Abstract
Disruption of intracellular calcium homeostasis via abnormal and excessive activation of ryanodine receptors plays an important role in the neuropathology of Alzheimer’s disease. We investigated the therapeutic effect of dantrolene, a ryanodine receptor antagonist, on cognitive dysfunction and neuropathology in the triple transgenic Alzheimer mouse model (3xTg-AD). 3xTg-AD mice were treated with dantrolene from 2 to 13 months of age. Learning and memory were measured with the Morris Water Maze at 6, 10, and 13 months of age. Amyloid and tau neuropathology and biomarkers for synaptic dysfunction and neurodegeneration were examined in the brain using immunoblotting or immunohistochemistry. Dantrolene treatment for 11 months significantly reduced both memory deficits and amyloid plaque load in the hippocampus in 13 month old 3xTg-AD mice. Dantrolene treatment, however, had no effect on phosphorylated tau, phosphorylated or total GSK-3β, synaptic markers, or mitochondrial or cytosolic cytochrome C. Our results suggest that dantrolene significantly improves cognition in a murine model of Alzheimer’s disease and is associated with a reduction in amyloid plaque burden, forming the basis for a novel therapeutic approach for Alzheimer’s disease.
Keywords: Alzheimer’s disease, Calcium, Ryanodine Receptors, Dantrolene, Memory and Learning, Amyloid, Tau, Apoptosis
Increasing evidence suggests that calcium signaling dysregulation plays an important role in synaptic dysfunction in Alzheimer’s disease (AD), well before amyloidopathy and cognitive decline [6;10]. Specifically, mutations in two genes associated with familial AD, presenilin-1 and presenilin-2, significantly increase the expression and activation of the ryanodine receptor (RYR), a calcium channel on the membrane of the endoplasmic reticulum (ER), resulting in aberrant calcium release from the ER and amplification of cytosolic calcium levels [3;6;10]. Interestingly, this abnormal ryanodine-mediated calcium increase appears to be driven by calcium influx through NMDA-type glutamate receptors [10]. Overactivation of the RYR in the AD brain increases calcium release from the ER and elevates resting levels of cytosolic calcium [16], which exacerbates other pathological changes, ultimately resulting in cognitive dysfunction. Thus, a drug that inhibits the ryanodine receptor should be able to ameliorate the above pathological changes and mitigate learning and memory deficits associated with AD [1].
Dantrolene is an antagonist of the RYR and is used clinically to treat malignant hyperthermia, neuroplaptic malignant syndrome, and muscle spasms [13;14]. Previous studies suggest that it inhibits calcium influx via the NMDA receptor and reverses glutamate-induced excitotoxicity [17;18]. Additionally, dantrolene has been found to protect against neuronal damage induced by various insults, including ischemia, hypoxia, seizure, sepsis, trauma and spinocerebellar ataxia [13] and Huntington’s disease [8]. Considering the noted inhibitory effects of dantrolene on RYRs, glutamate excitotoxicity, and oxidative stress [13;18;21], we hypothesize that dantrolene should ameliorate neuropathology and improve learning and memory in the triple transgenic mouse model of AD (3xTg-AD) [19].
Use of animals and all procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Pennsylvania. Homozygous triple transgenic Alzheimer (3xTg-AD) mice, with the presenilin-1 (PS1M146V), amyloid precursor protein (APPSwe), and taup301L transgenes, were bred using an initial 3xTg-AD breeding pair from Dr. Frank LaFerla [19]. Aged-matched C57BL/6 wild type mice were used as controls.
Four groups of mice were included in this study; 3xTg-AD mice treated with dantrolene and vehicle; 3xTg-AD mice vehicle only; 3xTg-AD and C57BL/6 mice with no treatment. Dantrolene (Sigma, St Louis, MO) was initially delivered to 2 month old 3xTg-AD mice by intracerebroventricular (ICV) injection for 3 months using an Alzet intracranial ventricular infusion system (Durect Corp, Cupertino, CA) following manufacture’s instruction. The pump was filled to its capacity (100µl) with dantrolene (25mM) dissolved in 25% DMSO (Sigma, St Louis, MO) in PBS (DMSO:PBS, 1:3) and implanted under the skin between the scapulae with a catheter tube inserted into the lateral ventricle on one side of the brain, with a constant flow rate of 0.11µL/hr for 28 days. In a pilot study, the dantrolene concentration in the brain, measured by HPLC (Beckman Coulter, Fullerton, CA), was in the range of 200-10090 ng/g, and dantrolene concentration seemed to increase with ICV treatment duration. Two month old vehicle control 3xTG-AD mice were implanted with the same type of Alzet pump containing DMSO in PBS (1:3). Pumps needed to be replaced once a month. Due to the high perioperative mortality during surgery for the replacement of the Alzet pumps, we discontinued the ICV infusion after 90 days. Thereafter, treatment was continued with subcutaneous (SQ) injections of dantrolene (5mg/kg) or vehicle 20% Dimethylformamide/Methanol in PBS (DMF/MeOH, 50:50, Sigma, St Louis, MO), 3 times a week for 8 months, until the mice reached 13 months of age (Fig. 1A). No animals exhibited signs of toxicity from the 11 months of treatment with dantrolene or vehicle.
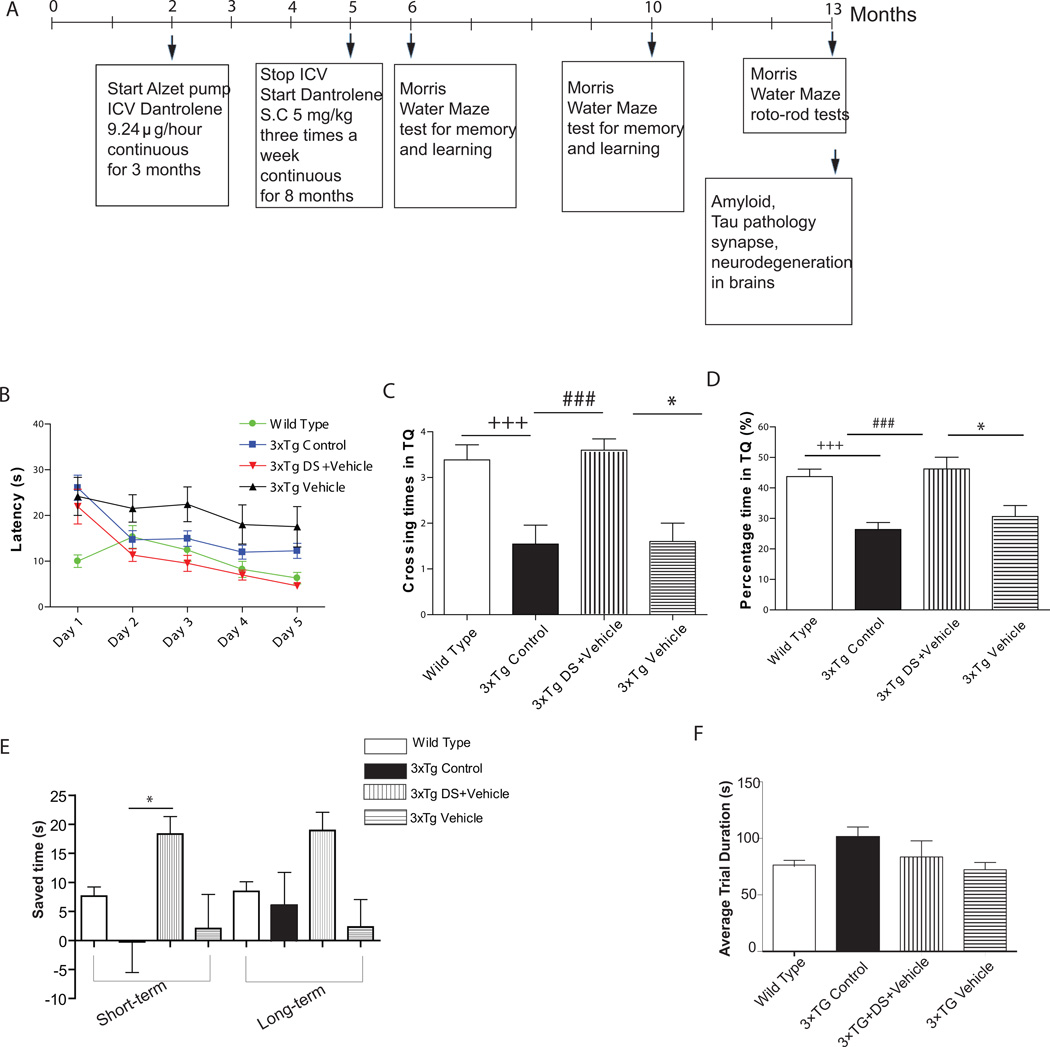
Figure 1. Dantrolene treatment blocks memory loss in 13 month old 3xTG-AD mice.
Memory and learning was tested using the Morris Water Maze. (A) Time line for dantrolene treatment and behavioral assessment. (B) The mean escape latency over 5 consecutive days of reference memory testing shows no significant differences between groups by repeated measures Two-way ANOVA. (C, D) A Probe test was performed 24h after last place test to determine memory retention. The hidden platform was removed from the pool and the number of times the target quadrant was crossed (C) and the percent time in the target quadrant (TQ) (D) were determined. (C) There was a significant genotype effect with wild type mice performing significantly better than the 3xTG-AD Controls (+P<0.05), and a significant dantrolene treatment effect compared to 3xTG-AD Controls (#P<0.05) and 3xTG-AD Vehicle (*P<0.05) measured by One-way ANOVA with Bonferroni post-hoc tests. (D) A genotype effect was also found for the percent time spent in the target quadrant with wild type performing significantly better than the 3xTG-AD controls (+++P<0.001) and a significant dantrolene treatment effect compared to 3xTG-AD controls (###P<0.001) and 3xTG Vehicle (*P<0.05) as measured by One-way ANOVA with Bonferroni post hoc tests. (E) Working memory test started 24h after completion of probe test and ran for 21 consecutive days. Data are plotted as mean time saved in matching trials 1 (short-term) and 2 (long-term) from sample trials over 21 consecutive days. Dantrolene treated 3xTG-AD mice saved significantly more than 3xTG-AD Controls (*P<0.05) and showed a very strong trend toward more saved time than 3xTG-AD Vehicle mice. (F) Motor function determined by the roto-rod test showed no significant difference among all experimental groups. All data represent mean ± SEM. WT: Wild Type; 3xTG-AD: triple transgenic mice; DS: Dantrolene; Vehicle: DMSO. WT Control (n=12), 3xTG-AD Control (n=10), 3xTG-AD + DS (n= 5) and 3xTG-AD + Vehicle (n=5).
Cognitive function was repeatedly assessed in all four experimental groups at 6, 10, and 13 months of age using the MWM test as we previously described [23] using the timeline in Figure 1A. For the reference memory testing, escape latencies were measured 4 trials per day for 5 consecutive days. The probe test was conducted 24 h after the last reference memory trial. The percent time spent in the target quadrant compared to other quadrants was measured as an indication of memory retention. Working memory testing began 1 day after the probe test, using the 21 day trial-dependent learning procedure previously described by Vorhees and Williams [20]. All MWM tests were recorded using a video tracking system (Actimetrics software, Wilmette, IL).
Motor skill was examined with the roto-rod test. All animals received two 60 s training trials on the Roto-rod (IITC Series 8, Life Sciences, Woodland hills, CA) at 9 rpm with a 30 min interval between trials. The mice then underwent three test trials for a maximum of 120 s with variable speed, 4–40 rpm, and the time spent on the roto-rod was recorded for each mouse. The interval between trials was 60 min.
After the roto-rod testing, all mice were anesthetized with an intraperitoneal injection of sodium pentobarbital (100mg/kg) and transcardially perfused with normal saline. The left hemisphere of the brain of each mouse was quickly frozen in liquid nitrogen for Western blot assays and the right hemisphere was fixed in 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) for immunohistochemical analyses.
Western blots were done with cortical lysates from the left brain hemisphere as we previously described [23]. Apoptosis was examined by determining the amount of ctyochrome C in the cytosol and mitochondrial fractions as described previously [15]. The blots were probed for APP, PSD-95, Synapsin 1a or 1b, phosphorylated or total GSK3β, phosphorylated or total tau, and cytochrome C, and then followed by horseradish peroxidase-conjugated secondary antibody. β-Actin protein was used as a loading control and the changes of targeted proteins were normalized to the level of β-Actin.
Right brain hemispheres were cryoprotected and sectioned, and serial sections (10µm) were processed for immunocytochemistry using the Vector M.O.M. immunodetection kit (Vector Laboratories, Brulingame, CA) with a similar protocol that we have described previously [23]. The percent area of the hippocampus occupied by Aβ plaques (antibody: 6E10) was quantified using IPLab Suite v4.0 Imaging Processing and Analysis software (Biovision Technologies, Exton, PA) and the number of phosphorylated tau positive neurons (AT8) per area of the hippocampus was quantified. The means were calculated from three consecutive slides per animal. All sections were quantified by two people blinded to the treatments.
Statistical analyses were performed using GraphPad Prism v5.04 software and are explained in the figure legends. P<0.05 was accepted as statistically significant.
No significant cognitive improvement was found in the dantrolene treated 3xTg-AD mice at either 6 or 10 months of age (data not shown). At 13 months of age, no significant differences were found in the escape latencies among all groups, although there was a trend for improved learning and memory in the dantrolene treated 3xTg-AD mice (Fig. 1B). Memory retention assessed by the probe test showed significant improvement in the dantrolene treated 3xTg-AD mice compared to untreated 3xTg-AD mice (Fig. 1C, 1D). In fact, 3xTg-AD mice treated with dantrolene performed at the same level as the wild type (WT) controls (Fig. 1C, 1D). Similarly, dantrolene treated 3xTg-AD mice exhibited significantly improved short-term working memory compared to untreated 3xTg-AD controls (Fig. 1E) and a strong trend toward improved short-term working memory compared to vehicle controls. Similarly, dantrolene-treated animals showed a trend for improved long-term working memory, though no statistical differences were noted (Fig. 1E). Motor function assessed by the roto-rod test showed no statistical differences among all experimental groups (Fig. 1F). Thus, chronic dantrolene treatment, starting in early adulthood, resulted in an almost complete inhibition of memory deficits in the 3xTg-AD mice at 13 months of age.
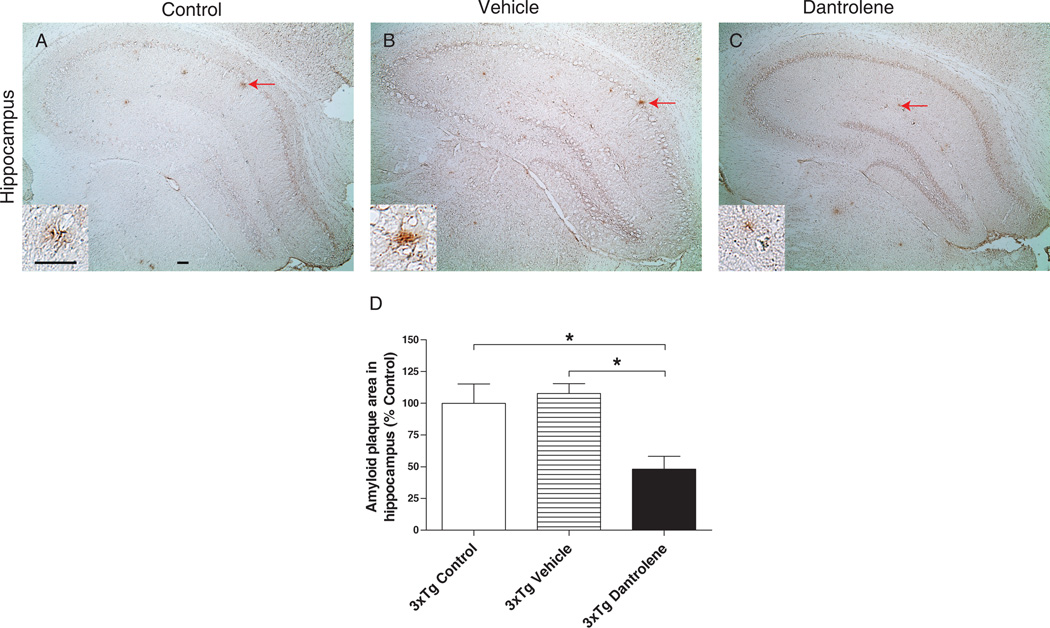
Amyloid plaques are apparent in the hippocampus of 3xTg-AD mice at 13 months of age, as shown in the 3xTg-AD controls and vehicle controls (Fig. 2A and B). Vehicle treatment did not significantly change the amyloid plaque load in the hippocampus of 3xTg-AD mice (Fig. 2B and D). However, dantrolene treatment significantly decreased the percent area of the hippocampus occupied by amyloid plaques in 3xTg-AD mice compared to both control groups (Fig. 2C and D).
Figure 2. Dantrolene significantly decreases amyloid plaque level in the hippocampus of 3xTG-AD mice.
Representative micrographs that show 6E10 immunoreactivity in the hippocampus. Arrows indicate typical characteristic of amyloid plaques (A and B). (D) The percentage area of the whole hippocampus covered by plaques in experimental mice. Aβ plaques covered significantly more hippocampal area in untreated and vehicle controls than dantrolene-treated 3xTg-AD mice (*P<0.05, Controls, n=6; Vehicle, n=5; Dantrolene, n=5). ALL data represent mean ± SEM and analyzed by One-way ANOVA with Bonferroni post-hoc tests (*P<0.05). Inlet in each picture (A, B and C) represents a sample plaque in higher power. Scale bar = 100µm.
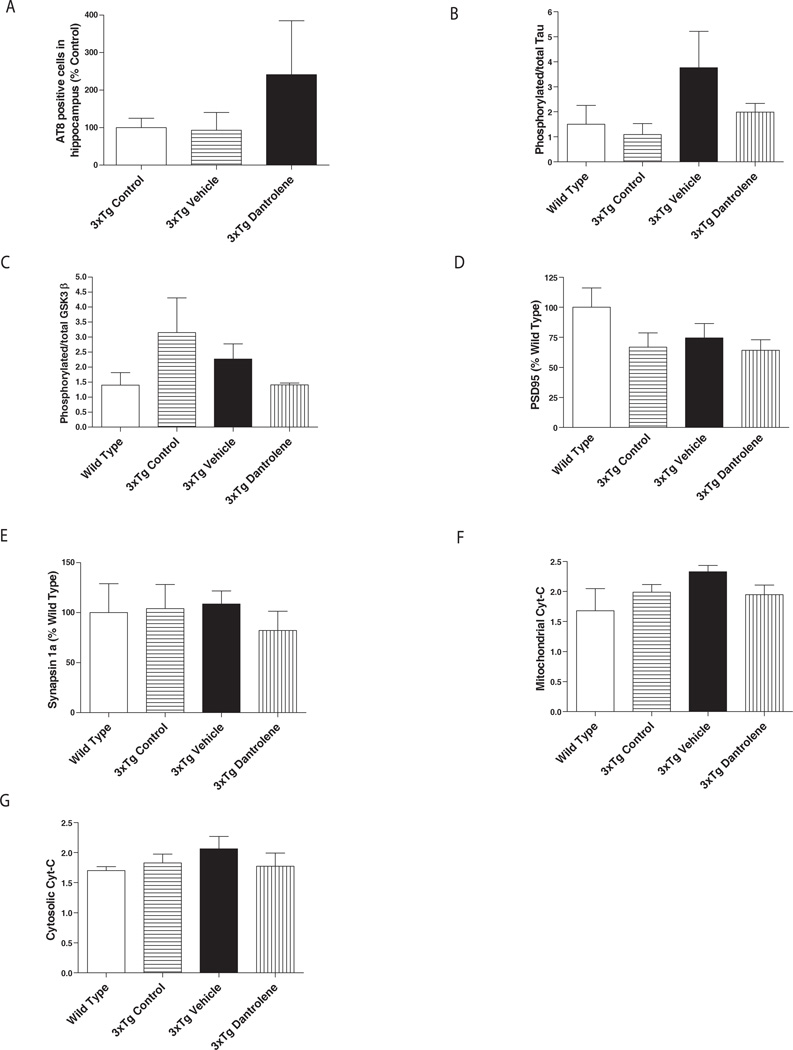
Immunohistochemical analysis of tau (AT8) in the hippocampus did not reveal any significant differences among the treatment groups (Fig. 3A). Similarly, no significant differences were found for phosphorylated tau, phosphorylated GSK-3β between experimental groups (Fig. 3 B and C) in the cortex. In addition, no significant differences in synaptic marker expression (PSD95 and Synapsin 1a) were found in the cerebral cortex (Fig. 3 D and E). We did not detect any significant effects on mitochondrial or cytoplasmic cytochrome-C expression by dantrolene in cortical tissues in any treatment groups (Fig, 3 F and G).
Figure 3. Effects of dantrolene treatment on Tau protein phosphorylation, expression of synaptic, and apoptotic proteins biomarkers in cerebral cortex of 3xTG- AD mice.
(A) Immunocytochemical analysis on cortical sections from right hemisphere was used to determine the number of AT8 positive cells. (B–G) Western blots were performed using brain tissues from the left hemisphere of the cerebral cortex to determine the protein expression levels of phyosphorylated Tau (B), phosphorylated GSK3β (C), PSD95 (D), synapsin 1a (E), mitochondrial (F) and cytosolic (G) cytochrome C. The animal numbers of Wild Type Control, 3xTG-AD Control, 3xTG Vehicle and 3xTG-AD Dantrolene groups were 4, 4, 4 and 4, respectively. Data are given as mean ± SEM and analyzed by One-way ANOVA, followed by Bonferroni post-hoc test.
Taken together, our results suggest that early and chronic dantrolene treatment largely prevents memory loss at 13 months of age in 3xTg-AD mice. This reduction in memory loss appears to correlate with a significant reduction in Aβ aggregation and plaque formation in the hippocampus. Previous immunohistochemical characterization of 3xTg-AD mice have shown Aβ deposits appearing as early as 6 months and intraneuronal tau pathology at 9 months of age in various brain regions [19]. This is in line with our results showing 6E10 and AT8 immunoreactivity in the hippocampus of 3xTg-AD mice at 13 months of age, and thus our study does not seem to be affected by the recently suggested drift in this model [12]. Interestingly, we only observe significant effects in learning and memory after 13 months (Fig. 1). It is not clear whether these delayed effects are due to developmental regulation of RYRs or calcium signaling pathways. It must be noted that our behavioral experiments were carried out in the same cohort of animals repeatedly at 6, 10, and 13 months of age, raising the possibility that previous learning experience in the MWM could have influenced age-related cognitive functions in our cohort. Although we cannot rule out this possibility, the experimental and control groups received the same amount of training and thus we believe that the improvement in the dantrolene group was due to the drug.
Although dantrolene treatment was associated with a reduction in the plaque load in the hippocampus, this study cannot mechanistically make the linkage. The role of amyloid pathology on neurodegeneration, synaptic dysfunction, and memory impairment in AD is controversial. It has even been proposed that amyloid protein may protect from toxic intermediates and oxidative stress and promote neurogenesis [4]. In this study, although dantrolene decreased amyloid aggregation, it did not significantly affect tau pathology, apoptosis or synaptic proteins, lending support to the idea that pathways associated with amyloidopathy are linked to cognitive status.
Another possible mechanism for cognitive improvement is that dantrolene treatment prevented the calcium release from the ER previously shown to be increased in 3xTg-AD mice. Ryanodine receptor over activation in presymptomatic 3xTG-AD mice has been shown to impair synaptic transmission [5], which may result in the later memory impairment. Dantrolene, a ryanodine receptor antagonist, inhibits the aberrant calcium release from the ER and restores both synaptic transmission and memory in 3xTG-AD mice, as suggested in this study (Fig. 1 C, D, E), although we would have expected to see improvements in synaptic markers.
Contrary to our findings, oral treatment of dantrolene in a double transgenic (APP/PS1) AD mouse model resulted in an increase in amyloid plaque load, a loss of synaptic markers, and neuronal atrophy in hippocampal and cortical brain regions [22]. The nature of the transgenic animals used in the two studies may be a contributive factor for the discrepancy. Many studies use single or double AD transgenic mouse models that mimic the amyloidopathology of the human disease. Even among these models, there are differences in timing and degree of neuropathology [9] and the onset of cognitive decline. Since cognitive function was not assessed in the study by Zhang et al.[22], it is difficult to correlate their findings with ours in that respect. While single or double mutations models are important for studying amyloidopathy, the interaction between amyloid and tau likely plays a key role in Alzheimer progression, where the amyloid beta peptide is thought to induce tau phosphorylation. With the triple transgenic AD mouse model, we found cognitive improvement and reduced plaque load, without significant changes in tauopathy or synaptic markers. It is possible that dantrolene treatment disrupts the amyloid beta downstream pathway, thereby reducing the tau phosphorylation and triggering compensatory responses to pathological conditions such as cell cycle dysregulation or oxidative stress [24]. These results are consistent with the idea that dantrolene can act as an anti-excitotoxic agent as previously demonstrated for mouse KI-PS1M146V hippocampal neurons [11] and spinocerebellar ataxia mice [7]. It is intriguing that chronic treatment of two different AD mouse models with dantrolene, by different delivery routes and with different concentrations, resulted in two different neuropathological outcomes. Recent work has suggested protective effects for dantrolene in Huntington’s disease [8]. Thus, the literature would suggest that neuroprotection is not unprecedented. Further studies are needed to provide experimental insight into these differences. This suggests that unique and intrinsic factors to the two transgenic mouse models affect AD neuropathology in response to prolonged RYR inactivity in different ways.
We found a significant accumulation of dantrolene in the brain with ICV infusions. Dantrolene was administered ICV initially because there has been controversy if dantrolene can pass blood brain barrier (BBB) or not. For example, oral administration produced reasonable blood levels [7], but the brain levels were uncertain, as those actually measured could be accounted for by the retained blood in the brain and dantrolene brain levels were not measured in the dantrolene fed AD transgenic mouse study [22]. Of course, our subcutaneous administration would be subject to the same limitations, but we believed this route would produce more consistent and stable blood concentrations than oral alone.
Despite the neuropathological and cognitive changes found in our study, there are limitations. One is that the number of dantrolene- and DMSO-treated animals (n=5 each) is small. We started the study with approximately equal numbers of mice in all groups (≥10), but had significant mortality in the DMSO- and dantrolene-treated groups when they underwent surgery for the Alzet pump replacement, primarily during the anesthetic induction. Excess mortality in this transgenic animal has been documented before [2]. This suggests that, given the loss of nearly the same number of vehicle and dantrolene treated animals, the 3xTg-AD mouse model may be more vulnerable to the effects of anesthesia. Due to the high perioperative mortality, we changed our dantrolene treatment from ICV to subcutaneous administration. Another limitation is that we did not design the study to include dantrolene-treated wild type mice to rule out the possibility that dantrolene may also improve learning and memory in wild type mice. Dantrolene has been used clinically for some time [14], but no benefits to cognition have been reported. Despite the above limitations, our data showed that early treatment with dantrolene significantly improved memory retention and short-term memory, while showing a trend toward improved spatial and long-term working memory. This suggests that dantrolene might serve as a probe compound for further development as an approach to treat Alzheimer’s disease.
Highlights.
Dantrolene nearly abolished memory loss in 3xTG Alzheimer’s disease (AD) mice.
Dantrolene significantly reduced amyloid burden in brains of 3xTG AD mice.
Dantrolene did not significantly affect tau, synaptic or apoptotic markers.
Acknowledgement
Supported by by NIH (GM-073224, GM084979, GM084979-02S1 to H.W.), and a March of Dimes Birth Defects Foundation Research Grant (#12-FY08-167 to H.W.). We appreciate the technical support from Roger Liu, and Dr. Junxia Tang. We thank Drs. Roderic Eckenhoff and Lee Fleisher for their invaluable discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Bezprozvanny I. Calcium signaling and neurodegenerative diseases. Trends in Molecular Medicine. 2009;15:89–100. doi: 10.1016/j.molmed.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bianchi SL, Caltagarone BM, LaFerla FM, Eckenhoff RG, Kelz MB. Inhaled anesthetic potency in aged Alzheimer mice. Anesth. Analg. 2010;110:427–430. doi: 10.1213/ANE.0b013e3181b5a292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruno AM, Huang JY, Bennett DA, Marr RA, Hastings ML, Stutzmann GE. Altered ryanodine receptor expression in mild cognitive impairment and Alzheimer's disease. Neurobiol. Aging. 2011 doi: 10.1016/j.neurobiolaging.2011.03.011. [EPub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castellani RJ, Lee HG, Siedlak SL, Nunomura A, Hayashi T, Nakamura M, Zhu X, Perry G, Smith MA. Reexamining Alzheimer's disease: evidence for a protective role for amyloid-beta protein precursor and amyloid-beta. J. Alzheimers. Dis. 2009;18:447–452. doi: 10.3233/JAD-2009-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chakroborty S, Goussakov I, Miller MB, Stutzmann GE. Deviant ryanodine receptor-mediated calcium release resets synaptic homeostasis in presymptomatic 3xTg-AD mice. J. Neurosci. 2009;29:9458–9470. doi: 10.1523/JNEUROSCI.2047-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan SL, Mayne M, Holden CP, Geiger JD, Mattson MP. Presenilin-1 mutations increase levels of ryanodine receptors and calcium release in PC12 cells and cortical neurons. J Biol. Chem. 2000;275:18195–18200. doi: 10.1074/jbc.M000040200. [DOI] [PubMed] [Google Scholar]
- 7.Chen X, Tang TS, Tu H, Nelson O, Pook M, Hammer R, Nukina N, Bezprozvanny I. Deranged calcium signaling and neurodegeneration in spinocerebellar ataxia type 3. J. Neurosci. 2008;28:12713–12724. doi: 10.1523/JNEUROSCI.3909-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen X, Wu J, Lvovskaya S, Herndon E, Supnet C, Bezprozvanny I. Dantroleneis neuroprotective in Huntington's disease transgenic mouse model. Mol. Neurodegener. 2011;6:81. doi: 10.1186/1750-1326-6-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Games D, Buttini M, Kobayashi D, Schenk D, Seubert P. Mice as models: transgenic approaches and Alzheimer's disease. J. Alzheimers. Dis. 2006;9:133–149. doi: 10.3233/jad-2006-9s316. [DOI] [PubMed] [Google Scholar]
- 10.Goussakov I, Miller MB, Stutzmann GE. NMDA-mediated Ca(2+) influx drives aberrant ryanodine receptor activation in dendrites of young Alzheimer's disease mice. J. Neurosci. 2010;30:12128–12137. doi: 10.1523/JNEUROSCI.2474-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo Q, Fu WM, Sopher BL, Miller MW, Ware CB, Martin GM, Mattson MP. Increased vulnerability of hippocampal neurons to excitotoxic necrosis inpresenilin-1 mutant knock-in mice. Nat. Med. 1999;5:101–106. doi: 10.1038/4789. [DOI] [PubMed] [Google Scholar]
- 12.Hirata-Fukae C, Li HF, Hoe HS, Gray AJ, Minami SS, Hamada K, Niikura T, Hua F, Tsukagoshi-Nagai H, Horikoshi-Sakuraba Y, Mughal M, Rebeck GW, LaFerla FM, Mattson MP, Iwata N, Saido TC, Klein WL, Duff KE, Aisen PS, Matsuoka Y. Females exhibit more extensive amyloid, but not tau, pathology inan Alzheimer transgenic model. Brain Res. 2008;1216:92–103. doi: 10.1016/j.brainres.2008.03.079. [DOI] [PubMed] [Google Scholar]
- 13.Inan S, Wei H. The cytoprotective effects of dantrolene: a ryanodine receptor antagonist. Anesth. Analg. 2010;111:1400–1410. doi: 10.1213/ANE.0b013e3181f7181c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krause T, Gerbershagen MU, Fiege M, Weisshorn R, Wappler F. Dantrolene--a review of its pharmacology, therapeutic use and new developments. Anaesthesia. 2004;59:364–373. doi: 10.1111/j.1365-2044.2004.03658.x. [DOI] [PubMed] [Google Scholar]
- 15.Li Q, Li H, Roughton K, Wang X, Kroemer G, Blomgren K, Zhu C. Lithium reduces apoptosis and autophagy after neonatal hypoxia-ischemia. Cell Death. Dis. 2010;1:e56. doi: 10.1038/cddis.2010.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopez JR, Lyckman A, Oddo S, LaFerla FM, Querfurth HW, Shtifman A. Increased intraneuronal resting [Ca2+] in adult Alzheimer's disease mice. J. Neurochem. 2008;105:262–271. doi: 10.1111/j.1471-4159.2007.05135.x. [DOI] [PubMed] [Google Scholar]
- 17.Makarewicz D, Zieminska E, Lazarewicz JW. Dantrolene inhibits NMDA-induced 45Ca uptake in cultured cerebellar granule neurons. Neurochem. Int. 2003;43:273–278. doi: 10.1016/s0197-0186(03)00012-3. [DOI] [PubMed] [Google Scholar]
- 18.Mody I, MacDonald JF. NMDA receptor-dependent excitotoxicity: the role of intracellular Ca2+ release. Trends in Pharmacological Sciences. 1995;16:356–359. doi: 10.1016/s0165-6147(00)89070-7. [DOI] [PubMed] [Google Scholar]
- 19.Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer's disease with plaques and tangles: Intracellular A beta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 20.Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei H, Leeds P, Chen RW, Wei W, Leng Y, Bredesen DE, Chuang DM. Neuronal apoptosis induced by pharmacological concentrations of 3-hydroxykynurenine: characterization and protection by dantrolene and Bcl-2 overexpression. J Neurochem. 2000;75:81–90. doi: 10.1046/j.1471-4159.2000.0750081.x. [DOI] [PubMed] [Google Scholar]
- 22.Zhang H, Sun S, Herreman A, De SB, Bezprozvanny I. Role of presenilins in neuronal calcium homeostasis. J. Neurosci. 2010;30:8566–8580. doi: 10.1523/JNEUROSCI.1554-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao Y, Liang G, Chen Q, Joseph DJ, Meng Q, Eckenhoff RG, Eckenhoff MF, Wei H. Anesthetic-induced neurodegeneration mediated via inositol 1,4,5-trisphosphate receptors. J. Pharmacol. Exp. Ther. 2010;333:14–22. doi: 10.1124/jpet.109.161562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu X, Raina AK, Perry G, Smith MA. Alzheimer's disease: the two-hit hypothesis. Lancet Neurol. 2004;3:219–226. doi: 10.1016/S1474-4422(04)00707-0. [DOI] [PubMed] [Google Scholar]