Abstract
Induction of terminal differentiation of neoplastic cells offers potential for a novel approach to cancer therapy. One of the agents being investigated for this purpose in preclinical studies is 1,25-dihydroxyvitamin D3 (1,25D), which can convert myeloid leukemia cells into normal monocyte-like cells, but the molecular mechanisms underlying this process are not fully understood. Here, we report that 1,25D upregulates the expression of hKSR-2, a new member of a small family of proteins that exhibit evolutionarily conserved function of potentiating ras signaling. The upregulation of hKSR-2 is direct, as it occurs in the presence of cycloheximide, and occurs primarily at the transcriptional level, via activation of vitamin D receptor, which acts as a ligand-activated transcription factor. Two VDRE-type motifs identified in the hKSR-2 gene bind VDR-RXR alpha heterodimers present in nuclear extracts of 1,25D-treated HL60 cells, and chromatin immunoprecipitation assays show that these VDRE motifs bind VDR in 1,25D-dependent manner in intact cells, coincident with the recruitment of RNA polymerase II to these motifs. Treatment of the cells with siRNA to hKSR-2 reduced the proportion of the most highly differentiated cells in 1,25D-treated cultures. These results demonstrate that hKSR-2 is a direct target of 1,25D in HL60 cells, and is required for optimal monocytic differentiation.
Keywords: KSR, Vitamin D, vitamin D receptor, si RNA, ras-signaling, differentiation
INTRODUCTION
Cell fate is regulated by developmental, intrinsic signals, and by an integration of environmental cues. The latter are transmitted to the nucleus either by sequential protein-protein interactions (such as the MAPK pathway) that are initially activated by cell surface events, or by a direct activation by a ligand of proteins, such as steroid receptors, that can function as ligand-activated nuclear transcription factors.
However, these modes of gene activation are not necessarily mutually exclusive. For instance in the case of 1,25D-induced monocytic differentiation of myeloid leukemia cells, 1,25D activates the vitamin D receptor (VDR), which then heterodimerizes with one of the isoforms of retinoid X receptor (RXR), usually the alpha isoform [1–3]. The heterodimer then binds to its cognate DNA sequences known as vitamin D response elements (VDREs), and induces the expression of 1,25D-responsive genes [1, 4]. Many genes known to respond to 1,25D-activated VDR are implicated in the regulation of calcium homeostasis or the degradation of 1,25D [5–9]. One of the exceptions is the gene which encodes kinase suppressor of Ras 1 (KSR-1) [10, 11], a primarily membrane-associated protein, that enhances the activity of ras-induced MAPK pathways [12–14]. Thus, we have suggested that the pleiotropic effects of 1,25D [15, 16] that result in monocytic differentiation of myeloid leukemia cells are, at least in part, mediated by modification by KSR-1 of membrane-associated signals to the MAPK pathways, which complement the initial activation of gene transcription in the nucleus [16–18].
KSR-1 has an interesting, if rather controversial, relationship to MAPK pathways [14, 19, 20]. Although originally described as a protein kinase [13, 21–24], the prevailing opinion is that KSR-1 functions as a scaffold which facilitates signal transduction from the cell membrane through the Raf-MEK-ERK kinase cascade to nuclear transcription factors [12, 25, 26]. Given that the intensity and the duration of ras-initiated signals impinging on the transcriptional apparatus in the nucleus may determine cell fate, ranging from accelerated proliferation to terminal differentiation ( e.g. [27, 28] ), modulation of these signals by KSR-1 may substantially contribute to cell fate determination. Indeed, we have previously shown that KSR-1 amplifies the signals for monocytic differentiation initiated by low, near physiological, concentrations of 1,25D [29], which is associated with increased phosphorylation of Raf-1 and p90RSK-1 [30]. Curiously, in this system the “classical” Raf-1 directed MAPK pathway becomes modified in the later stages of differentiation to bypass the MEK-ERK module while Raf-1 and p90RSK-1 continue to be activated [30]. Importantly, KSR-1 was shown to be a direct target of 1,25D-activated VDR, via a consensus VDRE motif upstream from the KSR-1 gene [17], providing a mechanistic link between 1,25D and the modulation of the MAP pathways, and thus of the events that lead to the monocytic phenotype.
A second KSR family gene, ksr-2, was first reported in C. elegans and shown to be required for ras-mediated signaling in germline meiosis, but to function redundantly with ksr-1 in the development of the excretory and genital systems [31]. Mechanistically, it was found that ksr-1 and ksr-2 are jointly required for ERK phosphorylation in C. elegans soma, suggesting that both KSR proteins act to promote the activation or maintenance of the Raf/MEK/ERK kinase cascade in this species. The human homolog, hKSR-2, has also been identified , with 66 % nucleotide and 61% amino acid identify with human KSR-1 [32]. Like the C. elegans ksr-2, hKSR-2 appears to be lacking the N-terminal conserved area 1 (CA1) [32], which is unique to the KSR proteins [10, 19]. However, in contrast to the C. elegans ksr-2, hKSR-2 is reported to be a negative regulator of the ERK pathway, attributed to its inhibitory interactions with the upstream MAPK regulators Tpl2/Cot-1 and MEKK3 [32, 33].
As part of our continuing efforts to unravel the signaling network through which 1,25D induces terminal differentiation of malignant human cells (e.g. [34–36]), we examined if hKSR-2 is expressed and regulated by 1,25D in a model of human myeloid leukemia, the HL60 cells. We found this to be the case, and that the two DR3-type VDREs in the hKSR-2 promoter regions can provide dual targets for vitamin D receptor to directly upregulate the expression of hKSR-2 gene.
Materials and Methods
Reagents
1,25D was a gift from Dr. Milan Uskokovic (Bio Xell, Inc., Nutley, NJ). ZK159222 was a gift from Schering AG (Berlin, Germany). RNA synthesis inhibitor actinomycin D and protein synthesis inhibitor cycloheximide were purchased from Sigma (St. Louis, MO).
Cell Culture
Human leukemia HL60-G cells, a subclone of cells originally derived from a patient with promyeloblastic leukemia [37, 38], were cultured at 37°C and 5% CO2 in RPMI 1640 medium (Sigma) supplemented with 10% heat-inactivated, iron-enriched bovine calf serum (HyClone, Logan, UT). Cell cultures were passaged every 2 to 3 days and screened routinely for Mycoplasma contamination. For experiments, the cells were seeded at 3 × 105 cells/mL in fresh medium. In specified experiments inhibitors were added to the cultures 1 h before the exposure to 1,25D.
Determination of markers of differentiation
To monitor 1,25D-induced differentiation, aliquots of 106 cells were harvested, washed twice with phosphate buffered saline (PBS), and suspended in 100 μL PBS. The cell suspensions were incubated for 45 minutes at room temperature with 0.5 μL MY4-RD-1 and 0.5 μL MO1-FITC (1:20 dilution of the stock antibodies) to analyze the expression of surface cell markers CD14 and CD11b, respectively [39]. The cells were then washed three times with ice-cold PBS, and resuspended in 1 mL PBS. Two-parameter analysis was performed using a FACSCalibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ). Isotypic mouse IgG1 was used to set threshold parameters. In addition, monocytic phenotype was confirmed by cytochemical demonstration of the cytoplasmic non-specific esterase (NSE) characteristic of the monocyte in hematopoietic cells, as described before [39].
Polymerase Chain Reactions (PCR)
Semi-quantitative measurements of hKSR-2, IMP and β-actin mRNA levels were carried out as described before [29]. Briefly, total RNA was extracted using RNeasy Kit, treated with DNase I, reverse transcribed and amplified by using Eppendorf gradient mastercycler using 35 cycles of amplification. The primer sequences used were hKSR-2: upstream primer (5-CCGACACAGAGGAGGATAAG-3), downstream primer (5- TTCAAAGGCCCAGCAGAAG-3); IMP: upstream primer (5-TCCAAACAGCTTCCAGACC-3), downstream primer (5-AAAAACTATCCGCATCAGCC-3); β-actin, upstream primer (5-TGACGGGGTCACCCACACTGTGCCCAGCTA-3), and downstream primer (5-CTAGAAGCATTTGCCGGTGGACGATGGAGGG-3). The RT-PCR products were separated in 2% agarose gels containing ethidium bromide (1 μg/mL). The intensities of signals of hKSR-2, IMP and β-actin bands were scanned and measured using Image QuaNT program (Molecular Dynamics, Sunnyvale, CA).
Real-time PCR was carried out by using a lightcycler with Faststart DNA SYBR Green PCR kit (Roche Diagnostics, Indianapolis, IN). cDNA was synthesized by using 1 μg DNase I-treated total RNA. Reverse transcription conditions were as follows: 42°C for 15 min, 95°C for 5 min and 5°C for 5 min (one cycle). Real time PCR was performed following the protocol provided by the manufacturer. Preincubation at 95°C for 7 min was followed by 40 cycles of 95°C for 10 s, 55°C for 10 s and 72°C for 10 s. mRNA-fold changes in hKSR-2 target gene relative to the RNA polymerase II control were calculated by relative quantification analysis. Primers used for real-time PCR were: KSR1: upstream 5’-AGCAAGTCCCATGAGTCTCA-3’), downstream 5’-CAACCTGCAATGCTTGCACT-3’, hKSR-2: same as for semi-quantitative procedure upstream primer 5-CCGACACAGAGGAGGATAAG -3, downstream primer 5-TTCAAAGGCCCAGCAGAAG-3; RNA Pol II: upstream 5-GCACC ACGTCCAATGACAT-3, downstream 5-GTGCGGCTGCTTCCATAA-3. Threshold cycle (Ct) is the cycle number where the fluorescence increases above a background threshold level. Relative expression levels were calculated by equation: . Ct value for KSR-1 is approximately 25 cycles and hKSR-2 approximately 27 cycles, so we set real time PCR reaction to 40 cycles. The quality of PCR product was monitored using post-PCR melting curve analysis.
Western Blot Analysis
The cells were harvested and washed twice with ice-cold 1 x PBS and whole cell extracts were prepared for immunoblotting. The washed cell pellets were solubilized with a lysis buffer containing 20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM sodium β-glycerophosphate, 1 mM Na3VO4, 1 mM PMSF, 1 μg/ml leupeptin, 1 μg/ml aprotinin, followed by centrifugation at 12,000g for 5 minutes. The protein concentrations of the extracts were determined using the Bio-Rad protein assay kit. Equal amounts of 3 × SDS sample buffer containing 150 mM Tris-HCl, pH 6.8, 30% glycerol, 3% SDS, 1.5 mg/ml bromophenol blue dye, and 100 mM dithiothreitol were then added to each sample. Whole cell extracts (40 μg of protein in each lane) were separated on 10% SDS-PAGE gels and transferred to nitrocellulose membranes (Amersham Pharmacia Biotech, Piscataway, NJ). The membranes were blocked with 5% milk in TBS/0.1% Tween-20 for 1 h, subsequently blotted with either KSR-1 (N-19, goat polyclonal antibody, Santa Cruz Biotechnology Inc, Santa Cruz, CA) or KSR-2 antibody (supplied by Affinity Bioreagents, Golden, CO) , and then the membranes were blotted with a horseradish-linked secondary antibody for 1 h. The protein bands, which migrated at approximately 90Kd (Fig 3), similar to the calculated MW of 93KD, were visualized with a chemiluminescence assay system (Amersham). The protein loading of the gel and efficiency of the transfer were monitored by stripping the membrane and reprobing for Crk-L (C-20, rabbit polyclonal antibody, Santa Cruz Biotechnology Inc), a constitutively expressed protein in HL60 cells.
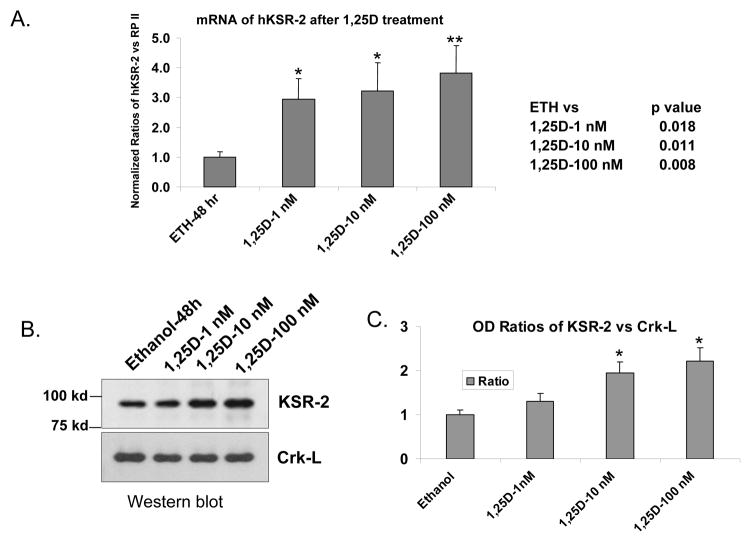
Figure 3.
1,25D increases cellular levels of hKSR-2 mRNA and protein in a concentration dependent manner. (A) HL60 cells were treated for 48 hours with the indicated concentrations of 1,25D, and hKSR-2 mRNA was determined by quantitative RT- qPCR. (B) hKSR-2 protein levels shown by Western blotting. (C) The ratios of optical density of KSR-2 to internal control Crk-L of Westerns illustrated in panel B. Means +/− SD, n=3. * indicates p < 0.05 compared to the vehicle control.
Electrophoretic Mobility Shift Assays (EMSA)
The nuclear extracts used for gel mobility shift assays were prepared as described before [40]. All steps were performed at 4°C. Briefly, 107 cells were harvested, washed twice with PBS, and resuspended in 0.2 ml of cell extraction buffer (10 mM HEPES-KOH, pH 7.9, 1.5 mM MgCl2, 10 mM KC1, 0.5 mM dithiothreitol (DTT), 0.2 mM phenylmethylsulfonyl fluoride (PMSF) and 10 μg/ml aprotinin). The cells were kept on ice for 10 min, vortexed for 10 seconds, and centrifuged at 16,000 g for 30 s. The pellet was resuspended in 30 μl of nuclear extraction buffer (20 mM HEPES-KOH, pH 7.9, 25% glycerol, 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM DTT, and 0.2 mM PMSF and 10 μg/ml aprotinin), placed on ice for 20 min, and centrifuged at 16,000 g for 2 min. The supernatant was saved as the nuclear extract and used for gel shift assay. Double-stranded oligonucleotides from promoter regions of hKSR-2 gene containing the putative hKSR-2 VDRE1 (-2521 to -2498 relative to the transcription start site, GAGCTCAGTTCAgcatGGTCAACA ), putative hKSR-2 VDRE2 (3180 to 3203 relative to the transcription start site, CTCCTGGGTTCAaacAGTTCTCCT), and mutant oligonucleotides with a underlined “GT”→“CA” substitution in the 5’ half element of the VDRE binding motif, were synthesized by the Molecular Resource Facility of the NJ Medical School. The oligonucleotides were 5’-end labeled using T4 polynucleotide kinase in the presence of [γ-32P]-ATP (Perkin Elmer, Shelter, CT). Ten μg of nuclear extracts were incubated with 50 pg (approximately 50,000 cpm) of 32P-labeled double-stranded oligonucleotide for 30 min at room temperature. Specificity of the VDRE binding was determined by competition with either a 50 x molar excess of the unlabeled double stranded VDRE nucleotide, or mutant VDRE double-stranded nucleotide added to parallel samples before the incubation period. Gel shift blocking assays were performed using antibodies against VDR or RXR isoforms purchased from Santa Cruz Biotechnology Inc. VDR (C-20) and RXRα (D-20) are concentrated forms suitable for gel shift analysis; c-fos antibody (D-1, Santa Cruz) was used as an irrelevant control. The samples were separated on 6% polyacrylamide gels under non-denaturing conditions with a constant current of 22 mA, for 3 h at 4°C. The gels were then dried and set up for autoradiography.
Chromatin immunoprecipitation (ChIP) assays
ChIP assays were performed essentially as described [41] with HL60 cell lysates immunoprecipitated with either normal rabbit IgG or anti-VDR (C-20) rabbit polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA). PCR amplifications were performed with primers: hKSR-2 VDRE1 (-2721/-2431), 5’: 5’-TCAAGTGCCACTTGTGGGGGC-3’, 3’: 5’-TCAGGTACCTGCAG GTCAGG-3’; hKSR-2 VDRE2 (+3242/+3413) 5’: 5’-TGCGACCTCGGCTCATTGTA-3’, 3’: 5’-GGTGTGATGGCTCACACTTG-3’; a control sequence without a VDRE motif 2 kb upstream from hKSR-2 VDRE1 (-4187/-4487) 5’: 5’-TAGTCCAGCGCTGTCCAGTG-3’, 3’: 5’-GGATCACAG GCATGAGCCAC-3’.
Transfection of small interfering (si) RNA
We designed double-stranded 21-mer siRNA targeting hKSR-2 at the sequence 5'-AAUGUCCACAUGGUCAGCACC-3' (3068-3088). Non-targeting siControl supplied by Dharmacon Corp.( Chicago, IL) was used as a negative control for siKSR-2. The siRNAs were transfected into HL60 cells using Amaxa nucleofector (Gaithersburg, MD) and incubated for 48 hrs before adding 1,25D.
Statistical Methods
All experiments were repeated at least three times. Significance of differences between mean values was assessed by student T-test. All computations were performed using an IBM personal computer using Microsoft EXCEL + ANALYSE-IT Program.
RESULTS
Expression of hKSR-2 is regulated by 1,25D in time and concentration-dependent manner
Currently there is only scanty information on the expression of hKSR-2 in human cells. The cDNA clones used for the identification of the predicted gene were generated from human testis cDNA library, studied in HEK-293T, HeLa and RAW 2647 cells lines, and Northern blots revealed that hKSR-2 is mainly expressed in mouse brain and kidney [32]. Since its expression in hematopoietic cells has not been previously reported, we investigated if hKSR-2 expression can be detected in HL60 cells, a model differentiation system, and if so, whether it is modulated by 1,25D, as we previously showed to be the case for the other KSR family member, KSR-1 [17]. Indeed, as Fig 1A, B and C demonstrates by semi-quantitative (panels A and B) and real-time RT-PCR (panel C), expression of hKSR-2 can be detected in cells not exposed to 1,25D, and the levels of the mRNA increase in the presence of 1,25D, reaching after 48 hours approximately 4-6 fold higher levels than in the vehicle treated cells. The semi-quantitative illustration of the results of this experiment (panel A) is validated by the quantitative real time procedure, and also shows that the expression of IMP (Impedes Mitogenic signal Propagation) gene, the protein product of which is implicated in the control of KSR-1 stability [42, 43], is not altered by the exposure of HL60 cells to 1,25D (Fig 1, A and B).
Figure 1.
1,25D increases hKSR-2 gene expression and differentiation of HL60 cells in a time-dependent manner. (A) HL60 cells were treated with 1 nM 1,25D for the indicated times, and the mRNA levels of hKSR-2 were determined by semi-quantitative RT-PCR. Note that while hKSR-2 transcript levels were increased after 6 h, mRNA levels of mRNA IMP, a negative regulator of KSR, were not significantly influenced by 1,25D. Determination of β-actin mRNA was used as the housekeeper gene control. (B) Quantitation of the experiments illustrated in panel A. The ratios of optical density of each gene mRNA to β-actin signal are shown in this bar chart. (C) Quantitative RT-qPCR supports the results obtained with semi-quantitative results summarized in panel B. (D) 1,25D-induced differentiation monitored by non-specific esterase staining. All bar charts show the mean +/− SD, n=3. * indicates p < 0.01 compared to the vehicle control.
The upregulation of hKSR-2 mRNA expression correlated with the induction by 1,25D of the monocytic phenotype, as shown here by the presence of NSE, an esterase typical of mature monocytes [44] ,which continued to increase during the period of observation (Fig 1D).
Western analysis (Fig 2, A and B) showed that 1,25D-induced hKSR-2 protein level increases are similar to, though slightly less marked than the increases in hKSR-2 mRNA, suggesting that expression of hKSR-2 is regulated by 1,25D principally at the transcriptional level. The regulation by 1,25D was further demonstrated when the expression of hKSR-2 was determined at both mRNA (Fig 3A) and protein levels (Fig 3B) at increasing concentrations of 1,25D and in a time-dependent manner, though lagging somewhat behind the upregulation of KSR-1 (Table 1). Together, these results clearly show that, similar to KSR-1, the expression of hKSR-2 can be regulated by 1,25D.
Figure 2.
1,25D increases hKSR-2 protein levels of HL60 cells in a time-dependent manner. (A) A Western blot showing the time-course of 1,25D-induced (1 nM) upregulation of hKSR-2 protein levels. (B) Quantitation of the experiments illustrated in panel B. All bar charts show the mean +/− SD, n=3. * indicates p < 0.05 compared to the vehicle control.
Table 1.
Comparison of the kinetics of increases in mRNA and protein levels of KSRs after exposure of HL60 cells to 1 nM 1,25D.
| mRNA | Protein | |||
|---|---|---|---|---|
| Treatment | KSR-1 | hKSR-2 | KSR-1 | hKSR-2 |
| Ethanol | 1.0 ± 0.2 | 1.0 ± 0.3 | 1.0 ± 0.2 | 1.0 ± 0.1 |
| 1,25D-6 h | 1.9 ± 0.2 | 1.4 ± 0.2 | 2.3 ± 0.5 | 1.7 ± 0.4 |
| 1,25D-12 h | 2.7 ± 0.5 | 2.2 ± 0.5 | 3.3 ± 0.3 | 2.2 ± 0.4 |
| 1,25D-24 h | 4.7 ± 0.5 | 3.1 ± 0.4 | 4.6 ± 0.9 | 2.4 ± 0.6 |
| 1,25D-48 h | 7.0 ± 1.1 | 4.1 ± 0.7 | 6.2 ± 0.8 | 2.9 ± 0.5 |
Note: The mRNA ined levels by quantitative real time PCR andof KSRs were determ normalized ratios relative to the RNA polymerase II control were ls of KSRs quantification were determined by westernanalysis. The protein leve blotting and scanned by Imagequant program.
1,25D directly induces transcription of the hKSR-2 gene
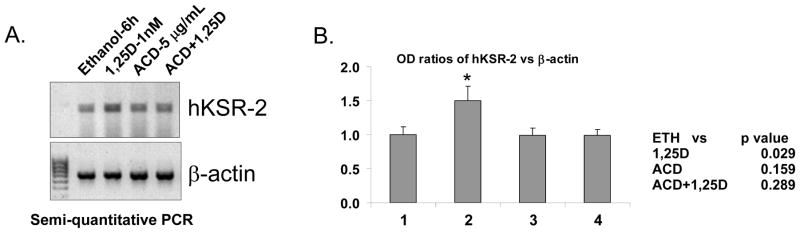
The results presented above showed that the exposure of HL60 cells to 1,25D increased cellular levels of hKSR-2 mRNA (Fig 2 and Fig 3). This can be due to an increased rate of transcription, or to mRNA stabilization. To distinguish between these possibilities we pretreated HL60 cells with high concentration of actinomycin D (ACD) which inhibits RNA polymerase II [45], and found that ACD blocked the 1,25D-induced increase in hKSR-2 mRNA (Fig 4). In this experiment the 1,25D/ACD exposure lasted only 6 hrs, as at 5 μg/ml ACD causes general cell toxicity at longer times. therefore no decreases in basal levels of hKSR-2 were seen in cells treated with ACD, indicating that hKSR-2 transcripts have a relatively long half life.
Figure 4.
Actinomycin D inhibits 1,25D-induced transcription of hKSR-2 mRNA. (A) HL60 cells were pretreated for 1hr with a high concentration of actinomycin D, an RNA synthesis inhibitor, then incubated for 6 hr in the presence or absence of 1,25D. The mRNA levels of hKSR-2 were determined by semi-quantitative RT-PCR. (B) The ratios of optical density of hKSR-2 to β-actin of the signals shown in panel A. Means +/− SD, n = 3. * indicates p < 0.05 compared to the vehicle control.
We also measured the ability of 1,25D to upregulate the expression of hKSR-2 when protein synthesis was inhibited by cycloheximide (CHX) at concentrations that were high but subtoxic at 6 h, and marginally toxic at 24 h, as determined by trypan blue exclusion (Fig 5A) . We first performed semi-quantitative RT-PCR experiments, as these can be illustrated by primary data (Fig 5A), and followed these with real-time RT-PCR determinations, as these provide more precise data (Fig 5B). It is evident in both parts of Fig 5 that the 1,25D-induced increase in hKSR-2 mRNA is not reduced by the presence of CHX, but the level of hKSR-2 mRNA actually somewhat increases in the presence of CHX (Fig 5B, columns 2 and 6), a phenomenon known as “superinduction” [e.g. ref 46]. A further increase in the levels of hKSR-2 mRNA was noted when CHX and 1,25D were present together. This indicates that regulation by 1,25D of hKSR-2 gene expression does not require de novo protein synthesis, and implies that hKSR-2 is a primary response gene for 1,25D [47]. Our data therefore show that upregulation of hKR-2 occurs primarily at transcriptional level and is direct.
Figure 5.
KSR-2 is a direct gene target of 1,25D. (A) HL60 cells were pretreated for 1 hr with 200 μg/mL cycloheximide, a protein synthesis inhibitor, prior to the induction to differentiation with 1 nM 1,25D for the indicated times. The levels of hKSR-2 mRNA were determined by semi-quantitative RT-PCR. Note that hKSR-2 transcripts were superinduced by CHX. Viabilities of the cells were determined by trypan blue exclusion to monitor cytotoxicity of CHX and are shown below each lane. (B) The levels of hKSR-2 mRNA determined by quantitative RT-qPCR were normalized to vehicle (ethanol) treated cells. Means +/- SD, n = 3. * indicates p< 0.05 compared to the untreated control.
The expression of hKSR-2 is inhibited by ZK 159222 (ZK), an antagonist of VDR transcriptional activity
Exposure of HL60 cells to ZK, a synthetic analog of 1,25D, has been shown to inhibit 1,25D-induced differentiation of HL60 cells [17]. We demonstrate here that the 1,25D-induced upregulation of hKSR-2 expression is also inhibited by this analog (Fig 6). This provides evidence that nuclear VDR mediates these effects of 1,25D, since ZK has been shown to compete with 1,25D for VDR binding, and inhibit the interaction of the liganded VDR with transcriptional coregulators when present in large excess (100-fold) over 1,25D [48].
Figure 6.
The VDR antagonist ZK159222 inhibits 1,25D-induced expression of KSR-2 gene in parallel with its inhibition of monocytic differentiation. (A) HL60 cells were pretreated for 1 hour with 100 nM ZK159222 (ZK) before adding 1,25D for the indicated times. The ratios of optical density of each RNA band to β-actin are shown underneath each panel. (B) ZK inhibited hKSR-2 differentiation induced by 1,25D in parallel with the inhibition of hKSR-2 expression. Mean values +/− SD, n = 3. * indicates p < 0.05 compared to the untreated control. The actual p values for key groups are shown below the bar graph.
Vitamin D response element (VDRE) motifs in the vicinity of hKSR-2 gene can bind VDR/RXR heterodimers in vitro
In a genome-wide response element screen, two motifs, designated hKSR-2 VDRE1 and hKSR-2 VDRE2, corresponding to DR3-type VDREs, each containing only a single nonconcensus nucleotide substitution, were identified at positions - 2501 (hKSR-2 VDRE1) and +3185 (hKSR-2 VDRE2) relative to the transcription start site of the hKSR-2 gene [41]. We used these double stranded sequences to perform electrophoretic mobility shift analyses (EMSA) designed to determine if these motifs can bind VDR complexes, and if so, the nature of these complexes. We found that the hKSR-2 VDRE1 sequence binds complexes which are most likely VDR-RXR alpha heterodimers, as shown by the block of binding to the oligonucleotide probe by antibodies to VDR or RXR alpha, but not by an irrelevant antibody to c-fos (Fig 7B, lane 5). The hKSR-2 VDRE2 sequence also bound VDR ( Fig 7C, lane 7), but with lower intensity than VDRE1, and the binding of RXR alpha was less clear (Fig 7C, lane 8). The specificity of binding was demonstrated by the efficient competition of probe binding by an unlabeled probe (Fig 7B, lane 3; Fig 7C, lane 5), but not when the competing probe was mutated in two positions (Fig 7B, lane 4, Fig 7C, lane 6). While these experiments do not prove the functionality of these VDRE elements, they strongly suggest that they can be recognized by the VDR.
Figure 7.
1,25D increases the in vitro binding of VDR and RXRα to the VDRE motifs in the promoter region of the hKSR-2 gene. (A) Schematic of two VDRE sequences at positions -2501 (hKSR-2 VDRE1) and +3185 (hKSR-2 VDRE2) relative to the transcription start site of the hKSR-2 gene. These sequences were used as double stranded oligonucleotides for gel shift mobility shift analyses. (B) Gel-shift analysis of hKSR-2 VDRE1 binding by proteins in nuclear extracts in HL60 cells treated for 48 h with 100 nM 1,25D, showing increased VDR and RXRα binding in the VDRE1 region. The specificity of VDRE binding was shown by its marked inhibition by a 50-fold excess of the unlabeled VDRE oligonucleotide, but not by an excess of the unlabeled mutated VDRE oligonucleotide. The mutated oligonucleotide did not bind nuclear proteins. Antibodies to VDR and RXRα were added to the reaction mixture for DNA binding blocking assay, and both antibodies blocked the VDRE binding, but the control antibody (to c-fos) did not block the binding . (C) Similar results were demonstrated using hKSR-2 VDRE2 oligonucleotide for the gel shift mobility assay, but the in vitro binding of VDR/RXR α was weaker to this DNA element, in contrast to in vivo binding (Fig 8).
Exposure to 1,25D increases binding of VDR and of RNA polymerase II to hKSR-2 VDRE1 and hKSR-2 VDRE2 in vivo
It has been previously reported that unliganded VDR can be found in cell nuclei prior to an exposure to 1,25D, and we found by ChIP assays that VDRs occupy some hKSR-2 VDRE1 and VDRE2 sites in proliferating HL60 cells (Fig 8B). This is consistent with the expression of hKSR-2 in cells not treated with 1,25D (Figs 1–6). However, exposure of the cells to 1,25D increases the VDR occupancy of VDRE1 and VDRE2 sites (Fig 8B), as well as of RNA polymerase II at these sites and at the transcription start site of the hKSR-2 gene (Fig 8C), consistent with their function as 1,25D-regulated enhancer elements. Importantly, the magnitude of the occupancy of VDR and RNA polymerase II, shown as in the table insert to Figure 8, is regulated by 1,25D in parallel, increasing 2–3 fold after exposure of the cells to 1,25D. Thus, we conclude that in HL60 cells induced to differentiate by 1,25D hKSR-2 is a primary response gene, and its expression is upregulated most likely via two novel VDRE motifs, one upstream and another downstream of the transcription start site.
Figure 8.
ChIP analysis of the effects of 1,25D on binding of VDR and RNA polymerase II in vivo to regions of the hKSR-2 gene containing VDRE motifs, and of RNA polymerase II to the transcription start site. DNA regions amplified are indicated in grey, and the positions of VDREs in black. Region 3 in the hKSR-2 gene contains no obvious VDRE motifs, and was amplified as a control for specificity of immunoprecipitation, while region 4 contains the transcription start site. The inset table shows OD quantitation of the signals shown in panels B and C.
Knock-down of hKSR-2 expression reduces the intensity of 1,25D-induced differentiation
To ascertain if hKSR-2 has a function in the process of cell differentiation we transfected siRNA to hKSR-2 into HL60 cells by electrophoresis, in parallel with control siRNA which does not affect gene expression. In cells subjected to electrophoresis with control siRNA hKSR-2 expression was increased by 1,25D, although to a lesser extent than in cells not subjected to the stress of electrophoresis, and the cells differentiated well when treated with 1 nM 1,25D. However, cells treated with siRNA to hKSR-2 had reduced levels of hKSR-2 protein (Fig 9B), and there was an absence of the most highly differentiated cells recognized by high expression of both CD14 and CD11b markers (Fig 9A). The lack of an effect of siRNA to hKSR-2 on the expression of KSR-1 and on the loading control protein Crk-L, demonstrated the specificity of this knock down (Fig 9B). Interestingly, although the number of CD14 positive cells was only slightly lower in the siRNA to hKSR-2 cultures than in the control transfection, the intensity of CD14 expression was clearly reduced, as shown by the preponderance of cells near the threshold on the vertical axis in Fig 9A. In contrast, CD11b positive cells were robustly reduced both in numbers and in staining intensity (Fig 9A). This demonstrates that hKSR-2 is particularly important for 1,25D-induced CD11b expression, and thus, is required for monocytic differentiation, apparently mostly in the late, advanced stages of this process.
Figure 9. Inhibition of hKSR-2 expression by silencing hKSR-2 RNA (siRaf-1) reduces the intensity of 1,25D-induced differentiation.
A. siRNAs were transfected into HL60 cells using Amaxa nucleofector and incubated for 48 hrs, then 1 nM 1,25D was added for 48 hrs. The cells were harvested to determine CD11b and CD14 differentiation markers. “siControl” is non specific siRNA used here as a control. B. Immunoblots for the KSR proteins after transfection with the different silencing RNAs showed that siRNAs effectively knocked down the target protein hKSR-2, but not KSR-1, or the loading control protein Crk-L. Optical densities of signals of the KSRs were determined by Imagequant software and are shown below each panel as normalized ratios to Crk-L.
DISCUSSION
This report , together with a previous study [17], shows that both known members of human KSR family are direct targets of 1,25D, a vitamin-hormone with differentiation-inducing properties in leukemia cells. The importance of these findings is that since both KSR proteins are also involved in evolutionarily conserved ras signaling [10–12, 31], we now have a plausible mechanistic explanation of what can initiate the previously described upregulation of MAPK pathways in leukemic cells exposed to 1,25D.
The upregulation of Raf/MEK/ERK MAPK pathway is already strongly linked to 1,25D-induced differentiation of a variety of myeloid leukemia cells. In human acute promyelocytic leukemia NB4 cells MAPK activity was observed within minutes after exposure to 1,25D and was considered to be the result of a direct membrane, non-genomic action of 1,25D [49]. An early, membrane-linked, sphingomyelinase-related effect of 1,25D was also noted in HL60 cells [50]. More prolonged, but not fully sustained ERK activation by 1,25D in HL60 cells was reported by several laboratories [51–53], and was considered to define the first phase of 1,25D-induced differentiation in this system [51]. Specifically, ERK 1/2 phosphorylation increased for the first 20 hours or so, then decreased to basal levels, while differentiation still continued to increase, to approximately 96 hours [51]. During this second period of induction of monocytic differentiation, other members of the MAPK cascades continue to show evidence of activation, and these include Raf-1, p90RSK-1, and JNK1/2 [30]. Our studies also showed that the activities of MEK-1 and ERK 1/2 were unlikely to be required for the later stages of differentiation, but the activity of Raf-1 was required [30].
It is also clear that the downstream kinases of MAPK pathways can activate nuclear transcription factors. For instance, p90RSK-1 can activate C/EBPβ [54], a transcription factor linked to the expression of the CD14 component of mature monocytes [55]. Also, JNK 1/2 kinases phosphorylate c-jun [56], a component of the AP-1 transcription factor shown in several studies to be required for 1,25D-induced monocytic differentiation [35, 57]. Thus, the downstream components of 1,25D signaling pathway seem clear, at least in outline.
The current focus of our studies is on the upstream components of this pathway. We have succeeded in showing that KSR-1 serves to amplify the differentiation signal provided by low, near-physiological concentrations of 1,25D in myeloid leukemia cells [29], presumably by modifying the intensity or branching of ras-related membrane events, in the background of basal stimulation by the growth factors present in the cell’s environment. However, the principal scaffolding model for KSR-1 function [58] fits only the initial steps in the 1,25D-induced differentiation, as phosphorylation of MEK-1 and ERK 1/2 was observed to be waning while Raf-1 phosphorylation continued to increase [29, 30, 51]. The data presented here suggest that hKSR-2 provides a function that is particularly essential for completion of cell differentiation, since the CD11bhi/CD14 hi subpopulation of differentiated cells was not present in cells subjected to hKSR-2 knock down (Fig 9). This is in keeping with the somewhat delayed induction of hKSR-2 following exposure to 1,25D (Fig 1, Table 1). The full elucidation of this function awaits additional, extensive, studies.
The two VDRE motifs identified in the hKSR-2 gene are not in the immediate vicinity of the transcription start site, and one (hKSR-2 VDRE2) lies within the long first intron. This is in keeping with the realization that DNA looping permits enhancer function over considerable distance (e.g. [59]), and that the presence of more than one binding site for a specific transcription factor favors a prominent response of the gene to the regulator [60].
To our knowledge, this is the first report of endogenous expression of the hKSR-2 product, a novel ras signaling-related protein, and its regulation in human cells. It also adds a new target gene to the relatively small repertoire of genes regulated by 1,25D that can reasonably be related to differentiation of malignant cells (e.g. [61-66]), and thus has implications for the approaches to the treatment of human cancer that are currently being investigated [67].
Acknowledgments
We thank Dr. M. Uskokovic for the gift of 1, 25-dihydroxyvitamin D3 and Dr. A. Steinmeyer for the gift of ZK159,222. This research was supported by USPHS NIH grant R01 CA 44722 from the National Cancer Institute to GPS, and the Canadian Institute of Health Research to JHW.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.MacDonald PN, Haussler CA, Terpening CM, Galligan MA, Reeder MC, Whitfield GK, Haussler MR. Baculovirus-mediated expression of the human vitamin D receptor. Functional characterization, vitamin D response element interactions, and evidence for a receptor auxiliary factor. J Biol Chem. 1991;266:18808–13. [PubMed] [Google Scholar]
- 2.Jones G, Strugnell SA, DeLuca HF. Current understanding of the molecular actions of vitamin D. Physiol Rev. 1998;78:1193–231. doi: 10.1152/physrev.1998.78.4.1193. [DOI] [PubMed] [Google Scholar]
- 3.Jurutka PW, Whitfield GK, Hsieh JC, Thompson PD, Haussler CA, Haussler MR. Molecular nature of the vitamin D receptor and its role in regulation of gene expression. Rev Endocr Metab Disord. 2001;2:203–16. doi: 10.1023/a:1010062929140. [DOI] [PubMed] [Google Scholar]
- 4.Jurutka PW, MacDonald PN, Nakajima S, Hsieh JC, Thompson PD, Whitfield GK, Galligan MA, Haussler CA, Haussler MR. Isolation of baculovirus-expressed human vitamin D receptor: DNA responsive element interactions and phosphorylation of the purified receptor. J Cell Biochem. 2002;85:435–57. doi: 10.1002/jcb.10134. [DOI] [PubMed] [Google Scholar]
- 5.Markose ER, Stein JL, Stein GS, Lian JB. Vitamin D-mediated modifications in protein-DNA interactions at two promoter elements of the osteocalcin gene. Proc Natl Acad Sci U S A. 1990;87:1701–5. doi: 10.1073/pnas.87.5.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noda M, Vogel RL, Craig AM, Prahl J, DeLuca HF, Denhardt DT. Identification of a DNA sequence responsible for binding of the 1,25-dihydroxyvitamin D3 receptor and 1,25-dihydroxyvitamin D3 enhancement of mouse secreted phosphoprotein 1 (SPP-1 or osteopontin) gene expression. Proc Natl Acad Sci U S A. 1990;87:9995–9. doi: 10.1073/pnas.87.24.9995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ozono K, Liao J, Kerner SA, Scott RA, Pike JW. The vitamin D-responsive element in the human osteocalcin gene. Association with a nuclear proto-oncogene enhancer. J Biol Chem. 1990;265:21881–8. [PubMed] [Google Scholar]
- 8.Breen EC, van Wijnen AJ, Lian JB, Stein GS, Stein JL. In vivo occupancy of the vitamin D responsive element in the osteocalcin gene supports vitamin D-dependent transcriptional upregulation in intact cells. Proc Natl Acad Sci U S A. 1994;91:12902–6. doi: 10.1073/pnas.91.26.12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohyama Y, Ozono K, Uchida M, Yoshimura M, Shinki T, Suda T, Yamamoto O. Functional assessment of two vitamin D-responsive elements in the rat 25-hydroxyvitamin D3 24-hydroxylase gene. J Biol Chem. 1996;271:30381–5. doi: 10.1074/jbc.271.48.30381. [DOI] [PubMed] [Google Scholar]
- 10.Therrien M, Chang HC, Solomon NM, Karim FD, Wassarman DA, Rubin GM. KSR, a novel protein kinase required for RAS signal transduction. Cell. 1995;83:879–88. doi: 10.1016/0092-8674(95)90204-x. [DOI] [PubMed] [Google Scholar]
- 11.Kornfeld K, Hom DB, Horvitz HR. The ksr-1 gene encodes a novel protein kinase involved in Ras-mediated signaling in C. elegans. Cell. 1995;83:903–13. doi: 10.1016/0092-8674(95)90206-6. [DOI] [PubMed] [Google Scholar]
- 12.Therrien M, Michaud NR, Rubin GM, Morrison DK. KSR modulates signal propagation within the MAPK cascade. Genes Dev. 1996;10:2684–95. doi: 10.1101/gad.10.21.2684. [DOI] [PubMed] [Google Scholar]
- 13.Roy F, Therrien M. MAP kinase module: the Ksr connection. Curr Biol. 2002;12:R325–7. doi: 10.1016/s0960-9822(02)00831-x. [DOI] [PubMed] [Google Scholar]
- 14.Kortum RL, Costanzo DL, Haferbier J, Schreiner SJ, Razidlo GL, Wu MH, Volle DJ, Mori T, Sakaue H, Chaika NV, Chaika OV, Lewis RE. The molecular scaffold kinase suppressor of Ras 1 (KSR1) regulates adipogenesis. Mol Cell Biol. 2005;25:7592–604. doi: 10.1128/MCB.25.17.7592-7604.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin R, White JH. The pleiotropic actions of vitamin D. Bioessays. 2004;26:21–8. doi: 10.1002/bies.10368. [DOI] [PubMed] [Google Scholar]
- 16.Studzinski GP, Wang X, Ji Y, Wang Q, Zhang Y, Kutner A, Harrison JS. The rationale for deltanoids in therapy for myeloid leukemia: role of KSR-MAPK-C/EBP pathway. J Steroid Biochem Mol Biol. 2005;97:47–55. doi: 10.1016/j.jsbmb.2005.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X, Wang TT, White JH, Studzinski GP. Induction of kinase suppressor of RAS-1(KSR-1) gene by 1, alpha25-dihydroxyvitamin D3 in human leukemia HL60 cells through a vitamin D response element in the 5'-flanking region. Oncogene. 2006;25:7078–85. doi: 10.1038/sj.onc.1209697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Studzinski GP, Garay E, Patel R, Zhang J, Wang X. Vitamin D receptor signaling of monocytic differentiation in human leukemia cells: role of MAPK pathways in transcription factor activation. Curr Top Med Chem. 2006;6:1267–71. doi: 10.2174/156802606777864935. [DOI] [PubMed] [Google Scholar]
- 19.Morrison DK. KSR: a MAPK scaffold of the Ras pathway? J Cell Sci. 2001;114:1609–12. doi: 10.1242/jcs.114.9.1609. [DOI] [PubMed] [Google Scholar]
- 20.Kolesnick R, Xing HR. Inflammatory bowel disease reveals the kinase activity of KSR1. J Clin Invest. 2004;114:1233–7. doi: 10.1172/JCI23441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Yao B, Delikat S, Bayoumy S, Lin XH, Basu S, McGinley M, Chan-Hui PY, Lichenstein H, Kolesnick R. Kinase suppressor of Ras is ceramide-activated protein kinase. Cell. 1997;89:63–72. doi: 10.1016/s0092-8674(00)80183-x. [DOI] [PubMed] [Google Scholar]
- 22.Xing HR, Kolesnick R. Kinase suppressor of Ras signals through Thr269 of c-Raf-1. J Biol Chem. 2001;276:9733–41. doi: 10.1074/jbc.M008096200. [DOI] [PubMed] [Google Scholar]
- 23.Yan F, Polk DB. Kinase suppressor of ras is necessary for tumor necrosis factor alpha activation of extracellular signal-regulated kinase/mitogen-activated protein kinase in intestinal epithelial cells. Cancer Res. 2001;61:963–9. [PubMed] [Google Scholar]
- 24.Xing HR, Campodonico L, Kolesnick R. The kinase activity of kinase suppressor of Ras1 (KSR1) is independent of bound MEK. J Biol Chem. 2004;279:26210–4. doi: 10.1074/jbc.M401323200. [DOI] [PubMed] [Google Scholar]
- 25.Michaud NR, Therrien M, Cacace A, Edsall LC, Spiegel S, Rubin GM, Morrison DK. KSR stimulates Raf-1 activity in a kinase-independent manner. Proc Natl Acad Sci U S A. 1997;94:12792–6. doi: 10.1073/pnas.94.24.12792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morrison DK, Davis RJ. Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annu Rev Cell Dev Biol. 2003;19:91–118. doi: 10.1146/annurev.cellbio.19.111401.091942. [DOI] [PubMed] [Google Scholar]
- 27.Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiol Rev. 2001;81:153–208. doi: 10.1152/physrev.2001.81.1.153. [DOI] [PubMed] [Google Scholar]
- 28.Ebisuya M, Kondoh K, Nishida E. The duration, magnitude and compartmentalization of ERK MAP kinase activity: mechanisms for providing signaling specificity. J Cell Sci. 2005;118:2997–3002. doi: 10.1242/jcs.02505. [DOI] [PubMed] [Google Scholar]
- 29.Wang X, Studzinski GP. Kinase suppressor of RAS (KSR) amplifies the differentiation signal provided by low concentrations 1,25-dihydroxyvitamin D3. J Cell Physiol. 2004;198:333–42. doi: 10.1002/jcp.10443. [DOI] [PubMed] [Google Scholar]
- 30.Wang X, Studzinski GP. Raf-1 signaling is required for the later stages of 1,25-dihydroxyvitamin D3-induced differentiation of HL60 cells but is not mediated by the MEK/ERK module. J Cell Physiol. 2006;209:253–60. doi: 10.1002/jcp.20731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohmachi M, Rocheleau CE, Church D, Lambie E, Schedl T, Sundaram MV. C. elegans ksr-1 and ksr-2 have both unique and redundant functions and are required for MPK-1 ERK phosphorylation. Curr Biol. 2002;12:427–33. doi: 10.1016/s0960-9822(02)00690-5. [DOI] [PubMed] [Google Scholar]
- 32.Channavajhala PL, Wu L, Cuozzo JW, Hall JP, Liu W, Lin LL, Zhang Y. Identification of a novel human kinase supporter of Ras (hKSR-2) that functions as a negative regulator of Cot (Tpl2) signaling. J Biol Chem. 2003;278:47089–97. doi: 10.1074/jbc.M306002200. [DOI] [PubMed] [Google Scholar]
- 33.Channavajhala PL, Rao VR, Spaulding V, Lin LL, Zhang YG. hKSR-2 inhibits MEKK3-activated MAP kinase and NF-kappaB pathways in inflammation. Biochem Biophys Res Commun. 2005;334:1214–8. doi: 10.1016/j.bbrc.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Zhang J, Studzinski GP. AKT pathway is activated by 1, 25- dihydroxyvitamin D3 and participates in its anti-apoptotic effect and cell cycle control in differentiating HL60 cells. Cell Cycle. 2006;5:447–51. doi: 10.4161/cc.5.4.2467. [DOI] [PubMed] [Google Scholar]
- 35.Wang X, Studzinski GP. The requirement for and changing composition of the activating protein-1 transcription factor during differentiation of human leukemia HL60 cells induced by 1,25-dihydroxyvitamin D3. Cancer Res. 2006;66:4402–9. doi: 10.1158/0008-5472.CAN-05-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marcinkowska E, Garay E, Gocek E, Chrobak A, Wang X, Studzinski GP. Regulation of C/EBPbeta isoforms by MAPK pathways in HL60 cells induced to differentiate by 1,25-dihydroxyvitamin D3. Exp Cell Res. 2006;312:2054–65. doi: 10.1016/j.yexcr.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gallagher R, Collins S, Trujillo J, McCredie K, Ahearn M, Tsai S, Metzgar R, Aulakh G, Ting R, Ruscetti F, Gallo R. Characterization of the continuous, differentiating myeloid cell line (HL-60) from a patient with acute promyelocytic leukemia. Blood. 1979;54:713–33. [PubMed] [Google Scholar]
- 38.Studzinski GP, Reddy KB, Hill HZ, Bhandal AK. Potentiation of 1-beta-D-arabinofuranosylcytosine cytotoxicity to HL-60 cells by 1,25-dihydroxyvitamin D3 correlates with reduced rate of maturation of DNA replication intermediates. Cancer Res. 1991;51:3451–5. [PubMed] [Google Scholar]
- 39.Wang X, Gardner JP, Kheir A, Uskokovic MR, Studzinski GP. Synergistic induction of HL60 cell differentiation by ketoconazole and 1-desoxy analogues of vitamin D3. J Natl Cancer Inst. 1997;89:1199–206. doi: 10.1093/jnci/89.16.1199. [DOI] [PubMed] [Google Scholar]
- 40.Andrews NC, Faller DV. A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acids Res. 1991;19:2499. doi: 10.1093/nar/19.9.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang TT, Tavera-Mendoza LE, Laperriere D, Libby E, MacLeod NB, Nagai Y, Bourdeau V, Konstorum A, Lallemant B, Zhang R, Mader S, White JH. Large-scale in silico and microarray-based identification of direct 1,25- dihydroxyvitamin D3 target genes. Mol Endocrinol. 2005;19:2685–95. doi: 10.1210/me.2005-0106. [DOI] [PubMed] [Google Scholar]
- 42.Matheny SA, Chen C, Kortum RL, Razidlo GL, Lewis RE, White MA. Ras regulates assembly of mitogenic signalling complexes through the effector protein IMP. Nature. 2004;427:256–60. doi: 10.1038/nature02237. [DOI] [PubMed] [Google Scholar]
- 43.Ory S, Morrison DK. Signal transduction: implications for Ras-dependent ERK signaling. Curr Biol. 2004;14:R277–8. doi: 10.1016/j.cub.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 44.Yam LT, Li CY, Crosby WH. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971;55:283–90. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]
- 45.Yu FL. Selective inhibition of rat liver nuclear RNA polymerase II by actinomycin D in vivo. Carcinogenesis. 1980;1:577–81. doi: 10.1093/carcin/1.7.577. [DOI] [PubMed] [Google Scholar]
- 46.Sakata Y, Yoshioka W, Tohyama C, Ohsako S. Internal genomic sequence of human CYP1A1 gene is involved in superinduction of dioxin-induced CYP1A1 transcription by cycloheximide. Biochem Biophys Res Commun. 2007;355:687–92. doi: 10.1016/j.bbrc.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 47.Lau LF, Nathans D. Expression of a set of growth-related immediate early genes in BALB/c 3T3 cells: coordinate regulation with c-fos or c-myc. Proc Natl Acad Sci U S A. 1987;84:1182–6. doi: 10.1073/pnas.84.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herdick M, Steinmeyer A, Carlberg C. Antagonistic action of a 25- carboxylic ester analogue of 1alpha, 25-dihydroxyvitamin D3 is mediated by a lack of ligand-induced vitamin D receptor interaction with coactivators. J Biol Chem. 2000;275:16506– 12. doi: 10.1074/jbc.M910000199. [DOI] [PubMed] [Google Scholar]
- 49.Zhou LX, Nemere I, Norman AW. 1,25-Dihydroxyvitamin D3 analog structure-function assessment of the rapid stimulation of intestinal calcium absorption (transcaltachia) J Bone Miner Res. 1992;7:457–63. doi: 10.1002/jbmr.5650070414. [DOI] [PubMed] [Google Scholar]
- 50.Okazaki T, Bielawska A, Domae N, Bell RM, Hannun YA. Characteristics and partial purification of a novel cytosolic, magnesium-independent, neutral sphingomyelinase activated in the early signal transduction of 1 alpha,25- dihydroxyvitamin D3-induced HL-60 cell differentiation. J Biol Chem. 1994;269:4070–7. [PubMed] [Google Scholar]
- 51.Wang X, Studzinski GP. Activation of extracellular signal-regulated kinases (ERKs) defines the first phase of 1,25-dihydroxyvitamin D3-induced differentiation of HL60 cells. J Cell Biochem. 2001;80:471–82. doi: 10.1002/1097-4644(20010315)80:4<471::aid-jcb1001>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 52.Marcinkowska E. Evidence that activation of MEK1,2/erk1,2 signal transduction pathway is necessary for calcitriol-induced differentiation of HL-60 cells. Anticancer Res. 2001;21:499–504. [PubMed] [Google Scholar]
- 53.Hughes PJ, Brown G. 1Alpha,25-dihydroxyvitamin D3-mediated stimulation of steroid sulphatase activity in myeloid leukaemic cell lines requires VDRnuc-mediated activation of the RAS/RAF/ERK-MAP kinase signalling pathway. J Cell Biochem. 2006;98:590–617. doi: 10.1002/jcb.20787. [DOI] [PubMed] [Google Scholar]
- 54.Buck M, Poli V, Hunter T, Chojkier M. C/EBPbeta phosphorylation by RSK creates a functional XEXD caspase inhibitory box critical for cell survival. Mol Cell. 2001;8:807–16. doi: 10.1016/s1097-2765(01)00374-4. [DOI] [PubMed] [Google Scholar]
- 55.Pan Z, Hetherington CJ, Zhang DE. CCAAT/enhancer-binding protein activates the CD14 promoter and mediates transforming growth factor beta signaling in monocyte development. J Biol Chem. 1999;274:23242–8. doi: 10.1074/jbc.274.33.23242. [DOI] [PubMed] [Google Scholar]
- 56.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–52. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 57.Wang Q, Salman H, Danilenko M, Studzinski GP. Cooperation between antioxidants and 1,25-dihydroxyvitamin D3 in induction of leukemia HL60 cell differentiation through the JNK/AP-1/Egr-1 pathway. J Cell Physiol. 2005;204:964–74. doi: 10.1002/jcp.20355. [DOI] [PubMed] [Google Scholar]
- 58.Nguyen A, Burack WR, Stock JL, Kortum R, Chaika OV, Afkarian M, Muller WJ, Murphy KM, Morrison DK, Lewis RE, McNeish J, Shaw AS. Kinase suppressor of Ras (KSR) is a scaffold which facilitates mitogen-activated protein kinase activation in vivo. Mol Cell Biol. 2002;22:3035–45. doi: 10.1128/MCB.22.9.3035-3045.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dekker J. The three 'C' s of chromosome conformation capture: controls, controls, controls. Nat Methods. 2006;3:17–21. doi: 10.1038/nmeth823. [DOI] [PubMed] [Google Scholar]
- 60.Saramaki A, Banwell CM, Campbell MJ, Carlberg C. Regulation of the human p21(waf1/cip1) gene promoter via multiple binding sites for p53 and the vitamin D3 receptor. Nucleic Acids Res. 2006;34:543–54. doi: 10.1093/nar/gkj460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jiang H, Lin J, Su ZZ, Collart FR, Huberman E, Fisher PB. Induction of differentiation in human promyelocytic HL-60 leukemia cells activates p21, WAF1/CIP1, expression in the absence of p53. Oncogene. 1994;9:3397–406. [PubMed] [Google Scholar]
- 62.Liu M, Lee MH, Cohen M, Bommakanti M, Freedman LP. Transcriptional activation of the Cdk inhibitor p21 by vitamin D3 leads to the induced differentiation of the myelomonocytic cell line U937. Genes Dev. 1996;10:142–53. doi: 10.1101/gad.10.2.142. [DOI] [PubMed] [Google Scholar]
- 63.Sinkkonen L, Malinen M, Saavalainen K, Vaisanen S, Carlberg C. Regulation of the human cyclin C gene via multiple vitamin D3-responsive regions in its promoter. Nucleic Acids Res. 2005;33:2440–51. doi: 10.1093/nar/gki502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Verlinden L, Verstuyf A, Convents R, Marcelis S, Van Camp M, Bouillon R. Action of 1,25(OH)2D3 on the cell cycle genes, cyclin D1, p21 and p27 in MCF-7 cells. Mol Cell Endocrinol. 1998;142:57–65. doi: 10.1016/s0303-7207(98)00117-8. [DOI] [PubMed] [Google Scholar]
- 65.Lin R, Nagai Y, Sladek R, Bastien Y, Ho J, Petrecca K, Sotiropoulou G, Diamandis EP, Hudson TJ, White JH. Expression profiling in squamous carcinoma cells reveals pleiotropic effects of vitamin D3 analog EB1089 signaling on cell proliferation, differentiation, and immune system regulation. Mol Endocrinol. 2002;16:1243–56. doi: 10.1210/mend.16.6.0874. [DOI] [PubMed] [Google Scholar]
- 66.Peng X, Mehta R, Wang S, Chellappan S, Mehta RG. Prohibitin is a novel target gene of vitamin D involved in its antiproliferative action in breast cancer cells. Cancer Res. 2006;66:7361–9. doi: 10.1158/0008-5472.CAN-06-1004. [DOI] [PubMed] [Google Scholar]
- 67.Campbell MJ, Adorini L. The vitamin D receptor as a therapeutic target. Expert Opin Ther Targets. 2006;10:735–48. doi: 10.1517/14728222.10.5.735. [DOI] [PubMed] [Google Scholar]