Abstract
Almost all human cancers display dysregulated expression and/or function of one or more receptor tyrosine kinases (RTKs). The strong causative association between altered RTK function and cancer progression has translated into novel therapeutic strategies that target these cell surface receptors in the treatment of cancer. Yet, the full spectrum of RTKs that may alter the oncogenic process is not completely understood. Accumulating evidence suggests that a unique set of RTKs known as the Discoidin Domain Receptors (DDRs) play a role in cancer progression by regulating the interactions of tumor cells with their surrounding collagen matrix. The DDRs are the only RTKs that specifically bind to, and are activated by collagen. Hence, the DDRs are part of the signaling networks that translate information from the extracellular matrix thereby acting as key regulators of cell-matrix interactions. Under physiological conditions, DDRs control cell and tissue homeostasis by acting as collagen sensors, transducing signals that regulate cell polarity, tissue morphogenesis, and cell differentiation. In cancer, DDRs are hijacked by tumor cells to disrupt normal cell-matrix communication and initiate pro-migratory and pro-invasive programs. Importantly, several cancer types exhibit DDR mutations, which are thought to alter receptor function and contribute to cancer progression. Other evidence suggests that the actions of DDRs in cancer are complex, either promoting or suppressing tumor cell behavior in a DDR type/isoform specific and context dependent manner. Thus, there is still a considerable gap in our knowledge of DDR actions in cancer tissues. This review summarizes the current knowledge on DDR expression and function in cancer and discusses the potential implications of DDRs in cancer biology. It is hoped that this effort will encourage more research into these poorly understood but unique RTKs, which have the potential of becoming novel therapeutics targets in cancer.
Keywords: discoidin domain receptor, tyrosine kinase, collagen, extracellular matrix, signaling, cell migration, metastasis
1 Introduction
A fundamental characteristic of metastatic cancers is the ability of tumor cells to acquire an invasive phenotype and disseminate to other organs. The acquisition of this malignant phenotype is accompanied by disruptions in the physiological interactions of tumor cells with their immediate microenvironment represented by the surrounding extracellular matrix (ECM). During normal conditions, cell-matrix interactions support cell polarity, differentiation, and survival via specific cell surface receptors that transduce cues from the ECM and activate signaling networks that support normal cellular function. In cancer, dysregulation of ECM-induced signaling disrupts normal tissue organization and contributes to the migratory and pro-invasive programs of cancer cells. The major ECM components known to interact with almost all cell types are collagens. There are twenty-eight different types of collagen. Collectively, they are the most abundant proteins in vertebrates. Besides their structural role as scaffolding proteins, collagens initiate cellular signaling events by activating specific cell surface receptors. The integrin and DDR families are the most widely expressed collagen receptors in vertebrates. Each activates unique signaling pathways in response to collagen, and elicits distinct cellular responses. Many of these pathways overlap and consist of common components, and thus their specificity is still unclear. A plethora of structural, functional, and clinical studies exist on integrins and on the role they play in cancer tissues. In contrast, little is known about the expression and function of DDRs in cancer progression. Given that DDRs are the only receptor tyrosine kinases (RTKs) that signal in response to collagen, and that RTKs are integral in cancer progression, the paucity of information on DDRs in cancer is remarkable. Therefore, in this review, we set to summarize the current knowledge on DDRs with an emphasis on their expression and functions in cancer.
2 Genomic and protein domain organization of DDRs
2.1 Genomic organization
In the early 90s, several groups reported the cloning of a novel class of transcripts, which turned out to encode proteins with an unusual N-terminal discoidin-I like domain and a C-terminal kinase domain that were about 45% identical to the neurotrophin receptor, TrkA [1–10]. These novel transcripts were given distinct names: DDR, TrkE, NEP, CAK, RTK-6, Ptk3, MCK-10, CCK-2, TKT and Tyro 10, and were found to predict unusual RTK protein structures. It was later recognized that the various transcripts were in fact either different isoforms of the same RTK or different homologs belonging to a novel family of RTKs. Based on homology of the N-terminal domain, the DDR, TrkE, CAK, RTK-6 and MCK-10 proteins were re-named as DDR1, while the CCK-2, TKT, and Tyro 10 proteins were re-named as DDR2 [11,12]. The DDR1 gene maps to human chromosome 6 (6p21.3) and is composed of 17 exons that are alternatively spliced to generate 5 different transcripts, which give rise to five distinct DDR1 isoforms. The DDR2 gene, which maps to human chromosome 1 (1q23.3), is made up of 19 exons and encodes a single transcript, with one protein.
2.2 Domain organization and function
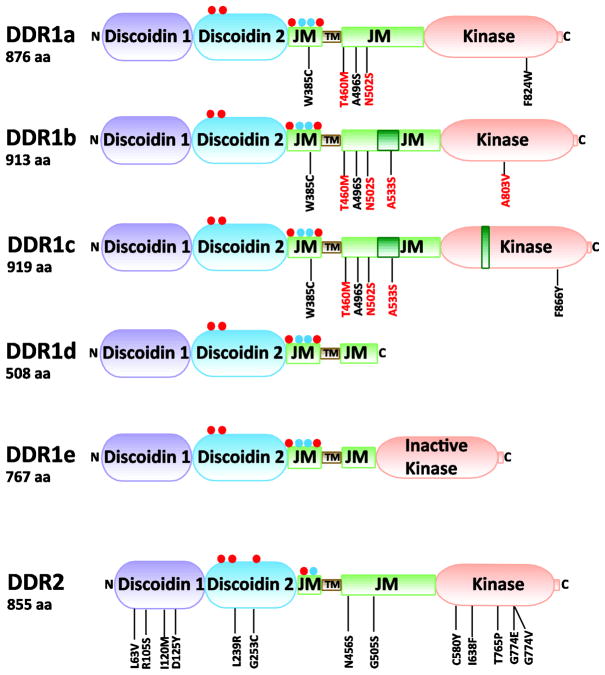
DDRs are type I transmembrane RTKs that display an overall structural organization that is similar to many members of the RTK family [13], albeit with unique features (Fig. 1). The N-terminal DDR discoidin domains are homologous to discoidin I, a secreted protein from the slime mold Dictyostelium discoideum (reviewed in [14]). In this organism, discoidin I functions as a lectin (a carbohydrate binding protein), playing a role in cell-cell aggregation and cytoskeletal organization [15]. Discoidin domains are found in several membrane and secreted proteins such as the blood coagulation factors V and VIII, and the milk proteins MFG-E8 and BA46, among others (reviewed in [16]). In both DDR1 and DDR2, the N-terminal discoidin domains, referred to here as discoidin 1 (Dr. Leitinger, unpublished) (Fig. 1), have been found to bind to various types of collagens (reviewed in [17]). Besides discoidin 1, the ectodomain of DDRs contains another globular domain that is predicted to belong to the same superfamily [13], referred to here as discoidin 2 (Fig. 1). This domain is followed by an extracellular juxtamembrane (JM) region of about 50 (DDR1) or 30 (DDR2) amino acids. A single transmembrane (TM) domain comes next, followed by an unusually large cytosolic juxtamembrane (JM) domain (up to 169 or 140 amino acids in DDR1 and DDR2, respectively). A catalytic kinase domain follows the cytosolic JM domain and at the very end comes a short C-terminal tail (Fig. 1). Within its intracellular region, DDR1 contains 15 tyrosine residues: 7 in the JM region and 8 in the kinase domain (Fig. 2). DDR2 contains 14 tyrosine residues: 4 in the JM region and 10 in the kinase domain.
Fig. 1.
Domain structure of DDRs. Residues that are added as a result of alternative splicing are indicated by dark green boxes within the corresponding domain. Red and blue circles indicate putative N-glycosylation and O-glycosylation sites, respectively. Mutated DDR residues, which were identified in samples of Non-Small Cell Lung Carcinomas (black) and Acute Myeloid Leukemia (red), are indicated. aa, amino acids; JM, juxtamembrane region, and TM, transmembrane region.
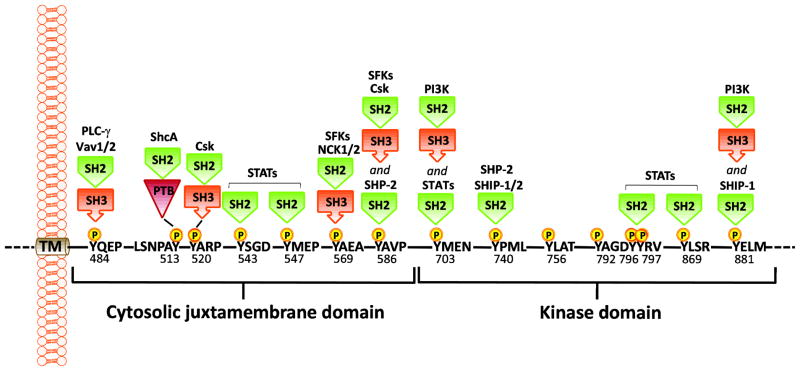
Fig. 2.
Identified phospho-DDR1 interactions. The PTB domain in ShcA and the SH2 domain in Csk and Nck2 directly interact with phospho-DDR1. In the case of PLC-γ, Vav1/2, SFKs, and PI3K, which contain both SH2 and SH3 domains, the interacting domain(s) is unknown. TM, transmembrane domain; SH2, Src homology 2 domain; SH3, Src homology 3 domain; PTB, phospho-tyrosine binding domain; PLCγ, phospholipase C γ; ShcA, SH2 containing transforming protein A; Csk, C-terminal Src kinase; SFKs, Src family tyrosine kinases; PI3K, phosphoinositide 3 kinase; SHP-2, SH2 containing protein tyrosine phosphatase 2; SHIP-1/2, SH2-containing inositol polyphosphate 5-phosphatase 1/2, and STATs, Signal transducer and activator of transcription. The indicated amino acid annotations refer to the DDR1b isoform.
Three of the five DDR1 isoforms, DDR1a, DDR1b, and DDR1c, encode full length, functional receptors while DDR1d and DDR1e encode truncated or kinase inactive receptors. DDR1a is generated as a result of deletion of exon 11 in the cytosolic JM domain [18]. DDR1c is generated as a result of the use of an alternate 5′ splice acceptor site at the 5′ intron/exon boundary of exon 14 within the kinase domain, giving rise to an additional 18 bp without ORF disruption [18]. The DDR1d isoform is generated through deletion of exons 11 and 12 in the cytosolic JM domain, resulting in a frame-shift mutation and a pre-maturely truncated protein [19]. In DDR1e, exons 11 and 12 are deleted, but the open reading frame is not disrupted due to an additional deletion at the beginning of exon 10 (via an alternate 5′ splice acceptor site in exon 10). However, in DDR1e the ATP binding site within the kinase domain (at the end of exon 12) is missing, rendering this isoform inactive [19].
2.3 Post-translational modifications of DDRs
The predicted molecular mass of the full length DDR1 isoforms ranges from ~97 to ~102 kDa. However, in immunoblots, DDR1 appears as a doublet of ~125 kDa, likely due to glycosylation [20]. Similarly, DDR2 runs at an apparent molecular mass that is much higher than that predicted by its amino acid sequence. Using software that predicts glycosylation sites (http://www.expasy.ch/tools/) we were able to identify several potential N- and O-glycosylation sites within the DDR1 and DDR2 discoidin 2 domains (Fig. 1). Whether those sites are in fact glycosylated in vivo and how they affect DDR activation remains to be determined.
RTKs are characterized by their ability to generate stable dimers, which is an essential requirement for receptor autophosphorylation [13]. While the majority of RTKs are single chain receptors that generate dimeric complexes upon ligand binding, some RTKs exist as pre-formed dimers. Ligand-induced RTK dimerization occurs via multiple interactions at distinct domains inducing conformational changes in the dimer that support phosphorylation of tyrosine residues in the kinase and JM domains. Studies by Curat et. al. found the discoidin 1 domain to be required for dimerization of DDR1 [20]. Interestingly, Agarwal et. al. and Leitinger later showed that dimerization of discoidin 1 domains is a prerequisite for ligand/collagen binding in both DDR1 and DDR2 [21–23]. Furthermore, ligand-independent DDR dimers were found to originate within the biosynthetic pathway and to be stable at the cell surface even in the absence of collagen [24–26]. Therefore, unlike the paradigm of the majority of RTKs whereby ligand binding precedes receptor dimerization, DDRs appear to exist in dimeric complexes prior to interaction with the ligand. Other studies found that neither the extracellular nor the intracellular regions of DDR1 are required for receptor dimerization. Instead, these studies proposed that dimerization occurs via the transmembrane (TM) region of DDR1, which was found to have a strong potential for self-association via the action of a leucine zipper [24]. Nonetheless, the structural and cellular mechanisms regulating DDR dimerization in the absence or presence of collagen are practically unknown.
3 Collagen binding of DDRs
3.1 Collagen specificity
DDRs are unique among RTKs because they are activated by an extracellular matrix protein, collagen (typical RTKs use soluble, peptide-like growth factors as their ligands). The DDRs only bind collagen in its native, triple-helical conformation and do not recognize heat-denatured collagen (gelatin) [11,23]. However, triple-helical peptides containing the collagen-binding motif of DDRs (described below) are able to induce receptor activation, indicating that the supramolecular structure of collagen is not required for DDR signaling [27]. Both DDRs display broad collagen specificity and are activated by many different collagen types, with fibrillar collagens acting as ligands for both receptors [11,12]. The DDRs have distinct preferences for certain types of collagens. DDR1, but not DDR2, binds to the basement membrane collagen IV [11,12], while DDR2 seems to preferentially bind collagen II [28] and collagen X [29]. DDR1 can also bind collagen VIII [30], but it is not known whether DDR2 shares this property. Similar to collagen-binding integrins, the DDRs recognize specific amino acid motifs in collagen. Initial mapping of DDR2 binding sites was performed with recombinant triple-helical variants of collagen II, which mapped a specific DDR2 binding site to the second quarter of its collagenous (COL) domain [28]. More detailed studies, utilizing libraries of triple-helical peptides, the so-called Collagen Toolkits [31], uncovered a six amino acid motif, GVMGFO (O is hydroxyproline), as a binding motif for both DDRs [27,32]. The GVMGFO motif is present in the fibrillar collagens I-III. DDR2 has additional binding motifs in collagen II and III [27,32], but their exact sequences have not yet been determined. The distinct preferences of DDRs for various collagen types suggest that migrating cancer cells may deploy different DDRs during invasive processes through different collagen matrices.
3.2 Collagen binding sites for DDRs
The DDR collagen binding sites are entirely contained within their discoidin 1 domains, as shown by in vitro collagen binding experiments using recombinant extracellular domains [23]. The DDR discoidin 1 domains show a high degree of conservation with 59% sequence identity. Initial mutagenesis experiments mapped the collagen binding sites to three spatially adjacent surface-exposed loops that are highly conserved between the DDRs [23,33]. The structure of the un-liganded DDR2 discoidin 1 domain was subsequently determined by NMR, and the collagen-binding site was identified by transferred cross-saturation experiments and mutagenesis [34]. The collagen-binding site is in the form of a trench created by five protruding loops at the “top” of the discoidin 1 domain, opposite the disulfide-linked chain termini. The identification of the GVMGFO motif as a DDR ligand enabled the crystal structure determination of a complex between the DDR2 discoidin 1 domain and a triple-helical peptide encompassing the GVMGFO motif [35]. This structure revealed the apolar GVMGFO motif to be accommodated in an amphiphilic binding pocket. The main collagen binding residues defined by this structure are strictly conserved in DDR1, which is consistent with both receptor types binding to fibrillar collagens. Several DDR2 residues at the periphery of the GVMGFO peptide-binding interface are not conserved in DDR1. These residues are responsible for the distinctive collagen binding specificity of the DDRs. Replacing these amino acids in DDR2 with the corresponding DDR1 residues created a DDR2 construct that was able to bind collagen IV [32].
4 DDR activation and signaling
4.1 Mechanisms of DDR activation/phosphorylation
Like all RTKs, the DDRs undergo receptor autophosphorylation upon ligand binding, but in the DDRs, contrary to most RTKs, this process is unusually slow and sustained [12,11]. However, the structural, biochemical and cellular mechanisms behind the slow rate of DDR activation remain unknown. Recently, Mihai et. al. proposed a model of receptor activation, which may partly explain the slow and sustained phosphorylation of DDR1. Using GFP or YFP-labeled DDR1 proteins, Mihai et. al. showed that collagen exposure induces rapid receptor aggregation, which is then followed by internalization of receptor into early endosome vesicles [25]. The internalized DDR1 then recycles back to the cell surface with a time frame suggestive of intracellular phosphorylation. Although this model provides an explanation for the unusually slow kinetics of DDR1 activation, the exact composition of the ligand-induced, higher order, DDR1 oligomers are yet to be defined. Moreover, the mechanisms that trigger DDR1 endocytosis and the nature of the endocytic pathways remain unknown.
How collagen binding to the DDR discoidin 1 domain leads to the activation of the cytosolic kinase domain is not clear. It is assumed that collagen-induced transmembrane signaling involves substantial conformational changes within the dimer. Structural studies showed that DDR1 activation requires a leucine zipper motif in the TM domain [24], and it is possible that the conformational change upon collagen binding could involve a rotation of the TM helices within the dimer. However, further structural studies will be necessary to understand the nature of these conformational changes. Other open questions are whether TM signaling requires one or both discoidin 1 domains within the preformed dimer to be occupied by ligand and how auto-inhibition of the inactive dimer is achieved. Although the observation that DDRs exist in preformed dimers is consistent with a model of auto-inhibition of phosphorylation, which can only be overcome by ligand binding, studies using the phosphatase inhibitor pervanadate showed a significant and rapid phosphorylation of DDR1 in the absence of added ligand [36,37]. These findings suggest a rapid turnover of DDR1 between phosphorylated and unphosphorylated states. Whether the observed rapid phosphorylation induced by the presence of pervanadate is due to the release of a phosphatase-mediated inhibition mechanism is not known. Collagen-evoked DDR1 activation is also stimulated by interacting signaling pathways. For instance, the Wnt5a/Frzld pathway regulated by TGFβ was shown to be necessary for maximal DDR1 phosphorylation in breast epithelial cells [38–40]. Additionally, Src has been shown to be required for DDR1 phosphorylation in breast epithelial and vascular smooth muscle (VSM) cells [39,41]. How these signaling molecules contribute to DDR1 activation is still unclear. In the case of DDR2, Src has been shown to contribute to receptor activation in COS7 cells [42]. In activated rat hepatic stellate (HS) cells, it was found that DDR2 constitutively interacts with Src and that this interaction increases following binding of the cells to collagen I [42]. Src phosphorylates DDR2 on three tyrosine residues, Y736, Y740, Y741, within the DDR2 kinase domain activation loop, which results in intramolecular autophosphorylation of DDR2 at other tyrosine residues [43]. Substitution of Y740 (within the activation loop of the DDR2) for phenylalanine, results in constitutive collagen-independent DDR2 kinase activity suggesting that this residue plays a role in blocking DDR2 autophosphorylation [43]. Thus DDR2-Src interactions may play a key role in DDR2-intiated signaling.
4.2 DDR binding partners
Upon collagen binding, DDRs undergo autophosphorylation at multiple tyrosine residues within the cytosolic JM and kinase domains. Phosphorylation of tyrosine residues in a precise order recruits cytoplasmic signaling molecules containing Src homology-2 (SH2) and phosphotyrosine-binding (PTB) domains, which in turn assemble protein complexes that serve to transduce receptor signals. In the case of DDR1b and DDR1c, the cytosolic JM region and the kinase domain possess 15 tyrosine residues that can potentially undergo phosphorylation upon receptor activation and serve as docking sites for SH2/3 or PTB containing adaptor proteins (described in Fig. 2). Several adaptor proteins have been identified that bind DDR1 in response to collagen stimulation of DDR1, and, in some instances, the phospho-tyrosine residues involved in binding were also identified (Fig. 2). For example, ShcA [11], the p85a subunit of PI3K [37,39], Nck1/2 [44], SHP-2 [45,44], C-Src tyrosine kinase [46] non-muscle myosin heavy chain (NMHC)-IIA [47], and the focal adhesion kinase (FAK) homolog, Pyk2 [48] were all found to associate with activated DDR1. Most of these interactions were confirmed in a recent proteomic study, which investigated the phospho-tyrosine interactome of DDR1 in pervanadate treated SF268 human glioblastoma cells overexpressing DDR1b and in human placenta using immobilized DDR1 phosphotyrosine peptides [36]. This study also showed that Stat1a/b, Stat3, and Stat5 directly bind to various phospho-tyrosine residues in DDR1, suggesting that activated DDR1 possibly brings SHP-2 and its substrates in close proximity. Other DDR1 binding proteins identified by this approach include RasGAP, the guanine nucleotide exchange factors Vav2 and Vav3, the adaptor protein CRKII and the phosphatase SHIP2 [36]. Several DDR1-interacting partners that bind to unphosphorylated DDR1 have also been identified. These include DARPP32 [49], KIBRA [50], Syk [51], Notch1 [52], E-cadherin [53,54], and the Par3/Par6 cell polarity proteins [54]. In the case of DDR2, ShcA was reported to bind at pY471 following binding of DDR2 to collagen I [43], and indirect evidence suggest that DDR2 may constitutively interact with Src [42], which may also be phosphorylated by DDR2 [55].
4.3 DDR initiated signaling
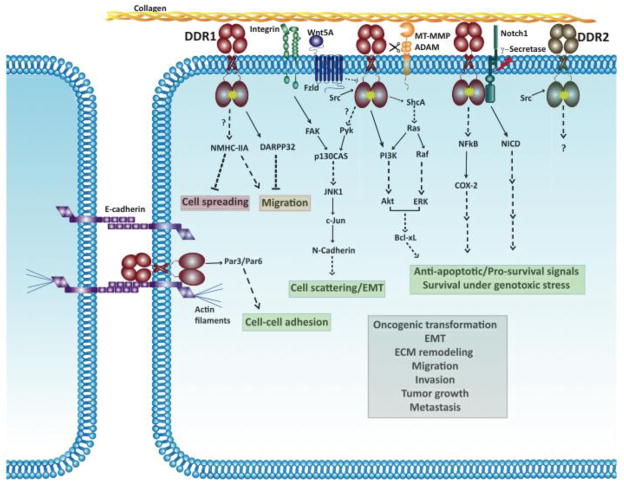
Figure 3 summarizes the downstream signaling events regulated by DDRs, with emphasis on those identified in cancer cells. For DDR signaling in non-malignant cells, the reader is encouraged to see the following studies [56,45,57–59,55,60–62]. In human breast and colon carcinoma cell lines, DDR1 activation triggers pro-survival Ras/Raf/ERK and PI3K/Akt pathways resulting in upregulation of anti-apoptotic Bcl-xL and survival under conditions of genotoxic stress [63]. DDR1 activation also increases NFkB DNA binding activity and cyclooxygenase (COX)-2 expression in human breast cancer cells, leading to increased chemoresistance [64]. In colon cancer cells, DDR1 forms a complex with Notch 1. DDR1 activation triggers Notch1 cleavage by γ-secretase, generating the Notch 1 Intracellular Domain (NICD), which translocates to the nucleus and upregulates pro-survival genes such as Hes1 and Hey2 [52]. In pancreatic cancer cells, DDR1 activation, in conjunction with integrin β1, triggers a p130CAS/JNK pathway that results in upregulation of N-cadherin and epithelial to mesenchymal transition (EMT)-like cell scattering [48]. Although there is a plethora of studies on DDR2 signaling in non-malignant mesenchymal cells (for example [55,60–62]), DDR2 signaling pathways in cancer are practically unknown.
Fig. 3.
Reported DDR-initiated signaling pathways in cancer cells. Solid lines indicate direct interactions or effects. Dashed lines represent indirect interactions or effects that are mediated through one or more intermediate steps. Unknown interactions (direct or indirect) are indicated with a question mark. Arrows pointing to DDRs indicate pathways involved in DDR activation and arrows pointing away from DDRs indicate pathways triggered by activated DDRs. Activated DDRs are indicated with a yellow star. The red box depicts processes that are suppressed by DDR signaling. The green box depicts processes that are promoted by DDRs. The orange box depicts processes that are either suppressed or promoted by DDR signaling under different contexts. The grey box depicts processes that are associated with DDR expression/function but the signaling pathways or mechanisms activated by DDRs in these processes have not been worked out yet. Some DDR1 interactions, e.g. with Nck1/2, SHP-2 and Csk, are not shown since downstream signaling pathways triggered by these interactions are unknown in the context of cancer.
4.4 DDR-integrin cross talk
As malignant cells navigate through the collagen matrix, they must adapt their response to the structural and biochemical nature of their new environment by commanding the activity of two completely different types of receptors: the integrins and the DDRs. It is therefore important to know what the specific contributions of these receptors are, and the potential contribution of receptor cross talk, in the response of cells to the collagen matrix. In some instances, integrins and DDRs may elicit antagonistic effects, which must be integrated to accomplish the proper cellular response. Because cells generally express both integrins and DDRs, several studies have investigated whether DDR activation is coupled with integrin signaling. Although it was observed that DDR1 activation by collagen does not require the activity of collagen-binding integrins [65], some of the downstream signaling pathways activated by DDR1 appear to intersect with integrin-activated pathways. In MDCK cells, DDR1 antagonizes integrin-FAK-Cdc42 mediated cell spreading [58], and integrin-STAT1/3-mediated cell migration [45]. In pancreatic cancer cells, DDR1 and integrin β1 signal co-operate to induce N-cadherin upregulation and cell scattering/EMT-like changes in response to collagen I [48]. In this system, DDR1 activates Pyk2 while integrin β1 activates FAK. p130CAS, a signaling scaffold known to bind to FAK and Pyk2, also binds DDR1 in pancreatic cancer cells. The Pyk/FAK-p130CAS complex activates JNK1, which then upregulates N-cadherin through c-Jun, leading to cell scattering (Fig. 3) [48]. Also, collagen I, through activation of integrin α2β1 and DDR1, plays a role in maintaining an undifferentiated state in mouse embryonic stem cells [66]. In this context, signals downstream of DDR1 and integrin co-operate to promote self-renewal via cell-cycle regulation. Specifically, integrin α2β1 activates integrin-linked kinase (ILK), which then triggers Notch cleavage and Gli-1 upregulation. DDR1 on the other hand activates PI3K/Akt and Ras/ERK. Gli-1 and ERK are both required to activate Bmi-1, which then downregulates p16 and upregulates pRb allowing cell cycle progression to occur in these stem cells [66]. Based on these examples, one can postulate that DDRs and integrins may play co-operating or antagonizing roles at different stages of cancer progression. Thus, in the future, it will be important to delineate the relative contributions of these two collagen receptors at various stages of cancer progression and in different cancer types in order to effectively design novel anti-tumor therapeutic regiments targeting these receptors.
5 Regulation of DDR expression
5.1 Transcriptional regulation
Transcriptional regulation of DDR1 expression is mediated in part by the Ras/Raf/ERK signaling pathway in a variety of normal and cancer cells. In human T cells, for example, T cell receptor activation induces DDR1 expression through Ras/Raf/ERK and protein kinase C dependent pathways [67]. In primary lung fibroblasts, collagen I upregulates DDR1 expression through a mechanism that is dependent on DDR2 activation, phospho-JAK2 recruitment to DDR2 and ERK1/2 activation, leading to increased recruitment of the polyoma enhancer A-binding protein 3 to the DDR1 promoter [68]. This suggests that the interactions of fibroblasts with collagen I require a coordinated regulation and function of both DDR receptors. DDR1 activation itself also results in Ras/Raf/ERK signaling and could therefore positively regulate its own expression, as shown in MCF7 breast cancer and HCT116 colon carcinoma cell lines [63]. Several reports have shown that induction of an EMT phenotype results in transcriptional downregulation of DDR1 in MDCK cells and in human bronchial and breast epithelial cells [69–71]. Consistent with these findings, the DDR1 promoter contains potential binding sites for the EMT-associated transcription factor, Zeb1 [71]. However, the relative role of EMT transcription factors, including Zeb 1, in the regulation of DDR1 expression needs to be further explored.
The DDR1 genomic sequence also contains a functional p53 regulatory element [72,63]. Genotoxic stress induced either by ionizing radiation or chemotherapy, upregulates DDR1 expression in a p53-dependent manner [72,63,64]. Similarly, overexpression of the DNA repair protein XRCC3, results in DDR1 upregulation by an as yet unknown mechanism [73]. A recent study in oligodendrocytes found that the DDR1 gene contains an hnRNP A2 response element-like sequence, which may be involved in alternative splicing and nuclear export of DDR1 mRNA [74]. Also, DDR1 mRNA is a direct target of the microRNA miR-199a-5p; levels of miR-199a-5p inversely correlate with DDR1 expression in human hepatocellular carcinoma cells [75].
Several transcription factors/complexes have been implicated in the regulation of DDR2 expression in different systems. During osteogenic differentiation, the ATF4-C/EBPb transcription factor complex regulates DDR2 upregulation [62]. In rat VSM cells, hypoxia or hyperbaric oxygen increases Myc-Max DNA binding activity in the DDR2 promoter region thereby increasing DDR2 expression [76,77]. In nasopharyngeal carcinomas commonly associated with Epstein Barr Virus (EBV) infection, upregulation of DDR2 has been linked to direct activation of the DDR2 promoter by the EBV-Z transactivator protein [78]. DDR2 mRNA and protein expression are upregulated in HS cells undergoing activation during liver injury [79]. Inhibition of HS cell activation by overexpression of miR-29b, which directly targets collagen I mRNA, results in the concomitant downregulation of DDR2 mRNA, suggesting a relationship between collagen I expression and DDR2 levels [80]. Acquisition of an EMT phenotype in MDCK and human breast epithelial cells has also been shown to induce DDR2 expression [69,71]. However, the transcription factors and mechanisms involved in regulation of DDR2 expression during EMT have not been investigated.
5.2 Posttranslational regulation: Protease-mediated regulation
The DDRs are type I transmembrane proteins and as such are regulated by a variety of biochemical and cellular mechanisms that govern transmembrane protein trafficking including insertion into membrane microdomains (lipid rafts), apical/basal targeting, endocytosis, recycling and degradation. At the time of this writing, there are no reports on these basic aspects of DDR regulation. There is evidence, however, on protease-mediated ectodomain shedding of DDRs, a process that can profoundly affect receptor function. A large number of membrane-anchored proteins including RTKs can be cleaved by proteases to release the extracellular domains [21,81]. This process known as “ectodomain shedding” provides an extra level of regulation to membrane-anchored proteins by depleting functional ectodomains from the cell surface. In the case of RTKs, ectodomain shedding can significantly alter signaling by reducing access of the ligand to its cognate receptor. In turn, the soluble, ligand-binding ectodomain may elicit a variety of biological functions in the extracellular space and possibly influence the membrane-anchored receptor in different ways. DDR1 was shown to undergo ectodomain shedding in a constitutive [10] and collagen-induced manner [82,83] in a process that requires metalloprotease activity. It was reported that DDR1 is cleaved within the extracellular JM region generating two phosphorylated C-terminal fragments of 58- and 62-kDa consistent with the existence of two cleavage sites [82]. Collagen-induced shedding of DDR1 in T47D breast cancer cells is reduced by a Src inhibitor suggesting a role for Src in this process [83]. The nature of the metalloprotease(s) involved in DDR1 shedding has yet to be identified. However, DDR1 cleavage is sensitive to inhibitors of MMPs and ADAMs [83], two metalloprotease families known for their ability to accomplish ectodomain shedding. Data from our laboratory implicate the transmembrane membrane type matrix metalloproteinase MT1-MMP (MMP14), MT2-MMP (MMP15) and MT3-MMP (MMP16) in the shedding of DDR1 in a process that is independent of collagen and involves cleavage within the extracellular JM region at two specific sites. Moreover, cleavage of DDR1 by these MT-MMPs blocks DDR1 phosphorylation by collagen (manuscript in preparation). So far, there are no reports on shedding of DDR2, and in our hands DDR2 is not shed by these MT-MMPs. This suggests that DDRs are differentially regulated by membrane-anchored collagenases, which may have implication for cell-collagen interactions during matrix remodeling. Although the functional consequences of DDR1 shedding need to be determined, this process may serve to control the pool of functional receptors at the cell surface thereby influencing collagen signaling. Alternatively, soluble DDR1 ectodomains may alter collagen fibrillogenesis and organization [22,84,85]. In addition to accomplishing ectodomain shedding, collagenases like the MT-MMPs may also influence DDR activation by modulating the structural integrity of the collagen matrix at areas of cell-matrix contact. Indeed, an early study showed that collagen degradation inhibits DDR1 activation [11] consistent with this hypothesis. Thus, the ability of metalloproteases to regulate DDR integrity and function highlights a potential DDR/protease axis, which may play a key role during intense proteolytic remodeling of the matrix by migrating cancer cells.
6 DDR expression and mutations in cancer tissues
The identification of DDRs and the realization that they are unique RTKs promoted investigation of their expression, phosphorylation, and mutational status in cancer. Valuable information exists on the level of DDRs in cancer tissues, as determined by immunohistochemistry or analyses of RNA expression. However, these methods have intrinsic limitations for evaluation of DDR contribution to cancer progression. For instance, due to the limitations of the currently available antibodies, immunohistochemical methods cannot provide information on receptor activation status, expression of distinct DDR1 isoforms and/or presence of mutated DDRs. Analyses of RNA expression by real-time PCR or searches in current databases such as Oncomine (Compendia Bioscience, Ann Arbor, MI) provide a glimpse on the associations between DDR expression and a variety of cancer related parameters. However, these analyses are limited because posttranslational regulation of DDRs is a key determinant of receptor function. Analyses of receptor phosphorylation status by phospho-proteomic approaches provide information on the pool of phosphorylated receptor within the sample. However, these approaches may be limited for DDR detection because DDRs exhibit very slow kinetics of phosphorylation (discussed in section 4.1), which may influence receptor capture. Mutations in tyrosine kinases are a common feature of most cancers, and are known to play a major role in disease development and progression. While not all the mutations detected in tyrosine kinases have functional consequences, some of these substitutions occur at critical residues within kinase domain activation loops, auto-inhibitory juxtamembrane regions, and/or ligand binding domains, and thus can dysregulate kinase function. Depending on the relative contribution of these mutations to cancer progression, they are classified as “driver” or “passenger” mutations. “Driver” mutations are thought to be responsible for the pro-oncogenic activity of tyrosine kinases whereas “passenger” mutations are those that arise as a consequence of dysregulated DNA-damage responses and cell-cycle check points in the transformed cells (reviewed in [86]). Determining whether mutations are “drivers” or “passengers” can have significant implications for targeted therapies. Thus, given the importance of tyrosine kinases in cancer, sequencing technologies have been applied to identify the mutational status of the human tyrosine kinome in different types of cancer. The results of these approaches are provided in the COSMIC database (http://www.sanger.ac.uk/cosmic/) and the reader is directed to this database for search of DDR mutations in various cancers. Several studies specifically addressed the mutational status of DDRs and in some cases the functional consequences of these mutations were addressed. However, whether these DDR mutations are “driver” or “passenger” mutations remains unclear. With these issues in mind, we summarize the results of published studies that have specifically focused on DDR expression in different cancer types (below and Table 1).
Table 1.
Summary of DDR expression in human cancer tissues
| Cancer | DDR1 | DDR2 | ||
|---|---|---|---|---|
| High | Low | High | Low | |
| Breast | Primary tumors and metastasis-containing lymph nodesa [95]. Invasive ductal carcinomab [97,175,54]. | Invasive lobular carcinomab[97,175]. Middle to high grade carcinomasa[96] | NR | NR |
| Lung | Non-small cell lung carcinomas a[88], b[91,89,90] | Non-small cell lung carcinomasa[88]. | ||
| Brain | High grade primary neuroepithelial and metastatic tumorsa[99]. High grade gliomasa,b [101]. GH- or PRL-producing pituitary adenomas and macroadenomasb [102]. Malignant GBMsa,b [100]. | ACTH- or TSH-producing pituitary adenomas, non-functioning adenomas and microadenomasb [102]. | NR | NR |
| Gynecological | Malignant ovarian tumors (moderate, poorly and well-differentiated)a[8]. Tumor cells of ovarian papillary adenocarcinomaa [10]. Borderline, low-grade and stage epithelial ovarian tumors, epithelial inclusion cystsb [103]. High grade and stage serous ovarian cancera,b[104]. Endometrial tumorsa[105] | Benign and borderline ovarian tumorsa [8]. Low grade and stage serous ovarian cancersa,b [104] | Stromal cells around a papillary adenocarcinomaa [10]. | NR |
| Esophageal | Tumors and lymph node metastases a,b[106]. | NR | NR | |
| Head and Neck | Primary tongue tumorsa[107]. Head and neck squamous cell carcinomasb[54]. | Primary and metastatic nasopharyngeal carcinoma, other head and neck carcinomasa,b [78]. | ||
| Liver and Pancreatic | Advanced stage, poorly differentiated hepatocellular carcinomasa[75]. Primary cholangiocarcinomasb[108]. Malignant pancreatic endocrine tumors with metastasisa[109]. | Benign pancreatic endocrine tumorsa[109]. | Primary cholangiocarcnomas b[108]. | NR |
| Prostate | Preneoplastic and malignant tumorsb[110]. | NR | NR | |
| Thyroid | NR | NR | Aneuploid papillary thyroid cancera[111]. | |
| Mesenchymal tumors | Solitary fibrous tumorsa [112]. | NR | NR | |
| Lymphoma and Leukemia | Acute Myeloid Leukemiaa[114,115]. Primary CNS lymphomaa[176]. | Non-CNS diffuse large B-cell lymphomaa[176]. | Primary Hodgkin’s lymphomaa,b[116,117]. AMLa [114] | B-cell non-Hodgkins lymphomab[116]. Anaplastic large cell lymphomaa,b[117]. |
NR: Not Reported,
RNA,
Protein.
6.1 Lung cancer
Among the human cancers, most of the advances in DDR research have been made in lung cancer, in particular in Non-Small Cell Lung Carcinomas (NSCLCs). Indeed, several studies reported dysregulated expression, phosphorylation, and mutations of DDRs in NSCLCs. Moreover, recent evidence suggests that DDR2 may represent a new therapeutic target in squamous cell carcinoma (SCC) of the lung [87]. Early studies by Ford et. al. [88] in 146 primary NSCLC and an independent set of 23 matched tumor and normal lung tissue samples showed that DDR1 mRNA was upregulated in tumor vs. normal tissue, whereas DDR2 was downregulated. Moreover, DDR1 was found to be an independent favorable prognostic marker for early-stage NSCLC patients while no association between DDR2 mRNA expression and prognosis or patient survival was observed [88]. In contrast, an immunohistochemical study using 171 NSCLC samples showed that DDR1 expression was associated with lymph node metastasis, and poor overall survival [89]. Also, a recent study using a cohort of 83 patients with NSCLC found that tumors with high DDR1 protein levels (as determined by immunohistochemistry) were associated with poor survival [90]. Thus, two independent studies [89,90] reported results that conflict with those of the Ford et. al. study, in regard to the association of DDRs with lung cancer progression. However, dysregulated DDR function in NSCLC may be caused by altered receptor activation or the presence of mutations. Using a phospho-proteomic approach Rikova et. al [91] found that DDR1 and DDR2 are among the top twenty RTKs that are highly phosphorylated in a set of 150 NSCLC tumors. Specifically, DDR1 was the third most phosphorylated tyrosine kinase (following Met and Alk) while DDR2 was at position thirteen. Thus, DDR activation may be associated with development and/or progression of NSCLC. Also, several somatic DDR1 and DDR2 mutations have been identified in primary NSCLC samples. Davies et. al. screened 26 primary NSCLC patient samples for somatic mutations in protein kinases and identified one somatic mutation in the DDR1 gene, A496S, in one patient with squamous cell carcinoma, and one somatic mutation in the DDR2 gene, R105S, in one patient with large cell carcinoma [92]. These mutations map to the cytosolic JM region in DDR1 and the discoidin 1 domain in DDR2 (Fig. 1). No further validation or functional analyses of these mutations were performed. In another study, Ding et. al. screened 188 primary lung adenocarcinoma samples for somatic mutations in 623 genes comprising known oncogenes, tumor suppressors and protein kinase families and found two novel somatic mutations in the DDR1 gene, W385C and F866Y, and one in DDR2, N456S, with each mutation occurring once in the cohort [93]. These mutations map to the extracellular JM and kinase domains in DDR1 and to the cytosolic JM domain in DDR2 (Fig. 1). A recent study conducted to determine the mutational status of the entire tyrosine kinome of lung SCC using a set of 290 tumor samples and cell lines identified somatic mutations in the DDR2 gene [87]. These analyses indicated a frequency of DDR2 mutations at a rate of 3.8% in all samples (tumors and cell lines) and 3.2% in primary lung SCC samples. Overall, 11 mutations in DDR2 were found throughout the entire gene and located in various DDR2 domains (Fig. 1). These include mutations at L63V, I120M and D125Y within the collagen-binding discoidin 1 domain; L239R and G253C within the discoidin 2 domain; G505S in the cytosolic JM domain and C580Y, I638F, T765P, G774E and G774V within the kinase domain. Experimental data showed that the DDR2 L239R and I638F mutations rendered SCC cell lines sensitive to the tyrosine kinase inhibitor dasatinib, and conferred an oncogenic gain of function phenotype to NIH-3T3 fibroblasts in a colony-forming assay in soft agar. Kinase active wild type DDR2 was also oncogenic in BA/F3 cells [87]. Interestingly, Hammerman and colleagues reported that a SCC patient with no evidence of EGFR kinase mutations and who exhibited good therapeutic response to a combination of dasatinib and erlotinib, harbored a DDR2 mutation, S768R, which, based on modeling studies, could alter DDR2 kinase activity. Based on these indirect findings the authors suggested that the patient’s response to the dasatinib/erlotinib regimen was due to a potential sensitivity to dasanitib caused by the mutant DDR2, since erlotinib was found to be a weaker inhibitor of DDR2. Together, these exciting results highlight the importance of further investigating the significance of dysregulated DDR function in the pathogenesis and treatment of NSCLC.
6.1 Breast cancer
Early studies in tissues, cell lines, and knockout mice suggest that altered DDR1 expression and/or function may be critical for breast cancer progression. One of the original studies that reported the cloning of DDR1 (from a full term human placental cDNA library) also reported that DDR1 mRNA and protein are abundantly expressed in the human breast carcinoma cell lines T47D and BT-20 [1]. Moreover, mice deficient in DDR1 exhibit an abnormal mammary phenotype, characterized by hyperproliferation of mammary epithelium and disruption of normal ductal architecture [94]. Studies examining the expression of DDR1 mRNA in primary human breast carcinoma tissues however, reported conflicting results. While one study reported abundant expression of DDR1 mRNA in primary invasive carcinoma and in lymph node metastases [95], another study showed a moderate reduction in DDR1 mRNA levels in the majority of intermediate to high-grade human breast carcinomas compared to normal mammary tissues [96]. These discrepancies may be due to differences in the histological type and/or tumor grade. For example, DDR1 mRNA and protein levels were significantly decreased in invasive lobular carcinomas when compared to invasive ductal carcinomas [97]. These intriguing results have potential clinical application in distinguishing between invasive ductal and lobular carcinomas and warrant further investigation. At present, whether DDR1 expression is associated with progression in breast cancer remains unknown. DDR2 expression in human breast epithelial cells has been associated with induction of EMT [71], but the expression pattern and association of DDR2 with disease progression in breast cancer are unknown. Future studies will have to focus on determining DDR1 and DDR2 expression in the different subtypes of breast cancer and in the transition from in situ to invasive carcinoma.
6.3 Brain cancer
DDR1 mRNA was found to be highly expressed in both high-grade pediatric [98] and adult brain tumors regardless of cell type (glioblastoma multiforme, anaplastic atrocytoma, anaplastic mixed glioma, primitive neuroectodermal tumors, ependymoma, and meningeal sarcoma) [99]. DDR1 protein expression is also elevated in gliomas compared to normal brain tissue [100] and significantly correlates with poor clinical outcome [101]. Since the collagen matrix within the brain parenchyma is limited to the vascular and perivascular areas, the high expression of DDR1 in brain tumors may play a role in the invasiveness of glioma cells along the perivascular matrix. In pituitary adenomas, DDR1 protein is widely expressed, and its level correlates with the hormonal background of the tumor [102]. Specifically, DDR1 protein expression is significantly higher in growth hormone- and prolactin-producing adenomas compared to adrenocorticotropic-producing and non-functioning adenomas. DDR1 protein expression was also found to be higher in macroadenomas compared to microadenomas [102].
6.4 Gynecological cancers
An early study reported that DDR1 mRNA was expressed in moderately or poorly differentiated ovarian tumors, while benign or borderline tumors showed very little expression [8]. In a later study, high levels of DDR1 protein were found in all histological types of epithelial ovarian cancers and in ovarian epithelial inclusion cysts, which are thought to be sites of initial oncogenic transformation. However, no significant correlation between DDR1 protein expression and prognosis was observed [103]. Another study in serous ovarian tumors found high levels of DDR1 protein in high grade and advanced stage tumors compared to low grade and early stage tumors, and a significant correlation between DDR1 protein expression and poor outcome [104]. Thus, two independent studies [103,104] suggest that DDR1 may be a useful target and biomarker for early [103] as well as advanced [104] ovarian cancer. A study in endometrial cancer reported that DDR1 mRNA was significantly upregulated in tumor tissues and in uterine aspirates. This suggests that DDR1 could serve as a novel early diagnostic marker for this type of cancer [105].
6.5 Esophageal cancer
DDR1 mRNA and protein are highly expressed in esophageal carcinoma tissues [106]. In these tumors, DDR1 mRNA levels were found to significantly correlate with tumor cell proliferation. In addition, DDR1 protein was detected in infiltrating foci and lymph node metastases, suggesting a role for DDR1 in invasion and metastasis of this cancer type [106].
6.6 Head and neck cancers
DDR1 mRNA was found to be consistently upregulated in primary tongue tumors when compared to normal tongue mucosa [107]. Head and neck squamous carcinoma tissues were reported to express high levels of DDR1 protein [54]. Studies in nasopharyngeal carcinomas found high levels of DDR2 (mRNA and protein), but not DDR1, in the cancer cells of primary and metastatic tumors [78]. The high expression of DDR2 in these tumors was attributed in part to transcriptional activation of the DDR2 promoter by the EBV-Z-transactivator (discussed in section 5.1) [78].
6.7 Liver and pancreatic cancer
High levels of DDR1 mRNA were found in hepatocellular carcinomas, which significantly correlated with advanced tumor stage [75]. The increased expression of DDR1 mRNA in these tumors was associated with downregulation of miR-199-5p, a miRNA that directly targets the 3′ UTR of DDR1 mRNA [75]. In cholangiocarcinoma tissues, both DDRs were identified in a phospho-proteomic screen for highly phosphorylated tyrosine kinases, with DDR1 being the third most phosphorylated tyrosine kinase in these tumors [108]. In pancreatic endocrine tumor tissues, DDR1 was identified as one of the 72 genes that were significantly upregulated in malignant versus benign pancreatic endocrine tumors [109]. Considering the dismal survival of pancreatic cancer patients, the role of DDRs in this devastating cancer needs to be further examined.
6.8 Prostate cancer
DDR1 and PCA-1 (prostate cancer antigen -1) proteins were found to be highly expressed in prostate cancer tissues and to significantly correlate with hormone independence, suggesting that these two proteins may play important roles in the development of the hormone refractory state in prostatic tumors [110]. Moreover, expression of PCA-1 was found to regulate DDR1 mRNA and protein expression in the human prostate cancer cell lines PC3 and DU145 [110]. Since advanced prostate cancer is characterized by the development bone metastasis, it will be interesting to examine the functional contribution of DDRs to this important clinical problem.
6.9 Thyroid cancer
Microarray analyses in aneuploid papillary thyroid carcinomas revealed that DDR2 is one of the few genes that are highly expressed in patients with metastatic disease at time of diagnosis [111]. Moreover, in the same study, analysis of tumors from patients that had died from this disease revealed that DDR2 was one of the most overexpressed genes in this group as well [111]. While these microarray results were validated by quantitative PCR, no further protein expression or functional analyses were performed.
6.10 Solitary fibrous tumors
Solitary fibrous tumors (SFT) are rare mesenchymal neoplasms that arise from soft tissues, and are characterized by intense proliferation of spindle, fibroblast-like cells within a collagen stroma. A microarray study found upregulated DDR1 mRNA in SFT samples, regardless of anatomical location. However, sequencing of 8 SFT samples showed no mutations in the DDR1 gene [112]. Considering the role that DDRs play in regulating the interactions of mesenchymal cells with collagen, these results suggest that DDR1 may be involved in the development of SFT. Moreover, the high expression of DDR1 in a mesenchymal tumor disputes the notion that DDR1 and DDR2 should be classified as epithelial or mesenchymal receptors, respectively.
6.11 Lymphomas and leukemias
Dysregulated tyrosine kinase function is implicated in the pathogenesis of hematological malignancies. In fact, most of the targeted therapies in these cancer types are directed against kinases including RTKs. Therefore, a great effort has been invested in defining the signatures of kinases that are altered in hematological tumors. These searches identified the DDRs as one set of RTKs that are either overexpressed and/or mutated in hematological cancers. A study aimed at characterizing gene expression signatures in adult acute lymphocytic leukemia (ALL) reported that DDR1 was one of the highly expressed kinases in cases without evidence of molecular rearrangements, and in ALL cases with BCR/ABL rearrangements. These studies suggested that dysregulated activation of DDR1 might play a role in the transformation of a subset of ALL [113]. Two studies reported the identification of DDR1 mutations in leukemias. Re-sequencing of twenty-six selected cytoplasmic tyrosine kinase and receptor tyrosine kinase genes in patients with de novo AML identified an A803V somatic mutation of DDR1 in 1 out of the 188 patients tested [114]. This mutation maps on to the predicted activation loop within the DDR1 kinase domain suggesting the possibility that DDR1 activation is altered in AML [114]. However, the effects of this mutation on DDR1 activity and in the pathogenesis of AML remain to be determined. In another study, Loriaux et. al. [115] used high-throughput DNA sequencing to screen exons encoding the activation loop and cytosolic JM domains of eighty-five tyrosine kinase genes in 188 AML patients without FLT3 or c-KIT mutations. These analyses identified five AML patients with non-synonymous somatic mutations in the DDR1 JM domain: T460M (1 sample), N502S (3 samples) and A533S (1 sample). However, these DDR1 mutations did not affect the kinetics or the extent of collagen-induced receptor phosphorylation when compared to wild type DDR1 upon expression of these receptors in murine pro-B Ba/F3 cells. Also, these mutations failed to transform Ba/F3 cells into cells that exhibit hypersensitivity to interleukin-3-stimulated growth. However, due to the inherent limitations of the Ba/F3 assay [115], the potential involvement of DDR1, alone, or in combination with other dysregulated RTKs, in AML cannot be ruled out and needs to be further explored.
A study in Hodgkin’s Lymphoma (HL) demonstrated enhanced expression of DDR2 protein in 30–70% of the Hodgkin/Reed-Sternberg (HRS) cells present in the lymph nodes of HL patients whereas the normal B cells showed no expression [116]. Interestingly, collagen I was detected near the HRS cells within the lymph nodes of HL patients, suggesting that DDR2 can potentially be activated in these neoplastic cells. The high expression of DDR2 in HL was later confirmed in an immunohistochemical analysis of lymph node tissue infiltrated by HL, which showed high DDR2 in 44% of the HL samples [117]. Thus, DDR2 is emerging as one of the RTKs that are upregulated in HL. Collectively, these studies suggest that, as in solid tumors, dysregulated DDR expression or activity may contribute to the pathogenesis of hematological cancers, with DDR1 and DDR2 associated with leukemias and lymphomas, respectively.
7 Function of DDRs in normal and cancer tissues
7.1 Functions of DDRs as revealed in knockout and mutant mice (summarized in Table 2)
Table 2.
Insights into DDR function from Knockout Mice and Mutations in Humans
| DDR1 knockout mice | DDR2 knockout mice | DDR2 mutations in mice (slie) and humans (SMED-SL) | |
|---|---|---|---|
| Skeletal system | Smaller in size, poorly mineralized fibula bone [94]. | Mouse: Dwarfism and shorter long bones due to reduced chondrocyte proliferation [118]. |
Mouse: Dwarfism [121]. Human: Multiple skeletal defects in SMED-SL (Short-limb abnormal calcification) [119], due to DDR2 trafficking defects or defects in collagen-binding activity [120]. |
| Mammary Gland | Lactational defect, delayed mammary gland ductal outgrowth, hyper-proliferative epithelium, abnormal ductal branching, enlarged terminal end buds [94]. Reduced Stat5 activation and β-casein expression upon lactogenic stimulation in primary mammary epithelial cells [124]. | NR | NR |
| Kidney | Thickening of glomerular basement membrane, focal loss of split diaphragms, proteinuria [125]. Reduced adhesion of primary mesangial cells to ECM, increased mesangial proliferation due to increased MAPK activity [126]. | NR | NR |
| Vascular System | Reduced neointimal thickening after vascular injury [30]. Reduced adhesion and migration to collagen I and MMP secretion by smooth muscle (SM) cells [147]. Reduced atherosclerotic calcifications in Ldlr−/− background [177]. Increased matrix secretion, proliferation and migration of vessel wall SM cells[135]. | No effect on adhesion, migration, or proliferation of vascular SM cells [152]. | NR |
| Inflammatory/Fibrotic response/Immune system function | Reduced inflammatory response to LPS, reduced glomerular fibrosis and inflammation in hypertension [178]. Impaired macrophage migration and invasion in response to MCP-1 [157,150]. Reduced inflammatory and fibrotic response to bleomycin-induced lung injury, no p-38 MAPK activation [179]. Reduced macrophage recruitment, inflammation, and fibrosis in atherogenesis in Ldlr−/− background [180]. Reduced proinflammatory and profibrotic cells in kidney in hereditary collagen IV disease (COL4A3−/− background) [181]. Reduced inflammation and fibrosis in obstructed kidneys [157]. | Increased inflammatory and proliferative response of hepatic stellate cells and macrophages to chronic liver injury [129]. | NR |
| Skin | NR | Reduced proliferation, invasion through basement membrane and MMP activity of dermal fibroblasts [128]. Impaired dermal wound healing due to defective ECM remodeling by skin fibroblasts [55]. | NR |
| Ear | Unable to control ear movement [94]. Loss of auditory function and profound structural changes throughout the cochlear duct [127]. | NR | NR |
| Reproduction | Impaired blastocyst implantation into uterine wall [94]. | NR | Mouse: Impaired spermatogenesis [121,123] and ovulation [121,122] in slie mice. |
| Susceptibility/Resistance to diseases | Resistant to bleomycin-induced lung fibrosis [179]. Resistant to hypertension-induced renal failure [178]. Decreased atherosclerosis in Ldlr−/− background [180,177]. Delayed renal fibrosis, inflammation, and death in hereditary collagen IV disease (COL4A3 background) [181]. | Increased susceptibility of hepatic tissue to colon carcinoma metastasis [130]. Increased susceptibility to hepatic fibrosis in chronic liver injury [129]. | NR |
NR: Not reported
While both DDR1 and DDR2 knockout mice are viable, they are small in size compared to wild type littermates [94,118]. Not surprisingly for collagen receptors, DDRs likely play critical roles in skeletal development. DDR1 knockout mice have poorly mineralized fibula bones [94]. In DDR2 knockout mice, dwarfism has been linked to shorter long bones that arise due to reduced chondrocyte proliferation [118]. In humans, DDR2 mutations are associated with multiple skeletal defects, including short limbs and abnormal calcification. Some of these effects have been attributed to defects in DDR2 trafficking or collagen binding activity [119,120]. Besides being smaller in size, DDR knockout/mutant mice exhibit defects in reproduction. DDR1 knockout mice have impaired blastocyst implantation into the uterine wall while DDR2 mutant mice display impaired spermatogenesis and ovulation [94,121–123]. DDR1 knockout mice are unable to lactate due to aberrant mammary gland morphogenesis, as manifested by delayed ductal elongation, abnormal secondary branching, hyperproliferative ductal epithelium, enlarged terminal end buds, and increased collagen deposition around ducts [94]. Primary mammary epithelial cells from DDR1 knockout mice exhibit impaired Stat5 activation and β-casein expression when exposed to lactogenic stimuli [124]. Additionally, DDR1 knockout mice exhibit altered kidney structure [125] and impaired primary mesangial cell adhesion to ECM along with hyperproliferation due to increased MAPK/ERK activation [126]. These mice are also unable to control their ear movements [94] and show loss of auditory function with profound structural changes throughout the cochlear duct [127]. DDR2 knockout mice, in contrast, show no defects in lactation, kidney structure, or auditory function. Instead these mice display impaired dermal wound healing due to defective proliferation, invasion, proteolytic activity, and ECM remodeling by skin fibroblasts [128,55]. In addition, the DDR knockout mice exhibit altered responses to various insults and pathological conditions. For instance, DDR1 knockout mice exhibit reduced neointimal thickening and altered VSM cell function following vascular injury. Also, these mice have reduced inflammatory and fibrotic responses in obstructive nephropathy, renal hypertension, bleomycin-induced lung injury, atherogenesis, and hereditary collagen IV disease (see Table 2 for details and references). Taken together, these findings suggest that DDR1 is involved in regulation of inflammatory and fibrotic responses in lung, vascular, and renal tissues. Similarly, DDR2 promotes HS cell activation and fibrosis in acute liver injury [79]. However, in chronic liver injury DDR2 likely plays a suppressing role, since DDR2 knockout mice are more susceptible to inflammatory responses under this condition [129]. A recent report also found that DDR2 knockout mice are more prone to metastasis of colon carcinoma cells to the liver, presumably due to altered HS cell responses leading to a pro-metastatic environment within the liver [130].
7.2 Functions of DDRs as revealed in cultured primary cells and cell lines (summarized in Table 3)
Table 3.
DDR functions reported in cultured cells
| DDR1 | DDR2 | |||
|---|---|---|---|---|
| Promoting | Suppressing | Promoting | Suppressing | |
| Survival and Chemoresistance |
Non-malignant cells: MDCK cells [56] Malignant cells: Human prostate cancer cells (PC3, DU145, LNCaP) [110], human breast cancer cells (MCF7 [63], T47D [64]), human colon cancer cells (HCT116) [63,52] |
NR | NR | |
| Proliferation and colony forming ability |
Non-malignant cells: Murine vascular smooth muscle (VSM) cells [30], primary human bronchial epithelial cells (HBECs) [131], T cells [132] Malignant cells: Human glioma cells (U251, GI-1, T98G) [101], HCT116 (in vitro colony forming assay) [52] |
Non-malignant cells: Primary murine mesangial cells [126], murine VSM cells [135] |
Non-malignant cells: Rat hepatic stellate (HS) cells [79], murine skin fibroblasts [128,118], rat VSM cells [133], murine chondrocytes [118], Slug-MDCK cells [69], NIH-3T3 fibroblasts can be oncogenically transformed by DDR2 L63V and I638F mutations [87] Malignant cells: Human melanoma cells (A375), hepatoma cells (SK-HEP), colon carcinoma cells (HT-29) [134], human lung squamous cell carcinoma (H2286, HCC366) [87] |
Non-malignant cells: Murine HS cells after chronic liver injury [129], 3T3-L1 preadipocytes [140] Malignant cells: Fibrillar collagen induced proliferation in human melanoma cells (M24met, A2058), human fibrosarcoma cells (HT1080) [136] |
| Differentiation | Non-malignant cells: Murine granule neuron axon formation [137], osteogenic potential in human mesenchymal stem cells [85], epithelial differentiation in porcine kidney epithelial cells (LLC-PK1) and murine mammary epithelial cells (NMuMG) [59], murine myoblast (C2C12) differentiation/formation of myofibers [65]. | Non-malignant cells: Maintenance of undifferentiated state in murine embryonic stem cells[66]. | Non-malignant cells: Differentiation in murine osteoblasts [62,61], and chondrocytes [61]. | Non-malignant cells: Differentiation of murine pre-adipocytes to functional adipocytes [140]. |
| EMT | Malignant cells: Collagen I-induced cell spreading in human pancreatic cancer cells (BxPC3) [48], TGFβ/Collagen I-induced EMT in human lung carcinoma cells (A549) [144] | Non-malignant cells: DDR1 expression suppresses mesenchymal genes in LLC-PK1 and NMuMG [59]. |
Non-malignant cells: TGFβ/Collagen I-induced EMT in renal proximal tubule cells (HK-2) [144]. Malignant cells: TGFβ/Collagen I-induced EMT in A549 [144]. |
|
| Cell-Cell contact |
Non-malignant cells: MDCK [53,57], LLC-PK1, NMuMG [59]. Malignant cells: Human oral squamous carcinoma cells (A431, SCC12) [54]. |
NR | NR | |
| Adhesion |
Non-malignant cells: Murine VSM cells [30,147], human mammary epithelial cells (HB2)[38,39], human monocytic cells (THP-1)[148], primary murine mesangial cells[126], primary human melanocytes [149], murine macrophages [150]. Malignant cells: Human pituitary adenoma cells (HP-75) [102], human glioma cells (G140) [100], human hepatoma cells (Huh7) [151]. |
NR | NR | |
| Spreading |
Non-malignant cells: NIH3T3 fibroblasts [47], human mesenchymal stem cells [85], MDCK [57,58]. Malignant cells: MCF7 [47]. |
NR | NR | |
| Migration |
Non-malignant cells: Murine VSM cells ([30], [147,41]), THP-1 cells [148], human T cells [156,67], NIH3T3 fibroblasts, mouse embryonic fibroblasts [47], murine macrophages [157], HBECs and human bronchial epithelial cells (BEAS-2B) [131]. Malignant cells: G140 cells [100], human hepatocellular carcinoma cells (HLE, Huh 7) [158], human lung adenocarcinoma cells (A549, H538) [89], human breast carcinoma cells (MDA-MB-231) [159] (T47D, MDA-MB-468) [96], (MCF7) [47]. |
Non-malignant cells: HB2 cells [38], MDCK [56,45], LLC-PK1 [59], murine VSM cells [135]. Malignant cells: Huh7 cells [151], MCF7 (when DARPP32 is expressed), MDA-MB-231 (when DDR1 and DARPP32 are expressed) [49]. |
Non-malignant cells: Murine skin fibroblasts [128], rat VSM cells [76,133,77]. Malignant cells: A375, SK-Hep and HT-29 cells [134]. |
Non-malignant cells: Murine HS cells and macrophages from chronically injured liver [129]. |
| Invasion |
Non-malignant cells: Murine macrophages [150]. Malignant cells: HP-75 cells [102], A431 and SCC12 cells [54], G140 cells [100], U251, GI-1 and T98G cells [101], HLE and Huh 7 cells [158], human hepatoma cells (HEPG2, SNU-182) [75] PC3, DU145 and LNCaP [110], A549 and H538 cells [89], MDA-MB-231 cells [161]. |
Non-malignant cells: Rat HS cells [79]. | ||
| Collagen remodeling | Non-malignant cells: Organization of naïve collagen I matrix by human mesenchymal stem cells [85]. | Non-malignant cells: Soluble DDR1 extracellular domains suppress collagen I fibrillogenesis [84]. | Non-malignant cells: Human VSM cells (collagen I degradation) [182], murine skin fibroblasts (collagen I deposition) [128,55]. | Non-malignant cells: Soluble DDR2 extracellular domains suppress collagen I fibrillogenesis [84,173,183]. |
| MMP expression |
Non-malignant cells: Murine VSM cells (MMP2, 9) ([30] [147]), human VSM cells (MMP1) [182], LDLR−/− murine macrophages (MMP2, 9, 14) [180], HBECs and BEAS-2B cells (MMP7) [131]. Malignant cells: HP-75 cells (MMP2, 9) [102], G140 cells (MMP2) [100], HLE and Huh 7 cells (MMP2, 9) [158], PC3, DU145 and LNCaP cells (MMP9) [110], H538 cells (MMP9) [89], MDA-MB-231 cells (MMP-2, 9) [161]. |
Non-malignant cells: Rat HS cells (MMP2) [79], murine skin fibroblasts (MMP2 secretion) [128,55], rat VSM cells (MMP2) [133,77], human VSM cells (MMP1, MMP2)[182], osteoarthritic chondrocytes in collagen XI−/− mice (MMP13) [184], murine chondrocyte cell line C-28/I2 (MMP13) [185], human rheumatoid arthritis synovial fibroblasts (MMP1) [186], (MMP13) [187]), NIH-3T3 cells (MMP-1) [186], murine synoviocytes and 293T cells (MMP13) [187], primary goat articular chondrocytes (MMP13) [60]. Malignant cells: A375 cells (MMP2, 9) [134]. |
||
| Tumor growth and Metastasis | Malignant cells: HCT116 (xenografts) [52], human lung large cell carcinoma (H460) metastasis to bone [90]. | Malignant cells: A375 metastasis to liver [134]. | Malignant cells: Increased metastasis to liver of DDR2 knockout mice [130]. | |
NR: Not reported
7.2.1 Cell proliferation and survival
Both DDR1 and DDR2 can exhibit pro- [131,30,79,132,101,133,118,128,69,134,87] and anti- [135,126,94,129,136] proliferative activities in a cell type and context-dependent manner. However, the mechanisms by which each DDR regulates cell proliferation are unknown but are likely to require kinase activity [79]. In the case of DDR2, mutations of the receptor were shown to confer cell growth promoting activity in NIH3T3 cells, while both wild type and mutated DDR2 were able to promote cell growth in Ba/F3 cells [87] suggesting that both wild type and dysregulated DDR2 stimulate cell proliferation and have oncogenic potential. The DDR1 genomic sequence contains a consensus p53 tumor suppressor response element and DDR1 was shown to be a direct transcriptional target of p53 [72,63]. Consistently, exposure of breast and colon cancer cell lines to DNA-damaging agents results in p53-dependent upregulation of DDR1 expression and kinase activity, which promotes cell survival via the Ras/MEK/MAPK and Akt pathways [63]. Likewise, downregulation of DDR1 expression significantly enhances the chemosensitivity of breast cancer cells to genotoxic drugs [64]. Studies in human HCT116 colon carcinoma cells showed that the pro-survival effects of DDR1 in response to genotoxic drugs are partly mediated by autocrine activation of Notch1 signaling in response to collagen-induced activation of DDR1 [52].
7.2.2 Differentiation and EMT
DDRs have been shown to play key roles in regulation of cell differentiation in various cellular systems. DDR1 signaling is required for granule neurite outgrowth in vitro and extension of granule cell parallel fibers in situ in immature granule cells [137]. DDR1 is one of the transcripts that are upregulated during neuronal differentiation of mouse embryonic stem cells [138]. DDR1 expression strongly correlates with oligodendroglial differentiation both in vitro and in vivo in a mouse model of remyelination [139]. Inhibition of DDR1 expression is associated with decreased osteogenic potential of human mesenchymal stem cells [85]. DDR1 promotes differentiation into myotubes of the skeletal muscle cell line, C2C12, in a process that requires kinase activity [65]. DDR1 expressing porcine LLC-PK1 kidney epithelial cells form microvilli even in the presence of collagen I (which promotes de-differentiation), while cells expressing a dominant negative DDR1 have severely impaired microvilli formation [59]. Moreover, DDR1 expressing normal murine breast epithelial cells (NMuMG) and LLC-PK1 cells exhibit higher levels of E-cadherin and lower levels of the mesenchymal markers fibronectin, integrin β1, and α-smooth muscle actin, suggesting a role for DDR1 in maintenance of the epithelial phenotype [59]. DDR1 signaling in response to collagen I contributes to the maintenance of the de-differentiated state and to self-renewal in mouse embryonic stem cells, possibly by co-operating with integrin α2β1 [66]. DDR2 promotes osteogenic differentiation in murine bone marrow stromal cells [61], pre-osteoblast MC3T3-E1 cells [62,61], pluripotent C3H10T1/2 cells and C3C12 cells [62]. DDR2 promotes chondrogenic differentiation in the murine chondrogenic cell line ATDC5 [61]. Transactivation of the transcription factor Runx2, by DDR2, is a key event in both osteogenic and chondrogenic differentiation in these systems [62,61]. DDR2 downregulation is important during the differentiation from pre-adipocytes to functional adipocytes [140]. Thus, whether DDRs promote or inhibit differentiation appears to be cell context dependent. Regardless, given the roles that DDRs play in normal cell differentiation, it will be important to determine whether DDR signaling is involved in differentiation of cancer stem cells.
During normal development, certain epithelial cells acquire the ability to express mesenchymal properties in a phenomenon known as epithelial to mesenchymal transition (EMT). EMT occurs during such processes as gastrulation, neural crest formation, pancreas, liver and reproductive tract development, atrioventricular (AV) canal formation in the heart and during physiological responses to injury, just to name a few (reviewed in [141]). EMT is characterized by the activation of specific transcription factors, which, among other effects, induce a switch in cadherin expression (from E-cadherin in the epithelial state to N-cadherin in the mesenchymal state) and a loss of cell polarity. EMT has been associated with increased cell motility and invasiveness, induction and maintenance of stem cell properties, prevention of apoptosis and senescence, resistance to immuno/chemotherapy and escape from immune surveillance in pathological conditions such as fibrosis and tumor progression [reviewed in [141]]. Induction of an EMT in various cellular systems has been found to correlate with a switch in DDR expression; from DDR1 (epithelial) to DDR2 (mesenchymal). For instance, EMT during heart development in chicken embryos is associated with high levels of DDR2 mRNA and protein [142]. In mice, elevated DDR2 mRNA was found in epicardium-derived cells undergoing EMT during development of the annulus fibrosis of the heart [143]. EMT induction in MDCK cells by Slug causes a switch from DDR1 to DDR2, which is thought to be responsible for the increased proliferation of the Slug-MDCK cells within collagen I gels [69]. TGF-β-induced EMT in primary human bronchial epithelial cells reduces DDR1 protein levels while DDR2 levels are unchanged [70]. Likewise, TGF-β induces DDR2 expression in renal proximal tubule epithelial cells, and knocking down DDR2 expression suppresses TGF-β or collagen I induced EMT in these cells [144]. Expression of EMT inducers (Goosecoid, Snail, Twist, Slug and TGF-β) in a non-transformed human mammary epithelial cell line (HMLE) induces an “EMT core signature”, which includes upregulation and downregulation of DDR2 and DDR1, respectively [71]. Downregulation of DDR1 was attributed to the presence of Zeb1 binding sites within the DDR1 promoter. Zeb1 is a transcription factor that is overexpressed downstream of the above-mentioned EMT inducers, and plays a critical role in mediating gene expression changes during EMT, particularly in E-cadherin downregulation [145]. Gene expression analyses in human breast cancer cell lines showed that DDR1 mRNA is lower in the more mesenchymal and invasive Basal B type cell lines when compared to the Basal A and luminal breast cell cancer lines [146]. While these studies suggest that acquisition of a more mesenchymal-like phenotype is associated with expression of DDR2, other studies suggest that, depending on the cell type, both DDRs can support EMT. For instance, knockdown of either DDR1 or DDR2 inhibits TGF-β- or collagen I-induced EMT in human A549 lung carcinoma cells [144] while in a pancreatic cancer cell line, Bx-PC3, DDR1 cooperates with integrins to mediate collagen-I induced EMT [48]. Thus, although EMT may be associated with a switch from DDR1 to DDR2, the relationship between induction or maintenance of EMT and DDRs cannot be generalized and may be context-dependent. The roles of DDRs in development and maintenance of EMT in various cellular contexts are still unknown. However, given the ability of DDRs to signal in response to collagen matrices, and that EMT is partly regulated by collagen, DDRs may activate signal transduction pathways that contribute to the adaptation of cells to the new collagenous environment upon activation of EMT-induced migration programs.
7.2.3 Cell adhesion and spreading
Given the ability of DDRs to bind various collagen types, it is not surprising that regulation of cell adhesion is a major biological function of these receptors. Indeed, a plethora of studies demonstrated that both DDR1a and DDR1b support adhesion to collagen I in a variety of cells types [30,147,148,126,39]. Moreover, DDR1-mediated cell adhesion to collagen appears to be essential for tissue and cellular functions in normal and pathological processes. For instance, in melanocytes, DDR1-mediated adhesion to collagen IV plays a key role in the localization of these cells to the BM/basal layer of the epidermis [149]. In macrophages, DDR1 supports monocyte chemotactic protein (MCP)-1 induced adhesion to and invasion through collagen IV, which may contribute to macrophage infiltration and accumulation during atherogenesis [150]. In cancer, DDR1 plays a role in adhesion to collagen of a variety of malignant cells including pituitary adenoma [102], glioma [100], and hepatoma cells [151], which may support tumor cell migration. In the case of DDR2, its contribution to cell adhesion is still unclear. For example, one study showed that DDR2 is not required for adhesion of VSM cells to collagen I [152], while another study showed that DDR2 expression and activation plays an important role in regulating FAK levels in VSM cells attaching to collagen I [153]. In human bronchial epithelial cells [131] and activated human T cells [67], knockdown of DDR1 expression or blocking DDR1 function using a soluble DDR1 ectodomain did not significantly affect adhesion to collagen.
The role of DDR phosphorylation (and the subsequent activation of downstream effectors) in cell adhesion has not been studied in detail and thus is barely known. A study using murine VSM cells showed that adhesion to collagen I is effectively mediated by kinase inactive DDR1, suggesting that in this system, kinase activity of the receptor is not critical [147]. However, whether this is the case for other cell types and collagens, remains to be determined. Regardless, while molecular interactions between DDRs and collagen are not dependent on receptor phosphorylation [21–23], it is reasonable to postulate that meaningful cellular adhesion to collagen (as defined by the establishment of cell-matrix contacts, changes in cell morphology, and activation of downstream effectors) requires receptor structural changes and phosphorylation of specific tyrosine residues.
After adhesion to collagen, cells undergo changes in morphology as they spread over the substratum. At the molecular level, the interaction of adhesion receptors with the ECM initiates a cascade of signaling events, which subsequently triggers actin polymerization and myosin contraction, resulting in active cell spreading [154,155]. To date, all reports show that DDR1 activity suppresses cell spreading in a variety of cell types [57,58,85,47]. In MDCK cells, inhibition of cell spreading requires DDR1 activation by collagen; expressing kinase inactive DDR1 in these cells suppresses this inhibition, thereby promoting cell spreading [57]. It has been proposed that inhibition of α2β1 integrin-mediated Cdc42 activity is involved in the suppressive activity of DDR1 in cell spreading [58]. In other systems, including NIH3T3 fibroblasts and a human breast carcinoma cell line (MCF7), DDR1 appears to regulate cell spreading through its interaction with the non-muscle myosin IIA (NMHC-IIA), a ubiquitously expressed contractile protein that has been implicated in the regulation of cell spreading and directional migration in response to various stimuli [47]. At present, the role of DDR2 in cell spreading is unknown.
7.2.4 Cell-cell adhesion
Evidence suggests that DDR1 plays a role in supporting cell-cell interactions in epithelial cells independently of its ability to bind collagen. DDR1 was found to localize to cell-cell junctions, usually with E-cadherin, in a variety of normal [57,53,59,54] and malignant [54] cell lines and tissues. Both DDR1 and DDR2 were found to be able to interact to E-cadherin via their extracellular domains [53]. In the case of DDR1, a complex with E-cadherin abrogates collagen-induced DDR1 activation, possibly by sequestering the receptor away from its ligand [57]. Conversely, DDR1 expression stabilizes E-cadherin at the cell surface and promotes cell-cell aggregation [53] by negatively regulating Cdc42 [59]. DDR1 also promotes cell-cell adhesion by reducing actomyosin contractility at cell-cell junctions, independent of DDR1 activation since neither the kinase inactive mutant (K618A), nor the collagen binding mutant (R105A) has any effect on this function [54]. Following recruitment to cell-cell junctions by E-cadherin, DDR1 associates with the Par3/Par6 proteins via its C-terminal, PDZ interacting domains. The Par3/Par6 complex in turn, through RhoE recruitment, abrogates RhoA/ROCK activity and actomyosin levels at the junctions. This function of DDR1 was found to be indispensable for collective invasion by human oral squamous carcinoma cells [54]. The physiological significance for the ability of DDR2 to interact with E-cadherin is not known at present.
7.2.5 Cell migration
DDRs regulate cell migration in a cell type and isoform dependent manner. Thus, both pro- and anti-migratory effects of DDRs have been reported. For instance, in activated human T cells, DDR1 supports ameboid migration within 3D collagen I lattices [156,67]. Likewise, overexpression of DDR1a, but not DDR1b, enhances migration of THP-1 monocytic cells through 3D collagen I [148]. DDR1 is also required for the migration of primary human bronchial epithelial cells and the human bronchial epithelial cell line BEAS-2B [131]. In addition, DDR1 expression increases the migration of mouse embryonic fibroblasts and NIH3T3 fibroblasts, toward collagen I [47]. Consistent with a pro-migratory activity of DDR1, macrophages isolated from DDR1 knockout mice exhibit impaired chemotactic migration in response to MCP-1 [157], and VSM cells derived from these mice exhibit impaired migration toward collagen I, which is restored by expression of DDR1b [30,147,41]. DDR2 also supports the migration of rat VSM cells under hypoxic conditions via the p38 MAPK pathway [77,76] and re-expression of DDR2 in DDR2-deficient murine fibroblasts restores cell migration through Matrigel [128]. Promotion of cell migration by DDR1 was also reported in various cancer cell lines including glioma [100], hepatocarcinoma [158], lung [89], and breast [159,47,96] cancer. In some breast cancer cell lines (T47D and MDA-MB-468), DDR1-dependent promotion of cell migration was ascribed to regulation of the migration suppressor Syk kinase by DDR1 [96]. DDR2 was shown to support the migration of human A375 melanoma cells, SK-HEP hepatoma cells, and HT-29 colon carcinoma cells through collagen I [134].
Other studies showed that DDRs suppress cell migration. Overexpression of DDR1a or DDR1b in MDCK cells markedly reduces collagen I induced migration, in a process that involves inhibition of Stat1 and Stat3 tyrosine phosphorylation [45,56]. Knockdown of DDR1 in MDCK cells or expression of dominant negative DDR1 in the porcine kidney epithelial cells, LLC-PK1, dramatically increases migration towards collagen I [59]. In the hepatoma cell line, Huh7, DDR1a overexpression reduces migration toward collagen I, fibronectin, and Matrigel [151]. In non-malignant human mammary epithelial cells, Wnt-5a inhibits migration possibly through activation of DDR1 [38] while in the breast cancer cell lines MCF7 and MDA-MB-231, DDR1 apparently suppresses migration only when co-expressed with its interacting partner, the phosphoprotein DARPP32 [49]. Interestingly, both Wnt-5a and DARPP32 are highly expressed in non-malignant mammary epithelial cells, while expression is reduced or absent in malignant breast cell lines such as MCF7 and MDA-MB-231 [38,49]. These observations suggest that the effect of DDR1 on cell migration, at least in mammary epithelial cell lines, is dependent on expression of co-factors such as Wnt-5a and DARPP32.
7.2.6 In vitro cell invasion
The ability to invade through ECM and, consequently, to disseminate to other organs is a characteristic of malignant cancer cells. Invasion of ECM is a complex process requiring the activation of multiple genes but eventually depends on the action of key molecules such as ECM receptors and ECM-degrading proteases. Most studies investigating the role of DDRs in invasion utilize Matrigel, a tumor-derived basement membrane extract. Although Matrigel is rich in laminin and collagen IV, two major components of basement membranes, its relevance as a model to study cell invasion has been questioned, particularly due to the lack of a covalently cross-linked collagen IV network, as is the case in natural basement membranes [160]. Therefore, more studies are required to determine the relative contribution of DDRs in models of cell invasion that better mimic the structure and composition of natural ECMs, which may influence DDR signaling. Nevertheless, accumulating evidence using Matrigel as matrix barriers suggests that DDR1 plays a promoting role in invasion of a variety of human cancer cell lines including prostate [110], breast [161], lung [89], pituitary adenoma [102], oral squamous cell carcinoma [54], hepatocellular carcinoma [158,75], and glioma [101,100] cell lines. The pro-invasive activity of DDR1 in the Matrigel assay appears to be mediated by upregulation of MMP expression, in particular MMP-2 and MMP-9, which may contribute to degradation of matrix components [101,100,102,158,161,110,89]. Thus, DDR1 cooperates with the proteolytic machinery of the cells to facilitate invasiveness. However, how DDR1 regulates MMP expression and activity is unknown. Comparison of the respective roles of DDR1a and DDR1b showed that while in glioma cell lines DDR1a but not DDR1b is necessary for cellular invasion [100], both isoforms support the invasion of lung [89] and hepatocellular carcinoma cell lines [158]. The differential effects of DDR1 isoforms on invasion may be due to the activation of distinct signaling networks, which consequently may control invasive activity in a cell type specific and context-dependent manner. However, the role of DDR1 phosphorylation and the downstream effectors involved in regulating DDR1 function in tumor cell invasion have not been studied in detail and remain to be elucidated. A recent study suggested that the ability of DDR1 to support collective cell invasion of human A431 oral squamous cell carcinoma cells does not require receptor signaling and is independent of collagen binding. In these cells, DDR1 supports collective cell migration by its ability to regulate actomyosin contractility at sites of cell-cell junctions via formation of a complex with E-cadherin. Consequently, downregulation of DDR1 leads to a loss of cell-cell adhesion and decreased collective cell invasion [54]. In this mode of invasive behavior, DDR1 may contribute to cell invasion by acting as a scaffolding protein to sustain cell-cell interactions, independent of its ability to signal in response to collagen. Regardless, it is expected that the impact of DDR1 on the invasive phenotype of cancer cells will be rather complex involving both collagen dependent and independent mechanisms and will depend on the cell’s genetic background, the mode of cell invasion (single or collective), and the physical properties and composition of the ECM. DDRs may not act solely to transduce outside signals, but also to actively participate in modification and remodeling of the tumor ECM via their extracellular domains [22,84]. The ability of DDR1 to bind and signal in response to both collagen IV (basement membrane) and collagen I (interstitial matrix) supports the hypothesis that DDR1 plays a role in the transition from in situ to invasive carcinoma, an essential part of the invasion program, particularly during development of EMT. In this regard, the reported switch from DDR1 to DDR2 observed in some cell types upon induction of EMT [69,71] may facilitate the mesenchymal mode of migratory and invasive activities of carcinoma cells through interstitial ECM, which is dominated by fibrillar collagens. However, at present, the contribution of DDR2 in tumor cell invasion has not been reported.
7.2.7 Tumor growth and metastasis
Most studies on DDRs have focused on their role in cancer cell migration and invasion. Thus, there is a significant lack of information on the role the ability of DDRs to regulate cellular transformation and their role in tumor growth and metastasis, particularly in relevant animal models. Up to this writing, three studies have addressed the ability of DDRs to promote tumor growth and metastasis in mice. Downregulation of DDR1 expression in HCT116 human colon cancer cells reduced size and weight of subcutaneous tumors in athymic mice [52]. Similarly, downregulation of DDR1 in human H460 lung large cell carcinoma cells substantially reduced tumor metastasis to bone and significantly decreased bone tumor burden after intratibial injection [90]. Given the osseous abundance of the DDR ligand, collagen, that DDRs play a significant role in tumor bone metastasis is an exciting hypothesis that is beginning to gain credibility [90]. Indeed, this new study has shown, for the first time, that DDR1 promotes homing of tumor cells to the bone, survival and colonization of tumor cells within the osseous environment and increases osteolytic lesions, possibly by promoting osteoclastogenic activity [90]. However, how DDR1 regulates tumor-derived osteoclastogenesis remains unclear. Also, whether these effects of DDR1 are at play in other models of tumor bone metastasis will require further investigation in the future. In the case of DDR2, downregulation in A375 human melanoma cells reduced the amount of tumor burden in livers after intrasplenic inoculation of the cells [134]. Interestingly, while tumor-derived DDR2 appears to support the ability of melanoma to metastasize to the liver [134], host (hepatic) derived DDR2 appears to elicit a metastatic suppressive phenotype [130]. Lack of DDR2 promoted an immune suppressive and profibrogenic response in the liver of DDR2 knockout mice resulting in the generation of a pro-metastatic niche and increased liver metastasis of MCA38 mouse colon cancer cells following intrasplenic inoculation [130]. While the significance of these studies in cancer patients needs to be established, these findings highlight the potential contribution of stromal derived DDRs to cancer progression.
A few studies in human cancer tissues examined the association of DDR expression with disease progression and metastasis. Yang et. al. found that DDR1 expression in 171 samples of non-small cell lung carcinomas significantly correlated with lymph node metastasis and with reduced overall survival [89]. Similarly, DDR2 expression in aneuploidy papillary thyroid carcinomas was found to associate with distant metastasis and death from disease [111]. It is evident that more studies are required to establish the association, and relative contribution of DDRs to cancer progression both in human cancers and in experimental models of cancer. Such studies will have to make a distinction between DDR1 isoforms and DDRs with specific mutations, which may generate different outcomes in different tumor types. Moreover, the use of transgenic and DDR knockout mice with altered expression of known oncogenes and tumor suppressors in different tissues will provide a glimpse into the relationship and cross talk between cancer-initiating genes and DDR signaling at various stages of cancer progression.
8. DDRs as therapeutic targets in cancer
Given the accumulating evidence implicating DDRs in cancer progression, these receptors may represent promising new targets for specific tyrosine kinase inhibitors (TKIs). Approaches that target tyrosine kinase activity and function involve small chemical molecules designed to disrupt intracellular kinase activity or blocking monoclonal antibodies designed to interfere with ectodomain function. Small molecule TKIs are mostly ATP-competitive inhibitors, which bind to either the active (type I inhibitors) or inactive (type II inhibitors) conformation of the kinase thereby interfering with the transfer of the terminal phosphate of ATP to proteins containing a tyrosine residue [162,163]. The conserved structure of the kinase domain represents a significant challenge for the design of small molecule TKIs and thus many of the TKIs used today in the clinic are known to target multiple kinases including DDRs [164–166]. Chemical proteomic profiling studies identified imatinib [165], nilotinib [164], and dasatinib [164], as DDR1 inhibitors (Table 4). While these inhibitors were originally developed to target ABL kinases, they also exhibit potent anti-DDR activity. These TKIs inhibit collagen-induced activation of DDR1b and DDR2 expressed in HEK293 cells with IC50 values in the low nanomolar range [167]. Immobilized dasatinib has also been shown to preferentially capture DDR1 from lung cancer tissues and cell lines [168]. Moreover, dasatinib was shown to inhibit mutated DDR2 in lung cancer cells both in vitro and in vivo [87], suggesting that lung cancer patients harboring mutated DDR2 may benefit from dasatinib therapy. Another small TKI of DDRs is bafetanib (INNO-406), an inhibitor of the BCR-ABL, PDGFR, KIT, and LYN kinases, and a structural analogue of imatinib and nilotinib, [169]. LCB 03-0110, a thienopyridine derivative, was identified from a chemical library using the kinase domain of DDR2, and has been shown to inhibit collagen-induced DDR1 and DDR2 activation in HEK293 cells. However, this compound is also an effective inhibitor for other RTKs [170]. Together, these studies show that DDRs are high-affinity targets of many established and new small molecule TKIs and it is therefore reasonable to assume that some of the anti-tumor activities, as well as some of the side effects [171], of small molecule TKIs currently used in cancer patients are due in part to interference of DDR signaling. However, mutation of the “gatekeeper” residue [163] in the kinase domain of DDR1 did not confer resistance to dasatinib in glioma [172] and lung cancer [168] cells, which are driven by members of the Src family of kinases (which are high-affinity targets of dasatinib). Nonetheless, these dasatinib-resistant DDR “gatekeeper” mutants [172,168] represent new and valuable tools to evaluate the role of DDRs in various cancers. However, assessment of the relative contribution of DDRs to cancer progression will require development of highly selective DDR kinase inhibitors. This effort will require elucidation of the unique structural features of the kinase domain of DDRs and an understanding of their structural/functional dynamics in a cellular context that includes collagen as the ligand. In addition, kinase inhibitors for DDRs will have to be examined in tumors harboring defined DDR mutations [87,114,115,92,93].
Table 4.
Reported DDR inhibitors
| Inhibitor | Type | Target |
|---|---|---|
| Dasatinib [164] | Kinase type I inhibitor | DDR1 and DDR2 |
| Nilotinib [164] | Kinase type II inhibitor | DDR1 and DDR2 |
| Imatinib (Gleevec®) [165] | Kinase type II inhibitor | DDR1 and DDR2 |
| Bafetinib (INNO-406) [169] | Kinase type II inhibitor | DDR1 and DDR2 |
| LCB 03-0110 [170] | thienopyridine derivative | DDR1 and DDR2 |
| Actinomysin D [174] | Antibiotic | DDR2 |
| DDR1 (48B3): sc-21790 [100] | Blocking antibody | DDR1 |
Inhibition of kinase activity is not the only option to block DDR function in cancer. The soluble ectodomains of DDRs have been shown to interfere with endogenous receptor function [156] and to exhibit collagen binding and biological functions independent of membrane anchoring [25,173,22,84,17]. Thus, targeting the ectodomain of DDRs is a viable approach to neutralize receptor function in cancer cells. A search of chemical compounds able to inhibit the interactions of collagen type I with DDR2 expressed in insect cells led to the identification of actinomycin D as an inhibitor of DDR2 [174]. Actinomycin D blocked collagen-induced DDR2 activation in HEK293 cells but had no effect on DDR1 activation. The inhibitory mechanism of DDR2 activation by actinomycin D remains unclear. Although a rational effort to produce function-blocking monoclonal antibodies against DDRs has not been reported, a commercially available monoclonal antibody was shown to block DDR1a-mediated invasion of Matrigel and collagen adhesion of glioma cells [100] (Table 4). However, the epitope of the antibody has not been described.
10 Conclusions
In light of the fundamental aspect of DDR function; to impart cells with the ability to recognize their ECM microenvironment, and their unique characteristics as regulators of cell migration and invasion, it is surprising that these RTKs have received such limited attention by the cancer research community, especially when compared to other cell adhesion receptors such as integrins, and other RTKs. Indeed, many basic aspects of DDR biology remain unknown. Recent discoveries in DDR actions, and the observation that DDRs can be mutated in cancers has raised considerable interest in these receptors, particularly as potential therapeutic targets. Although new and exciting findings in the DDR field are now beginning to emerge, many questions remain unanswered and many challenges lie ahead. A few examples include: 1) What is the profile of DDR expression in various cancer types? 2) Does DDR expression and/or mutational status correlate with disease progression and survival? 3) Can we utilize DDR mutational studies to predict patient outcome and determine treatment? 4) What is the contribution of DDRs (wild type and mutated) to tumor growth and metastasis in preclinical models of cancer? 5) What is the relative functional contribution of DDR1 isoforms in cancer progression? 6) What underlies the unusual kinetics of DDR phosphorylation and what are the signaling pathways activated by wild type and mutated DDRs in response to the different collagen microenvironments? 7) What is the profile of the protein interactome that regulates DDR activity in cancer cells? 8) What are the structural features of DDRs that define their downstream signaling effectors? 9) What are the posttranslational mechanisms (such as trafficking, endocytosis, and proteolysis) that regulate DDR activity in cancer cells? 10) Is there a DDR switch during EMT? 11) Are DDRs viable therapeutic targets in cancer? And if so, what kind of DDR specific inhibitors can we develop? The answers to these questions will shed light into the action of a unique set of RTKs that have the promise to become novel therapeutic targets in cancer.
Acknowledgments
This work is supported by NIH/National Cancer Institute grant CA-61986 and Department of Defense grant PC100274 (to RF), R01 CA125577 and CA107469 (to CGK), Medical Research Council UK grant G0701121 and Biotechnology and Biological Sciences Research Council UK grant BB/IO11226/1 (to BL) and a postdoctoral fellowship from the Karmanos Cancer Institute and Wayne State University, Office of the Vice President for Research (to RRV). We thank Dr. Anjum Sohail and Mr. Richard Arkwright for their helpful comments. This review is dedicated to the memory of Dr. Wolfgang Vogel, who pioneered the DDR field.
Footnotes
The authors declare that they have no conflict of interest.
References
- 1.Johnson JD, Edman JC, Rutter WJ. A receptor tyrosine kinase found in breast carcinoma cells has an extracellular discoidin I-like domain. Proc Natl Acad Sci U S A. 1993;90(22):10891. doi: 10.1073/pnas.90.22.10891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Marco E, Cutuli N, Guerra L, Cancedda R, De Luca M. Molecular cloning of trkE, a novel trk-related putative tyrosine kinase receptor isolated from normal human keratinocytes and widely expressed by normal human tissues. The Journal of biological chemistry. 1993;268(32):24290–24295. [PubMed] [Google Scholar]
- 3.Zerlin M, Julius MA, Goldfarb M. NEP: a novel receptor-like tyrosine kinase expressed in proliferating neuroepithelia. Oncogene. 1993;8(10):2731–2739. [PubMed] [Google Scholar]
- 4.Karn T, Holtrich U, Brauninger A, Bohme B, Wolf G, Rubsamen-Waigmann H, et al. Structure, expression and chromosomal mapping of TKT from man and mouse: a new subclass of receptor tyrosine kinases with a factor VIII-like domain. Oncogene. 1993;8(12):3433–3440. [PubMed] [Google Scholar]
- 5.Perez JL, Shen X, Finkernagel S, Sciorra L, Jenkins NA, Gilbert DJ, et al. Identification and chromosomal mapping of a receptor tyrosine kinase with a putative phospholipid binding sequence in its ectodomain. Oncogene. 1994;9(1):211–219. [PubMed] [Google Scholar]
- 6.Perez JL, Jing SQ, Wong TW. Identification of two isoforms of the Cak receptor kinase that are coexpressed in breast tumor cell lines. Oncogene. 1996;12(7):1469–1477. [PubMed] [Google Scholar]
- 7.Lai C, Lemke G. Structure and expression of the Tyro 10 receptor tyrosine kinase. Oncogene. 1994;9(3):877–883. [PubMed] [Google Scholar]
- 8.Laval S, Butler R, Shelling AN, Hanby AM, Poulsom R, Ganesan TS. Isolation and characterization of an epithelial-specific receptor tyrosine kinase from an ovarian cancer cell line. Cell growth & differentiation: the molecular biology journal of the American Association for Cancer Research. 1994;5(11):1173–1183. [PubMed] [Google Scholar]
- 9.Sanchez MP, Tapley P, Saini SS, He B, Pulido D, Barbacid M. Multiple tyrosine protein kinases in rat hippocampal neurons: isolation of Ptk-3, a receptor expressed in proliferative zones of the developing brain. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(5):1819–1823. doi: 10.1073/pnas.91.5.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alves F, Vogel W, Mossie K, Millauer B, Hofler H, Ullrich A. Distinct structural characteristics of discoidin I subfamily receptor tyrosine kinases and complementary expression in human cancer. Oncogene. 1995;10(3):609–618. [PubMed] [Google Scholar]
- 11.Vogel W, Gish GD, Alves F, Pawson T. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell. 1997;1(1):13–23. doi: 10.1016/s1097-2765(00)80003-9. [DOI] [PubMed] [Google Scholar]
- 12.Shrivastava A, Radziejewski C, Campbell E, Kovac L, McGlynn M, Ryan TE, et al. An orphan receptor tyrosine kinase family whose members serve as nonintegrin collagen receptors. Mol Cell. 1997;1(1):25–34. doi: 10.1016/s1097-2765(00)80004-0. [DOI] [PubMed] [Google Scholar]
- 13.Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141(7):1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiedzierska A, Smietana K, Czepczynska H, Otlewski J. Structural similarities and functional diversity of eukaryotic discoidin-like domains. Biochim Biophys Acta. 2007;1774(9):1069–1078. doi: 10.1016/j.bbapap.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Alexander S, Sydow LM, Wessels D, Soll DR. Discoidin proteins of Dictyostelium are necessary for normal cytoskeletal organization and cellular morphology during aggregation. Differentiation. 1992;51(3):149–161. doi: 10.1111/j.1432-0436.1992.tb00691.x. [DOI] [PubMed] [Google Scholar]
- 16.Baumgartner S, Hofmann K, Chiquet-Ehrismann R, Bucher P. The discoidin domain family revisited: new members from prokaryotes and a homology-based fold prediction. Protein Sci. 1998;7(7):1626–1631. doi: 10.1002/pro.5560070717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leitinger B. Transmembrane collagen receptors. Annual review of cell and developmental biology. 2011;27:265–290. doi: 10.1146/annurev-cellbio-092910-154013. [DOI] [PubMed] [Google Scholar]
- 18.Playford MP, Butler RJ, Wang XC, Katso RM, Cooke IE, Ganesan TS. The genomic structure of discoidin receptor tyrosine kinase. Genome Res. 1996;6(7):620–627. doi: 10.1101/gr.6.7.620. [DOI] [PubMed] [Google Scholar]
- 19.Alves F, Saupe S, Ledwon M, Schaub F, Hiddemann W, Vogel WF. Identification of two novel, kinase-deficient variants of discoidin domain receptor 1: differential expression in human colon cancer cell lines. The FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2001;15(7):1321–1323. doi: 10.1096/fj.00-0626fje. [DOI] [PubMed] [Google Scholar]
- 20.Curat CA, Eck M, Dervillez X, Vogel WF. Mapping of epitopes in discoidin domain receptor 1 critical for collagen binding. J Biol Chem. 2001;276(49):45952–45958. doi: 10.1074/jbc.M104360200. [DOI] [PubMed] [Google Scholar]
- 21.Agarwal G, Kovac L, Radziejewski C, Samuelsson SJ. Binding of discoidin domain receptor 2 to collagen I: an atomic force microscopy investigation. Biochemistry. 2002;41(37):11091–11098. doi: 10.1021/bi020087w. [DOI] [PubMed] [Google Scholar]
- 22.Agarwal G, Mihai C, Iscru DF. Interaction of discoidin domain receptor 1 with collagen type 1. J Mol Biol. 2007;367(2):443–455. doi: 10.1016/j.jmb.2006.12.073. [DOI] [PubMed] [Google Scholar]
- 23.Leitinger B. Molecular analysis of collagen binding by the human discoidin domain receptors, DDR1 and DDR2. Identification of collagen binding sites in DDR2. J Biol Chem. 2003;278(19):16761–16769. doi: 10.1074/jbc.M301370200. [DOI] [PubMed] [Google Scholar]
- 24.Noordeen NA, Carafoli F, Hohenester E, Horton MA, Leitinger B. A transmembrane leucine zipper is required for activation of the dimeric receptor tyrosine kinase DDR1. J Biol Chem. 2006;281(32):22744–22751. doi: 10.1074/jbc.M603233200. [DOI] [PubMed] [Google Scholar]
- 25.Mihai C, Chotani M, Elton TS, Agarwal G. Mapping of DDR1 distribution and oligomerization on the cell surface by FRET microscopy. Journal of molecular biology. 2009;385(2):432–445. doi: 10.1016/j.jmb.2008.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abdulhussein R, Koo DH, Vogel WF. Identification of disulfide-linked dimers of the receptor tyrosine kinase DDR1. The Journal of biological chemistry. 2008;283(18):12026–12033. doi: 10.1074/jbc.M704592200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Konitsiotis AD, Raynal N, Bihan D, Hohenester E, Farndale RW, Leitinger B. Characterization of High Affinity Binding Motifs for the Discoidin Domain Receptor DDR2 in Collagen. J Biol Chem. 2008;283(11):6861–6868. doi: 10.1074/jbc.M709290200. [DOI] [PubMed] [Google Scholar]
- 28.Leitinger B, Steplewski A, Fertala A. The D2 period of collagen II contains a specific binding site for the human discoidin domain receptor, DDR2. J Mol Biol. 2004;344(4):993–1003. doi: 10.1016/j.jmb.2004.09.089. [DOI] [PubMed] [Google Scholar]
- 29.Leitinger B, Kwan AP. The discoidin domain receptor DDR2 is a receptor for type X collagen. Matrix Biol. 2006;25(6):355–364. doi: 10.1016/j.matbio.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 30.Hou G, Vogel W, Bendeck MP. The discoidin domain receptor tyrosine kinase DDR1 in arterial wound repair. J Clin Invest. 2001;107(6):727–735. doi: 10.1172/JCI10720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farndale RW, Lisman T, Bihan D, Hamaia S, Smerling CS, Pugh N, et al. Cell-collagen interactions: the use of peptide Toolkits to investigate collagen-receptor interactions. Biochem Soc Trans. 2008;36(Pt 2):241–250. doi: 10.1042/BST0360241. [DOI] [PubMed] [Google Scholar]
- 32.Xu H, Raynal N, Stathopoulos S, Myllyharju J, Farndale RW, Leitinger B. Collagen binding specificity of the discoidin domain receptors: binding sites on collagens II and III and molecular determinants for collagen IV recognition by DDR1. Matrix biology: journal of the International Society for Matrix Biology. 2011;30(1):16–26. doi: 10.1016/j.matbio.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abdulhussein R, McFadden C, Fuentes-Prior P, Vogel WF. Exploring the collagen-binding site of the DDR1 tyrosine kinase receptor. J Biol Chem. 2004;279(30):31462–31470. doi: 10.1074/jbc.M400651200. [DOI] [PubMed] [Google Scholar]
- 34.Ichikawa O, Osawa M, Nishida N, Goshima N, Nomura N, Shimada I. Structural basis of the collagen-binding mode of discoidin domain receptor 2. Embo J. 2007;26(18):4168–4176. doi: 10.1038/sj.emboj.7601833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carafoli F, Bihan D, Stathopoulos S, Konitsiotis AD, Kvansakul M, Farndale RW, et al. Crystallographic insight into collagen recognition by discoidin domain receptor 2. Structure. 2009;17(12):1573–1581. doi: 10.1016/j.str.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lemeer S, Bluwstein A, Wu Z, Leberfinger J, Muller K, Kramer K, et al. Phosphotyrosine mediated protein interactions of the discoidin domain receptor 1. Journal of proteomics. 2011 doi: 10.1016/j.jprot.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 37.L’Hote CG, Thomas PH, Ganesan TS. Functional analysis of discoidin domain receptor 1: effect of adhesion on DDR1 phosphorylation. Faseb J. 2002;16(2):234–236. doi: 10.1096/fj.01-0414fje. [DOI] [PubMed] [Google Scholar]
- 38.Jonsson M, Andersson T. Repression of Wnt-5a impairs DDR1 phosphorylation and modifies adhesion and migration of mammary cells. J Cell Sci. 2001;114(Pt 11):2043–2053. doi: 10.1242/jcs.114.11.2043. [DOI] [PubMed] [Google Scholar]
- 39.Dejmek J, Dib K, Jonsson M, Andersson T. Wnt-5a and G-protein signaling are required for collagen-induced DDR1 receptor activation and normal mammary cell adhesion. Int J Cancer. 2003;103(3):344–351. doi: 10.1002/ijc.10752. [DOI] [PubMed] [Google Scholar]
- 40.Roarty K, Serra R. Wnt5a is required for proper mammary gland development and TGF-beta-mediated inhibition of ductal growth. Development. 2007;134(21):3929–3939. doi: 10.1242/dev.008250. [DOI] [PubMed] [Google Scholar]
- 41.Lu KK, Trcka D, Bendeck MP. Collagen stimulates discoidin domain receptor 1-mediated migration of smooth muscle cells through Src. Cardiovascular pathology: the official journal of the Society for Cardiovascular Pathology. 2011;20(2):71–76. doi: 10.1016/j.carpath.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 42.Ikeda K, Wang LH, Torres R, Zhao H, Olaso E, Eng FJ, et al. Discoidin domain receptor 2 interacts with Src and Shc following its activation by type I collagen. J Biol Chem. 2002;277(21):19206–19212. doi: 10.1074/jbc.M201078200. [DOI] [PubMed] [Google Scholar]
- 43.Yang K, Kim JH, Kim HJ, Park IS, Kim IY, Yang BS. Tyrosine 740 phosphorylation of discoidin domain receptor 2 by Src stimulates intramolecular autophosphorylation and Shc signaling complex formation. J Biol Chem. 2005;280(47):39058–39066. doi: 10.1074/jbc.M506921200. [DOI] [PubMed] [Google Scholar]
- 44.Koo DH, McFadden C, Huang Y, Abdulhussein R, Friese-Hamim M, Vogel WF. Pinpointing phosphotyrosine-dependent interactions downstream of the collagen receptor DDR1. FEBS letters. 2006;580(1):15–22. doi: 10.1016/j.febslet.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 45.Wang CZ, Su HW, Hsu YC, Shen MR, Tang MJ. A discoidin domain receptor 1/SHP-2 signaling complex inhibits alpha2beta1-integrin-mediated signal transducers and activators of transcription 1/3 activation and cell migration. Mol Biol Cell. 2006;17(6):2839–2852. doi: 10.1091/mbc.E05-11-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang G, Li Q, Ren S, Lu X, Fang L, Zhou W, et al. Proteomic, functional and motif-based analysis of C-terminal Src kinase-interacting proteins. Proteomics. 2009;9(21):4944–4961. doi: 10.1002/pmic.200800762. [DOI] [PubMed] [Google Scholar]
- 47.Huang Y, Arora P, McCulloch CA, Vogel WF. The collagen receptor DDR1 regulates cell spreading and motility by associating with myosin IIA. Journal of cell science. 2009;122(Pt 10):1637–1646. doi: 10.1242/jcs.046219. [DOI] [PubMed] [Google Scholar]
- 48.Shintani Y, Fukumoto Y, Chaika N, Svoboda R, Wheelock MJ, Johnson KR. Collagen I-mediated up-regulation of N-cadherin requires cooperative signals from integrins and discoidin domain receptor 1. J Cell Biol. 2008;180(6):1277–1289. doi: 10.1083/jcb.200708137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hansen C, Greengard P, Nairn AC, Andersson T, Vogel WF. Phosphorylation of DARPP-32 regulates breast cancer cell migration downstream of the receptor tyrosine kinase DDR1. Experimental cell research. 2006;312(20):4011–4018. doi: 10.1016/j.yexcr.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 50.Hilton HN, Stanford PM, Harris J, Oakes SR, Kaplan W, Daly RJ, et al. KIBRA interacts with discoidin domain receptor 1 to modulate collagen-induced signalling. Biochim Biophys Acta. 2008;1783(3):383–393. doi: 10.1016/j.bbamcr.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 51.Dejmek J, Leandersson K, Manjer J, Bjartell A, Emdin SO, Vogel WF, et al. Expression and signaling activity of Wnt-5a/discoidin domain receptor-1 and Syk plays distinct but decisive roles in breast cancer patient survival. Clin Cancer Res. 2005;11(2 Pt 1):520–528. [PubMed] [Google Scholar]
- 52.Kim HG, Hwang SY, Aaronson SA, Mandinova A, Lee SW. DDR1 Receptor Tyrosine Kinase Promotes Prosurvival Pathway through Notch1 Activation. The Journal of biological chemistry. 2011;286(20):17672–17681. doi: 10.1074/jbc.M111.236612. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 53.Eswaramoorthy R, Wang CK, Chen WC, Tang MJ, Ho ML, Hwang CC, et al. DDR1 regulates the stabilization of cell surface E-cadherin and E-cadherin-mediated cell aggregation. Journal of cellular physiology. 2010;224(2):387–397. doi: 10.1002/jcp.22134. [DOI] [PubMed] [Google Scholar]
- 54.Hidalgo-Carcedo C, Hooper S, Chaudhry SI, Williamson P, Harrington K, Leitinger B, et al. Collective cell migration requires suppression of actomyosin at cell-cell contacts mediated by DDR1 and the cell polarity regulators Par3 and Par6. Nature cell biology. 2011;13(1):49–58. doi: 10.1038/ncb2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Olaso E, Lin HC, Wang LH, Friedman SL. Impaired dermal wound healing in discoidin domain receptor 2-deficient mice associated with defective extracellular matrix remodeling. Fibrogenesis & tissue repair. 2011;4(1):5. doi: 10.1186/1755-1536-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang CZ, Hsu YM, Tang MJ. Function of discoidin domain receptor I in HGF-induced branching tubulogenesis of MDCK cells in collagen gel. J Cell Physiol. 2005;203(1):295–304. doi: 10.1002/jcp.20227. [DOI] [PubMed] [Google Scholar]
- 57.Wang CZ, Yeh YC, Tang MJ. DDR1/E-cadherin complex regulates the activation of DDR1 and cell spreading. American journal of physiology Cell physiology. 2009;297(2):C419–429. doi: 10.1152/ajpcell.00101.2009. [DOI] [PubMed] [Google Scholar]
- 58.Yeh YC, Wang CZ, Tang MJ. Discoidin domain receptor 1 activation suppresses alpha2beta1 integrin-dependent cell spreading through inhibition of Cdc42 activity. Journal of cellular physiology. 2009;218(1):146–156. doi: 10.1002/jcp.21578. [DOI] [PubMed] [Google Scholar]
- 59.Yeh YC, Wu CC, Wang YK, Tang MJ. DDR1 triggers epithelial cell differentiation by promoting cell adhesion through stabilization of E-cadherin. Molecular biology of the cell. 2011;22(7):940–953. doi: 10.1091/mbc.E10-08-0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vonk LA, Doulabi BZ, Huang C, Helder MN, Everts V, Bank RA. Collagen-induced expression of collagenase-3 by primary chondrocytes is mediated by integrin α1 and discoidin domain receptor 2: a protein kinase C-dependent pathway. Rheumatology. 2011;50(3):463–472. doi: 10.1093/rheumatology/keq305. [DOI] [PubMed] [Google Scholar]
- 61.Zhang Y, Su J, Yu J, Bu X, Ren T, Liu X, et al. An essential role of discoidin domain receptor 2 (DDR2) in osteoblast differentiation and chondrocyte maturation via modulation of Runx2 activation. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2011;26(3):604–617. doi: 10.1002/jbmr.225. [DOI] [PubMed] [Google Scholar]
- 62.Lin KL, Chou CH, Hsieh SC, Hwa SY, Lee MT, Wang FF. Transcriptional upregulation of DDR2 by ATF4 facilitates osteoblastic differentiation through p38 MAPK-mediated Runx2 activation. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2010;25(11):2489–2503. doi: 10.1002/jbmr.159. [DOI] [PubMed] [Google Scholar]
- 63.Ongusaha PP, Kim JI, Fang L, Wong TW, Yancopoulos GD, Aaronson SA, et al. p53 induction and activation of DDR1 kinase counteract p53-mediated apoptosis and influence p53 regulation through a positive feedback loop. Embo J. 2003;22(6):1289–1301. doi: 10.1093/emboj/cdg129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Das S, Ongusaha PP, Yang YS, Park JM, Aaronson SA, Lee SW. Discoidin domain receptor 1 receptor tyrosine kinase induces cyclooxygenase-2 and promotes chemoresistance through nuclear factor-kappaB pathway activation. Cancer Res. 2006;66(16):8123–8130. doi: 10.1158/0008-5472.CAN-06-1215. [DOI] [PubMed] [Google Scholar]
- 65.Vogel W, Brakebusch C, Fassler R, Alves F, Ruggiero F, Pawson T. Discoidin domain receptor 1 is activated independently of beta(1) integrin. J Biol Chem. 2000;275(8):5779–5784. doi: 10.1074/jbc.275.8.5779. [DOI] [PubMed] [Google Scholar]
- 66.Suh HN, Han HJ. Collagen I regulates the self-renewal of mouse embryonic stem cells through alpha2beta1 integrin- and DDR1-dependent Bmi-1. Journal of cellular physiology. 2011 doi: 10.1002/jcp.22697. [DOI] [PubMed] [Google Scholar]
- 67.Chetoui N, El Azreq MA, Boisvert M, Bergeron ME, Aoudjit F. Discoidin domain receptor 1 expression in activated T cells is regulated by the ERK MAP kinase signaling pathway. Journal of cellular biochemistry. 2011;112(12):3666–3674. doi: 10.1002/jcb.23300. [DOI] [PubMed] [Google Scholar]
- 68.Ruiz PA, Jarai G. Collagen I induces discoidin domain receptor (DDR) 1 expression through DDR2 and a JAK2-ERK1/2-mediated mechanism in primary human lung fibroblasts. The Journal of biological chemistry. 2011;286(15):12912–12923. doi: 10.1074/jbc.M110.143693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maeyama M, Koga H, Selvendiran K, Yanagimoto C, Hanada S, Taniguchi E, et al. Switching in discoid domain receptor expressions in SLUG-induced epithelial-mesenchymal transition. Cancer. 2008;113(10):2823–2831. doi: 10.1002/cncr.23900. [DOI] [PubMed] [Google Scholar]
- 70.Camara J, Jarai G. Epithelial-mesenchymal transition in primary human bronchial epithelial cells is Smad-dependent and enhanced by fibronectin and TNF-alpha. Fibrogenesis & tissue repair. 2010;3(1):2. doi: 10.1186/1755-1536-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Taube JH, Herschkowitz JI, Komurov K, Zhou AY, Gupta S, Yang J, et al. Core epithelial-to-mesenchymal transition interactome gene-expression signature is associated with claudin-low and metaplastic breast cancer subtypes. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(35):15449–15454. doi: 10.1073/pnas.1004900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sakuma S, Saya H, Tada M, Nakao M, Fujiwara T, Roth JA, et al. Receptor protein tyrosine kinase DDR is up-regulated by p53 protein. FEBS Lett. 1996;398(2–3):165–169. doi: 10.1016/s0014-5793(96)01234-3. [DOI] [PubMed] [Google Scholar]
- 73.Martinez-Marignac VL, Rodrigue A, Davidson D, Couillard M, Al-Moustafa AE, Abramovitz M, et al. The effect of a DNA repair gene on cellular invasiveness: XRCC3 over-expression in breast cancer cells. PloS one. 2011;6(1):e16394. doi: 10.1371/journal.pone.0016394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Roig B, Moyano S, Martorell L, Costas J, Vilella E. The Discoidin domain receptor 1 gene has a functional A2RE sequence. Journal of neurochemistry. 2011 doi: 10.1111/j.1471-4159.2011.07580.x. [DOI] [PubMed] [Google Scholar]
- 75.Shen Q, Cicinnati VR, Zhang X, Iacob S, Weber F, Sotiropoulos GC, et al. Role of microRNA-199a-5p and discoidin domain receptor 1 in human hepatocellular carcinoma invasion. Molecular cancer. 2010;9:227. doi: 10.1186/1476-4598-9-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen SC, Wang BW, Wang DL, Shyu KG. Hypoxia induces discoidin domain receptor-2 expression via the p38 pathway in vascular smooth muscle cells to increase their migration. Biochemical and biophysical research communications. 2008;374(4):662–667. doi: 10.1016/j.bbrc.2008.07.092. [DOI] [PubMed] [Google Scholar]
- 77.Shyu KG, Wang BW, Chang H. Hyperbaric oxygen activates discoidin domain receptor 2 via tumour necrosis factor-alpha and the p38 MAPK pathway to increase vascular smooth muscle cell migration through matrix metalloproteinase 2. Clinical science. 2009;116(7):575–583. doi: 10.1042/CS20080215. [DOI] [PubMed] [Google Scholar]
- 78.Chua HH, Yeh TH, Wang YP, Huang YT, Sheen TS, Lo YC, et al. Upregulation of discoidin domain receptor 2 in nasopharyngeal carcinoma. Head Neck. 2008;30(4):427–436. doi: 10.1002/hed.20724. [DOI] [PubMed] [Google Scholar]
- 79.Olaso E, Ikeda K, Eng FJ, Xu L, Wang LH, Lin HC, et al. DDR2 receptor promotes MMP-2-mediated proliferation and invasion by hepatic stellate cells. J Clin Invest. 2001;108(9):1369–1378. doi: 10.1172/JCI12373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sekiya Y, Ogawa T, Yoshizato K, Ikeda K, Kawada N. Suppression of hepatic stellate cell activation by microRNA-29b. Biochemical and biophysical research communications. 2011;412(1):74–79. doi: 10.1016/j.bbrc.2011.07.041. [DOI] [PubMed] [Google Scholar]
- 81.van Kilsdonk JW, van Kempen LC, van Muijen GN, Ruiter DJ, Swart GW. Soluble adhesion molecules in human cancers: sources and fates. European journal of cell biology. 2010;89(6):415–427. doi: 10.1016/j.ejcb.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 82.Vogel WF. Ligand-induced shedding of discoidin domain receptor 1. FEBS Lett. 2002;514(2–3):175–180. doi: 10.1016/s0014-5793(02)02360-8. [DOI] [PubMed] [Google Scholar]
- 83.Slack BE, Siniaia MS, Blusztajn JK. Collagen type I selectively activates ectodomain shedding of the discoidin domain receptor 1: involvement of Src tyrosine kinase. J Cell Biochem. 2006;98(3):672–684. doi: 10.1002/jcb.20812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Flynn LA, Blissett AR, Calomeni EP, Agarwal G. Inhibition of collagen fibrillogenesis by cells expressing soluble extracellular domains of DDR1 and DDR2. Journal of molecular biology. 2010;395(3):533–543. doi: 10.1016/j.jmb.2009.10.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lund AW, Stegemann JP, Plopper GE. Mesenchymal Stem Cells Sense Three Dimensional Type I Collagen through Discoidin Domain Receptor 1. The open stem cell journal. 2009;1:40–53. doi: 10.2174/1876893800901010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Torkamani A, Verkhivker G, Schork NJ. Cancer driver mutations in protein kinase genes. Cancer letters. 2009;281(2):117–127. doi: 10.1016/j.canlet.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hammerman PS, et al. Mutations in the DDR2 Kinase Gene Identify a Novel Therapeutic Target in Squamous Cell Lung Cancer. Cancer Discovery. 2011;1(1):78–89. doi: 10.1158/2159-8274.CD-11-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ford CE, Lau SK, Zhu CQ, Andersson T, Tsao MS, Vogel WF. Expression and mutation analysis of the discoidin domain receptors 1 and 2 in non-small cell lung carcinoma. Br J Cancer. 2007;96(5):808–814. doi: 10.1038/sj.bjc.6603614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang SH, Baek HA, Lee HJ, Park HS, Jang KY, Kang MJ, et al. Discoidin domain receptor 1 is associated with poor prognosis of non-small cell lung carcinomas. Oncology reports. 2010;24(2):311–319. doi: 10.3892/or_00000861. [DOI] [PubMed] [Google Scholar]
- 90.Valencia K, Ormazabal C, Zandueta C, Luis-Ravelo D, Anton I, Pajares MJ, et al. Inhibition of collagen receptor Discoidin Domain Receptor-1 (DDR1) Reduces Cell Survival, Homing and Colonization in Lung Cancer Bone Metastasis. Clinical cancer research: an official journal of the American Association for Cancer Research. 2012 doi: 10.1158/1078-0432.CCR-11-1686. [DOI] [PubMed] [Google Scholar]
- 91.Rikova K, Guo A, Zeng Q, Possemato A, Yu J, Haack H, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131(6):1190–1203. doi: 10.1016/j.cell.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 92.Davies H, Hunter C, Smith R, Stephens P, Greenman C, Bignell G, et al. Somatic mutations of the protein kinase gene family in human lung cancer. Cancer research. 2005;65(17):7591–7595. doi: 10.1158/0008–5472.CAN-05–1855. [DOI] [PubMed] [Google Scholar]
- 93.Ding L, Getz G, Wheeler DA, Mardis ER, McLellan MD, Cibulskis K, et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;455(7216):1069–1075. doi: 10.1038/nature07423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vogel WF, Aszodi A, Alves F, Pawson T. Discoidin domain receptor 1 tyrosine kinase has an essential role in mammary gland development. Mol Cell Biol. 2001;21(8):2906–2917. doi: 10.1128/MCB.21.8.2906-2917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Barker KT, Martindale JE, Mitchell PJ, Kamalati T, Page MJ, Phippard DJ, et al. Expression patterns of the novel receptor-like tyrosine kinase, DDR, in human breast tumours. Oncogene. 1995;10(3):569–575. [PubMed] [Google Scholar]
- 96.Neuhaus B, Buhren S, Bock B, Alves F, Vogel WF, Kiefer F. Migration inhibition of mammary epithelial cells by Syk is blocked in the presence of DDR1 receptors. Cellular and molecular life sciences: CMLS. 2011 doi: 10.1007/s00018-011-0676-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Turashvili G, Bouchal J, Baumforth K, Wei W, Dziechciarkova M, Ehrmann J, et al. Novel markers for differentiation of lobular and ductal invasive breast carcinomas by laser microdissection and microarray analysis. BMC cancer. 2007;7:55. doi: 10.1186/1471-2407-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Weiner HL, Rothman M, Miller DC, Ziff EB. Pediatric brain tumors express multiple receptor tyrosine kinases including novel cell adhesion kinases. Pediatric neurosurgery. 1996;25(2):64–71. doi: 10.1159/000121099. ; discussion 71–62. [DOI] [PubMed] [Google Scholar]
- 99.Weiner HL, Huang H, Zagzag D, Boyce H, Lichtenbaum R, Ziff EB. Consistent and selective expression of the discoidin domain receptor-1 tyrosine kinase in human brain tumors. Neurosurgery. 2000;47(6):1400–1409. [PubMed] [Google Scholar]
- 100.Ram R, Lorente G, Nikolich K, Urfer R, Foehr E, Nagavarapu U. Discoidin domain receptor-1a (DDR1a) promotes glioma cell invasion and adhesion in association with matrix metalloproteinase-2. J Neurooncol. 2006;76(3):239–248. doi: 10.1007/s11060-005-6874-1. [DOI] [PubMed] [Google Scholar]
- 101.Yamanaka R, Arao T, Yajima N, Tsuchiya N, Homma J, Tanaka R, et al. Identification of expressed genes characterizing long-term survival in malignant glioma patients. Oncogene. 2006;25(44):5994–6002. doi: 10.1038/sj.onc.1209585. [DOI] [PubMed] [Google Scholar]
- 102.Yoshida D, Teramoto A. Enhancement of pituitary adenoma cell invasion and adhesion is mediated by discoidin domain receptor-1. J Neurooncol. 2007;82(1):29–40. doi: 10.1007/s11060-006-9246-6. [DOI] [PubMed] [Google Scholar]
- 103.Heinzelmann-Schwarz VA, Gardiner-Garden M, Henshall SM, Scurry J, Scolyer RA, Davies MJ, et al. Overexpression of the cell adhesion molecules DDR1, Claudin 3, and Ep-CAM in metaplastic ovarian epithelium and ovarian cancer. Clin Cancer Res. 2004;10(13):4427–4436. doi: 10.1158/1078-0432.CCR-04-0073. [DOI] [PubMed] [Google Scholar]
- 104.Quan J, Yahata T, Adachi S, Yoshihara K, Tanaka K. Identification of Receptor Tyrosine Kinase, Discoidin Domain Receptor 1 (DDR1), as a Potential Biomarker for Serous Ovarian Cancer. International journal of molecular sciences. 2011;12(2):971–982. doi: 10.3390/ijms12020971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Colas E, Perez C, Cabrera S, Pedrola N, Monge M, Castellvi J, et al. Molecular markers of endometrial carcinoma detected in uterine aspirates. International journal of cancer Journal international du cancer. 2011;129(10):2435–2444. doi: 10.1002/ijc.25901. [DOI] [PubMed] [Google Scholar]
- 106.Nemoto T, Ohashi K, Akashi T, Johnson JD, Hirokawa K. Overexpression of protein tyrosine kinases in human esophageal cancer. Pathobiology. 1997;65(4):195–203. doi: 10.1159/000164123. [DOI] [PubMed] [Google Scholar]
- 107.Squire JA, Bayani J, Luk C, Unwin L, Tokunaga J, MacMillan C, et al. Molecular cytogenetic analysis of head and neck squamous cell carcinoma: By comparative genomic hybridization, spectral karyotyping, and expression array analysis. Head Neck. 2002;24(9):874–887. doi: 10.1002/hed.10122. [DOI] [PubMed] [Google Scholar]
- 108.Gu TL, Deng X, Huang F, Tucker M, Crosby K, Rimkunas V, et al. Survey of tyrosine kinase signaling reveals ROS kinase fusions in human cholangiocarcinoma. PloS one. 2011;6(1):e15640. doi: 10.1371/journal.pone.0015640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Couvelard A, Hu J, Steers G, O’Toole D, Sauvanet A, Belghiti J, et al. Identification of potential therapeutic targets by gene-expression profiling in pancreatic endocrine tumors. Gastroenterology. 2006;131(5):1597–1610. doi: 10.1053/j.gastro.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 110.Shimada K, Nakamura M, Ishida E, Higuchi T, Yamamoto H, Tsujikawa K, et al. Prostate cancer antigen-1 contributes to cell survival and invasion though discoidin receptor 1 in human prostate cancer. Cancer Sci. 2008;99(1):39–45. doi: 10.1111/j.1349-7006.2007.00655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rodrigues R, Roque L, Espadinha C, Pinto A, Domingues R, Dinis J, et al. Comparative genomic hybridization, BRAF, RAS, RET, and oligo-array analysis in aneuploid papillary thyroid carcinomas. Oncol Rep. 2007;18(4):917–926. [PubMed] [Google Scholar]
- 112.Hajdu M, Singer S, Maki RG, Schwartz GK, Keohan ML, Antonescu CR. IGF2 over-expression in solitary fibrous tumours is independent of anatomical location and is related to loss of imprinting. The Journal of pathology. 2010;221(3):300–307. doi: 10.1002/path.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chiaretti S, Li X, Gentleman R, Vitale A, Wang KS, Mandelli F, et al. Gene expression profiles of B-lineage adult acute lymphocytic leukemia reveal genetic patterns that identify lineage derivation and distinct mechanisms of transformation. Clinical cancer research: an official journal of the American Association for Cancer Research. 2005;11(20):7209–7219. doi: 10.1158/1078-0432.CCR-04-2165. [DOI] [PubMed] [Google Scholar]
- 114.Tomasson MH, Xiang Z, Walgren R, Zhao Y, Kasai Y, Miner T, et al. Somatic mutations and germline sequence variants in the expressed tyrosine kinase genes of patients with de novo acute myeloid leukemia. Blood. 2008;111(9):4797–4808. doi: 10.1182/blood-2007-09-113027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Loriaux MM, Levine RL, Tyner JW, Frohling S, Scholl C, Stoffregen EP, et al. High-throughput sequence analysis of the tyrosine kinome in acute myeloid leukemia. Blood. 2008;111(9):4788–4796. doi: 10.1182/blood-2007-07-101394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Renne C, Willenbrock K, Kuppers R, Hansmann ML, Brauninger A. Autocrine- and paracrine-activated receptor tyrosine kinases in classic Hodgkin lymphoma. Blood. 2005;105(10):4051–4059. doi: 10.1182/blood-2004-10-4008. [DOI] [PubMed] [Google Scholar]
- 117.Willenbrock K, Kuppers R, Renne C, Brune V, Eckerle S, Weidmann E, et al. Common features and differences in the transcriptome of large cell anaplastic lymphoma and classical Hodgkin’s lymphoma. Haematologica. 2006;91(5):596–604. [PubMed] [Google Scholar]
- 118.Labrador JP, Azcoitia V, Tuckermann J, Lin C, Olaso E, Manes S, et al. The collagen receptor DDR2 regulates proliferation and its elimination leads to dwarfism. EMBO Rep. 2001;2(5):446–452. doi: 10.1093/embo-reports/kve094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bargal R, Cormier-Daire V, Ben-Neriah Z, Le Merrer M, Sosna J, Melki J, et al. Mutations in DDR2 gene cause SMED with short limbs and abnormal calcifications. American journal of human genetics. 2009;84(1):80–84. doi: 10.1016/j.ajhg.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ali BR, Xu H, Akawi NA, John A, Karuvantevida NS, Langer R, et al. Trafficking defects and loss of ligand binding are the underlying causes of all reported DDR2 missense mutations found in SMED-SL patients. Human molecular genetics. 2010;19(11):2239–2250. doi: 10.1093/hmg/ddq103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kano K, Marin de Evsikova C, Young J, Wnek C, Maddatu TP, Nishina PM, et al. A novel dwarfism with gonadal dysfunction due to loss-of-function allele of the collagen receptor gene, Ddr2, in the mouse. Molecular endocrinology. 2008;22(8):1866–1880. doi: 10.1210/me.2007–0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Matsumura H, Kano K, Marin de Evsikova C, Young JA, Nishina PM, Naggert JK, et al. Transcriptome analysis reveals an unexpected role of a collagen tyrosine kinase receptor gene, Ddr2, as a regulator of ovarian function. Physiological genomics. 2009;39(2):120–129. doi: 10.1152/physiolgenomics.00073.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kano K, Kitamura A, Matsuwaki T, Morimatsu M, Naito K. Discoidin domain receptor 2 (DDR2) is required for maintenance of spermatogenesis in male mice. Molecular reproduction and development. 2010;77(1):29–37. doi: 10.1002/mrd.21093. [DOI] [PubMed] [Google Scholar]
- 124.Faraci-Orf E, McFadden C, Vogel WF. DDR1 signaling is essential to sustain Stat5 function during lactogenesis. Journal of cellular biochemistry. 2006;97(1):109–121. doi: 10.1002/jcb.20618. [DOI] [PubMed] [Google Scholar]
- 125.Gross O, Beirowski B, Harvey SJ, McFadden C, Chen D, Tam S, et al. DDR1-deficient mice show localized subepithelial GBM thickening with focal loss of slit diaphragms and proteinuria. Kidney Int. 2004;66(1):102–111. doi: 10.1111/j.1523-1755.2004.00712.x. [DOI] [PubMed] [Google Scholar]
- 126.Curat CA, Vogel WF. Discoidin domain receptor 1 controls growth and adhesion of mesangial cells. J Am Soc Nephrol. 2002;13(11):2648–2656. doi: 10.1097/01.asn.0000032419.13208.0c. [DOI] [PubMed] [Google Scholar]
- 127.Meyer zum Gottesberge AM, Gross O, Becker-Lendzian U, Massing T, Vogel WF. Inner ear defects and hearing loss in mice lacking the collagen receptor DDR1. Lab Invest. 2008;88(1):27–37. doi: 10.1038/labinvest.3700692. [DOI] [PubMed] [Google Scholar]
- 128.Olaso E, Labrador JP, Wang L, Ikeda K, Eng FJ, Klein R, et al. Discoidin domain receptor 2 regulates fibroblast proliferation and migration through the extracellular matrix in association with transcriptional activation of matrix metalloproteinase-2. J Biol Chem. 2002;277(5):3606–3613. doi: 10.1074/jbc.M107571200. [DOI] [PubMed] [Google Scholar]
- 129.Olaso E, Arteta B, Benedicto A, Crende O, Friedman SL. Loss of Discoidin Domain Receptor 2 Promotes Hepatic Fibrosis after Chronic Carbon Tetrachloride through Altered Paracrine Interactions between Hepatic Stellate Cells and Liver-Associated Macrophages. The American journal of pathology. 2011;179(6):2894–2904. doi: 10.1016/j.ajpath.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Badiola I, Olaso E, Crende O, Friedman SL, Vidal-Vanaclocha F. Discoidin domain receptor 2 deficiency predisposes hepatic tissue to colon carcinoma metastasis. Gut. 2011 doi: 10.1136/gutjnl-2011–300810. [DOI] [PubMed] [Google Scholar]
- 131.Roberts ME, Magowan L, Hall IP, Johnson SR. Discoidin domain receptor 1 regulates bronchial epithelial repair and matrix metalloproteinase production. The European respiratory journal: official journal of the European Society for Clinical Respiratory Physiology. 2011;37(6):1482–1493. doi: 10.1183/09031936.00039710. [DOI] [PubMed] [Google Scholar]
- 132.Dang N, Hu J, Liu X, Li X, Ji S, Zhang W, et al. CD167 acts as a novel costimulatory receptor in T-cell activation. Journal of immunotherapy. 2009;32(8):773–784. doi: 10.1097/CJI.0b013e3181acea46. [DOI] [PubMed] [Google Scholar]
- 133.Shyu KG, Wang BW, Kuan P, Chang H. RNA interference for discoidin domain receptor 2 attenuates neointimal formation in balloon injured rat carotid artery. Arteriosclerosis, thrombosis, and vascular biology. 2008;28(8):1447–1453. doi: 10.1161/ATVBAHA.108.165993. [DOI] [PubMed] [Google Scholar]
- 134.Badiola I, Villace P, Basaldua I, Olaso E. Downregulation of discoidin domain receptor 2 in A375 human melanoma cells reduces its experimental liver metastasis ability. Oncology reports. 2011;26(4):971–978. doi: 10.3892/or.2011.1356. [DOI] [PubMed] [Google Scholar]
- 135.Franco C, Ahmad PJ, Hou G, Wong E, Bendeck MP. Increased cell and matrix accumulation during atherogenesis in mice with vessel wall-specific deletion of discoidin domain receptor 1. Circulation research. 2010;106(11):1775–1783. doi: 10.1161/CIRCRESAHA.109.213637. [DOI] [PubMed] [Google Scholar]
- 136.Wall SJ, Werner E, Werb Z, DeClerck YA. Discoidin domain receptor 2 mediates tumor cell cycle arrest induced by fibrillar collagen. J Biol Chem. 2005;280(48):40187–40194. doi: 10.1074/jbc.M508226200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bhatt RS, Tomoda T, Fang Y, Hatten ME. Discoidin domain receptor 1 functions in axon extension of cerebellar granule neurons. Genes Dev. 2000;14(17):2216–2228. doi: 10.1101/gad.821600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Suzuki N, Ando S, Sumida K, Horie N, Saito K. Analysis of altered gene expression specific to embryotoxic chemical treatment during embryonic stem cell differentiation into myocardiac and neural cells. The Journal of toxicological sciences. 2011;36(5):569–585. doi: 10.2131/jts.36.569. [DOI] [PubMed] [Google Scholar]
- 139.Franco-Pons N, Tomas J, Roig B, Auladell C, Martorell L, Vilella E. Discoidin domain receptor 1, a tyrosine kinase receptor, is upregulated in an experimental model of remyelination and during oligodendrocyte differentiation in vitro. Journal of molecular neuroscience: MN. 2009;38(1):2–11. doi: 10.1007/s12031-008-9151-x. [DOI] [PubMed] [Google Scholar]
- 140.Zurakowski H, Gagnon A, Landry A, Layne MD, Sorisky A. Discoidin domain receptor 2 impairs insulin-stimulated insulin receptor substrate-1 tyrosine phosphorylation and glucose uptake in 3T3-L1 adipocytes. Horm Metab Res. 2007;39(8):575–581. doi: 10.1055/s-2007-985132. [DOI] [PubMed] [Google Scholar]
- 141.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 142.Goldsmith EC, Zhang X, Watson J, Hastings J, Potts JD. The collagen receptor DDR2 is expressed during early cardiac development. Anatomical record. 2010;293(5):762–769. doi: 10.1002/ar.20922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhou B, von Gise A, Ma Q, Hu YW, Pu WT. Genetic fate mapping demonstrates contribution of epicardium-derived cells to the annulus fibrosis of the mammalian heart. Developmental biology. 2010;338(2):251–261. doi: 10.1016/j.ydbio.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Walsh LA, Nawshad A, Medici D. Discoidin domain receptor 2 is a critical regulator of epithelial-mesenchymal transition. Matrix biology: journal of the International Society for Matrix Biology. 2011;30(4):243–247. doi: 10.1016/j.matbio.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Eger A, Aigner K, Sonderegger S, Dampier B, Oehler S, Schreiber M, et al. DeltaEF1 is a transcriptional repressor of E-cadherin and regulates epithelial plasticity in breast cancer cells. Oncogene. 2005;24(14):2375–2385. doi: 10.1038/sj.onc.1208429. [DOI] [PubMed] [Google Scholar]
- 146.Blick T, Hugo H, Widodo E, Waltham M, Pinto C, Mani SA, et al. Epithelial mesenchymal transition traits in human breast cancer cell lines parallel the CD44(hi/)CD24 (lo/-) stem cell phenotype in human breast cancer. Journal of mammary gland biology and neoplasia. 2010;15(2):235–252. doi: 10.1007/s10911-010-9175-z. [DOI] [PubMed] [Google Scholar]
- 147.Hou G, Vogel WF, Bendeck MP. Tyrosine kinase activity of discoidin domain receptor 1 is necessary for smooth muscle cell migration and matrix metalloproteinase expression. Circ Res. 2002;90(11):1147–1149. doi: 10.1161/01.res.0000022166.74073.f8. [DOI] [PubMed] [Google Scholar]
- 148.Kamohara H, Yamashiro S, Galligan C, Yoshimura T. Discoidin domain receptor 1 isoform-a (DDR1alpha) promotes migration of leukocytes in three-dimensional collagen lattices. Faseb J. 2001;15(14):2724–2726. doi: 10.1096/fj.01-0359fje. [DOI] [PubMed] [Google Scholar]
- 149.Fukunaga-Kalabis M, Martinez G, Liu ZJ, Kalabis J, Mrass P, Weninger W, et al. CCN3 controls 3D spatial localization of melanocytes in the human skin through DDR1. J Cell Biol. 2006;175(4):563–569. doi: 10.1083/jcb.200602132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Franco C, Britto K, Wong E, Hou G, Zhu SN, Chen M, et al. Discoidin domain receptor 1 on bone marrow-derived cells promotes macrophage accumulation during atherogenesis. Circulation research. 2009;105(11):1141–1148. doi: 10.1161/CIRCRESAHA.109.207357. [DOI] [PubMed] [Google Scholar]
- 151.Song S, Shackel NA, Wang XM, Ajami K, McCaughan GW, Gorrell MD. Discoidin domain receptor 1: isoform expression and potential functions in cirrhotic human liver. The American journal of pathology. 2011;178(3):1134–1144. doi: 10.1016/j.ajpath.2010.11.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hou G, Wang D, Bendeck MP. Deletion of discoidin domain receptor 2 does not affect smooth muscle cell adhesion, migration, or proliferation in response to type I collagen. Cardiovascular pathology: the official journal of the Society for Cardiovascular Pathology. 2011 doi: 10.1016/j.carpath.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 153.Bhadriraju K, Chung KH, Spurlin TA, Haynes RJ, Elliott JT, Plant AL. The relative roles of collagen adhesive receptor DDR2 activation and matrix stiffness on the downregulation of focal adhesion kinase in vascular smooth muscle cells. Biomaterials. 2009;30(35):6687–6694. doi: 10.1016/j.biomaterials.2009.08.036. [DOI] [PubMed] [Google Scholar]
- 154.Zamir E, Geiger B. Components of cell-matrix adhesions. Journal of cell science. 2001;114(Pt 20):3577–3579. doi: 10.1242/jcs.114.20.3577. [DOI] [PubMed] [Google Scholar]
- 155.Etienne J, Duperray A. Initial dynamics of cell spreading are governed by dissipation in the actin cortex. Biophysical journal. 2011;101(3):611–621. doi: 10.1016/j.bpj.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hachehouche LN, Chetoui N, Aoudjit F. Implication of discoidin domain receptor 1 in T cell migration in three-dimensional collagen. Molecular immunology. 2010;47(9):1866–1869. doi: 10.1016/j.molimm.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 157.Guerrot D, Kerroch M, Placier S, Vandermeersch S, Trivin C, Mael-Ainin M, et al. Discoidin domain receptor 1 is a major mediator of inflammation and fibrosis in obstructive nephropathy. The American journal of pathology. 2011;179(1):83–91. doi: 10.1016/j.ajpath.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Park HS, Kim KR, Lee HJ, Choi HN, Kim DK, Kim BT, et al. Overexpression of discoidin domain receptor 1 increases the migration and invasion of hepatocellular carcinoma cells in association with matrix metalloproteinase. Oncol Rep. 2007;18(6):1435–1441. [PubMed] [Google Scholar]
- 159.Castro-Sanchez L, Soto-Guzman A, Navarro-Tito N, Martinez-Orozco R, Salazar EP. Native type IV collagen induces cell migration through a CD9 and DDR1-dependent pathway in MDA-MB-231 breast cancer cells. European journal of cell biology. 2010;89(11):843–852. doi: 10.1016/j.ejcb.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 160.Rowe RG, Weiss SJ. Navigating ECM barriers at the invasive front: the cancer cell-stroma interface. Annual review of cell and developmental biology. 2009;25:567–595. doi: 10.1146/annurev.cellbio.24.110707.175315. [DOI] [PubMed] [Google Scholar]
- 161.Castro-Sanchez L, Soto-Guzman A, Guaderrama-Diaz M, Cortes-Reynosa P, Salazar EP. Role of DDR1 in the gelatinases secretion induced by native type IV collagen in MDA-MB-231 breast cancer cells. Clinical & experimental metastasis. 2011;28(5):463–477. doi: 10.1007/s10585-011-9385-9. [DOI] [PubMed] [Google Scholar]
- 162.Gschwind A, Fischer OM, Ullrich A. The discovery of receptor tyrosine kinases: targets for cancer therapy. Nature reviews Cancer. 2004;4(5):361–370. doi: 10.1038/nrc1360. [DOI] [PubMed] [Google Scholar]
- 163.Zhang J, Yang PL, Gray NS. Targeting cancer with small molecule kinase inhibitors. Nature reviews Cancer. 2009;9(1):28–39. doi: 10.1038/nrc2559. [DOI] [PubMed] [Google Scholar]
- 164.Rix U, Hantschel O, Durnberger G, Remsing Rix LL, Planyavsky M, Fernbach NV, et al. Chemical proteomic profiles of the BCR-ABL inhibitors imatinib, nilotinib, and dasatinib reveal novel kinase and nonkinase targets. Blood. 2007;110(12):4055–4063. doi: 10.1182/blood-2007-07-102061. [DOI] [PubMed] [Google Scholar]
- 165.Bantscheff M, Eberhard D, Abraham Y, Bastuck S, Boesche M, Hobson S, et al. Quantitative chemical proteomics reveals mechanisms of action of clinical ABL kinase inhibitors. Nature biotechnology. 2007;25(9):1035–1044. doi: 10.1038/nbt1328. [DOI] [PubMed] [Google Scholar]
- 166.Hantschel O, Rix U, Superti-Furga G. Target spectrum of the BCR-ABL inhibitors imatinib, nilotinib and dasatinib. Leukemia & lymphoma. 2008;49(4):615–619. doi: 10.1080/10428190801896103. [DOI] [PubMed] [Google Scholar]
- 167.Day E, Waters B, Spiegel K, Alnadaf T, Manley PW, Buchdunger E, et al. Inhibition of collagen-induced discoidin domain receptor 1 and 2 activation by imatinib, nilotinib and dasatinib. European journal of pharmacology. 2008;599(1–3):44–53. doi: 10.1016/j.ejphar.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 168.Li J, Rix U, Fang B, Bai Y, Edwards A, Colinge J, et al. A chemical and phosphoproteomic characterization of dasatinib action in lung cancer. Nature chemical biology. 2010;6(4):291–299. doi: 10.1038/nchembio.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rix U, Remsing Rix LL, Terker AS, Fernbach NV, Hantschel O, Planyavsky M, et al. Leukemia: official journal of the Leukemia Society of America. 1. Vol. 24. Leukemia Research Fund; U K: 2010. A comprehensive target selectivity survey of the BCR-ABL kinase inhibitor INNO-406 by kinase profiling and chemical proteomics in chronic myeloid leukemia cells; pp. 44–50. [DOI] [PubMed] [Google Scholar]
- 170.Sun X, Phan TN, Jung SH, Kim SY, Cho JU, Lee H, et al. LCB 03-0110, a novel pan-DDR/c-Src family tyrosine kinase inhibitor, suppresses scar formation by inhibiting fibroblast and macrophage activation. The Journal of pharmacology and experimental therapeutics. 2011 doi: 10.1124/jpet.111.187328. [DOI] [PubMed] [Google Scholar]
- 171.Yang X, Huang Y, Crowson M, Li J, Maitland ML, Lussier YA. Kinase inhibition-related adverse events predicted from in vitro kinome and clinical trial data. Journal of biomedical informatics. 2010;43(3):376–384. doi: 10.1016/j.jbi.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Du J, Bernasconi P, Clauser KR, Mani DR, Finn SP, Beroukhim R, et al. Bead-based profiling of tyrosine kinase phosphorylation identifies SRC as a potential target for glioblastoma therapy. Nature biotechnology. 2009;27(1):77–83. doi: 10.1038/nbt.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mihai C, Iscru DF, Druhan LJ, Elton TS, Agarwal G. Discoidin domain receptor 2 inhibits fibrillogenesis of collagen type 1. J Mol Biol. 2006;361(5):864–876. doi: 10.1016/j.jmb.2006.06.067. [DOI] [PubMed] [Google Scholar]
- 174.Siddiqui K, Kim GW, Lee DH, Shin HR, Yang EG, Lee NT, et al. Actinomycin D identified as an inhibitor of discoidin domain receptor 2 interaction with collagen through an insect cell based screening of a drug compound library. Biological & pharmaceutical bulletin. 2009;32(1):136–141. doi: 10.1248/bpb.32.136. [DOI] [PubMed] [Google Scholar]
- 175.Turashvili G, Bouchal J, Ehrmann J, Fridman E, Skarda J, Kolar Z. Novel immunohistochemical markers for the differentiation of lobular and ductal invasive breast carcinomas. Biomedical papers of the Medical Faculty of the University Palacky, Olomouc, Czechoslovakia. 2007;151(1):59–64. doi: 10.5507/bp.2007.010. [DOI] [PubMed] [Google Scholar]
- 176.Tun HW, Personett D, Baskerville KA, Menke DM, Jaeckle KA, Kreinest P, et al. Pathway analysis of primary central nervous system lymphoma. Blood. 2008;111(6):3200–3210. doi: 10.1182/blood-2007-10-119099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ahmad PJ, Trcka D, Xue S, Franco C, Speer MY, Giachelli CM, et al. Discoidin domain receptor-1 deficiency attenuates atherosclerotic calcification and smooth muscle cell-mediated mineralization. The American journal of pathology. 2009;175(6):2686–2696. doi: 10.2353/ajpath.2009.080734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Flamant M, Placier S, Rodenas A, Curat CA, Vogel WF, Chatziantoniou C, et al. Discoidin domain receptor 1 null mice are protected against hypertension-induced renal disease. J Am Soc Nephrol. 2006;17(12):3374–3381. doi: 10.1681/ASN.2006060677. [DOI] [PubMed] [Google Scholar]
- 179.Avivi-Green C, Singal M, Vogel WF. Discoidin domain receptor 1-deficient mice are resistant to bleomycin-induced lung fibrosis. Am J Respir Crit Care Med. 2006;174(4):420–427. doi: 10.1164/rccm.200603-333OC. [DOI] [PubMed] [Google Scholar]
- 180.Franco C, Hou G, Ahmad PJ, Fu EY, Koh L, Vogel WF, et al. Discoidin domain receptor 1 (ddr1) deletion decreases atherosclerosis by accelerating matrix accumulation and reducing inflammation in low-density lipoprotein receptor-deficient mice. Circulation research. 2008;102(10):1202–1211. doi: 10.1161/CIRCRESAHA.107.170662. [DOI] [PubMed] [Google Scholar]
- 181.Gross O, Girgert R, Beirowski B, Kretzler M, Kang HG, Kruegel J, et al. Loss of collagen-receptor DDR1 delays renal fibrosis in hereditary type IV collagen disease. Matrix biology: journal of the International Society for Matrix Biology. 2010;29(5):346–356. doi: 10.1016/j.matbio.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 182.Ferri N, Carragher NO, Raines EW. Role of discoidin domain receptors 1 and 2 in human smooth muscle cell-mediated collagen remodeling: potential implications in atherosclerosis and lymphangioleiomyomatosis. Am J Pathol. 2004;164(5):1575–1585. doi: 10.1016/S0002-9440(10)63716-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Blissett AR, Garbellini D, Calomeni EP, Mihai C, Elton TS, Agarwal G. Regulation of collagen fibrillogenesis by cell-surface expression of kinase dead DDR2. Journal of molecular biology. 2009;385(3):902–911. doi: 10.1016/j.jmb.2008.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Xu L, Peng H, Wu D, Hu K, Goldring MB, Olsen BR, et al. Activation of the discoidin domain receptor 2 induces expression of matrix metalloproteinase 13 associated with osteoarthritis in mice. J Biol Chem. 2005;280(1):548–555. doi: 10.1074/jbc.M411036200. [DOI] [PubMed] [Google Scholar]
- 185.Xu L, Peng H, Glasson S, Lee PL, Hu K, Ijiri K, et al. Increased expression of the collagen receptor discoidin domain receptor 2 in articular cartilage as a key event in the pathogenesis of osteoarthritis. Arthritis Rheum. 2007;56(8):2663–2673. doi: 10.1002/art.22761. [DOI] [PubMed] [Google Scholar]
- 186.Wang J, Lu H, Liu X, Deng Y, Sun T, Li F, et al. Functional analysis of discoidin domain receptor 2 in synovial fibroblasts in rheumatoid arthritis. J Autoimmun. 2002;19(3):161–168. doi: 10.1006/jaut.2002.0606. [DOI] [PubMed] [Google Scholar]
- 187.Su J, Yu J, Ren T, Zhang W, Zhang Y, Liu X, et al. Discoidin domain receptor 2 is associated with the increased expression of matrix metalloproteinase-13 in synovial fibroblasts of rheumatoid arthritis. Molecular and cellular biochemistry. 2009;330(1–2):141–152. doi: 10.1007/s11010-009-0127-0. [DOI] [PubMed] [Google Scholar]