Abstract
Endocytosis and endosomal trafficking play a multitude of roles in cellular function beyond regulating entry of essential nutrients. In this review, we discuss the cell biological principles of endosomal trafficking, the neuronal adaptations to endosomal organization, and the role of endosomal trafficking in neural development. In particular, we consider how cell fate decisions, polarity, migration, and axon outgrowth and guidance are influenced by five endosomal tricks: dynamic modulation of receptor levels by endocytosis and recycling, cargo-specific responses via cargo-specific endocytic regulators, cell type-specific endocytic regulation, ligand-specific endocytic regulation, and endosomal regulation of ligand processing and trafficking.
Keywords: Endocytosis, endosomes, recycling, degradation, signaling endosomes, axon outgrowth, neuronal migration, integrin, Wnt, L1, Notch, numb, clathrin adaptor, lipid raft endocytosis
Old and new tricks of endosomes to regulate neural development
Many developmental processes are dependent on endocytosis, endosomal recycling, and degradation (Shilo and Schejter, 2011), and the endocytic machinery has been shown to be important in a number of neurodevelopmental processes. Given the importance of endocytosis for essential housekeeping functions, this comes as no surprise. Further, the integration of a large number of receptor systems is critical for directing cell behavior during neural development. Clearly, which receptors are present where, when, at what levels, and for how long will determine the outcome of these various signaling events. Endocytosis at its most fundamental influences just that, the temporal and spatial distribution of membrane receptors. The details of endosomal regulation of nervous system development, including initial cell fate decisions, neuronal polarity, neuronal migration, and axon outgrowth and guidance are increasingly being uncovered (Sann et al., 2009). In this review, we begin by introducing some of the basic cell biology of endocytosis and endosomal trafficking and then discuss neuronal-specific adaptations to the endosomal system. Finally, we will emphasize the tricks of the endosome that are utilized during neural development to organize, regulate, and orchestrate the myriad of ligand-receptor based signaling systems that play parts in building the nervous system. As it is not possible to provide a fully comprehensive review of endocytic and endosomal roles in neural development, we focus on recent cases where mechanistic insights into the regulatory roles of endocytosis and endosomal trafficking have been discovered.
The cell biology of endosomes – a primer
Endocytosis via multiple distinct pathways
All cells are capable of internalizing molecules from the extracellular environment by endocytosis (reviewed in Doherty and McMahon, 2009; Kelly and Owen, 2011; Mellman, 1996; Mukherjee et al., 1997). Cells internalize molecules via several distinct endocytic entry routes, including the well-described clathrin-mediated pathway and less well understood clathrin-independent mechanisms (for excellent reviews see Conner and Schmid, 2003; Doherty and McMahon, 2009; Ewers and Helenius, 2011; Sandvig et al., 2011). Clathrin-mediated endocytosis is often referred to as “receptor-mediated endocytosis” because it is initiated by membrane receptors that bind to and recruit adaptor complexes (AP-2 in particular) and clathrin. Other endocytic pathways can be receptor-mediated, but are not necessarily clathrin-dependent. These include the less well understood “lipid raft” pathways, such as caveolar- and flotillin-mediated endocytosis. In these pathways, the internalized structure is a small, membrane-bounded vesicle and contains only small amounts of extracellular fluid. Membrane receptors, on the other hand, become clustered and enriched in the invaginating vesicle. Other internalization pathways, such as macropinocytosis and phagocytosis, involve large regions of the plasma membrane (Flannagan et al., 2011; Kerr and Teasdale, 2009). Macropinocytosis in neurons has been described in multiple contexts (Bonanomi et al., 2008; Kabayama et al., 2011; Shao et al., 2002), but the extent of phagocytosis carried out by neuronal cell types is not well established, and might be very restricted (Bowen et al., 2007; Lu et al., 2011). The presence of several independent endocytic pathways allows preferential internalization of some receptors and exclusion of others. Entry via distinct pathways can also change the trafficking fate of the receptor, and extent and lifetime of signaling. In principle, endocytosis can regulate receptor surface expression in a spatially and temporally precise fashion.
Postendocytic trafficking via multiple endosomal compartments
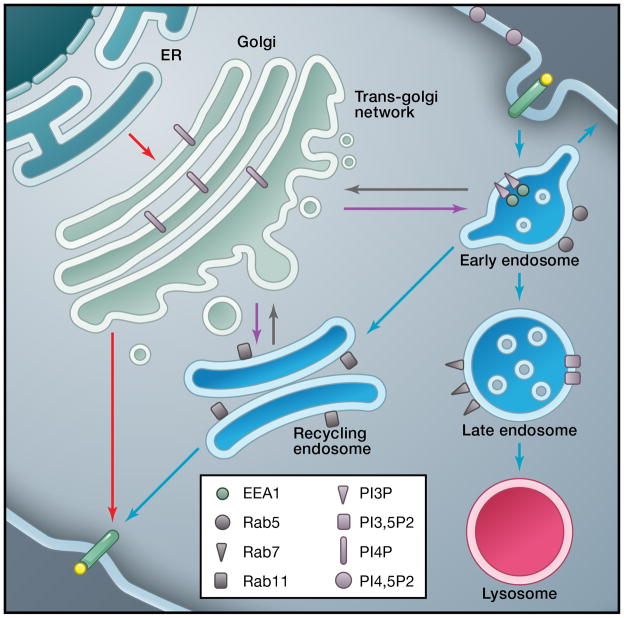
After endocytosis, cargo molecules are transported through a complex endosomal system that sorts them to be degraded, stored, or recycled back to the plasma membrane (Figure 1). Transport to the trans-Golgi-network (TGN), or even back to the Golgi and endoplasmic reticulum, can also occur under some circumstances. At its simplest, proteins can be endocytosed and removed from their current location and then transported to the lysosome for degradation. Alternatively, endocytosed proteins can be recycled back to the plasma membrane (reviewed in Huotari and Helenius, 2011). Even though the biosynthetic pathway and endocytic pathway are conceptualized as separate entities, it is clear that the two systems are interconnected (Schmidt and Haucke, 2007). There is retrograde transport from endosomes back to the TGN, and there is also transport of newly-made, biosynthetic cargo from the TGN to various endosomes before delivery to the plasma membrane (Ang et al., 2004; Folsch et al., 2009).
Figure 1. Membrane traffic in non-polarized cells.
Eukaryotic cells contain a large number of endomembrane compartments, dedicated to biosynthetic transport of molecules to the surface as well as internalization from the surface. These compartments are dynamically interconnected via vesicular carriers and/or via maturation of earlier into later compartments. Compartment identity is ensured by distinct lipid composition and regulatory proteins. The phosphoinositides enriched on each compartment as well as the most commonly used protein markers for Golgi, trans-Golgi network (TGN), early endosomes (EE), recycling endosomes (RE), late endosomes (LE), and lysosomes (lys) are indicated. Arrows denote some of the well-studied transport steps between compartments. Not shown: Rab8, Rab13, and Rab10 are protein markers for the TGN.
Several distinct types of endosomal compartments have been identified (Figure 1): Early endosomes (EE), recycling endosomes (RE), late endosomes (LE), and lysosomes (lys) (Mukherjee et al., 1997). This simple classification, however, does not do justice to the complexities of the endosome, even in non-polarized cells. The main endosomal compartments can be distinguished either by functional criteria or by co-localization with markers. Because several proteins are highly enriched in some of these compartments, proteins are frequently used as markers: rab4 and rab5 for EE, rab11 for RE, rab7 for LE (Zerial and McBride, 2001). Caution is necessary, though. Commonly used markers are usually in more than one compartment, since the compartments are continuously formed and consumed with constant flux among them (Huotari and Helenius, 2011).
Most (but not all) endocytosed cargos first enter cells in endosomal carrier vesicles that fuse with EEs. Endosomal carriers can also fuse with each other to create EEs. As cargo enters the endosome, the lumenal pH is rapidly and progressively acidified (pH of EE ~ pH 6) with the lowest pH found in lysosomes (pH < 5). Acidification plays an important functional role as it affects binding affinities for ligands in the lumen as well as the activity of lumenal enzymes (Van Dyke, 1996). From the EE, cargos can be trafficked to LE and lysosomes via multivesicular bodies (MVBs), to REs via tubular intermediates, or back to the plasma membrane directly from the EE (Jovic et al., 2009). Recycling to the plasma membrane, therefore, can occur from both the EE as well as the RE. The EE usually returns endocytosed receptors rapidly to the same place from where they were first endocytosed. Recycling from the RE is slower and returns internalized cargos to multiple locations on the surface. The regulated routing through these various endosomes endows endosomes with the capacity to finely tune the distribution of receptors and the extent of signaling (Huotari and Helenius, 2011; Figure 2).
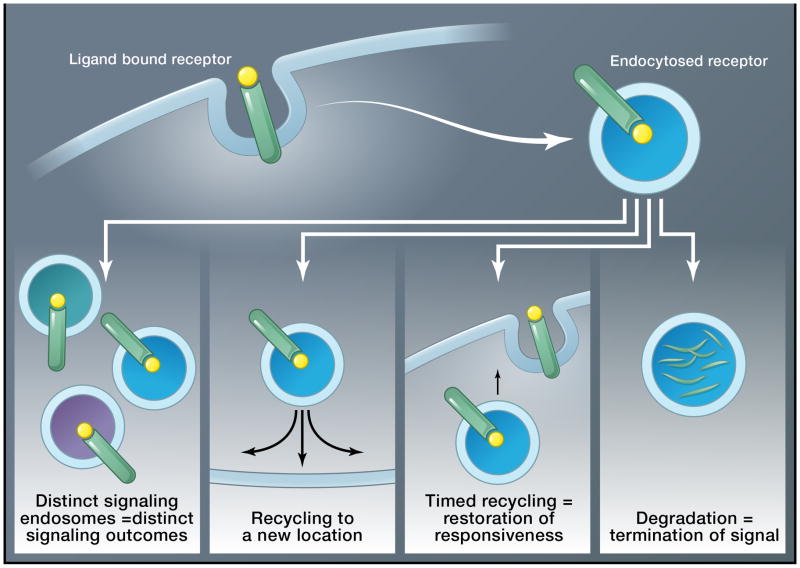
Figure 2. Tricks of the endosome.
After ligands bind to their receptors, they initiate signaling at the PM. Frequently, receptors can be endocytosed. Once endocytosed, they can either traffic to lysosomes for degradation and signal termination, or recycle in a temporally and spatially controlled fashion back to the PM. Recycling can restore responsiveness to an extracellular cue and/or deliver a ligand or signal to a different membrane domain. Endocytosed receptors can also generate various signaling endosomes where they encounter signaling components. Depending on the particular signaling endosome and the components recruited to it or present there, distinct signaling cascades can be initiated by receptors at endosomes.
The operational logic of the endosome
How do endosomes maintain compartment identity in the face of continuous flux of their components? How is directionality and specificity of transport ensured and how is polarized sorting to distinct endosomal compartments regulated? Why do late endosomes not fuse with the nucleus or another inappropriate compartment? The answer to these questions is complex, but some answers are becoming apparent. A large number of protein families are necessary to ensure correct vesicular transport of membrane cargos, such as the small GTPase families Arfs and rabs, tethering proteins such as exocyst complex, actin cytoskeleton regulators, and others. The coordinated action of these proteins ensures specificity and directionality of fission, transport and fusion. Excellent reviews of the detailed molecular mechanisms unraveled to date exist on these different classes of proteins (Brett and Traub, 2006; Brunger, 2005; Di Paolo and De Camilli, 2006; Folsch, 2005; Grant and Caplan, 2008; Huotari and Helenius, 2011; Miaczynska et al., 2004; Myers and Casanova, 2008; Prinz and Hinshaw, 2009; Schafer, 2004), and we will only touch on some of them as a way of exemplifying overarching ideas. Some of the mechanisms thought to impose specificity and selectivity upon a transport step include phosphoinositide composition, regulated membrane deformation, and the sequential assembly of regulatory platforms (Krauss and Haucke, 2011). All of these mechanisms are in effect throughout cellular membrane transport processes, not just the endosome.
Modifying lipid composition and partitioning into distinct lipid domains are common mechanisms for creating distinct compartments. The lipid composition, especially in terms of phosphoinositides, is distinct for different compartments (Di Paolo and De Camilli, 2006). The enzymes making and turning over these specific phosphoinositides are spatially segregated, and their activity is regulated by membrane regulatory proteins, such as Arfs and rabs. For instance, activated Arf6 binds the PI(4)P-5 kinase (such as the neuronally expressed PIPKIγ661) which synthesizes PI(4,5)P2. AP-2 adaptor is then recruited to the plasma membrane by binding PI(4,5)P2 (Bairstow et al., 2006; Krauss et al., 2006). Modulating lipid composition aids in regulating vesicle budding. Vesicle or tubule formation requires deformation of the lipid bilayer and curvature. For a small diameter vesicle (diameter of ~50 nm), the curvature is substantial and not all lipids can be accommodated into such a curved configuration: Lipids which cannot be accommodated into a small vesicle are therefore systematically excluded from forming vesicles. Additionally, regulation of lipid composition provides a possible mechanism for regulating cargo entry, as some proteins associate preferentially with certain types of lipids. The best studied examples of these are “lipid raft” lipids, including cholesterol and glycosphingolipids (Rajendran and Simons, 2005), which contain straight saturated fatty acid chains and prefer planar membranes over highly curved ones. The lipid raft concept has been controversial since it was introduced several decades ago (Munro, 2003). Nevertheless, consensus has emerged that lipids are not homogeneously distributed in membranes and that domains with differing lipid and protein composition coexist side-by-side in cellular membranes (Simons and Gerl, 2010; Simons and Ikonen, 1997).
Curvature of membranes into small vesicles does not occur spontaneously, and several classes of proteins have been found to bend membranes (often using banana-shaped BAR domains) and induce tubulation of liposomes in vitro. These proteins play important roles in vesicle transport. A growing number of gene families are implicated in membrane bending, among them the EHD protein family (Daumke et al., 2007), the sorting nexins, and others (Prinz and Hinshaw, 2009). Once the membrane is deformed by the action of such proteins, other proteins preferentially bind to membranes of a certain curvature and are therefore recruited to nascent vesicles or tubules. It was shown a few years ago that the enzymatic activity of some proteins, such as Arf1, is dependent on membrane curvature (Antonny, 2011; Bigay et al., 2003). Some regulators are only active on planar membranes or only on highly curved vesicle membranes. It is easily seen how linking enzymatic activity to lipid composition and curvature can restrict activity to a small specific subsets of membranes and guard against random fission and fusion events.
Subsequently, regulatory platforms consisting of multiple interacting proteins are assembled in a sequential fashion. The GTP-bound form of the Rab GTPases bind effectors that mediate the downstream action of the pathway (Horgan and McCaffrey, 2011). Interestingly, rabs often work in networks that regulate other rab proteins downstream in the transport cascade (Hutagalung and Novick, 2011). For instance, rab4 acts downstream of rab5 in endosomal transport, and activated rab5 controls the spatial and temporal properties of rab4 activation by regulating its GTPase exchange factor (Miaczynska et al., 2004). Multiple rabs in a pathway associate and dissociate from endosomes in a regulated, sequential manner, and not surprisingly, many of the regulatory families interact and regulate one another, thereby forming interconnected regulatory networks. The simultaneous action of these mechanisms contributes to compartment identity and ensures vectorial transport.
The power of live imaging: watching endosome dynamics
Owing to the advancement in imaging techniques, especially high-resolution live cell imaging, it is now clear that the endosomal system is very dynamic and more complex than previously anticipated (Kirchhausen, 2009; Lakadamyali et al., 2006; Mattheyses et al., 2011; Sonnichsen et al., 2000; Zoncu et al., 2009). One long-standing question in the field of membrane trafficking is the stability of endosomal compartments in time and space. For the biosynthetic system, a “stable compartment” model has been favored in which enduring ER and Golgi compartments are connected via mobile small vesicular carriers that deliver and remove cargos (Pfeffer, 2010; Polishchuk et al., 2003). Membrane-associated regulators required for directed fusion (such as SNAREs) are then temporarily found in the “incorrect” compartment after fusion and need to be recycled back to their compartment of origin. In the endosomal system, evidence supporting a “maturation” model has emerged in which earlier compartments in the pathway recruit successively new regulators (such as rabs and lipid-modifying enzymes), which change the compartment identity over time (Poteryaev et al., 2010; Zoncu et al., 2009).
Live cell imaging of endosomal compartments labeled with different endosomal regulators showed that pre-early endosomal compartments (APPL-positive) convert to early endosomes (EEA1-positive) (Zoncu et al., 2009), by shedding APPL and recruiting EEA1 to the same pre-existing endosome. Little is known about how the conversion of one compartment to another is achieved. For the switch from the APPL-positive preEE to the EEA1-positive EE, rab5 activity and accumulation of a different phosphoinositide species, PI-3P, are required (Zoncu et al., 2009). Rab 5 is at some point rapidly removed from the early endosome and replaced by rab7 (Rink et al., 2005) in a coordinated “rab conversion” event. Similar rab conversions also occur on other compartments as they mature. Rab7-containing endosomes will mature towards the late endosomal fate by recruiting additional machinery, such as ESCRT complex (Henne et al., 2011). How rab5 converts to a rab7-positive LE is not known, but rab activity and phosphoinositides likely play a role here as well. Most likely, neither the biosynthetic nor the endosomal system are either pure “stable compartments” or pure “maturing compartments”, but fall on a continuum with both mechanisms going on at the same time to various extents. Importantly then, cargos take charge of their own destiny and play an active role in their own trafficking by recruiting specific regulators. To use an analogy, cargos are often less like passengers on a subway train with a predetermined route, but more like taxi cab riders who direct the driver where to go.
Signaling from endosomes
For many receptor classes, signaling is not restricted to the plasma membrane. Rather, upon ligand binding, the receptor is internalized and continues to signal from endosomes (Cosker et al., 2008; Murphy et al., 2009; Platta and Stenmark, 2011; Sadowski et al., 2009). Often, the signals generated in endosomes are distinct from those generated at the plasma membrane (Figure 2), due to the endosomal localization of signaling components (for review see Hupalowska and Miaczynska, 2012; Murphy et al., 2009; Dobrowolski and De Robertis, 2011). This mechanism was first demonstrated for EGF receptor signaling in cell lines (Vieira et al., 1996), and it is now known that similar signaling on endosomes occurs for other tyrosine kinases, including Trks which play crucial roles in the nervous system (Cosker et al., 2008; Howe and Mobley, 2004; Ibanez, 2007). G-protein coupled receptors also signal from endosomes. β-arrestin-mediated endocytosis of GPCRs into endosomes recruits G-protein independent signaling components and elicits additional signaling in endosomes. Depending on the particular GPCR, β-arrestin affinity varies, thereby, changing the extent and nature of signaling (for review see Murphy et al., 2009).
The entry route of receptors during endocytosis can also influence the subsequent endosomal trafficking and the nature of endosomal signaling. TGF-β receptors, for instance, can enter cells either via clathrin-mediated endocytosis or via clathrin-independent caveolar endocytosis (Di Guglielmo et al., 2003; Le Roy and Wrana, 2005). When entering through caveolae, activated TGF-β receptors associate with Smad7 and Smurf2 and enter a degradative endosomal compartment. When entering through clathrin-mediated endocytosis, TGF-β receptors associate with Sara and Smad2 and elicit signaling in early endosomes.
Endosomes are thus essential locales for signal transduction. The term “signaling endosomes” has been coined for the retrogradely transporting endosomes containing activated neurotrophin receptors (Howe and Mobley, 2004), but arguably many different kinds of signaling endosomes can be generated by different ligand/receptor system and result in a large range of different signaling responses. Much work is still needed to understand the kinds of signaling endosomes generated downstream of different receptors and their regulation.
Endosomes in neurons
Adaptations of the neuronal endosome
Endosomes regulate a large number of processes in neurons: retrograde neurotrophic signaling, turnover and degradation of proteins, axonal pathfinding during development, synaptic vesicle recycling, synaptic plasticity, and more (Dittman and Ryan, 2009; Itofusa and Kamiguchi, 2011; Kennedy and Ehlers, 2006; Winckler and Mellman, 2010). Endosomal trafficking plays a role in neurological pathologies resulting from disturbances of membrane traffic, such as the lysosomal storage diseases Batten’s, Tay Sachs, Gaucher’s and Niemann Pick disease (reviewed in Aridor and Hannan, 2000; Aridor and Hannan, 2002).
It is clear that the endosomal system in polarized cells (both epithelial and neuronal cells) is much more diverse than that of non-polarized cells and contains unique compartments and molecular players in particular locations of the cell. For instance, we know that REs of polarized cells (such as MDCK) and non-polarized cells (such as CHO cells) differ in their sorting ability, and in their recruitment of rab proteins and adaptors (Folsch et al., 2009; Thompson et al., 2007). Neuronal endosomes, therefore, likely need to be “polarized” in order to accomplish diverse sorting and recycling tasks. Endosomes in neurons are not yet well characterized. Neuronal endosomes involved in synaptic vesicle recycling, in carrying out retrograde transport of neurotrophic signals, and at dendritic spines for recycling AMPARs (reviewed in Howe and Mobley, 2004; Kennedy and Ehlers, 2006; Schweizer and Ryan, 2006) are under active investigation by many labs, and new insights are emerging constantly. For other sites and other cargo molecules, still relatively little is known.
It is clear that striking differences exist between axonal and somatodendritic endosomes (Mundigl et al., 1993). For instance, the early endosomal regulator EEA1, a rab5 effector thought to be essential for fusion of early endosomes, is only present on somatodendritic endosomes and not in axonal endosomes (Wilson et al., 2000). The morphology of REs also differs from that in non-neuronal cells. Whereas in non-neuronal cells REs are clustered tightly near the nucleus in close proximity of the TGN, in neurons REs, labeled with transferrin or rab11, are spread throughout soma, dendrites and axons (Ascano et al., 2009; Park et al., 2006; Prekeris et al., 1999; Thompson et al., 2007). This distribution likely serves the diverse spatial demands of the neuron.
Interestingly, many membrane trafficking regulators are highly enriched in brain or even expressed in a brain-specific fashion. It is therefore likely that neurons contain a more elaborate endosomal system that makes use of common regulators and mechanisms and adapts them to specific neuronal functions by adding neuron-specific components. Delineating the components and their neuronal roles is still in the beginning stages. Besides the long-range trafficking of proteins to the correct locations using endosomal trafficking (for review see Lasiecka and Winckler, 2011), there is also constant local recycling and degradation of proteins within specific domains (such as synapses or axon growth cones). These local endosomal pathways dynamically regulate the number and availability of plasma membrane receptors and adhesion molecules (Itofusa and Kamiguchi, 2011).
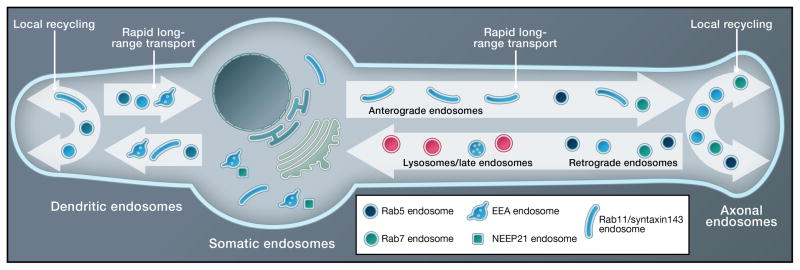
Neurons are much larger than most other cell types. The neuronal soma is roughly the size of an epithelial cell, but the vast extent of neuronal processes creates unique spatial challenges (Figure 3). Endosomes have been implicated in long-distance communication between axon terminals and the soma. Trafficking of endosomes containing endocytosed cargos along the axon occurs primarily in the retrograde direction towards the cell soma. For degradative cargos, endosomes acidify as they move proximally along the axon (Overly and Hollenbeck, 1996). Retrograde axonal transport has received particular attention since it is crucial for neurotrophic signaling and neuronal survival (reviewed in Howe and Mobley, 2004; Ibanez, 2007). These endosomes escape acidification (Lalli and Schiavo, 2002). Endosomal trafficking along the axon in the anterograde direction is less well established, but was observed for endosomes containing L1/NgCAM axonal adhesion molecules (Yap et al., 2008), Trk receptors (Ascano et al., 2009), and integrins (Eva et al., 2010), as well as endosomal regulators, such as syntaxin13 (Prekeris et al., 1999) and rab 11 (Ascano et al., 2009). Since biosynthetic cargos can enter endosomes in other cell types, endosomes containing biosynthetic cargos might also be transported anterogradely down the axon in neurons. Vesicular transport in dendrites is also bi-directional and occurs presumably for both TGN-derived as well as endosomally-derived carriers. For instance, endosomes containing the endosomal regulator EHD1 or rab11 traffic bi-directionally along dendrites (Lasiecka et al., 2010). Some of the known compartmental organization in soma, dendrites, and axons are depicted in Figure 3.
Figure 3. Polarized endosomes in neurons.
The large size and spatial heterogeneity of neurons necessitates a far-flung endosomal system that differs in many aspects from non-polarized cells. Somatic, dendritic, axonal, spine (not depicted) and synaptic (not depicted) endosomes have common and distinct regulators associated and show distinct motility profiles. In addition to local recycling, endosomes in axons and dendrites also undergo long-range anterograde and retrograde motility. Compartment maturation also occurs as endosomes travel retrogradely in axons and acidify.
Neuronal-specific functions of endosomal regulators
The importance of endosomal regulation to neuronal function a priori is expected since neurons have to solve many of the same problems as all other cell types. The ubiquitous endosomal regulators EHD1/Rme1, as well as the RE regulators rab11 and syntaxin13, were shown to be important for local AMPA receptor recycling at postsynaptic sites (Park et al., 2004) and in transcytotic trafficking of L1/NgCAM (Yap et al., 2010). Rab11 is also important for anterograde axonal trafficking of Trk in endosomes in sympathetic neurons (Ascano et al., 2009). Other rabs, such as rab5 and rab7, are important in regulating endosomal trafficking at postsynaptic sites (Brown et al., 2005), for retrograde trafficking along the axon (Deinhardt et al., 2006), and for the migration of newborn neurons in the neocortex (Kawauchi et al., 2010).
In order to solve specific neuronal demands, neurons express neuronal-specific endosomal regulators and general endosomal machinery plays modified roles in neurons. Many membrane trafficking regulators are highly enriched in the brain or expressed in a brain-specific fashion. For instance, the neuronal early endosome protein NEEP21 (originally identified as Neural Specific Gene 1, Nsg1; Saberan-Djoneidi et al., 1998; Sutcliffe et al., 1983) is expressed primarily in neurons and is found in an early endosomal population largely distinct from EEA1-positive endosomes (Steiner et al., 2002). NEEP21 interacts with the SNARE protein syntaxin13 and localizes to rab4-positive, but rab5-negative domains of early endosomes (Steiner et al., 2002). NEEP21-positive endosomes accumulate endocytosed L1/NgCAM adhesion molecules, as well as AMPA receptors (Steiner et al., 2005; Steiner et al., 2002; Yap et al., 2008) and are involved in trafficking of multiple cargos (Alberi et al., 2005; Debaigt et al., 2004; Steiner et al., 2005; Steiner et al., 2002; Yap et al., 2008). NEEP21 also binds to GRIP1, an interaction important for GluR2 trafficking (Steiner et al., 2005). Recently, NEEP21 was shown to interact with and affect proteolytic processing of βAPP (Norstrom et al., 2010). The precise mode of NEEP21 action, the role of its interaction with syntaxin13, and what neuronal-specific role it might play, are still unknown. Another neuronal-specific protein is GRASP-1, which is an effector of Rab4 and an important component of the molecular machinery that coordinates RE maturation in dendrites. GRASP-1 is also necessary for AMPAR recycling, maintenance of spine morphology, and synaptic plasticity (Hoogenraad et al., 2010). It will be important to elucidate how these neuronal-specific components modify the canonical machinery to achieve neuron-specific functions.
Some canonical endosomal regulators have specialized functions in neurons. For example, in non-neuronal cells members of the EHD family, EHD1-EHD4, regulate trafficking through early and recycling endosomes (Grant and Caplan, 2008). EHD1 associates with pre-existing tubules in fibroblasts (Sharma et al., 2009), but in neurons tubular EHD1-containing compartments are virtually absent. Rather, EHD1 co-localizes predominantly with round EEA1-positive EEs. Live imaging showed that EHD1 precedes EEA1 on EEs and often persists even after EEA1 has dissociated (Yap et al., 2010). Interestingly, in neurons EHD4 (also called pincher) and EHD1 are involved in endocytosis (Shao et al., 2002), rather than (or in addition to) recycling (Sharma et al., 2008). For instance, Nogo-A, a repulsive cue for axon growth cones, was shown to be endocytosed by an EHD4/pincher pathway (Joset et al., 2010). L1/NgCAM uses an EHD1/EHD4-dependent pathway for endocytosis. This pathway is cargo-specific and cell type-specific (Yap et al., 2010). EHD4 (possibly as a hetero-dimer with EHD1) thus mediates a specialized internalization pathway in neurons. Since EHD proteins interact with multiple trafficking regulators via their C-terminal EH domains (Naslavsky and Caplan, 2011), they regulate and coordinate recruitment and activation of other effectors classes, such as rabs (Jovic et al., 2009). The cell-type specific expression of these EH-domain binding proteins likely contributes to cell type-specific functions of the EHD family proteins.
Tricks of the endosome and how they are used to regulate neural development
We highlight here five “endosomal tricks” and discuss how they contribute to regulating neural development: dynamic modulation of receptor levels, cargo specific responses, cell type specific responses, ligand specific responses, and regulation of ligand processing and trafficking.
Modulating receptor levels dynamically by endocytosis and recycling
The most immediate role of endocytosis in neurodevelopment is to dynamically modulate levels of receptors in time and space. The location, levels, and residence time of adhesion and guidance receptors crucially influence their functional activity (Long and Lemmon, 2000) and hence axon guidance and growth. Little is known about the endosomes from which adhesion receptors, such as L1, signal. L1 endocytosis is dependent on multiple regulators and might be spatially controlled in different ways in different locales. For instance, local endocytosis of L1 at axon growth cones is mediated by numb and AP-2 (Kamiguchi et al., 1998; Nishimura et al., 2003). In dendrites, L1 also uses a highly specialized endocytic pathway, requiring EHD1 and EHD4 (Yap et al., 2010). It is possible that the endocytic pathway taken by L1 in different locales results in different signaling outcomes (Kamiguchi and Yoshihara, 2001).
Growth cone advance is in many ways analogous to cell migration, and endocytosis plays multiple crucial roles in this process. For instance, recruitment of L1 to the edge of the growth cone via directed endosomal recycling and re-insertion powers L1-mediated growth cone advance (Kamiguchi and Lemmon, 2000). When L1 binds ligand at the growth cone edge, it engages retrograde actin flow to advance the growth cone (Gil et al., 2003). When L1 reaches the central portion of the growth cone, it is endocytosed, and signals from endosomes in the growth cone through the MAP kinase (and possibly other) pathways (Kamiguchi and Lemmon, 2000; Schaefer et al., 1999). Not surprisingly then, L1-mediated outgrowth is impaired when endocytosis or MAP kinase signaling are inhibited.
Adhesive contacts mediated by adhesion molecules provide the tension to generate traction force for movement. Asymmetric distribution of adhesion receptors that contribute to traction leads to asymmetric forces in the growth cone and therefore growth cone guidance. Directional growth cone steering can be accomplished by differential exocytosis and endocytosis of adhesion receptors, such as integrins (Tojima et al., 2007; Tojima et al., 2010), and the regulation of this directional membrane trafficking of guidance receptors is downstream of second messenger systems that are activated by the ligand-receptor interaction itself (Tojima et al., 2011). The details of how local receptor ligation causes increased exo- or endocytosis are currently not fully understood. In the case of L1, phosphorylation of a tyrosine-based endocytosis signal in the cytoplasmic tail downstream of src signaling decreases binding to the clathrin adaptor AP-2 (Schaefer et al., 2002). Interestingly, L1 ligation causes dephosphorylation of the L1 endocytosis motif and triggers endocytosis (Schaefer et al., 2002), and similarly, exocytosis can be elicited downstream of L1 ligation (Alberts et al., 2003; Dequidt et al., 2007), pointing to the essential role of signaling in regulated membrane trafficking.
Detachment of traction force-generating adhesion sites at the cell’s rear, or at the non-turning side of the growth cone, is also essential for cell and growth cone motility (Broussard et al., 2008). Detachment is often accomplished by endocytic removal of adhesion receptors (for example Bechara et al., 2008; Chao and Kunz, 2009; Ezratty et al., 2009), which leads to weakening and ultimately disassembly of adhesive contacts. Endocytosis and re-insertion also play important roles in the “gain control” necessary for enabling continued migration up a concentration gradient by continuously adjusting receptor levels to maintain differential sensitivity (Piper et al., 2005). Endocytosis, signaling, and subsequent disassembly of focal adhesions therefore leads to growth cone collapse downstream of Sema3A (Tojima et al., 2011). L1 endocytosis has been shown to be important to this process. For example, L1 is required for sema3A-mediated growth cone collapse (Castellani et al., 2004), and L1 endocytosis is involved in downregulating the levels of the semaphorin3A co-receptor, neuropilin (Bechara et al., 2007). The ability of L1 to bind ERM proteins via its cytoplasmic tail is important in these semaphorin-mediated events (Mintz et al., 2008). The endocytosis of the L1-neuropilin1 complex also leads to local signaling and disassembly of focal adhesions (Bechara et al., 2008).
Recently, several papers have begun to analyze the requirement for endocytosis and endosomes in neuronal migration. The Hoshino lab demonstrated that rab proteins known to be involved in endosomal trafficking and recycling (rab5 and rab11) are important for normal migration (Kawauchi et al., 2010). It is reasonable to assume that the trafficking of many receptors is altered by downregulation of rab5 or rab11, and many receptor systems are likely affected. The authors demonstrated that N-cadherin surface levels were slightly elevated on the surface of migratory neurons expressing less rab5; β1-integrin distribution, on the other hand, was not obviously disturbed. Downregulation of N-cadherin phenocopied the migration defect of rab downregulation and partially rescued the simultaneous downregulation of rab5. Interestingly, expressing too much N-cadherin also caused migration defects. These observations support the model that precise control of surface distribution of N-cadherin and its recycling are important for normal migration (Kawauchi et al., 2010). Interestingly, rab7 was not needed for most of the migration, but only for the terminal translocation steps to position the soma in the cortical plate. This finding raises the intriguing possibility that regulated degradative trafficking might be crucial for the ultimate correct somal positioning in the cortical plate.
With ex vivo and in vivo experiments, McConnell and colleagues demonstrated that endocytosis is essential for neuronal migration (Shieh et al., 2011). They found that active endocytosis takes place preferentially in the leading process close to the soma and coincides with an enlarged cytoplasmic swelling of the leading process. Inhibition of endocytosis was shown to increase levels of integrins and lead to defects in rear detachments in migrating neurons. This is in agreement with insights gained from other migratory cells over many years (Huttenlocher and Horwitz, 2011), but was here shown beautifully for migratory neurons. Crucial roles of a number of integrins in neuronal migration in vivo have been described over the years from knock-out mouse models (Schmid et al., 2004; Stanco et al., 2009). Evidence from in vitro studies has implicated integrin endocytosis (coordinately with L1 (Thelen et al., 2002)) in migratory neurons. Future studies will shed more light on the question of whether rab5- and rab11-dependent endocytic events are specifically required for N-cadherin mediated steps, and whether integrin endocytosis is required for neuronal migratory events in a rab-dependent manner.
The current experiments in this field have focused our attention on the importance of regulating receptor levels via endocytosis in order to regulate adhesive strength and to obtain correct morphology and migratory patterns. It is likely that most of the mechanisms that have been discovered for growth cone guidance, including ligand-triggered spatially precise exo- and endocytosis, extensive regulated signaling from endosomes, and differential recycling of multiple receptors are all operative during neuronal migration as well. Given the obvious importance of the correct morphology of the leading process in migrating neurons, the frequent defects in correct migration coinciding with aberrant branching of the leading process, and the complex relationship between migration, fate, and morphology at different stages of neurogenesis and migration, it will be fascinating to uncover how membrane trafficking of particular receptors and signaling control of trafficking contributes to these complex neurodevelopmental behaviors.
Regulating cargo-specific responses via cargo-specific endocytic regulators
Not all receptors capable of endocytosis are endocytosed constitutively at all times and in all places. Rather, different receptors/cargos behave in highly specific and regulated ways. Even cargos that use the clathrin adaptor AP-2 for endocytosis are subject to further regulation via other protein interactions. A great example of this is numb, an evolutionarily conserved protein originally identified as a cell fate determinant during peripheral and CNS development in Drosophila (Uemura et al., 1989). Numb functions in maintaining progenitor cell numbers (Shen and Temple, 2002; Shen et al., 2002; Verdi et al., 1999; Zhong et al., 2000), and is also involved in promoting neurogenesis and differentiation (Klein et al., 2004; Wakamatsu et al., 1999; Zilian et al., 2001).
Numb is a membrane-associated adaptor protein containing multiple protein-binding motifs, and numb associates with a number of other proteins. Via its specific binding to AP-2 and other endocytic proteins (such as Eps15 and EHD4), numb plays roles in regulated endocytosis of receptors, including those of the Notch, integrin, L1, and Trk families. Many of the biological effects of numb on neurodevelopment are therefore likely related to regulating endocytosis of specific receptors (Santolini et al., 2000).
Interaction of numb with integrin promotes endocytosis and directional cell migration (Nishimura and Kaibuchi, 2007). Par3-dependent phosphorylation of numb by aPKC regulates polarized localization of numb and its association with clathrin coated structures (Smith et al., 2007). Negative regulation of numb by aPKC appears to play an important role in integrin endocytosis and integrin-based cell migration (Nishimura and Kaibuchi, 2007).
Numb has also been reported to play a critical role in cerebellar granule cell polarization during migration via a distinct mechanism (Zhou et al., 2011). Numb interacts with activated TrkB and promotes TrkB endocytosis and polarization. BDNF-induced phosphorylation of numb by aPKC increases binding to TrkB and promotes chemotactic responses to BDNF. Thus, in the context of TrkB endocytosis, phosphorylation by aPKC increases affinity of numb for its cargo.
In addition to its role in mediating receptor internalization, numb has a novel function in endosomal recycling of receptors. For example, numb has been implicated in regulating the post-endocytic trafficking of Notch1. In mammalian cell lines, overexpression of numb promotes trafficking and degradation of Notch 1, whereas depletion of numb facilitates recycling of Notch1. Numb mutants defective in binding to endocytic proteins such as α-adaptin, fail to promote Notch 1 degradation, suggesting numb suppresses Notch activity by regulating post-endocytic sorting pathways that lead to Notch degradation (McGill et al., 2009; McGill and McGlade, 2003).
Regulating cell type-specific responses via endocytic specificity
Neurons frequently show cell type-specific responses to the same environment. One mechanism of cell type-specificity involves differential expression of adhesion and guidance receptors (Kolodkin and Tessier-Lavigne, 2010; Stein and Tessier-Lavigne, 2001). Interestingly, neurons can have different responses even when expressing the same receptors. Subsets of cortical neurons, identified by differential expression of the transcription factors Satb2 or Ctip2 respond differentially to Sema3A cues even though both populations similarly express the Neuropilin1, L1, and plexinA4 receptors (Carcea et al., 2010). Differential capacity for Sema3A endocytosis seems to be the mechanism underlying these distinct responses and there is evidence that distinct modes of internalization are involved. In particular, downregulation of the lipid raft organizing protein Flotillin (also known as Reggie (Otto and Nichols, 2011)) renders neurons insensitive to Sema3A-mediated growth cone collapse (Carcea et al., 2010). There are multiple examples of the need for lipid raft partitioning for directional responsiveness to guidance cues (Guirland and Zheng, 2007), and endocytosis could be one of the cellular responses that differ for receptors found in rafts or not in rafts. These results suggest the possibility that neurons can use an “internalization switch” such that intrinsic differences in signaling responses downstream of common guidance cues regulates extent of endocytosis and thus responsiveness to guidance cues (Carcea et al., 2010). One can also envision how the same cell could throw the internalization switch differently at different developmental junctures, or how different parts of the same neuron could respond differently to the same cues (for example Polleux et al., 2002; Shelly et al., 2011) using an internalization switch.
Regulating ligand-specific responses via endocytic specificity
Many receptors that have more than one ligand show ligand-specific responses upon activation. What could be a cellular mechanism explaining this observation? A recent study of differential signaling outcomes resulting from NGF and NT-3 binding to the TrkA receptor provides a beautiful example (Harrington et al., 2011). NGF and NT-3 both bind and activate TrkA receptor to promote axonal extension (Kuruvilla et al., 2004), and activate multiple known downstream effectors of TrkA activation. NT-3 is secreted by intermediate targets of sympathetic neurons and mediates signaling important for local axon extension, while NGF is produced in final target fields of sympathetic neurons and supports neuronal survival via retrograde signaling. Only NGF-induced internalized NGF/TrkA endosomes are capable of eliciting retrograde survival signaling. Harrington et al. discovered that NGF/TrkA endosomes, but not NT-3/TrkA endosomes, recruit and activate rac1 and cofilin, a microfilament-depolymerizing factor (Harrington et al., 2011). Activation of rac1 on early endosomes and activation of cofilin are necessary and sufficient for maturation of TrkA-containing early endosomes to retrogradely-transporting signaling endosomes. The authors also showed that NT-3 binds inefficiently to Trk under acidic environment, such as that in the early endosome, and by a mechanism that remains to be defined, dissociation of NT-3 from TrkA in the endosome prevents recruitment of rac1, even though activation of other signaling cascades is sustained. These data suggest that differential sensitivity to endosomal acidification underlies the differences in the capability of NGF/Trk and NT-3/Trk endosomes to elicit retrograde survival signaling and beautifully highlight the regulatory power of post-endocytic events in signaling endosomes.
Endosomal regulation of ligand processing and trafficking – interplay between biosynthetic and endosomal pathways
Many ligands active in neural development are secreted and require processing for activation, such as cleavage of pro-domains, cleavage of extracellular domains, and other posttranslational modifications. Some of the processing events take place in endo-compartments. The trafficking and endosomal compartmentalization of required processing components for many potent ligands (such as EGF ligands, TGFβ-ligands, Wnt, Notch and others) fine-tunes when and where active ligand reaches the surface. Endosomal regulation of ligand processing and trafficking is certain to impact many neurodevelopmental processes (for review see Shilo and Schejter, 2011).
Shilo and co-workers discovered a striking mechanism by which the generation of active ligand is tightly controlled by subcompartmentalization of a processing component (Yogev et al., 2008). The EGF ligand Spitz (Spi) controls multiple developmental pathways in Drosophila, including fate decisions in the developing eye. Spi is synthesized in a pro-form in the ER and requires proteolytic processing by the protease rhomboid for activity. A complex, regulated interplay between Spi, its ER chaperone Star, and rhomboid allows for precise regulation of generation and secretion of active Spi. Star ensures traffic of pro-Spi to a rab4/rab14-positive endosomal compartment, where it encounters rhomboid, is cleaved, and then secreted as an active ligand. Subsequent cleavage of Star by rhomboid presumably bestows directionality to Spi transport.
Wnt signaling is also regulated by multiple factors and trafficking is emerging as an important node for both ligand transport to the surface (Coudreuse and Korswagen, 2007) and signaling. Wnt signaling is dependent on retromer (Coudreuse et al., 2006; Prasad and Clark, 2006), a complex of proteins needed for retrograde transport from endosomes to the TGN. Why would Wnt signaling depend on retromer function? It was shown in multiple beautiful studies that Wnt requires the membrane receptor Wingless (Wls) for Golgi exit. Retromer function is then required to return Wls from the cell surface via endosomes to the Golgi where it can mediate another round of Wnt trafficking (Belenkaya et al., 2008; Franch-Marro et al., 2008; Pan et al., 2008; Port et al., 2008; Yang et al., 2008). These examples highlight the intimate interplay between biosynthetic and endosomal trafficking.
Conclusion
Neural development and neuronal function in the adult nervous system are regulated by large numbers of membrane receptors that signal upon ligand binding. The biology of the receptors, the ligands, and the signaling cascades is complex and only incompletely understood. In this review, we focused on the roles of endocytosis and subsequent endosomal trafficking in regulating this biology. The first and most studied role of endocytosis is to regulate the distribution in time and space of various receptors on the cell surface. The surface distribution contributes to setting responsiveness to extracellular cues and therefore influences the strength of signaling. Secondly, post-endocytic sorting decisions along either recycling or degradative routes have profound impact on receptors distribution and half-life of signaling. Thirdly, signaling per se takes place in endosomes, and the nature of the signaling, in the addition to the duration of the signal, depends on the identity of the endosome. It is not only receptor identity that is responsible for eliciting different signaling outcomes, regulated endocytosis and differences in post-endocytic sorting also play a role. Endosomal mechanisms therefore contribute to ligand specificity via the same receptor, cell type specificity via the same ligand-receptor system, and developmental switches in responsiveness, among others. We focused our discussion on neurons, but without a doubt other cell type in the nervous system also have an endosomal trick or two up their sleeves to accomplish the important roles they play in development and nervous system function.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alberi S, Boda B, Steiner P, Nikonenko I, Hirling H, Muller D. The endosomal protein NEEP21 regulates AMPA receptor-mediated synaptic transmission and plasticity in the hippocampus. Mol Cell Neurosci. 2005;29:313–319. doi: 10.1016/j.mcn.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Alberts P, Rudge R, Hinners I, Muzerelle A, Martinez-Arca S, Irinopoulou T, Marthiens V, Tooze S, Rathjen F, Gaspar P, et al. Cross talk between tetanus neurotoxin-insensitive vesicle-associated membrane protein-mediated transport and L1-mediated adhesion. Mol Biol Cell. 2003;14:4207–4220. doi: 10.1091/mbc.E03-03-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang AL, Taguchi T, Francis S, Folsch H, Murrells LJ, Pypaert M, Warren G, Mellman I. Recycling endosomes can serve as intermediates during transport from the Golgi to the plasma membrane of MDCK cells. Journal of Cell Biology. 2004;167:531–543. doi: 10.1083/jcb.200408165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonny B. Mechanisms of membrane curvature sensing. Annu Rev Biochem. 2011;80:101–123. doi: 10.1146/annurev-biochem-052809-155121. [DOI] [PubMed] [Google Scholar]
- Aridor M, Hannan LA. Traffic Jam: A Compendium of Human Diseases that Affect Intracellular Transport Processes. Traffic. 2000;1:836–851. doi: 10.1034/j.1600-0854.2000.011104.x. [DOI] [PubMed] [Google Scholar]
- Aridor M, Hannan LA. Traffic jams II: an update of diseases of intracellular transport. Traffic. 2002;3:781–790. doi: 10.1034/j.1600-0854.2002.31103.x. [DOI] [PubMed] [Google Scholar]
- Ascano M, Richmond A, Borden P, Kuruvilla R. Axonal targeting of Trk receptors via transcytosis regulates sensitivity to neurotrophin responses. J Neurosci. 2009;29:11674–11685. doi: 10.1523/JNEUROSCI.1542-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bairstow SF, Ling K, Su X, Firestone AJ, Carbonara C, Anderson RA. Type Igamma661 phosphatidylinositol phosphate kinase directly interacts with AP2 and regulates endocytosis. J Biol Chem. 2006;281:20632–20642. doi: 10.1074/jbc.M601465200. [DOI] [PubMed] [Google Scholar]
- Bechara A, Falk J, Moret F, Castellani V. Modulation of semaphorin signaling by Ig superfamily cell adhesion molecules. Adv Exp Med Biol. 2007;600:61–72. doi: 10.1007/978-0-387-70956-7_6. [DOI] [PubMed] [Google Scholar]
- Bechara A, Nawabi H, Moret F, Yaron A, Weaver E, Bozon M, Abouzid K, Guan JL, Tessier-Lavigne M, Lemmon V, et al. FAK-MAPK-dependent adhesion disassembly downstream of L1 contributes to semaphorin3A-induced collapse. Embo J. 2008;27:1549–1562. doi: 10.1038/emboj.2008.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belenkaya TY, Wu Y, Tang X, Zhou B, Cheng L, Sharma YV, Yan D, Selva EM, Lin X. The retromer complex influences Wnt secretion by recycling wntless from endosomes to the trans-Golgi network. Dev Cell. 2008;14:120–131. doi: 10.1016/j.devcel.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Bigay J, Gounon P, Robineau S, Antonny B. Lipid packing sensed by ArfGAP1 couples COPI coat disassembly to membrane bilayer curvature. Nature. 2003;426:563–566. doi: 10.1038/nature02108. [DOI] [PubMed] [Google Scholar]
- Bonanomi D, Fornasiero EF, Valdez G, Halegoua S, Benfenati F, Menegon A, Valtorta F. Identification of a developmentally regulated pathway of membrane retrieval in neuronal growth cones. J Cell Sci. 2008;121:3757–3769. doi: 10.1242/jcs.033803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen S, Ateh DD, Deinhardt K, Bird MM, Price KM, Baker CS, Robson JC, Swash M, Shamsuddin W, Kawar S, et al. The phagocytic capacity of neurones. Eur J Neurosci. 2007;25:2947–2955. doi: 10.1111/j.1460-9568.2007.05554.x. [DOI] [PubMed] [Google Scholar]
- Brett TJ, Traub LM. Molecular structures of coat and coat-associated proteins: function follows form. Curr Opin Cell Biol. 2006;18:395–406. doi: 10.1016/j.ceb.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Broussard JA, Webb DJ, Kaverina I. Asymmetric focal adhesion disassembly in motile cells. Curr Opin Cell Biol. 2008;20:85–90. doi: 10.1016/j.ceb.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Brown TC, Tran IC, Backos DS, Esteban JA. NMDA receptor-dependent activation of the small GTPase Rab5 drives the removal of synaptic AMPA receptors during hippocampal LTD. Neuron. 2005;45:81–94. doi: 10.1016/j.neuron.2004.12.023. [DOI] [PubMed] [Google Scholar]
- Brunger AT. Structure and function of SNARE and SNARE-interacting proteins. Q Rev Biophys. 2005;38:1–47. doi: 10.1017/S0033583505004051. [DOI] [PubMed] [Google Scholar]
- Carcea I, Ma’ayan A, Mesias R, Sepulveda B, Salton SR, Benson DL. Flotillin-mediated endocytic events dictate cell type-specific responses to semaphorin 3A. J Neurosci. 2010;30:15317–15329. doi: 10.1523/JNEUROSCI.1821-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani V, Falk J, Rougon G. Semaphorin3A-induced receptor endocytosis during axon guidance responses is mediated by L1 CAM. Mol Cell Neurosci. 2004;26:89–100. doi: 10.1016/j.mcn.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Chao WT, Kunz J. Focal adhesion disassembly requires clathrin-dependent endocytosis of integrins. FEBS Lett. 2009;583:1337–1343. doi: 10.1016/j.febslet.2009.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- Cosker KE, Courchesne SL, Segal RA. Action in the axon: generation and transport of signaling endosomes. Curr Opin Neurobiol. 2008 doi: 10.1016/j.conb.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coudreuse D, Korswagen HC. The making of Wnt: new insights into Wnt maturation, sorting and secretion. Development. 2007;134:3–12. doi: 10.1242/dev.02699. [DOI] [PubMed] [Google Scholar]
- Coudreuse DY, Roel G, Betist MC, Destree O, Korswagen HC. Wnt gradient formation requires retromer function in Wnt-producing cells. Science. 2006;312:921–924. doi: 10.1126/science.1124856. [DOI] [PubMed] [Google Scholar]
- Daumke O, Lundmark R, Vallis Y, Martens S, Butler PJ, McMahon HT. Architectural and mechanistic insights into an EHD ATPase involved in membrane remodelling. Nature. 2007;449:923–927. doi: 10.1038/nature06173. [DOI] [PubMed] [Google Scholar]
- Debaigt C, Hirling H, Steiner P, Vincent JP, Mazella J. Crucial role of neuron-enriched endosomal protein of 21 kDa in sorting between degradation and recycling of internalized G-protein-coupled receptors. J Biol Chem. 2004;279:35687–35691. doi: 10.1074/jbc.M402751200. [DOI] [PubMed] [Google Scholar]
- Deinhardt K, Reversi A, Berninghausen O, Hopkins CR, Schiavo G. Neurotrophins Redirect p75NTR from a clathrin-independent to a clathrin-dependent endocytic pathway coupled to axonal transport. Traffic. 2007;8:1736–1749. doi: 10.1111/j.1600-0854.2007.00645.x. [DOI] [PubMed] [Google Scholar]
- Deinhardt K, Salinas S, Verastegui C, Watson R, Worth D, Hanrahan S, Bucci C, Schiavo G. Rab5 and Rab7 control endocytic sorting along the axonal retrograde transport pathway. Neuron. 2006;52:293–305. doi: 10.1016/j.neuron.2006.08.018. [DOI] [PubMed] [Google Scholar]
- Dequidt C, Danglot L, Alberts P, Galli T, Choquet D, Thoumine O. Fast turnover of L1 adhesions in neuronal growth cones involving both surface diffusion and exo/endocytosis of L1 molecules. Mol Biol Cell. 2007;18:3131–3143. doi: 10.1091/mbc.E06-12-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Guglielmo GM, Le Roy C, Goodfellow AF, Wrana JL. Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat Cell Biol. 2003;5:410–421. doi: 10.1038/ncb975. [DOI] [PubMed] [Google Scholar]
- Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- Dittman J, Ryan TA. Molecular circuitry of endocytosis at nerve terminals. Annu Rev Cell Dev Biol. 2009;25:133–160. doi: 10.1146/annurev.cellbio.042308.113302. [DOI] [PubMed] [Google Scholar]
- Dobrowolski R, De Robertis EM. Endocytic control of growth factor signalling: multivesicular bodies as signalling organelles. Nat Rev Mol Cell Biol. 2011;13:53–60. doi: 10.1038/nrm3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- Eva R, Dassie E, Caswell PT, Dick G, ffrench-Constant C, Norman JC, Fawcett JW. Rab11 and its effector Rab coupling protein contribute to the trafficking of beta 1 integrins during axon growth in adult dorsal root ganglion neurons and PC12 cells. J Neurosci. 2010;30:11654–11669. doi: 10.1523/JNEUROSCI.2425-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewers H, Helenius A. Lipid-mediated endocytosis. Cold Spring Harb Perspect Biol. 2011;3:a004721. doi: 10.1101/cshperspect.a004721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezratty EJ, Bertaux C, Marcantonio EE, Gundersen GG. Clathrin mediates integrin endocytosis for focal adhesion disassembly in migrating cells. J Cell Biol. 2009;187:733–747. doi: 10.1083/jcb.200904054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannagan RS, Jaumouille V, Grinstein S. The Cell Biology of Phagocytosis. Annu Rev Pathol. 2011 doi: 10.1146/annurev-pathol-011811-132445. [DOI] [PubMed] [Google Scholar]
- Folsch H. The building blocks for basolateral vesicles in polarized epithelial cells. Trends Cell Biol. 2005;15:222–228. doi: 10.1016/j.tcb.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Folsch H, Mattila PE, Weisz OA. Taking the Scenic Route: Biosynthetic Traffic to the Plasma Membrane in Polarized Epithelial Cells. Traffic. 2009;10:972–981. doi: 10.1111/j.1600-0854.2009.00927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franch-Marro X, Wendler F, Guidato S, Griffith J, Baena-Lopez A, Itasaki N, Maurice MM, Vincent JP. Wingless secretion requires endosome-to-Golgi retrieval of Wntless/Evi/Sprinter by the retromer complex. Nat Cell Biol. 2008;10:170–177. doi: 10.1038/ncb1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil OD, Sakurai T, Bradley AE, Fink MY, Cassella MR, Kuo JA, Felsenfeld DP. Ankyrin binding mediates L1CAM interactions with static components of the cytoskeleton and inhibits retrograde movement of L1CAM on the cell surface. J Cell Biol. 2003;162:719–730. doi: 10.1083/jcb.200211011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BD, Caplan S. Mechanisms of EHD/RME-1 Protein Function in Endocytic Transport. Traffic. 2008;9:2043–2052. doi: 10.1111/j.1600-0854.2008.00834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guirland C, Zheng JQ. Membrane lipid rafts and their role in axon guidance. Adv Exp Med Biol. 2007;621:144–155. doi: 10.1007/978-0-387-76715-4_11. [DOI] [PubMed] [Google Scholar]
- Harrington AW, St Hillaire C, Zweifel LS, Glebova NO, Philippidou P, Halegoua S, Ginty DD. Recruitment of actin modifiers to TrkA endosomes governs retrograde NGF signaling and survival. Cell. 2011;146:421–434. doi: 10.1016/j.cell.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henne WM, Buchkovich NJ, Emr SD. The ESCRT pathway. Dev Cell. 2011;21:77–91. doi: 10.1016/j.devcel.2011.05.015. [DOI] [PubMed] [Google Scholar]
- Hoogenraad CC, Popa I, Futai K, Martinez-Sanchez E, Wulf PS, van Vlijmen T, Dortland BR, Oorschot V, Govers R, Monti M, et al. Neuron specific Rab4 effector GRASP-1 coordinates membrane specialization and maturation of recycling endosomes. PLoS Biol. 2010;8:e1000283. doi: 10.1371/journal.pbio.1000283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horgan CP, McCaffrey MW. Rab GTPases and microtubule motors. Biochem Soc Trans. 2011;39:1202–1206. doi: 10.1042/BST0391202. [DOI] [PubMed] [Google Scholar]
- Howe CL, Mobley WC. Signaling endosome hypothesis: A cellular mechanism for long distance communication. J Neurobiol. 2004;58:207–216. doi: 10.1002/neu.10323. [DOI] [PubMed] [Google Scholar]
- Huotari J, Helenius A. Endosome maturation. Embo J. 2011;30:3481–3500. doi: 10.1038/emboj.2011.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hupalowska A, Miaczynska M. The new faces of endocytosis in signaling. Traffic. 2012;13:9–18. doi: 10.1111/j.1600-0854.2011.01249.x. [DOI] [PubMed] [Google Scholar]
- Hutagalung AH, Novick PJ. Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev. 2011;91:119–149. doi: 10.1152/physrev.00059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher A, Horwitz AR. Integrins in cell migration. Cold Spring Harb Perspect Biol. 2011;3:a005074. doi: 10.1101/cshperspect.a005074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez CF. Message in a bottle: long-range retrograde signaling in the nervous system. Trends Cell Biol. 2007;17:519–528. doi: 10.1016/j.tcb.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Itofusa R, Kamiguchi H. Polarizing membrane dynamics and adhesion for growth cone navigation. Mol Cell Neurosci. 2011;48:332–338. doi: 10.1016/j.mcn.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Joset A, Dodd DA, Halegoua S, Schwab ME. Pincher-generated Nogo-A endosomes mediate growth cone collapse and retrograde signaling. J Cell Biol. 2010;188:271–285. doi: 10.1083/jcb.200906089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovic M, Sharma M, Rahajeng J, Caplan S. The early endosome: a busy sorting station for proteins at the crossroads. Histol Histopathol. 2009;25:99–112. doi: 10.14670/hh-25.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabayama H, Takeuchi M, Taniguchi M, Tokushige N, Kozaki S, Mizutani A, Nakamura T, Mikoshiba K. Syntaxin 1B suppresses macropinocytosis and semaphorin 3A-induced growth cone collapse. J Neurosci. 2011;31:7357–7364. doi: 10.1523/JNEUROSCI.2718-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiguchi H, Lemmon V. Recycling of the cell adhesion molecule L1 in axonal growth cones. J Neurosci. 2000;20:3676–3686. doi: 10.1523/JNEUROSCI.20-10-03676.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiguchi H, Long KE, Pendergast M, Schaefer AW, Rapoport I, Kirchhausen T, Lemmon V. The neural cell adhesion molecule L1 interacts with the AP-2 adaptor and is endocytosed via the clathrin-mediated pathway. J Neurosci. 1998;18:5311–5321. doi: 10.1523/JNEUROSCI.18-14-05311.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiguchi H, Yoshihara F. The role of endocytic l1 trafficking in polarized adhesion and migration of nerve growth cones. J Neurosci. 2001;21:9194–9203. doi: 10.1523/JNEUROSCI.21-23-09194.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawauchi T, Sekine K, Shikanai M, Chihama K, Tomita K, Kubo K, Nakajima K, Nabeshima Y, Hoshino M. Rab GTPases-dependent endocytic pathways regulate neuronal migration and maturation through N-cadherin trafficking. Neuron. 2010;67:588–602. doi: 10.1016/j.neuron.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Kelly BT, Owen DJ. Endocytic sorting of transmembrane protein cargo. Curr Opin Cell Biol. 2011;23:404–412. doi: 10.1016/j.ceb.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Kennedy MJ, Ehlers MD. Organelles and trafficking machinery for postsynaptic plasticity. Annu Rev Neurosci. 2006;29:325–362. doi: 10.1146/annurev.neuro.29.051605.112808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr MC, Teasdale RD. Defining macropinocytosis. Traffic. 2009;10:364–371. doi: 10.1111/j.1600-0854.2009.00878.x. [DOI] [PubMed] [Google Scholar]
- Kirchhausen T. Imaging endocytic clathrin structures in living cells. Trends Cell Biol. 2009;19:596–605. doi: 10.1016/j.tcb.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein AL, Zilian O, Suter U, Taylor V. Murine numb regulates granule cell maturation in the cerebellum. Dev Biol. 2004;266:161–177. doi: 10.1016/j.ydbio.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Kolodkin AL, Tessier-Lavigne M. Mechanisms and molecules of neuronal wiring: a primer. Cold Spring Harb Perspect Biol. 2010:3. doi: 10.1101/cshperspect.a001727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss M, Haucke V. Shaping membranes for endocytosis. Rev Physiol Biochem Pharmacol. 2011;161:45–66. doi: 10.1007/112_2008_2. [DOI] [PubMed] [Google Scholar]
- Krauss M, Kukhtina V, Pechstein A, Haucke V. Stimulation of phosphatidylinositol kinase type I-mediated phosphatidylinositol (4,5)-bisphosphate synthesis by AP-2mu-cargo complexes. Proc Natl Acad Sci U S A. 2006;103:11934–11939. doi: 10.1073/pnas.0510306103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuruvilla R, Zweifel LS, Glebova NO, Lonze BE, Valdez G, Ye H, Ginty DD. A neurotrophin signaling cascade coordinates sympathetic neuron development through differential control of TrkA trafficking and retrograde signaling. Cell. 2004;118:243–255. doi: 10.1016/j.cell.2004.06.021. [DOI] [PubMed] [Google Scholar]
- Lakadamyali M, Rust MJ, Zhuang X. Ligands for clathrin-mediated endocytosis are differentially sorted into distinct populations of early endosomes. Cell. 2006;124:997–1009. doi: 10.1016/j.cell.2005.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalli G, Schiavo G. Analysis of retrograde transport in motor neurons reveals common endocytic carriers for tetanus toxin and neurotrophin receptor p75NTR. J Cell Biol. 2002;156:233–239. doi: 10.1083/jcb.200106142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasiecka ZM, Winckler B. Mechanisms of polarized membrane trafficking in neurons -- focusing in on endosomes. Mol Cell Neurosci. 2011;48:278–287. doi: 10.1016/j.mcn.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasiecka ZM, Yap CC, Caplan S, Winckler B. Neuronal early endosomes require EHD1 for L1/NgCAM trafficking. J Neurosci. 2010;30:16485–16497. doi: 10.1523/JNEUROSCI.3127-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Roy C, Wrana JL. Clathrin- and non-clathrin-mediated endocytic regulation of cell signalling. Nat Rev Mol Cell Biol. 2005;6:112–126. doi: 10.1038/nrm1571. [DOI] [PubMed] [Google Scholar]
- Long KE, Lemmon V. Dynamic regulation of cell adhesion molecules during axon outgrowth. J Neurobiol. 2000;44:230–245. doi: 10.1002/1097-4695(200008)44:2<230::aid-neu12>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Lu Z, Elliott MR, Chen Y, Walsh JT, Klibanov AL, Ravichandran KS, Kipnis J. Phagocytic activity of neuronal progenitors regulates adult neurogenesis. Nat Cell Biol. 2011;13:1076–1083. doi: 10.1038/ncb2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maday S, Wallace KE, Holzbaur EL. Autophagosomes initiate distally and mature during transport toward the cell soma in primary neurons. J Cell Biol. 2012;196:407–417. doi: 10.1083/jcb.201106120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallard F, Tang BL, Galli T, Tenza D, Saint-Pol A, Yue X, Antony C, Hong W, Goud B, Johannes L. Early/recycling endosomes-to-TGN transport involves two SNARE complexes and a Rab6 isoform. J Cell Biol. 2002;156:653–664. doi: 10.1083/jcb.200110081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maness PF, Schachner M. Neural recognition molecules of the immunoglobulin superfamily: signaling transducers of axon guidance and neuronal migration. Nat Neurosci. 2007;10:19–26. doi: 10.1038/nn1827. [DOI] [PubMed] [Google Scholar]
- Mattheyses AL, Atkinson CE, Simon SM. Imaging single endocytic events reveals diversity in clathrin, dynamin and vesicle dynamics. Traffic. 2011;12:1394–1406. doi: 10.1111/j.1600-0854.2011.01235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MA, Dho SE, Weinmaster G, McGlade CJ. Numb regulates post-endocytic trafficking and degradation of Notch1. J Biol Chem. 2009;284:26427–26438. doi: 10.1074/jbc.M109.014845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MA, McGlade CJ. Mammalian numb proteins promote Notch1 receptor ubiquitination and degradation of the Notch1 intracellular domain. J Biol Chem. 2003;278:23196–23203. doi: 10.1074/jbc.M302827200. [DOI] [PubMed] [Google Scholar]
- Mellman I. Endocytosis and molecular sorting. Annu Rev Cell Dev Biol. 1996;12:575–625. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- Miaczynska M, Pelkmans L, Zerial M. Not just a sink: endosomes in control of signal transduction. Curr Opin Cell Biol. 2004;16:400–406. doi: 10.1016/j.ceb.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Mintz CD, Carcea I, McNickle DG, Dickson TC, Ge Y, Salton SR, Benson DL. ERM proteins regulate growth cone responses to Sema3A. J Comp Neurol. 2008;510:351–366. doi: 10.1002/cne.21799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Ghosh RN, Maxfield FR. Endocytosis. Physiol Rev. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- Mundigl O, Matteoli M, Daniell L, Thomas-Reetz A, Metcalf A, Jahn R, DeCamilli P. Synaptic Vesicle Proteins and Early Endosomes in Cultured Hippocampal Neurons: Differential Effects of Brefeldin A in Axon and Dendrites. Journal of Cell Biology. 1993;122:1207–1221. doi: 10.1083/jcb.122.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S. Lipid rafts: elusive or illusive? Cell. 2003;115:377–388. doi: 10.1016/s0092-8674(03)00882-1. [DOI] [PubMed] [Google Scholar]
- Murphy JE, Padilla BE, Hasdemir B, Cottrell GS, Bunnett NW. Endosomes: a legitimate platform for the signaling train. Proc Natl Acad Sci U S A. 2009;106:17615–17622. doi: 10.1073/pnas.0906541106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KR, Casanova JE. Regulation of actin cytoskeleton dynamics by Arf-family GTPases. Trends Cell Biol. 2008;18:184–192. doi: 10.1016/j.tcb.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naslavsky N, Caplan S. EHD proteins: key conductors of endocytic transport. Trends Cell Biol. 2011;21:122–131. doi: 10.1016/j.tcb.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T, Fukata Y, Kato K, Yamaguchi T, Matsuura Y, Kamiguchi H, Kaibuchi K. CRMP-2 regulates polarized Numb-mediated endocytosis for axon growth. Nat Cell Biol. 2003;5:819–826. doi: 10.1038/ncb1039. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Kaibuchi K. Numb controls integrin endocytosis for directional cell migration with aPKC and PAR-3. Dev Cell. 2007;13:15–28. doi: 10.1016/j.devcel.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Norstrom EM, Zhang C, Tanzi R, Sisodia SS. Identification of NEEP21 as a ss-amyloid precursor protein-interacting protein in vivo that modulates amyloidogenic processing in vitro. J Neurosci. 2010;30:15677–15685. doi: 10.1523/JNEUROSCI.4464-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto GP, Nichols BJ. The roles of flotillin microdomains - endocytosis and beyond. J Cell Sci. 2011;124:3933–3940. doi: 10.1242/jcs.092015. [DOI] [PubMed] [Google Scholar]
- Overly CC, Hollenbeck PJ. Dynamic organization of endocytic pathways in axons of cultured sympathetic neurons. J Neurosci. 1996;16:6056–6064. doi: 10.1523/JNEUROSCI.16-19-06056.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan CL, Baum PD, Gu M, Jorgensen EM, Clark SG, Garriga G. C. elegans AP-2 and retromer control Wnt signaling by regulating mig-14/Wntless. Dev Cell. 2008;14:132–139. doi: 10.1016/j.devcel.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M, Penick EC, Edwards JG, Kauer JA, Ehlers MD. Recycling endosomes supply AMPA receptors for LTP. Science. 2004;305:1972–1975. doi: 10.1126/science.1102026. [DOI] [PubMed] [Google Scholar]
- Park M, Salgado JM, Ostroff L, Helton TD, Robinson CG, Harris KM, Ehlers MD. Plasticity-induced growth of dendritic spines by exocytic trafficking from recycling endosomes. Neuron. 2006;52:817–830. doi: 10.1016/j.neuron.2006.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer SR. How the Golgi works: a cisternal progenitor model. Proc Natl Acad Sci U S A. 2010;107:19614–19618. doi: 10.1073/pnas.1011016107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper M, Salih S, Weinl C, Holt CE, Harris WA. Endocytosis-dependent desensitization and protein synthesis-dependent resensitization in retinal growth cone adaptation. Nat Neurosci. 2005;8:179–186. doi: 10.1038/nn1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platta HW, Stenmark H. Endocytosis and signaling. Curr Opin Cell Biol. 2011;23:393–403. doi: 10.1016/j.ceb.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Polishchuk EV, Di Pentima A, Luini A, Polishchuk RS. Mechanism of constitutive export from the golgi: bulk flow via the formation, protrusion, and en bloc cleavage of large trans-golgi network tubular domains. Mol Biol Cell. 2003;14:4470–4485. doi: 10.1091/mbc.E03-01-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polleux F, Ghosh AJ. The slice overlay assay: a versatile tool to study the influence of extracellular signals on neuronal development. Sci STKE. 2002;136:PL9. doi: 10.1126/stke.2002.136.pl9. [DOI] [PubMed] [Google Scholar]
- Port F, Kuster M, Herr P, Furger E, Banziger C, Hausmann G, Basler K. Wingless secretion promotes and requires retromer-dependent cycling of Wntless. Nat Cell Biol. 2008;10:178–185. doi: 10.1038/ncb1687. [DOI] [PubMed] [Google Scholar]
- Poteryaev D, Datta S, Ackema K, Zerial M, Spang A. Identification of the switch in early-to-late endosome transition. Cell. 2010;141:497–508. doi: 10.1016/j.cell.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Prasad BC, Clark SG. Wnt signaling establishes anteroposterior neuronal polarity and requires retromer in C. elegans. Development. 2006;133:1757–1766. doi: 10.1242/dev.02357. [DOI] [PubMed] [Google Scholar]
- Prekeris R, Foletti DL, Scheller RH. Dynamics of tubulovesicular recycling endosomes in hippocampal neurons. J Neurosci. 1999;19:10324–10337. doi: 10.1523/JNEUROSCI.19-23-10324.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz WA, Hinshaw JE. Membrane-bending proteins. Crit Rev Biochem Mol Biol. 2009;44:278–291. doi: 10.1080/10409230903183472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendran L, Simons K. Lipid rafts and membrane dynamics. J Cell Sci. 2005;118:1099–1102. doi: 10.1242/jcs.01681. [DOI] [PubMed] [Google Scholar]
- Rink J, Ghigo E, Kalaidzidis Y, Zerial M. Rab conversion as a mechanism of progression from early to late endosomes. Cell. 2005;122:735–749. doi: 10.1016/j.cell.2005.06.043. [DOI] [PubMed] [Google Scholar]
- Saberan-Djoneidi D, Picart R, Escalier D, Gelman M, Barret A, Tougard C, Glowinski J, Levi-Strauss M. A 21-kDa polypeptide belonging to a new family of proteins is expressed in the Golgi apparatus of neural and germ cells. J Biol Chem. 1998;273:3909–3914. doi: 10.1074/jbc.273.7.3909. [DOI] [PubMed] [Google Scholar]
- Sadowski L, Pilecka I, Miaczynska M. Signaling from endosomes: location makes a difference. Exp Cell Res. 2009;315:1601–1609. doi: 10.1016/j.yexcr.2008.09.021. [DOI] [PubMed] [Google Scholar]
- Sandvig K, Pust S, Skotland T, van Deurs B. Clathrin-independent endocytosis: mechanisms and function. Curr Opin Cell Biol. 2011;23:413–420. doi: 10.1016/j.ceb.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Sann S, Wang Z, Brown H, Jin Y. Roles of endosomal trafficking in neurite outgrowth and guidance. Trends Cell Biol. 2009;19:317–324. doi: 10.1016/j.tcb.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Santolini E, Puri C, Salcini AE, Gagliani MC, Pelicci PG, Tacchetti C, Di Fiore PP. Numb is an endocytic protein. J Cell Biol. 2000;151:1345–1352. doi: 10.1083/jcb.151.6.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer AW, Kamei Y, Kamiguchi H, Wong EV, Rapoport I, Kirchhausen T, Beach CM, Landreth G, Lemmon SK, Lemmon V. L1 endocytosis is controlled by a phosphorylation-dephosphorylation cycle stimulated by outside-in signaling by L1. J Cell Biol. 2002;24:1223–1232. doi: 10.1083/jcb.200203024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer AW, Kamiguchi H, Wong EV, Beach CM, Landreth G, Lemmon V. Activation of the MAPK signal cascade by the neural cell adhesion molecule L1 requires L1 internalization. J Biol Chem. 1999;274:37965–37973. doi: 10.1074/jbc.274.53.37965. [DOI] [PubMed] [Google Scholar]
- Schafer DA. Regulating actin dynamics at membranes: a focus on dynamin. Traffic. 2004;5:463–469. doi: 10.1111/j.1600-0854.2004.00199.x. [DOI] [PubMed] [Google Scholar]
- Schmid RS, Shelton S, Stanco A, Yokota Y, Kreidberg JA, Anton ES. alpha3beta1 integrin modulates neuronal migration and placement during early stages of cerebral cortical development. Development. 2004;131:6023–6031. doi: 10.1242/dev.01532. [DOI] [PubMed] [Google Scholar]
- Schmidt MR, Haucke V. Recycling endosomes in neuronal membrane traffic. Biol Cell. 2007;99:333–342. doi: 10.1042/BC20070007. [DOI] [PubMed] [Google Scholar]
- Schweizer FE, Ryan TA. The synaptic vesicle: cycle of exocytosis and endocytosis. Curr Opin Neurobiol. 2006;16:298–304. doi: 10.1016/j.conb.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Shao Y, Akmentin W, Toledo-Aral JJ, Rosenbaum J, Valdez G, Cabot JB, Hilbush BS, Halegoua S. Pincher, a pinocytic chaperone for nerve growth factor/TrkA signaling endosomes. J Cell Biol. 2002;157:679–691. doi: 10.1083/jcb.200201063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M, Jovic M, Kieken F, Naslavsky N, Sorgen P, Caplan S. A model for the role of EHD1-containing membrane tubules in endocytic recycling. Commun Integr Biol. 2009;2:431–433. doi: 10.4161/cib.2.5.9157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M, Naslavsky N, Caplan S. A role for EHD4 in the regulation of early endosomal transport. Traffic. 2008;9:995–1018. doi: 10.1111/j.1600-0854.2008.00732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelly M, Cancedda L, Lim BK, Popescu AT, Cheng PL, Gao H, Poo MM. Semaphorin3A regulates neuronal polarization by suppressing axon formation and promoting dendrite growth. Neuron. 2011;71:433–446. doi: 10.1016/j.neuron.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Temple S. Creating asymmetric cell divisions by skewing endocytosis. Sci STKE. 2002;2002:pe52. doi: 10.1126/stke.2002.162.pe52. [DOI] [PubMed] [Google Scholar]
- Shen Q, Zhong W, Jan YN, Temple S. Asymmetric Numb distribution is critical for asymmetric cell division of mouse cerebral cortical stem cells and neuroblasts. Development. 2002;129:4843–4853. doi: 10.1242/dev.129.20.4843. [DOI] [PubMed] [Google Scholar]
- Shieh JC, Schaar BT, Srinivasan K, Brodsky FM, McConnell SK. Endocytosis regulates cell soma translocation and the distribution of adhesion proteins in migrating neurons. PLoS One. 2011;6:e17802. doi: 10.1371/journal.pone.0017802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilo BZ, Schejter ED. Regulation of developmental intercellular signalling by intracellular trafficking. Embo J. 2011;30:3516–3526. doi: 10.1038/emboj.2011.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K, Gerl MJ. Revitalizing membrane rafts: new tools and insights. Nat Rev Mol Cell Biol. 2010;11:688–699. doi: 10.1038/nrm2977. [DOI] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Smith CA, Dho SE, Donaldson J, Tepass U, McGlade CJ. The cell fate determinant numb interacts with EHD/Rme-1 family proteins and has a role in endocytic recycling. Mol Biol Cell. 2004;15:3698–3708. doi: 10.1091/mbc.E04-01-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CA, Lau KM, Rahmani Z, Dho SE, Brothers G, She YM, Berry DM, Bonneil E, Thibault P, Schweisguth F, et al. aPKC-mediated phosphorylation regulates asymmetric membrane localization of the cell fate determinant Numb. Embo J. 2007;26:468–480. doi: 10.1038/sj.emboj.7601495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnichsen B, De Renzis S, Nielsen E, Rietdorf J, Zerial M. Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J Cell Biol. 2000;149:901–914. doi: 10.1083/jcb.149.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanco A, Szekeres C, Patel N, Rao S, Campbell K, Kreidberg JA, Polleux F, Anton ES. Netrin-1-alpha3beta1 integrin interactions regulate the migration of interneurons through the cortical marginal zone. Proc Natl Acad Sci U S A. 2009;106:7595–7600. doi: 10.1073/pnas.0811343106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein E, Tessier-Lavigne M. Hierarchical organization of guidance receptors: silencing of netrin attraction by slit through a Robo/DCC receptor complex. Science. 2001;291:1928–1938. doi: 10.1126/science.1058445. [DOI] [PubMed] [Google Scholar]
- Steiner P, Alberi S, Kulangara K, Yersin A, Sarria JC, Regulier E, Kasas S, Dietler G, Muller D, Catsicas S, et al. Interactions between NEEP21, GRIP1 and GluR2 regulate sorting and recycling of the glutamate receptor subunit GluR2. EMBO J. 2005;24:2873–2884. doi: 10.1038/sj.emboj.7600755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner P, Sarria JC, Glauser L, Magnin S, Catsicas S, Hirling H. Modulation of receptor cycling by neuron-enriched endosomal protein of 21 kD. J Cell Biol. 2002;157:1197–1209. doi: 10.1083/jcb.200202022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe JG, Milner RJ, Shinnick TM, Bloom FE. Identifying the protein products of brain-specific genes with antibodies to chemically synthesized peptides. Cell. 1983;33:671–682. doi: 10.1016/0092-8674(83)90010-7. [DOI] [PubMed] [Google Scholar]
- Thelen K, Kedar V, Panicker AK, Schmid RS, Midkiff BR, Maness PF. The neural cell adhesion molecule L1 potentiates integrin-dependent cell migration to extracellular matrix proteins. J Neurosci. 2002;22:4918–4931. doi: 10.1523/JNEUROSCI.22-12-04918.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson A, Nessler R, Wisco D, Anderson E, Winckler B, Sheff D. Recycling Endosomes of Polarized Epithelial Cells Actively Sort Apical and Basolateral Cargos into Separate Subdomains. Mol Biol Cell. 2007;18:2687–2697. doi: 10.1091/mbc.E05-09-0873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tojima T, Akiyama H, Itofusa R, Li Y, Katayama H, Miyawaki A, Kamiguchi H. Attractive axon guidance involves asymmetric membrane transport and exocytosis in the growth cone. Nat Neurosci. 2007;10:58–66. doi: 10.1038/nn1814. [DOI] [PubMed] [Google Scholar]
- Tojima T, Hines JH, Henley JR, Kamiguchi H. Second messengers and membrane trafficking direct and organize growth cone steering. Nat Rev Neurosci. 2011;12:191–203. doi: 10.1038/nrn2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tojima T, Itofusa R, Kamiguchi H. Asymmetric clathrin-mediated endocytosis drives repulsive growth cone guidance. Neuron. 2010;66:370–377. doi: 10.1016/j.neuron.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Uemura T, Shepherd S, Ackerman L, Jan LY, Jan YN. numb, a gene required in determination of cell fate during sensory organ formation in Drosophila embryos. Cell. 1989;58:349–360. doi: 10.1016/0092-8674(89)90849-0. [DOI] [PubMed] [Google Scholar]
- Van Dyke RW. Acidification of lysosomes and endosomes. Subcell Biochem. 1996;27:331–360. doi: 10.1007/978-1-4615-5833-0_10. [DOI] [PubMed] [Google Scholar]
- Verdi JM, Bashirullah A, Goldhawk DE, Kubu CJ, Jamali M, Meakin SO, Lipshitz HD. Distinct human NUMB isoforms regulate differentiation vs. proliferation in the neuronal lineage. Proc Natl Acad Sci U S A. 1999;96:10472–10476. doi: 10.1073/pnas.96.18.10472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira AV, Lamaze C, Schmid SL. Control of EGF receptor signaling by clathrin-mediated endocytosis. Science. 1996;274:2086–2089. doi: 10.1126/science.274.5295.2086. [DOI] [PubMed] [Google Scholar]
- Wakamatsu Y, Maynard TM, Jones SU, Weston JA. NUMB localizes in the basal cortex of mitotic avian neuroepithelial cells and modulates neuronal differentiation by binding to NOTCH-1. Neuron. 1999;23:71–81. doi: 10.1016/s0896-6273(00)80754-0. [DOI] [PubMed] [Google Scholar]
- Wilson JM, de Hoop M, Zorzi N, Toh BH, Dotti CG, Parton RG. EEA1, a tethering protein of the early sorting endosome, shows a polarized distribution in hippocampal neurons, epithelial cells, and fibroblasts. Mol Biol Cell. 2000;11:2657–2671. doi: 10.1091/mbc.11.8.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winckler B, Mellman I. Trafficking guidance receptors. Cold Spring Harb Perspect Biol. 2010;2:a001826. doi: 10.1101/cshperspect.a001826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang PT, Lorenowicz MJ, Silhankova M, Coudreuse DY, Betist MC, Korswagen HC. Wnt signaling requires retromer-dependent recycling of MIG-14/Wntless in Wnt-producing cells. Dev Cell. 2008;14:140–147. doi: 10.1016/j.devcel.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Yap CC, Lasiecka ZM, Caplan S, Winckler B. Alterations of EHD1/EHD4 protein levels interfere with L1/NgCAM endocytosis in neurons and disrupt axonal targeting. J Neurosci. 2010;30:6646–6657. doi: 10.1523/JNEUROSCI.5428-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap CC, Wisco D, Kujala P, Lasiecka ZM, Cannon JT, Chang MC, Hirling H, Klumperman J, Winckler B. The somatodendritic endosomal regulator NEEP21 facilitates axonal targeting of L1/NgCAM. J Cell Biol. 2008;180:827–842. doi: 10.1083/jcb.200707143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogev S, Schejter ED, Shilo BZ. Drosophila EGFR signalling is modulated by differential compartmentalization of Rhomboid intramembrane proteases. Embo J. 2008;27:1219–1230. doi: 10.1038/emboj.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- Zhong W, Jiang MM, Schonemann MD, Meneses JJ, Pedersen RA, Jan LY, Jan YN. Mouse numb is an essential gene involved in cortical neurogenesis. Proc Natl Acad Sci U S A. 2000;97:6844–6849. doi: 10.1073/pnas.97.12.6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Alfaro J, Chang EH, Zhao X, Porcionatto M, Segal RA. Numb links extracellular cues to intracellular polarity machinery to promote chemotaxis. Dev Cell. 2011;20:610–622. doi: 10.1016/j.devcel.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilian O, Saner C, Hagedorn L, Lee HY, Sauberli E, Suter U, Sommer L, Aguet M. Multiple roles of mouse Numb in tuning developmental cell fates. Curr Biol. 2001;11:494–501. doi: 10.1016/s0960-9822(01)00149-x. [DOI] [PubMed] [Google Scholar]
- Zoncu R, Perera RM, Balkin DM, Pirruccello M, Toomre D, De Camilli P. A phosphoinositide switch controls the maturation and signaling properties of APPL endosomes. Cell. 2009;136:1110–1121. doi: 10.1016/j.cell.2009.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]