Abstract
Objectives
To evaluate clinical, laboratory, and quantitative cerebrospinal fluid (CSF) cryptococcal cell counts for associations with in-hospital outcomes of HIV-infected patients with cryptococcal meningitis.
Design
Retrospective study.
Methods
98 HIV-infected adult patients with CSF culture-proven cryptococcal meningitis admitted between January 2006 and June 2008 at a referral center in Sao Paulo, Brazil.
Results
Cryptococcal meningitis was the first AIDS-defining illness in 69% of whom 97% (95/98) had known prior HIV-infection. The median CD4+ T cell count was 39 cells/mcL (IQR: 17–87 cells/mcL). Prior antiretroviral therapy (ART) was reported in 50%. Failure to sterilize the CSF by 7–14 days was associated with baseline fungal burden of ≥10 yeasts/mcL by quantitative CSF microscopy (OR=15.3, 95% CI: 4.1–56.7;P<.001) and positive blood cultures (OR=11.5, 95% CI:1.2–109;P=.034). At 7–14 days, ≥10 yeasts/mcL CSF was associated with positive CSF cultures in 98% vs. 36% when <10 yeasts/mcL CSF (P<.001). In-hospital mortality was 30% and associated with symptoms duration for >14 days, altered mental status (P<.001), CSF WBC counts <5 cells/mcL (P=.027), intracranial hypertension (P=.011), viral loads >50,000 copies/mL (P=.036), ≥10 yeasts/mcL CSF at 7–14 days (P=.038), and intracranial pressure >50 cmH20 at 7–14 days (P=.007).
Conclusion
Most patients were aware of their HIV-status. Fungal burden of ≥10 yeasts/mcL by quantitative CSF microscopy predicted current CSF culture status and may be useful to customize the induction therapy. High uncontrolled intracranial pressure was associated with mortality.
Introduction
Cryptococcal meningitis causes significant overall mortality among HIV-infected patients from low and middle-income regions (Satishchandra et al., 2000; Harrison, 2009; Ganiem et al., 2009; Prado et al., 2009; Jarvis et al., 2010). Most published studies have been reported from sub-Saharan Africa, South Asia, and Southeast Asia, but data are scarce from South America (Park et al., 2009).
AIDS-associated cryptococcosis has decreased less dramatically in Brazil (Guimarães, 2000; Pappalardo et al., 2003), a middle-income country with universal access to antiretroviral therapy (ART) (Greco et al., 2007), when compared to developed countries (Mirza et al., 2003; Jarvis et al., 2007). Cryptococcal meningitis continues to be a common complication in Brazil (Leimann et al., 2009), representing the primary cause of opportunistic meningitis and the second most frequent neurologic opportunistic infection in HIV-infected patients (Oliveira et al., 2006; Vidal et al., 2008).
In this study, we report clinical and laboratory characteristics associated with clinical outcomes among HIV-infected patients with cryptococcal meningitis at a referral center in Sao Paulo, Brazil.
Patients and Methods
We retrospectively studied HIV-infected adult patients with a first diagnosis of cryptococcal meningitis admitted between January 2006 and June 2008 at the Emilio Ribas Institute of Infectious Diseases, Sao Paulo, Brazil. This hospital is a 250-bed tertiary teaching center and is the main public referral institution for HIV-infected patients in the Sao Paulo State. The institute serves primarily a population with low socioeconomic status. In these patients, HIV infection was diagnosed by enzyme-linked immunosorbent assay (ELISA) and confirmed by western blot. The diagnosis of cryptococcal meningitis was based on Cryptococcus neoformans cultured from cerebrospinal fluid (CSF). Quantitative CSF cultures were not performed, but quantitative CSF yeast cell counts were performed by direct microscopic exam with a Fuchs-Rosenthal cell counting chamber, quantifying how many yeast cells/mcL of CSF were present (the protocol is showed in Table 1).
Table 1.
The Fuchs-Rosenthal counting chamber can be used for cerebrospinal fluid (CSF) cell counts. This is the normal cell counting chamber that is often used to measure WBC total and differential counts. We adapted this method to quantitatively count yeasts in patients with cryptococcal meningitis.
| 1) PREPARING THE SAMPLE | ||
| The CSF collected in a sterile test tube should contain no visible trace of blood. To place a cover slide in the chamber. After carefully manual homogenization the CSF is filled by capillary attraction into the chamber using a pipette. The CSF will be filled into the chamber without having to be stained first. Care is to be given to make sure that just enough CSF is drawn up with no air bubbles and that the CSF fluid does not spill over the edges of the chamber. After a sedimentation period of approximately one minute, the counting of the yeasts can begin. | ||
| 2) CALCULATING THE YEAST DENSITY |  |
|
| A) SETTING ONE: LOW BURDEN | ||
| Computing the volume | ||
| “A” = total area has 16 large squares sub-divided in 256 small squares | ||
| “AR” = Area of each small square: 0.0625 mm2 | ||
| “P” = Depth of each one of the small square: 0.2 mm | ||
| “VR” = Volume of each small square: | ||
| Calculating formula: | ||
| “VT” = Total volume of the chamber: | ||
| Calculating formula: | ||
| Calculating the number of yeasts (yeasts/mm3) | ||
| Count all the chamber (256 small squares) and divide by “VT” (3.2 mm3) = yeasts/mm3 or mcL | ||
| B) SETTING TWO: HIGH BURDEN | ||
| If there are more than 10 yeasts in each small square, to do a mean (M) in 10 small squares and to multiply by a correction factor (F). The result will be expressed in yeasts/mm3or mL | ||
| Example: M=19, thereby: 19X80=1520 yeasts/mm3or mcL | ||
At the Emilio Ribas Institute of Infectious Diseases, routine clinical care consists typically of all HIV-infected patients receiving a brain computed tomography (CT) scan prior to lumbar puncture. After diagnosis, the patients usually received induction therapy with amphotericin B deoxycholate (0.7 mg/kg/day) for 4–6 weeks. If necessary, more prolonged induction therapy was used. Consolidation therapy was with oral fluconazole 400–800mg per day for at least 4–6 weeks.
Disseminated cryptococcosis was defined as the involvement of ≥2 noncontiguous sites. Increased ICP was defined as opening CSF pressure >20 cm H2O, measured via lumbar puncture (LP). In cases of opening ICP ≥25 cm H2O, the routine practice included repeat therapeutic LPs daily until the CSF opening pressure and symptoms were stabilized. Neurosurgery was pursued for refractory elevated ICP if therapeutic LPs failed to normalize pressures, typically after >14 days. Routine CSF diagnostic studies including culture were performed weekly while hospitalized. Patients were routinely discharged after two sterile CSF cultures.
For this retrospective cohort study, we extracted demographic, clinical, and laboratory data from medical records using a standardized questionnaire. CSF findings were classified in two groups: ‘diagnostic CSF’ collected at initial hospital admission and ‘follow up CSF’ collected 7–14 days after admission. Analysis was primarily descriptive. The primary analysis focused on risk factors associated with in-hospital mortality. Statistical testing between groups (survivors vs. non-survivors) was assessed by Mann-Whitney U test for continuous variables with distributions presented as median and interquartile range (IQR). Fischer’s exact test was used for categorical variables. Factors associated with mortality in a univariate model with P<0.2 were considered as potential covariates in a multivariate logistic regression model (SPSS 18.0, IBM, Chicago, IL, USA). All P-values were two-sided. Variables with P ≤ 0.05 remained in the multivariate model with risk presented as an adjusted odds ratio (OR). The study was approved by the ethical and scientific boards of the Emilio Ribas Institute of Infectious Diseases.
Results
Demographic, clinical and laboratory characteristics
During the 30-month study period, 113 patients had CSF cultures positive for C. neoformans. Eight (7%) records were unavailable, and seven (6%) cases did not meet the inclusion criteria (six HIV-negative, and one child). Of the 98 adults included in the study, 76 (78%) were male with a median age of 38 (range: 18–56) years.
HIV diagnoses were most often not new with 97% (95/98) having a prior HIV diagnosis, 31% (30/98) having previous AIDS-defining disease, and 50% (46/98) having prior ART use, though ART adherence was unknown. The time between HIV diagnosis and cryptococcal meningitis was a median of 84 months (IQR: 12–120 months). The median CD4+ T cell count (n=85) was 36 cells/mcL (IQR: 17–87 cells/mcL). Sixty five percent of patients (n=55) had CD4+ T cell count ≤50 cells/mcL and 14% (n=12) had 51–100 cells/mcL. However, cryptococcosis also occurred at higher absolute CD4+ T cell counts: 12% (n=10) had 101–200 cells/mcL and 9% (n=8) had >200 cells/mcL. CD4+ T cell counts were similar among patients with and without prior ART use with a median of 30 cells/mcL (IQR: 16–72 cells/mcL) in ART-naïve vs. 39 cells/mcL (IQR: 19–96 cells/mcL) with prior ART use (P=.42). Of 34 persons prescribed ART who had a viral load measured, only 6 (18%) had HIV-1 viral loads <400 copies/mL of whom only 2 had <50 copies/mL. Data were unavailable on duration of ART use and ART adherence.
Presenting symptoms were typical for cryptococcal meningitis. The most frequent symptoms were headache in 91% (87/96), nausea/vomiting in 56% (54/96), and fever in 55% (53/96). Altered mental status was observed in 31% (30/96) cases with a GCS<15, but coma was reported in only 3% (3/96) cases. Meningeal signs were documented in 12% (11/93) and seizures present in 17% (16/96). Neurologic symptoms were present at admission for a median of 14 days (IQR: 5–26 days). At admission, increased ICP was present in 54 (55%) cases. The median ICP was 31 cm H2O (IQR: 20 – 48 cm H2O). Serum cryptococcal antigen detection was positive in 37 (90%) of 41 patients tested.
Disseminated disease in addition to meningitis was reported in 52% (49/94) of cases: bloodstream fungaemia in 28% (n=26), pulmonary infection in 11% (n=10), bone marrow in 4% (n=4), and dermatologic manifestations in 2% (n=2). Concomitant pulmonary and bloodstream infection were observed in 6% (n=6), and concomitant pulmonary, bloodstream, skin and bone marrow in 1 person. Brain CT abnormalities were observed in 26 (31%) of 83 persons who received a CT and were: brain atrophy (n=20), pseudocysts (n=10), cryptococcoma(s) (n=7), and diffuse brain edema (n=2).
Quantitative CSF Microscopy
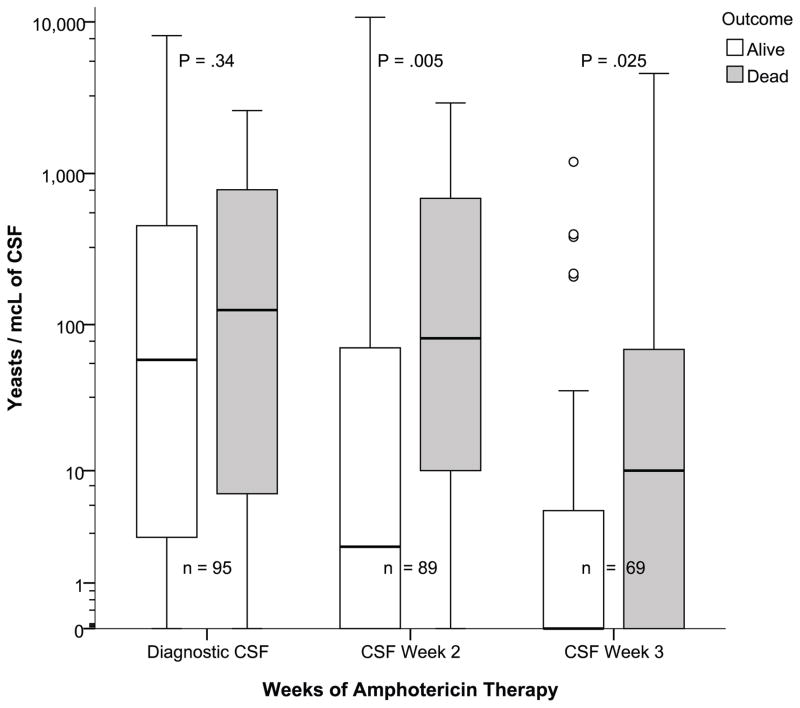
Quantitative CSF yeast counts were routinely performed. The diagnostic CSF specimen collected at admission had a median of 71 yeast/mcL of CSF (IQR: 3–512; range: 0 – 8160) and declined during amphotericin therapy with follow up CSF collected 7–14 days having a median of 11 yeast/mcL (IQR: 0 to 108, max:10720, P<.0001) with 55% of follow up CSF remaining culture-positive (Figure 1). On the follow up CSF, survivors had fewer yeast than non-survivors (median 6 vs. 81 yeast/mcL, P=.035) at 7–14 days. By three weeks, the quantitative yeast burden had decreased (median 1.0 yeast/mcL, IQR: 0 to 11, max: 4,566, P<.001).
Figure 1.
Temporal change in quantitative cerebrospinal fluid yeast counts.
We analyzed variables associated with lack of CSF culture sterility at 7–14 days. In univariate analysis, CD4 cell count <100 cells/mcL (P=.008), disseminated disease (P=.003), fungemia (P=.003), baseline fungal burden ≥ 10 yeast/mcL of CSF (P<.001), and CSF WBCs ≤25 cells/mcL (P=.022) were statistically associated with a positive follow up CSF culture. In a multivariate model, only baseline CSF fungal burden ≥10 yeast/mcL by quantitative microscopy (OR=15.3, 95% CI: 4.1–56.8; P<.001) and baseline fungemia (OR=11.5, 95% CI: 1.2–109; P=.034) remained associated with a positive follow up CSF culture at 7–14 day of amphotericin therapy. As all subjects received similar antifungal treatment, the burden of infection was the predominant factor affecting the timing of culture sterility.
The quantitative microscopy yeast count was useful in predicting the week two culture-status. Persons with quantitative CSF yeast counts <10/mcL at 7–14 days had positive CSF cultures in 37.5% (12/32) as compared to 98% (41/42) positive CSF cultures when ≥10 yeasts/mcL (P<.001). Similarly, among those with LPs performed between 15–21 days, quantitative microscopy of <5 yeasts/mcL CSF had 16% (6/37) CSF culture positivity vs. 57% (8/14) CSF culture positivity when CSF yeasts were ≥5 yeasts/mcL CSF (P=.011).
Intracranial Pressure
Management of increased ICP was not always optimal and/or there was not documentation of follow up therapeutic LPs. Of 38 patients with known initial increased ICP of >25 cmH2O, 68% (26/38) had documentation of receiving a follow up therapeutic LP. Of 34 patients who did not have an initial opening pressure documented, only 26% (9/34) had therapeutic LPs similar to the 26% (5/19) incidence when the ICP was initially measured as <25 cmH2O. Seven persons had missing documentation on therapeutic LPs. We analyzed variables associated persistently increased ICP at 7–14 days of treatment among 54 persons with a second LP with an opening pressure measured. Only CSF fungal burden ≥10 yeast/mcL collected between 7–14 days was associated with persistently elevated ICP >20 cmH2O between 7–14 days (OR=6.5, 95% CI: 1.7–24.4; P=.006).
Neurosurgery was performed in 18% (18/94). In 17 patients, the surgical indication was failure to control increased ICP after repeated therapeutic lumbar punctures, typically after ≥2 week of daily LPs. Interventions included: ventriculoperitoneal shunt (n=9), external lumbar drainage (n=6), external ventricular drainage (n=2), and lumbar peritoneal shunt (n=1). One of these patients first underwent external lumbar drainage which was then later converted to a ventriculoperitoneal shunt. In the one additional case without refractory increased ICP, the surgical indication was hydrocephalus at admission. Median time from admission to neurosurgery was 25 days (IQR: 16–42 days). Survival was 67% (6/9) in ventriculoperitoneal shunts and 17% (1/5) in external lumbar drains.
Outcomes and characteristics associated with mortality
The in-hospital case-fatality rate was 30% (n=29). The attribute cause of death was cryptococcal meningitis in 52% of deaths (n=15) and other nosocomial infections in 48% (n=14). Among patients undergoing neurosurgery, the case-fatality rate was 56% (10/18). Demographic, clinical, and laboratory features in survivors and nonsurvivors patients are shown in Table 2.
Table 2.
Demographic, clinical and laboratory features in survivors and non-survivors in 98 HIV-infected patients with cryptococcal meningitis.
| Variable, median (IQR) | Survivors n=69 | Non-survivors n=29 | P - value |
|---|---|---|---|
| Age years | 39 (32.5–44) | 38 (35– 42.5) | 0.63 |
| Gender (male, %) | 52 (75%) | 24 (83%) | 0.30 |
| Previous HIV diagnosis, n (%) | 66 (96%) | 29 (100%) | 0.55 |
| Previous AIDS-defining illness, n (%) | 22 (35%) | 8(32%) | 0.81 |
| Prior ART use, n (%) | 32 (48%) | 14 (48%) | 0.99 |
| Duration of HIV diagnosis in months | 96 (25.5–120) | 60 (15–120) | 0.52 |
| Symptoms duration in days | 14 (5–20) | 16.5 (7.25–30) | 0.030 |
| Disseminated disease, n (%) | 35 (54%) | 14 (52%) | 0.99 |
| Blood culture positivity, n (%) | 18 (27%) | 8 (30%) | 0.80 |
| Increased ICP, n (%), n=89 | 33 (51%) | 21 (87%) | 0.003 |
| Abnormal mental status | 14 (20%) | 16 (55%) | 0.001 |
| Seizures, n (%) | 7 (10%) | 9 (31%) | 0.012 |
| Need for CSF shunt / drainage, n (%) | 8 (12%) | 10 (34.5%) | 0.011 |
| HIV RNA log10 copies/mL | 4.1 (3.2–5.3) | 5.5 (5.0–5.7) | 0.004 |
| CD4 count cells/mcL, n=85 | 38 (21–106) | 44 (16 –110) | 0.97 |
| Hemoglobin g/dL | 12.0 (10.6 – 13.5) | 11.9 (10.0– 13.0) | 0.60 |
| Opening pressure of CSF, cm H2O | |||
| CSF at admission, n=43,16 | 30 (18–41) | 36 (22 – 71) | 0.23 |
| CSF at 7–14 days, n-40, 14 | 21 (13.5– 38) | 48 (22 – 75) | 0.027 |
| CSF at >14 days, n=24, 8 | 17.5 (10–25) | 28.5 (22–58) | 0.013 |
| CSF quantative yeasts/mcL count | |||
| CSF at admission, n=68, 27 | 58.5 (3 – 453) | 125 (8 – 782) | 0.34 |
| CSF at 7–14 days, n=66, 23 | 3 (0 – 70) | 81 (10 – 690) | 0.005 |
| CSF at 15–21 days, n=50, 19 | 0 (0 – 5) | 10 (0 – 90) | 0.025 |
ART = antiretroviral therapy; CSF = cerebrospinal fluid. ICP = intracranial pressure. Non-parametric data are presented as median and interquartile range (IQR). Categorical and nominal data are presented as n(%). Statistical testing is by Fisher’s exact test for categorical variables, Mann-Whitney U testing for non-parametric data.
We identified a number of demographic features associated with all-cause mortality (Table 3). Most notably, independent risk factors for mortality included: antecedent symptom duration >14 days, abnormal mental status, HIV-1 viral load >50,000 copies/mL, normal CSF WBC counts <5 cells/mcL, increased ICP, persistently increased ICP between 7–14 days.
Table 3.
Multivariate risk factors for all-cause mortality in 98 HIV-infected patients with cryptococcal meningitis.
| Variable | Adjusted Odds Ratio | 95% CI | P-value |
|---|---|---|---|
| Abnormal mental status | 18.0 | 3.8 – 84 | < 0.001 |
| Symptoms duration | |||
| <14 days | 1.0 | Ref | Ref |
| 15–28 days | 3.9 | 0.7 – 22.5 | 0.12 |
| >28 days | 10.8 | 2.0 – 57.3 | 0.005 |
| CSF WBC count | |||
| ≤ 5 cells/mcL | 4.6 | 1.1 – 18.9 | 0.027 |
| 6–25 cells/mcL | 2.3 | 0.3 – 20.1 | 0.40 |
| >25 cells/mcL | 1.0 | Ref | Ref |
| CNS shunt placement / drainage | 2.8 | 0.6 – 12.8 | 0.19 |
| Increased intracranial pressure | 15.0 | 2.1 –107 | 0.007 |
| Viral load > 50,000 copies/mL | 5.8 | 1.1 – 30 | 0.037 |
| At day 7–14 lumbar puncture 1 | |||
| CSF quantitative yeast count ≥10/mcL (n=89) | 6.9 | 1.1 – 43.0 | 0.038 |
| Opening pressure ≥50 cm H20 (n=14) | 14.6 | 1.7 – 124 | 0.014 |
| Opening pressure not documented (n=35) | 5.7 | 0.5 – 63.4 | 0.154 |
| Opening pressure <50 cm H20 (n=40) | 1.0 | Ref | Ref |
Day 7–14 lumbar puncture variables are adjusted for baseline predictors but not vice versa.
Of the 89 persons who had a second LP, death was associated with a quantitative fungal burden ≥10 yeast/mcL on CSF collected 7–14 days (OR=6.9, 95% CI: 1.1–43.0; P=.038) and an opening pressure of >50 cmH2O (OR=14.6, 95% CI: 1.7–124, P=.014) as adjusted for baseline independent mortality risk factors listed above. For increased ICP, only opening pressures >50 cmH2O at 7–14 days were associated with increased mortality of 50% (7/14), as persons with ICP between 20–49 cmH2O had minimal increased mortality of 21% (5/24) (adjusted OR=1.1, P=.96) compared with normal ICP <20 cmH2O mortality of 12.5% (2/16). Of the 36 persons who did not have an opening pressure measured on their second LP, they also trended toward increased mortality of 25% (9/36) (adjusted OR=1.8, P=.51) as compared to the 16 persons with normal ICP <20 cmH2O, suggesting that a subset likely had markedly increased ICP. Thus while, the initial baseline fungal burden was not associated with in-hospital mortality, the follow up burden during week two was associated with mortality as was ongoing uncontrolled ICP of >50 cmH2O during week two.
Discussion
This study revealed three important clinical findings. First, quantitative CSF microscopy was a simple and useful tool which beyond 7 days was associated with CSF culture status and survival. Second, initial ICP was not associated with outcome; however uncontrolled ICP of >50 cm H2O after >7 days was associated with high mortality (≥50%). Third, we found a continued high case-fatality rate among Brazilian HIV-infected patients with cryptococcal meningitis. At admission, most patients were aware of their HIV-status, although cryptococcal meningitis was their first AIDS-defining condition. The majority of patients presented with features of severe disease including: abnormal mental status, increased ICP, disseminated disease, neurologic manifestations, and severe immunosuppression.
First, this study systematically utilized quantitative CSF microscopy to estimate fungal burden. This is a simple laboratory technique which can be processed while measuring a CSF WBC differential. Although quantitative CSF cultures can also identify fungal burden and the rate of clearance (Bicanic et al., 2009a; Bicanic et al., 2009b; Bicanic et al., 2011), quantitative cultures are not often used in routine practice, and there is an intrinsic delay in reporting. Thus, rapid and simple methods to estimate fungal burden remain clinically useful worldwide. In Brazil, several tertiary laboratories use the direct exam with the Fuchs-Rosenthal cell counting chamber to estimate the quantitative burden of infection. We evaluated several cut-offs (data not shown) but the best cutoff was ≥10 yeasts/mcL in CSF collected after 7 days of treatment which was independently associated with CSF culture positivity and all-cause mortality. A very useful clinical test was ≥10 yeasts/mcL in CSF at 7–14 days was associated with 98% CSF culture positivity. This cut-off is clinically useful to predict who should be targeted for prolonged induction amphotericin therapy or higher dose consolidation therapy starting at fungicidal doses of fluconazole at ≥800mg/day.
Second, only very high opening pressure in the CSF of >50 cm H2O when collected ≥7days after admission were associated with mortality. These findings confirm the value of ICP management during cryptococcal meningitis treatment in that lack of ICP control is associated with mortality. Similar findings have been reported previously in three African locations (Bicanic et al., 2009a; Bicanic et al., 2009b, Bicanic et al., 2011; Longley et al., 2008, Kambugu et al., 2008). Bicanic et al. reported a significant association between day 14 CSF fungal burden and day 14 opening pressure, suggesting that improved clearance of infection may help prevent persistence of raised ICP (Bicanic et al., 2009a). We also identified a significant association between increased ICP and ≥10 yeasts/mcL in CSF collected 7 to 14 days after admission.
Third, the present study reported an in-hospital mortality of 30%, despite being performed in a referral center with a reasonable infrastructure. There is scarce information about case-fatality rate of HIV-infected patients with cryptococcal meningitis from South America in routine practice. In unselected hospital-based studies performed in Brazil and Argentina, case-fatality rates have ranged from 31.5% to 62.5% (Metta et al., 2002; Pasqualotto et al., 2004; Moreira et al., 2006; Pappalardo et al., 2007; Mónaco et al., 2008; Lindenberg et al., 2008; Mora et al, 2011). Taken together, these studies confirm that acute mortality of cryptococcal meningitis remains unacceptably high in South America despite the administration of amphotericin B yet with frequent non-optimal measures of ICP control.
Limitations
Limitations include problems with a retrospective study including the absence of a standardized history, lab information, and neurologic examination as well as long-term follow up data. ,Although this is a large series of AIDS-associated cryptococcal meningitis of a single institution, we these results are representative of outcomes in routine practice in Brazil with the available sample size allowing for the detection of the most significant clinical associations with mortality.
In conclusion, AIDS-associated cryptococcal meningitis remains an important cause of mortality in Brazil, despite the increasing ART availability. We report that quantitative CSF microscopy is a useful intervention to predict CSF culture status and thereby potentially to tailor anti-fungal therapy to an individual patient. We believe that quantitative CSF microcopy is a simple tool which is broadly implementable. Future challenges in our setting include: optimizing adjunct antifungal treatment (e.g. addition of 5-flucytosine or fluconazole ≥800mg/day to amphotericin-based therapy), evaluating the role of alternative approaches (e.g. immunotherapy such as adjunct interferon-gamma), evaluating standardized ICP management, and finally determining the most efficient public health strategy to avoid severe immunosuppression in HIV-infection patients who are aware of their HIV-status.
Acknowledgments
We would like to thank Thomas Harrison, Professor of Infectious Diseases and Medicine, St George’s University of London, for helpful advice and discussions. DRB is supported by the U.S. National Institutes of Health, NIAID (K23AI073192).
Footnotes
Conflicts of Interest: For the remaining authors none were declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bicanic T, Brouwer AE, Meintjes G, Rebe K, Limmathurotsakul D, Chierakul W, et al. Relationship of cerebrospinal fluid pressure, fungal burden and outcome in patients with cryptococcal meningitis undergoing serial lumbar punctures. AIDS. 2009a;23:701–706. doi: 10.1097/QAD.0b013e32832605fe. [DOI] [PubMed] [Google Scholar]
- Bicanic T, Muzoora C, Brouwer AE, Meintjes G, Longley N, Taseera K, et al. Independent association between rate of clearance of infection and clinical outcome of HIV-associated cryptococcal meningitis: analysis of a combined cohort of 262 patients. Clin Infect Dis. 2009b;49:702–709. doi: 10.1086/604716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicanic T, Jarvis J, Loyse A, Jackson A, Muzoora C, Wilson D, et al. Determinants of acute outcome and long-term survival in HIV-associated cryptococcal meningitis: results from a combined cohort of 523 patients. Abstract 892. 18th Conference on Retroviruses and Opportunistic Infections; Boston. 2011. [Google Scholar]
- Ganiem AR, Parwati I, Wisaksana R, van der Zanden A, van de Beek D, Sturm P, et al. The effect of HIV infection on adult meningitis in Indonesia: a prospective cohort study. AIDS. 2009;23:2309–2316. doi: 10.1097/QAD.0b013e3283320de8. [DOI] [PubMed] [Google Scholar]
- Guimarães MD. Temporal study in AIDS-associated disease in Brazil, 1980–1999. Cad Saude Publica. 2000;16(suppl 1):S21–S36. [PubMed] [Google Scholar]
- Greco DB, Simão M. Brazilian policy of universal access to AIDS treatment: sustainability challenges and perspectives. AIDS. 2007;21(Suppl 4):37–45. doi: 10.1097/01.aids.0000279705.24428.a3. [DOI] [PubMed] [Google Scholar]
- Harrison TS. The burden of HIV-associated cryptococcal disease. AIDS. 2009;23:531–532. doi: 10.1097/QAD.0b013e328322ffc3. [DOI] [PubMed] [Google Scholar]
- Jarvis JN, Harrison TS. HIV-associated cryptococcal meningitis. AIDS. 2007;21:2119–2129. doi: 10.1097/QAD.0b013e3282a4a64d. [DOI] [PubMed] [Google Scholar]
- Jarvis JN, Meintjes G, Williams A, Brown Y, Crede T, Harrison TS. Adult meningitis in a setting of high HIV and TB prevalence: findings from 4961 suspected cases. BMC Infect Dis. 2010;10:67. doi: 10.1186/1471-2334-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambugu A, Meya DB, Rhein J, O'Brien M, Janoff EN, Ronald, et al. Outcomes of cryptococcal meningitis in Uganda before and after the availability of highly active antiretroviral therapy. Clin Infect Dis. 2008;46:1694–1701. doi: 10.1086/587667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leimann BCQ, Koifman RJ. Official information systems for cryptococcal meningitis, state of Rio de Janeiro, Southeastern Brazil. Rev Saúde Pública. 2009;43:1–4. doi: 10.1590/s0034-89102009005000029. [DOI] [PubMed] [Google Scholar]
- Lindenberg Ade S, Chang MR, Paniago AM, Lazéra Mdos S, Moncada PM, Bonfim GF, Nogueira SA, Wanke B. Clinical and epidemiological features of 123 cases of cryptococcosis in Mato Grosso do Sul, Brazil. Rev Inst Med Trop Sao Paulo. 2008;50:75–78. doi: 10.1590/s0036-46652008000200002. [DOI] [PubMed] [Google Scholar]
- Longley N, Muzoora C, Taseera K, Mwesigye J, Rwebembera J, Chakera A, et al. Dose response effect of high-dose fluconazole for HIV-associated cryptococcal meningitis in southwestern Uganda. Clin Infect Dis. 2008;47:1556–1561. doi: 10.1086/593194. [DOI] [PubMed] [Google Scholar]
- Metta HA, Corti ME, Negroni R, Helou S, Arechavala A, Soto I, et al. Disseminated cryptococcosis in patients with AIDS. Clinical, microbiological, and immunologoical analysis of 51 patients. Rev Argent Microbiol. 2002;34:117–123. [PubMed] [Google Scholar]
- Mirza SA, Phelan M, Rimland D, Graviss E, Hamill R, Brandt ME, et al. The changing epidemiology of cryptococcosis: an update from population-based active surveillance in 2 large metropolitan areas. Clin Infect Dis. 2003;36:789–794. doi: 10.1086/368091. [DOI] [PubMed] [Google Scholar]
- Mónaco LS, Tamayo Antabak N. Cryptococcosis in AIDS patients: case study from 1996 to 2006 in Paroissien Hospital. Rev Argent Microbiol. 2008;40:218–221. [PubMed] [Google Scholar]
- Mora DJ, da Cunha Colombo ER, Ferreira-Paim K, Andrade-Silva LE, Nascentes GA, Silva-Vergara ML. Clinical, epidemiological and outcome features of patients with cryptococcosis in Uberaba, Minas Gerais, Brazil. Mycopathologia. 2011 Dec 1; doi: 10.1007/s11046-011-9504-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Moreira TA, Ferreira MS, Ribas RM, Borges AS. Cryptococcosis: clinical epidemiological laboratorial study and fungi varieties in 96 patients. Rev Soc Bras Med Trop. 2006;39:255–258. doi: 10.1590/s0037-86822006000300005. [DOI] [PubMed] [Google Scholar]
- Pappalardo MCSM, Melhem MSC. Cryptococcosis: a review of the Brazilian experience for the disease. Rev Inst Med Trop S Paulo. 2003;45:299–305. doi: 10.1590/s0036-46652003000600001. [DOI] [PubMed] [Google Scholar]
- Pappalardo MCSM, Paschoal RC, Melhem MSC. AIDS-associated central nervous system cryptococcosis: a Brazilian case study. AIDS. 2007;21:1971–1983. doi: 10.1097/01.aids.0000287549.79368.33. [DOI] [PubMed] [Google Scholar]
- Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23:525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- Pasqualotto AC, Severo CB, Oliveira FM, Severo LC. Cryptococcemia. An analysis of 28 cases with emphasis on the clinical outcome and its etiologic agent. Rev Iberoam Micol. 2004;21:143–146. [PubMed] [Google Scholar]
- Prado M, Silva MB, Laurenti R, Travassos LR, Taborda CP. Mortality due to systemic mycoses as a primary cause of death or in association with AIDS in Brazil: a review from 1996 to 2006. Mem Inst Oswaldo Cruz. 2009;104:513–521. doi: 10.1590/s0074-02762009000300019. [DOI] [PubMed] [Google Scholar]
- Satishchandra P, Nalini A, Gourie-Devi M, Khanna N, Santosh V, Ravi V, et al. Profile of neurologic disorders associated with HIV/AIDS from Bangalore, south India (1989–96) Indian J Med Res. 2000;111:14–23. [PubMed] [Google Scholar]
- Oliveira JF, Greco DB, Oliveira GC, Christo PP, Guimarães MD, Oliveira RC. Neurological disease in HIV-infected patients in the era of highly active antiretroviral treatment: a Brazilian experience. Rev Soc Bras Med Trop. 2006;39:146–151. doi: 10.1590/s0037-86822006000200002. [DOI] [PubMed] [Google Scholar]
- Vidal JE, Penalva de Oliveira AC, Fink MC, Pannuti CS, Trujillo JR. AIDS related progressive multifocal leukoencephalopathy: a retrospective study in a referral center in São Paulo, Brazil. Rev Inst Med Trop Sao Paulo. 2008;50:209–212. doi: 10.1590/s0036-46652008000400004. [DOI] [PubMed] [Google Scholar]