Abstract
AIM: To study computed tomography (CT) features of abdominal malignant fibrous histiocytoma (MFH) in various rare locations.
METHODS: We retroprospectively identified cases of MFH involving the abdominal cavity. Particular attention was paid to details regarding imaging features and histological types.
RESULTS: The study population consisted of seven men and one woman, with a mean age of 52.5 years. Seven patients had some physical symptoms, while one was incidentally detected. The sites of origin were liver (n = 3), greater omentum (n = 1), superior mesentery (n = 1), ileum (n = 1), right psoas muscle (n = 1) and right kidney (n = 1). With the exception of the ileum lesion, all were of huge size. The contour of the lesions was more or less clear. Foci of necrosis were present in six lesions (n = 6). On plain CT scan, all lesions were hypo to iso dense. The lesion in the greater omentum was cystic. One lesion (n = 1) showed significant enhancement and the cystic lesion showed mild peripheral enhancement. An abundance of blood vessels surrounding the mass was seen in two lesions (n = 2) and both were of the inflammatory variety. Pathological examination revealed storiform-pleomorphic variety (n = 4), inflammatory variety (n = 3) and myxoid variety (n = 1). Two of the patients with inflammatory MFH had a clinical presentation of fever and one was afebrile, however, blood investigations in all three showed leukocytosis.
CONCLUSION: Primary MFHs of the abdominal viscera and gastrointestinal tract are generally huge soft tissue masses containing areas of low attenuation and mild to moderate contrast enhancement.
Keywords: Abdomen, Computed tomography, Malignant fibrous histiocytoma, Soft tissue sarcoma
INTRODUCTION
Malignant fibrous histiocytoma (MFH) is a pleomorphic sarcoma. It was first described as malignant histiocytoma and fibrous xanthoma by Ozello et al in 1963[1], and was established by O’Brien and Stout as a soft tissue sarcoma arising from fibroblasts and histiocytes in 1964[1,2]. MFH is a relatively rare tumor that occurs throughout the body[3]. However, it is also the most common sarcoma appearing in late adult life (6th-7th decades) and men are more often affected than women. The most frequent site of MFH is the extremities (lower extremity 49%, upper extremity 19%). The second most prevalent site is the retroperitoneum (16%) and peritoneal cavity (5%-10%)[4]. Occurrence of this lesion in visceral organs and the gastrointestinal tract is very rare, and there is a marked paucity of reports describing MFH in these locations. Our purpose was to study the computed tomography (CT) features of abdominal MFH in various rare locations.
MATERIALS AND METHODS
Subjects
Cases of MFH of the abdominal cavity registered in the pathological department of our institution from 2000 to 2010 were identified. The search yielded 9 cases, the reports of which were reviewed. One was excluded due to unavailability of clinical data. For the remaining 8 cases, complete hospital records (history, diagnostic imaging, operative findings and pathological reports) were reviewed. Particular attention was paid to details regarding imaging features and histological types. Clinical data were reviewed for age, sex, and presenting complaints. Ethical approval from the institutional review board at our hospital was obtained to conduct this study.
CT scanning and image analysis
CT data were available on PACS (picture archiving and communication system, Marotech) of all patients which were studied by two radiologists. All CT examinations were performed using a 16 slice CT scanner (Light speed, GE Health Care, USA). Scans were obtained with a tube voltage of 120 kV, tube current of 280-320 mAs, slice thickness 5 mm and section interval of 5 mm. With the exception of the patient with the ileum mass, all underwent a contrast scan. For the contrast-enhanced scan, 80-120 mL of non-ionic contrast agent (Ultravist) was injected at the rate of 3.5-4 mL/s. All images were reviewed on a PACS monitor and were retrospectively analyzed with regard to the location of the lesions, tumor morphology and enhancement pattern.
Pathologic analysis
With the exception of the patient with a mesenteric lesion who only underwent CT-guided biopsy, the other patients were treated surgically. Each specimen was examined microscopically and immunohistochemically to confirm the diagnosis. The pathology records of each patient were reviewed to establish the histological subtypes of the lesions.
RESULTS
The study population consisted of seven men and one woman, who had an age range of 23-79 years (mean 52.5 years). Seven patients had some physical symptoms, while one was incidentally detected during a regular health check-up. The sites of origin were liver (n = 3), greater omentum (n = 1), superior mesentery (n = 1), ileum (n = 1), right psoas muscle (n = 1) and right kidney (n = 1) (Table 1). With the exception of the ileum lesion, all were of huge size. The contours of the lesions were more or less clear, except one, in the left lobe of the liver. Foci of necrosis were present in six lesions (n = 6). On plain CT scan, all lesions were hypo to iso dense, seven lesions were soft tissue masses of density ranging from 30-47 HU in the plain scan. The lesion in the greater omentum was cystic with a density of 15-23 HU. Contrast scans showed significant enhancement in one lesion (n = 1) and the cystic lesion showed mild peripheral enhancement. The central necrotic zone in the mesenteric lesion was more evident in the contrast scan. An abundance of blood vessels surrounding the mass was seen in two lesions (n = 2), both of which were the inflammatory variety. The lesion in the ileum had peritoneal metastasis at the time of diagnosis. The greater omentum lesion recurred within 3 mo of surgery. The patient with the mesentery lesion could not undergo surgery due to multiple distant metastatic foci. Pathological examination revealed storiform-pleomorphic variety (n = 4), inflammatory variety (n = 3) and myxoid variety (n = 1). Two patients with inflammatory MFH had a clinical presentation of fever and one was afebrile, however, blood investigations in all three showed leukocytosis.
Table 1.
Clinical, computed tomography and pathological findings of cases
| S. No. | Age (yr)/sex | Clinical presentations | Location | Size (cm) | Necrosis |
CT |
Histological subtypes | |
| Plain | Contrast | |||||||
| 1 | 31/F | Fever, abdominal pain | Liver lt. lobe | 9.7 × 5.8 × 3.6 | - | Hypodense | Significant | Inflammatory |
| 2 | 47/M | Upper abdominal pain | Liver rt. lobe | 7.9 × 8.8 × 3.3 | + | Hypodense | Mild | Storiform-pleomorphic |
| 3 | 54/M | Jaundice, upper abdominal pain | Liver lt. lobe | 7.5 × 6.2 × 1.2 | + | Hypodense | Mild | Myxoid |
| 4 | 34/M | Fever, polyuria | Greater omentum | 12.0 × 11.0 × 22.6 | + | Hypodense | Mild peripheral | Inflammatory |
| 5 | 64/M | Abdominal pain, weight loss | Mesentery | 6.3 × 4.1 × 2.4 | + | Hypodense | Mild in equilibrium phase | Inflammatory |
| 6 | 79/M | Abdominal discomfort | Ileum | 3.3 × 2.3 × 5.0 | - | Hypodense | - | Storiform-pleomorphic |
| 7 | 59/M | Occasional Lower abdominal pain | Retroperitoneal - psoas muscle | 9.4 × 7.7 × 10.0 | + | Isoodense | Mild | Storiform-pleomorphic |
| 8 | 23/M | Health check up | Rt. Kidney | 7.0 × 6.4 × 1.8 | + | Hypodense | Mild in equilibrium phase | Storiform-pleomorphic |
CT: Computed tomography; +: Present; -: Absent.
DISCUSSION
MFH is a well-established sarcoma arising in the adult population. It is considered to arise from primitive mesenchymal cells and can occur in any part of the body. Despite this ubiquity, involvement of the visceral organs and peritoneal cavity is uncommon. The cause of its origin is not clear. However, it has been recognized as a complication resulting from radiation, chronic postoperative repair, trauma, surgical incisions or burn scars[1], but this would explain only a minority of cases. Sun exposure does not seem to be an important risk factor[5]. MFH mainly involves extremities followed by the retroperitoneum. Uncommon locations include the head and neck region, dura mater, brain, lung, heart, aorta, pancreas, liver, spleen, breast, intestine and mesentery[6-10].
MFH pathological and clinical features
MFH is composed of both histiocytic and fibrous elements, according to their content and arrangement, MFH was divided into five major groups by Enzinger et al[11]: storiform-pleomorphic, myxoid, giant cells, inflammatory and angiomatoid. The first two types, namely storiform-pleomorphic and myxoid types are usually high grade, whereas the others tend to be lower grade[1]. Of these types, the storiform-pleomorphic type is most common, accounting for two thirds of cases. It is composed of spindle-shaped cells arranged in short fascicles in a cartwheel or storiform pattern, along with plump histiocytic cells showing mitotic features[4]. The myxoid subtype is the second most common variant, with definite metastatic potential, but a relatively indolent natural history compared with the storiform-pleomorphic variant[12]. The inflammatory MFH was first described by Kyriakos et al[13] in 1976. Unlike other types of MFHs, inflammatory MFH is more likely to develop in the retroperitoneum than in extremities[14]. Although the mechanism is poorly understood, patients often present with striking features of infection such as fever and pathologically acute inflammatory infiltration mainly by neutrophils, together with a small number of other types of inflammatory cells[15]. On immunohistochemistry examination, MFH frequently expresses a positive reaction to vimentin, actin, CD-68, and α 1-antitrypsin[1,16].
Liver MFH
Liver is an exceptional site of involvement. Since the first report in 1985, only about 30 cases of hepatic MFH have been reported in the English literature[17-20]. There is no predilection for lobe or the site of origin. The prognosis is poor, with the overall 2-year survival rate approximately 60%[18]. Unenhanced CT findings of hepatic MFH include poorly demarcated or a well circumscribed, large or multinodular mass showing heterogeneous low attenuation density, with frequent areas of necrosis and occasional intratumoral calcification[19-22]. Smaller tumors may show a solid mass without prominent internal necrosis[21]. Following contrast injection, heterogeneous mild to moderate enhancement of the mass with several necrotic areas may be evident, and rarely enhancing peripheral pseudo-capsule or significant enhancement is noted[17]. Although the vascularity of MFH is variable, the majority of lesions are moderately hypervascular with the tumor supply derived from multiple surrounding vessels[23].
One of three patients with inflammatory MFH in our study showed marked enhancement and abundant blood vessels surrounding the tumor mass (Figure 1), and in the other two cases the enhancement was mild (Figure 2). The patient with a mass in the left liver lobe showed a poorly demarcated tumor margin (Figure 3), while the other two had well demarcated margins. The tumors may involve the liver capsule and adjacent organs, but there is no evidence of portal vein invasion, bile duct obstruction, or regional lymph node metastasis[17,21]. A report of a mass undergoing complete necrosis and significant intratumoral hemorrhage involving the hemoperitoneum has also been published[23]. The differential diagnosis of liver MFH includes hepatocellular carcinoma and other sarcomas such as leiomyosarcoma, fibrosarcoma and angiosarcoma.
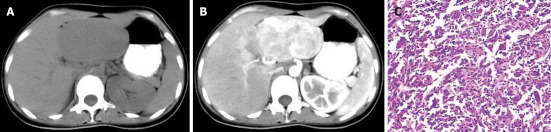
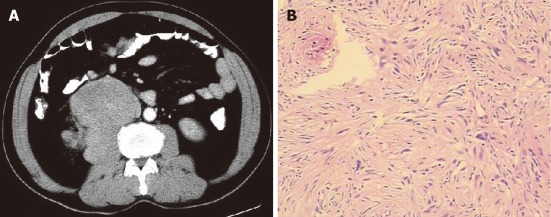
Figure 1.
A 31-year-old female with liver malignant fibrous histiocytoma. A: Plain computed tomography scan shows well demarcated mass with direct contact to the left lobe of liver and uniform density of 38 HU; B: Contrast scan arterial phase shows heterogeneous enhancement of mass compare to normal liver parenchyma with surrounding blood vessels; C: Pathological specimen HE (× 400) showing multinucleated spindle shape tumor cells with inflammatory cells.
Figure 2.
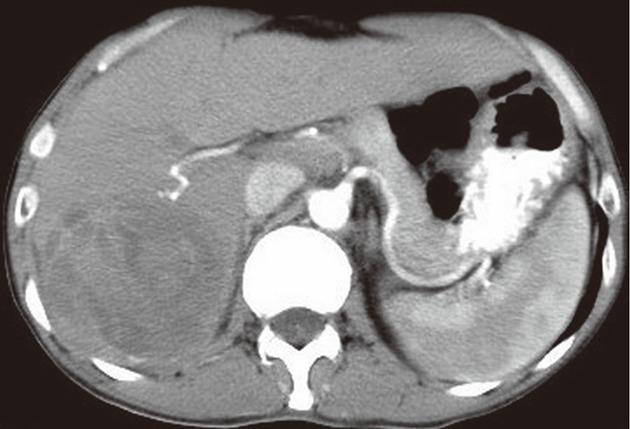
A 47-year-old male with liver malignant fibrous histiocytoma. Contrast-enhanced computed tomography scan arterial phase shows a mass with a clear boundary in the right-posterior lobe of liver segment 6 and 7. No obvious enhancement with areas of hypodensity is evident.
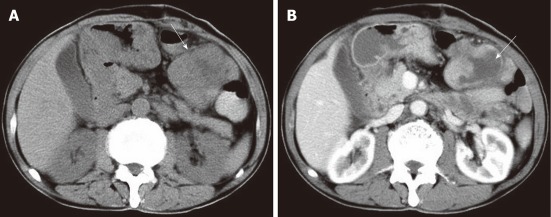
Figure 3.
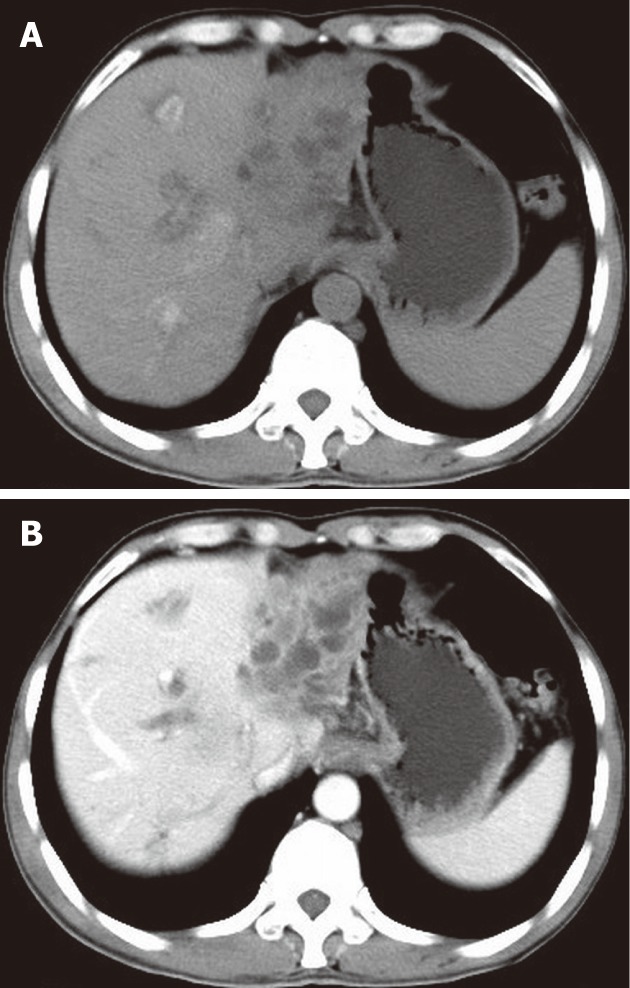
A 54-year-old male with liver malignant fibrous histiocytoma. A: Non-contrast computed tomography shows ill defined lesion of density 30 HU in the left lobe with dilated intrahepatic biliary duct; B: Contrast scan portal vein phase shows low enhancement compared to normal liver parenchyma and multiple areas of low attenuation.
Greater omentum MFH
Despite the ubiquity of MFHs, involvement of the omentum is uncommon. Involvement of the peritoneal cavity is seen in 5%-10% of MFHs[4,24]. During a MEDLINE search we found only 5 cases of MFH in the English literature with specific occurrence in the omentum including ours. On CT, MFH has a density predominantly equivalent to muscle[2,6]. Hypodense areas represent necrosis which frequently exist within the lesions and these are more apparent on post-contrast study. It is difficult to determine the true origin of omentum MFH because the tumor is usually huge. A few authors have even described the mass undergoing complete necrosis and resembling a cyst or abscess in the imaging study[6,20]. Approximately 5% of MFHs may eventually develop spontaneous intratumoral hemorrhage, especially when the MFH arises from the peritoneum or omentum. The intratumoral hemorrhage can be so extensive as to obscure the underlying neoplasm and could create a large cyst-like space that might be mistaken for a hematoma, abscess or cystic tumor[23]. Similarly, in our case, the mass was completely cystic with a density of 15 to 23 HU extending to the pelvic cavity and it was very difficult to determine its site of origin, which corresponded to the findings of Huang et al[6]. Therefore, the preoperative diagnosis was difficult (Figure 4). However, due to the size and presence of abundant blood vessels surrounding the mass, a malignant lesion was suspected. The patient underwent preoperative embolization of the feeding vessels to minimize the risk of bleeding. Intra-operative findings revealed a greater omentum cystic mass with adherence to the small bowel and sigmoid colon. The pathological diagnosis revealed the inflammatory variety of MFH. The tumor recurred during the subsequent 3-mo follow-up, which was inconsistent with the previous report commenting on the inflammatory variety which had a low rate of recurrence and metastasis[2]. Differential diagnosis in cystic omental MFH includes gastrointestinal stromal tumors, cystic mesothelioma, lymphangioma, hematoma and abscess.
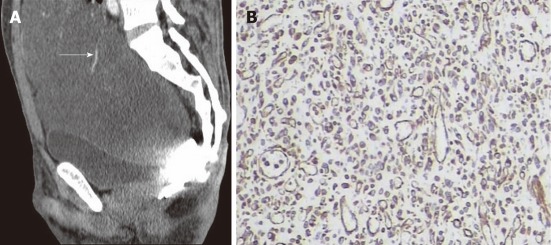
Figure 4.
A 34-year-old male with greater omentum malignant fibrous histiocytoma. A: Contrast-enhanced computed tomography scan sagittal view shows a well demarcated cystic mass extending from the abdominal to the pelvic cavity, note the blood vessel on the surface (arrow); B: Immunohistochemistry shows positive staining to vimentin (× 400).
Superior mesentery MFH
MFH of the mesentery is an extremely rare and highly malignant neoplasm with early metastatic spread. There are only a few reported cases of MFH of the superior mesentery. During a MEDLINE search we found only 14 reported cases in the English literature including ours. On CT, MFH typically presents as a poorly marginated or well circumscribed soft tissue mass, with hypodense areas due to necrosis[9,25]. Eccentrically located lumpy and ring-like calcifications due to osteoid and chondroid metaplasia have been reported on CT in 16% of abdominal MFHs[6]. As in our patient, the mass was of soft tissue density with central necrotic areas which were more evident on the contrast scan. CT-guided biopsy was performed, and pathological findings revealed tissue composed of fibroblastic and histiocytic elements with marked inflammatory cell infiltrates. The area of low attenuation corresponded to necrosis and cystic degeneration (Figure 5). The diagnosis of mesenteric MFH must be differentiated from gastrointestinal stromal tumors which account for most primary mesenchymal tumors of the gastrointestinal tract. Other differential diagnoses include leiomyoma, leiomyosarcoma, fibrosarcoma, and liposarcoma[26].
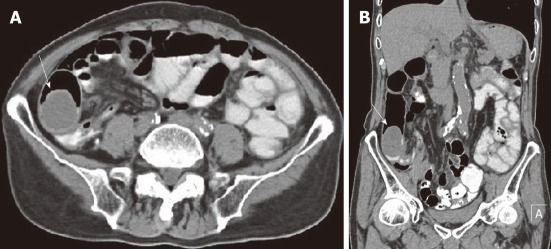
Figure 5.
A 64-year-old male with superior mesentery malignant fibrous histiocytoma. A: Non-contrast computed tomography scan shows mass (arrow) in the peritoneal cavity of 33 HU with a clear margin and central area of hypodensity; B: Contrast-enhanced scan venous phase shows mild enhancement with more pronounced central necrotic zone (arrow).
Ileum MFH
Primary malignant mesenchymal tumors of the small intestine are seldom found in clinical practice. The occurrence of MFH in the alimentary tract is less well documented in the English literature. In our MEDLINE search we could only yield 11 cases of primary ileum MFH including ours[1,27-29]. Compared to MFHs in other parts of the body, small intestinal MFHs are relatively smaller in size. Patients mainly present with features of obstruction such as abdominal pain, vomiting and melena due to dynamic obstruction by mass or by intussusception. By reviewing previous reports we found that the ileum is the preferred site compared with the duodenum and jejunum. The prognosis associated with ileum MFH is unclear, but most of the reports suggest that it is poor. According to Kobayashi et al[29], the 1-year survival rate is 53.6% and 2-year survival rate is 42.9%. Primary MFH must also be distinguished from gastrointestinal metastasis or invasions from other parts of the body. We regarded the tumor in our patient to be a primary ileum lesion, rather than an extension of a mesenteric or retroperitoneal tumor, because of the exophytic nature of the lesion with intraluminal and intramural growth (Figure 6). Although this tumor was smaller than the average visceral MFH, CT showed dilatation of the small intestine causing incomplete obstruction with nodules in the pelvic peritoneum which were later confirmed as metastases. A correct diagnosis of MFH in the small intestine is difficult before surgery, because it usually presents as a submucosal tumor. The diagnosis of ileum MFH depends on an accurate differential diagnosis from primary small intestinal carcinoma and other sarcomas such as pleomorphic liposarcoma and rhabdomyosarcoma.
Figure 6.
A 79-year-old male with ileum malignant fibrous histiocytoma, plain computed tomography scan abdomen. A, B: Axial (A) and coronal view (B) show intraluminal and intramural soft tissue mass of density 34 HU in the ilio-cecal region (arrows) without significant areas of necrosis. Note the oral contrast stagnation in the proximal loop of the small intestine and dilatation of the ascending colon.
Retroperitoneal MFH
Retroperitoneal sarcomas represent between 10% to 15% of all soft tissue sarcomas in adults[30,31]. Previous studies emphasize that most retroperitoneal MFHs are de novo. MFH is often referred to as the most usual type of soft-tissue sarcoma. Fletcher et al[30,31] suggested that it may only represent a common morphologic appearance shared by various soft-tissue sarcomas during their tumoral progression[32]. The most frequent tumors encountered in this location are liposarcoma, well-differentiated or dedifferentiated types, followed by leiomyosarcoma and MFH. Dedifferentiated liposarcomas mainly occur in the retroperitoneal space, and the most common pattern of dedifferentiated areas consists of high grade pleomorphic MFH or storiform fibroblastic MFH[33-35]. A recent study reported that a subgroup of MFH was associated with a specific genetic pattern similar to that of dedifferentiated liposarcomas[36], particularly when they are located in the retroperitoneum. In the retroperitoneal space, MFH represents 7% to 30% of sarcomas[33]. In our case, CT showed a mass in the right retroperitoneal space, a fat plane between the mass and the adjacent psoas muscle was not visible. The mass was in direct relation to the psoas muscle with an area of necrosis (Figure 7), and contrast enhancement was mild. Pathological examination revealed the storiform-pleomorphic variety of MFH. With respect to differential diagnosis, differentiation from rhabdomayosarcoma, neurofibroma, lipomatous tumour, leiyomayosarcoma, lymphoma and lymph node metastasis may also be difficult. When imaging alone is not conclusive, CT-guided biopsy may be a better alternative due to convenience in the retroperitoneal space.
Figure 7.
A 59-year-old male with retroperitoneal psoas muscle malignant fibrous histiocytoma. A: Contrast-enhanced computed tomography scan arterial phase shows mass in retroperitoneum with clear margin, no obvious enhancement of mass and area of low density corresponding to necrosis. Note that the fat plane between the mass and psoas muscle is not evident, and the mass seems to be in direct contact with the right psoas muscle; B: Pathological specimen HE stain (× 400) shows spindle to oval tumor cells arranged in a criss-cross fashion.
Kidney MFH
Primary renal MFH is a rare tumor of kidney. To date, there are only 54 reported cases of MFH arising from kidney[37,38]. The other reported sites of MFH occurring in the genitourinary system are the urinary bladder, prostate and spermatic cord[39-42]. Kidney MFH is clinically and radiologically indistinguishable from renal cell carcinoma. Patients usually present with abdominal or flank pain, generalized weakness and weight loss. An abdominal mass and hematuria may be evident but are less common. In contrast, these symptoms are seen in renal cell carcinoma and preoperative differentiation can be difficult[37]. However, selective renal arteriography usually reveals a hypovascular tumor as compared to renal cell carcinoma[38]. Our case showed a soft tissue mass with a density of 38 HU on the upper pole of the right kidney (Figure 8). The mass appeared encapsulated with peripheral areas of necrosis and mild enhancement on equilibrium phase.
Figure 8.
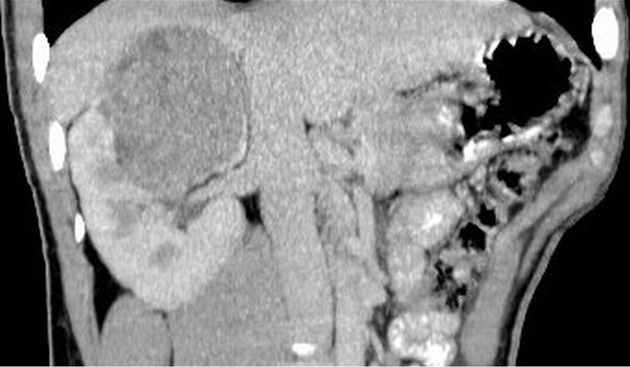
A 23-year-old male with right kidney malignant fibrous histiocytoma. Non-contrast computed tomography scan coronal view shows soft tissue mass of 47 HU in the upper pole of the right kidney extending toward the renal pelvis.
MFH treatment and prognosis
MFH is an aggressive tumor, with a high local recurrence rate and a significant metastasis rate[43]. Distant metastasis may spread via blood (30%) and the lymphatic system (12%). The risk of local recurrence and distant metastasis correlates with the depth and size of the primary tumor[4]. The liver is the most common site of sarcoma metastasis, occurring in 64%-70% of patients[44]. Recurrence of the tumor is not uncommon even when the resection margins are tumor free[43]. Most reports suggest that the prognosis associated with abdominal MFH is poorer than tumors in the extremities, due to late detection owing to location. Weiss and Einzinger’s analysis of MFH (1,2) showed that the 2-year survival rate with the storiform-pleomorphic type of MFH is 60% and the rate of metastases is 42%. Tumor site, location, size, and histology are not significant determinants of final outcome[45]. For metastasis and relapse, the major determinants are histology (myxoid) and tumor size. Myxoid tumors have low metastatic propensity (13%, 10-year metastatic rate) compared to non-myxoid tumors (40%, 10-year metastatic rate)[1]. Depending on the stage of disease and the depth of invasion by the tumor, surgical resection is the treatment of choice. Radiotherapy, chemotherapy, and immunotherapy are other therapeutic modalities. Long term follow-up with regular chest X-rays and CT scans of the abdomen to detect tumor recurrence, metastasis and any lymph node involvement are mandatory[4]. Postoperative recurrent leukocytosis and elevated CRP level may be predictors for recurrence of inflammatory MFH[1].
In summary, we present a series of patients with MFHs occurring as primary tumors in the abdominal cavity. Although our study consisted of a small sample size, our cases exhibited variable morphological findings at CT. However, signs suggestive of malignancy such as huge size, heterogeneous density, necrotic areas and heterogeneous mild to moderate enhancement were present. On plain CT, most lesions were hypointense and showed areas of low attenuation corresponding to necrosis. On contrast scan, solid components showed variable enhancement, depending on tumor vascularity and extent of necrosis. The intensity of enhancement may be dependent on the amount of fibrosis. Unlike previous findings, there was no evidence of calcification in our cases. Inflammatory varieties were more vascular and had a high metastatic propensity. Thus, we conclude that primary MFH of the abdominal viscera and gastrointestinal tract can be suggested as a diagnosis in a patient with a huge soft tissue mass containing areas of low attenuation and mild to moderate contrast enhancement.
COMMENTS
Background
Malignant fibrous histiocytoma (MFH) is a relatively rare tumor with very limited data which mainly focus on treatment. Thus, the authors retrospectively reviewed the computed tomography (CT) appearance of pathologically diagnosed cases of abdominal MFH to investigate CT features, which may help in early differentiation and the planning of necessary treatment modalities.
Research frontiers
Knowledge of the CT features of MFH is essential for correct identification and accurate differentiation, which are necessary for effective treatment.
Innovations and breakthroughs
The CT characteristics of abdominal MFH were described. We believe that CT which is readily available can better characterize the lesion and identify local and distant metastasis.
Applications
As a starting point in the abdominal imaging work-up, CT always follows ultrasound. This is a very important imaging modality because of its availability and depiction of the tumor mass, which is very important for planning treatment modalities and assessing prognosis. Contrast-enhanced scans should always be added which better delineate tumor vascularity and malignant potential. We hope that this illustration of the appearance of MFH helps radiologists and clinicians to achieve an appropriate imaging interpretation and clinical decision.
Peer review
This is not a scientific paper, but a pictorial essay. Abdominal MFH is rare disease and this pictorial essay is therefore important.
Footnotes
Peer reviewers: Vlastimil Valek, Professor, MD, CSc, MBA, Department of Radiology, University Hospital Brno and Med. Faculty Masaryk University Brno, Brno 62800, Czech Republic; Hiroshi Yoshida, Department of Surgery, Nippon Medical School Tama Nagayama Hospital, Tokyo 205-8512, Japan
S- Editor Cheng JX L- Editor Webster JR E- Editor Zheng XM
References
- 1.Fu DL, Yang F, Maskay A, Long J, Jin C, Yu XJ, Xu J, Zhou ZW, Ni QX. Primary intestinal malignant fibrous histiocytoma: two case reports. World J Gastroenterol. 2007;13:1299–1302. doi: 10.3748/wjg.v13.i8.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiss SW, Enzinger FM. Malignant fibrous histiocytoma: an analysis of 200 cases. Cancer. 1978;41:2250–2266. doi: 10.1002/1097-0142(197806)41:6<2250::aid-cncr2820410626>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 3.Kearney MM, Soule EH, Ivins JC. Malifnant fibrous histiocytoma: a retrospective study of 167 cases. Cancer. 1980;45:167–178. doi: 10.1002/1097-0142(19800101)45:1<167::aid-cncr2820450127>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 4.Atmatzidis KS, Pavlidis TE, Galanis IN, Papaziogas BT, Papaziogas TB. Malignant fibrous histiocytoma of the abdominal cavity: report of a case. Surg Today. 2003;33:794–796. doi: 10.1007/s00595-003-2577-4. [DOI] [PubMed] [Google Scholar]
- 5.Stadler FJ, Scott GA, Brown MD. Malignant fibrous tumors. Semin Cutan Med Surg. 1998;17:141–152. doi: 10.1016/s1085-5629(98)80007-2. [DOI] [PubMed] [Google Scholar]
- 6.Huang CC, Ko SF, Ng SH, Lee TY, Wan YL, Lin JW, Chen WJ. Cystic malignant fibrous histiocytoma of the gastrocolic ligament. Br J Radiol. 2001;74:651–653. doi: 10.1259/bjr.74.883.740651. [DOI] [PubMed] [Google Scholar]
- 7.Ros PR, Viamonte M, Rywlin AM. Malignant fibrous histiocytoma: mesenchymal tumor of ubiquitous origin. AJR Am J Roentgenol. 1984;142:753–759. doi: 10.2214/ajr.142.4.753. [DOI] [PubMed] [Google Scholar]
- 8.Bruneton JN, Drouillard J, Rogopoulos A, Laurent F, Normand F, Balu-Maestro C, Monticelli J. Extraretroperitoneal abdominal malignant fibrous histiocytoma. Gastrointest Radiol. 1988;13:299–305. doi: 10.1007/BF01889085. [DOI] [PubMed] [Google Scholar]
- 9.Ko SF, Wan YL, Lee TY, Ng SH, Lin JW, Chen WJ. CT features of calcifications in abdominal malignant fibrous histiocytoma. Clin Imaging. 1998;22:408–413. doi: 10.1016/s0899-7071(98)00066-7. [DOI] [PubMed] [Google Scholar]
- 10.Castillo M, Davis PC, Takei YD, Schwartzberg DG, Hoffman JC. Intracranial cystic malignant fibrous histiocytoma in a child: sonographic and CT findings. Pediatr Radiol. 1990;20:194–195. doi: 10.1007/BF02012974. [DOI] [PubMed] [Google Scholar]
- 11.Enzinger FM, Weiss SW. Malignant fibrohistiocytic tumors. In: Stamathis G, editor. Soft Tissue Tumors, 2nd ed. St. Louis, MO: Mosby; 1988. pp. 269–300. [Google Scholar]
- 12.Ajisaka H, Maeda K, Uchiyama A, Miwa A. Myxoid malignant fibrous histiocytoma of the breast: report of a case. Surg Today. 2002;32:887–890. doi: 10.1007/s005950200173. [DOI] [PubMed] [Google Scholar]
- 13.Kyriakos M, Kempson RL. Inflammatory fibrous histiocytoma. An aggressive and lethal lesion. Cancer. 1976;37:1584–1606. doi: 10.1002/1097-0142(197603)37:3<1584::aid-cncr2820370349>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 14.Kato T, Kojima T, Shimizu T, Sasaki H, Abe M, Okushiba S, Kondo S, Kato H, Sato H. Inflammatory malignant fibrous histiocytoma of the gallbladder: report of a case. Surg Today. 2002;32:81–85. doi: 10.1007/s595-002-8121-z. [DOI] [PubMed] [Google Scholar]
- 15.Murata I, Makiyama K, Miyazaki K, Kawamoto AS, Yoshida N, Muta K, Itsuno M, Hara K, Nakagoe T, Tomita M. A case of inflammatory malignant fibrous histiocytoma of the colon. Gastroenterol Jpn. 1993;28:554–563. doi: 10.1007/BF02776955. [DOI] [PubMed] [Google Scholar]
- 16.Rosenberg AE. Malignant fibrous histiocytoma: past, present, and future. Skeletal Radiol. 2003;32:613–618. doi: 10.1007/s00256-003-0686-1. [DOI] [PubMed] [Google Scholar]
- 17.Yu RS, Chen Y, Jiang B, Wang LH, Xu XF. Primary hepatic sarcomas: CT findings. Eur Radiol. 2008;18:2196–2205. doi: 10.1007/s00330-008-0997-7. [DOI] [PubMed] [Google Scholar]
- 18.Ding GH, Wu MC, Yang JH, Cheng SQ, Li N, Liu K, Dai BH, Cong WM. Primary hepatic malignant fibrous histiocytoma mimicking cystadenocarcinoma: a case report. Hepatobiliary Pancreat Dis Int. 2006;5:620–623. [PubMed] [Google Scholar]
- 19.Anagnostopoulos G, Sakorafas GH, Grigoriadis K, Kostopoulos P. Malignant fibrous histiocytoma of the liver: a case report and review of the literature. Mt Sinai J Med. 2005;72:50–52. [PubMed] [Google Scholar]
- 20.Ferrozzi F, Bova D. Hepatic malignant fibrous histiocytoma: CT findings. Clin Radiol. 1998;53:699–701. doi: 10.1016/s0009-9260(98)80298-5. [DOI] [PubMed] [Google Scholar]
- 21.Yu JS, Kim KW, Kim CS, Yoon KH, Jeong HJ, Lee DG. Primary malignant fibrous histiocytoma of the liver: imaging features of five surgically confirmed cases. Abdom Imaging. 1999;24:386–391. doi: 10.1007/s002619900520. [DOI] [PubMed] [Google Scholar]
- 22.Wunderbaldinger P, Schima W, Harisinghani M, Saini S. Primary malignant fibrous histiocytoma of the liver: CT and MR findings. AJR Am J Roentgenol. 1998;171:900–901. doi: 10.2214/ajr.171.3.9725359. [DOI] [PubMed] [Google Scholar]
- 23.Chen HC, Chen CJ, Jeng CM, Yang CM. Malignant fibrous histiocytoma presenting as hemoperitoneum mimicking hepatocellular carcinoma rupture. World J Gastroenterol. 2007;13:6441–6443. doi: 10.3748/wjg.v13.i47.6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kweon JH, Choi CS, Im CJ, Seo GS, Choi SC. Malignant fibrous histiocytoma arising from the omentum presenting as hemoperitoneum. Gut Liver. 2010;4:241–244. doi: 10.5009/gnl.2010.4.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldman SM, Hartman DS, Weiss SW. The varied radiographic manifestations of retroperitoneal malignant fibrous histiocytoma revealed through 27 cases. J Urol. 1986;135:33–38. doi: 10.1016/s0022-5347(17)45507-8. [DOI] [PubMed] [Google Scholar]
- 26.Kim HC, Lee JM, Kim SH, Kim KW, Lee M, Kim YJ, Han JK, Choi BI. Primary gastrointestinal stromal tumors in the omentum and mesentery: CT findings and pathologic correlations. AJR Am J Roentgenol. 2004;182:1463–1467. doi: 10.2214/ajr.182.6.1821463. [DOI] [PubMed] [Google Scholar]
- 27.Hasegawa S, Kawachi H, Kurosawa H, Obi Y, Yamanaka K, Nakamura K, Abe T. Malignant fibrous histiocytoma in the ileum associated with intussusception. Dig Dis Sci. 2004;49:1156–1160. doi: 10.1023/b:ddas.0000037804.91628.8d. [DOI] [PubMed] [Google Scholar]
- 28.Kotan C, Kosem M, Alici S, Ilhan M, Tuncer I, Harman M. Primary malignant fibrous histiocytoma of the small intestine presenting as an intussusception: report of a case. Surg Today. 2002;32:1091–1095. doi: 10.1007/s005950200221. [DOI] [PubMed] [Google Scholar]
- 29.Kobayashi K, Narita H, Morimoto K, Hato M, Ito A, Sugiyama K. Primary malignant fibrous histiocytoma of the ileum: report of a case. Surg Today. 2001;31:727–731. doi: 10.1007/s005950170080. [DOI] [PubMed] [Google Scholar]
- 30.Fletcher CD, Gustafson P, Rydholm A, Willén H, Akerman M. Clinicopathologic re-evaluation of 100 malignant fibrous histiocytomas: prognostic relevance of subclassification. J Clin Oncol. 2001;19:3045–3050. doi: 10.1200/JCO.2001.19.12.3045. [DOI] [PubMed] [Google Scholar]
- 31.Fletcher CD. Pleomorphic malignant fibrous histiocytoma: fact or fiction? A critical reappraisal based on 159 tumors diagnosed as pleomorphic sarcoma. Am J Surg Pathol. 1992;16:213–228. [PubMed] [Google Scholar]
- 32.Fabre-Guillevin E, Coindre JM, Somerhausen Nde S, Bonichon F, Stoeckle E, Bui NB. Retroperitoneal liposarcomas: follow-up analysis of dedifferentiation after clinicopathologic reexamination of 86 liposarcomas and malignant fibrous histiocytomas. Cancer. 2006;106:2725–2733. doi: 10.1002/cncr.21933. [DOI] [PubMed] [Google Scholar]
- 33.Coindre JM, Mariani O, Chibon F, Mairal A, De Saint Aubain Somerhausen N, Favre-Guillevin E, Bui NB, Stoeckle E, Hostein I, Aurias A. Most malignant fibrous histiocytomas developed in the retroperitoneum are dedifferentiated liposarcomas: a review of 25 cases initially diagnosed as malignant fibrous histiocytoma. Mod Pathol. 2003;16:256–262. doi: 10.1097/01.MP.0000056983.78547.77. [DOI] [PubMed] [Google Scholar]
- 34.McCormick D, Mentzel T, Beham A, Fletcher CD. Dedifferentiated liposarcoma. Clinicopathologic analysis of 32 cases suggesting a better prognostic subgroup among pleomorphic sarcomas. Am J Surg Pathol. 1994;18:1213–1223. doi: 10.1097/00000478-199412000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Henricks WH, Chu YC, Goldblum JR, Weiss SW. Dedifferentiated liposarcoma: a clinicopathological analysis of 155 cases with a proposal for an expanded definition of dedifferentiation. Am J Surg Pathol. 1997;21:271–281. doi: 10.1097/00000478-199703000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Chibon F, Mariani O, Derré J, Malinge S, Coindre JM, Guillou L, Lagacé R, Aurias A. A subgroup of malignant fibrous histiocytomas is associated with genetic changes similar to those of well-differentiated liposarcomas. Cancer Genet Cytogenet. 2002;139:24–29. doi: 10.1016/s0165-4608(02)00614-3. [DOI] [PubMed] [Google Scholar]
- 37.Kim SJ, Ahn BC, Kim SR, Kim YB, Joo HJ, Lee KB, Kim YS. Primary malignant fibrous histiocytoma of the kidney. Yonsei Med J. 2002;43:399–402. doi: 10.3349/ymj.2002.43.3.399. [DOI] [PubMed] [Google Scholar]
- 38.Matsui Y, Kobayashi S, Sugino Y, Iwamura H, Oka H, Fukuzawa S, Takeuchi H. [Malignant fibrous histiocytoma originating in a renal capsule: a case report] Hinyokika Kiyo. 2001;47:727–729. [PubMed] [Google Scholar]
- 39.Goodman AJ, Greaney MG. Malignant fibrous histiocytoma of the bladder. Br J Urol. 1985;57:106–107. doi: 10.1111/j.1464-410x.1985.tb08996.x. [DOI] [PubMed] [Google Scholar]
- 40.Zhang G, Chen KK, Manivel C, Fraley EE. Sarcomas of the retroperitoneum and genitourinary tract. J Urol. 1989;141:1107–1110. doi: 10.1016/s0022-5347(17)41184-0. [DOI] [PubMed] [Google Scholar]
- 41.Kulmala RV, Seppänen JH, Vaajalahti PJ, Tammela TL. Malignant fibrous histiocytoma of the prostate. Case report. Scand J Urol Nephrol. 1994;28:429–431. doi: 10.3109/00365599409180527. [DOI] [PubMed] [Google Scholar]
- 42.Glazier DB, Vates TS, Cummings KB, Pickens RL. Malignant fibrous histiocytoma of the spermatic cord. J Urol. 1996;155:955–957. [PubMed] [Google Scholar]
- 43.Lee YT. Leiomyosarcoma of the gastro-intestinal tract: general pattern of metastasis and recurrence. Cancer Treat Rev. 1983;10:91–101. doi: 10.1016/0305-7372(83)90007-5. [DOI] [PubMed] [Google Scholar]
- 44.Qureshi NA, Hallissey MT, Fielding JW, Gourevitch D. Primary intra-abdominal malignant fibrous histiocytoma presenting as pyrexia of unknown origin--report of a case with review of literature. Int Semin Surg Oncol. 2006;3:15. doi: 10.1186/1477-7800-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zagars GK, Mullen JR, Pollack A. Malignant fibrous histiocytoma: outcome and prognostic factors following conservation surgery and radiotherapy. Int J Radiat Oncol Biol Phys. 1996;34:983–994. doi: 10.1016/0360-3016(95)02262-7. [DOI] [PubMed] [Google Scholar]