Abstract
Background
High grade primary bone sarcomas are rare cancers that affect mostly children and young adults. Osteosarcoma and Ewing sarcoma are the most common histological subtypes in this age group, with current multimodality treatment strategies achieving 55-70% overall survival. As there remains an urgent need to develop new therapeutic interventions, we have reviewed published phase I/II trials that have been reported for osteosarcoma and Ewing sarcoma in the last twenty years.
Results
We conducted a literature search for clinical trials between 1990 and 2010, either for trials enrolling bone sarcoma patients as part of a general sarcoma indication or trials specifically in osteosarcoma and Ewing sarcoma. We identified 42 clinical trials that fulfilled our search criteria for general sarcoma that enrolled these patient groups, and eight and twenty specific trials for Ewing and osteosarcoma patients, respectively. For the phase I trials which enrolled different tumour types our results were incomplete, because the sarcoma patients were not mentioned in the PubMed abstract. A total of 3,736 sarcoma patients were included in these trials over this period, 1,114 for osteosarcoma and 1,263 for Ewing sarcoma. As a proportion of the worldwide disease burden over this period, these numbers reflect a very small percentage of the potential patient recruitment, approximately 0.6% for Ewing sarcoma and 0.2% for osteosarcoma. However, these data show an increase in recent activity overall and suggest there is still much room for improvement in the current trial development structures.
Conclusion
Lack of resources and commercial investment will inevitably limit opportunity to develop sufficiently rapid improvements in clinical outcomes. International collaboration exists in many well founded co-operative groups for phase III trials, but progress may be more effective if there were also more investment of molecular and translational research into disease focused phase I/II clinical trials. Examples of new models for early translational and early phase trial collaboration include the European based EuroBoNeT network, the Sarcoma Alliance for Research through Collaboration network (SARC) and the new European collaborative translational trial network, EuroSarc.
Keywords: Ewing sarcoma, osteosarcoma, early phase trials, translational research
Background
Primary bone sarcomas are rare, and account for approximately 6% of all childhood malignancies, with Ewing sarcoma and osteosarcoma accounting for approximately 3% of tumours arising in teenagers. Here we focus on osteosarcoma and Ewing sarcoma, the two most common bone sarcomas, whose treatments are similar as they involve multimodality treatment with dose intensive and toxic chemotherapy, combined with potentially mutilating surgery.
Osteosarcoma is the most common primary malignant tumour arising from bone. It is a pleiomorphic tumour of bone, and based on animal model systems, thought to arise in mesenchymal stem cells in which the mutant proliferating spindle cells produce osteoid or immature bones [1]. The EMEA published data in 2009 estimating approximately five people in 1,000,000 in the European Union (EU) are affected by osteosarcoma. Recently, data from the RARECARE surveillance network revealed an incidence of 0.23 per 100,000, amounting to approximately 1135 new cases per year in EU27 [2]. Osteosarcoma mostly affects children and young adults with the median age of diagnosis being fifteen. Seventy-five percent of patients are between eight and twenty-five years old. Osteosarcoma is often located in the extremities of long bones near metaphyseal growth plates. Current treatments for osteosarcoma achieve 60-70% event-free survival (EFS) for patients who present with localized disease and approximately 20% EFS for patients with clinically detectable metastatic disease [3]. Of all patients, 20% have clinically detectable metastatic disease at first presentation. Surgical resection of all clinically detectable sites of disease and systemic therapy to control microscopic metastatic disease is currently the therapy of choice for early stage osteosarcoma. Since the introduction of neo-adjuvant and adjuvant chemotherapy to surgery in the early 1980s, the long term survival of patients with osteosarcoma has remained stable at about 60-65%. In terms of chemotherapy, several agents have demonstrated activity in osteosarcoma including cisplatin, doxorubicin, high-dose methotrexate with leucovorin rescue (HDMTX) and ifosfamide. For current treatment options these agents are combined. Since the early 1980s, trials have been conducted in which the variations in doses and scheduling between these four agents were tested, but these have not result in improvement of EFS [4]. Since the introduction of ifosfamide, more than two decades ago, the only new agent that has been approved is muramyl tripeptide, a drug that activates the innate immune system [5]. Thus, despite surgical resection of the primary tumour and aggressive adjuvant chemotherapy, 30%-40% still die of metastases that are resistant to conventional therapies [6-8]. For osteosarcoma patients with resectable pulmonary metastases it has become more standard to treat these patients with metastectomy. This has been shown to improve relapse free survival and a subgroup of patients may even be cured [9].
Ewing Sarcoma/Primitive Neuroectodermal (PNET) are the second most common bone malignancy after osteosarcoma in children and young adults with a peak incidence at age fifteen. Ewing sarcoma are diagnostically defined by a Ewing sarcoma EWS (Chromosome 22) translocation resulting in fusion with an ETS transcription factor, the commonest abnormality (85%) being EWS-FLI1 (Chromosome 11) or rarely with a non ETS family partner [10,11]. Although claimed in the past that the transcript type reflected a difference in prognoses [12] this proved not to be the case in prospective randomised trials [13]. RARECARE estimates an EU27 incidence rate for bone and soft tissue Ewing sarcoma of 0.13 per 100,000 and 0.05 per 100,000, respectively [2]. This translates to approximately 647 bone and 263 soft tissue new Ewing sarcoma diagnoses per year (EU27), with a predicted 65-75% 5 year survival for non-metastatic disease using conventional chemotherapy, radiotherapy, high dose chemotherapy and peripheral blood stem cell transplant (PBSCT). However, survival for the 25% of patients that present with metastatic disease is approximately 20% [14], and for those who develop relapsed and/or refractory disease, the survival is no more that 10%. To date, studies in the patient population requiring salvage treatment have been confined to chemotherapy combinations and high dose chemotherapy, with variable response rates and little or no impact on survival. During the last two decades, the outcome has improved in patients with localized disease. This has been achieved by dose intensification and standardisation of conventional therapeutics and radiotherapy. With the use of multidisciplinary treatments, such as chemotherapy (including vincristine, doxorubicin, cyclophosphamide, ifosfamide and etoposide), surgery and radiation therapy, the five year overall survival probability exceeds 75% for patients with non metastatic low volume Ewing sarcoma. However, advances in the treatment of Ewing sarcoma have not impacted on the outcome of patients with large volume and metastatic disease [15].
In the last two decades the treatment outcome for these bone sarcomas has not improved greatly, even though some new treatment interventions have been successfully tested. Large scale phase III trials with long durations of recruitment have established material for prognostic and treatment related correlative studies with survival and toxicity outcomes, and importantly, have formed the basis of international collaboration. However, the smaller phase I/II studies are also important in order to develop proof of principle single agent and combination treatments, particularly with newer molecular and biological based interventions that directly test disease specific molecular mechanisms.
We wished to establish the effective level of phase I/II activity that has been reported in peer reviewed publications in the last twenty years. We report an overview of the phase I/II trials that have been conducted for osteosarcoma and Ewing sarcoma, where we detail the kind of drugs that have been tested, what the published study outcomes were and what interventions have progressed to testing in phase III trials. The results show an improvement in overall activity, but that the number of studies and International collaborations in early phase trials remain at a low and limited level. We discuss potential routes to improve the number and quality of early phase trials in Ewing and osteosarcoma.
Methods
Search strategy
We report data available in the public domain only. Publications were identified from searches of PubMed, the Cochrane Controlled Trials Register, American Society of Clinical Oncology (ASCO) abstracts and ClinicalTrials.gov. databases for the period 1990-2010. The search strategies used terms for osteosarcoma; (osteosarcoma) AND (phase I OR phase II) AND (clinical trial), and was supplemented with a text word search. For Ewing sarcoma the search algorithm was; (Ewing sarcoma) AND (phase I OR phase II) AND (clinical trial). To validate the search we broadened the search algorithm to; (sarcoma) AND (phase I OR phase II) AND (clinical trial), and compared the results from the narrow search with the ones from the broad search. The latest search was performed in April 2010.
Whenever multiple reports from the same trial were published, we used only the report with the longest follow-up to avoid any duplication of information. Publications were eligible if they: (1) described (or cited a paper that described) osteosarcoma or Ewing sarcoma study of early phase clinical trials; (2) were published in English; and (3) came from industrialized countries. All types of evaluation were accepted (full papers, conference abstracts, reports) as long as results (including data) were presented.
Data extraction
Data extraction was conducted independently by two authors (A. B. and A.M. van M.). We used a systematic method for the search normally used for meta-analysis [16]. Differences in data extraction were resolved by consensus with a third author (A.B. H.). From each eligible trial we recorded authors' names, journal and year of publication, number of patients enrolled, number of osteosarcoma or Ewing sarcoma patients, study phase and the outcome of the trial.
Results and discussion
Eligible trials
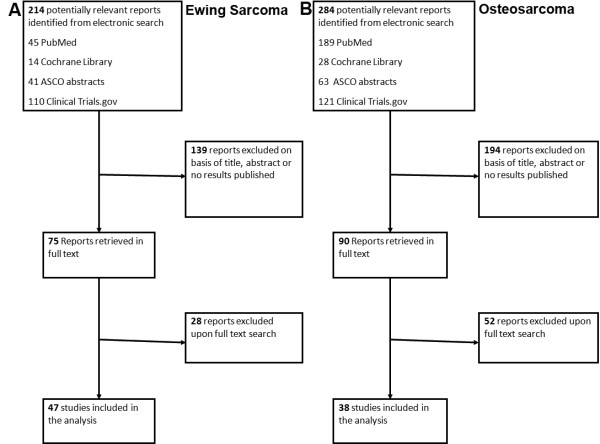
A flow-chart indicating the identification of clinical trials for inclusion in the analysis is reported for Ewing sarcoma (Figure 1A) and osteosarcoma (Figure 1B). During the search many reports had to be excluded because no results were published. When we searched the reports using full text, we had to exclude some papers because neither osteosarcoma nor Ewing sarcoma patients were included in these studies. When we combined the results, we identified 42 trials enrolling patients with any histological diagnosis of sarcoma that were eligible for our study, in that they included osteosarcoma and Ewing sarcoma. Of the 42 clinical trials twenty-one were phase I, two were phase I/II and nineteen were phase II trials. We found eight clinical trials which included only Ewing sarcoma patients; of this group two were phase I and six were phase II trials. We identified twenty trials that included only osteosarcoma patients. There were two phase I, sixteen were phase II and two were phase I/II trials. A total of 3,736 patients were included in all the clinical trials, of which 1,263 were Ewing sarcoma and 1,114 were osteosarcoma patients.
Figure 1.
Flowchart diagrams of the clinical trial selection criteria outcomes for Ewing sarcoma (A) and osteosarcoma (B).
Primary Outcome
Tables 1 and 2 show the search results for clinical trials conducted in patients with the wider diagnosis of general sarcoma, and specifically the trials that included either osteosarcoma (Table 3) or Ewing sarcoma patients only (Table 4). Table 1 and 2 are trials testing either chemotherapy or biological treatments, respectively. Analysis of the number of trials conducted which included only osteosarcoma or Ewing sarcoma patients from 1990 to the present, (Figure 2A) it was clear that the number of trials reported for osteosarcoma has been stable since 1995, with approximately five trials in five years. For Ewing sarcoma there has been an increase in the number of trials published, with the no early phase trials reported between 1990 and 1999, an increasing number of trials in the period 2000-2005 and even more between 2006-2010.
Table 1.
Characteristics of eligible trials for general sarcoma with chemotherapy based treatment for phase I and phase II
| Author | Year | Intervention | No Osteo patients | No Ewing Patients | Phase | Outcome |
|---|---|---|---|---|---|---|
| Pratt et al, Cancer, 74: 2593-8 [22] | 1994 | Leucovorin, 5-Fluorouracil | 22 | 12 | II | Ewing: 3 (25%) SD, Osteo: 1 (5%) SD |
| Kushner et al, J Clin Oncol, 13 (11): 2796-804 [23] | 1995 | Cyclo, Doxo, Etop, Ifos, Vin | Not mentioned | Not mentioned | II | 6 (17%) CR, 2 (6%) PR, 34 (95%) CIR |
| Antman et al, Cancer, 82 (7): 1288-95 [24] | 1998 | Doxo, Dacarbazine, Ifos | 31 | 13 | II | 10 (12%) CR, 34 (42%) PR |
| Blaney et al, Clin Cancer Res, 4 (2): 357-60 [25] | 1998 | Topotecan | 18 | 25 | II | 1 (1%) CR, 1 (1%) PR, 8 (9%) SD |
| Lucidarme et al, Bone Marrow Transplant, 22 (6): 535-40 [26] | 1998 | Thiotepa combined with SCR | 7 | 3 | II | 11 (50%) PR |
| Berg et al, J Pediatric Hematol Oncol, 22 (6): 506-9 [27] | 2000 | Pyrazoloacridine | 8 | 10 | II | 0% response |
| Delaloge et al, J Clin Oncol, 19 (5): 1248-55 [28] | 2001 | Trabectedin | 3 | 1 | I | 4 (14%) CR, 10 (35%) SD |
| Saylors et al, J Clin Oncol, 19 (15): 3463-9 [29] | 2001 | Cyclo and Topotecan | 18 | 17 | II | 6 (35%) OR |
| Wagner et al, Clin Cancer Res, 10 (3): 840-8 [30] | 2004 | Temozolomide, Irinotecan | 0 | 7 | I | 1 (8%) CR, 2 (17%) PR |
| Lau et al, Clin Cancer Res, 11: 672-7 [31] | 2005 | Trabectedin | 4 | 3 | I | 1 (8%) CR |
| van Winkle et al, Pediatr Blood Cancer, 44 (4): 338-47 [32] | 2005 | Ifos, Carboplatin and Etop | 35 | 22 | I/II | Not mentioned |
| Hawkins et al, Pediatr Blood Cancer, 47 (6): 328-37 [33] | 2006 | Topotecan | 11 | 20 | II | 2 (4%) PR |
| Navid et al, Cancer, 106 (8): 1846-56 [34] | 2006 | Vin, Doxo, Cyclo, Ifos and Etop | 0 | 11 | II | 47 (66%) CR |
| Wagner-Bohn et al, Cancer, 46 (2): 262 [35] | 2006 | Gemcitabine | 2 | 4 | II | 2 (10%) SD |
| Zaucha et al, Int J Rad Oncol Biol Phys, 64 (1): 227-34 [36] | 2006 | Total body radiation after high-dose chemotherapy | 0 | 10 | II | 13 (25%) CR, 10 (19%) PR |
| Zwerdling et al, Cancer, 106 (8): 1821-8 [37] | 2006 | Docetaxel | 23 | 21 | II | 2 (1%) CR, 6 (3%) PR, 17 (10%) SD |
| Geoerger et al, J Clin Oncol, 26: 4394-400 [38] | 2008 | Oxaliplatin | 6 | 2 | I | 2 (4%) PR, 17 (6%) SD |
| Geler et al, Pediatr Blood Cancer, 52 | 2009 | Paclitaxel, Ifos | 1 | 3 | I | 3 (20%) PD, 5 (33%) SD |
| Wagner et al, ASCO abstract | 2009 | Vin, Irinotecan, Temozolomide | 3 | 5 | I | 1 (3%) PR,2 (6%) OR |
| McGregor et al, Cancer, 115 (8): 1765-75 [39] | 2009 | Oxaliplatin, Irinotecan | 6 | 1 | I | 1 (8%) CR, 1 (8%) SD |
Abbreviations: ClR = Clinical Response; CR = Complete Response; Cyclo = cyclophosphamide; Doxo = doxorubicine; EFS = Event Free Survival; Etop = etoposide; Ewing = Ewing Sarcoma; Ifos = ifosfamide; No = number; OR = Objective Response; Osteo = Osteosarcoma; PD = Progressive Disease; PR = Partial Response; RR = Response Rate; SCR = Stem Cell Rescue; SD = Stabile Disease; TMR = Total Marrow Radiation; Vin = vincristine
Table 2.
Characteristics of eligible trials for general sarcoma with biologically based treatment for phase I and phase II
| Author | Year | Intervention | No Osteo Patients | No Ewing Patients | Phase | Outcome |
|---|---|---|---|---|---|---|
| Daw et al, J Clin Oncol, 23 (25): 6172-80 [40] | 2005 | Gefitinib | 6 | 3 | I | 1 (4%) PR, 4 (16%) SD |
| Maki et al, Cancer, 103 (7): 1431-8 [41] | 2005 | Bortezomib | 1 | 2 | II | 1 (4%) OR |
| Villablanca et al, J Clin Oncol, 24 (21): 3423-30 [42] | 2006 | Fenretinide | 2 | 5 | I | 1 (2%) CR, 13 (24%) SD |
| Biron et al, ASCO abstract | 2007 | Gimatecan | 0 | Not mentioned | II | 3 (8%) SD |
| Bagatell et al, Clin Cancer Res, 13 (6): 1783-8 [43] | 2007 | Tanespimycin | 6 | 2 | I | 0% OR |
| Jimeno et al, Pediatr Blood Cancer, 49 (3): 352-7 [44] | 2007 | Gefitinib | 3 | 1 | I | 1 (7%) PR, 3 (20%) SD |
| Kramer et al, J Clin Oncol, 25 (34): 5465-70 [45] | 2007 | Monoclonal antibody(131-I-3F8) | 0 | 2 | I | 3 (23%) ClR |
| Bond et al, Pediatr Blood Cancer, 50 (2): 254-8 [46] | 2008 | Imatinib mesylate | 10 | 24 | II | 1 (1%) PR |
| Chao et al, ASCO abstract | 2008 | Imatinib mesylate | 0 | 7 | II | 1 (14%) PR |
| Fox et al, Clin Cancer Res, 14 (4): 1111-5 [47] | 2008 | Tubulin Inhibitor (ABT-751) | 3 | 3 | I | 0% OR |
| Jakacki et al, J Clin Oncol, 26 (30): 4921-7 [48] | 2008 | Erlotinib, Temozolomide | 1 | 0 | I | 1 (2%) SD |
| Langevin et al, Pediatr Blood Cancer, 50 (3): 577-80 [49] | 2008 | Rebeccamycin | 16 | 14 | II | % OR |
| Mita et al, J Clin Oncol, 26 (3): 361-7 [50] | 2008 | Deforolimus | 1 | 1 | I | 4 (13%) PR, 18 (56%) SD |
| Olmos et al, ASCO abstract | 2008 | Figitumumab | 0 | 9 | I | 1 (5%) PR, 6 (27%) SD |
| Chawla et al, Mol Ther, 17 (9): 1651-7 [51] | 2009 | Rexin-G | 3 | 1 | I/II | 13 (65%) SD |
| Chugh et al, J Clin Oncol, 27 (19): 3148-53 [52] | 2009 | Imatinib | 27 | 13 | II | 1 (0.5%) CR, 3 (2%) PR |
| Malempati et al, ASCO abstract | 2009 | Cixutumumab | Not mentioned | 10 | I | 1 (4%) PR |
| Patel et al, ASCO abstract | 2009 | IGF-1R antibody (R1507) | 43 | 71 | II | Not mentioned |
| Widemann et al, J Clin Oncol, 27 (4): 550-6 [53] | 2009 | Ixabepilone | 3 | 2 | I | 4 (21%) SD |
| Jacobs et al, Clin Cancer Res, 16 (2): 750-4 [54] | 2010 | Ixabepilone | 11 | 9 | II | 0% OR |
| Kurzrock et al, Clin Cancer Res, 16 (8): 2458-65 [55] | 2010 | IGF-1R antibody (R1507) | 0 | 9 | I | 2 (5%) PR, 13 (35%) SD |
| Olmos et al, Lancet Oncol, 11: 129-35 [56] | 2010 | Figitumumab | 0 | 16 | I | 1 (3%) CR, 1 (3%) PR, 8 (28%) SD |
Abbreviations:, ClR = Clinical Response; CR = Complete Response; Cyclo = cyclophosphamide; Doxo = doxorubicin; EFS = Event Free Survival; Etop = etoposide; Ewing = Ewing Sarcoma; Ifos = ifosfamide; No = number; OR = Objective Response; Osteo = Osteosarcoma; PR = Partial Response; RR = Response Rate; SCR = Stem Cell Rescue; SD = Stabile Disease; TMR = Total Marrow Radiation; Vin = vincristine
Table 3.
Characteristics of eligible trials for osteosarcoma only
| Author | Year | Intervention | Phase | No of Patients | Outcome |
|---|---|---|---|---|---|
| Salesh RA., et al., Cancer 65:861-5. | 1990 | Etoposide 72-h i.v 600 mg/m2. Cyclophosphamide 300 mg/m2 every 12 hours for a total dose of 1800 mg/m2. | II | 17 | 15 (88%) CR or PR |
| Kleinerman ES, et al J. Clin. Oncol. 10, 1310-6. [57] | 1992 | L-MTP-PE 2 mg/m2 infused during a 1-h period twice a wk for 12 wks, then once a wk for 12 wks. | II | 16 | Not mentioned |
| Kleinerman ES, et al Am J Clin Oncol. 18(2):93-9. [58] | 1995 | L-MTP-PE, 2 mg/m2, i.v over a 1-h twice a wk for 12 wks in 12 pts (Group 1). 16 pts (Group 2) had 2 mg/m2 L-MTP-PE twice a wk for 12 wks, then once a wk for 12 wks, for a total of 24 wks. | II | 36 | 2 (6%) SD |
| Kleinerman ES, et al J. Immunother. Emphasis Tumor Immunol., 17, 181-93. [59] | 1995 | IFO 1.8 g/m2 for 5 days every 21 days for up to 8 cycles. L-MTP-PE twice weekly for 12 weeks, then once weekly for 12 weeks | IIb | 9 | Not mentioned |
| Harris MB, et al Med Ped Oncol 24, 87-92. [60] | 1995 | Two courses of ifosfamide (2400 mg/m2 × 5 days) administered 3 wks apart | II | 33 | Stratum 1: 1 (11%) CR, 8 (24%) PR Stratum 2: 1 (3%) CR, 2 (7%) PR |
| Patel SR, et al Cancer; 78:741-4. [61] | 1996 | Paclitaxel 175 mg/m2 24-h i.v | II | 15 | 0% OR |
| Gentet JC, et al Eur J Cancer, Vol. 33, No. 2, 232-7 [62] | 1997 | Two courses of IFO 3 g/m2/day and etoposide 75 mg/m2/day for 4 days. | II | 27 | 6 (23%) CR, 7 (25%) PR, 5 (23%) SD |
| Worth LL, et al Clin Cancer Res. 3(10):1721-9. [63] | 1997 | IL-1alpha followed by ICE daily for 5 days/3 wks. | II | 9 | 3 (34%) PR, 1 (11%) SD |
| Voûte PA, et al Annals of Oncology 10: 1211-8. [64] | 1999 | IFO 3 g/m2/dl-2, DOX 25 mg/m2/dl-3 i.v. bolus and CDDP 100 mg/m2/dl. | II | 103 | 5 year survival was 62% in limb-non-metastatic, 41% in axial skeletal and 16% in limb metastatic patients. |
| Fagioli F, et al Journal of Clinical Oncology, Vol 20, Issue 8:2150-6 [65] | 2002 | High-dose chemotherapy consisted of carboplatin and etoposide followed by stem-cell rescue. | I | 32 | 25 (78%) CR, 6 (19%) PD, 3 year OS 20% 3 year DFS 12% |
| Laverdiere C, et al Cancer; 98:832-40. [66] | 2003 | Trabectedin 1500 micro g/m2 as a 24-h i.v every 3 wks. | II | 25 | 0% OR |
| McTiernan A, et al. Sarcoma, Vol. 8, No. 2/3, 71-6. [67] | 2004 | Docetaxel 100 mg/m2 1-h i.v every 3 wks | II | 14 | 1 (7%) PR, 2 (14%) SD |
| Ferrari S et al J Clin Oncol 23:8845-52. [68] | 2005 | Two blocks of high-dose IFO (15 g/m2), MTX (12 g/m2), CDDP (120 m g/m2), and DOX (75 m g/m2) | II | 182 | 5-year EFS 64% and OS 77% |
| Arpaci F, et al Cancer; 104:1058-65. [69] | 2005 | 2 cycles of CDDP, DOX, and IFO followed by HDC and APBSCT. | II | 22 | 3 year: OS was 83% & 70% DFS |
| McTiernan A, et al Pediatr Blood Cancer. 46(3):345-50. [70] | 2006 | IFO 2.5 g/m2 etoposide 150 mg/m2 and DOX 20 mg/m2 on days 1-3, every 21 days, with interval MTX 12 g/m2 given on day 14, for a maximum of 8 cycles. | I/II | 13 | 0% OR |
| Seibel NL, et al Cancer 2007; 109:1646-53. [71] | 2007 | Topotecan daily x5 followed by chemotherapy (IFO, carboplatin, ICE, alternating with CDDP and CD. | I | 28 | 1 (6.6%) PR, 1 (6.6%) CLR 2- and 5-year EFS rates 7% & 4%, resp, 2- and 5-year OS rates 44% and 22%, resp. |
| Basaran M, et al Oncology;72:255-60 [72] | 2007 | Epirubicin 90 mg/m2 cisplatin 100 mg/m2 on day 1 and IFO 2.0 g/m2 day with an equivalent dose of mesna on days 2-4, repeated every 21 days | II | 38 | 10 (26%) CR, 5 year DFS 41.9%, OS 48.2%. |
| Iwamoto Y, et al J Orthop Sci 14:397-04. [73] | 2009 | Preoperative chemotherapy: HD-MTX, CDDP, and ADR. | II | 113 | 5 year OS 77.9% 5 year EFS 65.5% |
| Berger M, et al Cancer 115: 2980 -7. [74] | 2009 | Cyclophosphamide 4 g/m2 on Day 1 followed by etoposide at 200 mg/m2 on Days 2, 3, and 4. | II | 26 | 9 (35%) SD, OS at 1 yr 50%, PFS 42% |
| Chawla S P, et al Mol Ther. 17:1651-7. [51] | 2009 | Escalating doses of Rexin-G i.v from 8 × 1011 to 24 × 1011 colony forming units (cfu)/cycle. | II I/II |
Osteo = 22 Sar = 20 Osteo = 0 |
10 (58.8%) SD 3 (50%) SD(lowest dose) 10 (71%) SD(higher dose) |
Abbreviations- ADR = adriamycin; APBSCT = autologous peripheral blood stem cell support transplantation; CDDP = cisplatin; Cfu = colony forming units; CR = complete response; DOX = doxorubicin; EFS = event free survival; GCSF = Granulocyte colony-stimulating factor; HD-MTX = High-dose Methotrexate; ICE = etoposide; IFO = ifosfamide; IL-6 = interleukin -6; IL-8 = interleukin-8; L-MTP-PE = liposomal muramyl tripeptide phosphatidyl ethanolamine; MTX = methotrexate; OR = objective response; PD = progressive disease; PFS = progression free survival; OS = overall survival; RDI = relative dose intensity; resp = respectively; Sar = sarcoma; TNF = tumour necrosis factor
Table 4.
Characteristics of eligible trials for Ewing sarcoma only
| Author | Year | Intervention | Phase | No of Patients | Outcome |
|---|---|---|---|---|---|
| Hawkins et al, Med Pediatric Oncol, 34 (5): 328-37 [33] | 2000 | Myeloablative therapy followed by HSCT. | I | 16 | 3-year EFS 36% |
| Kolb et al, J Clin Oncol, 21 (18): 3423-30 [75] | 2003 | 7 cycles of chemotherapy consisted of Cyclo, Doxo, Vin, Ifos and Etop | II | 68 | 29 (43%) CR, 13 (19%) PD or SD |
| Meyer et al, Sarcoma, 7 (1): 13-7 [76] | 2003 | Doc thrice weekly for a maximum of six cycles. | II | 14 | 1 (14%) PR, 2 (28%) SD |
| Bernstein et al, J Clin Oncol, 24 (1): 152-9 [77] | 2006 | High-dose induction therapy followed by window period. Randomization between topo, topo + cyclo or no treatment. No window treatment patients received Amifostine | II | 117 | 45 (43%) CR, 41 (39%) PR, 14 (14%) SD |
| Womer et al, ASCO abstract | 2008 | Vin, Doxo and Cyclo alternating with Ifos and Etop, for 14 cycles. Regimen A: 3 weeks cycle, regimen B: 2 week cycle. Primary tumor treatment was scheduled to begin week 13. | II | 587 | EFS at a median of 3 years was 65% in Regimen A and 76% in Regimen B. |
| Rosenthal et al, Bone Marrow Transplantation, 42: 311-8 [78] | 2008 | HDT followed by HSCT. | I | 22 | 3-year EFS 47%, 3-year OS 45% |
| DuBois et al, Pediatric Blood Cancer, 52 (3): 324-7 [79] | 2009 | Cytarabine. | II | 10 | 1 (10%) SD |
| Casey et al, Pediatric Blood Cancer, 53 (6): 1029-34 [80] | 2009 | Irinotecan and Temozolomide. | II | 20 | 5 (25%) CR, 7 (36%) PR |
| Olmos et al, Lancet Oncol, 11: 129-35 [56] | 2010 | A subgroup of Ewing Sarcoma patients were treated with Figitumumab | I | 15 | 1 (6%) CR, 1 (6%) PR, 6 (37%) SD |
Abbreviations: CR = complete response; Cyclo = cyclophosphamide; Doc = docetaxel; Doxo = doxorubucine; EFS = event free survival; Etop = etoposide; G-CSF = granulocyte-colony stimulating factor; HDT = high-dose chemotherapy; HSCT = hematopoietic stem cell transplantation; Ifos = ifosfamide; OR = overall response; OS = overall survival; PD = progressive disease; PR = partial response; SD = stabile disease; Topo = topotecan; Vin = vincristine
Figure 2.
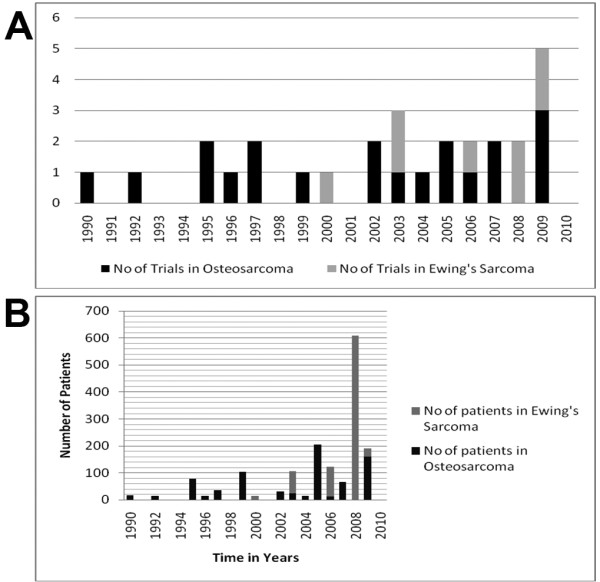
Distribution of the number of trials published that have recruited either osteosarcoma or Ewing sarcoma patients between 1990-2010 (A). The total number of either osteosarcoma or Ewing Sarcoma patients entered in phase I/II trials published between 1990-2010 (B).
For the number of patients enrolled in the eligible trials the results were disappointing (Figure 2B), with an almost stable number of patients in osteosarcoma trials, except for 1999, 2005 and 2009 when the results from larger phase II trials were published (Table 3). For Ewing sarcoma there were increasing numbers of patients in early phase trials, especially in 2008 when the result of the largest trial in Ewing sarcoma patients was published (Table 4). Out of all the 780 osteosarcoma patients enrolled in phase I/II trials including only osteosarcoma patients, 762 were evaluable and 58 patients (8%) achieved complete response (CR), 21 (2.8%) showed partial response (PR) and 30 (4%) developed stable disease (SD). For the trials enrolling only Ewing sarcoma patients, 869 were recruited and 840 were evaluable, 80 had CR (9.5%), 63 (7.5%) showed PR and 23 (3%) developed SD.
Looking at the trials subdivided for chemotherapy or biological treatment (Tables 1 and 2) in the past twenty years, there seems to have been a shift towards biologically based treatments instead of chemotherapy. Even so most of the phase III trials are still chemotherapy based treatments.
For current ongoing phase I and phase II studies we found 156 trials that are open and recruiting sarcoma patients. These trials recruit sarcoma patients in general or patients with solid tumours or soft tissue sarcoma. Studies enrolling only osteosarcoma or Ewing sarcoma patients are rare. For osteosarcoma we found four trials that are recruiting patients and two that are already active but not yet recruiting patients (Table 5). Of the trials two were testing chemotherapy treatment, three biologically agents and one a combination of both. For Ewing sarcoma we found no trials that enrol only Ewing patients and two that are still active but not currently enrolling patients.
Table 5.
Ongoing phase I or II trials in osteosarcoma (Assessed from www.ClinicalTrials.gov)
| ClinicalTrials.gov Identifier | Sponsors | Title | Status |
|---|---|---|---|
| NCT00102531 | Transave | Phase Ib/IIa non-randomised study of SLIT Cisplatin by inhalation in the treatment of patients with relapsed/progressive osteosarcoma metastatic to the lung | Recruiting |
| NCT01002092 | Shandong Simcere-Medgenn Bio-pharmaceutical Co., Ltd |
A randomized, controlled multicenter trial of Endostar combined with chemotherapy for treatment of osteosarcoma (phase II) | Recruiting |
| NCT00889057 | Italian Sarcoma Group | Phase II, open label, non-randomized study of second or third line treatment with Sorafenib (BAY 43-9006) in patients affected by relapsed high-grade osteosarcoma | Recruiting |
|
NCT00752206 NCT00923286 |
Sarcoma Alliance for Research through Collaboration (SARC) | A randomized, double-blinded, placebo-controlled, multi-institutional, phase II study of AZD0530, a selective Src kinase inhibitor, in patients with recurrent osteosarcoma localized to the lung | Recruiting |
| NCT01005043 | University of Heidelberg | Phase I/II therapy non-randomised trial to determine the safety and efficacy of heavy ion radiotherapy in patients with osteosarcoma | Not yet recruiting |
| NCT00902044 | Baylor College of Medicine | Administration of Her2 Chimeric antigen receptor expressing T Cells for subjects with advanced osteosarcoma (HEROS) (phase I) | Not yet recruiting |
When searching for studies that are enrolling sarcoma patients we found a number of trials that were preliminary stopped. For example SARC011, this is a phase II trial of R1507, a recombinant human monoclonal antibody to the Insulin-like growth factor-1 receptor for the treatment of patients with recurrent or refractory Ewing sarcoma, osteosarcoma, synovial sarcoma, rhabdomyosarcoma and other sarcomas. In December 2009, Roche/Genentech decided to discontinue the development of their IGF-1R antibody although the drug had shown important clinical benefit http://www.cancer.gov/ncicancerbulletin/111610/page5. Roche said the decision was due to the available clinical data, the large number of molecules targeting the same pathway that are presently in development and the prioritization of the Roche portfolio. The decision was not as a result of safety concerns. Both this study, and a Phase I/II with figitumumab (Pfizer) have been published since completing this survey, and show low but durable response rates in a subset of relapsed Ewing patients (10-20%) [17,18].
Bone sarcoma is fatal in approximately one third of the children and young adults in whom it is diagnosed. This mortality rate has not changed greatly in the two decades since the initial introduction of surgery and chemotherapy. The primary cause of death in treated patients is recurrent metastatic disease often to the lung. In the group of patients who present either with metastatic or relapsed disease, it is especially important to conduct biologically based trials with small groups of patients from which quantitative and quick answers of whether a treatment intervention is either working or not can be obtained. Importantly, tumour material obtained may provide informative clues for future studies especially if analysed with genomic technologies.
It is difficult to do trials in an orphan disease, mainly because of the problems of recruiting enough eligible patients for the trial. In our literature search, we identified 42 phase I/II trials for sarcoma patients which were published between 1990 and 2010. However the phase I trials for general sarcoma (Table 1) are not complete, this is because or our search criteria. With a PubMed search you only search the abstract of the article, so if sarcoma is not mentioned there we didn't find the article. In phase I trials quite a few different tumour types are sometimes enrolled and only the one who show results are mentioned in the abstract. Some of these articles we found in the Cochrane Controlled Trial Register or the ClinicalTrials.gov database, but not all trials are registered in these databases. Except with a very wide PubMed search using (phase 1) as only search criteria we don't think that it is possible to find all phase I trials enrolling sarcoma patients.
Of the trials we did found with our search criteria twenty-one were phase I, two phase I/II and nineteen phase II trials. Only eight clinical trials included only Ewing sarcoma patients; of this group two were phase I and six were phase II trials. We found only twenty trials which included only osteosarcoma patients. There were two phase I, sixteen phase II, and two phase I/II trials. A total of 3,736 patients were included in the clinical trials, of which 1,263 were Ewing sarcoma and 1,114 were osteosarcoma patients. From the identified trials, the results are not convincing for benefit and most of the time even disappointing, from osteosarcoma trials we found 8% CR, 2.8% PR and 4% SD. For Ewing sarcoma the results are 9.5% CR, 7.5% PR and 3% SD. And if the drugs seem to be effective, a lot of toxicity is reported (Tables 1, 2, 3, 4). Looking at the number of trials we found for sarcoma patients, the results seem encouraging as a high number of patients were included. However, if we make a calculation of the number of new Ewing sarcoma patients expected in the last twenty years, assuming a world population of five billion and an incidence of 2 in 1.000,000, approximately 200,000 new cases would be expected in twenty years world-wide. Of these patients, we could only find 1,263 reported and included in published clinical trials. For osteosarcoma the number is even worse, with 500,000 new cases expected in twenty years and only 1,114 reported in trials.
To improve these results changes in conducting trials for orphan state diseases have to be made. It is important to collaborate between nations; the Sarcoma Alliance for Research through Collaboration (SARC) is a good example based principally in North America. SARC is a non-profit organization started in 2003. Funding is provided by generous donations, sponsors and grants. SARC works with healthcare professionals as part of a collaborative multidisciplinary team from over 35 institutions in the USA. It works with a number of international institutions and provides the infrastructure for collaboration between physicians, researchers, and medical institutions from across the worlds who are engaged in clinical research for development of new standards of sarcoma patient treatment, education, and prevention. This organization facilitates dialogue and collaboration among sarcoma researchers and clinicians, assists in the development and dissemination of protocols for clinical trials and information relating to sarcoma research and the results of clinical trials, administers research grants and funding for clinical trials, and acts as a primary resource for those treating patients with sarcoma. The EORTC Soft Tissue and Bone Sarcoma Group is one of SARC's European counterpart, conducting multicenter academic studies in sarcoma, including some early stage translational studies, although it's main contribution in the past has been in the field of larger phase III trials soft tissue sarcoma. The European Osteosarcoma Intergroup, EURAMOS and EuroEwing consortium are examples of groups acting primarily as platforms for phase III studies in bone sarcomas.
The EC-granted European network, EuroBoNeT http://www.eurobonet.eu, was the first central organized network of excellence for integrated bone sarcoma research and staff exchange, in order to increase and disseminate knowledge of primary bone tumours at the molecular level for development of new tools for patient care. This integration, exchange of material (virtual BioBank), standard operating protocols and the use of technology platforms enabled researchers to obtain statistically significant datasets, otherwise not achievable due to the rareness of the condition and the large number of sub-entities. Without these kinds of collaborations, the development of new treatment strategies in osteosarcoma and Ewing sarcoma is going to be very difficult. But perhaps most importantly is that phase I/II clinical trials have to be conducted. To achieve this funding needs to be established from industry and governments to feed through these collaborative structures over time frames that might result in enduring progress. Recent progress has also been made with the FP7 funding of EuroSarc, European trials in rare sarcomas within an integrated translational trial network (2011-2016). In this new translational trial network, the challenges of combining high quality disease research into clinical trials in being addressed, and progresses the field beyond conventional trials and the existing clinical trial networks.
In addition to the challenge of identifying the most promising agents for clinical trials in bone sarcoma, obstacles inherent to this disease further complicate the successful design and completion of trials of novel agents. In evaluating the efficacy of novel agents, the standard approach is to use objective response criteria, such as Response Evaluation Criteria in Solid Tumours (RECIST) [19], to compare the size and/or volume of lesions pre-treatment and at regular intervals during and post-treatment. New methods for tumour response which may be more precise are radiological response based on new criteria, e.g. 18FDG-PET-CT and contrast enhanced MRI. For a patient to be eligible for a trial using this approach, he or she must have measurable disease. The traditional approach for patients with relapsed bone sarcoma, in particular pulmonary relapse, has been surgical resection, which at least temporarily renders a patient free of radiographic measurable disease and thus, ineligible for most trials of novel agents. Unfortunately novel targeted therapies may be most effective in the setting of minimal residual disease. The use of creative surrogate end points to estimate response and perhaps render the combination of surgical resection and treatment on trials of new agents not mutually exclusive would allow for better evaluation of new therapies in patients with bone sarcoma [20].
When conducting the literature search we found that the results of many trials are not published. We can only guess what the reason for this is, but is seems logical that the trial results were disappointing. From the phase I/II trials only a very limited number of treatments proceeded to phase III trial. For Ewing sarcoma we found three clinical trials conducted in the last twenty years and two trials which are being recruiting at this moment and for osteosarcoma we found twenty-two phase III clinical trials and four active trials currently recruiting patients and one is ongoing trial but not recruiting.
Most of the phase I/II trials that have been conducted recruited patients with general sarcoma or solid tumours. In these trials it is very difficult to say anything about disease specific response and it is therefore almost impossible to translate the results to clinical practise and patients with a specific sort of cancer. Of course it is understandable why researchers test a drug in different patient populations in the same trial, especially is rare cancers like bone sarcoma, but it makes it more difficult to proceed to a disease specific phase II or even phase III trial.
While the current standard of care continues to be multi-agent chemotherapy and surgical resection, it is clear that further intensification of traditional chemotherapy regimens is limited by toxicity, and new approaches are needed. Some promising novel agents are currently in or are entering into phase II trials in children with relapsed bone sarcoma. For example, Muramyl Tripeptide Phosphatidylethanolamine (MTP-PE) is the first drug to be approved for osteosarcoma in Europe for ten years [21]. In Ewing sarcoma the identification of the Insulin-like Growth Factor 1 Receptor (IGF1R) pathway deregulation, as a consequence of the EWS-FLI1 translocation seems to be a new treatment strategy to be explored. The incorporation of these new treatments into the up-front therapy for bone sarcoma in the near future holds promise for improving outcomes.
Lists of abbreviations
EMEA: European Medicines (Evaluation) Agency; EFS: Event free survival; IGF1R: Insulin-like Growth Factor 1 Receptor; MTP-PE: Muramyl Tripeptide Phosphatidylethanolamine; OS: Overall survival; PET: Positron emission tomography; PFS: Progression free survival; RECIST: Response Evaluation Criteria in Solid Tumours; SARC: Sarcoma Alliance for Research through Collaboration network.
Competing interests
All authors have read and approved the final manuscript, and have no competing financial interests in the publication of this manuscript. No organisation is funding or implicated in the manuscripts analysis and interpretation. Academic interests of the authors are to improve the outcome of patients with sarcoma, and this publication forms part of the deliverable output from EU FP7 funding from EuroBoNeT.
Authors' contributions
ABH conceived the study. AVM and AP collected data with ABH. Data extraction was conducted independently by two authors (AB and AVM). Differences in data extraction were resolved by consensus with a third author (ABH). AVM and ABH wrote the paper and AP, PC and AG made comments. All authors have read and approved the final manuscript.
Contributor Information
Annemiek M van Maldegem, Email: annemiek.maldegem@medonc.ox.ac.uk.
Aparna Bhosale, Email: aparna.bhosale@medonc.ox.ac.uk.
Hans J Gelderblom, Email: a.j.gelderblom@lumc.nl.
Pancras CW Hogendoorn, Email: p.c.w.hogendoorn@lumc.nl.
Andrew B Hassan, Email: bass.hassan@path.ox.ac.uk.
Acknowledgements
We thank EuroBoNeT FP6 for funding translational developments, and EuroSarc FP7 for supporting early phase trials in Ewing and osteosarcoma. We also thank the Oncology Theme of the Oxford Biomedical Research Centre for support (ABH).
References
- Mohseny AB, Szuhai K, Romeo S, Buddingh EP, Briaire-de Bruijn I, de Jong D, van Pel M, Cleton-Jansen AM, Hogendoorn PC. Osteosarcoma originates from mesenchymal stem cells in consequence of aneuploidization and genomic loss of Cdkn2. J Pathol. 2009;219:294–305. doi: 10.1002/path.2603. [DOI] [PubMed] [Google Scholar]
- Gatta G, van der Zwan JM, Casali PG, Siesling S, Dei Tos AP, Kunkler I, Otter R, Licitra L, Mallone S, Tavilla A, Trama A, Capocaccia R. Rare cancers are not so rare: The rare cancer burden in Europe. Eur J Cancer. 2011;47:2493–2511. doi: 10.1016/j.ejca.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Bacci G, Fabbri N, Balladelli A, Forni C, Palmerini E, Picci P. Treatment and prognosis for synchronous multifocal osteosarcoma in 42 patients. J Bone Joint Surg Br. 2006;88:1071–1075. doi: 10.1302/0301-620X.88B8.17809. [DOI] [PubMed] [Google Scholar]
- Lewis IJ, Nooij MA, Whelan J, Sydes MR, Grimer R, Hogendoorn PC, Memon MA, Weeden S, Uscinska BM, van Glabbeke M, Kirkpatrick A, Hauben EI, Craft AW, Taminiau AH. Improvement in histologic response but not survival in osteosarcoma patients treated with intensified chemotherapy: a randomized phase III trial of the European Osteosarcoma Intergroup. J Natl Cancer Inst. 2007;99:112–128. doi: 10.1093/jnci/djk015. [DOI] [PubMed] [Google Scholar]
- Chou AJ, Kleinerman ES, Krailo MD, Chen Z, Betcher DL, Healey JH, Conrad EU, Nieder ML, Weiner MA, Wells RJ, Womer RB, Meyers PA. Addition of muramyl tripeptide to chemotherapy for patients with newly diagnosed metastatic osteosarcoma: a report from the Children's Oncology Group. Cancer. 2009;115:5339–5348. doi: 10.1002/cncr.24566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilber F, Giuliano A, Eckardt J, Patterson K, Moseley S, Goodnight J. Adjuvant chemotherapy for osteosarcoma: a randomized prospective trial. J Clin Oncol. 1987;5:21–26. doi: 10.1200/JCO.1987.5.1.21. [DOI] [PubMed] [Google Scholar]
- Goorin AM, Perez-Atayde A, Gebhardt M, Andersen JW, Wilkinson RH, Delorey MJ, Watts H, Link M, Jaffe N, Frei E. et al. Weekly high-dose methotrexate and doxorubicin for osteosarcoma: the Dana-Farber Cancer Institute/the Children's Hospital--study III. J Clin Oncol. 1987;5:1178–1184. doi: 10.1200/JCO.1987.5.8.1178. [DOI] [PubMed] [Google Scholar]
- Link MP, Goorin AM, Miser AW, Green AA, Pratt CB, Belasco JB, Pritchard J, Malpas JS, Baker AR, Kirkpatrick JA. et al. The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity. N Engl J Med. 1986;314:1600–1606. doi: 10.1056/NEJM198606193142502. [DOI] [PubMed] [Google Scholar]
- Buddingh EP, Anninga JK, Versteegh MI, Taminiau AH, Egeler RM, van Rijswijk CS, Hogendoorn PC, Lankester AC, Gelderblom H. Prognostic factors in pulmonary metastasized high-grade osteosarcoma. Pediatr Blood Cancer. 2010;54:216–221. doi: 10.1002/pbc.22293. [DOI] [PubMed] [Google Scholar]
- Riggi N, Stamenkovic I. The Biology of Ewing sarcoma. Cancer Lett. 2007;254:1–10. doi: 10.1016/j.canlet.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Szuhai K, Ijszenga M, de Jong D, Karseladze A, Tanke HJ, Hogendoorn PC. The NFATc2 gene is involved in a novel cloned translocation in a Ewing sarcoma variant that couples its function in immunology to oncology. Clin Cancer Res. 2009;15:2259–2268. doi: 10.1158/1078-0432.CCR-08-2184. [DOI] [PubMed] [Google Scholar]
- de Alava E, Lozano MD, Patino A, Sierrasesumaga L, Pardo-Mindan FJ. Ewing family tumors: potential prognostic value of reverse-transcriptase polymerase chain reaction detection of minimal residual disease in peripheral blood samples. Diagn Mol Pathol. 1998;7:152–157. doi: 10.1097/00019606-199806000-00005. [DOI] [PubMed] [Google Scholar]
- Le Deley MC, Delattre O, Schaefer KL, Burchill SA, Koehler G, Hogendoorn PC, Lion T, Poremba C, Marandet J, Ballet S, Pierron G, Brownhill SC, Nesslbock M, Ranft A, Dirksen U, Oberlin O, Lewis IJ, Craft AW, Jurgens H, Kovar H. Impact of EWS-ETS fusion type on disease progression in Ewing's sarcoma/peripheral primitive neuroectodermal tumor: prospective results from the cooperative Euro-E.W.I.N.G. 99 trial. J Clin Oncol. 2010;28:1982–1988. doi: 10.1200/JCO.2009.23.3585. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Galindo C, Navid F, Liu T, Billups CA, Rao BN, Krasin MJ. Prognostic factors for local and distant control in Ewing sarcoma family of tumors. Ann Oncol. 2008;19:814–820. doi: 10.1093/annonc/mdm521. [DOI] [PubMed] [Google Scholar]
- Bernstein ML, Devidas M, Lafreniere D, Souid AK, Meyers PA, Gebhardt M, Stine K, Nicholas R, Perlman EJ, Dubowy R, Wainer IW, Dickman PS, Link MP, Goorin A, Grier HE. Intensive therapy with growth factor support for patients with Ewing tumor metastatic at diagnosis: Pediatric Oncology Group/Children's Cancer Group Phase II Study 9457--a report from the Children's Oncology Group. J Clin Oncol. 2006;24:152–159. doi: 10.1200/JCO.2005.02.1717. [DOI] [PubMed] [Google Scholar]
- Hamada C. The role of meta-analysis in cancer clinical trials. Int J Clin Oncol. 2009;14:90–94. doi: 10.1007/s10147-008-0876-x. [DOI] [PubMed] [Google Scholar]
- Juergens H, Daw NC, Geoerger B, Ferrari S, Villarroel M, Aerts I, Whelan J, Dirksen U, Hixon ML, Yin D, Wang T, Green S, Paccagnella L, Gualberto A. Preliminary efficacy of the anti-insulin-like growth factor type 1 receptor antibody figitumumab in patients with refractory ewing sarcoma. J Clin Oncol. 2011;29:4534–4540. doi: 10.1200/JCO.2010.33.0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappo AS, Patel SR, Crowley J, Reinke DK, Kuenkele KP, Chawla SP, Toner GC, Maki RG, Meyers PA, Chugh R, Ganjoo KN, Schuetze SM, Juergens H, Leahy MG, Geoerger B, Benjamin RS, Helman LJ, Baker LH. R1507, a Monoclonal Antibody to the Insulin-Like Growth Factor 1 Receptor, in Patients With Recurrent or Refractory Ewing Sarcoma Family of Tumors: Results of a Phase II Sarcoma Alliance for Research Through Collaboration Study. J Clin Oncol. 2011;29:4541–4547. doi: 10.1200/JCO.2010.34.0000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Le Cesne A, Van Glabbeke M, Verweij J, Casali PG, Findlay M, Reichardt P, Issels R, Judson I, Schoffski P, Leyvraz S, Bui B, Hogendoorn PC, Sciot R, Blay JY. Absence of progression as assessed by response evaluation criteria in solid tumors predicts survival in advanced GI stromal tumors treated with imatinib mesylate: the intergroup EORTC-ISG-AGITG phase III trial. J Clin Oncol. 2009;27:3969–3974. doi: 10.1200/JCO.2008.21.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers PA. Muramyl tripeptide (mifamurtide) for the treatment of osteosarcoma. Expert Rev Anticancer Ther. 2009;9:1035–1049. doi: 10.1586/era.09.69. [DOI] [PubMed] [Google Scholar]
- Pratt CB, Meyer WH, Howlett N, Douglass EC, Bowman LC, Poe D, Mounce K, Kun LE, Houghton JA. Phase II study of 5-fluorouracil/leucovorin for pediatric patients with malignant solid tumors. Cancer. 1994;74:2593–2598. doi: 10.1002/1097-0142(19941101)74:9<2593::AID-CNCR2820740930>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Kushner BH, Meyers PA, Gerald WL, Healey JH, La Quaglia MP, Boland P, Wollner N, Casper ES, Aledo A, Heller G. et al. Very-high-dose short-term chemotherapy for poor-risk peripheral primitive neuroectodermal tumors, including Ewing's sarcoma, in children and young adults. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1995;13:2796–2804. doi: 10.1200/JCO.1995.13.11.2796. [DOI] [PubMed] [Google Scholar]
- Antman K, Crowley J, Balcerzak SP, Kempf RA, Weiss RB, Clamon GH, Baker LH. A Southwest Oncology Group and Cancer and Leukemia Group B phase II study of doxorubicin, dacarbazine, ifosfamide, and mesna in adults with advanced osteosarcoma, Ewing's sarcoma, and rhabdomyosarcoma. Cancer. 1998;82:1288–1295. doi: 10.1002/(SICI)1097-0142(19980401)82:7<1288::AID-CNCR11>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Blaney SM, Needle MN, Gillespie A, Sato JK, Reaman GH, Berg SL, Adamson PC, Krailo MD, Bleyer WA, Poplack DG, Balis FM. Phase II trial of topotecan administered as 72-hour continuous infusion in children with refractory solid tumors: a collaborative Pediatric Branch, National Cancer Institute, and Children's Cancer Group Study. Clinical cancer research: an official journal of the American Association for Cancer Research. 1998;4:357–360. [PubMed] [Google Scholar]
- Lucidarme N, Valteau-Couanet D, Oberlin O, Couanet D, Kalifa C, Beaujean F, Lapierre V, Hartmann O. Phase II study of high-dose thiotepa and hematopoietic stem cell transplantation in children with solid tumors. Bone marrow transplantation. 1998;22:535–540. doi: 10.1038/sj.bmt.1701395. [DOI] [PubMed] [Google Scholar]
- Berg SL, Blaney SM, Sullivan J, Bernstein M, Dubowy R, Harris MB. Phase II trial of pyrazoloacridine in children with solid tumors: a Pediatric Oncology Group phase II study. Journal of pediatric hematology/oncology. 2000;22:506–509. doi: 10.1097/00043426-200011000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaloge S, Yovine A, Taamma A, Riofrio M, Brain E, Raymond E, Cottu P, Goldwasser F, Jimeno J, Misset JL, Marty M, Cvitkovic E. Ecteinascidin-743: a marine-derived compound in advanced, pretreated sarcoma patients--preliminary evidence of activity. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2001;19:1248–1255. doi: 10.1200/JCO.2001.19.5.1248. [DOI] [PubMed] [Google Scholar]
- Saylors RL, Stine KC, Sullivan J, Kepner JL, Wall DA, Bernstein ML, Harris MB, Hayashi R, Vietti TJ. Cyclophosphamide plus topotecan in children with recurrent or refractory solid tumors: a Pediatric Oncology Group phase II study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2001;19:3463–3469. doi: 10.1200/JCO.2001.19.15.3463. [DOI] [PubMed] [Google Scholar]
- Wagner LM, Crews KR, Iacono LC, Houghton PJ, Fuller CE, McCarville MB, Goldsby RE, Albritton K, Stewart CF, Santana VM. Phase I trial of temozolomide and protracted irinotecan in pediatric patients with refractory solid tumors. Clinical cancer research: an official journal of the American Association for Cancer Research. 2004;10:840–848. doi: 10.1158/1078-0432.CCR-03-0175. [DOI] [PubMed] [Google Scholar]
- Lau L, Supko JG, Blaney S, Hershon L, Seibel N, Krailo M, Qu W, Malkin D, Jimeno J, Bernstein M, Baruchel S. A phase I and pharmacokinetic study of ecteinascidin-743 (Yondelis) in children with refractory solid tumors. A Children's Oncology Group study. Clinical cancer research: an official journal of the American Association for Cancer Research. 2005;11:672–677. [PubMed] [Google Scholar]
- Van Winkle P, Angiolillo A, Krailo M, Cheung YK, Anderson B, Davenport V, Reaman G, Cairo MS. Ifosfamide, carboplatin, and etoposide (ICE) reinduction chemotherapy in a large cohort of children and adolescents with recurrent/refractory sarcoma: the Children's Cancer Group (CCG) experience. Pediatric blood & cancer. 2005;44:338–347. doi: 10.1002/pbc.20227. [DOI] [PubMed] [Google Scholar]
- Hawkins D, Barnett T, Bensinger W, Gooley T, Sanders J. Busulfan, melphalan, and thiotepa with or without total marrow irradiation with hematopoietic stem cell rescue for poor-risk Ewing-Sarcoma-Family tumors. Medical and pediatric oncology. 2000;34:328–337. doi: 10.1002/(SICI)1096-911X(200005)34:5<328::AID-MPO3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Navid F, Santana VM, Billups CA, Merchant TE, Furman WL, Spunt SL, Cain AM, Rao BN, Hale GA, Pappo AS. Concomitant administration of vincristine, doxorubicin, cyclophosphamide, ifosfamide, and etoposide for high-risk sarcomas: the St. Jude Children's Research Hospital experience. Cancer. 2006;106:1846–1856. doi: 10.1002/cncr.21810. [DOI] [PubMed] [Google Scholar]
- Wagner-Bohn A, Henze G, von Stackelberg A, Boos J. Phase II study of gemcitabine in children with relapsed leukemia. Pediatric blood & cancer. 2006;46:262. doi: 10.1002/pbc.20632. [DOI] [PubMed] [Google Scholar]
- Zaucha RE, Buckner DC, Barnett T, Holmberg LA, Gooley T, Hooper HA, Maloney DG, Appelbaum F, Bensinger WI. Modified total body irradiation as a planned second high-dose therapy with stem cell infusion for patients with bone-based malignancies. International journal of radiation oncology, biology, physics. 2006;64:227–234. doi: 10.1016/j.ijrobp.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Zwerdling T, Krailo M, Monteleone P, Byrd R, Sato J, Dunaway R, Seibel N, Chen Z, Strain J, Reaman G. Phase II investigation of docetaxel in pediatric patients with recurrent solid tumors: a report from the Children's Oncology Group. Cancer. 2006;106:1821–1828. doi: 10.1002/cncr.21779. [DOI] [PubMed] [Google Scholar]
- Geoerger B, Doz F, Gentet JC, Mayer M, Landman-Parker J, Pichon F, Chastagner P, Rubie H, Frappaz D, Le Bouil A, Gupta S, Vassal G. Phase I study of weekly oxaliplatin in relapsed or refractory pediatric solid malignancies. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2008;26:4394–4400. doi: 10.1200/JCO.2008.16.7585. [DOI] [PubMed] [Google Scholar]
- McGregor LM, Spunt SL, Furman WL, Stewart CF, Schaiquevich P, Krailo MD, Speights R, Ivy P, Adamson PC, Blaney SM. Phase 1 study of oxaliplatin and irinotecan in pediatric patients with refractory solid tumors: a children's oncology group study. Cancer. 2009;115:1765–1775. doi: 10.1002/cncr.24175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw NC, Furman WL, Stewart CF, Iacono LC, Krailo M, Bernstein ML, Dancey JE, Speights RA, Blaney SM, Croop JM, Reaman GH, Adamson PC. Phase I and pharmacokinetic study of gefitinib in children with refractory solid tumors: a Children's Oncology Group Study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2005;23:6172–6180. doi: 10.1200/JCO.2005.11.429. [DOI] [PubMed] [Google Scholar]
- Maki RG, Kraft AS, Scheu K, Yamada J, Wadler S, Antonescu CR, Wright JJ, Schwartz GK. A multicenter Phase II study of bortezomib in recurrent or metastatic sarcomas. Cancer. 2005;103:1431–1438. doi: 10.1002/cncr.20968. [DOI] [PubMed] [Google Scholar]
- Villablanca JG, Krailo MD, Ames MM, Reid JM, Reaman GH, Reynolds CP. Phase I trial of oral fenretinide in children with high-risk solid tumors: a report from the Children's Oncology Group (CCG 09709) Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2006;24:3423–3430. doi: 10.1200/JCO.2005.03.9271. [DOI] [PubMed] [Google Scholar]
- Bagatell R, Gore L, Egorin MJ, Ho R, Heller G, Boucher N, Zuhowski EG, Whitlock JA, Hunger SP, Narendran A, Katzenstein HM, Arceci RJ, Boklan J, Herzog CE, Whitesell L, Ivy SP, Trippett TM. Phase I pharmacokinetic and pharmacodynamic study of 17-N-allylamino-17-demethoxygeldanamycin in pediatric patients with recurrent or refractory solid tumors: a pediatric oncology experimental therapeutics investigators consortium study. Clinical cancer research: an official journal of the American Association for Cancer Research. 2007;13:1783–1788. doi: 10.1158/1078-0432.CCR-06-1892. [DOI] [PubMed] [Google Scholar]
- Jimeno A, Daw NC, Amador ML, Cusatis G, Kulesza P, Krailo M, Ingle AM, Blaney SM, Adamson P, Hidalgo M. Analysis of biologic surrogate markers from a Children's Oncology Group Phase I trial of gefitinib in pediatric patients with solid tumors. Pediatric blood & cancer. 2007;49:352–357. doi: 10.1002/pbc.20753. [DOI] [PubMed] [Google Scholar]
- Kramer K, Humm JL, Souweidane MM, Zanzonico PB, Dunkel IJ, Gerald WL, Khakoo Y, Yeh SD, Yeung HW, Finn RD, Wolden SL, Larson SM, Cheung NK. Phase I study of targeted radioimmunotherapy for leptomeningeal cancers using intra-Ommaya 131-I-3F8. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2007;25:5465–5470. doi: 10.1200/JCO.2007.11.1807. [DOI] [PubMed] [Google Scholar]
- Bond M, Bernstein ML, Pappo A, Schultz KR, Krailo M, Blaney SM, Adamson PC. A phase II study of imatinib mesylate in children with refractory or relapsed solid tumors: a Children's Oncology Group study. Pediatric blood & cancer. 2008;50:254–258. doi: 10.1002/pbc.21132. [DOI] [PubMed] [Google Scholar]
- Fox E, Maris JM, Widemann BC, Goodspeed W, Goodwin A, Kromplewski M, Fouts ME, Medina D, Cohn SL, Krivoshik A, Hagey AE, Adamson PC, Balis FM. A phase I study of ABT-751, an orally bioavailable tubulin inhibitor, administered daily for 21 days every 28 days in pediatric patients with solid tumors. Clinical cancer research: an official journal of the American Association for Cancer Research. 2008;14:1111–1115. doi: 10.1158/1078-0432.CCR-07-4097. [DOI] [PubMed] [Google Scholar]
- Jakacki RI, Hamilton M, Gilbertson RJ, Blaney SM, Tersak J, Krailo MD, Ingle AM, Voss SD, Dancey JE, Adamson PC. Pediatric phase I and pharmacokinetic study of erlotinib followed by the combination of erlotinib and temozolomide: a Children's Oncology Group Phase I Consortium Study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2008;26:4921–4927. doi: 10.1200/JCO.2007.15.2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langevin AM, Bernstein M, Kuhn JG, Blaney SM, Ivy P, Sun J, Chen Z, Adamson PC. A phase II trial of rebeccamycin analogue (NSC #655649) in children with solid tumors: a Children's Oncology Group study. Pediatric blood & cancer. 2008;50:577–580. doi: 10.1002/pbc.21274. [DOI] [PubMed] [Google Scholar]
- Mita MM, Mita AC, Chu QS, Rowinsky EK, Fetterly GJ, Goldston M, Patnaik A, Mathews L, Ricart AD, Mays T, Knowles H, Rivera VM, Kreisberg J, Bedrosian CL, Tolcher AW. Phase I trial of the novel mammalian target of rapamycin inhibitor deforolimus (AP23573; MK-8669) administered intravenously daily for 5 days every 2 weeks to patients with advanced malignancies. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2008;26:361–367. doi: 10.1200/JCO.2007.12.0345. [DOI] [PubMed] [Google Scholar]
- Chawla SP, Chua VS, Fernandez L, Quon D, Saralou A, Blackwelder WC, Hall FL, Gordon EM. Phase I/II and phase II studies of targeted gene delivery in vivo: intravenous Rexin-G for chemotherapy-resistant sarcoma and osteosarcoma. Molecular therapy: the journal of the American Society of Gene Therapy. 2009;17:1651–1657. doi: 10.1038/mt.2009.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugh R, Wathen JK, Maki RG, Benjamin RS, Patel SR, Meyers PA, Priebat DA, Reinke DK, Thomas DG, Keohan ML, Samuels BL, Baker LH. Phase II multicenter trial of imatinib in 10 histologic subtypes of sarcoma using a bayesian hierarchical statistical model. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27:3148–3153. doi: 10.1200/JCO.2008.20.5054. [DOI] [PubMed] [Google Scholar]
- Widemann BC, Goodspeed W, Goodwin A, Fojo T, Balis FM, Fox E. Phase I trial and pharmacokinetic study of ixabepilone administered daily for 5 days in children and adolescents with refractory solid tumors. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27:550–556. doi: 10.1200/JCO.2008.17.6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs S, Fox E, Krailo M, Hartley G, Navid F, Wexler L, Blaney SM, Goodwin A, Goodspeed W, Balis FM, Adamson PC, Widemann BC. Phase II trial of ixabepilone administered daily for five days in children and young adults with refractory solid tumors: a report from the children's oncology group. Clinical cancer research: an official journal of the American Association for Cancer Research. 2010;16:750–754. doi: 10.1158/1078-0432.CCR-09-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzrock R, Patnaik A, Aisner J, Warren T, Leong S, Benjamin R, Eckhardt SG, Eid JE, Greig G, Habben K, McCarthy CD, Gore L. A phase I study of weekly R1507, a human monoclonal antibody insulin-like growth factor-I receptor antagonist, in patients with advanced solid tumors. Clinical cancer research: an official journal of the American Association for Cancer Research. 2010;16:2458–2465. doi: 10.1158/1078-0432.CCR-09-3220. [DOI] [PubMed] [Google Scholar]
- Olmos D, Postel-Vinay S, Molife LR, Okuno SH, Schuetze SM, Paccagnella ML, Batzel GN, Yin D, Pritchard-Jones K, Judson I, Worden FP, Gualberto A, Scurr M, de Bono JS, Haluska P. Safety, pharmacokinetics, and preliminary activity of the anti-IGF-1R antibody figitumumab (CP-751,871) in patients with sarcoma and Ewing's sarcoma: a phase 1 expansion cohort study. The lancet oncology. 2010;11:129–135. doi: 10.1016/S1470-2045(09)70354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinerman ES, Jia SF, Griffin J, Seibel NL, Benjamin RS, Jaffe N. Phase II study of liposomal muramyl tripeptide in osteosarcoma: the cytokine cascade and monocyte activation following administration. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1992;10:1310–1316. doi: 10.1200/JCO.1992.10.8.1310. [DOI] [PubMed] [Google Scholar]
- Kleinerman ES, Gano JB, Johnston DA, Benjamin RS, Jaffe N. Efficacy of liposomal muramyl tripeptide (CGP 19835A) in the treatment of relapsed osteosarcoma. American journal of clinical oncology. 1995;18:93–99. doi: 10.1097/00000421-199504000-00001. [DOI] [PubMed] [Google Scholar]
- Kleinerman ES, Meyers PA, Raymond AK, Gano JB, Jia SF, Jaffe N. Combination therapy with ifosfamide and liposome-encapsulated muramyl tripeptide: tolerability, toxicity, and immune stimulation. Journal of immunotherapy with emphasis on tumor immunology: official journal of the Society for Biological Therapy. 1995;17:181–193. doi: 10.1097/00002371-199504000-00007. [DOI] [PubMed] [Google Scholar]
- Harris MB, Cantor AB, Goorin AM, Shochat SJ, Ayala AG, Ferguson WS, Holbrook T, Link MP. Treatment of osteosarcoma with ifosfamide: comparison of response in pediatric patients with recurrent disease versus patients previously untreated: a Pediatric Oncology Group study. Medical and pediatric oncology. 1995;24:87–92. doi: 10.1002/mpo.2950240205. [DOI] [PubMed] [Google Scholar]
- Patel SR, Papadopoulos NE, Plager C, Linke KA, Moseley SH, Spirindonidis CH, Benjamin R. Phase II study of paclitaxel in patients with previously treated osteosarcoma and its variants. Cancer. 1996;78:741–744. doi: 10.1002/(SICI)1097-0142(19960815)78:4<741::AID-CNCR8>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Gentet JC, Brunat-Mentigny M, Demaille MC, Pein F, Avet-Loiseau H, Berger C, De Lumley L, Pacquement H, Schmitt C, Sariban E, Pillon P, Bernard JL, Kalifa C. Ifosfamide and etoposide in childhood osteosarcoma. A phase II study of the French Society of Paediatric Oncology. European journal of cancer. 1997;33:232–237. doi: 10.1016/S0959-8049(96)00439-X. [DOI] [PubMed] [Google Scholar]
- Worth LL, Jaffe N, Benjamin RS, Papadopoulos NE, Patel S, Raymond AK, Jia SF, Rodriguez C, Gano J, Gianan MA, Kleinerman ES. Phase II study of recombinant interleukin 1alpha and etoposide in patients with relapsed osteosarcoma. Clinical cancer research: an official journal of the American Association for Cancer Research. 1997;3:1721–1729. [PubMed] [Google Scholar]
- Voute PA, Souhami RL, Nooij M, Somers R, Cortes-Funes H, van der Eijken JW, Pringle J, Hogendoorn PC, Kirkpatrick A, Uscinska BM, van Glabbeke M, Machin D, Weeden S. A phase II study of cisplatin, ifosfamide and doxorubicin in operable primary, axial skeletal and metastatic osteosarcoma. European Osteosarcoma Intergroup (EOI) Annals of oncology: official journal of the European Society for Medical Oncology/ESMO. 1999;10:1211–1218. doi: 10.1023/A:1008361612767. [DOI] [PubMed] [Google Scholar]
- Fagioli F, Aglietta M, Tienghi A, Ferrari S, Brach del Prever A, Vassallo E, Palmero A, Biasin E, Bacci G, Picci P, Madon E. High-dose chemotherapy in the treatment of relapsed osteosarcoma: an Italian sarcoma group study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2002;20:2150–2156. doi: 10.1200/JCO.2002.08.081. [DOI] [PubMed] [Google Scholar]
- Laverdiere C, Kolb EA, Supko JG, Gorlick R, Meyers PA, Maki RG, Wexler L, Demetri GD, Healey JH, Huvos AG, Goorin AM, Bagatell R, Ruiz-Casado A, Guzman C, Jimeno J, Harmon D. Phase II study of ecteinascidin 743 in heavily pretreated patients with recurrent osteosarcoma. Cancer. 2003;98:832–840. doi: 10.1002/cncr.11563. [DOI] [PubMed] [Google Scholar]
- McTiernan A, Whelan JS. A Phase II Study of Docetaxel for the Treatment of Recurrent Osteosarcoma. Sarcoma. 2004;8:71–76. doi: 10.1155/2004/762736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S, Smeland S, Mercuri M, Bertoni F, Longhi A, Ruggieri P, Alvegard TA, Picci P, Capanna R, Bernini G, Muller C, Tienghi A, Wiebe T, Comandone A, Bohling T, Del Prever AB, Brosjo O, Bacci G, Saeter G. Neoadjuvant chemotherapy with high-dose Ifosfamide, high-dose methotrexate, cisplatin, and doxorubicin for patients with localized osteosarcoma of the extremity: a joint study by the Italian and Scandinavian Sarcoma Groups. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2005;23:8845–8852. doi: 10.1200/JCO.2004.00.5785. [DOI] [PubMed] [Google Scholar]
- Arpaci F, Ataergin S, Ozet A, Erler K, Basbozkurt M, Ozcan A, Komurcu S, Ozturk B, Celasun B, Kilic S, Kuzhan O. The feasibility of neoadjuvant high-dose chemotherapy and autologous peripheral blood stem cell transplantation in patients with nonmetastatic high grade localized osteosarcoma: results of a phase II study. Cancer. 2005;104:1058–1065. doi: 10.1002/cncr.21279. [DOI] [PubMed] [Google Scholar]
- McTiernan A, Meyer T, Michelagnoli MP, Lewis I, Whelan JS. A phase I/II study of doxorubicin, ifosfamide, etoposide and interval methotrexate in patients with poor prognosis osteosarcoma. Pediatric blood & cancer. 2006;46:345–350. doi: 10.1002/pbc.20562. [DOI] [PubMed] [Google Scholar]
- Seibel NL, Krailo M, Chen Z, Healey J, Breitfeld PP, Drachtman R, Greffe B, Nachman J, Nadel H, Sato JK, Meyers PA, Reaman GH. Upfront window trial of topotecan in previously untreated children and adolescents with poor prognosis metastatic osteosarcoma: children's Cancer Group (CCG) 7943. Cancer. 2007;109:1646–1653. doi: 10.1002/cncr.22553. [DOI] [PubMed] [Google Scholar]
- Basaran M, Bavbek ES, Saglam S, Eralp L, Sakar B, Atalar AC, Bilgic B, Ozger H, Onat H. A phase II study of cisplatin, ifosfamide and epirubicin combination chemotherapy in adults with nonmetastatic and extremity osteosarcomas. Oncology. 2007;72:255–260. doi: 10.1159/000113017. [DOI] [PubMed] [Google Scholar]
- Iwamoto Y, Tanaka K, Isu K, Kawai A, Tatezaki S, Ishii T, Kushida K, Beppu Y, Usui M, Tateishi A, Furuse K, Minamizaki T, Kawaguchi N, Yamawaki S. Multiinstitutional phase II study of neoadjuvant chemotherapy for osteosarcoma (NECO study) in Japan: NECO-93J and NECO-95J. Journal of orthopaedic science: official journal of the Japanese Orthopaedic Association. 2009;14:397–404. doi: 10.1007/s00776-009-1347-6. [DOI] [PubMed] [Google Scholar]
- Berger M, Grignani G, Ferrari S, Biasin E, Brach del Prever A, Aliberti S, Saglio F, Aglietta M, Fagioli F. Phase 2 trial of two courses of cyclophosphamide and etoposide for relapsed high-risk osteosarcoma patients. Cancer. 2009;115:2980–2987. doi: 10.1002/cncr.24368. [DOI] [PubMed] [Google Scholar]
- Kolb EA, Kushner BH, Gorlick R, Laverdiere C, Healey JH, LaQuaglia MP, Huvos AG, Qin J, Vu HT, Wexler L, Wolden S, Meyers PA. Long-term event-free survival after intensive chemotherapy for Ewing's family of tumors in children and young adults. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2003;21:3423–3430. doi: 10.1200/JCO.2003.10.033. [DOI] [PubMed] [Google Scholar]
- Meyer T, McTiernan A, Whelan J. A Phase II Study of Docetaxel in Patients with Relapsed and Refractory Ewing's Tumours. Sarcoma. 2003;7:13–17. doi: 10.1080/1357714031000114192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein ML, Devidas M, Lafreniere D, Souid AK, Meyers PA, Gebhardt M, Stine K, Nicholas R, Perlman EJ, Dubowy R, Wainer IW, Dickman PS, Link MP, Goorin A, Grier HE. Intensive therapy with growth factor support for patients with Ewing tumor metastatic at diagnosis: Pediatric Oncology Group/Children's Cancer Group Phase II Study 9457--a report from the Children's Oncology Group. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2006;24:152–159. doi: 10.1200/JCO.2005.02.1717. [DOI] [PubMed] [Google Scholar]
- Rosenthal J, Bolotin E, Shakhnovits M, Pawlowska A, Falk P, Qian D, Oliver C, Sato J, Miser J, Forman S. High-dose therapy with hematopoietic stem cell rescue in patients with poor prognosis Ewing family tumors. Bone marrow transplantation. 2008;42:311–318. doi: 10.1038/bmt.2008.169. [DOI] [PubMed] [Google Scholar]
- DuBois SG, Krailo MD, Lessnick SL, Smith R, Chen Z, Marina N, Grier HE, Stegmaier K. Phase II study of intermediate-dose cytarabine in patients with relapsed or refractory Ewing sarcoma: a report from the Children's Oncology Group. Pediatric blood & cancer. 2009;52:324–327. doi: 10.1002/pbc.21822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey DA, Wexler LH, Merchant MS, Chou AJ, Merola PR, Price AP, Meyers PA. Irinotecan and temozolomide for Ewing sarcoma: the Memorial Sloan-Kettering experience. Pediatric blood & cancer. 2009;53:1029–1034. doi: 10.1002/pbc.22206. [DOI] [PubMed] [Google Scholar]