Abstract
Background
Patients with human immunodeficiency virus (HIV) infection have an increased risk of cardiovascular diseases. Previous publications described pericardial effusion as one of the most common HlV-associated cardiac affiliations. The aim of the current study was to investigate if pericardial effusion still has a relevant meaning of HIV-infected patients in the era of antiretroviral therapy.
Methods
The HIV-HEART (HIV-infection and HEART disease) study is a cardiology driven, prospective and multicenter cohort study. Outpatients with a known HIV-infection were recruited during a 20 month period in a consecutive manner from September 2004 to May 2006. The study comprehends classic parameters of HIV-infection, comprising CD4-cell count (cluster of differentiation) and virus load, as well as non-invasive tests of cardiac diseases, including a thorough transthoracic echocardiography.
Results
802 HIV-infected patients (female: 16.6%) with a mean age of 44.2 ± 10.3 years, were included. Duration of HIV-infection since initial diagnosis was 7.6 ± 5.8 years. Of all participants, 85.2% received antiretroviral therapy. Virus load was detectable in 34.4% and CD4 - cell count was in 12.4% less than 200 cells/μL. Pericardial effusions were present in only two patients of the analysed population. None of the participants had signs of a relevant cardiovascular impairment by pericardial effusion.
Conclusions
Our results demonstrate that the era of antiretroviral therapy goes along with low rates of pericardial effusions in HIV-infected outpatients. Our findings are in contrast to the results of publications, performed before the common use of antiretroviral therapy.
Keywords: pericardial effusion, HIV-infection, AIDS, antiretroviral therapy
Introduction
HIV-infection is often associated with cardiac disorders [1]. Nevertheless, cardiac involvement in this patient population was frequently underdiagnosed or attributed incorrectly to other non-cardiac disease processes [2].
In particular symptoms, such as fatigue or reduced exercise intolerance, are common in this patient population and could belong to chronic cardiac disorders, such as pericardial effusion. In fact, previous studies described, that a pericardial effusion is present in up to 11% of cases [3] and, therefore, it was supposed to be one of the most common cardiac disorder in HIV-infected subjects [4].
However, the development of antiretroviral therapeutics dramatically changed the natural history of the HIV-infection. Although today most of the patients infected with the HI-virus receive antiretroviral therapy, there is still a lack of knowledge about its effects on cardiac disorders and in particular the extend of pericardial effusion.
The current study investigates the frequency of pericardial effusion and gives an answer to the question, if it still is one of the main relevant HlV-associated cardiac diseases in the era of antiretroviral therapy.
Methods
Data from the HIV-HEART (HIV-infection and HEART disease) Study were used for the following analyses. This study is a cross-sectional study conducted as part of the HIV-HEART prospective study designed to define the prevalence and natural history of cardiovascular diseases in HIV-infected individuals with and without antiretroviral therapy. All relevant facts of the HIV-HEART Study, including design, inclusion and exclusion criteria, diagnostic techniques and questionnaires, have been published previously [5]. In short, main aspects for the present analyses are described as follows:
Patients
During a recruiting time of 20 month from September 2004 to May 2006, more than 800 consecutive patients were recruited. All patients had to fulfil the inclusion criteria, in particular a known infection with the human immunodeficiency virus and a stable health condition within 4 weeks before inclusion. Furthermore, an appropriate written informed consent and an age over 18 years were requested for inclusion in this prospective trial. Patients were recruited from a multicenter network, which has been founded for the present study. The network combined physicians specialized in the diagnostic and treatment of HIV-infected patients and contained all regional forms of out-patient medical care institutions, medical practices, health care centers, and outpatient departments.
Study protocol
The study protocol included a standardized assessment of medical history and physical examination. Blood was drawn for comprehensive laboratory tests. Furthermore, non-invasive tests of the present study were performed during the initial visit. These tests included subsequent heart rate and blood pressure measurements, resting electrocardiogram (ECGs), a functional capacity test (six-minute walk test), and transthoracic echocardiography.
Echocardiography was performed under stable conditions in a quiet environment and according to a previously defined standardized operation protocol. To get the best cardiac view, the patient laid on the left side. Echocardiography protocol included a parasternal, apical, substernal echocardiography of the heart. The presence of pericardial effusion was documented and separation of pericardium was measured in at the end of diastole at the right- and left ventricular chambers of the heart. The examinations were performed in accordance with the standard operating procedures (SOPs) of the German Heart Failure Network and the ESC-Guidelines. If a pericardial effusion was identified in 2D- Echocardiography or M-Mode- Echocardiography the size was graded as: (1) small (echo-free space in diastole < 10 mm), (2) moderate (10-20 mm), (3) large (> 20 mm), or (4) very large (≥ 20 mm and compression of the heart) corresponding to the Horowitz Class B to D [6].
Ethical Considerations and Statistics
The protocol was approved by the Institutional Review Board of our center and was performed in accordance with institutional guidelines and the Declaration of Helsinki. Statistical analysis was performed by the statistical package SPSS Version 17.0 (SPSS Inc.). Variables were presented as mean and standard deviation. In addition, median, whole range and interquartile range (IQR) from 25%75% quartile are obtained for selected variables.
Figure 1.
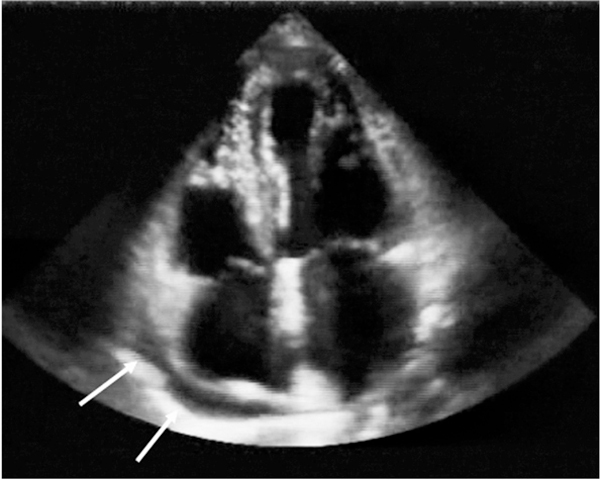
Pericardial effusion of an asymptomatic HIV-infected subject. The pericardial effusion was first identified by echocardiography in the present study.
Results
802 participants (83.4% male) were analysed for the present study. The mean age in this population was 44.2 ± 10.3 years (Median: 43 y). At the date of initial HIV-diagnosis, the age of the participants was 36.5 ± 10.7 years. Hence, the HIV-infection was diagnosed for 91.6 ± 69 months (= 7.6 ± 5.8 years) when patients were included into the study. Concerning the severity of the disease, more than 30% of the included persons had a status C (AIDS level) in the CDC-Classification [7]. Further information about demographics and HIV-specific parameters are presented in Table 1.
Table 1.
Demographics and HIV-specific variables.
| Patient characteristics | Mean ± SD |
|---|---|
| Age (years) | 44.2 ± 10.3 |
| Gender (n (%Male)) | 669 (83.4) |
| BMI (kg/m2) | 24.1 ± 3.7 |
| Heart rate at rest (beats/min) | 70.6 ± 12.2 |
| Systolic blood pressure at rest (mm Hg) | 128.4 ± 20.7 |
| Diastolic blood pressure at rest (mm Hg) | 83.2 ± 12.4 |
| HIV-specific variables | |
| Duration of HIV infection (month) | 91.6 ± 69 |
| Mean CD4 cell count (cells/mm3) | 508.8 ± 300.6 |
| CD4/CD8 ratio | 0.62 ± 0.53 |
| Mean HIV-1Viral load (RNA copies/mL) | 33419 ± 71827 |
| Way of infection (n (%)) | |
| • MSM | 477 (59.5) |
| • Heterosex. Contact | 163 (20.3) |
| • IVDA | 75 (9.4) |
| • Epidemic Areas | 56 (7.0) |
| • Blood Transfusion | 13 (1.6) |
| Patients receiving ART (% of all) | 85.2% |
| Patients antiretroviral therapy naive (% of all) | 10.8% |
| Patients in treatment interruption (% of all) | 4.0% |
| HIV specific treatment (% of ART) | |
| • NNRTI | 45.7% |
| • NRTI | 96.8% |
| • PI | 48.5% |
| Hepatitis B (% of all) | 30.5% |
| Hepatitis C (% of all) | 10.5% |
Abbreviations: BMI - body mass index, RNA - Ribonucleic acid, MSM - Men who have Sex with Men, IVDA - intravenous drug abuse, ART - anti-retroviral therapy, NNRTI non-nucleoside reverse transcriptase inhibitors; NRTI - nucleoside reverse transcriptase inhibitors; PI - protease inhibitors.
A number of patients presented with clinical symptoms suspicious for pericardial effusion. In particular 147 (18.3%) patients declared different stages of exercise-induced dyspnoea. Furthermore 29.4% exhibited a reduced physical activity (< 350 m in 6 minute walk test). Other signs of pericardial effusion, that could be a result of a reduction in right heart filling, such as oedema were seen in 33 (4.1%) participants.
In addition to clinical symptoms, patients also exhibited cardiovascular parameters, that can be associated with pericardial effusion or tamponade, such as an increased heart rate of more than 90 beats per minute (6.2% of patients) or a low systolic blood pressure of less than 100 mmHg (4.3% of patients).
On the other hand the included patient population also exhibited HIV-associated and non HIV-associated diseases predisposing for the development of pericardial effusion. One of the main aspects for the development of pericardial effusion is infectious disease. While all included subjects had a chronic infection with the HI-virus, co-infections with Hepatitis B and C were also common (30.5% and 10.5%, respectively). Other diseases, that could induce pericardial effusion, were renal dysfunction demonstrated by a creatinine value of more than 1.3 mg/dl in 6.2% of analysed patients. Liver cirrhosis was seen in 1.3% of all analysed patients.
We could even see a number of participants combining a severe CDC-classification with symptoms and diseases suggestive for development of pericardial effusion. However the rate of the subjects who actually developed a pericardial effusion was rather small. Only in two of the 802 (0.25%) HIV-infected patients pericardial effusion could be seen by echocardiography. None of these patients exhibited tamponade or signs of cardiovascular impairment such as swinging heart.
The two patients identified with pericardial effusion were male subjects at the age of 41 and 55 years, both receiving antiretroviral therapy. Small pericardial effusion (< 10 mm) corresponding to Type B pericardial effusion [8] could be identified on both patients. No clinical signs of pericardial tamponade [6] like oedema as a sign for right heart failure, hypotension, tachycardia, pulsus paradoxus could be documented. Both patients presented with normal left ventricular ejection fraction. In the younger patient HIV-infection (CDC stage C3), liver cirrhosis, co-infection with Hepatitis B and C, chronic renal failure grade 2 [9] was present and improved the assumption of extra-cardiac reasons for pericardial effusion. Follow-up data of this patient is not available. The other HIV-positive patient (CDC stage B3) presenting with pericardial effusion had no other known diseases. We therefore assume that HIV-infection was the cause for the documented small pericardial effusion. In the 2-year follow up of the second patient no remaining pericardial effusion could be documented.
Discussion
Our results demonstrate a low rate of pericardial effusion in HIV-infected patients in the era of antiretroviral therapy. This finding is in contrast to previously published results. Heidenreich et al. stated in 1995 that pericardial effusion is known to be common among patients infected with HIV [3]. Velasquez et al. even went one step further and declared this cardiac affiliation to be the most common cardiovascular manifestation of HIV infection [10].
The assumptions of the above authors were based on a variety of echocardiographic studies on HIV-infected subjects, mostly performed in the pre-HAART (Highly Active Anti-Retroviral Therapy) era between 1985 and 1995. The prevalence of pericardial effusion rates published in three major trials range from 5% to 45% in different HIV-infected patient collectives [11-13]. Despite different inclusion criteria and different patient collectives, one of them only including patients with AIDS [13], they illustrate the tremendous impact on morbidity of pericardial effusion in HIV-infected individuals.
Also in developing countries a high incidence of pericardial effusion was described [14]. Pericardial effusions in these patient collectives were mainly seen in combination with tuberculosis, which is associated with an increased mortality as previously described by Mayosi et al. [15,16].
In our patients presenting with pericardial effusion we could not identify a specific bacterial infection being the reason for pericardial effusion. Also other previously described reasons like neoplasm or a cardiac trauma [17-20] could be ruled out in our patients. One patient presented with liver cirrhosis based on hepatitis infection and renal disorder which lead to the suggestion that these diseases were responsible for development of pericardial effusion rather than the HIV-Infection.
Although antiretroviral drugs might diminish the incidence of pericardial effusion due to opportunistic infections, other causes for the development of this cardiac disorder might have become more frequent. In particular, the improvement of the immune defence by the use of antiretroviral drugs could induce pericardial effusions in the context of an immune reconstitution inflammatory syndromes [21]. This was not the case in our study, however.
The reason, why the rate of pericardial effusion is significantly reduced in the era of antiretroviral therapy is yet unknown and could not definitively be answered by our work. Nevertheless, it seems to be obvious, that the effective blockade of virus replication and a reduced rate of opportunistic infections might have major effects on the low pericardial effusion rate.
Limitations of the Study
There are limitations due to the single cross-sectional design of the study. Cross-sectional studies are carried out at one time point or over a short period of time and are usually conducted to estimate the prevalence of the outcome of interest for a given population. In the case of longer lasting diseases like HIV, any risk factor might be underrepresented among those with the disease. Hence the data have to be addressed carefully.
Due to a high specificity (100%) and sensitivity (94%) [8,22] transthoracic echocardiography is recommended for detection of pericardial effusion [6]. The present study was performed by a trained staff according to a standardized echocardiography protocol and demonstrated a very low intra-observer variability. However in patients with poor echo window, e.g. in patients with COPD image quality might not be optimal. This was not the case in our patient collective. Therefore, we believe that the present results are reliable and reflect reality.
Another limitation might be the exclusion of hospitalized patients. In fact, a hospitalized group of HIV patients might have lead to an increased rate of pericardial effusion. However, the topic of our work was to analyse the impact of pericardial effusion in the group of outpatients that is the largest cohort of individuals in HIV-community since an effective antiretroviral therapy was developed.
Conclusion
Summarizing our data, pericardial effusion in HIV-infected patients is less frequent in the era of antiretroviral drug therapy, compared to the pre-HAART era. Clinical symptoms such as dyspnoea, exercise intolerance or oedema are suspicious for pericardial effusion and should lead to further investigation by transthoracic echocardiography being a reliable screening tool. This should be done in any patient suffering from the above symptoms independent from an infection with the Hi-Virus.
Competing interests
The authors declare that they have no competing interests.
Acknowledgements
This work was supported by the Competence Network of Heart Failure funded by the Federal Ministry of Education and Research (BMBF), FKZ 01GI0205. The authors give their respect and thanks to all participating and supporting persons of the present study, including the staff of the recruiting centers, i.e. the team of Dr. Becker-Boost (Duisburg), Dr. Rudolph (Essen), Dr. Hower (Dortmund), Prof. Dr. Brockmeyer (Bochum), and Dr. Esser (Essen).
References
- Fisher SD, Lipshultz SE. In: Heart disease: A text book of cardiovascular medicine. Braunwald E, editor. Philadelphia: WB Saunders; 2001. Cardiovascular abnormalities in hiv-infected individuals; pp. 2211–21. [Google Scholar]
- Jacob AJ, Boon NA. HIV cardiomyopathy: A dark cloud with a silver lining? Br Heart J. 1991;66(1):1–2. doi: 10.1136/hrt.66.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidenreich PA, Eisenberg MJ, Kee LL, Somelofski CA, Hollander H, Schiller NB, Cheitlin MD. Pericardial effusion in AIDS. Incidence and survival. Circulation. 1995;92(11):3229–34. doi: 10.1161/01.cir.92.11.3229. [DOI] [PubMed] [Google Scholar]
- Fink L, Reichek N, Sutton MG. Cardiac abnormalities in acquired immune deficiency syndrome. Am J Cardiol. 1984;54(8):1161–3. doi: 10.1016/S0002-9149(84)80178-2. [DOI] [PubMed] [Google Scholar]
- Neumann T, Esser S, Potthoff A, Pankuweit S, Neumann A, Breuckmann F. et al. Prevalence and natural history of heart failure in outpatient hiv-infected subjects: Rationale and design of the HIV-HEART study. Eur J Med Res. 2007;12(6):243–8. [PubMed] [Google Scholar]
- Maisch B, Seferovic PM, Ristic AD, Erbel R, Rienmüller R, Adler Y. et al. Guidelines on the diagnosis and management of pericardial diseases executive summary; the task force on the diagnosis and management of pericardial diseases of the european society of cardiology. Eur Heart J. 2004;25(7):587–610. doi: 10.1016/j.ehj.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41(RR-17):1–19. [PubMed] [Google Scholar]
- Horowitz MS, Schultz CS, Stinson EB, Harrison DC, Popp RL. Sensitivity and specificity of echocardiographic diagnosis of pericardial effusion. Circulation. 1974;50(2):239–47. doi: 10.1161/01.cir.50.2.239. [DOI] [PubMed] [Google Scholar]
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–266. [PubMed] [Google Scholar]
- Velasquez EM, Glancy DL. Cardiovascular disease in patients infected with the human immunodeficiency virus. J La State Med Soc. 2003;155(6):314–24. [PubMed] [Google Scholar]
- Himelman RB, Chung WS, Chernoff DN, Schiller NB, Hollander H. Cardiac manifestations of human immunodeficiency virus infection: A two-dimensional echocardiographic study. J Am Coll Cardiol. 1989;13(5):1030–6. doi: 10.1016/0735-1097(89)90256-8. [DOI] [PubMed] [Google Scholar]
- Hecht SR, Berger M, Van Tosh A, Croxson S. Unsuspected cardiac abnormalities in the acquired immune deficiency syndrome. An echocardiographic study. Chest. 1989;96(4):805–8. doi: 10.1378/chest.96.4.805. [DOI] [PubMed] [Google Scholar]
- Akhras F, Dubrey S, Gazzard B, Noble MI. Emerging patterns of heart disease in HIV infected homosexual subjects with and without opportunistic infections; a prospective colour flow doppler echocardiographic study. Eur Heart J. 1994;15(1):68–75. doi: 10.1093/oxfordjournals.eurheartj.a060382. [DOI] [PubMed] [Google Scholar]
- Cegielski JP, Ramiya K, Lallinger GJ, Mtulia IA, Mbaga IM. Pericardial disease and human immunodeficiency virus in dar es salaam, tanzania. Lancet. 1990;335(8683):209–12. doi: 10.1016/0140-6736(90)90288-G. [DOI] [PubMed] [Google Scholar]
- Mayosi BM. Contemporary trends in the epidemiology and management of cardiomyopathy and pericarditis in sub-saharan africa. Heart. 2007;93(10):1176–83. doi: 10.1136/hrt.2007.127746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayosi BM, Wiysonge CS, Ntsekhe M, Gumedze F, Volmink JA, Maartens G. et al. Mortality in patients treated for tuberculous pericarditis in sub-saharan africa. S Afr Med J. 2008;98(1):36–40. [PubMed] [Google Scholar]
- Chyu KY, Birnbaum Y, Naqvi T, Fishbein MC, Siegel RJ. Echocardiographic detection of kaposi's sarcoma causing cardiac tamponade in a patient with acquired immunodeficiency syndrome. Clin Cardiol. 1998;21(2):131–3. doi: 10.1002/clc.4960210217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer E, Mischke U, Stömmer P, Harrer T, Stoll R. Kaposi's sarcoma with pericardial tamponade in AIDS. Dtsch Med Wochenschr. 1988;113(30):1187–90. doi: 10.1055/s-2008-1067791. [DOI] [PubMed] [Google Scholar]
- Stotka JL, Good CB, Downer WR, Kapoor WN. Pericardial effusion and tamponade due to kaposi's sarcoma in acquired immunodeficiency syndrome. Chest. 1989;95(6):1359–61. doi: 10.1378/chest.95.6.1359. [DOI] [PubMed] [Google Scholar]
- Theodossiades G, Tsevrenis V, Bellia M, Avgeropoulou A, Nomikos J, Kontopoulou-Griva I. Cardiac tamponade due to post-cardiac injury syndrome in a patient with severe haemophilia A and HIV-1 infection. Haemophilia. 2000;6(5):584–7. doi: 10.1046/j.1365-2516.2000.00399.x. [DOI] [PubMed] [Google Scholar]
- Rogers JS, Zakaria S, Thom KA, Flammer KM, Kanno M, Mehra MR. Immune reconstitution inflammatory syndrome and human immunodeficiency virus-associated myocarditis. Mayo Clin Proc. 2008;83(11):1275–9. doi: 10.4065/83.11.1275. [DOI] [PubMed] [Google Scholar]
- Friedman MJ, Sahn DJ, Haber K. Two-Dimensional echocardiography and b-mode ultrasonography for the diagnosis of loculated pericardial effusion. Circulation. 1979;60(7):1644–9. doi: 10.1161/01.cir.60.7.1644. [DOI] [PubMed] [Google Scholar]


