected YFP-γ3 in HeLa cells before and after activation of endogenous α2-adrenergic receptor activation with 10 µm norepinephrine (n=6). (D) YFP intensity changes in the internal membranes. (E) Front view of a cross section of 3D images of YFP-γ3 transfected HeLa cells (from C) before and after receptor activation. Overlay with an image of GalT-dsRed coexpressed in the same cell shows colocalization of YFP-γ3. (F) 3D images of a HeLa cell coexpressing αo-CFP (red) and YFP-γ3 (green), overlaid before and after α2AR activation. Scale bars = 10 µm.
We then examined γ4, γ7, γ8 and γ12 subunit types which had previously been thought to be incapable of translocation. The result of activating G proteins in HeLa cells with SDF-1α and norepinephrine showed that these subunits were also capable of translocation (Table I). When the rate of translocation of each of the twelve members of the γ subunit family was examined (Table I) the t1/2 varied widely from 10 sec in the case of γ9 to 290 sec in the case of γ3. For each γ subunit we examined the reverse translocation as well by deactivating the receptor with an antagonist. The ability of all γ subunit types to reverse translocate at any point of time shows that translocation is not likely to be due to permanent alterations to the γ subunit such as proteolysis or removal of the prenyl moiety attached to the C terminus.
Table I. Rates of forward and reverse translocation of γ subunit types on activation of endogenous receptors in HeLa cells.
t1/2 values are in seconds. F - forward translocation. Increase in intracellular membrane FP-γ subunit fluorescence intensity on agonist activation was plotted and t1/2 determined. R- reverse translocation. Decrease in intracellular membrane FP-γ subunit fluorescence intensity on antagonist addition was plotted and t1/2 determined. CXCR4 receptors were activated with 100 ng/ml SDF-1α and deactivated with 20 µM AMD 3100. α2-AR receptors were activated with 10 µM norepinephrine and deactivated with 60 µM yohimbine.
| CXCR4 | α2-AR | CXCR4 | α2-AR | CXCR4 | α2-AR | |||
|---|---|---|---|---|---|---|---|---|
| γ9-F γ9-R |
10±2(10) 19±2 |
14±2(7) 19±4 |
γ5-F γ5-R |
66±5(5) 167± |
65±3(9) 127±8 |
γ8-F γ8-R |
123±5(6) 111±3 |
N.D. N.D. |
| γ1-F γ1-R |
20±1(6) 22±1 |
N.D. N.D. |
γ12-F γ12-R |
89±2(6) 104±4 |
61±3(10) 87±11 |
γ4-F γ4-R |
118±6(7) 143±19 |
N.D. N.D. |
| γ11F γ11-R |
36±3(7) 65±9 |
15±3(4) 28±1 |
γ10-F γ10-R |
95±3(8) 95±3 |
48±5(7) 60±9 |
γ2-F γ2-R |
161±13(6) 145±5 |
191±25(6) N.D. |
| γ7-F γ7-R |
45±1(5) 74±3 |
58±12(6) 100±27 |
γ13-F γ13-R |
100±2(7) 212±2 |
56±7(6) 53±3 |
γ3-F γ3-R |
286±17(10) 296±9 |
219±61(6) N.D. |
Mean ± SEM (no. of cells).
N.D. - not done. Cells were from multiple dishes.
When translocation rates specific to different receptors, CXCR4 and α2AR were compared (Table I), the overall trend for the different γ subunit types was the same suggesting that the rate of translocation of a γ subunit is an intrinsic property of a particular subunit that may not be influenced by the receptor type.
We then confirmed that translocation of the βγ complex was selective with the α subunit being retained on the plasma membrane after receptor activation. In HeLa cells expressing αo-CFP and YFP-γ3, the emission from both αo-CFP and YFP-γ3 was observed on the plasma membrane in the basal state and overlaid images confirmed this distribution (Fig. 1F). On addition of norepinephrine to activate α2AR, YFP-γ3 emission increased in intracellular membranes but the overlay with an image of αo-CFP showed that αo-CFP was retained on the plasma membrane. Consistent with previous results for fast translocating subunits [10,11], this result shows that slow translocating βγ also dissociate from the α subunit prior to movement to internal membranes.
3.2. G protein β and γ subunits translocate as a complex
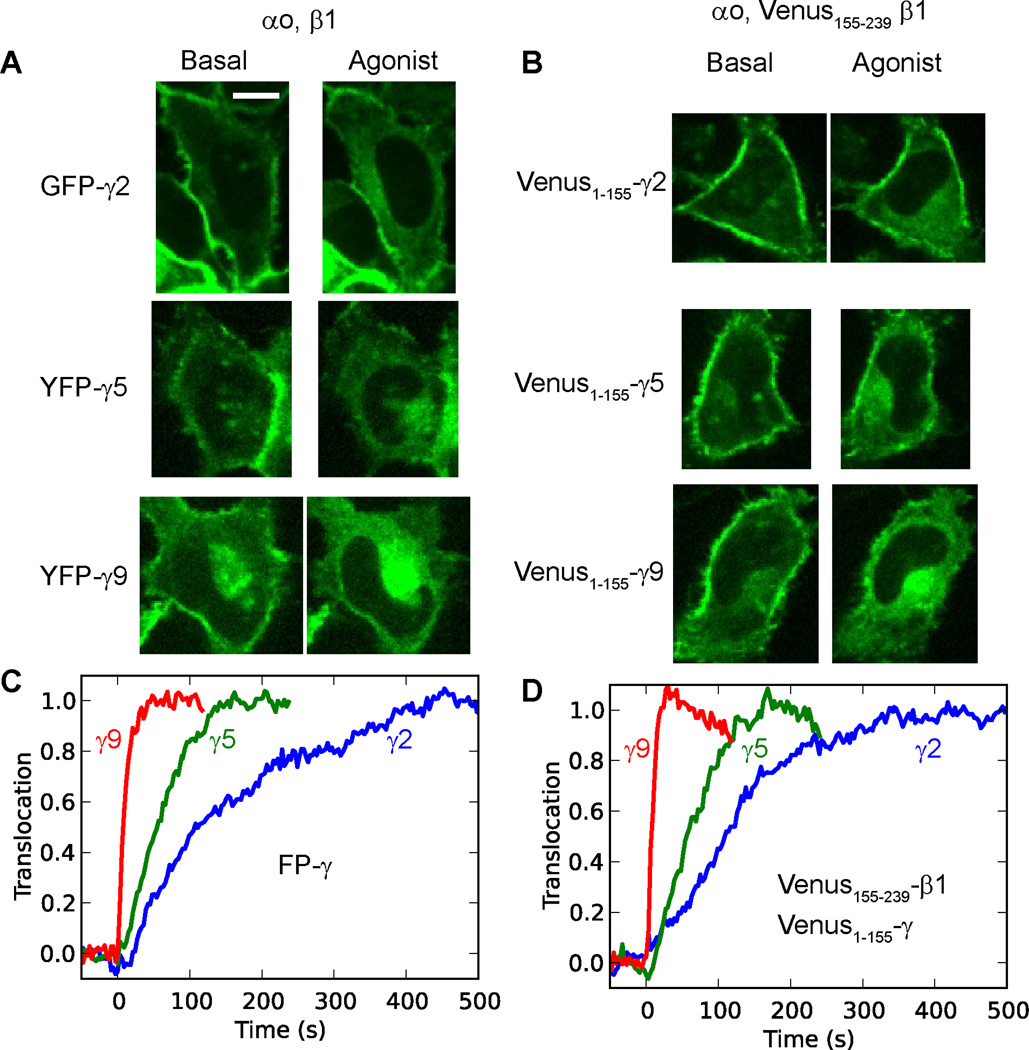
To directly demonstrate that the β and γ subunits translocate as a βγ dimer, we used fluorescence complementation of a split Venus fluorescent protein [15]. βγ translocation was imaged on activation of endogenous α2AR in HeLa cells cotransfected with either αo, β1, and a specific FP-γ subunit type (GFP-γ2, YFP-γ5 or YFP-γ9) (Fig. 2A), or αo, Venus155–239-β1 and a specific Venus1–155-γ subunit (γ2, γ5 or γ9) (Fig.2B). The results showed that regardless of whether the βγ complex was labeled by fluorescence complementation or by a FP-γ subunit, translocation from the plasma membrane to internal membranes was detected. Notably, the translocation kinetics showed the same γ subunit dependence for both labeling methods (Fig. 2C, D). These results showed that βγ translocation rates measured using FP tagged γ subunits are the same as those measured for the βγ complex based on fluorescence complementation between the β and γ subunits. These results confirmed that the translocation of FP tagged γ subunits observed in our live cell imaging assays is due to the translocation of the βγ complex. This result is consistent with the well-known stable association of β and γ subunits under physiological conditions [18].
Fig.2. G protein β and γ subunits translocate as a βγ dimer.
(A) Confocal images of HeLa cells transfected with αo, β1, and a FP-tagged γ subunit. (B) Images of cells transfected with αo, Venus155–239-β1, and a Venus1–155-γ subunit. (C, D) Translocation kinetics for the cells (in A) are shown in (C) and cells in (B) in (D). Images were captured in a single confocal plane at a rate of 1 image every 3 sec. All images are shown at the same scale (n=3). Endogenous α2 adrenergic receptors were activated with 10 µM norepinephrine. Scale bar = 20 µm.
3.3. Translocation of endogenous G protein γ subunits in HeLa cells, hippocampal neurons and HL-1 cardiomyocytes
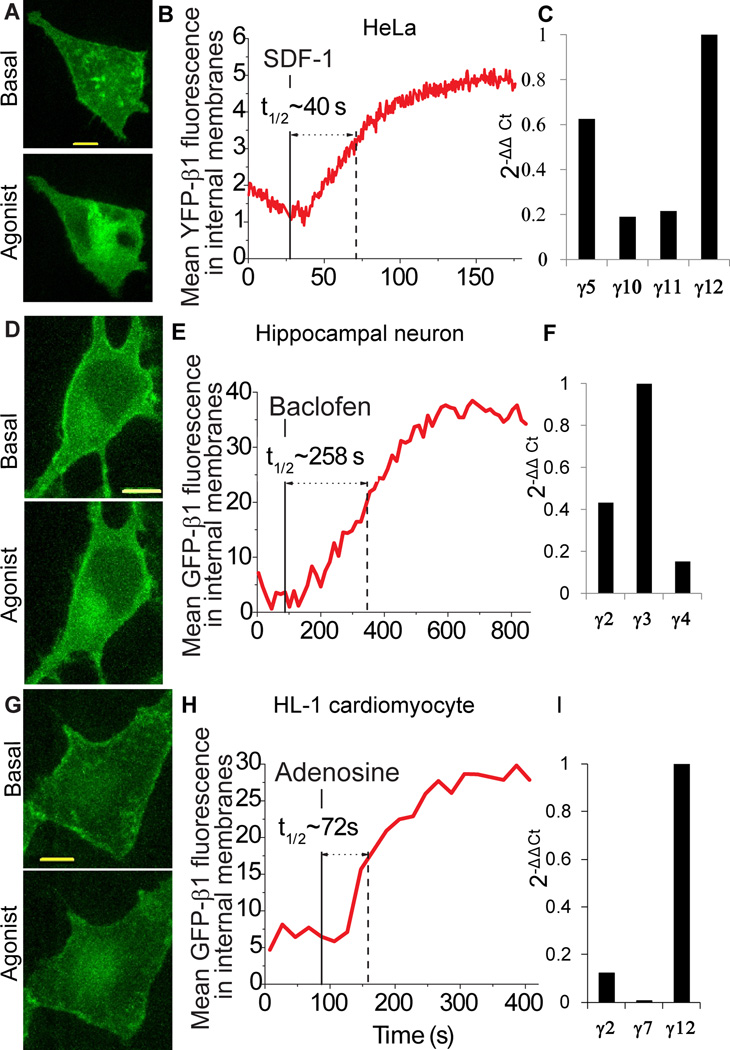
Since FP-β1 binds to endogenous γ subunits, we used Z stacking of confocal images to determine if endogenous γ subunits translocate. When the translocation kinetics of endogenous γ subunits in HeLa cells was determined by expressing FP-β1, the t1/2 for translocation was 40 sec on CXCR4 activation (Fig. 3A, B). When quantitative real time PCR was used to quantitate the levels of expression of Gγ subunit types in HeLa cells, the most abundant transcripts were those encoding γ12, γ5 and γ11 (Fig. 3C). The translocation rates of the endogenous γ subunits in HeLa cells thus reflect an approximation of the properties of the predominant native γ subunit types (Table I).
Fig.3. Endogenous γ subunits translocate in HeLa cells, hippocampal neurons and cardiomyocytes.
(A) Representative 3D images of HeLa cells expressing YFP-β1 before and after addition of SDF-1α (100 ng/ml) (n = 7). Image after agonist addition is representative of images where intensity in the intracellular membranes has reached maximum. B Representative plot showing the increase in internal membrane YFP fluorescence after receptor activation. C Quantitative real-time PCR profile of the most abundant γ subunit types in HeLa cells (D) Representative 3D images of rat hippocampal neurons expressing GFP-β1 before and after addition of baclofen. 6 day old neuronal cultures were transiently transfected with GFP-β1 and untagged αo. Cells were imaged before and after exposure to the GABA-B receptor agonist, baclofen (10 µM) (n = 20). Image after agonist addition is representative of images where intensity in the intracellular membranes has reached maximum. (E) Corresponding plot showing the increase in internal membrane GFP fluorescence after receptor activation. (F) Quantitative real-time PCR profile of major γ subunit types in neurons. (G) Representative 3D images of HL-1 cells expressing GFP-β1 before and after activation of endogenous Gi/o coupled Adenosine A1 receptor with 10 µM N6 cyclopentyladenosine (n=3). (H) Representative plot showing the increase in internal membrane GFP fluorescence after receptor activation. (I) Quantitative real-time PCR profile of major γ subunit types in HL-1 cells. Scale bars = 10 µm.
We then examined receptor induced βγ translocation in rat hippocampal neurons after activation of endogenous Gi/o coupled GABAB receptors with baclofen. Introduced β1 translocation was clearly detectable in the cell body of the neuron (Fig. 3D). The t1/2 of endogenous γ subunit translocation was 258 sec (Fig. 3E). This translocation rate was consistent with the preponderant expression of γ2 and γ3 transcripts in mouse hippocampal neurons, (Fig. 3F). Pure mouse neurons were used to determine the levels of γ subunit transcripts since rat neurons were cultured with astrocytes.
Finally, we observed β1 translocation in HL-1 cardiomyocytes after endogenous A1 adenosine receptor activation (Fig. 3G). Similar to HeLa cells and hippocampal neurons the translocation rate (72 sec) (Fig. 3H) reflected the previously identified properties of the predominant γ subunit type, γ12 (Table I), present in these cells based on quantitative real time PCR (Fig. 3 I).
These results showed that γ subunit types endogenous to various cell types translocate at rates that are similar to their corresponding transfected FP tagged counterparts indicating that the translocation properties of subunit types is not altered by the FP tags. Additionally these results showed that different cell types may express distinct γ subunit types which result in the overall kinetics of receptor mediated βγ translocation being strikingly different.
3.4. Slow translocating γ3 and fast translocating γ9 have differential effects on muscarinic receptor activation of GIRK channels
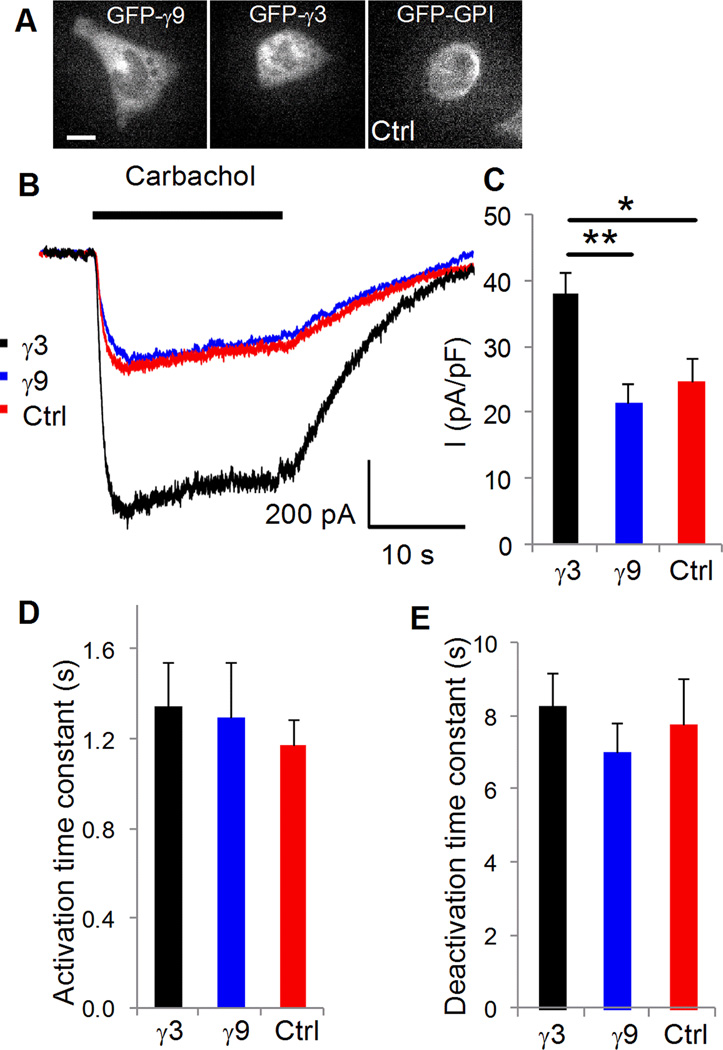
One possible role for the differential translocation kinetics of G protein βγ subunits is that they regulate downstream signaling dynamics differentially. Previous results from phospholipase C β activity in intact cells [13] suggests such a role. Here, we examined the effect of slow translocating γ3 and γ9 on the properties of GIRK channel activation by muscarinic receptors in HL-1 cardiomyocytes. In HL-1 cells activation of an endogenous muscarinic receptor has been shown to activate an endogenous GIRK channel [19]. We ensured that cells expressing the same levels of FP tagged γ3 or γ9 were assayed (Fig. 4A). As a control, cells expressing GPI-GFP were examined. Results (Fig. 4B) show that the amplitude of GIRK current was similar in control (Ctrl) cells and cells expressing γ9. In contrast, cells expressing γ3 showed significantly higher current amplitude (Fig. 4B, C). There was no difference in the activation or deactivation kinetics (Fig. 4D, E). Although alternative explanations cannot be ruled out, these results are consistent with the more rapid translocation of βγ9 leading to depletion of available βγ for activation of the GIRK channel resulting in the lower amplitude of K+ current. In contrast, the significantly slower translocation of βγ3 may provide a sufficiently higher concentration of local βγ for channel activation during the time course of the experiment. Results here suggest that in general GPCR activation in any cell will result in the translocation of the activated βγ complex to intracellular membranes. Since all the results here have been obtained using endogenous receptors, they show that translocation is not a peculiarity of overexpressed receptors.
Fig. 4. Dependence of K+ current activation on G proteins in transfected HL-1 cells.
(A) Representative current traces recorded from a cell transfected with: GFP-GPI (control) (red: Cm=22.67 pF), GFP-γ9 (blue: Cm=18.16 pF) and GFP-γ3 (black: Cm=17.42 pF). Cells were clamped at −80 mV and 20 µM carbachol was applied for 20s. (B) Maximum mean current (normalized to cell Cm) after activation with the agonist. Cells transfected with GFP-GPI (n=11) and GFP-γ9 (n=10) evoked significantly smaller currents compared to GFP-γ3 (n=10) transfected cells (p<0.03 and p<0.002 respectively). (C) Comparison of kinetics of current activation and (D) deactivation. Scale bars = 20 µm.
The striking differences in the translocation kinetics of βγ subunit types found here introduce unexpected spatiotemporal complexity to G protein action. Results showing that endogenous γ subunit types translocate at the rates predicted by the translocation properties of introduced FP tagged γ subunits not only validate the results obtained using tagged proteins but also show that endogenous βγ complexes translocate at differential rates depending on cell type and expression profile of subunit types. This suggests that differential translocation kinetics of Gβγ subunits can contribute to the functional differences between cell types.
Translocation of activated βγ can play several different roles. It has been shown to regulate Golgi structure and secretion [20]. The loss of G protein subunits at differential rates from the plasma membrane can also have differential effects on the kinetic characteristics of downstream effectors. Previous results have shown such an effect on IP3 stimulation by a Gq coupled muscarinic receptor [13]. The differences seen here in the amplitude of GIRK channel current induced by Gi coupled muscarinic receptor activation suggest that the wide variation in the translocation kinetics of βγ subunit types may play an important regulatory role in the modulation of downstream effector activity.
Highlights.
G protein βγ subunit types translocate universally on receptor activation
They do so with widely different kinetics dependent on the γ subunit type
Translocation occurs as a βγ complex
Endogenous γ subunits translocate at predicted rates
Varying translocation rates have differential effects on GIRK K+ channel activity
Supplementary Material
Fig. S1. 3D image processing for the detection of βγ translocation to internal membranes.
Confocal images of YFP-γ and GalT-dsRed were Z-stacked to create 3D image. To measure fluorescence in internal membranes, the top few planes were removed to reduce interference from the plasma membrane (below).
Fig. S2. Translocation of γ4, γ7, γ8 and γ12.
Confocal images and corresponding plots showing the translocation of γ4 (A), γ7 (B), γ8 (C) and γ12 (D).
Acknowledgements
We thank W. Claycomb for HL-1 cells, S. Mennerick, Ann Benz and N. Ramanan for hippocampal neurons. We also thank N. Lambert for constructs and L. Giri for discussions. This work was supported by NIH grants (GM69027 and GM080558) to N.G. and a NRSA post doctoral fellowship (F32 GM099351) to P.R.O. P.M was supported by NIH grant GM081748 to C. Lingle.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Neves SR, Ram PT, Iyengar R. G protein pathways. Science. 2002;296:1636–1639. doi: 10.1126/science.1071550. [DOI] [PubMed] [Google Scholar]
- 2.Denker SP, McCaffery JM, Palade GE, Insel PA, Farquhar MG. Differential distribution of alpha subunits and beta gamma subunits of heterotrimeric G proteins on Golgi membranes of the exocrine pancreas. J Cell Biol. 1996;133:1027–1040. doi: 10.1083/jcb.133.5.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kino T, Tiulpakov A, Ichijo T, Chheng L, Kozasa T, Chrousos GP. G protein beta interacts with the glucocorticoid receptor and suppresses its transcriptional activity in the nucleus. J Cell Biol. 2005;169:885–896. doi: 10.1083/jcb.200409150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slessareva JE, Routt SM, Temple B, Bankaitis VA, Dohlman HG. Activation of the phosphatidylinositol 3-kinase Vps34 by a G protein alpha subunit at the endosome. Cell. 2006;126:191–203. doi: 10.1016/j.cell.2006.04.045. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Regalado A, Guzman-Hernandez ML, Ramirez-Rangel I, Robles-Molina E, Balla T, Vazquez-Prado J, Reyes-Cruz G. G protein-coupled receptor-promoted trafficking of Gbeta1gamma2 leads to AKT activation at endosomes via a mechanism mediated by Gbeta1gamma2-Rab11a interaction. Mol Biol Cell. 2008;19:4188–4200. doi: 10.1091/mbc.E07-10-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andreeva AV, Kutuzov MA, Voyno-Yasenetskaya TA. G alpha12 is targeted to the mitochondria and affects mitochondrial morphology and motility. FASEB J. 2008;22:2821–2831. doi: 10.1096/fj.07-104224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saini DK, Chisari M, Gautam N. Shuttling and translocation of heterotrimeric G proteins and Ras. Trends Pharmacol Sci. 2009;30:278–286. doi: 10.1016/j.tips.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chisari M, Saini DK, Kalyanaraman V, Gautam N. Shuttling of G protein subunits between the plasma membrane and intracellular membranes. J Biol Chem. 2007;282:24092–24098. doi: 10.1074/jbc.M704246200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsutsumi R, Fukata Y, Noritake J, Iwanaga T, Perez F, Fukata M. Identification of G protein alpha subunit-palmitoylating enzyme. Mol Cell Biol. 2009;29:435–447. doi: 10.1128/MCB.01144-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akgoz M, Kalyanaraman V, Gautam N. Receptor-mediated reversible translocation of the G protein betagamma complex from the plasma membrane to the Golgi complex. J Biol Chem. 2004;279:51541–51544. doi: 10.1074/jbc.M410639200. [DOI] [PubMed] [Google Scholar]
- 11.Saini DK, Kalyanaraman V, Chisari M, Gautam N. A family of G protein betagamma subunits translocate reversibly from the plasma membrane to endomembranes on receptor activation. J Biol Chem. 2007;282:24099–24108. doi: 10.1074/jbc.M701191200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clapham DE, Neer EJ. G protein beta gamma subunits. Annu Rev Pharmacol Toxicol. 1997;37:167–203. doi: 10.1146/annurev.pharmtox.37.1.167. [DOI] [PubMed] [Google Scholar]
- 13.Chisari M, Saini DK, Cho J-H, Kalyanaraman V, Gautam N. G Protein Subunit Dissociation and Translocation Regulate Cellular Response to Receptor Stimulation. PLoS ONE. 2009;4:e7797. doi: 10.1371/journal.pone.0007797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Azpiazu I, Gautam N. A Fluorescence Resonance Energy Transfer-based Sensor Indicates that Receptor Access to a G Protein Is Unrestricted in a Living Mammalian Cell. J Biol Chem. 2004;279:27709–27718. doi: 10.1074/jbc.M403712200. [DOI] [PubMed] [Google Scholar]
- 15.Hollins B, Kuravi S, Digby GJ, Lambert NA. The c-terminus of GRK3 indicates rapid dissociation of G protein heterotrimers. Cellular signalling. 2009;21:1015–1021. doi: 10.1016/j.cellsig.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Claycomb WC, Lanson NA, Jr, Stallworth BS, Egeland DB, Delcarpio JB, Bahinski A, Izzo NJ., Jr HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci U S A. 1998;95:2979–2984. doi: 10.1073/pnas.95.6.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mennerick S, Que J, Benz A, Zorumski CF. Passive and synaptic properties of hippocampal neurons grown in microcultures and in mass cultures. J Neurophysiol. 1995;73:320–332. doi: 10.1152/jn.1995.73.1.320. [DOI] [PubMed] [Google Scholar]
- 18.Gautam N, Downes GB, Yan K, Kisselev O. The G-protein betagamma complex. Cell Signal. 1998;10:447–455. doi: 10.1016/s0898-6568(98)00006-0. [DOI] [PubMed] [Google Scholar]
- 19.Nobles M, Sebastian S, Tinker A. HL-1 cells express an inwardly rectifying K+ current activated via muscarinic receptors comparable to that in mouse atrial myocytes. Pflugers Arch. 2010;460:99–108. doi: 10.1007/s00424-010-0799-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saini DK, Karunarathne WK, Angaswamy N, Saini D, Cho JH, Kalyanaraman V, Gautam N. Regulation of Golgi structure and secretion by receptor-induced G protein betagamma complex translocation. Proc Natl Acad Sci U S A. 2010;107:11417–11422. doi: 10.1073/pnas.1003042107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. 3D image processing for the detection of βγ translocation to internal membranes.
Confocal images of YFP-γ and GalT-dsRed were Z-stacked to create 3D image. To measure fluorescence in internal membranes, the top few planes were removed to reduce interference from the plasma membrane (below).
Fig. S2. Translocation of γ4, γ7, γ8 and γ12.
Confocal images and corresponding plots showing the translocation of γ4 (A), γ7 (B), γ8 (C) and γ12 (D).