Abstract
AIM: To investigate the role and potential mechanisms of bone marrow mesenchymal stem cells (MSCs) in severe acute peritonitis (SAP).
METHODS: Pancreatic acinar cells from Sprague Dawley rats were randomly divided into three groups: non-sodium deoxycholate (SDOC) group (non-SODC group), SDOC group, and a MSCs intervention group (i.e., a co-culture system of MSCs and pancreatic acinar cells + SDOC). The cell survival rate, the concentration of malonaldehyde (MDA), the density of superoxide dismutase (SOD), serum amylase (AMS) secretion rate and lactate dehydrogenase (LDH) leakage rate were detected at various time points. In a separate study, Sprague Dawley rats were randomly divided into either an SAP group or an SAP + MSCs group. Serum AMS, MDA and SOD, interleukin (IL)-6, IL-10, and tumor necrosis factor (TNF)-α levels, intestinal mucosa injury scores and proliferating cells of small intestinal mucosa were measured at various time points after injecting either MSCs or saline into rats. In both studies, the protective effect of MSCs was evaluated.
RESULTS: In vitro, The cell survival rate of pancreatic acinar cells and the density of SOD were significantly reduced, and the concentration of MDA, AMS secretion rate and LDH leakage rate were significantly increased in the SDOC group compared with the MSCs intervention group and the Non-SDOC group at each time point. In vivo, Serum AMS, IL-6, TNF-α and MAD level in the SAP + MSCs group were lower than the SAP group; however serum IL-10 level was higher than the SAP group. Serum SOD level was higher than the SAP group at each time point, whereas a significant between-group difference in SOD level was only noted after 24 h. Intestinal mucosa injury scores was significantly reduced and the proliferating cells of small intestinal mucosa became obvious after injecting MSCs.
CONCLUSION: MSCs can effectively relieve injury to pancreatic acinar cells and small intestinal epithelium, promote the proliferation of enteric epithelium and repair of the mucosa, attenuate systemic inflammation in rats with SAP.
Keywords: Bone marrow mesenchymal stem cells, Severe acute pancreatitis, Intestinal barricade function, Pancreatic acinar cells
INTRODUCTION
Acute pancreatitis (AP) is characterized by a rapid onset and disease progression, with high fatality. Severe acute pancreatitis (SAP) is extremely challenging to treat and the mortality rate is approximately 20%-40%[1]. Several studies currently suggest that the pathogenesis of AP involves complicated cascade reactions that start from the activation of pancreatin in pancreatic acinar cells. Pancreatin causes injury to the acinar cells and induces both local and systemic inflammation[2]. Inflammatory factors such as C-reactive protein, tumor necrosis factor (TNF), interleukin (IL)-1, IL-6, IL-8, nitric oxide (NO) and endothelin (among others) are thought to be involved in both the genesis and progression of AP and play a critical role in the progression from slight acute pancreatitis to severe acute pancreatitis[3]. Intestinal barricade function is significantly injured in SAP permitting bacteria to invade the enteric cavity and allowing endotoxin to enter the circulatory system thereby inducing a systemic inflammatory factor cascade reaction that aggravates the condition[4].
Mesenchymal stem cells (MSCs) are multipotent stem cells. One previous study demonstrated that MSCs had strong immunoregulatory effects and multidirectional differentiation potency[5]. Other recent studies found that MSCs also played a special role in inhibiting inflammatory reactions and promoting tissue repair[6]. For example, Hagiwara et al [7] found that rats with renal injury caused by ischemia-reperfusion induced a significant reduction in renal cell apoptosis after the injection of thymidine kinase-expressing MSCs (TK-MSCs). In addition, nitric oxide synthase (NOS) and NO levels were significantly reduced, which significantly inhibited: the infiltration of neutrophils and mononuclear macrophages; reduced the activity of peroxidase; delayed the production of peroxide, phosphorylation of p38 extracellular signal regulated kinase, the expression of TNF-α, and monocyte chemoattractant protein-1 cell adhesion. TK-MSCs also inhibited H2O2-induced cell apoptosis and increased Akt phosphorylation and cell activity in the periphery of the renal tubular cells. Tögel et al[8] administered MSCs to mice with acute renal failure for 24 h. The proinflammatory cytokines IL-1β, TNF-α, IFN-γ and NOS were all significantly reduced, whereas the anti-inflammatory factors IL-10, β fibroblast growth factor, TGF-α and B cell lymphoma-2 appeared highly expressed. In a pulmonary injured animal model, Iyer et al[9] found that MSCs attenuated a self-inflammatory reaction and enhanced the anti-inflammatory reaction by regulating the proliferation, differentiation, and delomorphous nature of immunocytes.
MSCs have also been demonstrated to have therapeutic effects in inflammatory diseases. For example, Imberti et al[10] injected MSCs in a cisplatin-induced acute renal injury model in mice and found that the MSCs enhanced mitosis. In addition, the production of insulin-like growth-factor-1 promoted the repair of renal tubules. In the treatment of chronic ischemic cardiomyopathy, MSCs were injected into ligate ramus descendens anterior arteril coronariae sinistrae. They were also injected into acute myocardial infarction regions. MSCs in both cases enhanced the contractile force of the cardiac muscle cells, regulated the contents and composition of collagen fibers in the tissue, and prevented the reconstruction of cardiac ventricles, thereby protecting the basic structure of cardiac muscle[11]. After intravenous injection of MSCs in experimental rats with spinal injury, MSCs assembled and survived in the host injury spinal cord and promoted the neural repair and recovery of nerve function[12]. In yet another study, rats with a radioactive intestinal injury were injected with labeled MSCs and the intestinal chorioepithelium regeneration occurred in the injured intestinal mucosa for 3 d and the radial related regions (e.g., kidney, spleen, stomach) also had MSCs[13]. Finally, after injecting MSCs into rats with an intestinal injury (ischemia/reperfusion), the permeability of the intestine was reduced and the injury to the intestinal villi was attenuated[14]. Together, these data indicate that MSCs can reduce the expression of various inflammatory factors and promote the repair of various tissues and organ injury.
Because the treatment of AP with stem cells has not been studied to date, and based on the ability of MSCs to inhibit inflammatory reactions and promote tissue repair, the purpose of this study was to explore the role, and the possible mechanisms, of MSCs in rats with SAP.
MATERIALS AND METHODS
Animals
Healthy Sprague Dawley rats weighing 200-300 g were provided by Shanghai SLK experimental animal Company [Batch No. SCXK (Shanghai) 2007-0005, China]. The study was approval by the Institutional Animal Care and Use Committee Fujian Medical University. The care and handling of all animals were in accordance with guidelines for animal ethics.
Drugs, reagents and instruments
The following reagents were used in the experiments: sodium deoxycholate (SDOC), 3-(4,5-dimethylthiazol-2-yl)-2-,5-diphenyltetrazolium bromide (MTT; Sigma), fetal bovine serum (Purpleflower holly leaf, Hangzhou), Dulbecco’s modified Eagle’s medium (DMEM; Thermo Fisher, United States), ethylenediaminetetraaceticacid (EDTA)-trypsin (Amersco Co., United States), 4,6-diamidino-2-phenylindole (DAPI; Roche, Switzerland), malonaldehyde (MDA), superoxide dismutase (SOD), amylase (AMS) secretion rate and lactate dehydrogenase (LDH) kits (Nanjing Jianchen Scientific Co. Ltd), transwell double layer culture dish (Corning Costar, United States), IL-10 enzyme-linked immunosorbent assay (ELISA) kit for rats, IL-10 ELISA for rats, and TNF-α ELISA kit for rats (all ELISAs from Wuhan Youer Bio-scientific Co., Ltd), and antibody against proliferating cell nuclear antigen (Shanghai Zhuokang Bio-scientific Co., Ltd).
Culture, identification, and labeling of mesenchymal stem cells
Male rats weighting about 200 g were humanely sacrificed by cervical dislocation. The bone marrow was aseptically collected and subsequently cultured using whole marrow differential adherence methods. MSCs were obtained by multiple digestions and passages. The cellular identification of the expression of MSCs surface markers (i.e., CD29, CD34, CD45 and CD90) were detected using flow cytometry. The cells were labeled with DAPI and observed under fluorescence microscope. Third generation MSCs was acquired for subsequent experimentation.
Cell experiments
Pancreatic acinar cells from the rats were separated using the collagenase method[15]. The cells were seeded in Hanks buffer solution containing 10% fetal calf serum at a density of 1 × 106 cells/mL. The purity of the pancreatic acinar cells was > 80% and the survival rate was > 90%). Cells were seeded in 35 mm × 35 mm culture dishes and incubated at 37 °C and 55% CO2 for 2 h. Cell morphology was examined using phase contrast microscopy. The cultured acinar cells were seeded in the underlayer of transwell double-deck culture dishes.
Pancreatic acinar cells were randomly divided into three groups: non-sodium deoxycholate group (non-SODC group), SDOC group, and a MSCs intervention group. In the SDOC group, the pancreatic acinar cells were seeded in the bottom of the transwell double-layer culture dishes and had a final concentration of 50 μmol/L SDOC. In the Non-SDOC group, the pancreatic acinar cells were seeded in the bottom of the transwell double-layer culture dishes and were not cultured with SDOC. In MSCs intervention group, the insert of the transwell plates was inserted into the poles and the third generation MSCs were seeded at a density of 1 × 106 cell/mL. The culture medium in the insert and the six-pole plate were fused, thereby establishing the co-culture system of MSCs and acinar cells. The co-culture medium was LG-DMEM with SDOC at a final concentration of 50 μmol/L. Subsequently, the cells in each group were incubated for 0.5 h, 1 h, 4 h and 10 h. Alterations in cell morphology were examined and cell survival was quantitatively detected by the MTT assay. The cell survival rate was expressed by the percentage in each group using the following equation: 100% × absorbance at 490 in each group/absorbance at 490 in the fresh separated pancreatic acinar culture medium.
The supernatants were also collected and the concentration of MDA was determined using the thiobarbituric acid method. The density of culture serum SOD was also determined using the xanthine oxidase method. The AMS secretion rate and LDH leakage rate of acinar cells were measured by enzyme kinetics methods. The AMS secretion rate was cell supernatant AMS/cell total AMS × 100% and the LDH leakage rate was cell supernatant LDH/cell total LDH × 100%.
Animal experiment
Thirty-six male rats were randomly divided into either the SAP group or the SAP + MSCs group. The SAP model was established by injecting deoxy-STC under the pancreatic capsule[16]. Specifically, following a peritoneal injection of 2.5% thiopental sodium, the pancreas of each rat was sufficiently exposed after entering into the abdomen via a median abdominal incision. Next, 1 mL of 3.8% STC was slowly injected into the inferior aspect of capsule using a No. 4 needle from the tail of pancreas, which made the entire pancreas swell. The pancreas was replaced 2 min later and the abdominal cavity was sutured closed routinely. In the SAP + MSCs group, 2 mL of the MSCs cell suspension (containing approximately 1 × 106 cells/mL determined via DAPI fluorescence immunity labeling) were injected into the caudal vein. In the SAP group, 2 mL of normal saline was injected. Six mice were randomly collected from both groups 6 h, 24 h and 72 h postinjection. Blood was collected from the apex of the heart and 5 cm of the small intestine (the section from the terminal ileum and extending distally) was obtained. Serum AMS was detected and the concentrations of serum IL-6, IL-10 and TNF-α were determined using ELISAs. Serum MDA concentration was determined using the thiobarbituric acid method, and the concentration of serum SOD was measured via the xanthine oxidase method.
The small intestinal tissue was flash frozen, and the number of DAPI positive cells was measured under fluorescence microscopy. Conventional hematoxylin and eosin staining was performed on sections of small intestine and injury to the intestinal mucosa was assessed in six different, randomly selected, high-power fields (original magnification × 400). According to the injury scoring criteria of Chiu’s intestinal tissue[17], injury to intestinal mucosa, infiltration of inflammatory cells, and degree of hemorrhage and hyperemia were scored. The proliferating cell nuclear antigen Ki-67 immunohistochemistry staining was performed to note any proliferation of intestinal mucosa cells. Again, six different high-power fields (× 400) were randomly selected and the number positive cells were counted.
Statistical analysis
All data were expressed by mean ± SD. The mono-factor variance analysis was applied for comparisons between groups. A P < 0.05 was considered statistically significant, and all analyses were performed using SPSS 13.0.
RESULTS
General morphology of mesenchymal stem cells and the expression of surface markers
Third generation of MSCs were examined under an inverted microscope. The cells assumed a fusiform and swirling colony (Figure 1). As shown in Figure 2, the positive rate of CD29 was 98.6% and the positive rate of CD90 was 99.6%. In contrast, CD34 and CD45 were negative (0.56% and 0.89%, respectively), demonstrating that the purity of the MSCs was > 95%.
Figure 1.
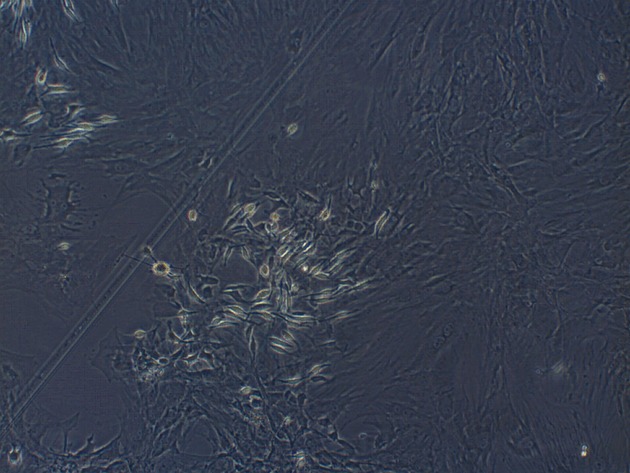
Third generation mesenchymal stem cells were spindle-shaped and formed spiral-like colonies (original magnification × 100).
Figure 2.
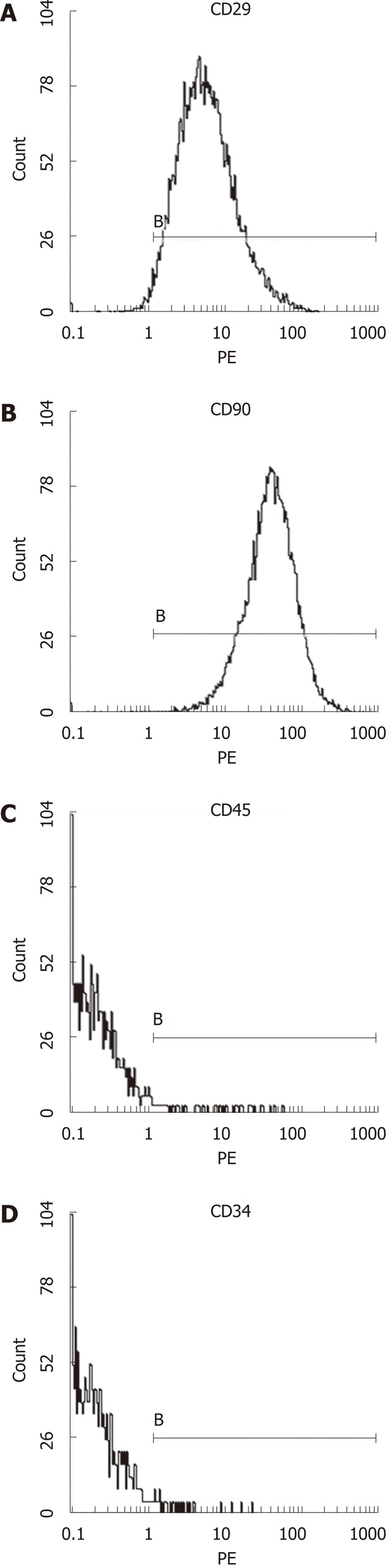
The expression of mesenchymal stem cells surface markers detected by flow cytometry. The proportion of CD29+ (A) cells was 98.6%, the proportion of CD90+ (B) cells was 99.6%, the proportion of CD45+ (C) cells was 0.89% and CD34+ (D) cells was 0.56%. B: The boundary of the cells.
Morphology of pancreatic acinar cells
After the pancreatic acinar cells were cultured for 2 h, no adherence was noted under the inverted microscope. Instead, the cells assumed a cluster formation and assembled with lumping. The boundary of the cells was clear and the refraction was strong. High density particles containing proenzymes could be seen in the cells (Figure 3).
Figure 3.
Separated pancreatic acinar cells (original magnification × 100).
The cell survival rate of fresh separated pancreatic acinar cell was comparatively high and that in each group was reduced. This reduction was most evident in the SDOC group. The cell survival rate at each time point in the MSCs intervention group was significantly increased compared with the SDOC group (Table 1).
Table 1.
Measurement of cell survival rate by the 3-(4,5-dimethylthiazol-2-yl)-2-,5-diphenyltetrazolium bromide assay of pancreatic acinar cells at various time points (mean ± SD)
| Group | 0.5 h | 1 h | 4 h | 10 h |
| Non-SODC group | 93.83% ± 3.13% | 89.00% ± 2.83% | 81.83% ± 3.06% | 75.00% ± 6.54% |
| SODC group | 87.83% ± 6.59%a | 77.50% ± 9.35%a | 65.83% ± 8.23%a | 39.17% ± 8.26%a |
| MSCs intervention group | 90.00% ± 3.41% | 82.17% ± 7.47% | 75.17% ± 5.85%c | 51.83% ± 6.79%c |
P < 0.05 vs the non-sodium deoxycholate (SDOC) group,
P < 0.05 vs the SDOC group. MSCs: Mesenchymal stem cells.
Amylase secretion and lactate dehydrogenase leakage rates
The AMS secretion rate and LDH leakage rate of pancreatic acinar cell in the SDOC group at each time point was significantly higher than the other two groups. The AMS secretion rate and LDH leakage rate in the MSCs intervention group at each time point was significantly reduced compared with the SDOC group (Table 2).
Table 2.
Changes in amylase secretion rate and lactate dehydrogenase leakage rate of pancreatic acinar cells at various time points (mean ± SD)
| Index | Group | 0.5 h | 1 h | 4 h | 10 h |
| AMS | Non-SDOC group | 7.47 ± 0.67 | 8.97 ± 0.69 | 20.32 ± 2.00 | 24.28 ± 2.47 |
| SDOC group | 11.75 ± 2.40a | 17.23 ± 2.43a | 40.88 ± 3.61a | 60.38 ± 4.01a | |
| MSCs intervention group | 10.18 ± 1.53 | 14.48 ± 1.74c | 29.33 ± 2.16c | 40.33 ± 4.27c | |
| LDH | Non-SDOC group | 3.00 ± 0.63 | 3.47 ± 0.59 | 13.17 ± 2.86 | 23.40 ± 2.55 |
| SDOC group | 7.65 ± 1.75a | 12.00 ± 3.17a | 39.02 ± 2.38a | 53.70 ± 6.73a | |
| MSCs intervention group | 5.35 ± 1.01c | 8.33 ± 3.08c | 27.67 ± 3.39c | 38.33 ± 3.20c |
P < 0.05 vs the non-sodium deoxycholate (SDOC) group;
P < 0.05 vs the SDOC group. MSCs: Mesenchymal stem cells; AMS:Amylase; LDH: Lactate dehydrogenase.
Oxidative stress
MDA and SOD were measured in the supernatants collected from each group (Table 3). In SDOC group, SOD activity significantly reduced and this difference was significant compared with the non-SDOC group (P < 0.05). With the extension of SDOC reaction time, the SOD activity in the cell culture supernatants was further reduced, which was also significantly lower than the non-SDOC group at the same time points (P < 0.05). However, MDA content in cell culture supernatants was significantly higher in the SDOC group than the non- SDOC group at the corresponding time points (P < 0.05). The SOD activity in the MSCs intervention group at each time point was significantly increased compared with the SDOC group, whereas MDA content was significantly lower than the SDOC group (P < 0.05).
Table 3.
Comparison of superoxide dismutase and malonaldehyde levels in pancreatic acinar cell culture supernatants at various time points (mean ± SD)
| Index | Group | 0.5 h | 1 h | 4 h | 10 h |
| Superoxide dismutase (U/mL) | Non-SDOC group | 194.83 ± 26.48 | 185.83 ± 37.79 | 170.00 ± 25.42 | 165.00 ± 31.72 |
| SDOC group | 116.17 ± 28.85a | 108.00 ± 41.52a | 102.00 ± 33.45a | 90.67 ± 33.55a | |
| MSCs intervention group | 125.50 ± 39.20 | 138.50 ± 42.03 | 147.67 ± 37.25c | 139.00 ± 46.22c | |
| Malonaldehyde (μmol/L) | Non-SDOC group | 3.50 ± 5.84 | 4.17 ± 0.75 | 4.33 ± 1.27 | 4.67 ± 1.21 |
| SDOC group | 4.40 ± 1.33 | 6.33 ± 1.63a | 7.33 ± 1.21a | 8.00 ± 1.10a | |
| MSCs intervention group | 3.97 ± 0.89 | 5.00 ± 1.41 | 5.33 ± 1.63c | 5.83 ± 2.04c |
P < 0.05 vs the non-sodium deoxycholate (SDOC) group;
P < 0.05 vs the SDOC group.
Permanent planting of mesenchymal stem cells in small intestine
Blue fluorescing cells (DAPI positive cells) were observed in sections of small intestinal tissue of rats that were flash frozen in the rats included in the SAP + MSCs group (Figure 4).
Figure 4.
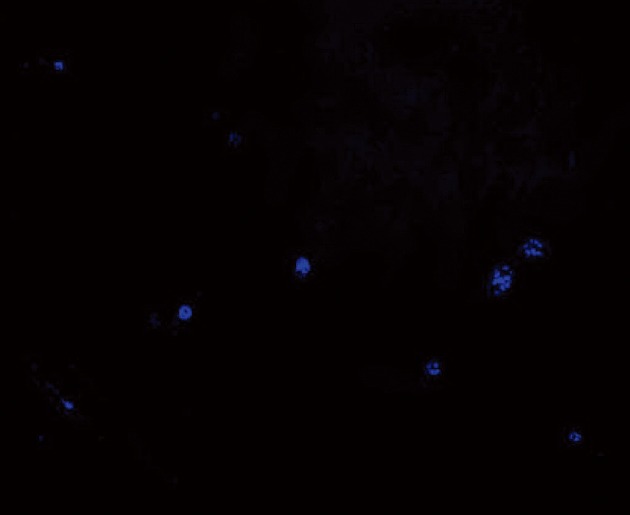
Transplanted mesenchymal stem cells were stained with 4,6-diamidino-2-phenylindole in advance, flash-frozen then observed under fluorescence microscope. The blue fluorescent 4,6-diamidino-2-phenylindole-positive cells (mesenchymal stem cells) were noted in the small intestinal tissue.
Effect of mesenchymal stem cells transplantation on serum amylase levels and oxidative stress
Serum AMS levels of rats in the SAP group at each time point was significantly enhanced compared with those measured in the SAP + MSCs group (P < 0.05, Table 4). Serum MDA and SOD levels have been summarized in Table 5. Serum MDA levels tended to initially increase, but were then reduced following the injection of MSCs. Serum MDA levels at each time point in the SAP + MSCs group were significantly lower than in the SAP group (P < 0.05). Serum SOD levels in the SAP + MSCs group was higher than in the SAP group, whereas a significant between-group difference in SOD level was only noted after 24 h (P < 0.01).
Table 4.
Comparisons of serum amylase levels (U/L) in rats that were or were not treated with mesenchymal stem cells following establishment of an severe acute pancreatitis model (n = 36, mean ± SD)
| Group |
Post-MSCs or saline injection (h) |
||
| 6 | 24 | 72 | |
| SAP | 3753.83 ± 791.65 | 5344.67 ± 649.63 | 7762.50 ± 977.30 |
| SAP + MSCs | 2671.33 ± 547.57a | 4235.83 ± 554.57a | 5615.17 ± 809.30b |
P < 0.05,
P < 0.01 vs the severe acute pancreatitis (SAP) group. MSCs: Mesenchymal stem cells.
Table 5.
Comparison of serum malonaldehyde, superoxide dismutase levels, interleukin-6, interleukin-10 and tumor necrosis factor-α measured at various time points after injecting either mesenchymal stem cells or saline into rats after establishing an severe acute pancreatitis model (n = 36, mean ± SD)
| Index | Group |
Post-mesenchymal stem cells or saline injection (h) |
||
| 6 | 24 | 72 | ||
| Malonaldehyde (nmol/mL) | Severe acute pancreatitis group | 4.89 ± 0.97 | 5.20 ± 1.21 | 4.43 ± 0.42 |
| Severe acute pancreatitis + mesenchymal stem cells group | 3.68 ± 0.38a | 3.89 ± 0.59a | 3.36 ± 0.98a | |
| Superoxide dismutase (U/mL) | Severe acute pancreatitis group | 43.16 ± 6.94 | 48.13 ± 3.93 | 45.83 ± 4.72 |
| Severe acute pancreatitis + mesenchymal stem cells group | 48.05 ± 3.83 | 61.29 ± 7.81b | 50.75 ± 7.59 | |
| Interleukin-6 (pg/mL) | Severe acute pancreatitis group | 107.70 ± 13.08 | 128.52 ± 8.52 | 134.06 ± 13.12 |
| Severe acute pancreatitis + mesenchymal stem cells group | 90.16 ± 9.55a | 107.33 ± 12.13b | 143.24 ± 12.11 | |
| Interleukin-10 (pg/mL) | Severe acute pancreatitis group | 31.08 ± 6.64 | 45.02 ± 4.28 | 40.11 ± 8.39 |
| Severe acute pancreatitis + mesenchymal stem cells group | 40.84 ± 7.05a | 52.08 ± 5.79a | 41.76 ± 3.37 | |
| TNF-α (pg/mL) | Severe acute pancreatitis group | 106.15 ± 9.01 | 132.62 ± 8.64 | 122.42 ± 13.44 |
| Severe acute pancreatitis + mesenchymal stem cells group | 91.47 ± 10.00a | 119.47 ± 10.83a | 110.91 ± 9.92 | |
P < 0.05,
P < 0.01 vs the severe acute pancreatitis group. TNF-α: Tumor necrosis factor-α.
Regulation of mesenchymal stem cells transplantation on inflammatory factors
Serum IL-6, IL-10 and TNF-α levels in the two groups have been summarized in Table 5. Serum IL-10 and TNF-α after MSCs transplantation tended to increase then decrease. IL-6 was persistently elevated and was obvious in the SAP + MSCs group. After MSCs transplantation, serum IL-6 and TNF-α levels were significantly lower than in the SAP group (P < 0.05), Further, serum IL-10 was significantly higher in the SAP + MSCs group than the SAP group (P < 0.05). After 72 h, each cytokine was not significantly different between the two groups.
Assessment and scoring of intestinal tissues at different time points after mesenchymal stem cells transplantation
Using a conventional light microscope, the intestinal mucosa was clearly damaged in the SAP group. Specifically, the lamina propria was destroyed, the blood capillary network was exposed, there was bulk infiltration of neutrophils, local regions of hemorrhage, there was a depopulation of intestinal villi, and the glands of the lamina propria showed a variable degree of destruction. In contrast, these changes were rarely noted in the SAP + MSCs group. The main changes noted were neutrophil infiltration of the proper layer and engorgement of the capillaries. The Chiu intestinal tissue damage score in the SAP + MSCs group was significantly lower than that in the SAP group after 6 h (36.33 ± 5.72, P = 0.045), 24 h (46.33 ± 2.80, P < 0.05), and 72 h (26.67 ± 3.08, P < 0.05) as described in Table 6.
Table 6.
Comparison of intestinal mucosa injury scores (each slice/score) and proliferating cells of small intestinal mucosa (each slice/number) determined at various time points after injecting either mesenchymal stem cells or saline after establishment of an severe acute pancreatitis model (n = 36, mean ± SD)
| Group |
Post-MSCs or saline injection (h) |
|||||
|
6 |
24 |
72 |
||||
| Intestinal mucosa injury scores | proliferating cells number | Intestinal mucosa injury scores | proliferating cells number | Intestinal mucosa injury scores | proliferating cells number | |
| SAP group | 43.33 ± 4.84 | 39.50 ± 5.09 | 52.83 ± 5.27 | 59.67 ± 6.80 | 32.17 ± 4.17 | 81.50 ± 7.89 |
| SAP + MSCs group | 36.33 ± 5.72a | 40.83 ± 5.12 | 46.33 ± 2.80a | 68.00 ± 3.22a | 26.67 ± 3.08a | 101.00 ± 11.58b |
P < 0.05,
P < 0.01 vs the severe acute pancreatitis (SAP) group. MSCs: Mesenchymal stem cells.
Cell proliferation in the small intestinal mucosa at different time points following mesenchymal stem cells transplantation
Cellular regeneration in the small intestinal mucosa in the SAP + MSCs group was more obvious than that in the SAP group, which was in accordance with conventional pathology (Figure 5). For 6 h after transplantation, neither of the two groups had any evidence of proliferation. Then the cell proliferation of small intestinal mucosa in the SAP + MSCs group became significant different than the SAP group (P < 0.05) as shown in Table 6 and Figure 6.
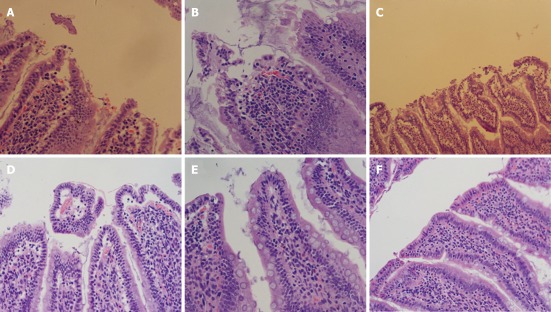
Figure 5.
Description of the intestinal pathologic manifestations 6 h after mesenchymal stem cells transplantation. A: Extensive injury of the intestinal mucosa was obvious in the severe acute pancreatitis (SAP) group; B: The dissection of the upper cortex of the intestinal mucosa was noted in the SAP + marrow mesenchymal stem cells (MSCs) group; C, D: Injury to the intestinal mucosa in the SAP (C) and SAP + MSCs groups (D) 24 h after MSCs transplantation were more severe than at 6 h; E, F: Repair of the intestinal mucosa was seen in the SAP (E) and SAP + MSCs groups (F) (HE staining, original magnification × 200).
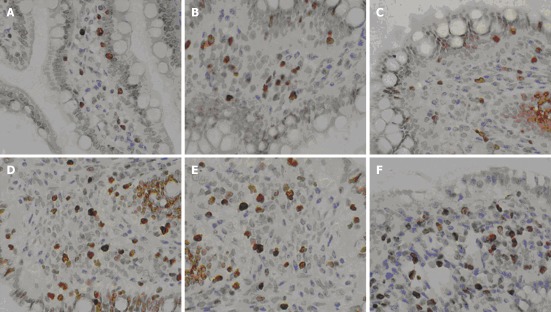
Figure 6.
The immunohistochemical staining of proliferating cell nuclear antigen Ki-067 after mesenchymal stem cells transplantation at 6 h (A, B), 24 h (C, D) and 72 h (E, F). Cell proliferation (brown cells) was obvious. The number of stained (brown) cells in the severe acute pancreatitis (SAP) + mesenchymal stem cells group (B, D, F) were significantly higher than the SAP group. Cell numbers gradually increased with time (original magnification × 400).
DISCUSSION
The goal of this study was to explore the role of bone marrow MSCs in a model of SAP to provide a new, practical basis for the intervention of this often fatal disease. The results of this study are supported by a recently published study on the inhibition of inflammation and reduction of acute pancreatitis in rats by human bone marrow-derived clonal mesenchymal stem cells[18]. Other studies have also demonstrated that SAP induces functional disturbances of the intestinal barrier, resulting in the displacement of bacteria in enteric cavity. Endotoxin subsequently enters the blood and induces a systemic inflammatory factors cascade reaction that aggravates the pathogenic condition. In this course, impairment of free radicals is thought to be one of the most important links between endotoxin and inflammatory reaction. Specifically, oxygen-derived free radicals can induce lipid peroxidation of biological membranes, change the activity of proteins and enzymes, and directly assault DNA and injure the mitochondria, etc., thereby elevating oxidative stress levels in cells[19,20].
Previous studies have also demonstrated that MSCs reduce oxygen-derived free radical levels in the body via multiple pathways and maintain the stability of membranes. Exogenous MSCs protected vascular endothelial cells to avoid the damage of oxidative stress[21], and relieve the oxidative damage of neuroblastoma[22]. In one study[23], Kallikrein-modified MSCs were transplanted into the renal tissues of rats with ischemic/reperfusion injury. Those MSCs inhibited the infiltration of neutrophils and mononuclear macrophages, reduced the activity of myeloperoxidases, diminished the formation of superoxides, and relieved H2O2-induced apoptosis. Another study reported that MSCs reduced amylase and lipase levels in the serum of rats with injury to the pancreas and repaired the necrotic pancreatic tissue. MSCs might also inhibit inflammation and involve in the reaction by producing some soluble materials[24].
The production of MDA and lipid peroxidation are parallel; therefore, detecting MDA is thought to represent lipid peroxidation. In addition, SOD is a critical free radical scavenger in mammals and its concentration reflects the ability of the body to scavenge oxygen-derived free radicals[25,26]. In this study, serum MDA of rats in the SAP + MSCs group was reduced while SOD level was heighten, indicating that MSCs transplantation could reduce the oxidative stress level of SAP rats, relieve lipid peroxidation, protect the stability of the membranes, improve the scavenging ability of oxygen-derived free radicals, relieve oxygen-derived free radical-induced multiple injury, and protect SAP-induced intestinal tissue damage.
After functional damage of the intestinal barrier, the displacement of endotoxin has multiple pathologic and physiologic consequences. For instance, endotoxin induces pyrogenic reactions, activates the complement system, affects mononuclear macrophages and endothelial cells, induces the genesis of endogenous mediators including TNF, IL, oxygen-free radicals, interferons, etc.[27], resulting in the aggravation of pathogenic conditions or even death. The displacement of endotoxin impairs intestinal epithelial cells and increases intestinal permeability[25]. MSCs also inhibit multiple immunocytes, such as T lymphocytes[28], secrete inhibitory mediators of inflammation such as IL-4 and IL-10, parasecrete IL-10, HGF, VEGF, reduce apoptosis signals[29], and relieve endotoxin-induced inflammatory reactions[30]. In the current study, mediators of inflammation, including serum IL-6 and TNF-α of rats in the SAP + MSCs group was higher than the SAP group, whereas IL-10 levels (an anti-inflammatory mediator) were lower in the SAP + MSCs group than the SAP group. This result is similar to several previous studies[13,31] indicating that MSCs might have a role in immunosuppression by reducing the expression of inflammatory factors and promoting the expression of anti-inflammatory mediators. The study reported herein also found that the Chiu intestinal tissue injury scores at 6 h, 24 h and 72 h after transplantation were significantly lower in the SAP + MSCs group than the SAP group, and cellular regeneration in the small intestinal mucosa in the SAP + MSCs group was more evident than in the SAP group. Therefore, MSCs appear to relieve the degree of injury to the small intestinal epithelium, promote the repair of enteric epithelium of rats, and maintain the integrity of the barrier of the intestinal mucosa.
Pancreatic acinar cells are the functional unit for the external secretion of the pancreas, which accounts for 80% of pancreatic tissue. SAP is caused by a functional disorder and impairment of pancreatic acinar cells[32]. During the process of SAP, inflammatory mediators, metabolic products of arachidonic acid, and oxygen-derived free radicals might reduce the antioxidative ability of pancreatic cells[33], enhance vascular permeability, and cause tissue thrombosis and hemorrhage, thereby inducing necrosis of the pancreas[34]. Thus, maintaining the function of pancreatic cells has a critical significance in relieving the severity of SAP. In this study, MDA levels in the MSCs intervention group were lower than in the SDOC group; however, SOD levels in the MSCs intervention group were higher than the SDOC group indicating that MSCs could impact the oxidative stress level of pancreatic acinar cells of injury rats inducted by SDOC, abrogate lipid peroxidation, protect the stability of membranes, improve the scavenging ability of free radicals, relieve free radical-induced injury to protect pancreatic acinar cells.
In conclusion, this study found that MSCs could relieve injury to pancreatic acinar cells in rats with SAP, attenuate inflammation and injury in the small intestinal epithelium, promote the proliferation of enteric epithelium and repair of the mucosa, and maintain the integrity of the intestinal barrier function. Potential mechanisms might involve regulating the oxidative stress levels of rats with SAP, inhibiting the extensive release of mediators of inflammation and cytokines, promoting the secretion of mediators of inflammation, and scavenging oxygen-derived free radicals. The specific mechanisms remain worthy of further study.
COMMENTS
Background
Acute pancreatitis (AP) is characterized by a rapid onset and disease progression with high fatality. Severe acute pancreatitis (SAP) is extremely challenging to treat. Several studies currently suggest that the pathogenesis of AP involves complicated cascade reactions of inflammation. Mesenchymal stem cells (MSCs) are multipotent stem cells which had strong immunoregulatory effects and multidirectional differentiation potency. Recent studies found that MSCs also played a special role in inhibiting inflammatory reactions and promoting tissue repair in various inflammation-based diseases such as kidney disease in ischemia/reperfusion injury, collagen-induced arthritis, and acute renal failure. However, very few studies to date have investigated the potential role of cell therapy for pancreatitis.
Research frontiers
Inflammation plays an important role in the pathology of AP. Tumor necrosis factor (TNF)-α and interleukin (IL)-6 as proinflammatory cytokines are produced mainly during AP. The research hotspot is to explore whether MSCs could reduce the level of inflammatory factors and promote the repair of various tissues and organ injury in AP.
Innovations and breakthroughs
Recent reports have indicated that MSCs can reduce the expression of various inflammatory factors and promote the repair of tissues and organ injury. In the present study the authors found that MSCs can also effectively relieve injury to pancreatic acinar cells and small intestinal epithelium, promote the proliferation of enteric epithelium and repair of the mucosa, attenuate systemic inflammation in rats with SAP.
Applications
The study results suggest that MSCs have a special role in inhibiting inflammatory reactions and promoting tissue repair in rats with SAP that might be developed as a cell therapy for pancreatitis.
Terminology
Severe acute pancreatitis (SAP): SAP is a serous gastrointestinal disorder which caused by a functional disorder and impairment of pancreatic acinar cells. The disease is characterized by a rapid onset and disease progression which has a high fatality. Mesenchymal stem cells (MSCs): MSCs are multipotent stem cells which derived form bone marrow. They have strong immunoregulatory effects and multidirectional differentiation potency.
Peer review
The authous detected the cell survival rate, the concentration of malonaldehyde (MDA), the density of superoxide dismutase (SOD), serum amylase (AMS) secretion rate and lactate dehydrogenase leakage rate in pancreatic acinar cell experiments, and measured serum AMS, MDA and SOD, IL-6, IL-10, and TNF-α levels, intestinal mucosa injury scores and proliferating cells of small intestinal mucosa at various time points in animal experiments. The results are interesting and suggest that MSCs could relieve injury to pancreatic acinar cells in rats with SAP, attenuate inflammation and injury in the small intestinal epithelium, promote the proliferation of enteric epithelium and repair of the mucosa. It is believable that MSCs infusion might be a promising treatment method for AP or SAP.
Footnotes
Supported by Health and Medicine Scientific Research Foundation of Nanjing Military Area Command, No. 08Z029
Peer reviewer: Dr. Lucia Ricci Vitiani, Department of Hematology, Oncology and Molecular Medicine, Istituto Superiore di Sanità, Viale Regina Elena, 299, 00161 Rome, Italy
S- Editor Shi ZF L- Editor A E- Editor Xiong L
References
- 1.Bruno M. [Minimally invasive treatment for acute pancreatitis] Ned Tijdschr Geneeskd. 2010;154:A2131. [PubMed] [Google Scholar]
- 2.Mifkovic A, Skultety J, Pindak D, Pechan J. Specific aspects of acute pancreatitis. Bratisl Lek Listy. 2009;110:544–552. [PubMed] [Google Scholar]
- 3.Cappell MS. Acute pancreatitis: etiology, clinical presentation, diagnosis, and therapy. Med Clin North Am. 2008;92:889–923, ix-x. doi: 10.1016/j.mcna.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 4.Rychter JW, van Minnen LP, Verheem A, Timmerman HM, Rijkers GT, Schipper ME, Gooszen HG, Akkermans LM, Kroese AB. Pretreatment but not treatment with probiotics abolishes mouse intestinal barrier dysfunction in acute pancreatitis. Surgery. 2009;145:157–167. doi: 10.1016/j.surg.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 5.Friedenstein AJ, Chailakhyan RK, Gerasimov UV. Bone marrow osteogenic stem cells: in vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet. 1987;20:263–272. doi: 10.1111/j.1365-2184.1987.tb01309.x. [DOI] [PubMed] [Google Scholar]
- 6.Hanson SE, Gutowski KA, Hematti P. Clinical applications of mesenchymal stem cells in soft tissue augmentation. Aesthet Surg J. 2010;30:838–842. doi: 10.1177/1090820X10386364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hagiwara M, Shen B, Chao L, Chao J. Kallikrein-modified mesenchymal stem cell implantation provides enhanced protection against acute ischemic kidney injury by inhibiting apoptosis and inflammation. Hum Gene Ther. 2008;19:807–819. doi: 10.1089/hum.2008.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tögel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289:F31–F42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- 9.Iyer SS, Rojas M. Anti-inflammatory effects of mesenchymal stem cells: novel concept for future therapies. Expert Opin Biol Ther. 2008;8:569–581. doi: 10.1517/14712598.8.5.569. [DOI] [PubMed] [Google Scholar]
- 10.Imberti B, Morigi M, Tomasoni S, Rota C, Corna D, Longaretti L, Rottoli D, Valsecchi F, Benigni A, Wang J, et al. Insulin-like growth factor-1 sustains stem cell mediated renal repair. J Am Soc Nephrol. 2007;18:2921–2928. doi: 10.1681/ASN.2006121318. [DOI] [PubMed] [Google Scholar]
- 11.Chen S, Liu Z, Tian N, Zhang J, Yei F, Duan B, Zhu Z, Lin S, Kwan TW. Intracoronary transplantation of autologous bone marrow mesenchymal stem cells for ischemic cardiomyopathy due to isolated chronic occluded left anterior descending artery. J Invasive Cardiol. 2006;18:552–556. [PubMed] [Google Scholar]
- 12.Neuhuber B, Timothy Himes B, Shumsky JS, Gallo G, Fischer I. Axon growth and recovery of function supported by human bone marrow stromal cells in the injured spinal cord exhibit donor variations. Brain Res. 2005;1035:73–85. doi: 10.1016/j.brainres.2004.11.055. [DOI] [PubMed] [Google Scholar]
- 13.Sémont A, François S, Mouiseddine M, François A, Saché A, Frick J, Thierry D, Chapel A. Mesenchymal stem cells increase self-renewal of small intestinal epithelium and accelerate structural recovery after radiation injury. Adv Exp Med Biol. 2006;585:19–30. doi: 10.1007/978-0-387-34133-0_2. [DOI] [PubMed] [Google Scholar]
- 14.Jiang H, Qu L, Li Y, Gu L, Shi Y, Zhang J, Zhu W, Li J. Bone marrow mesenchymal stem cells reduce intestinal ischemia/reperfusion injuries in rats. J Surg Res. 2011;168:127–134. doi: 10.1016/j.jss.2009.07.035. [DOI] [PubMed] [Google Scholar]
- 15.Kitagawa M, Williams JA, De Lisle RC. Amylase release from streptolysin O-permeabilized pancreatic acini. Am J Physiol. 1990;259:G157–G164. doi: 10.1152/ajpgi.1990.259.2.G157. [DOI] [PubMed] [Google Scholar]
- 16.Su KH, Cuthbertson C, Christophi C. Review of experimental animal models of acute pancreatitis. HPB (Oxford) 2006;8:264–286. doi: 10.1080/13651820500467358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg. 1970;101:478–483. doi: 10.1001/archsurg.1970.01340280030009. [DOI] [PubMed] [Google Scholar]
- 18.Jung KH, Song SU, Yi T, Jeon MS, Hong SW, Zheng HM, Lee HS, Choi MJ, Lee DH, Hong SS. Human bone marrow-derived clonal mesenchymal stem cells inhibit inflammation and reduce acute pancreatitis in rats. Gastroenterology. 2011;140:998–1008. doi: 10.1053/j.gastro.2010.11.047. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki Y, Lu Q, Xu DZ, Szabó C, Haskó G, Deitch EA. Na+,K+-ATPase activity is inhibited in cultured intestinal epithelial cells by endotoxin or nitric oxide. Int J Mol Med. 2005;15:871–877. [PubMed] [Google Scholar]
- 20.Sakaguchi S, Furusawa S. Oxidative stress and septic shock: metabolic aspects of oxygen-derived free radicals generated in the liver during endotoxemia. FEMS Immunol Med Microbiol. 2006;47:167–177. doi: 10.1111/j.1574-695X.2006.00072.x. [DOI] [PubMed] [Google Scholar]
- 21.Zhang G, Qin L, Sheng H, Wang XL, Wang YX, Yeung DK, Griffith JF, Yao XS, Xie XH, Li ZR, et al. A novel semisynthesized small molecule icaritin reduces incidence of steroid-associated osteonecrosis with inhibition of both thrombosis and lipid-deposition in a dose-dependent manner. Bone. 2009;44:345–356. doi: 10.1016/j.bone.2008.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lanza C, Morando S, Voci A, Canesi L, Principato MC, Serpero LD, Mancardi G, Uccelli A, Vergani L. Neuroprotective mesenchymal stem cells are endowed with a potent antioxidant effect in vivo. J Neurochem. 2009;110:1674–1684. doi: 10.1111/j.1471-4159.2009.06268.x. [DOI] [PubMed] [Google Scholar]
- 23.Cassatella MA, Mosna F, Micheletti A, Lisi V, Tamassia N, Cont C, Calzetti F, Pelletier M, Pizzolo G, Krampera M. Toll-like receptor-3-activated human mesenchymal stromal cells significantly prolong the survival and function of neutrophils. Stem Cells. 2011;29:1001–1011. doi: 10.1002/stem.651. [DOI] [PubMed] [Google Scholar]
- 24.Huang Y, Kucia M, Hussain LR, Wen Y, Xu H, Yan J, Ratajczak MZ, Ildstad ST. Bone marrow transplantation temporarily improves pancreatic function in streptozotocin-induced diabetes: potential involvement of very small embryonic-like cells. Transplantation. 2010;89:677–685. doi: 10.1097/TP.0b013e3181c9dc7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Isik AT, Mas MR, Yamanel L, Aydin S, Comert B, Akay C, Erdem G, Mas N. The role of allopurinol in experimental acute necrotizing pancreatitis. Indian J Med Res. 2006;124:709–714. [PubMed] [Google Scholar]
- 26.Jung KH, Hong SW, Zheng HM, Lee HS, Lee H, Lee DH, Lee SY, Hong SS. Melatonin ameliorates cerulein-induced pancreatitis by the modulation of nuclear erythroid 2-related factor 2 and nuclear factor-kappaB in rats. J Pineal Res. 2010;48:239–250. doi: 10.1111/j.1600-079X.2010.00748.x. [DOI] [PubMed] [Google Scholar]
- 27.Romics L, Szabo G, Coffey JC, Wang JH, Redmond HP. The emerging role of toll-like receptor pathways in surgical diseases. Arch Surg. 2006;141:595–601. doi: 10.1001/archsurg.141.6.595. [DOI] [PubMed] [Google Scholar]
- 28.Zhang D, DU X, Geng SX, Weng JY, Xing HZ, Lu ZS, Lin QX. [Role of nitro oxide in immunosuppressive effect of human mesenchymal stem cells on allogenic proliferative response of lymphocytes] Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2009;17:1273–1277. [PubMed] [Google Scholar]
- 29.Weil BR, Markel TA, Herrmann JL, Abarbanell AM, Meldrum DR. Mesenchymal stem cells enhance the viability and proliferation of human fetal intestinal epithelial cells following hypoxic injury via paracrine mechanisms. Surgery. 2009;146:190–197. doi: 10.1016/j.surg.2009.03.031. [DOI] [PubMed] [Google Scholar]
- 30.Gupta N, Su X, Popov B, Lee JW, Serikov V, Matthay MA. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol. 2007;179:1855–1863. doi: 10.4049/jimmunol.179.3.1855. [DOI] [PubMed] [Google Scholar]
- 31.Kudo K, Liu Y, Takahashi K, Tarusawa K, Osanai M, Hu DL, Kashiwakura I, Kijima H, Nakane A. Transplantation of mesenchymal stem cells to prevent radiation-induced intestinal injury in mice. J Radiat Res. 2010;51:73–79. doi: 10.1269/jrr.09091. [DOI] [PubMed] [Google Scholar]
- 32.Leung PS, Ip SP. Pancreatic acinar cell: its role in acute pancreatitis. Int J Biochem Cell Biol. 2006;38:1024–1030. doi: 10.1016/j.biocel.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Bhatia M, Wong FL, Cao Y, Lau HY, Huang J, Puneet P, Chevali L. Pathophysiology of acute pancreatitis. Pancreatology. 2005;5:132–144. doi: 10.1159/000085265. [DOI] [PubMed] [Google Scholar]
- 34.Liu ZH, Peng JS, Li CJ, Yang ZL, Xiang J, Song H, Wu XB, Chen JR, Diao DC. A simple taurocholate-induced model of severe acute pancreatitis in rats. World J Gastroenterol. 2009;15:5732–5739. doi: 10.3748/wjg.15.5732. [DOI] [PMC free article] [PubMed] [Google Scholar]