Abstract
Background
Methoxycarbonyl etomidate is an ultra-rapidly metabolized etomidate analog. It is metabolized to methoxycarbonyl etomidate carboxylic acid (MOC-ECA), which has a hypnotic potency that is 350-fold lower than that of methoxycarbonyl etomidate. We explored the relationships between methoxycarbonyl etomidate infusion duration, recovery time, metabolite concentrations in blood and cerebrospinal fluid (CSF), and methoxycarbonyl etomidate metabolism in brain tissue and CSF to test the hypothesis that rapid metabolism of methoxycarbonyl etomidate may lead to sufficient accumulation of MOC-ECA in the brain to produce a pharmacological effect.
Methods
A closed-loop system with burst suppression ratio feedback was used to administer methoxycarbonyl etomidate infusions of varying durations to rats. After infusion, recovery of the electroencephalogram and righting reflexes were assessed. MOC-ECA concentrations were measured in blood and CSF during and after methoxycarbonyl etomidate infusion and the in vitro half-life of methoxycarbonyl etomidate was determined in rat brain tissue and CSF.
Results
Upon terminating continuous methoxycarbonyl etomidate infusions, the burst suppression ratio recovered in a biexponential manner with fast and slow components having time constants that differed by more than 100-fold and amplitudes that varied inversely with infusion duration. MOC-ECA concentrations reached hypnotic levels in the CSF with prolonged methoxycarbonyl etomidate infusion and then fell over several hours after infusion termination. The metabolic half-life of methoxycarbonyl etomidate in brain tissue and CSF was 11 and 20 min, respectively.
Conclusion
In rats, methoxycarbonyl etomidate metabolism is sufficiently fast to produce pharmacologically active MOC-ECA concentrations in the brain with prolonged methoxycarbonyl etomidate infusion.
Introduction
Methoxycarbonyl etomidate is a “soft” analog of etomidate that, similar to remifentanil and esmolol, is rapidly hydrolyzed by esterases.1–3 Its metabolite, methoxycarbonyl etomidate carboxylic acid (MOC-ECA), is a carboxylic acid whose hypnotic and γ-aminobutyric acid receptor modulatory potencies are 350-fold lower than that of methoxycarbonyl etomidate.4 Following single bolus administration to rats, methoxycarbonyl etomidate produces hypnosis of extremely short duration even when given at doses that far exceed its hypnotic ED50 because it is very rapidly metabolized.1
With many drugs (including sedative-hypnotics), the duration of action after terminating a continuous infusion increases with infusion duration.5,6 After a single bolus or brief infusion, drug is cleared from the effect site by redistribution and the recovery time is relatively insensitive to the terminal elimination rate. However with longer infusions that significantly fill peripheral compartments with drug, clearance from the effect site slows as drug diffuses from these compartments back to the effect site and recovery depends more heavily on terminal elimination. A drug’s duration of action may also increase with infusion duration if its metabolite possesses pharmacological activity and accumulates at the effect site during infusion. This mechanism is of particular relevance for soft analogs that may be given as prolonged continuous infusions, resulting in the production of relatively high quantities of metabolites that are often not completely devoid of pharmacological activity.7–9
In a previous study, we found that the electroencephalographic burst suppression ratio (BSR) of rats increased with methoxycarbonyl etomidate administration and then decreased rapidly once administration was stopped, consistent with the drug’s known hypnotic activity and rapid rate of metabolism.2 With intravenous boluses, the BSR promptly returned to its baseline value after methoxycarbonyl etomidate administration. However with prolonged infusions, the BSR remained above the baseline pre-infusion value for many minutes after infusion termination suggesting a residual pharmacological effect. In the current study, we explored the relationship between methoxycarbonyl etomidate infusion time, post-infusion recovery time, metabolite concentrations in blood and cerebrospinal fluid (CSF), and the rate of methoxycarbonyl etomidate metabolism in brain tissue and CSF in a rat model to test the hypothesis that prolonged methoxycarbonyl etomidate infusion can lead to sufficient MOC-ECA accumulation within the central nervous system to produce persistent electroencephalographic and hypnotic effects.
Materials and Methods
Animals
All studies were conducted in accordance with rules and regulations of the Subcommittee on Research Animal Care at the Massachusetts General Hospital, Boston, Massachusetts. Adult male Sprague-Dawley rats (230–350 gm) were purchased from Charles River Laboratories (Wilmington, MA) and housed in the Massachusetts General Hospital Center for Comparative Medicine animal care facility. Drugs were administered through a femoral venous catheter and blood draws were from a femoral arterial catheter. CSF was sampled from an intracisternal cannula. All catheters and cannulae were pre-implanted by the vendor prior to animal delivery to our animal care facility.
Drugs and Chemicals
Methoxycarbonyl etomidate was synthesized (>99% purity) by Aberjona Laboratories (Beverly, MA) as previously described.1 MOC-ECA was synthesized as previously reported.2 Methoxycarbonyl etomidate and MOC-ECA were diluted in normal saline for infusion. Isoflurane was purchased from Baxter (Deerfield, IL). Bupivicaine and heparin were from APP Pharmaceuticals (Schaumburg, IL).
Methodological Overview
In the first series of experiments, rats implanted with electroencephalographic electrodes were sedated with 1% isoflurane and administered methoxycarbonyl etomidate by closed-loop continuous infusion for varying durations of time (5, 15, or 30 minutes). The purpose of these experiments was to (1) determine the methoxycarbonyl etomidate dosing protocol required to maintain a constant hypnotic depth (80% BSR); (2) define the rate of electroencephalographic recovery following methoxycarbonyl etomidate infusion termination; and (3) measure the blood concentrations of MOC-ECA achieved during and after methoxycarbonyl etomidate infusion. Our rationale for using BSR as an objective measure of hypnotic depth has been previously discussed. 2 In the second series of experiments, rats were sedated with 1% isoflurane and administered either methoxycarbonyl etomidate or MOC-ECA for 30 minutes (using the protocol defined in the first series of experiments) and the concentrations of MOC-ECA and methoxycarbonyl etomidate achieved in CSF were determined. In the third series of experiments, rats were administered methoxycarbonyl etomidate by continuous infusion (again using the protocol defined in the first series of experiments) without isoflurane for either 5 or 30 minutes and the time required for righting reflexes to return after infusion termination was determined. In the fourth series of experiments, the in vitro metabolic half-life of methoxycarbonyl etomidate was defined in rat blood and CSF.
Electroencephalographic Electrode Placement and Recording
Electroencephalographic electrodes were placed as previously described.2,10 Briefly, rats were anesthetized with 2–3% inhaled isoflurane in 100% oxygen and placed in a stereotactic frame fitted with a nose cone. The skin was infiltrated with 0.5% bupivicaine containing epinephrine 1:200,000, the skull was exposed, the periosteum removed, and four 1.59 mm OD, 3.2 mm long bone anchor screws (Stoelting, Wood Dale, IL) with attached 0.010” Teflon-coated stainless steel wire (A-M Systems, Sequim, WA) were inserted through the bone and reinforced with dental acrylic at the stereotactic coordinates described by Vijn and Sneyd.10 The wires were connected to a P511 AC preamplifier (Grass Technologies, West Warwick, RI). The electroencephalographic signal was amplified 5000-fold, filtered (low frequency pass: 0.3 Hz, high frequency pass 0.03 kHz), digitized at 128 Hz using a USB-6009 data acquisition board (National Instruments, Austin, TX), and the BSR measured in real time with LabView Software (version 8.5 for Macintosh OS X; National Instruments) to provide feedback for a closed-loop infusion system and to monitor BSR recovery after infusion termination.
BSR Extraction and Closed-loop Infusion of Methoxycarbonyl Etomidate
Methods described in Rampil and Laster, Vijn and Sneyd, and Cotten et al. were used to continuously estimate BSR, where BSR is the percent time the electroencephalographic signal spent in suppression during each 6 s time epoch.2,10,11 Temporal differentiation (the difference between two successive data samples in the digitized electroencephalographic signal) was used to enhance BSR sensitivity.10 Suppression was defined as an interval during which the time-differentiated electroencephalographic signal amplitude stayed within a suppression voltage window for at least 100 ms. To account for variable signal noise among rats, this suppression voltage window was defined individually in each rat as previously described.2 Rats were then equilibrated with 1% inhaled isoflurane delivered through a tight-fitting nose cone for at least 45 minutes until the BSR stabilized prior to study. Unless indicated otherwise, studies were done in a background of 1% inhaled isoflurane to facilitate BSR measurements and blood and CSF sampling.
A KDS Model 200 Series infusion pump (KD Scientific, Holliston, MA) was used for continuous methoxycarbonyl etomidate or MOC-ECA infusion. The pump was controlled remotely via its RS 232 serial port by a Macintosh computer using a Keyspan USB-Serial port adapter (Tripp Lite, Chicago, IL). A LabView 8.5 instrument driver using Virtual Instrument Software Architecture protocols provided computer-to-pump communication. For closed-loop methoxycarbonyl etomidate infusions, we used the algorithm described by Vijn & Sneyd.10 In this approach, the methoxycarbonyl etomidate infusion rate is increased (if the current BSR is < 80%) or decreased (if the current BSR is >80%) every 6 seconds. The magnitude of the change in the infusion rate is dependent upon the difference between the current BSR measured in the rat and our target BSR of 80%. The algorithm was modified with a maximum infusion rate of 60 mg/kg/min to prevent inadvertent over-dosage and a minimum rate of 5 mg/kg/min to assure continuous methoxycarbonyl etomidate infusion. Because it was not possible to place electroencephalographic electrodes in rats with intracisternal cannulae, we used an infusion protocol determined from previous closed-loop infusion experiments for in vivo studies to determine CSF MOC-ECA and methoxycarbonyl etomidate concentrations. This approach was also used for studies to define the duration of loss of righting reflexes following methoxycarbonyl etomidate infusion.
Analysis of BSR Recovery After Terminating Closed-Loop Infusion of Methoxycarbonyl Etomidate
Because the fast and slow components of the BSR recovery occurred over vastly different time scales, each component was analyzed separately by fitting the time-dependent change in BSR to an exponential equation over the appropriate time scale. Thus, the fast component was analyzed by fitting the first 5-minutes of BSR data recorded immediately after methoxycarbonyl etomidate infusion termination. The slow component was analyzed by fitting the BSR data beginning 2 minutes after infusion termination (to exclude the fast component) and ending at least 3.5 hours later.
Measurement of Blood and CSF Concentrations of MOC-ECA and Methoxycarbonyl Etomidate
Before, during, and after closed-loop methoxycarbonyl etomidate infusion, blood samples (200 ul/sample) were intermittently collected through the femoral arterial catheter and immediately mixed with an acetonitrile (200 μl). To minimize dehydration, this blood was replaced with an equal volume of normal saline. The samples were centrifuged and the resultant plasma was collected and stored at −20 °C until analyzed. After thawing, the MOC-ECA concentration in each sample was determined by high performance liquid chromatography using a Varian Prostar system with a 4.6 × 250 mm Proto 300 C18 column (Nest Group, Southborough, MA) with the UV detector set at 240nm. A linear gradient 20% to 90% acetonitrile in water with 0.05% trifluoroacetic acid (Thermo Scientific, Rockford, IL) over 30 minutes was used with a flow rate of 1 ml/min. MOC-ECA standards were prepared in rat blood and processed identically to experimental samples.
Before, during, and after methoxycarbonyl etomidate and MOC-ECA infusion, CSF samples (25 μl/sample) were collected through the intracisternal catheter, immediately mixed with acetonitrile (50 μl), centrifuged, and the supernatant stored at −20 °C until analyzed. The concentrations of MOC-ECA and methoxycarbonyl etomidate in CSF were determined by high performance liquid chromatography as described in the previous paragraph for blood using standards prepared in methanol.
The lower limit of quantitation, precision, and accuracy of our chromatographic analyses were determined generally as described by Hubbard et al. 12 For the lower limit of quantitation, we used samples within the lower range of our calibration curve (1, 3, 5, and 10 μM MOC-ECA or methoxycarbonyl etomidate) and defined a lower limit of quantitation of 3 μM for both compounds. We also obtained lower limit of quantitation estimates of 2.1 μM for MOC-ECA and 3.7 μM for methoxycarbonyl etomidate, respectively, based on the standard deviation of the responses and the slopes of the calibration curves. 13 Intra-day precision and accuracy were determined at 100 μM and 1 mM MOC-ECA and 10 μM methoxycarbonyl etomidate. At these concentrations, the relative standard deviations were less than 5% and the accuracy of all samples was within 15% of their nominal values. Standard curves were linear across the entire concentration ranges with r2>0.99.
Measurement of Methoxycarbonyl Etomidate In Vitro Metabolic Half-life in Brain Tissue and CSF
Rat brain homogenate (20mg/mL in phosphate buffered saline media pH 7.4) pooled from 4 rats was purchased from Bioreclamation LLC (Hicksville, NY). Methoxycarbonyl etomidate (100 μl from a 1 mM stock solution in saline) was added to 1 ml of the homogenate. After the desired incubation period at 37 °C, a 100 μl aliquot was removed and the metabolism was stopped by mixing with 100 μl of acetonitrile. The samples were then centrifuged and the supernatant collected and stored at −20 °C until analyzed by high performance liquid chromatography as described for blood.
CSF (150μl) was drawn from the pre-implanted intracisternal cannula of a rat sedated with 1% isoflurane. Methoxycarbonyl etomidate (16.5 μl from a 1 mM stock solution in saline) was added to the CSF and after the desired incubation period at 37 °C, a 25 μl aliquot was removed and mixed with 50 μl of acetonitrile. The samples were then centrifuged and the supernatant was collected and stored at −20 °C until analyzed by high performance liquid chromatography as described for blood.
Recovery of Righting Reflexes Following Infusion of Methoxycarbonyl Etomidate
Methoxycarbonyl etomidate was infused through a femoral venous catheter for either 5 or 30 minutes. Three minutes after starting the infusion, rats were turned supine. The time to recovery of righting reflexes following methoxycarbonyl etomidate infusion was defined as the time from infusion termination until spontaneous righting onto all four legs.
Statistical Analysis
All data are reported as mean +/− SD. Statistical analyses were done using Prism v5.0 for the Macintosh (GraphPad Software, Inc., LaJolla, CA) or Igor Pro 6.1 (Wavemetrics, Lake Oswego, OR). For multiple comparisons, we performed either a one-way or a two-way analysis of variance followed respectively by a Dunnett or Bonferroni post–test. P < 0.05 was considered statistically significant. Igor Pro 6.1’s built-in fitting functionality was used to fit data to an exponential equation.
Results
The Relationship between the Duration of Methoxycarbonyl etomidate Infusion and the Kinetics of BSR Recovery
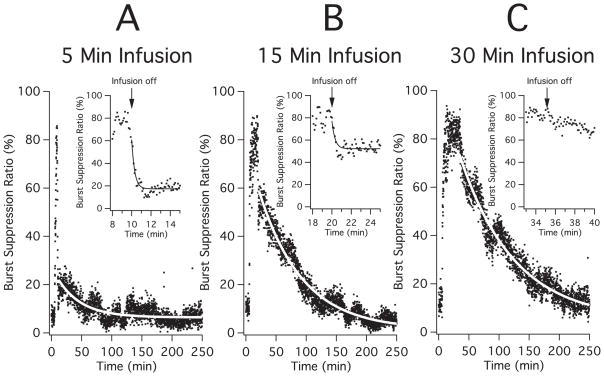
After equilibration with 1% isoflurane, rats were assigned to receive a 5-minute (n=4 rats), 15-minute (n=4 rats), or 30-minute (n=4 rats) closed-loop methoxycarbonyl etomidate infusion with a target BSR of 80%. In all experiments, the BSR was recorded for 5-minutes prior to beginning the methoxycarbonyl etomidate infusion, during infusion, and then for at least 3.5 hours after the infusion ended. Figure 1 shows the mean BSR recorded from rats prior to, during, and after 5-minute (panel A), 15-minute (panel B) or 30-minute (panel C) closed-loop methoxycarbonyl etomidate infusions. In these three groups of rats, baseline BSR values measured during the 5 minutes prior to methoxycarbonyl etomidate infusion averaged 7 ± 2%, 11 ± 2%, and 13 ± 3%, respectively. The BSR reached the target value of 80% approximately 3 minutes after beginning methoxycarbonyl etomidate infusion and remained near that value for the remainder of the closed-loop infusion period. After the infusion was complete, the BSR of rats in all three groups decreased from the 80% target towards the pre-infusion baseline value; however, the kinetics of this recovery varied with infusion duration.
Figure 1.
Mean burst suppression ratio (BSR) during each 6 sec epoch recorded from rats prior to, during, and after 5-minute (panel A; n=4 rats), 15-minute (panel B; n=4 rats) or 30-minute (panel C; n=4 rats) closed-loop methoxycarbonyl etomidate infusions. In each large panel, the curve is an exponential fit of the data to obtain the amplitude and time constant of the slow component of the BSR recovery. The inset in each panel expands the time period beginning 2 minutes prior to infusion termination until 5 minutes after infusion termination to better reveal the fast component of the recovery and the curve is an exponential fit of the data to obtain the amplitude and time constant of the fast component of the BSR recovery. Closed-loop infusions were begun after measuring the baseline BSR for five minutes. The target burst suppression ratio during methoxycarbonyl etomidate infusion was 80% for all experiments. Studies were done in a background of 1% isoflurane.
Upon terminating 5-minute infusions (figure 1A), the BSR recovered in a biexponential manner. There was a relatively large fast component that was complete within one minute of infusion termination and a smaller slow component that was barely perceptible above the signal noise and persisted for more than an hour. A fit of the fast component to an exponential equation yielded an amplitude of 45 ± 4% and a time constant of 18 ± 3 s (figure 1A inset) whereas a fit of the slow component yielded an amplitude of 15.5 ± 0.3% and a time constant of 40 ± 2 min.
Upon terminating 15-minute infusions (figure 1B), the BSR decayed in a manner similar to that observed upon terminating 5-minute infusions. However, the amplitude of the fast component was smaller and that of the slow component was larger. A fit of the fast component to an exponential equation yielded an amplitude of 31 ± 4% and a time constant of 12 ± 3 s (figure 1B inset) whereas a fit of the slow component yielded an amplitude of 57.5 ± 0.3% and a time constant 71 ± 1 min.
Upon terminating 30-minute infusions (figure 1C), there was no detectable fast component (figure 1C inset) and the BSR decayed over several hours. A fit of this slow decay to an exponential equation yielded an amplitude of 68.0 ± 0.4% and a time constant of 90 ± 1.7 min.
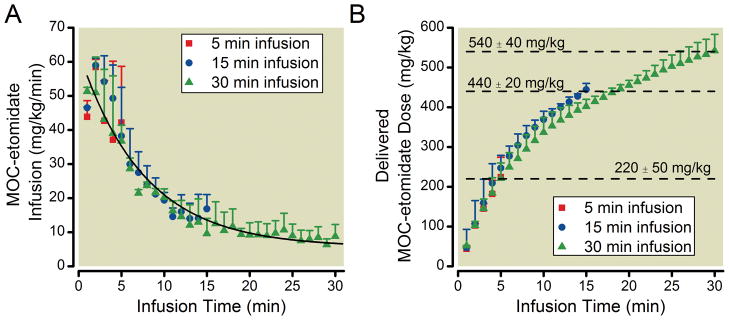
Figure 2A plots the average methoxycarbonyl etomidate dose delivered each minute by the closed-loop infusion system and shows that the methoxycarbonyl etomidate infusion rate required to maintain an 80% BSR decreased with infusion time. A fit of this infusion data to an exponential equation yielded a maximum of 58 ± 2 mg/kg, a plateau of 6 ± 2 mg/kg, and a time constant of 7.3 ± 0.8 min. Figure 2B plots the average cumulative methoxycarbonyl etomidate dose as a function of infusion time and shows that rats received total methoxycarbonyl etomidate doses of 220 ± 50 mg/kg, 440 ± 20 mg/kg, and 540 ± 40 mg/kg during 5-minute, 15-minute, and 30-minute closed-loop infusions respectively.
Figure 2.
(A) Infusion rate of methoxycarbonyl etomidate (MOC-etomidate) as a function of infusion time delivered during closed-loop infusions of 5-minute, 15-minute, or 30-minute duration. The curve is a fit of all three data sets to an exponential equation yielding an amplitude of 58 ± 2 mg/kg, a plateau of 6 ± 2 mg/kg, and a time constant of 7.3 ± 0.8 minutes. (B) Cumulative methoxycarbonyl etomidate doses as a function of infusion time delivered during closed-loop infusions of 5-minute, 15-minute, or 30-minute duration. All data represent the mean ± S.D from 4 rats.
MOC-ECA Concentrations in Blood with 30-Minute Intravenous Infusions of Methoxycarbonyl Etomidate
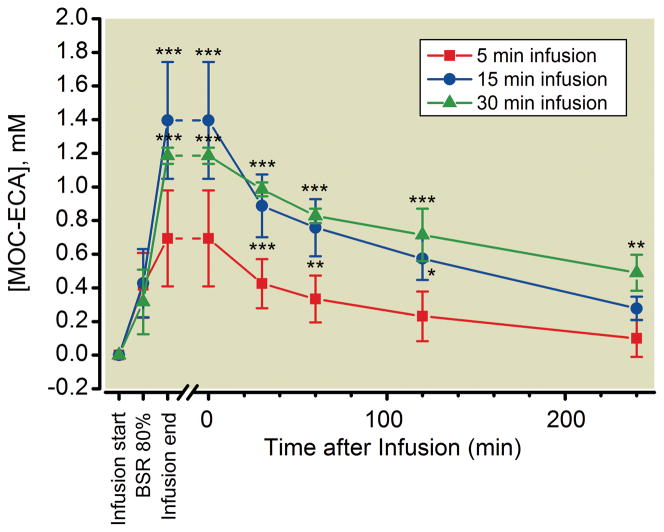
To assess the extent to which MOC-ECA accumulated in the blood during the closed-loop methoxycarbonyl etomidate infusions, we also drew arterial blood samples from each rat before starting the infusion, when the BSR first reached 80%, at the end of the infusion, and intermittently after the infusion was complete. Figure 3 reveals that the blood concentration of MOC-ECA in these samples progressively increased during methoxycarbonyl etomidate infusion. At the time that the BSR first reached 80%, the MOC-ECA concentration in the blood was not significantly different among the three groups of rats and averaged 0.39 ± 0.06 mM. In all three groups, the MOC-ECA concentration peaked at the end of the closed-loop methoxycarbonyl etomidate infusion with values of 0.7 ± 0.3 mM, 1.4 ± 0.4 mM, and 1.2 ± 0.1 mM, for infusion durations of 5 minutes, 15 minutes, and 30 minutes, respectively, and then decreased over the next several hours.
Figure 3.
Arterial blood concentrations of methoxycarbonyl etomidate’s carboxylic acid metabolite (MOC-ECA) before starting the closed-loop infusion (Infusion Start), when the burst suppression ratio first reached 80% (BSR 80%), at the end of the infusion (Infusion End), and intermittently after the infusion was complete. * P < 0.05, ** P < 0.01, *** P < 0.001 versus corresponding 5-minutes infusion value assessed using a two-way analysis of variance followed by a Bonferroni post–test where the factors were time and methoxycarbonyl etomidate infusion duration. There were no significant differences in MOC-ECA concentrations at analogous times with 15-minute and 30-minute infusions. All data represent the mean ± S.D from 4 rats.
MOC-ECA and Methoxycarbonyl Etomidate Concentrations in the CSF with 30-Minute Intravenous Infusions of Methoxycarbonyl Etomidate or MOC-ECA
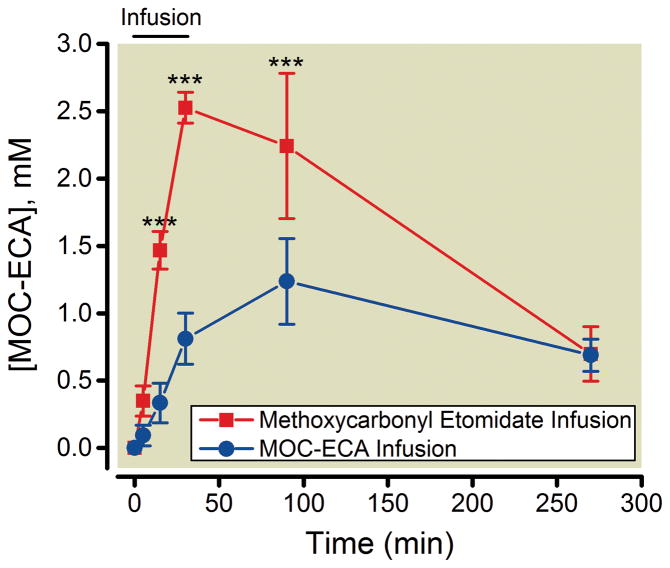
Using separate groups of rats, we also tested whether MOC-ECA accumulates within the central nervous system (i.e. CSF) with continuous infusion of methoxycarbonyl etomidate. Because the pre-implanted intracisternal catheters prevented our placing electroencephalographic electrodes, we could not use our closed-loop method for administering methoxycarbonyl etomidate. Instead, we used the average infusion protocol previously determined in rats without intracisternal catheters (shown in figure 2A) to administer 540 mg/kg methoxycarbonyl etomidate over 30 minutes. Figure 4 shows that during continuous infusion of methoxycarbonyl etomidate, the concentration of MOC-ECA in the CSF progressively increased and reached a peak value of 2.5 ± 0.11 mM at the end of the 30-minute infusion before falling to 0.7 ± 0.20 mM four hours after the infusion was complete. Figure 4 also shows the results of analogous experiments with continuous infusions of MOC-ECA (540 mg/kg over 30 minutes using the protocol in figure 2A). In these experiments, the concentration of MOC-ECA in the CSF also progressively increased; however the peak MOC-ECA concentration was significantly lower and occurred 60 minutes later than with infusions of methoxycarbonyl etomidate.
Figure 4.
Cerebrospinal fluid concentrations of methoxycarbonyl etomidate’s carboxylic metabolite (MOC-ECA) before, during, and after a 30-minute continuous infusion of either methoxycarbonyl etomidate or MOC-ECA. The total infused dose of methoxycarbonyl etomidate or MOC-ECA was 540 mg/kg, which was delivered using the infusion protocol defined from closed-loop infusion experiments. *** P < 0.001; two-way analysis of variance followed by a Bonferroni post–test where the factors were time and infusion duration. All data represent the mean ± S.D from 3 rats.
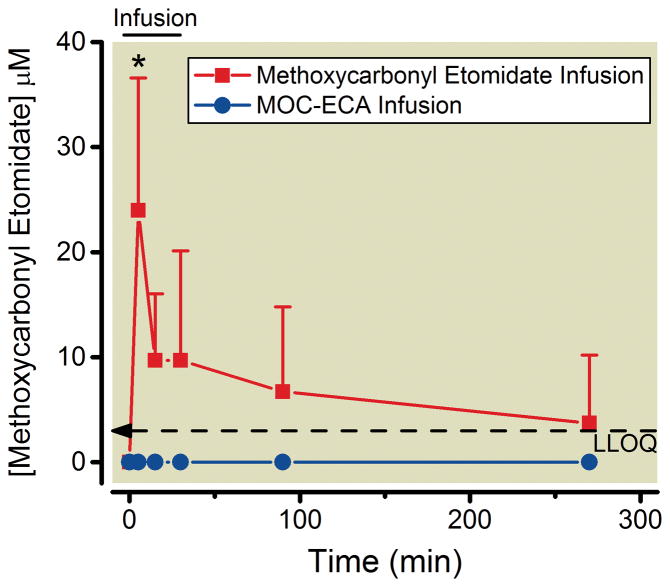
We also measured methoxycarbonyl etomidate concentrations in the CSF during the continuous infusion experiments described in the previous paragraph. Figure 5 shows that with methoxycarbonyl etomidate infusion, the methoxycarbonyl etomidate concentration in the CSF reached a peak value of 24 ± 13 μM five minutes into the infusion (i.e. when the methoxycarbonyl etomidate infusion rate was greatest) before decreasing to values that approached our lower limit of quantitation and were not significantly different from zero. As expected, no methoxycarbonyl etomidate was detected in the CSF at any time during or after MOC-ECA infusion.
Figure 5.
Cerebrospinal fluid concentrations of methoxycarbonyl etomidate before, during, and after 30-minute continuous infusions of methoxycarbonyl etomidate or methoxycarbonyl etomidate’s carboxylic acid metabolite (MOC-ECA). The total infused dose of methoxycarbonyl etomidate or MOC-ECA was 540 mg/kg, which was delivered using the infusion protocol defined from closed-loop infusion experiments. * P < 0.05 versus time 0; one-way analysis of variance followed by a Dunnett post–test. The dashed line indicates our lower limit of quantitation (LLOQ). All data represent the mean ± S.D from 3 rats.
In Vitro Determination of the Metabolic Half-Life of Methoxycarbonyl Etomidate in Rat Brain Tissue and CSF
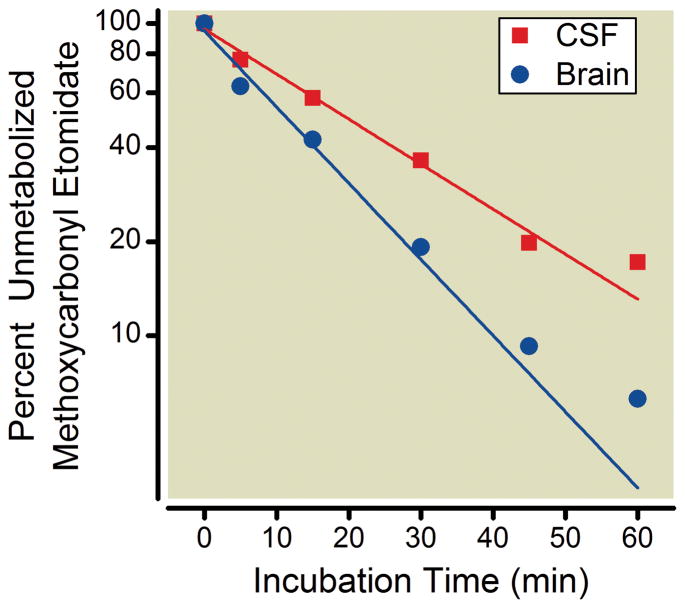
To assess the ability of methoxycarbonyl etomidate to be metabolized within the central nervous system of the rat, we added the sedative-hypnotic to pooled brain tissue or CSF (both from rats) and measured the incubation time-dependent reduction in methoxycarbonyl etomidate concentration. Figure 6 shows that the concentration of methoxycarbonyl etomidate decreased in an approximately first-order manner with preincubation time in both brain tissue and CSF. Methoxycarbonyl etomidate’s metabolic half-life in rat brain tissue was determined from a fit of the data to be 11 min (95% confidence intervals 10 – 13 min) whereas that in rat CSF was 20 min (95% confidence intervals 16 – 27 min).
Figure 6.
In vitro metabolism of methoxycarbonyl etomidate in pooled brain tissue and cerebrospinal fluid (CSF) from rats. Each brain tissue data point is the mean value from 3 experiments using a single source of pooled brain tissue derived from 4 rats. Each CSF data point is the mean value obtained from 4 separate experiments using CSF drawn from 4 rats. The lines are non-linear least squares fits of the data assuming a first-order process with the maxima and minima constrained to 100 and 0, respectively. The metabolic half-lives of methoxycarbonyl etomidate in brain tissue and CSF were determined to be 11 min (95% confidence intervals 10 to 13 min) and 20 min (95% confidence intervals 16 to 27 min), respectively.
Recovery of Righting Reflexes After Methoxycarbonyl Etomidate Infusions
To better understand the potential behavioral implications of MOC-ECA accumulation in the central nervous system and the slow electroencephalographic recovery that results from prolonged methoxycarbonyl etomidate infusion, we again used the infusion protocol shown in figure 2A to achieve and maintain approximately equivalent hypnotic depths for either 5 minutes or 30 minutes and measured the time required for rats to recover their righting reflexes after the infusion was complete. Because the goal of these studies was to assess a behavioral endpoint (i.e. recovery of righting reflexes), they were performed in the absence of isoflurane. All rats lost their righting reflexes within 3 minutes of beginning methoxycarbonyl etomidate infusion. Rats that received 5-minute infusions (n=4) recovered their righting reflexes 1.5 ± 0.40 min (range: 1.02 – 1.97 min) after the infusion was complete whereas those that received 30-minute infusions (n=4) recovered their righting reflexes after 30 ± 13 min (range: 20.2 – 50.5 min; p=0.0042 versus 5-minute infusion group).
Discussion
The current studies in rats demonstrate that upon terminating continuous closed-loop infusions of methoxycarbonyl etomidate, the electroencephalographic BSR recovers in a biexponential manner with fast and slow components that have time constants that differ by more than 100-fold and amplitudes that vary inversely with infusion duration.
The fast component dominated when the methoxycarbonyl etomidate infusion was brief (i.e. 5 minutes) and was similar to the BSR recovery that we previously observed after administering single methoxycarbonyl etomidate boluses.2 It’s time constant (~15 s) is 1–2 orders of magnitude faster than methoxycarbonyl etomidate’s in vitro metabolic half-life in rat brain tissue and CSF, but similar to methoxycarbonyl etomidate’s in vitro metabolic half-life in rat blood (20 s).14 Based on these results, we conclude that the fast component of the BSR recovery reflects the rapid elimination of methoxycarbonyl etomidate from the brain as the hydrophobic drug diffuses across the blood-brain barrier and is metabolized by esterases in the blood and/or other peripheral tissues.
The slow component dominated when the methoxycarbonyl etomidate infusion was long (i.e. 30 minutes). By the end of such infusions, the concentration of MOC-ECA in the CSF reached 2.5 ± 0.11 mM, which approximates MOC-ECA’s EC50 for loss of righting reflexes in tadpoles of 2.8 ± 0.64 mM.4 In addition, the methoxycarbonyl etomidate infusion rate required to maintain an 80% BSR decreased by an order of magnitude over the course of such infusions. These results suggest that with long methoxycarbonyl etomidate infusions, MOC-ECA reached sufficient concentrations in the brain to achieve (or at least contribute to) electroencephalographic burst suppression in our experiments. Upon discontinuing methoxycarbonyl etomidate infusion, the MOC-ECA concentration in the CSF decreased on approximately the same time scale (several hours) as the slow component of the BSR recovery (time constant ~ 1 hr). Based on these results, we conclude that the slow component reflects the slow elimination of MOC-ECA from the brain.
We considered two possible mechanisms by which MOC-ECA might have reached such high concentrations in the CSF with prolonged methoxycarbonyl etomidate infusion. The first possibility is that methoxycarbonyl etomidate was metabolized in the periphery (e.g. blood) and the resulting MOC-ECA diffused across the blood-brain barrier into the CSF. The second possibility is that that methoxycarbonyl etomidate was metabolized within the central nervous system, thus forming MOC-ECA in situ. Two lines of evidence favor the latter in situ mechanism as the major source of MOC-ECA in the CSF. First, the peak concentration of MOC-ECA in the CSF occurred 60 min after the MOC-ECA infusion ended, suggesting that MOC-ECA does not readily cross the blood-brain barrier. Slow penetration across the blood-brain barrier is expected for a charged compound and would explain our previous observation that MOC-ECA failed to significantly increase the BSR during a 15-minute intravenous infusion.2 Second, tissues from the rat central nervous system (i.e. brain tissue and CSF) not only metabolize methoxycarbonyl etomidate, they do so on the approximate time scale that we observed accumulation of hypnotic concentrations of MOC-ECA in the CSF during continuous methoxycarbonyl etomidate infusion.
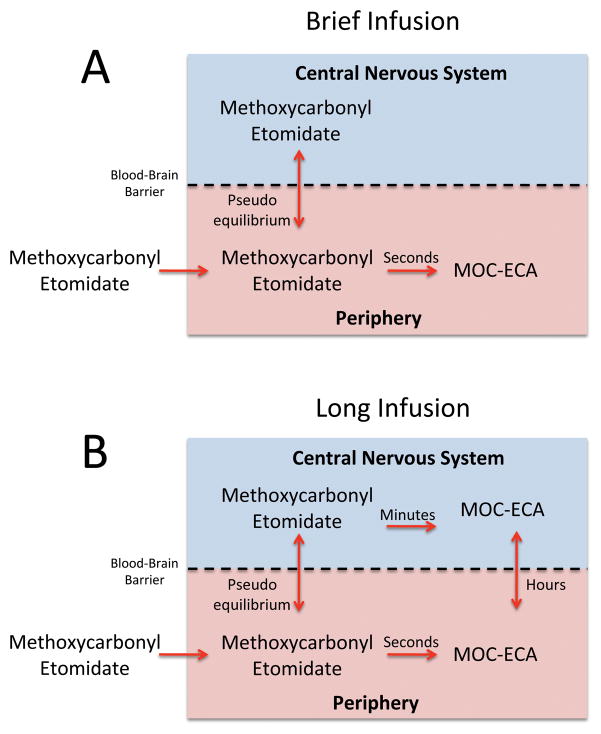
Figure 7 illustrates our current theory regarding the relationships between methoxycarbonyl etomidate infusion time, post-infusion recovery time, metabolite concentrations in the blood and CSF, and the rate of methoxycarbonyl etomidate metabolism in brain tissue and CSF. Panel A shows that with a single bolus or brief infusion, methoxycarbonyl etomidate rapidly equilibrates between the blood and brain to produce an increase in the BSR and loss-of-righting reflexes. Once administration has stopped, methoxycarbonyl etomidate concentrations in the blood fall rapidly as the drug is hydrolyzed to MOC-ECA. Because equilibration between the blood and brain is very fast, methoxycarbonyl etomidate concentrations in the brain fall in parallel with those in the blood. This results in rapid recovery of the BSR (i.e. the fast component) and the quick return of righting reflexes. Progressively longer methoxycarbonyl etomidate infusions lead to higher MOC-ECA concentrations in the brain because the charged metabolite is virtually trapped there after being formed in situ from methoxycarbonyl etomidate (figure 7B). As MOC-ECA accumulates in the brain, it makes a progressively greater contribution to the burst suppression and reduces the infusion rate of methoxycarbonyl etomidate required to maintain the BSR at 80%. By the end of a 30-minute methoxycarbonyl etomidate infusion, essentially all of the burst suppression (and hypnosis) in our rats is produced by accumulated metabolite alone. At this point, BSR recovery and the return of righting reflexes are slow because they are rate-limited by the slow transport of MOC-ECA out of the brain.
Figure 7.
Schematic illustration of our current theory regarding the relationships between methoxycarbonyl etomidate infusion time, post-infusion recovery time, methoxycarbonyl etomidate carboxylic acid (MOC-ECA) concentrations in the blood and cerebrospinal fluid, and the rate of methoxycarbonyl etomidate metabolism in brain tissue and cerebrospinal fluid in the rat. The approximate time scale for each process is given. (A) With brief methoxycarbonyl etomidate infusion (or single bolus), there is insufficient time for methoxycarbonyl etomidate to be metabolized to MOC-ECA within the central nervous system or for metabolite formed in the blood to diffuse into the central nervous system. Consequently, there is little MOC-ECA in the brain and essentially all of the burst suppression and hypnosis is produced by methoxycarbonyl etomidate alone. Upon terminating the infusion, the methoxycarbonyl etomidate concentration in the brain falls rapidly in parallel with that in the blood because methoxycarbonyl etomidate diffusion across the blood-brain barrier is very fast. Recovery is rate-limited by peripheral (e.g. blood) metabolism of methoxycarbonyl etomidate, which occurs on the time scale of seconds. (B) With longer infusions, significant quantities of MOC-ECA are formed from methoxycarbonyl etomidate within the central nervous system. This metabolic process occurs on the time scale of minutes. With sufficiently long infusions, essentially all of the burst suppression and hypnosis is produced by MOC-ECA. Upon terminating the infusion, the MOC-ECA concentration in the brain falls slowly because it crosses the blood-brain barrier and ultimately excreted from the body on the time scale of hours.
Within this theoretical construct, the relative amplitudes of the fast and slow components reflect the relative contributions made by methoxycarbonyl etomidate and MOC-ECA, respectively, to achieving our target BSR of 80%. Thus with a single bolus or brief infusion of methoxycarbonyl etomidate that results in a BSR recovery that is largely composed of the fast component, methoxycarbonyl etomidate itself is responsible for most of the burst suppression. By the end of a 15-minute methoxycarbonyl etomidate infusion when the fast and slow components have more similar amplitudes, methoxycarbonyl etomidate and MOC-ECA contribute more equally to achieving burst suppression. By the end of a 30-minute methoxycarbonyl etomidate infusion when only a slow component is observed, MOC-ECA is responsible for essentially all of the burst suppression. Our in vivo CSF results are consistent with this interpretation as the relative CSF concentrations of MOC-ECA measured after 5-minutes, 15-minutes, and 30-minutes of methoxycarbonyl etomidate infusion (0.14, 0.58, and 1, respectively) correlate reasonably well with the fraction of the BSR recovery that is represented by the slow component (0.26, 0.65. and 1, respectively) after 5-minute, 15-minute, and 30-minute closed-loop continuous methoxycarbonyl etomidate infusions.
The current studies were performed in rats, which are typically (but not always) less sensitive than humans to sedative-hypnotics and metabolize ester-containing drugs more rapidly.1,15–18 This suggests that MOC-ECA will not reach concentrations in humans that are as high as we observed in rats. However at this point, we cannot rule out the possibility that pharmacologically significant MOC-ECA concentrations could be achieved. In particular, patients with renal failure may achieve steady-state metabolite concentrations with prolonged infusion of rapidly metabolized drugs that are orders of magnitude higher than those with normal renal function.7,19 Such patients are predicted to be most vulnerable to the physiological actions of MOC-ECA which, based on the present studies, includes prolonged hypnotic action and slow recovery of the electroencephalographic after infusion termination.
Other recently developed soft sedative-hypnotics include CNS7056 (remimazolam) and AZD3043. 20 CNS7056 is a benzodiazepine that was developed as a shorter-acting alternative to midazolam whereas AZD3043 is a propranidid analog intended for use as an anesthetic induction and/or maintainence agent. Both drugs have reached clinical trials, but it has been reported that development of AZD3043 has been delayed by formulation difficulties. 21 Methoxycarbonyl etomidate is at an earlier stage of development and our laboratory is currently working on the design of other soft etomidate analogs that have somewhat longer hypnotic duration and/or higher hypnotic potency with the goal of reducing dosing requirements and any potential side effects related to metabolite accumulation.
In summary, MOC-ECA accumulates in rats with continuous methoxycarbonyl etomidate infusion and can reach sufficiently high concentrations in the brain to cause (or contribute to) electroencephalographic burst suppression with prolonged infusion. Because methoxycarbonyl etomidate and MOC-ECA are eliminated from the brain at very different rates, electroencephalographic recovery after terminating such infusions may occur as a biexponential process composed of rapid and slow components whose amplitude reflect the relative contributions that methoxycarbonyl etomidate and MOC-ECA make to achieving burst suppression. The time required for righting reflexes to recover similarly depends upon the duration of methoxycarbonyl etomidate infusion, suggesting that MOC-ECA concentrations in the brain may reach levels sufficient to produce hypnosis. Future studies in humans and human tissues will be necessary to determine the extent to which methoxycarbonyl etomidate is metabolized and accumulates in the human central nervous system and the role, if any, that accumulated metabolite plays in the recovery profile of methoxycarbonyl etomidate following prolonged continuous methoxycarbonyl etomidate infusion in man.
Summary.
What we already know about this topic
Methoxycarbonyl etomidate is an etomidate analog that produces hypnosis of short duration after bolus administration to rats.
It is rapidly metabolized by esterases to methoxycarbonyl etomidate carboxylic acid, which has less than 1% of its hypnotic potency
With prolonged methoxycarbonyl etomidate administration, the electroencephalographic burst suppression ratio remains elevated long after terminating the infusion
What this article tells us that is new
Prolonged methoxycarbonyl etomidate administration to rats produces brain methoxycarbonyl etomidate carboxylic acid concentrations that are sufficiently high to cause electroencephalographic burst suppression
Acknowledgments
Supported by grants R01-GM087316, R21-DA029253, and K08-GM083216 from the National Institutes of Health, Bethesda, MD and the Department of Anesthesia, Critical Care, and Pain Medicine, Massachusetts General Hospital, Boston, Massachusetts.
Footnotes
Conflict of Interest Statement: The Massachusetts General Hospital has submitted patent applications for methoxycarbonyl etomidate and related analogues. Three authors (Raines, Cotten, and Husain), and their respective laboratories, departments, and institutions could receive compensation related to the development or sale of methoxycarbonyl etomidate and related analogs. Dr. Raines is a consultant for and holds an equity interest in Annovation BioPharma Inc., which has licensed this technology for development.
References
- 1.Cotten JF, Husain SS, Forman SA, Miller KW, Kelly EW, Nguyen HH, Raines DE. Methoxycarbonyl-etomidate: A novel rapidly metabolized and ultra-short-acting etomidate analogue that does not produce prolonged adrenocortical suppression. Anesthesiology. 2009;111:240–9. doi: 10.1097/ALN.0b013e3181ae63d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cotten JF, Le Ge R, Banacos N, Pejo E, Husain SS, Williams JH, Raines DE. Closed-loop Continuous Infusions of Etomidate and Etomidate Analogs in Rats: A Comparative Study of Dosing and the Impact on Adrenocortical Function. Anesthesiology. 2011;115:764–73. doi: 10.1097/ALN.0b013e31821950de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bodor N, Buchwald P. Soft drug design: General principles and recent applications. Med Res Rev. 2000;20:58–101. doi: 10.1002/(sici)1098-1128(200001)20:1<58::aid-med3>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 4.Ge RL, Pejo E, Haburcak M, Forman SA, Raines DE. Pharmacological Studies of Methoxycarbonyl Etomidate’s Carboxylic Acid Metabolite. Anesth Analg. 2011 doi: 10.1213/ANE.0b013e318239c6ca. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eger EI, 2nd, Shafer SL. Tutorial: Context-sensitive decrement times for inhaled anesthetics. Anesth Analg. 2005;101:688–96. doi: 10.1213/01.ANE.0000158611.15820.3D. [DOI] [PubMed] [Google Scholar]
- 6.Hughes MA, Glass PS, Jacobs JR. Context-sensitive half-time in multicompartment pharmacokinetic models for intravenous anesthetic drugs. Anesthesiology. 1992;76:334–41. doi: 10.1097/00000542-199203000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Hoke JF, Shlugman D, Dershwitz M, Michalowski P, Malthouse-Dufore S, Connors PM, Martel D, Rosow CE, Muir KT, Rubin N, Glass PS. Pharmacokinetics and pharmacodynamics of remifentanil in persons with renal failure compared with healthy volunteers. Anesthesiology. 1997;87:533–41. doi: 10.1097/00000542-199709000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Egan TD, Minto CF, Hermann DJ, Barr J, Muir KT, Shafer SL. Remifentanil versus alfentanil: Comparative pharmacokinetics and pharmacodynamics in healthy adult male volunteers. Anesthesiology. 1996;84:821–33. doi: 10.1097/00000542-199604000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Kapila A, Glass PS, Jacobs JR, Muir KT, Hermann DJ, Shiraishi M, Howell S, Smith RL. Measured context-sensitive half-times of remifentanil and alfentanil. Anesthesiology. 1995;83:968–75. doi: 10.1097/00000542-199511000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Vijn PC, Sneyd JR. I.V. anaesthesia and EEG burst suppression in rats: Bolus injections and closed-loop infusions. Br J Anaesth. 1998;81:415–21. doi: 10.1093/bja/81.3.415. [DOI] [PubMed] [Google Scholar]
- 11.Rampil IJ, Laster MJ. No correlation between quantitative electroencephalographic measurements and movement response to noxious stimuli during isoflurane anesthesia in rats. Anesthesiology. 1992;77:920–5. doi: 10.1097/00000542-199211000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Hubbard KE, Schaiquevich P, Bai F, Fraga CH, Miller L, Panetta JC, Stewart CF. Application of a highly specific and sensitive fluorescent HPLC method for topotecan lactone in whole blood. Biomed Chromatogr. 2009;23:707–13. doi: 10.1002/bmc.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Validation of Analytical Procedures. Text and Methodology Q2(R1), International Conference of Harmonisation of Technical Requirements for Pharmaceuticals for Human Use; 1994. [Google Scholar]
- 14.Pejo E, Cotton JF, Kelly EW, Le Ge R, Cuny GD, Laha JK, Liu J, Lin XJ, Raines DE. In Vivo and In Vitro Pharmacological Studies of Methoxycarbonyl-Carboetomidate. Anesth Analg. 2011 doi: 10.1213/ANE.0b013e3182320559. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kissin I, Mason JO, 3rd, Bradley EL., Jr Pentobarbital and thiopental anesthetic interactions with midazolam. Anesthesiology. 1987;67:26–31. doi: 10.1097/00000542-198707000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Calvo R, Carlos R, Erill S. Etomidate and plasma esterase activity in man and experimental animals. Pharmacology. 1979;18:294–8. doi: 10.1159/000137268. [DOI] [PubMed] [Google Scholar]
- 17.Feldman PL, James MK, Brackeen MF, Bilotta JM, Schuster SV, Lahey AP, Lutz MW, Johnson MR, Leighton HJ. Design, synthesis, and pharmacological evaluation of ultrashort- to long-acting opioid analgetics. J Med Chem. 1991;34:2202–8. doi: 10.1021/jm00111a041. [DOI] [PubMed] [Google Scholar]
- 18.Quon CY, Mai K, Patil G, Stampfli HF. Species differences in the stereoselective hydrolysis of esmolol by blood esterases. Drug Metab Dispos. 1988;16:425–8. [PubMed] [Google Scholar]
- 19.Flaherty JF, Wong B, La Follette G, Warnock DG, Hulse JD, Gambertoglio JG. Pharmacokinetics of esmolol and ASL-8123 in renal failure. Clin Pharmacol Ther. 1989;45:321–7. doi: 10.1038/clpt.1989.35. [DOI] [PubMed] [Google Scholar]
- 20.Sneyd JR, Rigby-Jones AE. New drugs and technologies, intravenous anaesthesia is on the move (again) Br J Anaesth. 2010;105:246–54. doi: 10.1093/bja/aeq190. [DOI] [PubMed] [Google Scholar]
- 21.Sneyd JR. Recent advances in intravenous anaesthesia. Br J Anaesth. 2004;93:725–36. doi: 10.1093/bja/aeh253. [DOI] [PubMed] [Google Scholar]