Abstract
A 51-year-old Caucasian female with a 7-year history of intermittent abdominal pain and diarrhoea presented to our service. Before presentation, she had been successfully treated for Helicobacter pylori infection, but later developed new oesophageal ulcerations with exudative lesions that were positive for herpes simplex virus, and candida oesophagitis had developed. Biopsies showed chronic inactive gastritis with gastric intestinal metaplasia. MRI revealed a solid 3.4×3 cm lesion in the caudate lobe of the liver, with a 7-mm pancreatic cyst. The aspirated pancreatic cyst cytology was benign. On exploratory laporatomy, the lesion appeared confined to the caudate lobe, and a resection was performed. The pathology was consistent with a well-differentiated neuroendocrine carcinoma with vascular invasion and involvement of the liver capsule, although resection margins were negative. The patient had complete symptomatic improvement. This case re-affirms the high index of suspicion needed to make the diagnosis of gastrinoma. If caught in time, surgical removal of primary hepatic gastrinoma can be curative.
Background
Gastrinomas are the most common pancreatic neuroendocrine tumour; however, the estimated annual incidence is only one per million people. Current data suggest that duodenal gastrinomas are in fact more common than pancreatic gastrinomas, and together account for greater than 80% of gastrinomas. Lymph node primaries have also been described, and appear to represent 10% of all gastrinomas.1 The majority of gastrinomas are located within the ‘gastrinoma triangle’, defined by the junction between the cystic and common bile ducts, the second and third portions of the duodenum, and the head and tail of the pancreas. Cases of ectopic (ie, non-pancreaticoduodenal) and non-nodal gastrinomas are described in the literature, and their locations include, but are not limited to the ovaries, kidneys, stomach and liver. However, fewer than 20 cases of well-documented primary hepatic gastrinoma exist in the literature, with only four cases being published in the last 2 years.2–20 The current case study describes a patient with prolonged non-specific gastrointestinal complaints found to have a gastrinoma of hepatic origin.
Case presentation
A 51-year-old Caucasian female presented to our office for a second opinion regarding a 7-year history of intermittent abdominal pain and diarrhea which had become progressively worse over the past several months.
Four months before presentation, an oesophagogastroduodenoscopy was performed at an outside hospital (OSH), which showed diffuse, non-bleeding, erythematous, ulcerations in the antrum and duodenum. Biopsies for Helicobacter pylori were positive and the patient was treated with triple-therapy (lansoprazole/amoxicillin/clarithromycin); bacterial eradication was confirmed via faecal antigen. A lactose breath test had been suggestive of lactose intolerance. Additionally, she had endorsed a history of non-steroidal anti-inflammatory drug (NSAID) use. As instructed, she followed a lactose-free diet, avoided NSAIDs, and continued daily proton pump inhibitor therapy. Three months later, an enteroscopy was peformed at the OSH for persistent symptoms and showed new oesophageal ulcerations with exudative lesions in the lower third of the oesophagus. Biopsies were positive for herpes simplex virus (HSV) and candida oesophagitis. The duodenum and jejunum revealed persistent, non-bleeding, cratered ulcerations. Biopsies of the ulcers revealed patchy jejunitis with acute on chronic inflammation, scattered necrotic tissue and debris and no dysplasia. Treatment with fluconazole and acyclovir produced partial symptomatic improvement. Attempts at symptom control with the addition of loperamide, cholestyramine, diphenoxylate/atropine and narcotics were unsuccessful. Further stool studies for infectious aetiologies and malabsorption were negative. Basic metabolic panel, complete blood counts, liver function tests, thyroid function, B12, folate and iron studies were normal, and celiac serology and inflammatory markers were negative.
The patient then self-referred to our office.
Investigations
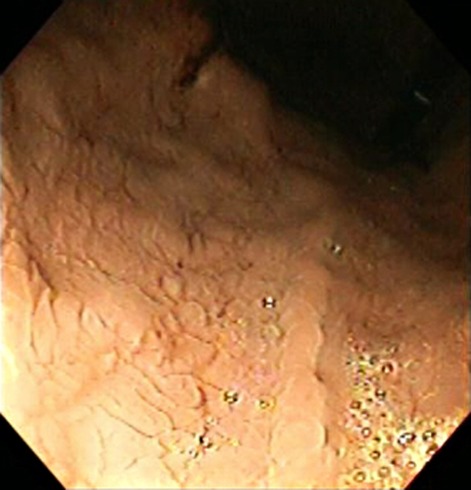
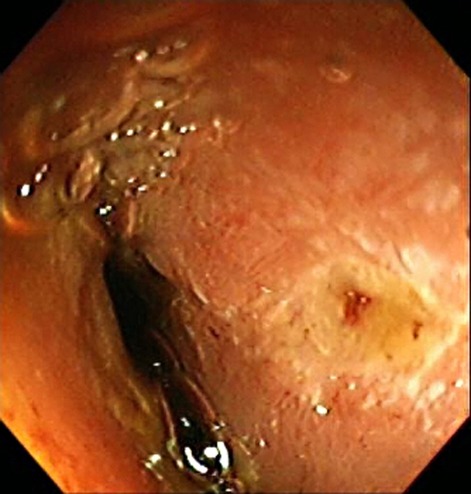
A repeat enteroscopy showed oedematous and congested distal oesophageal mucosa without frank ulceration or exudates; the biopsies and cultures were unremarkable. There was diffuse mucosal fissuring with a mosaic appearance, seen primarily in the fundus and proximal stomach, without ulcerations (figure 1). Biopsies showed chronic inactive gastritis with gastric intestinal metaplasia. The duodenum and jejunum appeared atrophic with scattered non-bleeding ulcerations ranging from 1 cm to 3 cm (figure 2). Biopsies showed villous shortening and few scattered intraepithelial lymphocytes; viral cultures for cytomegalovirus and HSV were negative. Due to the refractory ulcer disease, serum gastrin level was tested and found to be 36 357 pg/ml (normal <100 pg/ml). A secretin stimulation test was positive (gastrin level rose from 3423 pg/ml to a peak of 65 263 pg/ml within 2 min of administering a bolus of secretin). Parathyroid hormone was 26.9 pg/ml, prolactin was 28.7 ng/ml and calcium was 9.3 mg/dl, all within normal reference range.
Figure 1.
Retroflex view of stomach body and fundus with diffuse mucosal fissuring with a mosaic appearance without ulcerations.
Figure 2.
Atrophic jejunal mucosa with ulceration at 3 o’clock.
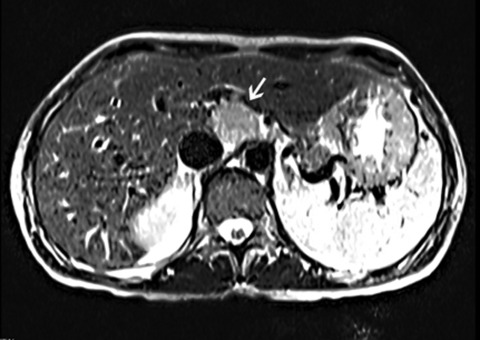
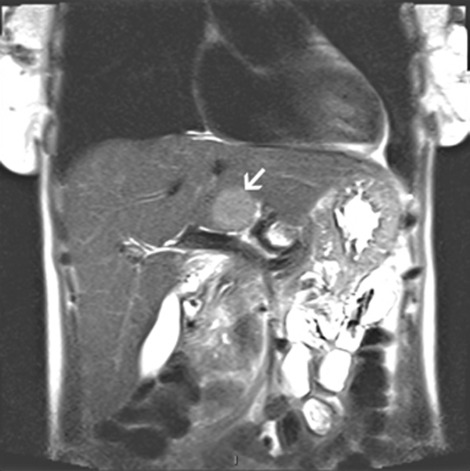
A CT scan of the abdomen and pelvis with contrast revealed diffuse mucosal enhancement of the duodenum and proximal jejunum and mild intrahepatic biliary dilation with poorly visualised extrahepatic biliary system due to adjacent bowel loops. A follow-up liver MRI/magnetic resonance cholangiopancreatography showed a solid 3.4 cm by 3 cm lesion in the caudate lobe of the liver with normal intra and extra-hepatic biliary system, along with a 7-mm pancreatic cyst. (figures 3 and 4) An octreotide scan showed a focal area of intense uptake in the liver correlating with the mass seen on MRI, but no other sites of uptake were noted (figure 5). Endoscopic ultrasound revealed a 12 mm by 5 mm cyst in the head of the pancreas. The rest of the pancreas was normal without evidence of a mass; the duodenum was visualised to the second portion which revealed a single superficial ulcer and a 5-mm paraduodenal node. The pancreatic cyst was aspirated, and the cytology was benign. An exploratory laporatomy was performed, and the duodenum was kocherised. Thorough exploration of the pancreas was done with opening of the lesser sac to improve visualisation of the pancreatic head, neck and body. The gastrinoma triangle was explored as well as the hepatoduodenal ligament and surrounding area. The duodenum was carefully examined and there was no evidence of any abnormalities within the wall of the duodenum. No additional extrahepatic tumour could be identified. An open abdominal ultrasound was performed and no extrahepatic or further intrahepatic tumour could be localised. The lesion appeared confined to the caudate lobe.
Figure 3.
MRI abdomen coronal image showing 3–4 cm solid lesion in the caudate lobe of the liver.
Figure 4.
MRI abdomen saggital image showing 3–4 cm solid lesion in the caudate lobe of the liver.
Figure 5.
Octreotide scan showed a focal area of intense uptake in the liver correlating with the mass seen on MRI abdomen.
Treatment
A surgical resection of the lesion was performed. The pathology was consistent with a well-differentiated neuroendocrine carcinoma with vascular invasion and involvement of the liver capsule, although resection margins were negative. The postoperative gastrin levels fell rapidly to normal values (38 pg/ml from 36 357 pg/ml).
Outcome and follow-up
The patient was followed as an outpatient and had complete symptomatic improvement. A follow-up secretin stimulation test 6 months later was within normal limits (gastrin level baseline 24 pg/ml with rise to a peak of 60 pg/ml within 2 min of administering a bolus of secretin).
Discussion
Due to the infrequent nature of primary hepatic gastrinoma, patients often do not undergo standardised laboratory and imaging studies or surgical exploration to evaluate for alternate primary sites. The major limitations that have been identified in these cases are short postoperative follow-up periods, failure to repeat fasting gastrin and secretin stimulation tests at regular intervals, and the failure to perform duodenotomy and lymph-node sampling at the time of surgery. A study by Norton et al in which patients with Zollinger-Ellison syndrome without multiple endocrine neoplasia type 1 underwent a surgical exploration for cure, found that despite extensive preoperative evaluation with multiple modalities, duodenotomy-identified duodenal gastrinomas were found in 62% of patients versus 18% in the non-duodenotomy group; further, these gastrinomas were often less than 1 cm.21 Endoscopic ultrasound lacks the sensitivity to reliably detect such lesions.21 22 Interestingly, however, the incidence of hepatic metastasis from duodenal primaries appears to be less than 10%, and as low as 3% by some reports.21 23
The latency to diagnosis in the present case was 7 years, which is in line with reports in the literature. It has been speculated that the common nature of the symptoms (abdominal pain, diarrhoea, heartburn, nausea and vomiting) may be the reason for the delay in diagnosis.24 This case re-affirms the high index of suspicion needed to make the diagnosis of gastrinoma. While this case does likely represent a primary hepatic gastrinoma, as evidenced by a precipitous drop in postoperative gastrin levels and normal secretin stimulation at 6 months, the follow-up period has been relatively short. It is important that clinicians be aware of this condition and should test for it if classic symptoms such as diarrhoea, uncontrolled reflux or non-healing mucosal ulcers are present with a negative preliminary investigation. If caught in time, surgical removal of primary hepatic gastrinoma can be curative, with a good probability of normal survival.
Learning points.
-
▶
A high index of suspicion needed to make the diagnosis of gastrinoma.
-
▶
It is important that clinicians be aware of this condition and should test for it if classic symptoms such as diarrhoea, uncontrolled reflux or non-healing mucosal ulcers are present with a negative preliminary investigation.
-
▶
If caught in time, surgical removal of primary hepatic gastrinoma can be curative, with a good probability of normal survival.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Norton JA, Alexander HR, Fraker DL, et al. Possible primary lymph node gastrinoma: occurrence, natural history, and predictive factors: a prospective study. Ann Surg 2003;237:650–7; discussion 657–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith AL, Auldist AW. Successful surgical resection of an hepatic gastrinoma in a child. J Pediatr Gastroenterol Nutr 1984;3:801–4 [DOI] [PubMed] [Google Scholar]
- 3.Evans JT, Nickles S, Hoffman BJ. Primary hepatic gastrinoma: an unusual case of zollinger-ellison syndrome. Gastroenterol Hepatol (N Y) 2010;6:53–6 [PMC free article] [PubMed] [Google Scholar]
- 4.Kuiper P, Biemond I, Verspaget H, et al. A case of recurrent gastrinoma in the liver with a review of “primary” hepatic gastrinomas. BMJ Case Rep 2009;10.1136/bcr.01.2009.1529 Published 11 June 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delgado J, Delgado B, Sperber AD, et al. Successful surgical treatment of a primary liver gastrinoma during pregnancy: a case report. Am J Obstet Gynecol 2004;191:1716–8 [DOI] [PubMed] [Google Scholar]
- 6.Kanakia RR, Sawant PD, Nanivadekar SA, et al. Primary gastrinoma of the liver. Indian Pediatr 1994;31:1137–40 [PubMed] [Google Scholar]
- 7.Díaz R, Aparicio J, Pous S, et al. Primary hepatic gastrinoma. Dig Dis Sci 2003;48:1665–7 [DOI] [PubMed] [Google Scholar]
- 8.Smyrniotis V, Kehagias D, Kostopanagiotou G, et al. Primary hepatic gastrinoma. Am J Gastroenterol 1999;94:3380–2 [DOI] [PubMed] [Google Scholar]
- 9.Ishikawa Y, Yoshida H, Mamada Y, et al. Curative resection of primary hepatic gastrinoma. Hepatogastroenterology 2008;55:2224–7 [PubMed] [Google Scholar]
- 10.Wu PC, Alexander HR, Bartlett DL, et al. A prospective analysis of the frequency, location, and curability of ectopic (nonpancreaticoduodenal, nonnodal) gastrinoma. Surgery 1997;122:1176–82 [DOI] [PubMed] [Google Scholar]
- 11.Krishnamurthy SC, Dutta V, Pai SA, et al. Primary carcinoid tumor of the liver: report of four resected cases including one with gastrin production. J Surg Oncol 1996;62:218–21 [DOI] [PubMed] [Google Scholar]
- 12.Tiomny E, Brill S, Baratz M, et al. Primary liver gastrinoma. J Clin Gastroenterol 1997;24:188–91 [DOI] [PubMed] [Google Scholar]
- 13.Moriura S, Ikeda S, Hirai M, et al. Hepatic gastrinoma. Cancer 1993;72:1547–50 [DOI] [PubMed] [Google Scholar]
- 14.Lee SR, Choi MC, Ahn KJ. A case of multiple endocrine neoplasia type 1 with primary liver gastrinoma. J Korean Med Sci 2010;25:953–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Norton JA, Doherty GM, Fraker DL, et al. Surgical treatment of localized gastrinoma within the liver: a prospective study. Surgery 1998;124:1145–52 [DOI] [PubMed] [Google Scholar]
- 16.Andreola S, Lombardi L, Audisio RA, et al. A clinicopathologic study of primary hepatic carcinoid tumors. Cancer 1990;65:1211–8 [DOI] [PubMed] [Google Scholar]
- 17.Gould VE, Banner BF, Baerwaldt M. Neuroendocrine neoplasms in unusual primary sites. Diagn Histopathol 1981;4:263–77 [PubMed] [Google Scholar]
- 18.Wolfe MM, Alexander RW, McGuigan JE. Extrapancreatic, extraintestinal gastrinoma: effective treatment by surgery. N Engl J Med 1982;306:1533–6 [DOI] [PubMed] [Google Scholar]
- 19.Thompson JC, Lewis BG, Wiener I, et al. The role of surgery in the Zollinger-Ellison syndrome. Ann Surg 1983;197:594–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson NW, Vinik AI, Eckhauser FE, et al. Extrapancreatic gastrinomas. Surgery 1985;98:1113–20 [PubMed] [Google Scholar]
- 21.Norton JA, Alexander HR, Fraker DL, et al. Does the use of routine duodenotomy (DUODX) affect rate of cure, development of liver metastases, or survival in patients with Zollinger-Ellison syndrome? Ann Surg 2004;239:617–25; discussion 626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Norton JA, McLean AM, Fairclogh PD. Endoscopic ultrasound in the localization of pancreatic islet cell tumors. Best Pract Res Clin Endocrinol Metab 2004;19:177–93 [DOI] [PubMed] [Google Scholar]
- 23.Thom AK, Norton JA, Axiotis CA, et al. Location, incidence, and malignant potential of duodenal gastrinomas. Surgery 1991;110:1086–91; discussion 1091–3 [PubMed] [Google Scholar]
- 24.Roy PK, Venzon DJ, Shojamanesh H, et al. Zollinger-Ellison syndrome. Clinical presentation in 261 patients. Medicine (Baltimore) 2000;79:379–411 [DOI] [PubMed] [Google Scholar]