1. INTRODUCTION
Since biological criteria are not available to define objective phenotypes in psychiatric disorders, clinical phenomenology has been used to classify psychiatric diagnoses. In recent years, traditional diagnostic constructs have been increasingly challenged, especially in the cases where prominent symptom dimensions cut across diagnostic boundaries. This is particularly evident in schizophrenia (SZ) and bipolar disorder (BD) where an extensive overlap exists in many clinical characteristics, including psychosis, cognition, mood and behavioral symptoms. Moreover, recent genetic (Wellcome Trust Case Control Consortium, 2007) and neurophysiological (Thaker, 2008) studies have provided evidence for putative molecular and biological markers that overlap the two diagnoses. These observations challenge the traditional dichotomous model of SZ and BD and advocate for dimensional distinctions to enable studies of biological disease criteria.
One of the approaches that seeks to define biological markers which support valid diagnosis is the identification of endophenotypes: the specific heritable characteristics of brain anatomy, biochemistry or function that provide a direct measure of brain behavior, and, perhaps, more direct molecular underpinnings for disease definition than does the clinical syndrome (Gottesman and Shields, 1973). One of the broadly studied putative endophenotypes in psychiatric syndromes is cognitive dysfunction, as suggested by Burdick et al. (2006) and Bora et al. (2009). The established heritability of cognitive function and the described neural networks mediating specific aspects of cognition, as well as the availability of objective measurement tools to assess cognitive performance provide support for examining cognitive function as a promising endophenotype for SZ and BD (Ivleva et al., 2010). Several cognitive endophenotypes have been described in probands with SZ (SZP) and BD (BDP), with deficits in executive function, declarative memory and processing speed consistently found in both diagnoses (Schretlen et al., 2007; Hill et al., 2008; Arts et al., 2008; Smith et al., 2009; Stefanopoulou et al., 2009). Deficits in working memory and sustained attention are common in SZP (Braff, 1993; Keefe et al., 1995; Cornblatt and Malhotra, 2001), whereas are less consistently reported in BDP (Clark et al., 2002; Glahn et al., 2010), with some describing them as state-dependent (Kumar et al., 2010). Cognition deficits in SZP are thought to be more severe and stable “trait” measures, while these deficits in BDP are described as less debilitating and partially state-dependent, although attenuated impairments remain during periods of euthymia (Ferrier et al., 1999; Rubinsztein et al., 2000; Thompson et al., 2005). In addition, deficits in executive function, working and declarative memory, and processing speed have been reported in biological relatives of SZP (SZR) (Faraone et al., 1995; Toomey et al., 1998; Cannon et al., 2000; Asarnow et al., 2002; Sitskoorn et al., 2004), as well as in relatives of BDP (BDR) (Glahn et al., 2004; Kieseppa et al., 2005; Hill et al., 2008; Glahn et al., 2010). Deficits in attention are well documented in SZR (Snitz et al., 2006; Sponheim et al., 2006), whereas reports in BDR are less consistent (Gourovitch et al., 1999; Bora et al., 2009; Kumar et al., 2010). Growing evidence suggests that BDP may have distinctive cognitive profiles depending on presence of lifetime psychosis; in that, probands with psychotic BD show deficits in domains of executive function, verbal and non-verbal declarative memory, working memory, processing speed and attention, similar to those found in SZP (Glahn et al., 2006; Glahn et al., 2007; Martinez-Aran et al., 2008; Reichenberg et al., 2009). Mild psychosis phenotypes (e.g., schizotypy) have been linked to poorer cognitive performance in SZR (Cannon et al., 1994; Niendam et al., 2007). Interestingly, a single study of BDR with lifetime subclinical psychotic-like symptoms showed better performance in motor speed, verbal declarative memory and attention compared to non-psychotic BDR (Jabben et al., 2009).
Overall, evidence suggests that psychosis may be a key clinical dimension associated with overlapping cognitive characteristics in SZ and BD. Therefore, in this study we examined putative cognitive endophenotypes within the SZ - psychotic BD boundary contrasting the two clinical paradigms: traditional dichotomous DSM-IV diagnoses (SZ, BD) and the psychosis dimension defined as manifestation of lifetime psychotic symptoms independent of the DSM-IV diagnoses. In accordance with the classic endophenotype criteria (Gottesman and Shields, 1973), we conducted this examination in a family sample of probands with SZ and psychotic BD, and in their first-degree biological relatives. Establishing similarities and differences in the endophenotypic signatures of SZ and BD families may provide important insights for future genetic studies, and may improve conceptualizations about the common and distinct aspects of pathophysiology, about clinical heterogeneity, and about clinical boundaries of the two psychotic disorders. We asked the question, whether there are aspects of cognitive dysfunction that distinguish the traditional diagnostic groups (SZ and BD), or whether cognitive dysfunction is common to probands and relatives across the SZ/BD psychosis dimension. We conducted two sets of analyses with the cognitive measures, one contrasting individuals with the two categorical diagnoses, and the other contrasting individuals with and without lifetime psychoses. The traditional DSM-IV diagnosis analyses examined cognitive performance in probands with SZ and BD, and their relatives in the four study groups: SZP, BDP, SZR and BDR. The psychosis dimension analyses examined cognitive performance in individuals with and without lifetime psychoses and contrasted the three study groups: 1) psychosis probands (SZP and BDP, combined), 2) relatives with psychosis spectrum disorders (defined in Methods), and 3) relatives without psychosis spectrum disorders. We hypothesized that: 1) within traditional diagnoses, SZP and BDP would show similar cognitive performance on measures of working memory, declarative memory, executive function and attention; SZR and BDR would show similar performance on these measures; whereas relatives would show higher cognitive performance than probands; and, 2) within the psychosis dimension, probands would show lower cognitive performance on the measures of working memory, declarative memory, executive function and attention compared to relatives without psychosis spectrum disorders; whereas relatives with psychosis spectrum disorders would show cognitive performance on these measures intermediate between probands and non-psychotic relatives. This is the first study to directly compare putative cognitive endophenotypes in probands and relatives from both diagnostic groups (SZ and psychotic BD) using a psychosis “diagnosis vs. dimension” contrast. The proband groups included stable on medications out-patients, similar in age, education, IQ and socio-economic status, providing a common baseline of relevant characteristics for cognitive performance comparisons.
2. METHODS
2.1. Subjects
The study recruited probands who met the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) (American Psychiatric Association, 1994) criteria for SZ or BD, type I with lifetime history of psychotic symptoms, and their eligible first-degree relatives with and without lifetime psychiatric diagnoses. All SZ and BD probands recruited into this study were clinically stable medicated out-patients with active psychosis and mood symptoms severity varying from remission/euthymic state to mild symptoms. Relatives who had lifetime psychiatric diagnoses were clinically stable and asymptomatic at the time of the study. Psychosis spectrum disorders in relatives were defined as lifetime psychotic DSM-IV Axis I diagnoses (e.g., major depressive disorder with psychotic features, substance-induced psychosis, etc.), or any SZ spectrum/Cluster A personality disorder. All volunteers were between ages 15 and 65 years, English-speaking, and had an IQ > 70. Probands were recruited through advertising and by referrals from community out-patient mental health centers within Dallas county and from the UT Southwestern out-patient psychiatric clinics; relatives were recruited with the probands’ consents. Individuals with a history of major neurological or decompensated medical illness, mental retardation, traumatic brain injury with loss of consciousness, substance abuse within the last month or substance dependence within the last three months were excluded from the study. The study was approved by the institutional review board of the UT Southwestern Medical Center and was consistent with standards for the ethical conduct of human research. All volunteers provided written informed consent after the study procedures had been fully explained.
Initially, a total of 131 volunteers were recruited for the study, including 76 probands (44 SZP and 32 BDP) and 55 relatives (30 SZR and 25 BDR). The few relatives who had diagnoses of SZ (n=2) or psychotic BD (n=2) were moved to the proband groups for both the traditional DSM-IV diagnosis and the psychosis dimension analyses. Therefore, the traditional diagnosis analysis groups included 1) SZP (n=46), 2) BDP (n=34), 3) SZR (n=29) and, 4) BDR (n=22); and the psychosis dimension analyses groups included 1) probands (n=80, including SZP and BDP); 2) psychotic relatives (n=22, including SZR and BDR with lifetime psychosis spectrum disorders: SZR [n=16, including 6 with psychotic major depressive disorder, 9 with cluster A personality disorder, and 1 with bipolar disorder, type II, and cluster A personality disorder] and BDR [n=6, including 1 with psychotic major depressive disorder, 1 with psychotic disorder not otherwise specified, and 4 with cluster A personality disorder]; and 3) non-psychotic relatives (n=29, including SZR and BDR without lifetime psychosis spectrum disorders: SZR [n=13, including 4 with no identifiable Axis I or II diagnoses, 3 with non-psychotic major depressive disorder, 1 with anxiety disorder, 2 with non-psychotic major depressive disorder and anxiety disorder, and 3 with substance abuse/dependence] and BDR [n=16, including 3 with no identifiable Axis I or II diagnoses, 1 with non-psychotic bipolar disorder not otherwise specified, 1 with non-psychotic major depressive disorder, 3 with non-psychotic major depressive disorder and anxiety disorder, 6 with substance abuse/dependence, and 2 with cluster C personality disorder].
2.2. Clinical Assessments
DSM-IV Axis I diagnoses in probands and relatives were determined using the Structured Clinical Interview for DSM-IV Axis I Disorders (First et al., 1996). DSM-IV Axis II diagnoses of cluster A/“SZ spectrum personality disorders” in relatives were determined based on the Structured Interview for DSM-IV Personality Disorders (Zanarini et al., 1996). In this study, more liberal criteria for SZ spectrum personality disorders were used in order to increase sensitivity for detecting mild psychosis phenotypes (Thaker et al., 1993): three DSM-IV criteria for schizoid and paranoid, and four criteria for schizotypal personality disorders were sufficient for the personality disorder diagnoses. The Brief Psychiatric Rating Scale (Overall and Gorham, 1962) was used to evaluate active symptoms severity. The Hollingshead Index of Social Position (Hollingshead, 1975) was used to evaluate the highest lifetime occupational/socio-economic level. In addition, psychiatric and medication history were collected in all study volunteers. Clinical assessments were administered by trained research clinicians who maintained inter-rater reliability at > 0.85.
2.3. Neuropsychological Testing
Study volunteers completed ten standardized neuropsychological measures that tapped four cognitive domains known to be most affected in psychosis: 1) working memory - Letter Number Sequencing and Spatial Span subtests from the Wechsler Memory Scale – Third edition (Wechsler, 1997b), and Digit Symbol Coding subtest of the Wechsler Adult Intelligence Scale – Third edition (Wechsler, 1997a); 2) declarative memory - Logical Memory II (delayed story recall) from the Wechsler Memory Scale – Third edition, the Word Recognition and the Face Recognition subtests from the Warrington Recognition Memory Test (Warrington, 1984); 3) executive function - perseverative response score from the Wisconsin Card Sorting Test (Heaton et al., 1993), Letter Fluency (PRW) total score from the Controlled Oral Word Association Test (Benton et al., 1994), and the Trail Making Test, Part B (Reitan and Wolfson, 1985); 4) attention - Trail Making Test, Part A (Reitan and Wolfson, 1985). In addition, an estimate of general intellectual level was obtained from the Wechsler Test of Adult Reading (Wechsler, 2001). Demographically corrected T-scores, which have a mean of 50 and standard deviation of 10, were used for the Wisconsin Card Sorting Test, Trail Making Test, Letter and Category Fluency (Heaton et al., 2004), and age-corrected scaled scores from the Wechsler Adult Intelligence Scale – Third edition and Wechsler Memory Scale – Third edition subtests were transformed to T-scores. T-scores for the Warrington Recognition Memory Test were obtained from the test manual (Warrington, 1984). The Wechsler Test of Adult Reading IQ estimate has a mean of 100 and standard deviation of 15. In addition, composite scores for the four cognitive domains were calculated as the average of the individual T-scores within each domain; a global neuropsychological composite score was also calculated as an average of means T-scores for all individual tests.
2.4. Statistical Analysis
A one-way analysis of variance (ANOVA) with a subsequent post hoc Tukey HSD test and Yates corrected chi-square test were used as appropriate for demographic and clinical variables. To test the a priori hypotheses, the primary analyses compared working memory, declarative memory, executive function, attention composites, and the global neuropsychological composite scores between the traditional DSM-IV diagnostic groups (SZP, BDP, SZR, BDR), and between the psychosis dimension groups (probands, psychotic relatives, and non-psychotic relatives). Each outcome was analyzed using a mixed-effects repeated measures analysis (PROC MIXED in SAS, SAS, Cary, North Carolina), applying Kenward–Roger approximation to calculate the appropriate denominator degrees of freedom. Correlations between members of the same family were accounted for in the error structure by inclusion of a random family effect. Subsequent post hoc pair wise comparisons between the diagnosis and psychosis dimension groups were conducted using PROC MIXED controlling the family-wise error rate by the simulation method of Edwards and Berry (Edwards and Berry, 1987). The magnitude of pair wise differences between the groups was also assessed using Cohen’s d. The analyses of neuropsychological composites (working memory, declarative memory, executive function and attention) were corrected for multiple comparisons using the Holm method (Holm, 1979) which allows for a less stringent correction for non-independent outcomes. In addition, an exploratory analysis of individual neuropsychological tests in each cognitive domain was conducted. Uncorrected probability values below 0.05 were considered significant.
3. RESULTS
3.1. Demographic and Clinical Characteristics
Age, gender, handedness, years of education, the Wechsler Test of Adult Reading estimated IQ, and lifetime occupational/social position status did not differ across proband and relative groups (Table 1). There was a higher proportion of African-American volunteers in the SZP group than in the BDP and BDR groups. No differences in the Brief Psychiatric Rating Scale scores were found between SZP and BDP, whereas probands expectedly had higher scores than relatives. Based on volunteers report, the majority of SZ and BD probands and a proportion of relatives had a past history of psychotropic medication use of varying duration. Most probands (33/46 of SZP and 26/34 of BDP) were actively treated with a combination of psychotropic medications including antipsychotic agents, mood stabilizers, antidepressants, and anxiolytics; only 1/46 of SZP and 1/34 of BDP were medication–free. In the relatives, 10/29 of SZR and 7/22 of BDR were treated with psychotropic agents, among which antidepressants and anxiolytics were most common. Since SZ and BD proband and relative groups were comparable with respect to medication status, cognitive outcomes were not adjusted for medication use.
Table 1.
Demographic and Clinical Characteristics of Study Sample
| Socio – demographic characteristics | SZP (n=46) | BDP (n=34) | SZR (n=29) | BDR (n=22) |
|---|---|---|---|---|
| Age, yrs; Mean (SD) | 40.58 (10.75) | 36.55 (10.68) | 42.06 (10.9) | 34.55 (14.29) |
| Gender/Male; n (%) | 27 (58.69) | 13 (38.23) | 12 (41.37) | 10 (45.45) |
| Left-handed; n (%) | 7 (15.22) | 4 (11.76) | 2 (6.89) | 2 (9.09) |
| Race; n (%) Black a | 15 (32.61) | 3 (8.82) | 8 (28.58) | 1 (4.54) |
| Education, yrs; Mean(SD) | 13.54 (2.53) | 13.35 (2.26) | 14.48 (2.38) | 13.68 (2.74) |
| WTAR IQ | 98.92 (13.61) | 101.35 (9.94) | 100.38 (12.58) | 101.50 (12.56) |
| Hollingshead occupational status; Mean (SD) | 4.25 (1.71) | 3.94 (1.38) | 3.07 (1.29) | 3.66 (1.82) |
| BPRS total scores; Mean (SD) b | 48.04 (11.68) | 44.06 (9.58) | 31.35 (8.94) | 28.4 (6.01) |
| Concomitant medications | ||||
| Off medications; n (%) | 1 (2.17) | 1 (2.95) | 19 (65.52) | 15 (68.18) |
| Typical antipsychotics; n (%) | 10 (21.74) | 1 (2.95) | - | - |
| Atypical antipsychotics; n (%) | 36 (78.26) | 19 (55.88) | 2 (6.9) | 2 (9.09) |
| Antidepressants; n (%) | 24 (52.17) | 17 (50.0) | 9 (31.04) | 3 (13.64) |
| Mood stabilizers; n (%) | 6 (13.04) | 26 (76.47) | 3 (10.34) | 1 (4.55) |
| Anxiolytics/Hypnotics; n (%) | 13 (28.26) | 14 (41.18) | 5 (17.24) | 4 (18.18) |
| Combined medications; n (%) | 33 (71.74) | 26 (76.47) | 5 (17.24) | 2 (9.09) |
SZP – probands with schizophrenia, BDP – probands with psychotic bipolar I disorder, SZR – relatives of SZP,
BDR – relatives of BDP, WTAR IQ – Wechsler Test of Adult Reading general intelligence estimate,
BPRS - the Brief Psychiatric Rating Scale
SZP vs. BDP, χ2(1, N = 80) = 5.05, P = 0.024; SZP vs. BDF, χ2(1, N = 68) = 5.05, P = 0.024
F(3,125) = 29.72, P < 0.001; SZP vs. SZR, P < 0.001; SZP vs. BDR, P < 0.001; BDP vs. SZR, P < 0.001; BDP vs. BDR, P < 0.001
3.2. Cognitive Performance
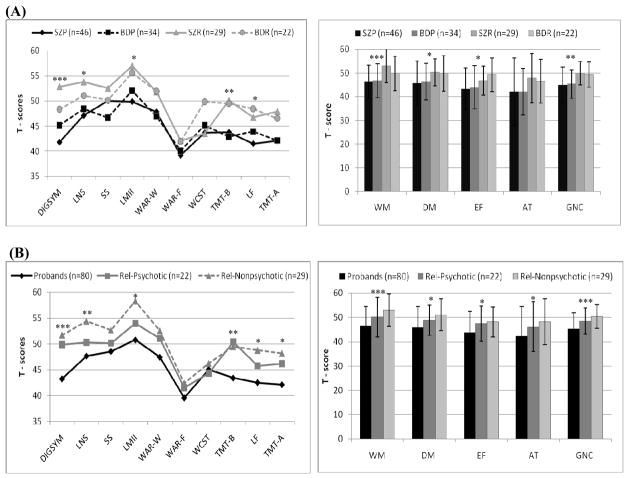
The mean scores for all neuropsychological tests in the proband and relative groups fell within one standard deviation of the normative mean using demographically corrected T-scores, except for performance on the Warrington Face Recognition test in SZP (39.20±11.03). Cognitive performance within the traditional diagnoses [SZP vs. BDP vs. SZR vs. BDR] failed to distinguish proband or relative diagnostic groups (Figure 1A). The diagnostic group contrasts showed differences in the global neuropsychological composite [F(3,96) = 5.6, P = 0.002], and in three of the four cognitive domain composites: working memory [F(3,93) = 8.2, P < 0.001], declarative memory [F(3,86) = 2.9, P = 0.038]), executive function [F(3,103) = 2.8, P = 0.043]), but not in attention [F(3,93) = 2.1, P = 0.107], with differences accounted for by probands vs. relatives performance. Differences in working memory remained significant after correction for multiple comparisons. Probands with SZ and BD did not differ on any composite measures (all P ≥ 0.95, d = 0.05 – 0.08 for pair wise comparisons) (Table 2) or individual tests (Supplementary Table 1). Similarly, SZ and BD relatives did not perform differently on any composite scores (all P ≥ 0.22, d = 0.7 – 0.44 for pair wise comparisons) or individual tests. Probands scored lower than relatives in the domains of working and declarative memory, on the global neuropsychological composite (all P < 0.49, d = 0.59 – 0.89 for pair wise comparisons), and on several individual tests.
Figure 1. Cognitive Profiles of Probands with Schizophrenia and Psychotic Bipolar I Disorder, and their First - Degree Relatives by Traditional Diagnoses (A) and Psychosis Dimension (B).
Between group differences based on a mixed - effect repeated measure analysis (PROC MED): * p < .05, ** p < .01, *** p < .001
SZP – probands with schizophrenia, BDP – probands with psychotic bipolar I disorder, SZR – relatives of SZP, BDR – relatives of BDP, Rel-Psychotic – relatives with psychosis spectrum disorders, Rel-Nonpsychotic – relatives without psychosis spectrum disorders; DIGSYM – Digit Symbol Coding, LNS – Letter Number Sequencing, SS – Spatial Span, LM II – Logical Memory II, WAR-W – Warrington Word Recognition, WAR-F – Warrington Face Recognition, WCST – Wisconsin Card Sort Test – Perseverative Response, TMT-B – Trial Making Test B Time, LF – Letter Fluency, TMT-A – Trial Making Test A Time; WM – Working Memory Composite, DM – Declarative Memory Composite, EF – Executive Function Composite, AT – Attention, GNC – Global Neuropsychological Composite
Table 2.
Cognitive Performance in the Traditional Diagnoses and the Psychosis Dimension Proband and Relative Groups
| Diagnosis [SZP (n=46) vs. BDP (n=34) vs. SZR (n=29) vs. BDR (n=22)] | ||||
|---|---|---|---|---|
| Composites | T-score, Mean(SD) | PROC MIXED Primary Analyses |
PROC MIXED Pair Wise Comparisons* |
Cohen’s d |
| WM | SZP, 46.30(8.71); BDP, 46.76(7.11) SZR, 53.10(7.19); BDR, 49.84(7.3) |
F(3,93) = 8.2, P < 0.001a |
SZP/SZR, P<0.001; BDP/SZR, P=0.01; SZP/BDP, P=0.95; SZR/BDR, P=0.33; BDP/BDR, P=0.50; SZP/BDR, P=0.33 |
SZP/SZR, d=0.84; BDP/SZR, d=0.89 SZP/BDP, d=0.05; SZR/BDR, d=0.44; BDP/BDR, d=0.43; SZP/BDR, d=0.43 |
| DM | SZP, 45.62(9.42); BDP, 46.37(7.73) SZR, 50.30(5.69); BDR, 49.84(7.46) |
F(3,86) = 2.9, P = 0.038 |
SZP/SZR, P=0.049; SZP/BDP, P=0.97; SZR/BDR, P=0.48; BDP/BDR, P=0.48; SZP/BDR, P=0.28; BDP/SZR, P=0.22 |
SZP/SZR, d=0.59; SZP/BDP, d=0.08; SZR/BDR, d=0.07; BDP/BDR, d=0.46; SZP/BDR, d=0.48; BDP/SZR, d=0.52 |
| EF | SZP, 43.27(8.81); BDP, 44.01(9.14) SZR, 46.78(6.16); BDR, 49.27(7.16) |
F(3,103) = 2.8, P = 0.043 | SZP/BDP, P=0.98; SZR/BDR, P=0.90; SZP/SZR, P=0.22; BDP/BDR, P=0.17; SZP/BDR, P=0.08; BDP/SZR, P=0.51 |
SZP/BDP, d=0.08; SZR/BDR, d=0.37; SZP/SZR, d=0.45; BDP/BDR, d=0.63; SZP/BDR, d=0.72; BDP/SZR, d=0.36 |
| AT | SZP, 42.10(14.23); BDP, 42.14(9.78) SZR, 47.89(10.31); BDR, 46.54(9.23) |
F(3,93) = 2.1, P = 0.107 | n/a | SZP/BDP, d=0.08; SZR/BDR, d=0.37; SZP/SZR, d=0.46; BDP/BDR, d=0.46; SZP/BDR, d=0.35; BDP/SZR, d=0.57 |
| GNC | SZP, 44.88(7.63); BDP, 45.35(6.01) SZR, 49.84(4.93); BDR, 49.34(5.35) |
F(3,96) = 5.6, P = 0.002 |
SZP/SZR, P=0.003; BDP/SZR, P=0.03; SZP/BDP, P=0.98; SZR/BDR, P=0.92; BDP/BDR, P=0.18; SZP/BDR, P=0.10 |
SZP/SZR, d=0.75; BDP/SZR, d=0.81; SZP/BDP, d=0.07; SZR/BDR, d=0.09; BDP/BDR, d=0.71; SZP/BDR, d =0.65 |
| Psychosis Dimension [Probands (n=80) vs. Psychotic Relatives (n=22) vs. Non-psychotic Relatives (n=29)] | ||||
| WM | Probands, 46.5(8.02) R-P, 50.10(8.11); R-NP, 52.91(6.6) |
F(2,83) = 10.0, P < 0.001a | Probands/R-NP, P<0.001; Probands/R-P, P=0.03; R-P/R-NP, P=0.61 | Probands/R-NP, d=0.84; Probands/R-P, d=0.45; R-P/R-NP, d=0.38 |
| DM | Probands, 45.94 (8.7) R-P, 48.83(6.18); R-NP, 51.07(6.59) |
F(2,102) = 4.5, P = 0.013a | Probands/R-NP, P=0.01; Probands/R-P, P=0.26; R-P/R-NP, P=0.67 | Probands/R-NP, d=0.63; Probands/R-P, d=0.36; R-P/R-NP, d=0.35 |
| EF | Probands, 43.59(8.91) R-P, 47.45(7.24); R-NP, 48.16(6.29) |
F(2,110) = 3.9, P = 0.022a | Probands/R-NP, P=0.051; Probands/R-P, P=0.10; R-P/R-NP, P=0.99 | Probands/R-NP, d=0.56; Probands/R-P, d=0.45; R-P/R-NP, d=0.11 |
| AT | Probands, 42.12(9.78) R-P, 46.18(10.27); R-NP, 48.17(9.49) |
F(2,111) = 3.3, P = 0.041a | Probands/R-NP, P=0.04; Probands/R-P, P=0.34; R-P/R-NP, P=0.78 | Probands/R-NP, d=0.52; Probands/R-P, d=0.34; R-P/R-NP, d=0.20 |
| GNC | Probands, 45.08(6.94) R-P, 48.53(5.35); R-NP, 50.46(4.76) |
F(2,106) = 8.4, P < 0.001 | Probands/R-NP, P<0.001; Probands/R-P, P=0.050; R-P/R-NP, P=0.63 | Probands/R-NP, d=0.84; Probands/R-P, d=0.52; R-P/R-NP, d=0.38 |
Adjusted probability values based on a simulation method in PROC MIXED reported for post hoc pair wise comparisons analysis.
Significant after Holm correction for multiple comparisons.
Statistically significant results are listed in bold.
SZP – probands with schizophrenia, BDP – probands with psychotic bipolar I disorder, SZR – relatives of SZP, BDR – relatives of BDP, R-P – relatives with psychosis spectrum disorders, R-NP – relatives without psychosis spectrum disorders; WM – Working Memory Composite, DM – Declarative Memory Composite, EF – Executive Function Composite, AT – Attention, GNC – Global Neuropsychological Composite
Analyses of cognitive performance across the psychosis dimension groups [probands vs. psychotic relatives vs. non-psychotic relatives] showed differences in all neuropsychological composites: working memory [F(2,83) = 10.0, P < 0.001], declarative function [F(2,102) = 4.5, P = 0.013], executive function [F(2,110) = 3.9, P = 0.022], attention [F(2,111) = 3.3, P = 0.041], and in the global neuropsychological composite [F(2,106) = 8.4, P < 0.001] (Figure 1B). These differences remained significant after correction for multiple comparisons. Specifically, probands showed lower performance than non-psychotic relatives in working and declarative memory, attention, in the global neuropsychological composite (all P < 0.4, d = 0.52 – 0.84 for pair wise comparisons) (Table 2), and on several individual tests (Supplementary Table 1). No differences emerged between probands and psychotic relatives, except for working memory composite (P = 0.3, d = 0.45), the Trail Making Test/B (P = 0.01), and the global neuropsychological composite (P = 0.50, d = 0.52) scores. No differences were found between psychotic and non-psychotic relatives, however the effect sizes for the global neuropsychological composite, working and declarative memory, and attention composite scores were small to medium (d = 0.20 – 0.38), supporting better cognitive performance in non-psychotic relatives.
4. DISCUSSION
The overall goal of this study was to examine whether probands with SZ and psychotic BD and their first-degree relatives differ in cognitive performance when segmented by traditional diagnostic boundaries or by psychosis dimension. We anticipated that these outcomes will contribute to the discussion of the distinctiveness of SZ and BD diagnoses. To our knowledge, this is the first study to directly compare cognitive function across several domains known to be affected in psychosis (working and declarative memory, executive function, and attention) in concurrently recruited probands with SZ and psychotic BD, along with their biological relatives. Probands recruited into this study were moderately symptomatic, stable, treated out-patients, similar in age, education, socio-economic status, and IQ. The relative groups were highly heterogeneous, allowing an exploration of cognitive endophenotypes across the psychosis dimension. There was a higher proportion of African-American individuals in the SZ proband and relative groups, compared to the BD groups. Prior reports have not shown differences in cognitive performance in African-American vs. Caucasian SZP across numerous cognitive domains after correction for level of education (Lewine and Caudle, 2000). In this sample, there were no differences in education between African-American and Caucasian subjects across the study groups (results not shown).
Cognitive performance within the DSM-IV diagnoses failed to distinguish SZP and BDP, or SZR and BDR, thus lending support to our first hypothesis. Indeed, probands with SZ and psychotic BD showed strikingly similar performance in all cognitive domains evaluated including working memory, declarative memory, executive function and attention. Similarly, SZR and BDR did not differ in performance across the four cognitive domains. As predicted, relatives had higher scores than probands in all domains (effect sizes ranging from .35 to .89), with differences in working and declarative memory being statistically significant. The pattern of cognitive performance was similar in proband and relative groups, consistent with previous reports (Cannon et al., 1994; Gur et al., 2007; Arts et al., 2008). These cognition findings from the diagnosis analysis add to the growing body of evidence suggesting that the two psychotic diagnoses are not distinguished based on cognitive characteristics (Glahn et al., 2007; Martinez-Aran et al., 2008; Reichenberg et al., 2009). By contrast, the psychosis dimension analysis showed that probands had poorer cognitive performance in working and declarative memory, executive function and attention, compared to non-psychotic relatives, whereas the psychotic relatives showed intermediate performance for SZ spectrum personality disorders, similar to Thaker et al. (Thaker et al., 1993), in order to capture across all cognitive domains. In this study, we used modestly less stringent diagnostic criteria mild psychosis phenotypes in relatives. This approach increased the proportion of relatives with SZ spectrum personality disorders two-fold, with the schizotypal phenotype being most sensitive to the reduced diagnostic criteria (results not shown). Thus, the liberalized diagnostic strategy significantly increased sensitivity for identifying individuals near the diagnostic threshold, and may benefit future translational and genetic studies in psychosis. Overall, our data suggest that individuals with lifetime psychosis, regardless of the formal DSM-IV diagnoses, manifest poorer cognitive function as defined by working memory, declarative memory, executive function and attention performance compared to their non-psychotic counterparts, lending support to the existence of a cognition endophenotype for psychosis, independent of diagnosis.
In this sample, SZP and BDP showed similar neuropsychological scores which challenges previous reports of more severe cognitive disturbance in SZP, compared to BDP (Seidman et al., 2002; Altshuler et al., 2004; Dickerson et al., 2004; Burdick et al., 2006). Characteristics of our sample may contribute to this finding, namely, this sample included only BDP with lifetime psychotic symptoms; whereas, the majority of previous studies combined psychotic and non-psychotic BD individuals (Pearlson et al., 1995; Potash, 2006; Glahn et al., 2006). This is consistent with previous reports of the negative effect of lifetime psychosis on cognitive function in BD (Glahn et al., 2007; Martinez-Aran et al., 2008). It is also worth noting that the estimated IQ scores for our groups were in the average range and did not differ across the groups. Therefore, this analysis compared SZP and BDP similar in overall intellectual functioning and was not affected by IQ differences. As such, our SZP sample may be less representative in general, but ideal for contrasting cognitive characteristics between diagnostic groups.
We should note several limitations of this study. First, the modest sample size limited statistical power of the cognitive analyses, despite our ability to show meaningful differences. Second, the specific neuropsychological battery chosen for this analysis could limit characterization of cognitive endophenotypes, although we selected measures commonly used in the literature that have good norms and psychometric properties to represent the cognitive domains of interest. Third, although probands and relatives included in this study were clinically stable and overall minimally symptomatic, the variation in the active symptom severity (e.i., higher BPRS scores in probands than in relatives) could have contributed to cognitive outcomes. Fourth, a significant proportion of relatives and most probands in this sample had a long history of medication use and were treated with a combination of various psychotropic agents while active in this study; therefore the effect of medication on cognitive performance cannot be ruled out. Future studies including larger samples of unaffected relatives could help to further characterize cognitive endophenotypes of psychosis free of illness and medication effect. Finally, our sample was delimited by the diagnostic boundaries of SZ and psychotic BD and thus does not reflect the full spectrum of psychotic disorders. As endophenotypes of psychosis become better characterized, it will be interesting to explore these measures in other psychoses.
4.1. Conclusions
Cognitive performance in the domains of working memory, declarative memory, executive function and attention was successful in distinguishing probands and relatives with and without lifetime psychosis (independent of their categorical diagnoses) but did not distinguish probands and their first-degree relatives within traditional SZ and psychotic BD diagnostic groups. These findings support the notion that the two disorders may present a clinical continuum with overlapping cognitive characteristics defining the psychosis phenotype. Future research examining cognitive and other endophenotypes and their genetic underpinnings in larger family psychosis samples may shed further light on a dimensional definition of psychosis and aid in the development of biology-based diagnostic classification and novel treatments.
Supplementary Material
Acknowledgments
This work was supported by NIMH (MH077851) and Stanley Medical Research Institute (05-RC-001). The NIMH and Stanley Medical Research Institute had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
We would like to thank Regena Mitschke, B.A., Judy Shaw M.S., Darwynn Cole, B.A., Ronald Chin, B.A., and Dorothy Denton, B.A. for assistance with data collection, analysis and the manuscript preparation; all clinicians for patients referral and patients themselves that took part in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Altshuler LL, Ventura J, van Gorp WG, Green MF, Theberge DC, Mintz J. Neurocognitive function in clinically stable men with bipolar I disorder or schizophrenia and normal control subjects. Biological Psychiatry. 2004;56:560–569. doi: 10.1016/j.biopsych.2004.08.002. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) 4. American Psychiatric Association; Washington, D.C: 1994. [Google Scholar]
- Arts B, Jabben N, Krabbendam L, van Os J. Meta-analyses of cognitive functioning in euthymic bipolar patients and their first-degree relatives. Psychological Medicine. 2008;38:771–785. doi: 10.1017/S0033291707001675. [DOI] [PubMed] [Google Scholar]
- Asarnow RF, Nuechterlein KH, Asamen J, Fogelson D, Subotnik KL, Zaucha K, Guthrie D. Neurocognitive functioning and schizophrenia spectrum disorders can be independent expressions of familial liability for schizophrenia in community control children: the UCLA family study. Schizophrenia Research. 2002;54:111–120. doi: 10.1016/s0920-9964(01)00358-9. [DOI] [PubMed] [Google Scholar]
- Benton AL, Hamsher Dd, Sivan AB. Multilingual Aphasia Examination. 3. Psychological Corporation; San Antonio, Texas: 1994. [Google Scholar]
- Bora E, Yucel M, Pantelis C. Cognitive endophenotypes of bipolar disorder: a meta-analysis of neuropsychological deficits in euthymic patients and their first-degree relatives. Journal of Affective Disorders. 2009;113:1–20. doi: 10.1016/j.jad.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Braff DL. Information processing and attention dysfunctions in schizophrenia. Schizophrenia Bulletin. 1993;19:233–259. doi: 10.1093/schbul/19.2.233. [DOI] [PubMed] [Google Scholar]
- Burdick KE, Goldberg JF, Harrow M, Faull RN, Malhotra AK. Neurocognition as a stable endophenotype in bipolar disorder and schizophrenia. The Journal of Nervous and Mental Disease. 2006;194:255–260. doi: 10.1097/01.nmd.0000207360.70337.7e. [DOI] [PubMed] [Google Scholar]
- Cannon T, Zorrila L, Shtasel D, Gur RE, Gur RC, Marco E, Moberg P, Price A. A neuropsychological functioning in siblings discordant for schizophrenia and healthy volunteers. Archives of General Psychiatry. 1994;51:561–661. doi: 10.1001/archpsyc.1994.03950080063009. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Huttunen MO, Lonnqvist J, Tuulio-Henriksson A, Pirkola T, Glahn D, Finkelstein J, Hietanen M, Kaprio J, Koskenvuo M. The inheritance of neuropsychological dysfunction in twins discordant for schizophrenia. American Journal of Human Genetics. 2000;67:369–382. doi: 10.1086/303006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L, Iversen SD, Goodwin GM. Sustained attention deficit in bipolar disorder. The British Journal of Psychiatry. 2002;180:313–319. doi: 10.1192/bjp.180.4.313. [DOI] [PubMed] [Google Scholar]
- Cornblatt BA, Malhotra AK. Impaired attention as an endophenotype for molecular genetic studies of schizophrenia. American Journal of Medical Genetics. 2001;105:11–15. [PubMed] [Google Scholar]
- Dickerson F, Boronow JJ, Stallings C, Origoni AE, Cole SK, Yolken RH. Cognitive functioning in schizophrenia and bipolar disorder: comparison of performance on the Repeatable Battery for the Assessment of Neuropsychological Status. Psychiatry Research. 2004;129:45–53. doi: 10.1016/j.psychres.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Edwards D, Berry JJ. The efficiency of simulation-based multiple comparisons. Biometrics. 1987;43:913–928. [PubMed] [Google Scholar]
- Faraone SV, Seidman LJ, Kremen WS, Pepple JR, Lyons MJ, Tsuang MT. Neuropsychological functioning among the nonpsychotic relatives of schizophrenic patients: a diagnostic efficacy analysis. Journal of Abnormal Psychology. 1995;104:286–304. doi: 10.1037//0021-843x.104.2.286. [DOI] [PubMed] [Google Scholar]
- Ferrier IN, Stanton BR, Kelly TP, Scott J. Neuropsychological function in euthymic patients with bipolar disorder. The British Journal of Psychiatry. 1999;175:246–251. doi: 10.1192/bjp.175.3.246. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders/Patient Edition (SCID-I/P) New York State Psychiatric Institute, Biometrics Research Department; New York, NY: 1996. [Google Scholar]
- Glahn DC, Almasy L, Barguil M, Hare E, Peralta JM, Kent JW, Jr, Dassori A, Contreras J, Pacheco A, Lanzagorta N, Nicolini H, Raventos H, Escamilla MA. Neurocognitive endophenotypes for bipolar disorder identified in multiplex multigenerational families. Archives of General Psychiatry. 2010;67:168–177. doi: 10.1001/archgenpsychiatry.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glahn DC, Bearden CE, Barguil M, Barrett J, Reichenberg A, Bowden CL, Soares JC, Velligan DI. The neurocognitive signature of psychotic bipolar disorder. Biological Psychiatry. 2007;62:910–916. doi: 10.1016/j.biopsych.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Bearden CE, Cakir S, Barrett JA, Najt P, Serap ME, Maples N, Velligan DI, Soares JC. Differential working memory impairment in bipolar disorder and schizophrenia: effects of lifetime history of psychosis. Bipolar Disorder. 2006;8:117–123. doi: 10.1111/j.1399-5618.2006.00296.x. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Bearden CE, Niendam TA, Escamilla MA. The feasibility of neuropsychological endophenotypes in the search for genes associated with bipolar affective disorder. Bipolar Disorder. 2004;6:171–182. doi: 10.1111/j.1399-5618.2004.00113.x. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Shields J. Genetic theorizing and schizophrenia. The British Journal of Psychiatry. 1973;122:15–30. doi: 10.1192/bjp.122.1.15. [DOI] [PubMed] [Google Scholar]
- Gourovitch ML, Torrey EF, Gold JM, Randolph C, Weinberger DR, Goldberg TE. Neuropsychological performance of monozygotic twins discordant for bipolar disorder. Biological Psychiatry. 1999;45:639–646. doi: 10.1016/s0006-3223(98)00148-6. [DOI] [PubMed] [Google Scholar]
- Gur RE, Nimgaonkar VL, Almasy L, Calkins ME, Ragland JD, Pogue-Geile MF, Kanes S, Blangero J, Gur RC. Neurocognitive endophenotypes in a multiplex multigenerational family study of schizophrenia. American Journal of Psychiatry. 2007;164:813–819. doi: 10.1176/ajp.2007.164.5.813. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G. Revised and Expanded Psychological. Assessment Resources, Inc; Odessa, FL: 1993. Wisconsin Card Sorting Test Manual. [Google Scholar]
- Heaton RK, Miller W, Taylor MJ, Grant I. Revised Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographically Adjusted Neuropsychological Norms for African American and Caucasian Adults Psychological. Assessment Resources, Inc; Odessa, FL: 2004. [Google Scholar]
- Hill SK, Harris MS, Herbener ES, Pavuluri M, Sweeney JA. Neurocognitive allied phenotypes for schizophrenia and bipolar disorder. Schizophrenia Bulletin. 2008;34:743–759. doi: 10.1093/schbul/sbn027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead A. Four Factor Index of Social Status. Yale University; New Heaven: 1975. [Google Scholar]
- Holm S. A Simple Sequentially Rejective Multiple Test Procedure. Scandinavian Journal of Statistics. 1979;6:65–70. [Google Scholar]
- Ivleva EI, Morris DW, Moates AF, Suppes T, Thaker GK, Tamminga CA. Genetics and Intermediate Phenotypes of the Schizophrenia-Bipolar Disorder Boundary. Neuroscience & Biobehavioral Reviews. 2010;34:897–921. doi: 10.1016/j.neubiorev.2009.11.022. [DOI] [PubMed] [Google Scholar]
- Jabben N, Arts B, Krabbendam L, van Os J. Investigating the association between neurocognition and psychosis in bipolar disorder: further evidence for the overlap with schizophrenia. Bipolar Disorder. 2009;11:166–177. doi: 10.1111/j.1399-5618.2009.00663.x. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Roitman SE, Harvey PD, Blum CS, DuPre RL, Prieto DM, Davidson M, Davis KL. A pen-and-paper human analogue of a monkey prefrontal cortex activation task: spatial working memory in patients with schizophrenia. Schiophrenia Research. 1995;17:25–33. doi: 10.1016/0920-9964(95)00027-j. [DOI] [PubMed] [Google Scholar]
- Kieseppa T, Tuulio-Henriksson A, Haukka J, Van Erp T, Glahn D, Cannon TD, Partonen T, Kaprio J, Lonnqvist J. Memory and verbal learning functions in twins with bipolar-I disorder, and the role of information-processing speed. Psychological Medicine. 2005;35:205–215. doi: 10.1017/s0033291704003125. [DOI] [PubMed] [Google Scholar]
- Kumar CT, Christodoulou T, Vyas NS, Kyriakopoulos M, Corrigall R, Reichenberg A, Frangou S. Deficits in visual sustained attention differentiate genetic liability and disease expression for Schizophrenia from Bipolar Disorder. Schizophrenia Research. 2010;124:152–160. doi: 10.1016/j.schres.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Lewine RR, Caudle J. Racial effects on neuropsychological functioning in schizophrenia. American Journal of Psychiatry. 2000;157:2038–2040. doi: 10.1176/appi.ajp.157.12.2038. [DOI] [PubMed] [Google Scholar]
- Martinez-Aran A, Torrent C, Tabares-Seisdedos R, Salamero M, Daban C, Balanza-Martinez V, Sanchez-Moreno J, Manuel GJ, Benabarre A, Colom F, Vieta E. Neurocognitive impairment in bipolar patients with and without history of psychosis. Journal of Clinical Psychiatry. 2008;69:233–239. doi: 10.4088/jcp.v69n0209. [DOI] [PubMed] [Google Scholar]
- Niendam TA, Bearden CE, Zinberg J, Johnson JK, O’Brien M, Cannon TD. The course of neurocognition and social functioning in individuals at ultra high risk for psychosis. Schizophrenia Bulletin. 2007;33:772–781. doi: 10.1093/schbul/sbm020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Reports. 1962;10:799–812. [Google Scholar]
- Pearlson GD, Wong DF, Tune LE, Ross CA, Chase GA, Links JM, Dannals RF, Wilson AA, Ravert HT, Wagner HN, Jr, Depaulo JR. In vivo D2 dopamine receptor density in psychotic and nonpsychotic patients with bipolar disorder. Archives of General Psychiatry. 1995;52:471–477. doi: 10.1001/archpsyc.1995.03950180057008. [DOI] [PubMed] [Google Scholar]
- Potash JB. Carving chaos: genetics and the classification of mood and psychotic syndromes. Harvard Review of Psychiatry. 2006;14:47–63. doi: 10.1080/10673220600655780. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Harvey PD, Bowie CR, Mojtabai R, Rabinowitz J, Heaton RK, Bromet E. Neuropsychological function and dysfunction in schizophrenia and psychotic affective disorders. Schizophrenia Bulletin. 2009;35:1022–1029. doi: 10.1093/schbul/sbn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan R, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: theory and clinical interpretation. Neuropsychology Press; Tucson, AZ: 1985. [Google Scholar]
- Rubinsztein JS, Michael A, Paykel ES, Sahakian BJ. Cognitive impairment in remission in bipolar affective disorder. Psychological Medicine. 2000;30:1025–1036. doi: 10.1017/s0033291799002664. [DOI] [PubMed] [Google Scholar]
- Schretlen DJ, Cascella NG, Meyer SM, Kingery LR, Testa SM, Munro CA, Pulver AE, Rivkin P, Rao VA, Diaz-Asper CM, Dickerson FB, Yolken RH, Pearlson GD. Neuropsychological functioning in bipolar disorder and schizophrenia. Biological Psychiatry. 2007;62:179–186. doi: 10.1016/j.biopsych.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman LJ, Kremen WS, Koren D, Faraone SV, Goldstein JM, Tsuang MT. A comparative profile analysis of neuropsychological functioning in patients with schizophrenia and bipolar psychoses. Schizophrenia Research. 2002;53:31–44. doi: 10.1016/s0920-9964(01)00162-1. [DOI] [PubMed] [Google Scholar]
- Sitskoorn MM, Aleman A, Ebisch SJ, Appels MC, Kahn RS. Cognitive deficits in relatives of patients with schizophrenia: a meta-analysis. Schizophrenia Research. 2004;71:285–295. doi: 10.1016/j.schres.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Smith MJ, Barch DM, Csernansky JG. Bridging the gap between schizophrenia and psychotic mood disorders: Relating neurocognitive deficits to psychopathology. Schizophrenia Research. 2009;107:69–75. doi: 10.1016/j.schres.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snitz BE, MacDonald AW, III, Carter CS. Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: a meta-analytic review of putative endophenotypes. Schizophrenia Bulletin. 2006;32:179–194. doi: 10.1093/schbul/sbi048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sponheim SR, McGuire KA, Stanwyck JJ. Neural anomalies during sustained attention in first-degree biological relatives of schizophrenia patients. Biological Psychiatry. 2006;60:242–252. doi: 10.1016/j.biopsych.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Stefanopoulou E, Manoharan A, Landau S, Geddes JR, Goodwin G, Frangou S. Cognitive functioning in patients with affective disorders and schizophrenia: a meta-analysis. International Review of Psychiatry. 2009;21:336–356. doi: 10.1080/09540260902962149. [DOI] [PubMed] [Google Scholar]
- Thaker G. Psychosis endophenotypes in schizophrenia and bipolar disorder. Schizophrenia Bulletin. 2008;34:720–721. doi: 10.1093/schbul/sbn055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaker GK, Adami H, Moran M, Lahti AC, Cassady SL. Psychiatric illnesses in families of subjects with schizophrenia- spectrum personality disorders: high morbidity risks for unspecified functional psychoses and schizophrenia. American Journal of Psychiatry. 1993;150:66–71. doi: 10.1176/ajp.150.1.66. [DOI] [PubMed] [Google Scholar]
- Thompson JM, Gallagher P, Hughes JH, Watson S, Gray JM, Ferrier IN, Young AH. Neurocognitive impairment in euthymic patients with bipolar affective disorder. The British Journal of Psychiatry. 2005;186:32–40. doi: 10.1192/bjp.186.1.32. [DOI] [PubMed] [Google Scholar]
- Toomey R, Faraone SV, Seidman LJ, Kremen WS, Pepple JR, Tsuang MT. Association of neuropsychological vulnerability markers in relatives of schizophrenic patients. Schizophrenia Research. 1998;31:89–98. doi: 10.1016/s0920-9964(98)00025-5. [DOI] [PubMed] [Google Scholar]
- Warrington EJ. Recognition Memory Test. Western Psychological Services; 1984. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. 3. The Psychological Corporation; San Antonio, TX: 1997a. [Google Scholar]
- Wechsler D. Wechsler Memory Scale. 3. The Psychological Corporation; San Antonio, TX: 1997b. [Google Scholar]
- Wechsler D. Wechsler Test of Adult Reading. The Psychological Corporation; San Antonio, TX: 2001. [Google Scholar]
- Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanarini MC, Frankenburg FR, Sickel AE, Yong L. The Diagnostic Interview for DSM-IV Personality Disorders (DIP DIV) McLean Hospital; Belmont: 1996. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.