Abstract
Several sporadic neurodegenerative diseases display phenomena that directly or indirectly relate to mitochondrial function. Data suggesting altered mitochondrial function in these diseases could arise from mitochondrial DNA (mtDNA) are reviewed. Approaches for manipulating mitochondrial function and minimizing the downstream consequences of mitochondrial dysfunction are discussed.
Keywords: cybrids, endophenotype, mitochondria, mitochondrial DNA, mitochondrial medicine, neurodegenerative disease
1. INTRODUCTION
Mitochondrial function is altered in several late-onset neurodegenerative diseases including Alzheimer’s disease (AD), Parkinson’s disease (PD), and amyotrophic lateral sclerosis (ALS) [1, 2]. When it comes to altered mitochondrial function in these conditions there are commonalities, such as the fact that changes are observed both within and beyond the nervous system and are therefore not simply a consequence of neurodegeneration [3, 4, 5]. There are also very specific differences that distinguish between the diseases. For example, AD subject mitochondria show reduced cytochrome oxidase (complex IV) activity, while PD subject mitochondria show reduced NADH:ubiquinone oxidoreductase (complex I) activity [6, 7, 8, 9].
Epidemiologic similarities also exist between AD, PD, and ALS. Each disease has relatively rare Mendelian variants and much more common sporadic variants [10, 11, 12]. It is important to note, though, that in this context “sporadic” does not mean genes do not contribute to disease risk or pathology. It simply means that recognizable autosomal dominant, autosomal recessive, or X-linked inheritance is not observed. Sporadic epidemiology also does not infer that a particular family is only allowed to have one affected member. It is well recognized that the offspring of an AD or PD-affected parent have an increased risk of developing AD or PD, and families in which there are both an affected parent and child are frequently ascertained [13, 14, 15]. Still, the percentage of the offspring of an AD parent that develop AD rarely reaches 50%, and the percentage of offspring of a PD parent that develop PD rarely reaches 50%. Autosomal dominant inheritance is therefore unlikely, or else if dominant mutations are responsible then they must have very low penetrance. Also, unless the responsible mutations are extremely common in the population, autosomal recessive inheritance would not readily account for kindreds that have affected members in different generations.
The non-autosomal, yet genetically-influenced epidemiology of the late-onset, sporadic neurodegenerative diseases suggest genetic factors that do not follow the rules of Mendelian genetics may play a role. Multiple lines of evidence indicate mtDNA may influence disease risk and also cause pathologic phenomena observed in the common sporadic variants of AD, PD, and ALS [16]. Understanding why and how mtDNA potentially contributes to these diseases is important because it yields mechanistic insights and identifies potential therapeutic targets. The first part of this review discusses evidence that argues mtDNA is relevant to various neurodegenerative diseases. The second part discusses therapeutic options from the perspective of an evolving field of medicine called mitochondrial medicine [17, 18, 19, 20].
2. MTDNA AND DISEASE
The human mtDNA consists of 16,569 nucleotides and includes 37 genes [21]. Thirteen of these genes encode electron transport chain (ETC) subunit proteins, two encode rRNA subunits used in mtDNA protein gene translation, and the remaining 22 encode tRNAs used in mtDNA protein gene translation. There is also a non-coding regulatory region that helps guide mtDNA replication and transcription [22].
Several diseases are recognized consequences of mtDNA mutations [1]. Some of the more common diseases include the mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes syndrome (MELAS); the myoclonic epilepsy and ragged red fiber disease syndrome (MERRF); the neuropathy, ataxia, and retinitis pigmentosa syndrome (NARP); and Leber’s hereditary optic neuropathy (LHON) [23, 24, 25, 26, 27]. MELAS often arises from an A3243G substitution in the tRNALeu(UUR) gene, MERRF often arises from a A8344G substitution in a tRNALys gene, NARP arises from a T8993G substitution in the ATPase 6 gene, and LHON can arise from mutation in several genes although the single most common mutation is a G11778A substitution in the ND4 gene. In most cases the causal mutations are present as high percentage heteroplasmies, meaning that the majority of mtDNA copies have the mutation, or the mutations are homoplasmic (all copies of mtDNA carry the mutation). Sometimes matrilineal inheritance patterns are discernable. Still, although these mutations are typically inherited from one generation to the next through maternal lines, matrilineal inheritance patterns are often not obvious.
Non-inherited mtDNA mutations may also mediate disease phenotypes. One example includes the mitochondrial neurogastrointestinal encephalopathy (MNGIE) syndrome, which is associated with mutations of the TYMP gene that encodes a thymidine phosphorylase enzyme [28]. TYMP mutation alters the intracellular nucleotide balance, which in turn causes the accumulation of mtDNA mutations and also mtDNA depletion. Although acquired mtDNA mutations are believed to drive MNGIE signs and symptoms, the disorder follows an autosomal recessive inheritance pattern since the ultimate cause is homozygous mutations in a nuclear gene. More recently, mutations in the nuclear gene that encodes the mtDNA polymerase gamma (POLG) protein required for mtDNA replication have been identified as a potential cause of PD [29, 30, 31, 32]. Impaired POLG function can cause an accelerated accumulation of mtDNA mutations, including point mutations and deletions [33, 34].
Several questions about the relationship between mtDNA mutation and human disease remain unresolved. One question relates to the issue of “threshold”. After all, an individual cell can contain thousands of mtDNA copies, and diverse heteroplasmic ratios are possible. A particular mtDNA mutation may reside in a cell at a very low level, and in some cases mutations may even reside on less than 1% of a cell’s mtDNA copies. Whether or not a heteroplasmic mutation causes a clinical phenotype is likely influenced by a combination of the mutation’s qualitative pathogenicity and its quantitative burden within an individual cell. For very deleterious mutations the burden of mutation required to affect biochemical function and thus surpass the disease threshold may be relatively low. For less deleterious mutations very high mutational burdens or perhaps even mutational homoplasmy may be needed to surpass the disease threshold. For the least deleterious mtDNA substitutions, potential clinical consequences may not always be obvious. For example, very mild mutations may only subtly affect a person’s risk for a particular disease rather than exert a deterministic effect, and act in conjunction with other factors to simply modify risk. Very mild mutations might also contribute to phenotypes that don’t readily manifest as a disease per se, but which instead manifest as accepted features of other conditions. Age-related functional changes might constitute one such example of this. It is in this context that mtDNA signatures may influence the risk of developing and also the progression of particular late-life neurodegenerative diseases.
A potential role for mtDNA in sporadic neurodegenerative diseases was almost simultaneously proposed by different investigators whose hypotheses were based on different assumptions. In the construct proposed by Parker, inherited mtDNA mutations were assumed to ultimately be critical [7, 35]. Parker postulated that in general mtDNA might influence the onset and progression of sporadic diseases that are nevertheless genetically influenced because mitochondrial genetic principles enable mtDNA to evade the rules of Mendelian genetics [16]. These unique mitochondrial genetic principles include maternal inheritance, heteroplasmy, threshold, and mitotic segregation; mitotic segregation refers to the fact that an individual’s different tissues, and within a tissue different cells, may accumulate different amounts of a heteroplasmic mutation. Figure 1 illustrates how these mitochondrial genetic principles let mtDNA contribute to sporadic-appearing diseases. According to an alternative hypothesis championed by several investigators including Wallace, an age-associated acquisition of mtDNA mutations in the central nervous system was assumed to represent the primary cause of late-onset, sporadic neurodegenerative diseases [36].
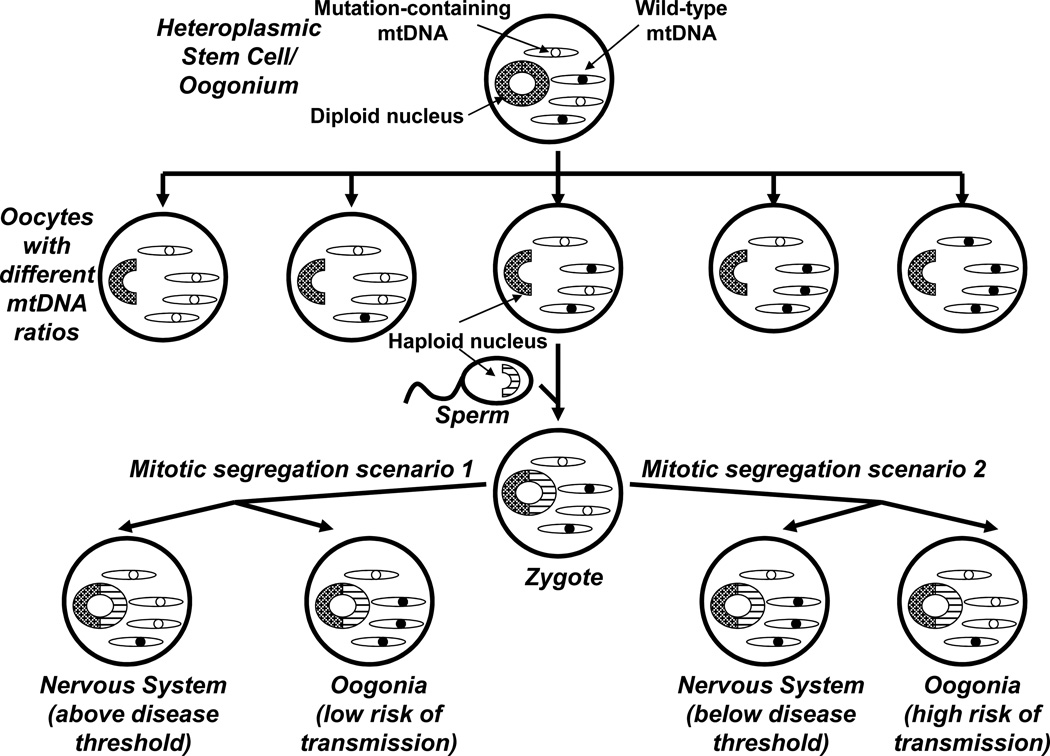
Figure 1. Maternal inheritance, heteroplasmy, mitotic segregation, and threshold effects distinguish mitochondrial genetics from Mendelian genetics.
During embryogenesis a female generates diploid oogonia, which produce haploid oocytes. An oogonium that contains a heteroplasmic mtDNA mutation may produce oocytes with different mtDNA mutation burdens; oocytes with low mutation levels are less likely to produce disease-affected offspring, while oocytes with high mutation levels are more likely to do so. Fertilization then produces a zygote whose mtDNA comes from the ooctye, with little or no sperm contribution. Because of mitotic segregation, heteroplasmic mtDNA mutations may end up having unequal tissue distributions. In scenario 1, the developing individual has a high mutational burden in the nervous system and low mutational burden in their germ cells. This individual has an elevated neurologic disease risk (surpasses the threshold) and low risk of passing on disease risk (if the individual is a male, there is no chance of passing on disease risk). In scenario 2, the developing individual ends up with a low mutational burden in the nervous system and high mutational burden in their germ cells. This individual does not have an elevated neurologic disease risk (below the threshold), but if the individual is female there is an increased risk of passing on the mtDNA mutation. Therefore, although mtDNA is maternally inherited diseases influenced by mtDNA-inheritance can appear sporadic.
In potential support of either view, it became increasingly apparent during the 1980s and 1990s that the mitochondrial ETC was altered in sporadic, late-onset neurodegenerative diseases and that these alterations were potentially relevant to the disease phenotype [2, 37, 38]. In some cases ETC dysfunction in a particular disease was rather specific. A systemic complex I defect was described in PD subjects, and the importance of this defect was emphasized by the fact that complex I inhibition had been found to cause parkinsonism in both humans and animals [3, 39, 40, 41, 42]. A systemic cytochrome oxidase defect was described in AD subjects, and the importance of this defect was emphasized through studies in which cytochrome oxidase inhibition was found to impair long term potentiation in rats [8, 38, 43]. The etiology of these disease-specific ETC defects, though, was not known. Exogenous toxins were considered as a possible cause, but no universal toxins that could explain the majority of disease cases were identified. Similarly, the possibility that endogenously generated ETC inhibitors might be responsible was considered but no such toxin could be reliably implicated. The fact that these ETC defects were systemic, and not simply a consequence of neurodegeneration, raised the question of whether they were gene-mediated.
This question could not be quickly answered through gene-sequencing studies. The ETC components are multi-unit holoenzymes; complex I alone contains 46 known subunits. Complexes I, III, IV, and V (complex V is not an ETC holoenzyme, although it is an indispensable component of the oxidative phosphorylation apparatus) are also bi-genomically encoded as they have subunits that are transcribed and translated from genes present on both nuclear and mtDNA. Further, the inclusion of mtDNA as a potential source of ETC miscoding introduced its own unique set of problems. MtDNA is highly variable between individuals, so it is not unusual to find mtDNA sequence deviations in disease-affected subjects [44]. Because by definition these diseases are sporadic, it is difficult to know whether the co-occurrence of a particular sequence deviation with a clinical phenotype is coincidental or etiologically relevant. Indeed, when evaluated from the context of an extended family, it is very likely a particular mtDNA polymorphism will not reliably segregate with disease. This indicates common mtDNA variants are very unlikely to cause disease, although it does not rule out a role for such variants in influencing disease risk. Also, until recently screening for low abundance heteroplasmic mutations was not routinely conducted and even now such surveys are rarely performed [45, 46, 47, 48, 49]. In the 1990s an alternative strategy, the cytoplasmic hybrid (cybrid) strategy, was therefore used to functionally assess whether unique mtDNA signatures might account for the known ETC defects present in AD and PD subjects [50].
3. DATA ADDRESSING A ROLE FOR MTDNA IN SPORADIC NEURODEGENERATIVE DISEASES
3.1 Cybrid Data
The cybrid technique was initially developed in the 1970s to assess the effects of particular mtDNAs on cell function [51]. In one of the earliest cybrid experiments, mtDNA from a chloromphanicol-resistant HeLa cell strain was transferred to a chloramphenicol-sensitive strain [52]. MtDNA transfer conferred chloramphenical resistance, and indicated chloramphenical resistance in HeLa cells is an mtDNA-determined trait. Over time, the technique was increasingly used to assess the consequences of known mtDNA mutations [53, 54, 55, 56, 57, 58]. This approach was assisted by the creation of mtDNA-depleted cell lines [59, 60, 61]. Since decades earlier mtDNA was commonly referred to as ρ DNA [62], these mtDNA-depleted cell lines were also called ρ0 cells [63]. ρ0 cells lack a functional ETC but can survive and expand in culture if appropriate metabolic support is provided. By transferring a known mtDNA sequence from an exogenous source to ρ0 cells, investigators were able to determine that sequence’s ability to restore ETC function and oxygen consumption rates [59]. The inability of a particular mutation-bearing mtDNA sequence to restore full function was assumed to reflect that mutation’s deleterious effects and potentially also its disease-relevance. Figure 2 summarizes the cybrid technique.
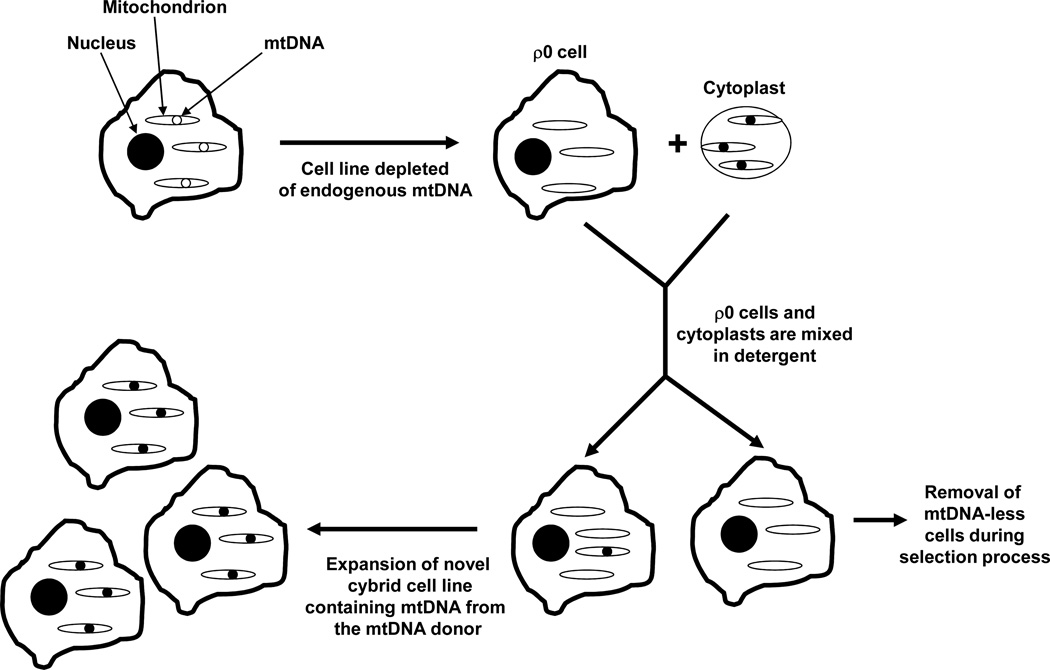
Figure 2. The cybrid technique.
The cybrid technique typically uses an mtDNA-depleted (ρ0) cell line and non-nucleated cytoplasts (such as platelets). A cybrid cell lines is produced by mixing the cytosolic contents of the ρ0 cells and cytoplasts. The mixing procedure, which is often facilitated by the temporary addition of a detergent, creates a population of cells that contain both a nucleus (from the ρ0 cell line) and mtDNA (from the cytoplast mitochondria). A selection process allows the isolation of these resulting cybrid cells, which can then be expanded in culture and used for biochemical and molecular analyses. The cybrid cell lines created from a common ρ0 cell nuclear background have equivalent nuclear genes, and the cell expansion environment is equivalent between cell lines. Therefore, biochemical or molecular differences between lines are expected to reflect differences between their mtDNA content.
Cybrid cell lines containing mtDNA from subjects with the common, sporadic forms of several late-onset neurodegenerative diseases have been generated and analyzed [50]. In most but not all of these studies subject mtDNA was obtained from platelets, since platelets are easily obtained and lack nuclei. Co-incubating platelets and ρ0 cells in the presence of the detergent polyethylene glycol allows platelet and ρ0 cell cytosolic contents to mix. After a brief period the detergent is quenched by dilution in a cell growth medium. A small percentage of cells that contain both a ρ0 cell nucleus and at least one platelet mitochondria will emerge. These cybrid cells are identified, isolated, and expanded. The result is a novel cell line that has the nucleus of the ρ0 cell line used to generate it, as well as the mtDNA from the platelet donor.
It is important to note the cytosolic transfer is not limited to mtDNA. Indeed, whole mitochondria transfer occurs. Because mitochondria undergo constant fusion and fission [64, 65], as the exogenous mtDNA replicates within the cell it reconstitutes the cell’s mtDNA content. During this period, the new cybrid cell line develops a unique mitochondrial population in which all its mitochondria’s nuclear-encoded proteins are generated by the nucleus from the ρ0 cell line, and all its mtDNA-encoded proteins are generated by its new mtDNA. Theoretically, the only difference between cybrid cell lines containing mtDNA from different donors is their mtDNA sequence. If different cybrid cell lines are functionally analyzed and found to have functional differences, as was discussed above the differences presumably reflect differences between mtDNA sequences.
Sporadic, late-onset neurodegenerative diseases that have been studied in depth include AD, PD, ALS, and progressive supranuclear palsy (PSP). Compared to cybrid cell lines prepared from control individuals, AD cybrid cell lines show reduced cytochrome oxidase activity, which recapitulates the systemic cytochrome oxidase activity reduction that was already known to exist in AD subjects [66, 67, 68]. Cybrid cell lines prepared from PD subjects have reduced complex I activity, which recapitulates the known PD subject complex I defect [69, 70, 71, 72]. ALS cybrids were found to have at least reduced complex I activity [73], which recapitulates a complex I activity reduction in ALS subject muscle [74, 75]. PSP cybrids have reduced complex I activity [76]. In each of these diseases the mtDNA-dependent ETC functional changes give rise to other abnormalities, including oxidative stress and protein aggregation phenomena [68, 72, 73, 77, 78, 79, 80, 81, 82, 83]. Results from most of the many cybrid studies published over the past 15 years were previously summarized in a number of comprehensive review articles [3, 4, 50, 84]. Table 1 summarizes the diseases in which cybrid studies have demonstrated specific respiratory chain (ETC or complex V) defects [3, 4, 50, 84, 85, 86, 87].
Table 1. Specific respiratory chain defects observed in cybrid models of human diseases.
This table summarizes the most consistently reported results from disease-oriented cybrid studies performed by different groups. Please note for some diseases there have been studies that report detectable abnormalities in alternative complexes or no detectable abnormalities. See text for details and references.
| Disease | Defect |
|---|---|
| Parkinson’s disease | Complex I |
| Alzheimer’s disease | Complex IV |
| Amyotrophic lateral sclerosis | Complex I |
| Progressive supranuclear palsy | Complex I |
| Leber’s hereditary optic neuropathy | Complex I |
| MELAS | Complex I with lower heteroplasmic burdens; Complexes I, III, and IV with higher heteroplasmic burdens |
| MERRF | Can include Complexes I, III, and IV |
| NARP / maternally inherited Leigh’s syndrome | Typically complex V; ETC enzymes may also be effected |
3.2 MtDNA Sequence Analysis Data
One limitation of the cybrid approach used to study late-onset, sporadic neurodegenerative diseases is that while disease-associated differences between disease and control cybrid cell lines imply disease-associated differences in mtDNA, the responsible mtDNA signature is not revealed. Various mtDNA sequence analysis studies have tried to address this knowledge gap [1, 3, 4, 38, 84, 88]. These studies often produce discordant results or reach different conclusions. It is nevertheless possible to venture several take-home messages.
First, it does not appear that for any of the diseases studied a single homoplasmic or nearly homoplasmic mtDNA deterministic mutation accounts for a significant percentage of cases. Second, studies that test for low abundance, heteroplasmic mtDNA mutations are uniformly positive. Some studies show deletion mutations, and some show point mutations. Whether these mutations are acquired, inherited, or both is not entirely clear. Such mutations are also found in control subjects. While some studies conclude there are no differences in either mutational burden or distribution, others report unique quantitative or qualitative signatures [45, 46, 47, 48, 49, 89, 90, 91, 92, 93]. Third, although deterministic homoplasmic mutations are an unlikely cause of disease, it may turn out that common homoplasmic mtDNA variations influence disease risk [44]. This is supported by a number of association studies that report particular mtDNA polymorphisms or haplogroups are more or less common in certain disease populations than they are in control populations [94, 95, 96, 97, 98, 99]. As is often the case with disease association studies, though, not all surveys yield positive results.
The big question at this time is not whether homoplasmic mtDNA variations or low-abundance heteroplasmic mutations are found in persons with diseases such as AD, PD, and ALS. They clearly are. Rather, the question now is whether these mtDNA variations or mutations influence disease risk, cause disease, or mediate at least some disease-associated pathologies. The ability of mitochondrial toxins to cause disease phenocopies and the presence of systemic mitochondrial dysfunction in disease-affected subjects further suggests altered mitochondrial function in persons with late-onset, sporadic neurodegenerative diseases is not simply an epiphenomenon. The fact that these functional mitochondrial alterations perpetuate in cultured cells following mtDNA transfer from affected subjects similarly argues mtDNA signatures are at least to some degree disease-relevant. In the case of AD a new line of investigation, endophenotype studies, is also consistent with a disease-relevant role for mtDNA.
3.3 Endophenotype Data
An “endophenotype” is a particular disease-associated characteristic or trait an individual demonstrates despite the fact that individual does not meet diagnostic criteria for the actual disease [4]. An endophenotype is typically demonstrable by biomarker studies or other types of approaches. The presence of a disease endophenotype does not necessarily mean the endophenotype carrier will develop the disease it is associated with, although it may imply the carrier individual has an increased risk of developing the disease. It often implies the affected individual has at least some genetic predisposition for the disease.
In AD, a family history of sporadic AD is a well-recognized risk factor as an individual’s risk of AD is increased if either parent has this disease [100]. Some degree of sporadic AD risk is certainly conferred via Mendelian genes. This is confirmed by the fact that particular Mendelian gene polymorphisms associate with AD. The most studied Mendelian associations are for two single nucleotide polymorphisms in the APOE gene on chromosome 19, which is found at the 19q13.2 region [101]. After almost 20 years of investigation it is still not entirely clear how the APOE gene product, apolipoprotein E protein, influences AD. Interestingly, polymorphisms in a neighboring gene that encodes the translocase of the outer mitochondrial membrane 40 (TOMM40) protein also reportedly influence AD risk [102, 103, 104, 105, 106, 107]. AD-associated TOMM40 and APOE gene polymorphisms are actually in linkage disequilibrium with each other. This means it is possible the TOMM40 protein, with its obvious role in mitochondrial function, is more relevant to AD than the apolipoprotein E protein.
Regardless of which gene or genes from the 19q13.2 drives AD risk, as a group middle aged, cognitively normal individuals with APOE4 alleles (the APOE variant associated with increased risk) have fluorodeoxyglucose positron emission tomography (FDG PET) scans that resemble those of AD subjects [108, 109]. Like the AD subjects, non-demented APOE4 carriers tend towards reduced glucose utilization in specific regions, including the parietal and posterior cingulate cortex. APOE4 carriers thus have a demonstrable AD endophenotype.
AD endophenotypes that are not observed in the children of AD-affected fathers are seen in the non-demented children of AD-diagnosed mothers [13]. Maternally inherited AD endophenotypes are demonstrable using various approaches. Compared to the children of AD fathers, the children of AD mothers have decreased glucose utilization on FDG PET [110, 111]. With brain amyloid imaging with Pittsburgh Compund B (PIB) PET, the children of AD mothers have more amyloid deposition [112]. Magnetic resonance imaging voxel based morphometry (MRI VBM) analyses reveal increased precuneus and parahippocampal atrophy and atrophy rates in those with a maternal AD family history [113, 114, 115]. As is the case with AD subjects, the children of AD mothers have low cerebrospinal fluid (CSF) Aβ42/Aβ40 ratios [116]. They also have increased CSF isoprostanes, which suggests increased oxidative stress is present [116]. Those with an AD maternal family history and an APOE4 allele have a unique AD endophenotype that is demonstrable through cognitive testing [117].
Maternally-inherited AD endophenotypes suggest a maternally inherited genetic factor such as mtDNA modifies AD risk. In this respect, it was recently reported that compared to those with an AD paternal family history, those with an AD maternal family history have lower platelet mitochondria cytochrome oxidase activities. Since cytochrome oxidase is partly encoded by mtDNA, this finding further suggests the responsible maternally inherited factor may be mtDNA.
4. MITOCHONDRIAL MEDICINE FOR NEURODEGENERATIVE DISEASES
4.1 Manipulating the Electron Transport Chain and Cell Energy Supplies
It is assumed ATP levels are reduced in at least the degenerating regions of neurodegenerative disease-affected brains. It is further presumed low ATP levels may alter cell physiology in ways that promote cell dysfunction and death. If correct, increasing cell ATP levels would seem to represent a reasonable therapeutic target. Several pathways and cycles that contribute to ATP production are shown in Figure 3.
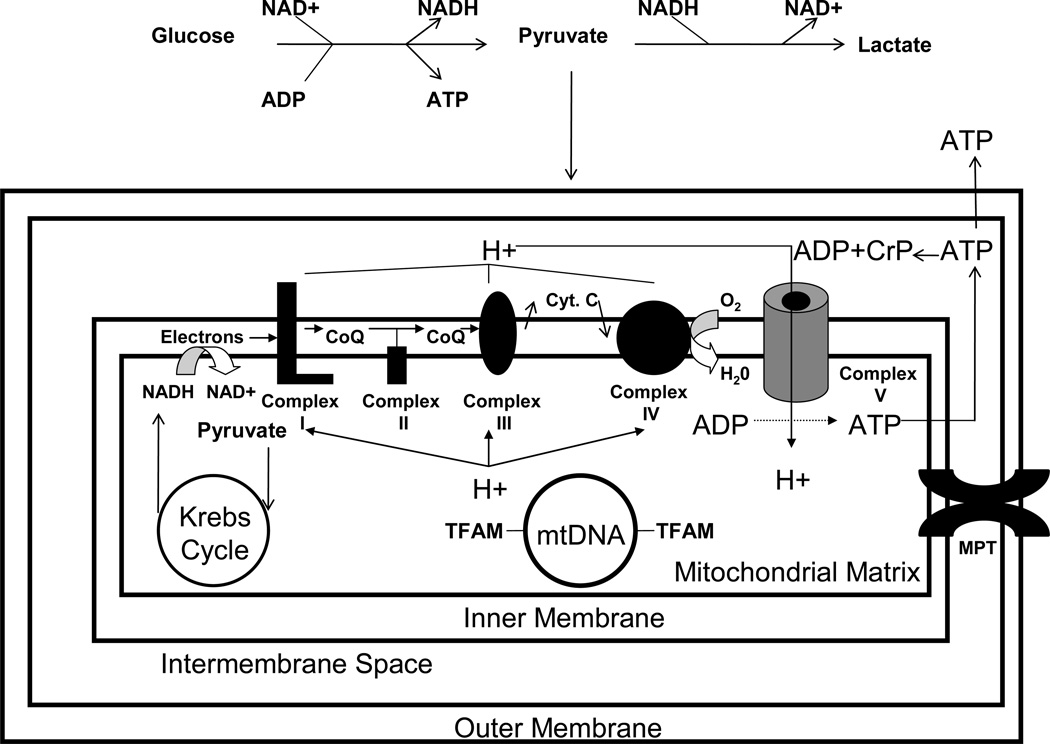
Figure 3. Simplified schematic of some pathways and cycles involved in ATP production.
Carbon intermediates from various sources, especially glycolysis, are imported into mitochondria and consumed to produce high energy electrons. These high energy electrons are transiently stored as reduced NADH. Energy harvested from the electrons fed to the inner mitochondrial membrane’s ETC creates a proton gradient; this energy is collected by the ATP synthase in the form of proton flux and used to phosphorylate ADP. High energy phosphate groups can also be stored as creatine phosphate. Also shown is the mtDNA, which is associated with TFAM protein (which helps mediate mtDNA replication and transcription), and the mitochondrial permeability transition (which is activated by mitochondrial membrane depolarization). CoQ=coenzyme; Cr-P=creatine phosphate; Cyt. C=cytochrome C.
Increasing electron flux through the ETC constitutes one potential therapeutic strategy. Various approaches have been used to attempt this. Dichloroacetate (DCA) inhibits pyruvate dehydrogenase kinase, which in turn activates pyruvate dehydrogenase complex [118]. The enzymatic conversion of pyruvate to acetyl CoA directly produces an NADH reducing equivalent, and should also increase Krebs cycle flux. While DCA has the ability to reduce blood lactate levels in MELAS patients [119], in the largest trial clinical benefits were not obvious and toxic side effects were noted [120].
Administering alternative electron carriers has been tried. The goal of this approach is essentially to bypass a known or suspected ETC block. For example, menadione and ascorbate used in combination allow ETC electron flux to circumvent a defective complex III enzyme [121]. While occasional anecdotal reports note a potential benefit in selected persons, in general this appears to have little impact. Methylene blue, which is used in the treatment of methemoglobinemia, is able to enhance cytochrome oxidase activity, possibly through a mechanism that involves a direct electron donation [122, 123, 124]. More recently, methylene blue has been advocated for the treatment of AD, either as a mitochondrial enhancing agent [125] or as an anti-neurofibrillary tangle agent [126]. In either case, if methylene blue is proven efficacious for the treatment of AD its ability to transfer electrons to cytochrome oxidase will need to be considered as a potential mechanism. The electron carriers coenzyme Q and idebenone, a water soluble ubiquinone analog, may also be included in this intervention category. Neither appear to have a big impact in subjects with late-onset, sporadic neurodegenerative diseases although a PD phase II trial did report over the course of the study those on coenzyme Q declined less than those on placebo [127]. Development of idebenone for AD was discontinued after a clinical trial showed an insufficient degree of benefit [128]. Idebenone may reduce ventricular hypertrophy and possibly confer a slight amount of neurologic improvement in persons with Friedreich’s ataxia (FA) [129, 130].
NADH and FADH2 supply high-energy electrons to the ETC. Nicotinic acid and nicotinamide, precursors of NADH, and riboflavin, an FADH2 precursor, have been tested in a variety of populations. The rationale is that supplementation with these precursors could increase mitochondrial reducing equivalents and ETC electron flux. Although riboflavin is sometimes used to reduce migraine frequency rates in persons with recurrent migraines [131], at this point none of these compounds have been shown to positively benefit persons with sporadic neurodegenerative diseases although results from clinical trials are either lacking or pending.
In terms of increasing energy stores, creatine supplementation has been tried in various neurodegenerative mitochondriopathies [132, 133]. The underlying rationale is that increasing creatine levels will enable cells to generate and store greater amounts of creatine phosphate. Under conditions of increased energy demand it is hoped the high energy phosphate of the creatine phosphate could be used to generate ATP. While creatine supplementation has not produced dramatic results in any clinical trials, and has had negative results in some [134, 135], at least in PD it currently cannot be concluded to constitute a futile approach [136, 137].
Supplementing brain bioenergetic metabolism with carbon fuels other than glucose represents yet another strategy for enhancing failing brain energy metabolism. The ketone bodies acetoacetate and β-hydroxybutyrate support neuron respiration [138]. It was empirically recognized decades ago that ketogenic diets, which boost acetoacetate and β-hydroxybutyrate levels, reduce seizure frequency in persons with epilepsy [139]. The effects this has on neuron respiration may contribute to this intervention’s efficacy. Using β-hydroxybutyrate as a way to enhance failing brain bioenergetics in AD was actually proposed in 1989 [140]. More recently this hypothesis was clinically tested. Clinical data from AD subjects are consistent with a possible benefit for at least some individuals, including in particular those without an APOE4 allele [141]. A supplement that increases β-hydroxybutyrate levels is currently available for the management of AD [142].
4.2 Manipulating Oxidative Stress
Oxidative stress is observed in multiple neurodegenerative disease states [143, 144]. This is demonstrable in the form of protein, lipid, or DNA oxidative modifications. In these cases oxidative stress is believed to arise as a consequence of free radical overproduction rather than reduced free radical scavenging. Free radicals are often cited as a potential cause of cell dysfunction, damage, and death and the mitochondrial ETC constitutes the single greatest source of cell free radical production [145]. Therefore, reducing free radicals and free-radical mediated chemistry has long been considered a reasonable mitochondrial medicine approach.
The earliest attempts to reduce oxidative stress focused on antioxidant vitamin supplements. For example, during the 1990s daily administration of a vitamin E, vitamin C, and beta carotene represented an often-used treatment for ALS despite a lack of objective supporting evidence [146]. High-dose vitamin E supplements were tried in PD without success [147]. In one prospective study, a group of AD patients taking 2000 IU per day of vitamin E took longer to reach predefined decline endpoints, such as nursing home placement, than a placebo group did [148]. While this was interpreted as a positive study, it is important to note the potential benefit was not completely straightforward because at the start of the trial the mini mental state exam (MMSE) score in the vitamin E group was slightly lower than that of the control group. The raw data from the study were subsequently adjusted to correct for this inequity. Without this mathematical adjustment there was no apparent advantage to the vitamin E treatment.
Multiple other compounds that accept electrons and could potentially reduce superoxide production have been evaluated. Idebenone was tested, without success, in AD [128]. As mentioned above, coenzyme Q was tested in a PD phase II and in that study a small benefit could not be excluded [127]. Coenzyme Q has also been evaluated in PSP [149].
More sophisticated antioxidant approaches have been developed and some have entered clinical trials. One important modification of the antioxidant approach includes the design of mitochondrially-targeted antioxidants [150]. This is accomplished by combining a cationic moiety with a classic antioxidant. MitoQ is perhaps the best known example of this [151]. MitoQ has been tested in PD subjects, but results to date are not encouraging [152].
Future antioxidant-based approaches may involve manipulations that enhance antioxidant enzyme expression [153, 154]. Investigators from the aging research field have thus far evaluated this approach. Results are mixed. In one study, expression of a mitochondrially-targeted catalase was found to extend lifespan in mice [155]. Other studies, though, in which antioxidant enzymes are over-expressed in transgenic mice have not produced lifespan extension [156, 157].
4.3 Manipulating Apoptosis
Evidence suggests apoptosis or apoptosis-related pathways or physiology may mediate cell death in neurodegenerative diseases [158]. Apoptotic signaling in these cases is believed to originate from mitochondria (the “internal” apoptotic pathway) [159, 160, 161, 162]. Efforts have focused on preventing the release of mitochondria-localized proteins that initiate apoptosis, such as cytochrome c. Strategies considered include preventing activation of the mitochondrial permeability transition (MPT) [163, 164], and preventing mitochondrial swelling. Manipulating ratios of proteins that regulate the MPT, especially ratios of Bcl-2 family proteins, constitutes another approach [165, 166]. Compounds that accomplish these ends are also sometimes referred to as mitochondrial stabilizing agents.
In cell culture and animal studies minocycline blocks the pore that is formed during MPT activation [167, 168]. It has been tested in ALS, but in a human trial minocycline-treated subjects actually did worse than placebo-treated subjects [169]. Rasagaline, which is approved as an MAO B inhibitor for the treatment of PD, has been shown under in vitro conditions to increase Bcl2/Bax ratios [170]. This helps mitigate toxin-induced mitochondrial depolarization and apoptosis [171, 172, 173, 174]. For these reasons it has been postulated rasagaline may have possible neuroprotective, or “disease modifying”, effects in PD. This possibility was tested in a clinical trial, but the results were not clear-cut [175]. In this trial the results from the low-dose rasagaline treatment group were consistent with a disease modifying effect, but data from the high-dose treatment group were not. R+ pramipexole, another reported mitochondrial stabilizing agent, is being evaluated in ALS clinical trials [176].
Latrepirdine, which was originally developed as an anithistamine, is reported by some to have mitochondrial stabilizing properties. This claim is based on a study that found latrepirdine reduced swelling of mitochondria exposed to Aβ, and a study in cultured cells that found it helped preserve mitochondrial membrane potentials under stress conditions [177, 178]. Its ability to mediate such effects in humans has been questioned [179]. In a phase II AD clinical study latrepirdine-treated subjects demonstrated improved function over placebo-treated subjects [180], but in a larger AD phase III trial latrepirdine had no effect [181]. Results from other phase III trials of latrepirdine in AD have yet to be reported.
4.4 Manipulating Mitochondrial Autophagy
Mitochondrial autophagy, also called mitophagy, refers to the intracellular removal of mitochondria through phagosome formation and subsequent lysosomal digestion [65, 182]. It provides a mechanism through which a cell can rid itself of failed or dysfunctional mitochondria [183]. Increased and altered mitochondrial autophagy is observed in AD, and evidence suggests autophagy is likely perturbed in other neurodegenerative disorders as well [184, 185, 186]. Further, mutations that cause Mendelian PD affect proteins that mediate autophagy, which implies autophagy plays a role in preventing PD while impaired autophagy contributes to PD [187, 188, 189, 190, 191].
From a conceptual standpoint, mitochondrial autophagy constitutes an attractive mitochondrial medicine approach since enhancing autophagy may help with mitochondrial quality control. Several years ago lithium was reported to prolong ALS patient survival, and the authors of that study also presented data that suggested its mechanism of action could involve mitophagy activation [192]. Unfortunately, additional clinical trials found lithium did not benefit ALS patients [193, 194]. Rapamycin, which has been shown to extend longevity in mice [195], is another drug being considered as a potential autophagy-inducing treatment for various neurodegenerative diseases [196, 197, 198]. Rapamycin activates autophagy through a mammalian target of rapamycin (mTOR)-dependent process [199, 200, 201].
4.5 Manipulating Mitochondrial Mass
Quantifying how many mitochondria a cell contains is challenging because they are physically dynamic organelles. A single mitochondrion may fission into individual, smaller mitochondria. Conversely, individual mitochondria can fuse together and even form a syncytium that extends throughout the cytosol [64, 65]. Mitochondrial fission and fusion are altered in AD brain and also AD fibroblasts [202, 203].
For this reason, considering how much mitochondria a cell contains may constitute a more physiologically meaningful parameter than how many mitochondria it has. Mitochondrial mass has been evaluated in different sporadic, late-onset neurodegenerative diseases. While results of studies that quantify total mitochondrial mass can vary, the mass of intact mitochondria is reduced in AD brain and also ALS spinal cord [185, 204, 205, 206]. In terms of developing mitochondrial medicine for the treatment of these and other related disorders, increasing mitochondrial mass is therefore a reasonable goal.
Mitochondrial mass is increased through the process of mitochondrial biogenesis. Mitochondrial biogenesis requires the synthesis of new mitochondrial protein, membrane, and mtDNA. This is accomplished through the coordinated efforts of transcriptional programs located within the nucleus [207]. In particular, the peroxisomal proliferator activated receptor gamma coactivator 1α (PGC1a) co-transcriptional factor in at least some tissues serves as a master regulator of mitochondrial mass [208, 209, 210]. PGC1a is itself regulated by various enzymes, transcription factors, and intracellular signaling pathways that monitor cell energy levels and metabolic states. Silent information regulator 1 (SIRT1), AMP kinase (AMPK), cyclic AMP responsive element binding protein (CREB), mTOR, the forkhead transcription factors (FOXOs), and AKT all associate with PGC1a in that they either directly or indirectly influence or are influenced by PGC1a [211, 212, 213, 214, 215, 216, 217, 218, 219, 220, 221, 222, 223, 224, 225, 226, 227]. Several of these relationships are summarized in Figure 4.
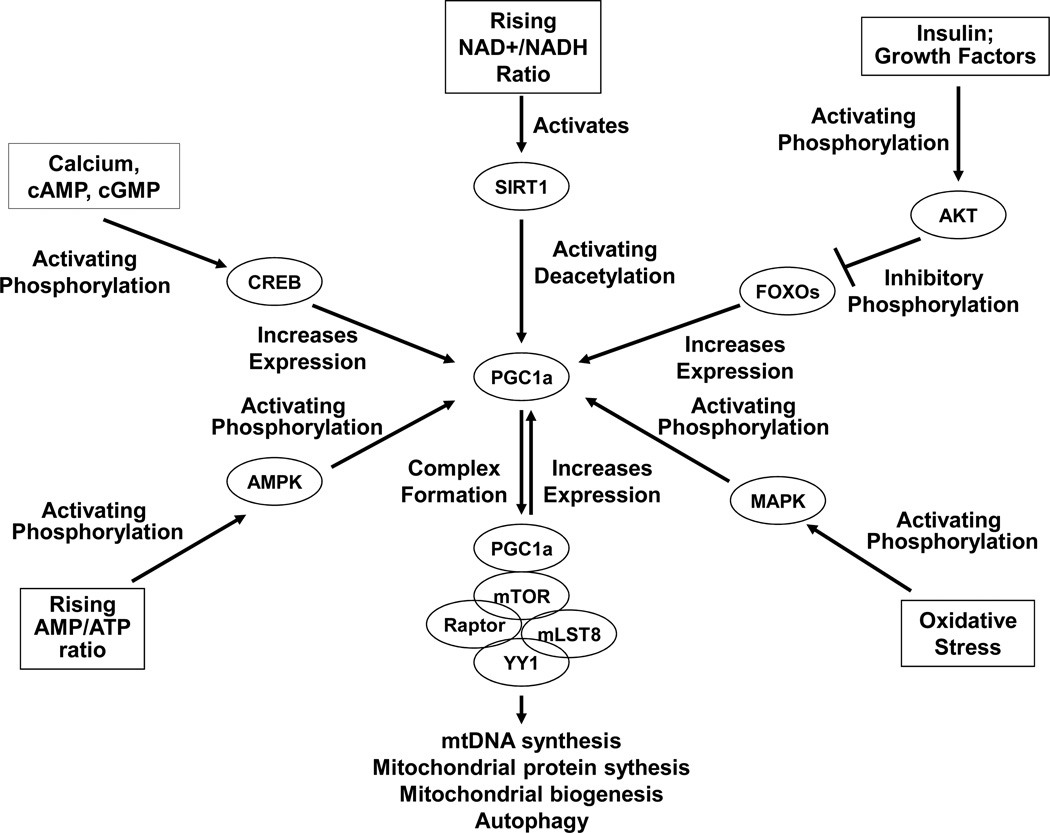
Figure 4. PGC1a, a key regulator of mitochondrial biogenesis, is regulated by various nutrient, energy, and stress-sensing pathways.
This diagram presents a schematic summary of different proteins and transcription factors that regulate PGC1a activity and levels. It is very simplified, and as more is learned about mitochondrial biogenesis the indicated relationships may change. Briefly, SIRT1-mediated PGC1a deacetylation and MAPK-mediated PGC1a phosphorylation induce PGC1a activation. AMPK also activates PGC1a through a direct phosphorylation event or indirectly through intermediates. The CREB transcription factor activates PGC1a expression. A FOXO transcription factor, FKHR, also appears to activate PGC1a expression; by retaining FOXOs in the cytosol, AKT phosphorylation of FOXOs would prevent this contribution. Activated PGC1a reportedly forms a super-complex with the mTOR-containing TORC1 complex (mTOR, raptor, and mLST8) and the YY1 transcription factor. This activated super-complex expresses genes needed to replicate mtDNA, produce mitochondrial proteins, accomplish mitochondrial biogenesis, and support autophagy. It also leads to the production of more PGC1a.
Certain drugs are known to increase PGC1a levels or increase its activity. Two thiazolidinedione drugs currently used to treat type II diabetes mellitus (DMII), pioglitazone and rosiglitazone, are examples [228, 229, 230]. In the case of these thiazolidinediones, PGC1a activation may actually contribute to their mode of action, which is a decrease in peripheral insulin resistance [231, 232]. Both these agents have been tested in AD, with results that range from no demonstrable benefit to a possible small benefit in selected treatment groups [233, 234, 235]. One limitation of these trials is that the thiazolidinediones poorly penetrate the blood-brain barrier (BBB). It seems unlikely that doses used in humans would generate brain levels that are high enough to induce a robust mitochondrial biogenesis [228]. Bezafibrate, another drug that activates PGC1a, has been tested in cell culture and animal models with mitochondrial dysfunction and in these models it appears to improve cell bioenergetics [236, 237].
When it comes to developing mitochondrial biogenesis induction approaches it may be useful to consider exercise and dietary restriction. Exercise is associated with improved health, and dietary restriction extends lifespan in invertebrate and vertebrate species [238, 239, 240, 241, 242, 243, 244]. Exercise increases muscle mitochondrial mass, and this likely mediates to some extent its physiologic benefits [245, 246, 247, 248, 249]. Dietary restriction induces liver mitochondrial biogenesis, and also has been reported to induce mitochondrial biogenesis in other tissues although its extra-hepatic impact may be less robust [250, 251, 252, 253, 254].
It is not surprising that exercise and dietary restriction should impact non-brain tissues more than they impact the brain. The brain is a metabolically privileged organ in the sense that the rest of the body has evolved to keep the brain in a state of bioenergetic homeostasis. For example, falling blood glucose levels activates liver gluconeogenesis and this helps ensure a steady delivery of glucose to the brain. In states of more extreme nutritional stress the liver will produce and secrete the ketone bodies acetoacetate and β-hydroxybutyrate, which are used in the brain as a bioenergetic supplement that fuels neuron respiration [138, 255]. Tight regulation of brain homeostasis may limit the degree to which exercise and dietary restriction can benefit the brain. This is not to say these interventions do not have a brain impact, as data certainly indicate that they do [254, 256, 257, 258]. These benefits, though, may represent relatively indirect sequellae that arise as a consequence of direct affects on other tissues. For example, both exercise and dietary restriction increase peripheral insulin sensitivity and reduce insulin production. This, in turn, should reduce brain insulin levels and brain insulin signaling [259]. Obviously, when it comes to the brain effects of exercise and dietary restriction, very complex relationships may exist. In any case, identifying effective ways to induce brain mitochondrial biogenesis by mimicking the effects of exercise on muscle or of dietary restriction on liver may prove useful.
4.6 Manipulating Redox State
For the purposes of this section redox state refers to the ratio of nicotinamide adenine dinucleotide’s (NAD) oxidized (NAD+) and reduced (NADH) states. In the cytosol NAD+ is reduced to form NADH during glycolysis, and in order for glycolysis to continue it is necessary to re-oxidize NADH back to NAD+. In the mitochondrial matrix NAD+ is reduced to NADH during the conversion of pyruvate to acetyl CoA and each fully executed run through the Krebs cycle generates another three NADH. Mitochondrial NADH, in turn, is used to feed high energy electrons into the ETC at complex I. Energy from these high energy electrons is used to pump protons from the matrix to the intermembrane space. This creates an electrochemical gradient that drives the ATP synthase proton flux and ADP phosphorylation.
A case has been made for increasing the cytosolic NAD+/NADH ratio [18, 260]. Activation of SIR2 in C. elegans and its mammalian homolog, SIRT1, is associated with life extension in dietary restriction [261]. NAD+ is consumed by sirtuin enzymes and an oxidized redox state activates sirtuins [262, 263, 264, 265]. It is therefore possible that increasing the cytosolic NAD+/NADH ratio may have sirtuin-mediated health benefits. Polyphenolic compounds such as resveratrol, which activate sirtuins, are currently being evaluated for the treatment of human diseases [266, 267, 268, 269, 270, 271]. Activating sirtuins could produce a number of downstream effects, including PGC1a activation. Increasing the cytosolic NAD+/NADH ratio, either through bioenergetic manipulation or by supplying NAD+ mimetics, should similarly accomplish this goal [272].
It is also possible that pushing the mitochondrial NAD pool to a more reduced state, or a lower NAD+/NADH ratio, could benefit brain bioenergetics in conditions where brain bioenergetics are impaired. In this respect Blass and Gibson have advocated malate, which may increase mitochondrial NADH levels, should constitute part of a bioenergetics-based approach to treating AD [273]. Malate is transported from the cytosol to the mitochondrial matrix, and inside the matrix the oxidation of malate to oxaloacete generates NADH.
4.7 Manipulating mtDNA
The most direct way of fixing an mtDNA-induced bioenergetic problem would be to correct the responsible mtDNA defect. Approaches to accomplish this have been considered. In the case of a deleterious heteroplasmic point mutation, it is possible under in vitro conditions to shift the heteroplasmic ratio towards a greater percentage of wild type mtDNA by selectively blocking replication of mtDNA molecules that contain the mutation [274]. This requires the ability to distinguish between mtDNA sequences. In the past, sequence-specific peptide nucleic acids which can bind complementary DNA sequences but which do not prime extension and which block new strand synthesis have been used to accomplish this [275].
As is the case with nuclear DNA, mtDNA associates with proteins. Transcription factor A of the mitochondria (TFAM) is the most important of these proteins and plays an important role in both mtDNA replication and transcription [276, 277, 278]. Increasing TFAM levels reportedly enhances mtDNA levels, mitochondrial biogenesis, respiration rates, or a combination of these events. One study reported that when TFAM was overexpressed, cell mtDNA levels increased but mitochondrial mass and mitochondrial respiration did not [279]. Another study found TFAM overexpression reduced age-associated complex I and IV reductions in mouse brain, and this was associated with reduced brain oxidative stress and behavioral testing benefits [280].
Administering an exogenous TFAM that is linked to a mitochondrial leader sequence and protein transduction domain reportedly increased respiration and mitochondrial biogenesis in cultured cells and mouse tissues [281]. This strategy may therefore provide a pharmacologic approach for increasing mtDNA replication and transcription in vivo, and increase both immediate and long term respiration through these effects. Further, because TFAM readily binds mtDNA, it may be possible to use this recombinant TFAM to deliver exogenous mtDNA molecules to mitochondria [282]. Given that it is already possible to deliver restriction enzymes to mitochondria [283, 284, 285], this could prove to be a powerful way to remove even homoplasmic mtDNA mutations from cells and replace the mutated mtDNA with mtDNA that does not contain the mutation [286].
5. MITOCHONDRIAL MEDICINE CONSIDERATIONS
5.1 Pros and Cons of Manipulating Cell Energy Supplies
It is assumed that increasing ATP levels in bioenergetically compromised neurons should benefit those neurons. While this is a straightforward assumption, it is necessary to consider data from the dietary restriction literature that in general suggest some degree of bioenergetic stress, or hormesis, can prove advantageous [287, 288]. This is not to suggest that reducing ATP levels to sub-normal levels is good. Rather, forcing neurons towards a more aerobic state could have wide ranging consequences, such AMPK and SIRT1 activation.
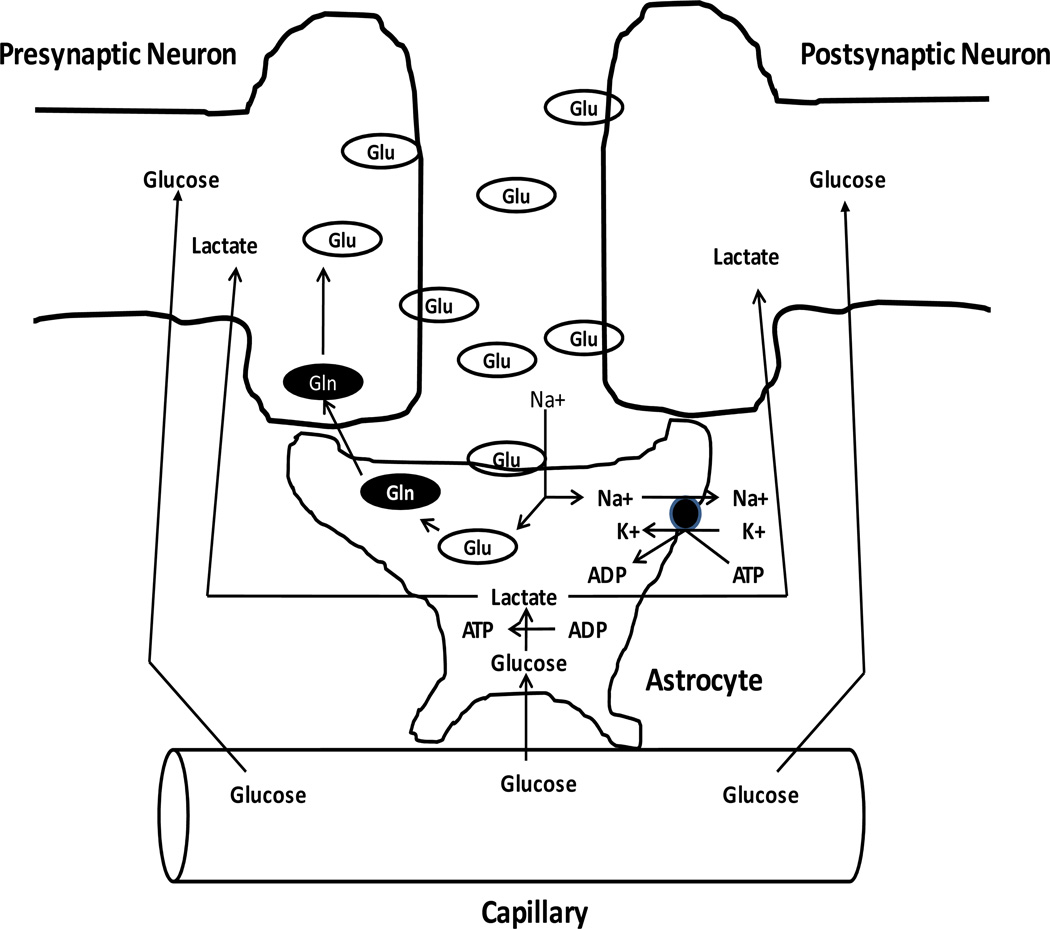
Another point to consider is that the brain is not a metabolically homogeneous entity. It consists of highly aerobic neurons and relatively anaerobic astrocytes. There is an emerging realization that neuron and astrocyte energy metabolism are intricately linked [289, 290, 291, 292, 293, 294]. As part of this relationship, glutamate release by active neurons is followed by astrocyte glutamate uptake. This uptake is coupled to and driven by sodium gradients. Exporting the acquired sodium is an energy dependent process that activates astrocyte aerobic glycolysis and produces lactate. The lactate is then exported to neurons where it is used to support mitochondrial respiration. This “tripartite synapse” model is illustrated in Figure 5. How manipulating brain energy metabolism may influence this dynamic relationship requires consideration.
Figure 5. The tripartite synapse.
An action potential induces glutamate release from a pre-synaptic neuron, which activates an excitatory post-synaptic potential. The glutamate signal is terminated by an astrocyte, which removes the glutamate from the synaptic cleft; this astrocyte glutamate uptake is driven by the plasma membrane sodium gradient. Astrocyte sodium-potassium ATPase pumps are activated by the sodium influx. The ATP this consumes is regenerated in the astrocyte through glycolysis, and generates lactate. The astrocyte lactate is exported by the astrocyte, imported by the neighboring neurons, and used by those neurons to fuel aerobic ATP production. This process also stimulates astrocyte uptake of capillary glucose, and provides for the return of the glutamate carbons, in the form of glutamine, to the presynaptic neuron.
5.2 Pros and Cons of Manipulating Oxidative Stress
It is assumed that decreasing oxidative stress in bioenergetically compromised neurons should benefit those neurons. Again, although this seems quite reasonable cautionary notes must be taken from other lines of investigation. Oxidative stress actually plays a crucial role in various intracellular signaling pathways [295, 296]. Indeed, dampening oxidative stress has been shown to abrogate the lifespan extension that results in C. elegans when the worms are treated with 2-deoxyglucose [287]. Antioxidants also minimize the endurance benefits that result from aerobic training [297, 298, 299]. In both cases, these adverse antioxidant effects associate with reduced mitochondrial biogenesis. It is also worth noting nitric oxide and superoxide signaling have been implicated in learning and memory [300, 301].
5.3 Pros and Cons of Manipulating Apoptosis
While it is tempting to assume blocking apoptosis in cells with mitochondrial dysfunction that is severe enough to activate apoptosis cannot have a downside, it is important to keep in mind that the pathways that mediate apoptosis do not exist in an all-or-none state. Some degree of caspase enzyme activity occurs in normal cells and likely has physiologically important and necessary roles [302, 303, 304]. Also, in the case of mtDNA-induced mitochondrial dysfunction apoptosis should represent a relatively downstream event. Changes that occur in the run-up to apoptosis, when occurring below the threshold needed to initiate cell death, may mediate part of a compensatory stress response. Interfering with compensatory adaptations can cause more harm than good. In this respect, it is interesting that minocycline treatment of ALS subjects actually accelerated mortality [169].
5.4 Pros and Cons of Manipulating Autophagy
The ability to selectively dispose of damaged or dysfunctional mitochondria is particularly attractive. Moreover, in dietary restriction mitophagy is increased [251, 305, 306]. This would certainly support the view that enhancing mitophagy beyond baseline levels could have therapeutic benefits. Still, for cases in which mtDNA mutation drives mitochondrial dysfunction, it is not clear that mitophagy mechanisms are dysfunctional or that these mechanisms require manipulation. Also, the consequences of enhancing mitophagy in cells with uniformly impaired mitochondria could prove complex. This point is emphasized by the prior finding that mitochondrial respiration itself can differentially influence mitophagy regulation and its functional outcomes [189].
5.5 Pros and Cons of Manipulating Mitochondrial Mass
Data suggest that in some tissues, increasing mitochondrial mass may constitute a normal response to age-associated declines in mitochondrial function [185, 307, 308]. This includes the brain. It may even be the case that with age-related diseases in which a particular biochemical or molecular phenomenon represents an exaggeration of a particular age-associated phenomenon, the changeover from aging to disease occurs when compensatory mechanisms cease to compensate [4, 84, 309]. For example, brain mitochondrial mass may increase with normal aging, but in AD itself the number of intact mitochondria is reduced [185]. If this decline contributes to the disease, then inducing mitochondrial biogenesis would seem to represent a logical therapeutic goal.
One potential downside is that increasing the mass of dysfunctional mitochondria within a cell may have adverse consequences. Increasing the mass of dysfunctional mitochondria within a compromised but still partly functional cell could push oxidative stress or apoptotic signaling above tolerable levels.
5.6 Pros and Cons of Manipulating Redox States
As discussed above, one line of reasoning presumes that increasing cytosolic NAD+/NADH ratios will enhance bioenergetic capacity. Increasing this ratio should increase glycolysis flux, which may in turn compensate for reduced mitochondrial ATP production. On the other hand, pushing cells that should be in a more aerobic state to a less aerobic state may have unintended consequences.
Another line of reasoning presumes that pushing the mitochondrial NAD pool towards reduction will benefit mitochondrial respiration. It is difficult to know, however, whether increasing mitochondrial NADH levels will increase respiration in functionally impaired mitochondria. A case can even be made for the opposite argument, which is that pushing the mitochondrial NAD pool towards oxidation could improve overall cell function by activating compensatory stress signaling pathways or compensation-enhancing transcription programs. A more oxidized mitochondrial NAD (or FAD) pool might also facilitate the transfer of reducing equivalents from the cytosol to the mitochondria, which could potentially increase glycolysis.
Finally, SIRT2 de-acetylates tubulin and this leads to microtubule depolymerization [310, 311, 312]. Excessive tubulin de-acetylation could potentially disrupt cytoskeletal infrastructure and secondarily impair mitochondrial axonal transport as well as the transport of aggregated proteins [313]. Indeed, in the PD cybrid model microtubule de-acetylation and alpha-synuclein oligomer levels directly correlate, and further data suggest tubulin de-acetylation may contribute to alpha synuclein oligomerization [314]. Consistent with this argument, reducing SIRT2 activity was found to be protective in cell and Drosophila PD models [315]. Similarly, an AD transgenic mouse model was shown to benefit from nicotinamide-induced sirtuin inhibition [316].
5.7 Pros and Cons of Manipulating mtDNA
It is possible that preventing replication of a particular mtDNA species, or eliminating a particular mtDNA species, could cause a transient or permanent state of relative mtDNA depletion. In such a case cleansing the cell of one form of mtDNA problem could introduce another type of mtDNA problem. Otherwise, when it comes to treating mtDNA-driven mitochondrial dysfunction through correction of a responsible mtDNA defect it is hard to think of a downside. Progress towards accomplishing this goal will hopefully continue.
6. CONCLUSIONS
A variety of pathologies observed in the late-onset, sporadic versions of several neurodegenerative diseases may arise from or relate to altered or deficient mitochondrial function. Some of these diseases, including AD, PD, and ALS, have relatively uncommon Mendelian forms in which nuclear gene mutations directly or indirectly induce mitochondrial dysfunction. What initiates mitochondrial dysfunction in the far more common sporadic versions is less clear, although data suggest in this circumstance mtDNA plays a role.
Given the existence of a mitochondrial nexus in common late-onset, sporadic neurodegenerative diseases, efforts targeted towards the therapeutic manipulation of mitochondria certainly seem reasonable. A number of approaches are probably worth pursuing, although two cautionary points are worth making. The first point is that when manipulating a specific aspect of mitochondrial function or mitochondria-related physiology, it is probably important not to extinguish or reverse potentially compensatory adaptations. The second point is that interventions which seem eminently rationale may have unforeseen negative consequences. Thus, the efficacy of a mitochondrial medicine approach may be maximized and the potential risks minimized by manipulating the most upstream identifiable cause of the observed mitochondrial dysfunction.
ACKNOWLEDGEMENTS
RHS is supported by a grant from the Morgan Family Foundation.
ABBREVIATIONS
- AD
Alzheimer’s disease
- ALS
amyotrophic lateral sclerosis
- AMPK
AMP kinase
- APOE
apolipoprotein E gene
- BBB
blood-brain barrier
- CoQ
coenzyme Q
- CREB
cyclic AMP responsive element binding protein
- CrP
creatine phosphate
- CSF
cerebrospinal fluid
- Cybrid
cytoplasmic hybrid
- Cyt. C
cytochrome c
- DCA
dichloroacetate
- DMII
diabetes mellitus type II
- ETC
electron transport chain
- FA
Friedreich’s ataxia
- FAD
flavin adenine dinucleotide
- FDG
fluorodeoxyglucose
- FKHR
forkhead homologue in rhabdomyosarcoma
- FOXO
forkhead O-box transcription factor
- LHON
Leber’s hereditary optic neuropathy
- MELAS
mitochondrial encephalopathy, lactic acid, and stroke syndrome
- MERRF
myoclonic epilepsy and ragged red fiber syndrome
- mLST8
target of rapamycin complex subunit LST8
- MAPK
mitogen activated protein kinase
- MMSE
mini-mental state exam
- MNGIE
mitochondrial neurogastrointestinal encephalopathy syndrome
- MPT
mitochondrial permeability transition
- MRI
magnetic resonance spectroscopy
- mtDNA
mitochondrial DNA
- mTOR
mammalian target of rapamycin
- NAD
nicotinamide adenine dinucleotide
- NAD+
nicotinamide adenine dinucleotide, oxidized
- NADH
nicotinamide adenine dinucleotide, reduced
- NARP
neuropathy, ataxia, and retinitis pigmentosa syndrome
- PD
Parkinson’s disease
- PET
positron emission tomography
- PGC1a
peroxisomal proliferator activated receptor gamma coactivator 1α
- PIB
Pittsburgh Compound B
- POLG
polymerase gamma
- PSP
progressive supranuclear palsy
- SIR(T)
Silent information regulator
- TOMM40
translocase of the outer mitochondrial membrane 40 gene
- TFAM
transcription factor A of the mitochondria
- TORC1
mTOR complex 1
- TYMP
thymidine phosphorylase gene
- VBM
voxel based morphometry
- YY1
yin-yang 1
REFERENCES
- 1.Swerdlow RH. The neurodegenerative mitochondriopathies. J Alzheimers Dis. 2009;17:737–751. doi: 10.3233/JAD-2009-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swerdlow RH. Treating neurodegeneration by modifying mitochondria: potential solutions to a "complex" problem. Antioxid Redox Signal. 2007;9:1591–1603. doi: 10.1089/ars.2007.1676. [DOI] [PubMed] [Google Scholar]
- 3.Swerdlow RH. Does mitochondrial DNA play a role in Parkinson's disease? A review of cybrid and other supportive evidence. Antioxid Redox Signal. 2011 doi: 10.1089/ars.2011.3948. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swerdlow RH, Burns JM, Khan SM. The Alzheimer's disease mitochondrial cascade hypothesis. J Alzheimers Dis. 2010;20(Suppl 2):S265–S279. doi: 10.3233/JAD-2010-100339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swerdlow RH, Parks JK, Pattee G, Parker WD., Jr Role of mitochondria in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:185–190. doi: 10.1080/14660820050515179. [DOI] [PubMed] [Google Scholar]
- 6.Kish SJ, Bergeron C, Rajput A, Dozic S, Mastrogiacomo F, Chang LJ, et al. Brain cytochrome oxidase in Alzheimer's disease. J Neurochem. 1992;59:776–779. doi: 10.1111/j.1471-4159.1992.tb09439.x. [DOI] [PubMed] [Google Scholar]
- 7.Parker WD, Jr, Boyson SJ, Parks JK. Abnormalities of the electron transport chain in idiopathic Parkinson's disease. Ann Neurol. 1989;26:719–723. doi: 10.1002/ana.410260606. [DOI] [PubMed] [Google Scholar]
- 8.Parker WD, Jr, Filley CM, Parks JK. Cytochrome oxidase deficiency in Alzheimer's disease. Neurology. 1990;40:1302–1303. doi: 10.1212/wnl.40.8.1302. [DOI] [PubMed] [Google Scholar]
- 9.Schapira AH, Cooper JM, Dexter D, Jenner P, Clark JB, Marsden CD. Mitochondrial complex I deficiency in Parkinson's disease. Lancet. 1989;1:1269. doi: 10.1016/s0140-6736(89)92366-0. [DOI] [PubMed] [Google Scholar]
- 10.Swerdlow RH. Is aging part of Alzheimer's disease, or is Alzheimer's disease part of aging? Neurobiol Aging. 2007;28:1465–1480. doi: 10.1016/j.neurobiolaging.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 11.Tanner CM, Goldman SM. Epidemiology of Parkinson's disease. Neurol Clin. 1996;14:317–335. doi: 10.1016/S0733-8619(05)70259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Majoor-Krakauer D, Willems PJ, Hofman A. Genetic epidemiology of amyotrophic lateral sclerosis. Clin Genet. 2003;63:83–101. doi: 10.1046/j.0009-9163.2002.00001.x. [DOI] [PubMed] [Google Scholar]
- 13.Mosconi L, Berti V, Swerdlow RH, Pupi A, Duara R, de Leon M. Maternal transmission of Alzheimer's disease: prodromal metabolic phenotype and the search for genes. Hum Genomics. 2010;4:170–193. doi: 10.1186/1479-7364-4-3-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swerdlow RH, Parker WD, Currie LJ, Bennett JP, Harrison MB, Trugman JM, et al. Gender ratio differences between Parkinson's disease patients and their affected relatives. Parkinsonism Relat Disord. 2001;7:129–133. doi: 10.1016/s1353-8020(00)00029-8. [DOI] [PubMed] [Google Scholar]
- 15.Swerdlow RH, Parks JK, Davis JN, 2nd, Cassarino DS, Trimmer PA, Currie LJ, et al. Matrilineal inheritance of complex I dysfunction in a multigenerational Parkinson's disease family. Ann Neurol. 1998;44:873–881. doi: 10.1002/ana.410440605. [DOI] [PubMed] [Google Scholar]
- 16.Swerdlow RH. Mitochondrial DNA--related mitochondrial dysfunction in neurodegenerative diseases. Arch Pathol Lab Med. 2002;126:271–280. doi: 10.5858/2002-126-0271-MDRMDI. [DOI] [PubMed] [Google Scholar]
- 17.Luft R. The development of mitochondrial medicine. Proc Natl Acad Sci U S A. 1994;91:8731–8738. doi: 10.1073/pnas.91.19.8731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swerdlow RH. Mitochondrial medicine and the neurodegenerative mitochondriopathies. Pharmaceuticals. 2009;2:150–167. doi: 10.3390/ph2030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haller RG, Vissing J. Drilling for energy in mitochondrial disease. Arch Neurol. 2009;66:931–932. doi: 10.1001/archneurol.2009.155. [DOI] [PubMed] [Google Scholar]
- 20.Armstrong JS. Mitochondrial medicine: pharmacological targeting of mitochondria in disease. Br J Pharmacol. 2007;151:1154–1165. doi: 10.1038/sj.bjp.0707288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson S, Bankier AT, Barrell BG, de Bruijn MH, Coulson AR, Drouin J, et al. Sequence and organization of the human mitochondrial genome. Nature. 1981;290:457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- 22.Clayton DA. Transcription and replication of mitochondrial DNA. Hum Reprod. 2000;15(Suppl 2):11–17. doi: 10.1093/humrep/15.suppl_2.11. [DOI] [PubMed] [Google Scholar]
- 23.Goto Y, Nonaka I, Horai S. A mutation in the tRNA(Leu)(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature. 1990;348:651–653. doi: 10.1038/348651a0. [DOI] [PubMed] [Google Scholar]
- 24.Shoffner JM, Lott MT, Lezza AM, Seibel P, Ballinger SW, Wallace DC. Myoclonic epilepsy and ragged-red fiber disease (MERRF) is associated with a mitochondrial DNA tRNA(Lys) mutation. Cell. 1990;61:931–937. doi: 10.1016/0092-8674(90)90059-n. [DOI] [PubMed] [Google Scholar]
- 25.Rojo A, Campos Y, Sanchez JM, Bonaventura I, Aguilar M, Garcia A, et al. NARP-MILS syndrome caused by 8993 T>G mitochondrial DNA mutation: a clinical, genetic and neuropathological study. Acta Neuropathol. 2006;111:610–616. doi: 10.1007/s00401-006-0040-5. [DOI] [PubMed] [Google Scholar]
- 26.Newman NJ. Leber's hereditary optic neuropathy. New genetic considerations. Arch Neurol. 1993;50:540–548. doi: 10.1001/archneur.1993.00540050082021. [DOI] [PubMed] [Google Scholar]
- 27.Wallace DC, Singh G, Lott MT, Hodge JA, Schurr TG, Lezza AM, et al. Mitochondrial DNA mutation associated with Leber's hereditary optic neuropathy. Science. 1988;242:1427–1430. doi: 10.1126/science.3201231. [DOI] [PubMed] [Google Scholar]
- 28.Lara MC, Valentino ML, Torres-Torronteras J, Hirano M, Marti R. Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE): biochemical features and therapeutic approaches. Biosci Rep. 2007;27:151–163. doi: 10.1007/s10540-007-9043-2. [DOI] [PubMed] [Google Scholar]
- 29.Davidzon G, Greene P, Mancuso M, Klos KJ, Ahlskog JE, Hirano M, et al. Early-onset familial parkinsonism due to POLG mutations. Ann Neurol. 2006;59:859–862. doi: 10.1002/ana.20831. [DOI] [PubMed] [Google Scholar]
- 30.Hudson G, Schaefer AM, Taylor RW, Tiangyou W, Gibson A, Venables G, et al. Mutation of the linker region of the polymerase gamma-1 (POLG1) gene associated with progressive external ophthalmoplegia and Parkinsonism. Arch Neurol. 2007;64:553–557. doi: 10.1001/archneur.64.4.553. [DOI] [PubMed] [Google Scholar]
- 31.Luoma P, Melberg A, Rinne JO, Kaukonen JA, Nupponen NN, Chalmers RM, et al. Parkinsonism, premature menopause, and mitochondrial DNA polymerase gamma mutations: clinical and molecular genetic study. Lancet. 2004;364:875–882. doi: 10.1016/S0140-6736(04)16983-3. [DOI] [PubMed] [Google Scholar]
- 32.Luoma PT, Eerola J, Ahola S, Hakonen AH, Hellstrom O, Kivisto KT, et al. Mitochondrial DNA polymerase gamma variants in idiopathic sporadic Parkinson disease. Neurology. 2007;69:1152–1159. doi: 10.1212/01.wnl.0000276955.23735.eb. [DOI] [PubMed] [Google Scholar]
- 33.Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, et al. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309:481–484. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- 34.Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429:417–423. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- 35.Parker WD. Sporadic neurologic disease and the electron transport chain: a hypothesis. In: Pascuzzi RM, editor. Proceedings of the 1989 Scientfic Meeting of the American Society for Neurological Investigation: New Developments in Neuromuscular Disease Bloomington. Indiana: Indiana University Printing Services; 1990. [Google Scholar]
- 36.Wallace DC. Mitochondrial genetics: a paradigm for aging and degenerative diseases? Science. 1992;256:628–632. doi: 10.1126/science.1533953. [DOI] [PubMed] [Google Scholar]
- 37.Parker WD, Jr, Swerdlow RH. Mitochondrial dysfunction in idiopathic Parkinson disease. Am J Hum Genet. 1998;62:758–762. doi: 10.1086/301812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swerdlow RH, Kish SJ. Mitochondria in Alzheimer's disease. Int Rev Neurobiol. 2002;53:341–385. doi: 10.1016/s0074-7742(02)53013-0. [DOI] [PubMed] [Google Scholar]
- 39.Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219:979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- 40.Nicklas WJ, Vyas I, Heikkila RE. Inhibition of NADH-linked oxidation in brain mitochondria by 1-methyl-4-phenyl-pyridine, a metabolite of the neurotoxin, 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. Life Sci. 1985;36:2503–2508. doi: 10.1016/0024-3205(85)90146-8. [DOI] [PubMed] [Google Scholar]
- 41.Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- 42.Swerdlow RH. Mitochondria and Parkinson's Disease. In: Chesselet M-F, editor. Molecular Mechanisms of Neurodegenerative Diseases. Totowa, New Jersey: Humana Press; 2000. [Google Scholar]
- 43.Bennett MC, Diamond DM, Stryker SL, Parks JK, Parker WD., Jr Cytochrome oxidase inhibition: a novel animal model of Alzheimer's disease. J Geriatr Psychiatry Neurol. 1992;5:93–101. doi: 10.1177/002383099200500206. [DOI] [PubMed] [Google Scholar]
- 44.Lu J, Wang K, Rodova M, Esteves R, Berry D, E L, et al. Polymorphic variation in cytochrome oxidase subunit genes. J Alzheimers Dis. 2010;21:141–154. doi: 10.3233/JAD-2010-100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin MT, Simon DK, Ahn CH, Kim LM, Beal MF. High aggregate burden of somatic mtDNA point mutations in aging and Alzheimer's disease brain. Hum Mol Genet. 2002;11:133–145. doi: 10.1093/hmg/11.2.133. [DOI] [PubMed] [Google Scholar]
- 46.Parker WD, Jr, Parks JK. Mitochondrial ND5 mutations in idiopathic Parkinson's disease. Biochem Biophys Res Commun. 2005;326:667–669. doi: 10.1016/j.bbrc.2004.11.093. [DOI] [PubMed] [Google Scholar]
- 47.Simon DK, Lin MT, Zheng L, Liu GJ, Ahn CH, Kim LM, et al. Somatic mitochondrial DNA mutations in cortex and substantia nigra in aging and Parkinson's disease. Neurobiol Aging. 2004;25:71–81. doi: 10.1016/s0197-4580(03)00037-x. [DOI] [PubMed] [Google Scholar]
- 48.Smigrodzki R, Parks J, Parker WD. High frequency of mitochondrial complex I mutations in Parkinson's disease and aging. Neurobiol Aging. 2004;25:1273–1281. doi: 10.1016/j.neurobiolaging.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 49.Cantuti-Castelvetri I, Lin MT, Zheng K, Keller-McGandy CE, Betensky RA, Johns DR, et al. Somatic mitochondrial DNA mutations in single neurons and glia. Neurobiol Aging. 2005;26:1343–1355. doi: 10.1016/j.neurobiolaging.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 50.Swerdlow RH. Mitochondria in cybrids containing mtDNA from persons with mitochondriopathies. J Neurosci Res. 2007;85:3416–3428. doi: 10.1002/jnr.21167. [DOI] [PubMed] [Google Scholar]
- 51.Bunn CL, Wallace DC, Eisenstadt JM. Cytoplasmic inheritance of chloramphenicol resistance in mouse tissue culture cells. Proc Natl Acad Sci U S A. 1974;71:1681–1685. doi: 10.1073/pnas.71.5.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wallace DC, Bunn CL, Eisenstadt JM. Cytoplasmic transfer of chloramphenicol resistance in human tissue culture cells. J Cell Biol. 1975;67:174–188. doi: 10.1083/jcb.67.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chomyn A, Martinuzzi A, Yoneda M, Daga A, Hurko O, Johns D, et al. MELAS mutation in mtDNA binding site for transcription termination factor causes defects in protein synthesis and in respiration but no change in levels of upstream and downstream mature transcripts. Proc Natl Acad Sci U S A. 1992;89:4221–4225. doi: 10.1073/pnas.89.10.4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.King MP, Koga Y, Davidson M, Schon EA. Defects in mitochondrial protein synthesis and respiratory chain activity segregate with the tRNA(Leu(UUR)) mutation associated with mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke like episodes. Mol Cell Biol. 1992;12:480–490. doi: 10.1128/mcb.12.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chomyn A, Lai ST, Shakeley R, Bresolin N, Scarlato G, Attardi G. Platelet-mediated transformation of mtDNA-less human cells: analysis of phenotypic variability among clones from normal individuals--and complementation behavior of the tRNALys mutation causing myoclonic epilepsy and ragged red fibers. Am J Hum Genet. 1994;54:966–974. [PMC free article] [PubMed] [Google Scholar]
- 56.Masucci JP, Davidson M, Koga Y, Schon EA, King MP. In vitro analysis of mutations causing myoclonus epilepsy with ragged-red fibers in the mitochondrial tRNA(Lys)gene: two genotypes produce similar phenotypes. Mol Cell Biol. 1995;15:2872–2881. doi: 10.1128/mcb.15.5.2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hofhaus G, Johns DR, Hurko O, Attardi G, Chomyn A. Respiration and growth defects in transmitochondrial cell lines carrying the 11778 mutation associated with Leber's hereditary optic neuropathy. J Biol Chem. 1996;271:13155–13161. doi: 10.1074/jbc.271.22.13155. [DOI] [PubMed] [Google Scholar]
- 58.Vergani L, Martinuzzi A, Carelli V, Cortelli P, Montagna P, Schievano G, et al. MtDNA mutations associated with Leber's hereditary optic neuropathy: studies on cytoplasmic hybrid (cybrid) cells. Biochem Biophys Res Commun. 1995;210:880–888. doi: 10.1006/bbrc.1995.1740. [DOI] [PubMed] [Google Scholar]
- 59.King MP, Attardi G. Human cells lacking mtDNA: repopulation with exogenous mitochondria by complementation. Science. 1989;246:500–503. doi: 10.1126/science.2814477. [DOI] [PubMed] [Google Scholar]
- 60.Miller SW, Trimmer PA, Parker WD, Jr, Davis RE. Creation and characterization of mitochondrial DNA-depleted cell lines with "neuronal-like" properties. J Neurochem. 1996;67:1897–1907. doi: 10.1046/j.1471-4159.1996.67051897.x. [DOI] [PubMed] [Google Scholar]
- 61.Binder DR, Dunn WH, Jr, Swerdlow RH. Molecular characterization of mtDNA depleted and repleted NT2 cell lines. Mitochondrion. 2005;5:255–265. doi: 10.1016/j.mito.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 62.Ephrussi B, Hottinger H, Chimenes A. Action de l'acriflavine sur les levures, I: la mutation "petite clonie". Ann Inst Pasteur. 1949;76:531. [Google Scholar]
- 63.Morais R, Desjardins P, Turmel C, Zinkewich-Peotti K. Development and characterization of continuous avian cell lines depleted of mitochondrial DNA. In Vitro Cell Dev Biol. 1988;24:649–658. doi: 10.1007/BF02623602. [DOI] [PubMed] [Google Scholar]
- 64.Chan DC. Mitochondrial fusion and fission in mammals. Annu Rev Cell Dev Biol. 2006;22:79–99. doi: 10.1146/annurev.cellbio.22.010305.104638. [DOI] [PubMed] [Google Scholar]
- 65.Chen H, Chan DC. Mitochondrial dynamics--fusion, fission, movement, and mitophagy--in neurodegenerative diseases. Hum Mol Genet. 2009;18:R169–R176. doi: 10.1093/hmg/ddp326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cardoso SM, Santana I, Swerdlow RH, Oliveira CR. Mitochondria dysfunction of Alzheimer's disease cybrids enhances Abeta toxicity. J Neurochem. 2004;89:1417–1426. doi: 10.1111/j.1471-4159.2004.02438.x. [DOI] [PubMed] [Google Scholar]
- 67.Sheehan JP, Swerdlow RH, Miller SW, Davis RE, Parks JK, Parker WD, et al. Calcium homeostasis and reactive oxygen species production in cells transformed by mitochondria from individuals with sporadic Alzheimer's disease. J Neurosci. 1997;17:4612–4622. doi: 10.1523/JNEUROSCI.17-12-04612.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Swerdlow RH, Parks JK, Cassarino DS, Maguire DJ, Maguire RS, Bennett JP, Jr, et al. Cybrids in Alzheimer's disease: a cellular model of the disease? Neurology. 1997;49:918–925. doi: 10.1212/wnl.49.4.918. [DOI] [PubMed] [Google Scholar]
- 69.Esteves AR, Domingues AF, Ferreira IL, Januario C, Swerdlow RH, Oliveira CR, et al. Mitochondrial function in Parkinson's disease cybrids containing an nt2 neuron-like nuclear background. Mitochondrion. 2008;8:219–228. doi: 10.1016/j.mito.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 70.Gu M, Cooper JM, Taanman JW, Schapira AH. Mitochondrial DNA transmission of the mitochondrial defect in Parkinson's disease. Ann Neurol. 1998;44:177–186. doi: 10.1002/ana.410440207. [DOI] [PubMed] [Google Scholar]
- 71.Shults CW, Miller SW. Reduced complex I activity in parkinsonian cybrids. Movement Disorders. 1998;13(suppl. 2):217. [Google Scholar]
- 72.Swerdlow RH, Parks JK, Miller SW, Tuttle JB, Trimmer PA, Sheehan JP, et al. Origin and functional consequences of the complex I defect in Parkinson's disease. Ann Neurol. 1996;40:663–671. doi: 10.1002/ana.410400417. [DOI] [PubMed] [Google Scholar]
- 73.Swerdlow RH, Parks JK, Cassarino DS, Trimmer PA, Miller SW, Maguire DJ, et al. Mitochondria in sporadic amyotrophic lateral sclerosis. Exp Neurol. 1998;153:135–142. doi: 10.1006/exnr.1998.6866. [DOI] [PubMed] [Google Scholar]
- 74.Vielhaber S, Kunz D, Winkler K, Wiedemann FR, Kirches E, Feistner H, et al. Mitochondrial DNA abnormalities in skeletal muscle of patients with sporadic amyotrophic lateral sclerosis. Brain. 2000;123(Pt 7):1339–1348. doi: 10.1093/brain/123.7.1339. [DOI] [PubMed] [Google Scholar]
- 75.Wiedemann FR, Winkler K, Kuznetsov AV, Bartels C, Vielhaber S, Feistner H, et al. Impairment of mitochondrial function in skeletal muscle of patients with amyotrophic lateral sclerosis. J Neurol Sci. 1998;156:65–72. doi: 10.1016/s0022-510x(98)00008-2. [DOI] [PubMed] [Google Scholar]
- 76.Swerdlow RH, Golbe LI, Parks JK, Cassarino DS, Binder DR, Grawey AE, et al. Mitochondrial dysfunction in cybrid lines expressing mitochondrial genes from patients with progressive supranuclear palsy. J Neurochem. 2000;75:1681–1684. doi: 10.1046/j.1471-4159.2000.0751681.x. [DOI] [PubMed] [Google Scholar]
- 77.Cassarino DS, Fall CP, Swerdlow RH, Smith TS, Halvorsen EM, Miller SW, et al. Elevated reactive oxygen species and antioxidant enzyme activities in animal and cellular models of Parkinson's disease. Biochim Biophys Acta. 1997;1362:77–86. doi: 10.1016/s0925-4439(97)00070-7. [DOI] [PubMed] [Google Scholar]
- 78.Khan SM, Cassarino DS, Abramova NN, Keeney PM, Borland MK, Trimmer PA, et al. Alzheimer's disease cybrids replicate beta-amyloid abnormalities through cell death pathways. Ann Neurol. 2000;48:148–155. [PubMed] [Google Scholar]
- 79.Domingues AF, Arduino DM, Esteves AR, Swerdlow RH, Oliveira CR, Cardoso SM. Mitochondria and ubiquitin-proteasomal system interplay: relevance to Parkinson's disease. Free Radic Biol Med. 2008;45:820–825. doi: 10.1016/j.freeradbiomed.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 80.Esteves AR, Arduino DM, Swerdlow RH, Oliveira CR, Cardoso SM. Oxidative Stress involvement in alpha-synuclein oligomerization in Parkinsons disease cybrids. Antioxid Redox Signal. 2008 doi: 10.1089/ars.2008.2247. [DOI] [PubMed] [Google Scholar]
- 81.Albers DS, Swerdlow RH, Manfredi G, Gajewski C, Yang L, Parker WD, Jr, et al. Further evidence for mitochondrial dysfunction in progressive supranuclear palsy. Exp Neurol. 2001;168:196–198. doi: 10.1006/exnr.2000.7607. [DOI] [PubMed] [Google Scholar]
- 82.Trimmer PA, Borland MK, Keeney PM, Bennett JP, Jr, Parker WD., Jr Parkinson's disease transgenic mitochondrial cybrids generate Lewy inclusion bodies. J Neurochem. 2004;88:800–812. doi: 10.1046/j.1471-4159.2003.02168.x. [DOI] [PubMed] [Google Scholar]
- 83.Trimmer PA, Swerdlow RH, Parks JK, Keeney P, Bennett JP, Jr, Miller SW, et al. Abnormal mitochondrial morphology in sporadic Parkinson's and Alzheimer's disease cybrid cell lines. Exp Neurol. 2000;162:37–50. doi: 10.1006/exnr.2000.7333. [DOI] [PubMed] [Google Scholar]
- 84.Swerdlow RH, Khan SM. The Alzheimer's disease mitochondrial cascade hypothesis: an update. Exp Neurol. 2009;218:308–315. doi: 10.1016/j.expneurol.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.D'Aurelio M, Vives-Bauza C, Davidson MM, Manfredi G. Mitochondrial DNA background modifies the bioenergetics of NARP/MILS ATP6 mutant cells. Hum Mol Genet. 2010;19:374–386. doi: 10.1093/hmg/ddp503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dunbar DR, Moonie PA, Zeviani M, Holt IJ. Complex I deficiency is associated with 3243G:C mitochondrial DNA in osteosarcoma cell cybrids. Hum Mol Genet. 1996;5:123–129. doi: 10.1093/hmg/5.1.123. [DOI] [PubMed] [Google Scholar]
- 87.Bornstein B, Mas JA, Patrono C, Fernandez-Moreno MA, Gonzalez-Vioque E, Campos Y, et al. Comparative analysis of the pathogenic mechanisms associated with the G8363A and A8296G mutations in the mitochondrial tRNA(Lys) gene. Biochem J. 2005;387:773–778. doi: 10.1042/BJ20040949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Swerdlow RH, Weaver B, Grawey A, Wenger C, Freed E, Worrall BB. Complex I polymorphisms, bigenomic heterogeneity, and family history in Virginians with Parkinson's disease. J Neurol Sci. 2006;247:224–230. doi: 10.1016/j.jns.2006.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Corral-Debrinski M, Horton T, Lott MT, Shoffner JM, McKee AC, Beal MF, et al. Marked changes in mitochondrial DNA deletion levels in Alzheimer brains. Genomics. 1994;23:471–476. doi: 10.1006/geno.1994.1525. [DOI] [PubMed] [Google Scholar]
- 90.Coskun PE, Beal MF, Wallace DC. Alzheimer's brains harbor somatic mtDNA control-region mutations that suppress mitochondrial transcription and replication. Proc Natl Acad Sci U S A. 2004;101:10726–10731. doi: 10.1073/pnas.0403649101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Coskun PE, Wyrembak J, Derbereva O, Melkonian G, Doran E, Lott IT, et al. Systemic mitochondrial dysfunction and the etiology of Alzheimer's disease and down syndrome dementia. J Alzheimers Dis. 2010;20(Suppl 2):S293–S310. doi: 10.3233/JAD-2010-100351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bender A, Krishnan KJ, Morris CM, Taylor GA, Reeve AK, Perry RH, et al. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet. 2006;38:515–517. doi: 10.1038/ng1769. [DOI] [PubMed] [Google Scholar]
- 93.Kraytsberg Y, Kudryavtseva E, McKee AC, Geula C, Kowall NW, Khrapko K. Mitochondrial DNA deletions are abundant and cause functional impairment in aged human substantia nigra neurons. Nat Genet. 2006;38:518–520. doi: 10.1038/ng1778. [DOI] [PubMed] [Google Scholar]
- 94.Mancuso M, Conforti FL, Rocchi A, Tessitore A, Muglia M, Tedeschi G, et al. Could mitochondrial haplogroups play a role in sporadic amyotrophic lateral sclerosis? Neurosci Lett. 2004;371:158–162. doi: 10.1016/j.neulet.2004.08.060. [DOI] [PubMed] [Google Scholar]
- 95.van der Walt JM, Dementieva YA, Martin ER, Scott WK, Nicodemus KK, Kroner CC, et al. Analysis of European mitochondrial haplogroups with Alzheimer disease risk. Neurosci Lett. 2004;365:28–32. doi: 10.1016/j.neulet.2004.04.051. [DOI] [PubMed] [Google Scholar]
- 96.van der Walt JM, Nicodemus KK, Martin ER, Scott WK, Nance MA, Watts RL, et al. Mitochondrial polymorphisms significantly reduce the risk of Parkinson disease. Am J Hum Genet. 2003;72:804–811. doi: 10.1086/373937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Autere J, Moilanen JS, Finnila S, Soininen H, Mannermaa A, Hartikainen P, et al. Mitochondrial DNA polymorphisms as risk factors for Parkinson's disease and Parkinson's disease dementia. Hum Genet. 2004;115:29–35. doi: 10.1007/s00439-004-1123-9. [DOI] [PubMed] [Google Scholar]
- 98.Ghezzi D, Marelli C, Achilli A, Goldwurm S, Pezzoli G, Barone P, et al. Mitochondrial DNA haplogroup K is associated with a lower risk of Parkinson's disease in Italians. Eur J Hum Genet. 2005;13:748–752. doi: 10.1038/sj.ejhg.5201425. [DOI] [PubMed] [Google Scholar]
- 99.Pyle A, Foltynie T, Tiangyou W, Lambert C, Keers SM, Allcock LM, et al. Mitochondrial DNA haplogroup cluster UKJT reduces the risk of PD. Ann Neurol. 2005;57:564–567. doi: 10.1002/ana.20417. [DOI] [PubMed] [Google Scholar]
- 100.Katzman R. Alzheimer's disease. N Engl J Med. 1986;314:964–973. doi: 10.1056/NEJM198604103141506. [DOI] [PubMed] [Google Scholar]
- 101.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 102.Bekris LM, Millard SP, Galloway NM, Vuletic S, Albers JJ, Li G, et al. Multiple SNPs within and surrounding the apolipoprotein E gene influence cerebrospinal fluid apolipoprotein E protein levels. J Alzheimers Dis. 2008;13:255–266. doi: 10.3233/jad-2008-13303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yu CE, Seltman H, Peskind ER, Galloway N, Zhou PX, Rosenthal E, et al. Comprehensive analysis of APOE and selected proximate markers for late-onset Alzheimer's disease: patterns of linkage disequilibrium and disease/marker association. Genomics. 2007;89:655–665. doi: 10.1016/j.ygeno.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Takei N, Miyashita A, Tsukie T, Arai H, Asada T, Imagawa M, et al. Genetic association study on in and around the APOE in late-onset Alzheimer disease in Japanese. Genomics. 2009;93:441–448. doi: 10.1016/j.ygeno.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 105.Bekris LM, Galloway NM, Montine TJ, Schellenberg GD, Yu CE. APOE mRNA and protein expression in postmortem brain are modulated by an extended haplotype structure. Am J Med Genet B Neuropsychiatr Genet. 2009;153B:409–417. doi: 10.1002/ajmg.b.30993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Potkin SG, Guffanti G, Lakatos A, Turner JA, Kruggel F, Fallon JH, et al. Hippocampal atrophy as a quantitative trait in a genome-wide association study identifying novel susceptibility genes for Alzheimer's disease. PLoS One. 2009;4:e6501. doi: 10.1371/journal.pone.0006501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Roses AD, Lutz MW, Amrine-Madsen H, Saunders AM, Crenshaw DG, Sundseth SS, et al. A TOMM40 variable-length polymorphism predicts the age of late-onset Alzheimer's disease. Pharmacogenomics J. 2009 doi: 10.1038/tpj.2009.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Reiman EM, Caselli RJ, Yun LS, Chen K, Bandy D, Minoshima S, et al. Preclinical evidence of Alzheimer's disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med. 1996;334:752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- 109.Small GW, Mazziotta JC, Collins MT, Baxter LR, Phelps ME, Mandelkern MA, et al. Apolipoprotein E type 4 allele and cerebral glucose metabolism in relatives at risk for familial Alzheimer disease. JAMA. 1995;273:942–947. [PubMed] [Google Scholar]
- 110.Mosconi L, Brys M, Switalski R, Mistur R, Glodzik L, Pirraglia E, et al. Maternal family history of Alzheimer's disease predisposes to reduced brain glucose metabolism. Proc Natl Acad Sci U S A. 2007;104:19067–19072. doi: 10.1073/pnas.0705036104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mosconi L, Mistur R, Switalski R, Brys M, Glodzik L, Rich K, et al. Declining brain glucose metabolism in normal individuals with a maternal history of Alzheimer disease. Neurology. 2009;72:513–520. doi: 10.1212/01.wnl.0000333247.51383.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mosconi L, Rinne JO, Tsui WH, Berti V, Li Y, Wang H, et al. Increased fibrillar amyloid-{beta} burden in normal individuals with a family history of late-onset Alzheimer's. Proc Natl Acad Sci U S A. 2010;107:5949–5954. doi: 10.1073/pnas.0914141107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Berti V, Mosconi L, Glodzik L, Li Y, Murray J, De Santi S, et al. Structural brain changes in normal individuals with a maternal history of Alzheimer's. Neurobiol Aging. 2011 doi: 10.1016/j.neurobiolaging.2011.01.001. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Honea RA, Swerdlow RH, Vidoni E, Burns JM. Progressive regional atrophy in normaladults with a maternal history of Alzheimer disease. Neurology. 2011;76:822–829. doi: 10.1212/WNL.0b013e31820e7b74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Honea RA, Swerdlow RH, Vidoni ED, Goodwin J, Burns JM. Reduced gray matter volume in normal adults with a maternal family history of Alzheimer disease. Neurology. 2010;74:113–120. doi: 10.1212/WNL.0b013e3181c918cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mosconi L, Glodzik L, Mistur R, McHugh P, Rich KE, Javier E, et al. Oxidative stress and amyloid-beta pathology in normal individuals with a maternal history of Alzheimer's. Biol Psychiatry. 2010;68:913–921. doi: 10.1016/j.biopsych.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Debette S, Wolf PA, Beiser A, Au R, Himali JJ, Pikula A, et al. Association of parental dementia with cognitive and brain MRI measures in middle-aged adults. Neurology. 2009;73:2071–2078. doi: 10.1212/WNL.0b013e3181c67833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Stacpoole PW, Kurtz TL, Han Z, Langaee T. Role of dichloroacetate in the treatment of genetic mitochondrial diseases. Adv Drug Deliv Rev. 2008;60:1478–1487. doi: 10.1016/j.addr.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Saitoh S, Momoi MY, Yamagata T, Mori Y, Imai M. Effects of dichloroacetate in three patients with MELAS. Neurology. 1998;50:531–534. doi: 10.1212/wnl.50.2.531. [DOI] [PubMed] [Google Scholar]
- 120.Kaufmann P, Engelstad K, Wei Y, Jhung S, Sano MC, Shungu DC, et al. Dichloroacetate causes toxic neuropathy in MELAS: a randomized, controlled clinical trial. Neurology. 2006;66:324–330. doi: 10.1212/01.wnl.0000196641.05913.27. [DOI] [PubMed] [Google Scholar]
- 121.Eleff S, Kennaway NG, Buist NR, Darley-Usmar VM, Capaldi RA, Bank WJ, et al. 31P NMR study of improvement in oxidative phosphorylation by vitamins K3 and C in a patient with a defect in electron transport at complex III in skeletal muscle. Proc Natl Acad Sci U S A. 1984;81:3529–3533. doi: 10.1073/pnas.81.11.3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Callaway NL, Riha PD, Bruchey AK, Munshi Z, Gonzalez-Lima F. Methylene blue improves brain oxidative metabolism and memory retention in rats. Pharmacol Biochem Behav. 2004;77:175–181. doi: 10.1016/j.pbb.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 123.Clifton J, 2nd, Leikin JB. Methylene blue. Am J Ther. 2003;10:289–291. doi: 10.1097/00045391-200307000-00009. [DOI] [PubMed] [Google Scholar]
- 124.Atamna H, Nguyen A, Schultz C, Boyle K, Newberry J, Kato H, et al. Methylene blue delays cellular senescence and enhances key mitochondrial biochemical pathways. FASEB J. 2008;22:703–112. doi: 10.1096/fj.07-9610com. [DOI] [PubMed] [Google Scholar]
- 125.Atamna H, Kumar R. Protective role of methylene blue in Alzheimer's disease via mitochondria and cytochrome c oxidase. J Alzheimers Dis. 2010;20(Suppl 2):S439–S452. doi: 10.3233/JAD-2010-100414. [DOI] [PubMed] [Google Scholar]
- 126.Wischik CM, Bentham P, Wischik DJ, Seng KM. Tau aggregation inhibitor (TAI) therapy with rember arrests disease progression in mild and moderate Alzheimer's disease over 50 weeks. Azheimer's and Dementia. 2008;4:T167. [Google Scholar]
- 127.Shults CW, Oakes D, Kieburtz K, Beal MF, Haas R, Plumb S, et al. Effects of coenzyme Q10 in early Parkinson disease: evidence of slowing of the functional decline. Arch Neurol. 2002;59:1541–1550. doi: 10.1001/archneur.59.10.1541. [DOI] [PubMed] [Google Scholar]
- 128.Thal LJ, Grundman M, Berg J, Ernstrom K, Margolin R, Pfeiffer E, et al. Idebenone treatment fails to slow cognitive decline in Alzheimer's disease. Neurology. 2003;61:1498–1502. doi: 10.1212/01.wnl.0000096376.03678.c1. [DOI] [PubMed] [Google Scholar]
- 129.Mariotti C, Solari A, Torta D, Marano L, Fiorentini C, Di Donato S. Idebenone treatment in Friedreich patients: one-year-long randomized placebo-controlled trial. Neurology. 2003;60:1676–1679. doi: 10.1212/01.wnl.0000055872.50364.fc. [DOI] [PubMed] [Google Scholar]
- 130.Di Prospero NA, Baker A, Jeffries N, Fischbeck KH. Neurological effects of high-dose idebenone in patients with Friedreich's ataxia: a randomised, placebo-controlled trial. Lancet Neurol. 2007;6:878–886. doi: 10.1016/S1474-4422(07)70220-X. [DOI] [PubMed] [Google Scholar]
- 131.Schoenen J, Jacquy J, Lenaerts M. Effectiveness of high-dose riboflavin in migraine prophylaxis. A randomized controlled trial. Neurology. 1998;50:466–470. doi: 10.1212/wnl.50.2.466. [DOI] [PubMed] [Google Scholar]
- 132.Adhihetty PJ, Beal MF. Creatine and its potential therapeutic value for targeting cellular energy impairment in neurodegenerative diseases. Neuromolecular Med. 2008;10:275–290. doi: 10.1007/s12017-008-8053-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tarnopolsky MA, Beal MF. Potential for creatine and other therapies targeting cellular energy dysfunction in neurological disorders. Ann Neurol. 2001;49:561–574. [PubMed] [Google Scholar]
- 134.Shefner JM, Cudkowicz ME, Schoenfeld D, Conrad T, Taft J, Chilton M, et al. A clinical trial of creatine in ALS. Neurology. 2004;63:1656–1661. doi: 10.1212/01.wnl.0000142992.81995.f0. [DOI] [PubMed] [Google Scholar]
- 135.Verbessem P, Lemiere J, Eijnde BO, Swinnen S, Vanhees L, Van Leemputte M, et al. Creatine supplementation in Huntington's disease: a placebo-controlled pilot trial. Neurology. 2003;61:925–930. doi: 10.1212/01.wnl.0000090629.40891.4b. [DOI] [PubMed] [Google Scholar]
- 136.Bender A, Koch W, Elstner M, Schombacher Y, Bender J, Moeschl M, et al. Creatine supplementation in Parkinson disease: a placebo-controlled randomized pilot trial. Neurology. 2006;67:1262–1264. doi: 10.1212/01.wnl.0000238518.34389.12. [DOI] [PubMed] [Google Scholar]
- 137.NINDS-NET PD Investigators. A randomized, double-blind, futility clinical trial of creatine and minocycline in early Parkinson disease. Neurology. 2006;66:664–671. doi: 10.1212/01.wnl.0000201252.57661.e1. [DOI] [PubMed] [Google Scholar]
- 138.Owen OE, Morgan AP, Kemp HG, Sullivan JM, Herrera MG, Cahill GF., Jr Brain metabolism during fasting. J Clin Invest. 1967;46:1589–1595. doi: 10.1172/JCI105650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kossoff EH, Zupec-Kania BA, Rho JM. Ketogenic diets: an update for child neurologists. J Child Neurol. 2009;24:979–988. doi: 10.1177/0883073809337162. [DOI] [PubMed] [Google Scholar]
- 140.Swerdlow R, Marcus DM, Landman J, Harooni M, Freedman ML. Brain glucose and ketone body metabolism in patients with Alzheimer's disease. Clin Res. 1989;37:461A. [Google Scholar]
- 141.Henderson ST, Vogel JL, Barr LJ, Garvin F, Jones JJ, Costantini LC. Study of the ketogenic agent AC-1202 in mild to moderate Alzheimer's disease: a randomized, double-blind, placebo-controlled, multicenter trial. Nutr Metab (Lond) 2009;6:31. doi: 10.1186/1743-7075-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Henderson ST. Ketone bodies as a therapeutic for Alzheimer's disease. Neurotherapeutics. 2008;5:470–480. doi: 10.1016/j.nurt.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 144.Perry G, Nunomura A, Hirai K, Zhu X, Perez M, Avila J, et al. Is oxidative damage the fundamental pathogenic mechanism of Alzheimer's and other neurodegenerative diseases? Free Radic Biol Med. 2002;33:1475–1479. doi: 10.1016/s0891-5849(02)01113-9. [DOI] [PubMed] [Google Scholar]
- 145.Shigenaga MK, Hagen TM, Ames BN. Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci U S A. 1994;91:10771–10778. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297:842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 147.Parkinsons's Study Group. Effect of deprenyl on the progression of disability in early Parkinson's disease. The Parkinson Study Group. N Engl J Med. 1989;321:1364–1371. doi: 10.1056/NEJM198911163212004. [DOI] [PubMed] [Google Scholar]
- 148.Sano M, Ernesto C, Thomas RG, Klauber MR, Schafer K, Grundman M, et al. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer's disease. The Alzheimer's Disease Cooperative Study. N Engl J Med. 1997;336:1216–1222. doi: 10.1056/NEJM199704243361704. [DOI] [PubMed] [Google Scholar]
- 149.Stamelou M, Reuss A, Pilatus U, Magerkurth J, Niklowitz P, Eggert KM, et al. Short-term effects of coenzyme Q10 in progressive supranuclear palsy: a randomized, placebo-controlled trial. Mov Disord. 2008;23:942–949. doi: 10.1002/mds.22023. [DOI] [PubMed] [Google Scholar]
- 150.Reddy PH. Mitochondrial oxidative damage in aging and Alzheimer's disease: implications for mitochondrially targeted antioxidant therapeutics. J Biomed Biotechnol. 2006;2006:31372. doi: 10.1155/JBB/2006/31372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kelso GF, Porteous CM, Coulter CV, Hughes G, Porteous WK, Ledgerwood EC, et al. Selective targeting of a redox-active ubiquinone to mitochondria within cells: antioxidant and antiapoptotic properties. J Biol Chem. 2001;276:4588–4596. doi: 10.1074/jbc.M009093200. [DOI] [PubMed] [Google Scholar]
- 152.Snow BJ, Rolfe FL, Lockhart MM, Frampton CM, O'Sullivan JD, Fung V, et al. A double-blind, placebo-controlled study to assess the mitochondria-targeted antioxidant MitoQ as a disease-modifying therapy in Parkinson's disease. Mov Disord. 2010;25:1670–1674. doi: 10.1002/mds.23148. [DOI] [PubMed] [Google Scholar]
- 153.Dumont M, Lin MT, Beal MF. Mitochondria and antioxidant targeted therapeutic strategies for Alzheimer's disease. J Alzheimers Dis. 2010;20(Suppl 2):S633–S643. doi: 10.3233/JAD-2010-100507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Greco T, Fiskum G. Neuroprotection through stimulation of mitochondrial antioxidant protein expression. J Alzheimers Dis. 2010;20(Suppl 2):S427–S437. doi: 10.3233/JAD-2010-100519. [DOI] [PubMed] [Google Scholar]
- 155.Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- 156.Perez VI, Van Remmen H, Bokov A, Epstein CJ, Vijg J, Richardson A. The overexpression of major antioxidant enzymes does not extend the lifespan of mice. Aging Cell. 2009;8:73–75. doi: 10.1111/j.1474-9726.2008.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jang YC, Remmen VH. The mitochondrial theory of aging: insight from transgenic and knockout mouse models. Exp Gerontol. 2009;44:256–260. doi: 10.1016/j.exger.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 158.Mattson MP. Apoptosis in neurodegenerative disorders. Nat Rev Mol Cell Biol. 2000;1:120–129. doi: 10.1038/35040009. [DOI] [PubMed] [Google Scholar]
- 159.Crompton M, Virji S, Doyle V, Johnson N, Ward JM. The mitochondrial permeability transition pore. Biochem Soc Symp. 1999;66:167–179. doi: 10.1042/bss0660167. [DOI] [PubMed] [Google Scholar]
- 160.Lemasters JJ, Theruvath TP, Zhong Z, Nieminen AL. Mitochondrial calcium and the permeability transition in cell death. Biochim Biophys Acta. 2009;1787:1395–1401. doi: 10.1016/j.bbabio.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Saelens X, Festjens N, Vande Walle L, van Gurp M, van Loo G, Vandenabeele P. Toxic proteins released from mitochondria in cell death. Oncogene. 2004;23:2861–2874. doi: 10.1038/sj.onc.1207523. [DOI] [PubMed] [Google Scholar]
- 162.Wang C, Youle RJ. The role of mitochondria in apoptosis*. Annu Rev Genet. 2009;43:95–118. doi: 10.1146/annurev-genet-102108-134850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 164.Zamzami N, Kroemer G. The mitochondrion in apoptosis: how Pandora's box opens. Nat Rev Mol Cell Biol. 2001;2:67–71. doi: 10.1038/35048073. [DOI] [PubMed] [Google Scholar]
- 165.Czabotar PE, Lessene G. Bcl-2 family proteins as therapeutic targets. Curr Pharm Des. 2010;16:3132–3148. doi: 10.2174/138161210793292429. [DOI] [PubMed] [Google Scholar]
- 166.Brunelle JK, Letai A. Control of mitochondrial apoptosis by the Bcl-2 family. J Cell Sci. 2009;122:437–441. doi: 10.1242/jcs.031682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Haroon MF, Fatima A, Scholer S, Gieseler A, Horn TF, Kirches E, et al. Minocycline, a possible neuroprotective agent in Leber's hereditary optic neuropathy (LHON): studies of cybrid cells bearing 11,778 mutation. Neurobiol Dis. 2007;28:237–250. doi: 10.1016/j.nbd.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 168.Zhu S, Stavrovskaya IG, Drozda M, Kim BY, Ona V, Li M, et al. Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature. 2002;417:74–78. doi: 10.1038/417074a. [DOI] [PubMed] [Google Scholar]
- 169.Gordon PH, Moore DH, Miller RG, Florence JM, Verheijde JL, Doorish C, et al. Efficacy of minocycline in patients with amyotrophic lateral sclerosis: a phase III randomised trial. Lancet Neurol. 2007;6:1045–1053. doi: 10.1016/S1474-4422(07)70270-3. [DOI] [PubMed] [Google Scholar]
- 170.Weinreb O, Amit T, Bar-Am O, Youdim MB. Rasagiline: a novel anti-Parkinsonian monoamine oxidase-B inhibitor with neuroprotective activity. Prog Neurobiol. 2010;92:330–344. doi: 10.1016/j.pneurobio.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 171.Yi H, Maruyama W, Akao Y, Takahashi T, Iwasa K, Youdim MB, et al. N-Propargylamine protects SH-SY5Y cells from apoptosis induced by an endogenous neurotoxin, N-methyl(R)salsolinol, through stabilization of mitochondrial membrane and induction of anti-apoptotic Bcl-2. J Neural Transm. 2006;113:21–32. doi: 10.1007/s00702-005-0299-z. [DOI] [PubMed] [Google Scholar]
- 172.Akao Y, Maruyama W, Shimizu S, Yi H, Nakagawa Y, Shamoto-Nagai M, et al. Mitochondrial permeability transition mediates apoptosis induced by N-methyl(R)salsolinol, an endogenous neurotoxin, and is inhibited by Bcl-2 and rasagiline, N-propargyl-1(R)-aminoindan. J Neurochem. 2002;82:913–923. doi: 10.1046/j.1471-4159.2002.01047.x. [DOI] [PubMed] [Google Scholar]
- 173.Youdim MB, Amit T, Falach-Yogev M, Bar Am O, Maruyama W, Naoi M. The essentiality of Bcl-2, PKC and proteasome-ubiquitin complex activations in the neuroprotective-antiapoptotic action of the anti-Parkinson drug, rasagiline. Biochem Pharmacol. 2003;66:1635–1641. doi: 10.1016/s0006-2952(03)00535-5. [DOI] [PubMed] [Google Scholar]
- 174.Youdim MB, Bar Am O, Yogev-Falach M, Weinreb O, Maruyama W, Naoi M, et al. Rasagiline: neurodegeneration, neuroprotection, and mitochondrial permeability transition. J Neurosci Res. 2005;79:172–179. doi: 10.1002/jnr.20350. [DOI] [PubMed] [Google Scholar]
- 175.Olanow CW, Rascol O, Hauser R, Feigin PD, Jankovic J, Lang A, et al. A double-blind, delayed-start trial of rasagiline in Parkinson's disease. N Engl J Med. 2009;361:1268–1278. doi: 10.1056/NEJMoa0809335. [DOI] [PubMed] [Google Scholar]
- 176.Wang H, Larriviere KS, Keller KE, Ware KA, Burns TM, Conaway MA, et al. R+ pramipexole as a mitochondrially focused neuroprotectant: initial early phase studies in ALS. Amyotroph Lateral Scler. 2008;9:50–58. doi: 10.1080/17482960701791234. [DOI] [PubMed] [Google Scholar]
- 177.Zhang S, Hedskog L, Petersen CA, Winblad B, Ankarcrona M. Dimebon (latrepirdine) enhances mitochondrial function and protects neuronal cells from death. J Alzheimers Dis. 2010;21:389–402. doi: 10.3233/JAD-2010-100174. [DOI] [PubMed] [Google Scholar]
- 178.Bachurin SO, Shevtsova EP, Kireeva EG, Oxenkrug GF, Sablin SO. Mitochondria as a target for neurotoxins and neuroprotective agents. Ann N Y Acad Sci. 2003;993:334–344. doi: 10.1111/j.1749-6632.2003.tb07541.x. discussion 45-9. [DOI] [PubMed] [Google Scholar]
- 179.Bezprozvanny I. The rise and fall of Dimebon. Drug News Perspect. 2010;23:518–523. doi: 10.1358/dnp.2010.23.8.1500435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Doody RS, Gavrilova SI, Sano M, Thomas RG, Aisen PS, Bachurin SO, et al. Effect of dimebon on cognition, activities of daily living, behaviour, and global function in patients with mild-to-moderate Alzheimer's disease: a randomised, double-blind, placebo-controlled study. Lancet. 2008;372:207–215. doi: 10.1016/S0140-6736(08)61074-0. [DOI] [PubMed] [Google Scholar]
- 181.Miller G. Pharmacology. The puzzling rise and fall of a dark-horse Alzheimer's drug. Science. 2010;327:1309. doi: 10.1126/science.327.5971.1309. [DOI] [PubMed] [Google Scholar]
- 182.Rabinowitz JD, White E. Autophagy and metabolism. Science. 2010;330:1344–1348. doi: 10.1126/science.1193497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. 2011;12:9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Banerjee R, Beal MF, Thomas B. Autophagy in neurodegenerative disorders: pathogenic roles and therapeutic implications. Trends Neurosci. 2010;33:541–549. doi: 10.1016/j.tins.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, et al. Mitochondrial abnormalities in Alzheimer's disease. J Neurosci. 2001;21:3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Nixon RA, Yang DS. Autophagy failure in Alzheimer's disease-locating the primary defect. Neurobiol Dis. 2011 doi: 10.1016/j.nbd.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bueler H. Impaired mitochondrial dynamics and function in the pathogenesis of Parkinson's disease. Exp Neurol. 2009;218:235–246. doi: 10.1016/j.expneurol.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 188.Chan NC, Salazar AM, Pham AH, Sweredoski MJ, Kolawa NJ, Graham RL, et al. Broad activation of the ubiquitin-proteasome system by Parkin is critical for mitophagy. Hum Mol Genet. 2011 doi: 10.1093/hmg/ddr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Gusdon AM, Chu CT. To Eat or Not to Eat: Neuronal Metabolism, Mitophagy and Parkinson's Disease. Antioxid Redox Signal. 2010 doi: 10.1089/ars.2010.3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Tong Y, Yamaguchi H, Giaime E, Boyle S, Kopan R, Kelleher RJ, 3rd, et al. Loss of leucine-rich repeat kinase 2 causes impairment of protein degradation pathways, accumulation of alpha-synuclein, and apoptotic cell death in aged mice. Proc Natl Acad Sci U S A. 2010;107:9879–9884. doi: 10.1073/pnas.1004676107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Vives-Bauza C, Zhou C, Huang Y, Cui M, de Vries RL, Kim J, et al. PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc Natl Acad Sci U S A. 2010;107:378–383. doi: 10.1073/pnas.0911187107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Fornai F, Longone P, Cafaro L, Kastsiuchenka O, Ferrucci M, Manca ML, et al. Lithium delays progression of amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 2008;105:2052–2057. doi: 10.1073/pnas.0708022105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Aggarwal SP, Zinman L, Simpson E, McKinley J, Jackson KE, Pinto H, et al. Safety and efficacy of lithium in combination with riluzole for treatment of amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2010;9:481–488. doi: 10.1016/S1474-4422(10)70068-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Chio A, Borghero G, Calvo A, Capasso M, Caponnetto C, Corbo M, et al. Lithium carbonate in amyotrophic lateral sclerosis: lack of efficacy in a dose-finding trial. Neurology. 2010;75:619–625. doi: 10.1212/WNL.0b013e3181ed9e7c. [DOI] [PubMed] [Google Scholar]
- 195.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zemke D, Azhar S, Majid A. The mTOR pathway as a potential target for the development of therapies against neurological disease. Drug News Perspect. 2007;20:495–499. doi: 10.1358/dnp.2007.20.8.1157618. [DOI] [PubMed] [Google Scholar]
- 197.Spilman P, Podlutskaya N, Hart MJ, Debnath J, Gorostiza O, Bredesen D, et al. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer's disease. PLoS One. 2010;5:e9979. doi: 10.1371/journal.pone.0009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Malagelada C, Jin ZH, Jackson-Lewis V, Przedborski S, Greene LA. Rapamycin protects against neuron death in in vitro and in vivo models of Parkinson's disease. J Neurosci. 2010;30:1166–1175. doi: 10.1523/JNEUROSCI.3944-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sudarsanam S, Johnson DE. Functional consequences of mTOR inhibition. Curr Opin Drug Discov Devel. 2010;13:31–40. [PubMed] [Google Scholar]
- 200.Jung CH, Ro SH, Cao J, Otto NM, Kim DH. mTOR regulation of autophagy. FEBS Lett. 2010;584:1287–1295. doi: 10.1016/j.febslet.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kapahi P, Chen D, Rogers AN, Katewa SD, Li PW, Thomas EL, et al. With TOR, less is more: a key role for the conserved nutrient-sensing TOR pathway in aging. Cell Metab. 2010;11:453–465. doi: 10.1016/j.cmet.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wang X, Su B, Fujioka H, Zhu X. Dynamin-like protein 1 reduction underlies mitochondrial morphology and distribution abnormalities in fibroblasts from sporadic Alzheimer's disease patients. Am J Pathol. 2008;173:470–482. doi: 10.2353/ajpath.2008.071208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Wang X, Su B, Lee HG, Li X, Perry G, Smith MA, et al. Impaired balance of mitochondrial fission and fusion in Alzheimer's disease. J Neurosci. 2009;29:9090–9103. doi: 10.1523/JNEUROSCI.1357-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.de la Monte SM, Luong T, Neely TR, Robinson D, Wands JR. Mitochondrial DNA damage as a mechanism of cell loss in Alzheimer's disease. Lab Invest. 2000;80:1323–1335. doi: 10.1038/labinvest.3780140. [DOI] [PubMed] [Google Scholar]
- 205.Wiedemann FR, Manfredi G, Mawrin C, Beal MF, Schon EA. Mitochondrial DNA and respiratory chain function in spinal cords of ALS patients. J Neurochem. 2002;80:616–625. doi: 10.1046/j.0022-3042.2001.00731.x. [DOI] [PubMed] [Google Scholar]
- 206.Brown AM, Sheu RK, Mohs R, Haroutunian V, Blass JP. Correlation of the clinical severity of Alzheimer's disease with an aberration in mitochondrial DNA (mtDNA) J Mol Neurosci. 2001;16:41–48. doi: 10.1385/JMN:16:1:41. [DOI] [PubMed] [Google Scholar]
- 207.Onyango IG, Lu J, Rodova M, Lezi E, Crafter AB, Swerdlow RH. Regulation of neuron mitochondrial biogenesis and relevance to brain health. Biochim Biophys Acta. 2010;1802:228–234. doi: 10.1016/j.bbadis.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 208.Finck BN, Kelly DP. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest. 2006;116:615–622. doi: 10.1172/JCI27794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev. 2006;27:728–735. doi: 10.1210/er.2006-0037. [DOI] [PubMed] [Google Scholar]
- 210.Houten SM, Auwerx J. PGC-1alpha: turbocharging mitochondria. Cell. 2004;119:5–7. doi: 10.1016/j.cell.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 211.Feige JN, Auwerx J. Transcriptional targets of sirtuins in the coordination of mammalian physiology. Curr Opin Cell Biol. 2008;20:303–309. doi: 10.1016/j.ceb.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Guarente L. Sirtuins in aging and disease. Cold Spring Harb Symp Quant Biol. 2007;72:483–488. doi: 10.1101/sqb.2007.72.024. [DOI] [PubMed] [Google Scholar]
- 213.Haigis MC, Guarente LP. Mammalian sirtuins--emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006;20:2913–2921. doi: 10.1101/gad.1467506. [DOI] [PubMed] [Google Scholar]
- 214.Nemoto S, Fergusson MM, Finkel T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1{alpha} J Biol Chem. 2005;280:16456–16460. doi: 10.1074/jbc.M501485200. [DOI] [PubMed] [Google Scholar]
- 215.Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- 216.Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Reznick RM, Shulman GI. The role of AMP-activated protein kinase in mitochondrial biogenesis. J Physiol. 2006;574:33–39. doi: 10.1113/jphysiol.2006.109512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Herzig S, Long F, Jhala US, Hedrick S, Quinn R, Bauer A, et al. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature. 2001;413:179–183. doi: 10.1038/35093131. [DOI] [PubMed] [Google Scholar]
- 219.Wu Z, Huang X, Feng Y, Handschin C, Gullicksen PS, Bare O, et al. Transducer of regulated CREB-binding proteins (TORCs) induce PGC-1alpha transcription and mitochondrial biogenesis in muscle cells. Proc Natl Acad Sci U S A. 2006;103:14379–14384. doi: 10.1073/pnas.0606714103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Daitoku H, Yamagata K, Matsuzaki H, Hatta M, Fukamizu A. Regulation of PGC-1 promoter activity by protein kinase B and the forkhead transcription factor FKHR. Diabetes. 2003;52:642–649. doi: 10.2337/diabetes.52.3.642. [DOI] [PubMed] [Google Scholar]
- 221.Nakae J, Oki M, Cao Y. The FoxO transcription factors and metabolic regulation. FEBS Lett. 2008;582:54–67. doi: 10.1016/j.febslet.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 222.Schieke SM, Phillips D, McCoy JP, Jr, Aponte AM, Shen RF, Balaban RS, et al. The mammalian target of rapamycin (mTOR) pathway regulates mitochondrial oxygen consumption and oxidative capacity. J Biol Chem. 2006;281:27643–27652. doi: 10.1074/jbc.M603536200. [DOI] [PubMed] [Google Scholar]
- 223.Cunningham JT, Rodgers JT, Arlow DH, Vazquez F, Mootha VK, Puigserver P. mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. Nature. 2007;450:736–740. doi: 10.1038/nature06322. [DOI] [PubMed] [Google Scholar]
- 224.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Koo SH, Satoh H, Herzig S, Lee CH, Hedrick S, Kulkarni R, et al. PGC-1 promotes insulin resistance in liver through PPAR-alpha-dependent induction of TRB-3. Nat Med. 2004;10:530–534. doi: 10.1038/nm1044. [DOI] [PubMed] [Google Scholar]
- 226.Canto C, Auwerx J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol. 2009;20:98–105. doi: 10.1097/MOL.0b013e328328d0a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Canto C, Auwerx J. AMP-activated protein kinase and its downstream transcriptional pathways. Cell Mol Life Sci. 2010;67:3407–3423. doi: 10.1007/s00018-010-0454-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Ghosh S, Patel N, Rahn D, McAllister J, Sadeghi S, Horwitz G, et al. The thiazolidinedione pioglitazone alters mitochondrial function in human neuron-like cells. Mol Pharmacol. 2007;71:1695–1702. doi: 10.1124/mol.106.033845. [DOI] [PubMed] [Google Scholar]
- 229.Strum JC, Shehee R, Virley D, Richardson J, Mattie M, Selley P, et al. Rosiglitazone induces mitochondrial biogenesis in mouse brain. J Alzheimers Dis. 2007;11:45–51. doi: 10.3233/jad-2007-11108. [DOI] [PubMed] [Google Scholar]
- 230.Bogacka I, Xie H, Bray GA, Smith SR. Pioglitazone induces mitochondrial biogenesis in human subcutaneous adipose tissue in vivo. Diabetes. 2005;54:1392–1399. doi: 10.2337/diabetes.54.5.1392. [DOI] [PubMed] [Google Scholar]
- 231.Bolten CW, Blanner PM, McDonald WG, Staten NR, Mazzarella RA, Arhancet GB, et al. Insulin sensitizing pharmacology of thiazolidinediones correlates with mitochondrial gene expression rather than activation of PPAR gamma. Gene Regul Syst Bio. 2007;1:73–82. [PMC free article] [PubMed] [Google Scholar]
- 232.Yki-Jarvinen H. Thiazolidinediones. N Engl J Med. 2004;351:1106–1118. doi: 10.1056/NEJMra041001. [DOI] [PubMed] [Google Scholar]
- 233.Risner ME, Saunders AM, Altman JF, Ormandy GC, Craft S, Foley IM, et al. Efficacy of rosiglitazone in a genetically defined population with mild-to-moderate Alzheimer's disease. Pharmacogenomics J. 2006;6:246–254. doi: 10.1038/sj.tpj.6500369. [DOI] [PubMed] [Google Scholar]
- 234.Geldmacher DS, Fritsch T, McClendon MJ, Landreth G. A randomized pilot clinical trial of the safety of pioglitazone in treatment of patients with Alzheimer disease. Arch Neurol. 2011;68:45–50. doi: 10.1001/archneurol.2010.229. [DOI] [PubMed] [Google Scholar]
- 235.Gold M, Alderton C, Zvartau-Hind M, Egginton S, Saunders AM, Irizarry M, et al. Rosiglitazone monotherapy in mild-to-moderate Alzheimer's disease: results from a randomized, double-blind, placebo-controlled phase III study. Dement Geriatr Cogn Disord. 2010;30:131–146. doi: 10.1159/000318845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Wenz T, Diaz F, Spiegelman BM, Moraes CT. Activation of the PPAR/PGC-1alpha pathway prevents a bioenergetic deficit and effectively improves a mitochondrial myopathy phenotype. Cell Metab. 2008;8:249–256. doi: 10.1016/j.cmet.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 237.Wenz T, Wang X, Marini M, Moraes CT. A metabolic shift induced by a PPAR panagonist markedly reduces the effects of pathogenic mitochondrial tRNA mutations. J Cell Mol Med. 2010 doi: 10.1111/j.1582-4934.2010.01223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Navarro A, Gomez C, Lopez-Cepero JM, Boveris A. Beneficial effects of moderate exercise on mice aging: survival, behavior, oxidative stress, and mitochondrial electron transfer. Am J Physiol Regul Integr Comp Physiol. 2004;286:R505–R511. doi: 10.1152/ajpregu.00208.2003. [DOI] [PubMed] [Google Scholar]
- 239.Pate RR, Pratt M, Blair SN, Haskell WL, Macera CA, Bouchard C, et al. Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA. 1995;273:402–407. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- 240.Ingram DK, Young J, Mattison JA. Calorie restriction in nonhuman primates: assessing effects on brain and behavioral aging. Neuroscience. 2007;145:1359–1364. doi: 10.1016/j.neuroscience.2006.10.031. [DOI] [PubMed] [Google Scholar]
- 241.Spindler SR. Biological effects of calorie restriction: From soup to nuts. Ageing Res Rev. 2009 doi: 10.1016/j.arr.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 242.Varady KA, Hellerstein MK. Alternate-day fasting and chronic disease prevention: a review of human and animal trials. Am J Clin Nutr. 2007;86:7–13. doi: 10.1093/ajcn/86.1.7. [DOI] [PubMed] [Google Scholar]
- 243.Weindruch R, Sohal RS. Seminars in medicine of the Beth Israel Deaconess Medical Center. Caloric intake and aging. N Engl J Med. 1997;337:986–994. doi: 10.1056/NEJM199710023371407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Greer EL, Brunet A. Different dietary restriction regimens extend lifespan by both independent and overlapping genetic pathways in C. elegans. Aging Cell. 2009;8:113–127. doi: 10.1111/j.1474-9726.2009.00459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Boveris A, Navarro A. Systemic and mitochondrial adaptive responses to moderate exercise in rodents. Free Radic Biol Med. 2008;44:224–229. doi: 10.1016/j.freeradbiomed.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 246.Fernstrom M, Tonkonogi M, Sahlin K. Effects of acute and chronic endurance exercise on mitochondrial uncoupling in human skeletal muscle. J Physiol. 2004;554:755–763. doi: 10.1113/jphysiol.2003.055202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Holloszy JO. Adaptation of skeletal muscle to endurance exercise. Med Sci Sports. 1975;7:155–164. [PubMed] [Google Scholar]
- 248.Hood DA. Mechanisms of exercise-induced mitochondrial biogenesis in skeletal muscle. Appl Physiol Nutr Metab. 2009;34:465–472. doi: 10.1139/H09-045. [DOI] [PubMed] [Google Scholar]
- 249.Hoppeler H, Fluck M. Plasticity of skeletal muscle mitochondria: structure and function. Med Sci Sports Exerc. 2003;35:95–104. doi: 10.1249/01.MSS.0000043292.99104.12. [DOI] [PubMed] [Google Scholar]
- 250.Civitarese AE, Smith SR, Ravussin E. Diet, energy metabolism and mitochondrial biogenesis. Curr Opin Clin Nutr Metab Care. 2007;10:679–687. doi: 10.1097/MCO.0b013e3282f0ecd2. [DOI] [PubMed] [Google Scholar]
- 251.Hepple RT. Why eating less keeps mitochondria working in aged skeletal muscle. Exerc Sport Sci Rev. 2009;37:23–28. doi: 10.1097/JES.0b013e3181877dc5. [DOI] [PubMed] [Google Scholar]
- 252.Lambert AJ, Wang B, Yardley J, Edwards J, Merry BJ. The effect of aging and caloric restriction on mitochondrial protein density and oxygen consumption. Exp Gerontol. 2004;39:289–295. doi: 10.1016/j.exger.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 253.Cassano P, Sciancalepore AG, Lezza AM, Leeuwenburgh C, Cantatore P, Gadaleta MN. Tissue-specific effect of age and caloric restriction diet on mitochondrial DNA content. Rejuvenation Res. 2006;9:211–214. doi: 10.1089/rej.2006.9.211. [DOI] [PubMed] [Google Scholar]
- 254.Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, et al. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310:314–317. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- 255.Owen OE, Felig P, Morgan AP, Wahren J, Cahill GF., Jr Liver and kidney metabolism during prolonged starvation. J Clin Invest. 1969;48:574–583. doi: 10.1172/JCI106016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Kempermann G, van Praag H, Gage FH. Activity-dependent regulation of neuronal plasticity and self repair. Prog Brain Res. 2000;127:35–48. doi: 10.1016/s0079-6123(00)27004-0. [DOI] [PubMed] [Google Scholar]
- 258.Witte AV, Fobker M, Gellner R, Knecht S, Floel A. Caloric restriction improves memory in elderly humans. Proc Natl Acad Sci U S A. 2009;106:1255–1260. doi: 10.1073/pnas.0808587106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Lu J, E L, Wang W, Frontera J, Zhu H, Wang WT, et al. Alternate day fasting impacts the brain insulin-signaling pathway of young adult male C57BL/6 mice. J Neurochem. 2011 doi: 10.1111/j.1471-4159.2011.07184.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Sauve AA. Pharmaceutical strategies for activating sirtuins. Curr Pharm Des. 2009;15:45–56. doi: 10.2174/138161209787185797. [DOI] [PubMed] [Google Scholar]
- 261.Fontana L, Partridge L, Longo VD. Extending healthy life span--from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Revollo JR, Grimm AA, Imai S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J Biol Chem. 2004;279:50754–50763. doi: 10.1074/jbc.M408388200. [DOI] [PubMed] [Google Scholar]
- 264.Blander G, Guarente L. The Sir2 family of protein deacetylases. Annu Rev Biochem. 2004;73:417–435. doi: 10.1146/annurev.biochem.73.011303.073651. [DOI] [PubMed] [Google Scholar]
- 265.Finkel T, Deng CX, Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature. 2009;460:587–591. doi: 10.1038/nature08197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Baur JA. Biochemical effects of SIRT1 activators. Biochim Biophys Acta. 2010;1804:1626–1634. doi: 10.1016/j.bbapap.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Anekonda TS, Reddy PH. Neuronal protection by sirtuins in Alzheimer's disease. J Neurochem. 2006;96:305–313. doi: 10.1111/j.1471-4159.2005.03492.x. [DOI] [PubMed] [Google Scholar]
- 268.Harikumar KB, Aggarwal BB. Resveratrol: a multitargeted agent for age-associated chronic diseases. Cell Cycle. 2008;7:1020–1035. doi: 10.4161/cc.7.8.5740. [DOI] [PubMed] [Google Scholar]
- 269.Barger JL, Kayo T, Vann JM, Arias EB, Wang J, Hacker TA, et al. A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS One. 2008;3:e2264. doi: 10.1371/journal.pone.0002264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 271.Singh M, Arseneault M, Sanderson T, Murthy V, Ramassamy C. Challenges for research on polyphenols from foods in Alzheimer's disease: bioavailability, metabolism, and cellular and molecular mechanisms. J Agric Food Chem. 2008;56:4855–4873. doi: 10.1021/jf0735073. [DOI] [PubMed] [Google Scholar]
- 272.Houtkooper RH, Canto C, Wanders RJ, Auwerx J. The secret life of NAD+: an old metabolite controlling new metabolic signaling pathways. Endocr Rev. 2010;31:194–223. doi: 10.1210/er.2009-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Blass JP, Gibson GE. Correlations of disability and biologic alterations in Alzheimer brain and test of significance by a therapeutic trial in humans. J Alzheimers Dis. 2006;9:207–218. doi: 10.3233/jad-2006-9212. [DOI] [PubMed] [Google Scholar]
- 274.Taylor RW, Chinnery PF, Turnbull DM, Lightowlers RN. Selective inhibition of mutant human mitochondrial DNA replication in vitro by peptide nucleic acids. Nat Genet. 1997;15:212–215. doi: 10.1038/ng0297-212. [DOI] [PubMed] [Google Scholar]
- 275.Taylor RW, Wardell TM, Smith PM, Muratovska A, Murphy MP, Turnbull DM, et al. An antigenomic strategy for treating heteroplasmic mtDNA disorders. Adv Drug Deliv Rev. 2001;49:121–125. doi: 10.1016/s0169-409x(01)00130-2. [DOI] [PubMed] [Google Scholar]
- 276.Kang D, Kim SH, Hamasaki N. Mitochondrial transcription factor A (TFAM): roles in maintenance of mtDNA and cellular functions. Mitochondrion. 2007;7:39–44. doi: 10.1016/j.mito.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 277.Scarpulla RC. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol Rev. 2008;88:611–638. doi: 10.1152/physrev.00025.2007. [DOI] [PubMed] [Google Scholar]
- 278.Parisi MA, Clayton DA. Similarity of human mitochondrial transcription factor 1 to high mobility group proteins. Science. 1991;252:965–969. doi: 10.1126/science.2035027. [DOI] [PubMed] [Google Scholar]
- 279.Ekstrand MI, Falkenberg M, Rantanen A, Park CB, Gaspari M, Hultenby K, et al. Mitochondrial transcription factor A regulates mtDNA copy number in mammals. Hum Mol Genet. 2004;13:935–944. doi: 10.1093/hmg/ddh109. [DOI] [PubMed] [Google Scholar]
- 280.Hayashi Y, Yoshida M, Yamato M, Ide T, Wu Z, Ochi-Shindou M, et al. Reverse of age-dependent memory impairment and mitochondrial DNA damage in microglia by an overexpression of human mitochondrial transcription factor a in mice. J Neurosci. 2008;28:8624–8634. doi: 10.1523/JNEUROSCI.1957-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Iyer S, Thomas RR, Portell FR, Dunham LD, Quigley CK, Bennett JP., Jr Recombinant mitochondrial transcription factor A with N-terminal mitochondrial transduction domain increases respiration and mitochondrial gene expression. Mitochondrion. 2009;9:196–203. doi: 10.1016/j.mito.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Keeney PM, Quigley CK, Dunham LD, Papageorge CM, Iyer S, Thomas RR, et al. Mitochondrial gene therapy augments mitochondrial physiology in a Parkinson's disease cell model. Hum Gene Ther. 2009;20:897–907. doi: 10.1089/hum.2009.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Tanaka M, Borgeld HJ, Zhang J, Muramatsu S, Gong JS, Yoneda M, et al. Gene therapy for mitochondrial disease by delivering restriction endonuclease SmaI into mitochondria. J Biomed Sci. 2002;9:534–541. doi: 10.1159/000064726. [DOI] [PubMed] [Google Scholar]
- 284.Alexeyev MF, Venediktova N, Pastukh V, Shokolenko I, Bonilla G, Wilson GL. Selective elimination of mutant mitochondrial genomes as therapeutic strategy for the treatment of NARP and MILS syndromes. Gene Ther. 2008;15:516–523. doi: 10.1038/gt.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Bacman SR, Williams SL, Garcia S, Moraes CT. Organ-specific shifts in mtDNA heteroplasmy following systemic delivery of a mitochondria-targeted restriction endonuclease. Gene Ther. 2010;17:713–720. doi: 10.1038/gt.2010.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Khan SM, Bennett JP., Jr Development of mitochondrial gene replacement therapy. J Bioenerg Biomembr. 2004;36:387–393. doi: 10.1023/B:JOBB.0000041773.20072.9e. [DOI] [PubMed] [Google Scholar]
- 287.Schulz TJ, Zarse K, Voigt A, Urban N, Birringer M, Ristow M. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 2007;6:280–293. doi: 10.1016/j.cmet.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 288.Mattson MP. Dietary factors, hormesis and health. Ageing Res Rev. 2008;7:43–48. doi: 10.1016/j.arr.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Magistretti PJ, Pellerin L. Cellular mechanisms of brain energy metabolism and their relevance to functional brain imaging. Philos Trans R Soc Lond B Biol Sci. 1999;354:1155–1163. doi: 10.1098/rstb.1999.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A. 1994;91:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Pellerin L, Magistretti PJ. Food for thought: challenging the dogmas. J Cereb Blood Flow Metab. 2003;23:1282–1286. doi: 10.1097/01.WCB.0000096064.12129.3D. [DOI] [PubMed] [Google Scholar]
- 292.Halassa MM, Haydon PG. Integrated brain circuits: astrocytic networks modulate neuronal activity and behavior. Annu Rev Physiol. 2010;72:335–355. doi: 10.1146/annurev-physiol-021909-135843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Perea G, Navarrete M, Araque A. Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci. 2009;32:421–431. doi: 10.1016/j.tins.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 294.Magistretti PJ. Neuron-glia metabolic coupling and plasticity. J Exp Biol. 2006;209:2304–2311. doi: 10.1242/jeb.02208. [DOI] [PubMed] [Google Scholar]
- 295.Finkel T. Reactive oxygen species and signal transduction. IUBMB Life. 2001;52:3–6. doi: 10.1080/15216540252774694. [DOI] [PubMed] [Google Scholar]
- 296.Finkel T. Oxidant signals and oxidative stress. Curr Opin Cell Biol. 2003;15:247–254. doi: 10.1016/s0955-0674(03)00002-4. [DOI] [PubMed] [Google Scholar]
- 297.Gomez-Cabrera MC, Domenech E, Romagnoli M, Arduini A, Borras C, Pallardo FV, et al. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am J Clin Nutr. 2008;87:142–149. doi: 10.1093/ajcn/87.1.142. [DOI] [PubMed] [Google Scholar]
- 298.McGinley C, Shafat A, Donnelly AE. Does Antioxidant Vitamin Supplementation Protect against Muscle Damage? Sports Med. 2009;39:1011–1032. doi: 10.2165/11317890-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 299.Ristow M, Zarse K, Oberbach A, Kloting N, Birringer M, Kiehntopf M, et al. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A. 2009;106:8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Katzoff A, Ben-Gedalya T, Susswein AJ. Nitric oxide is necessary for multiple memory processes after learning that a food is inedible in aplysia. J Neurosci. 2002;22:9581–9594. doi: 10.1523/JNEUROSCI.22-21-09581.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Levin ED, Brady TC, Hochrein EC, Oury TD, Jonsson LM, Marklund SL, et al. Molecular manipulations of extracellular superoxide dismutase: functional importance for learning. Behav Genet. 1998;28:381–390. doi: 10.1023/a:1021673703129. [DOI] [PubMed] [Google Scholar]
- 302.Lamb HM, Hardwick M. Noncanonical functions of BCL-2 proteins in the nervous system. Adv Exp Med Biol. 2010;687:115–129. doi: 10.1007/978-1-4419-6706-0_7. [DOI] [PubMed] [Google Scholar]
- 303.Danial NN, Gimenez-Cassina A, Tondera D. Homeostatic functions of BCL-2 proteins beyond apoptosis. Adv Exp Med Biol. 2010;687:1–32. doi: 10.1007/978-1-4419-6706-0_1. [DOI] [PubMed] [Google Scholar]
- 304.Autret A, Martin SJ. Bcl-2 family proteins and mitochondrial fission/fusion dynamics. Cell Mol Life Sci. 2010;67:1599–1606. doi: 10.1007/s00018-010-0286-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Bergamini E, Cavallini G, Donati A, Gori Z. The anti-ageing effects of caloric restriction may involve stimulation of macroautophagy and lysosomal degradation, and can be intensified pharmacologically. Biomed Pharmacother. 2003;57:203–208. doi: 10.1016/s0753-3322(03)00048-9. [DOI] [PubMed] [Google Scholar]
- 306.Kume S, Uzu T, Horiike K, Chin-Kanasaki M, Isshiki K, Araki S, et al. Calorie restriction enhances cell adaptation to hypoxia through Sirt1-dependent mitochondrial autophagy in mouse aged kidney. J Clin Invest. 2010;120:1043–1055. doi: 10.1172/JCI41376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Barrientos A, Casademont J, Cardellach F, Estivill X, Urbano-Marquez A, Nunes V. Reduced steady-state levels of mitochondrial RNA and increased mitochondrial DNA amount in human brain with aging. Brain Res Mol Brain Res. 1997;52:284–289. doi: 10.1016/s0169-328x(97)00278-7. [DOI] [PubMed] [Google Scholar]
- 308.Barrientos A, Casademont J, Cardellach F, Ardite E, Estivill X, Urbano-Marquez A, et al. Qualitative and quantitative changes in skeletal muscle mtDNA and expression of mitochondrial-encoded genes in the human aging process. Biochem Mol Med. 1997;62:165–171. doi: 10.1006/bmme.1997.2647. [DOI] [PubMed] [Google Scholar]
- 309.Swerdlow RH, Khan SM. A "mitochondrial cascade hypothesis" for sporadic Alzheimer's disease. Med Hypotheses. 2004;63:8–20. doi: 10.1016/j.mehy.2003.12.045. [DOI] [PubMed] [Google Scholar]
- 310.North BJ, Marshall BL, Borra MT, Denu JM, Verdin E. The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol Cell. 2003;11:437–444. doi: 10.1016/s1097-2765(03)00038-8. [DOI] [PubMed] [Google Scholar]
- 311.Li W, Zhang B, Tang J, Cao Q, Wu Y, Wu C, et al. Sirtuin 2, a mammalian homolog of yeast silent information regulator-2 longevity regulator, is an oligodendroglial protein that decelerates cell differentiation through deacetylating alpha-tubulin. J Neurosci. 2007;27:2606–2616. doi: 10.1523/JNEUROSCI.4181-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Suzuki K, Koike T. Mammalian Sir2-related protein (SIRT) 2-mediated modulation of resistance to axonal degeneration in slow Wallerian degeneration mice: a crucial role of tubulin deacetylation. Neuroscience. 2007;147:599–612. doi: 10.1016/j.neuroscience.2007.04.059. [DOI] [PubMed] [Google Scholar]
- 313.Arduino DM, Esteves AR, Oliveira CR, Cardoso SM. Mitochondrial Metabolism Modulation: A New Therapeutic Approach for Parkinson's Disease. CNS Neurol Disord Drug Targets. 2009 doi: 10.2174/187152710790966687. [DOI] [PubMed] [Google Scholar]
- 314.Esteves AR, Arduino DM, Swerdlow RH, Oliveira CR, Cardoso SM. Microtubule depolymerization potentiates alpha-synuclein oligomerization. Front Aging Neurosci. 2010;1:5. doi: 10.3389/neuro.24.005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Outeiro TF, Kontopoulos E, Altmann SM, Kufareva I, Strathearn KE, Amore AM, et al. Sirtuin 2 inhibitors rescue alpha-synuclein-mediated toxicity in models of Parkinson's disease. Science. 2007;317:516–519. doi: 10.1126/science.1143780. [DOI] [PubMed] [Google Scholar]
- 316.Green KN, Steffan JS, Martinez-Coria H, Sun X, Schreiber SS, Thompson LM, et al. Nicotinamide restores cognition in Alzheimer's disease transgenic mice via a mechanism involving sirtuin inhibition and selective reduction of Thr231-phosphotau. J Neurosci. 2008;28:11500–11510. doi: 10.1523/JNEUROSCI.3203-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]