Abstract
The most-severe form of congenital generalized lipodystrophy (CGL) is caused by mutations in BSCL2/seipin. Seipin is a homo-oligomeric integral membrane protein in the endoplasmic reticulum that concentrates at junctions with cytoplasmic lipid droplets (LDs). While null mutations in seipin are responsible for lipodystrophy, dominant mutations cause peripheral neuropathy and other nervous system pathologies. We first review the clinical aspects of CGL and the discovery of the responsible genetic loci. The structure of seipin, its normal isoforms, and mutations found in patients are then presented. While the function of seipin is not clear, seipin gene manipulation in yeast, flies, mice, and human cells has recently yielded a trove of information that suggests roles in lipid metabolism and LD assembly and maintenance. A model is presented that attempts to bridge these new data to understand the role of this fascinating protein.
Keywords: congenital generalized lipodystrophy, lipodystrophy, Berardinelli-Seip congenital lipodystrophy, lipid droplet, adipogenesis
Over the last 10 years, the genetic defects underlying most cases of congenital generalized lipodystrophy (CGL) have been identified. All but one of these are in genes that encode proteins known to be involved in neutral lipid synthesis or transport. The exceptions are mutations in BSCL2/seipin. How mutations in seipin attenuate adipogenesis, and how seipin normally functions in cells, are questions that are still unanswered. However, the effects of manipulation of expression at the tissue and cellular levels have offered intriguing hints to molecular function. In this review, we will first summarize lipodystrophy as a disease and present the known responsible genes. Then we will discuss the effects of seipin manipulation on function, from the level of intracellular organelles and lipid interconversions to the scale of tissues and organs. Two current hypotheses will be presented concerning seipin action, and we will conclude with a more-detailed mechanistic scenario.
CGL
The term “lipodystrophy” represents a wide spectrum of genetic and acquired syndromes characterized by an extreme paucity of functional adipose tissue, the most severe of which is that caused by hereditary seipin deficiency. Lipodystrophy may also involve the redistribution of adipose tissue. The first known report of a lipodystrophic syndrome was published in 1885 and described a patient with partial lipodystrophy of unknown etiology who experienced progressive wasting of fat tissue in the upper half of her body (1). In this and many other cases of partial or localized lipodystrophy in which only a subset of adipose depots are affected, the patient's chief concern is often cosmetic, since enough adipose tissue remains to avoid a major metabolic insult (2).
However, with more extreme fat loss, the patient's metabolic condition becomes more severe. The first thorough characterization of the effects of whole-body, or generalized, lipodystrophy was published by R. D. Lawrence in 1946 (3). This syndrome was often termed “lipoatrophic diabetes” because of the striking metabolic picture that accompanies fat loss. Patients with generalized or severe partial lipodystrophy experience extreme hypertriglyceridemia secondary to the loss of appropriate fat storage in adipose tissue. This hyperlipidemia may lead to eruptive xanthomas or recurring pancreatitis and is nearly always associated with ectopic lipid storage and insulin resistance. Lipid accumulation in nonadipose tissues, such as skeletal muscle and liver, gives patients a striking appearance marked by muscular hypertrophy with prominent veins and organomegaly with umbilical protrusion. This organomegaly often occurs with the eventual development of umbilical hernia, steatohepatitis, and cirrhosis. Insulin resistance, possibly caused by this ectopic lipid accumulation, leads to hyperinsulinemia, acanthosis nigricans, type II diabetes mellitus, and in female patients, polycystic ovarian syndrome. Generalized lipodystrophic patients also usually display a low HDL level, low levels of the adipocyte-derived hormones leptin and adiponectin, increased basal metabolic rate with increased body temperature and sweating, and a voracious appetite.
This clinical picture was first characterized for immune-mediated, progressive, acquired generalized lipodystrophy (AGL, sometimes called Seip-Lawrence syndrome) (3). In 1954 and 1959, W. Berardinelli and Martin Seip, respectively, reported and characterized the first cases of inherited syndromes of CGL, eventually termed Berardinelli-Seip syndrome or Berardinelli-Seip congenital lipodystrophy (BSCL) (4, 5). The diagnosis of CGL is usually apparent at birth or within the first 2 years of life by the child's strikingly lean, muscular appearance. Patients with CGL, as well as those patients who develop AGL at a particularly young age, suffer from, in addition to the features listed above, a set of problems unique to experiencing insulin resistance and hypertriglyceridemia from birth. These include accelerated growth and early growth arrest, mildly acromegaloid features, moderate genital enlargement, and pubertal onset of diabetes, all features that have been noted in other syndromes of infantile hyperinsulinemia (6). Additional frequently observed features include abundant curly hair and mild hypertrichosis. Partial and generalized forms of lipodystrophy have also been identified as features of a number of inherited syndromes with multisystem effects and as a long-term side effect of highly active anti-retroviral therapy for human immunodeficiency virus, as thoroughly reviewed elsewhere (2).
Treatment for lipodystrophy has necessarily focused on limiting hyperlipidemia and diabetic complications. Patients often require extremely high doses of insulin to achieve normoglycemia, and only a few oral antidiabetic or lipid-lowering medications have been useful, e.g., thiazolidinediones (TZDs), metformin, and fibrates (7). TZDs target the peroxisome proliferator-activated receptor γ (PPARγ), and the TZD troglitazone was found to be particularly effective in improving the metabolic profile in two patients with generalized lipodystrophy (8). Another successful method for controlling plasma lipid and glucose levels has been extreme dietary fat restriction. Adherence to diet has been aided by fenfluramine (used to treat a few of Seip's patients before its withdrawal from the market) or by leptin replacement to promote satiety (9–11). Additionally, independent of its effect in lowering caloric intake, leptin can also ameliorate diabetes and other metabolic disturbances that are caused or worsened by lipodystrophic leptin deficiency (9, 12).
None of these treatments, however, is capable of reversing the primary defect of fat loss. It was hoped that TZDs could reverse lipodystrophy by promoting differentiation of adipocytes because they were known to do so through stimulation of the PPARγ adipogenic program in vitro (13). However, troglitazone treatment produced only small increases in subcutaneous fat and no increases in visceral fat, resulting in an average 2.4% increase in total body fat after 6 months of treatment (8). It seems likely that development of a true lipodystrophy-reversing drug will require a much more specific understanding of the defects leading to adipocyte dysfunction in these patients.
IDENTIFICATION OF CGL GENES
From its initial discovery, CGL was found to be inherited in an autosomal recessive manner and has since been observed in over 250 families around the world, with an estimated prevalence of 1 in 10 million (14). Particularly significant clusters have been observed in Norway, Brazil, and Lebanon (15, 16). Forty-five years after the first report of CGL, the first genetic locus associated with the syndrome was identified and later found to be in AGPAT2 (17, 18). AGPAT2 encodes a 1-acylglycerol-3-phosphate-O-acyltransferase, which is known to catalyze the synthesis of phosphatidic acid (PA) from lysophosphatidic acid (19, 20). How loss-of-function mutations in this gene render adipose tissue dysfunctional remains unclear, but it has been speculated that AGPAT2 dysfunction may impair adipose triacylglycerol (TAG) synthesis, or the excess of PA and other phospholipids observed in AGPAT2 deficiency (synthesized either by other AGPAT isoforms or through an alternate monoacylglycerol-based pathway) may disrupt adipocyte signaling (21–23).
After the mapping of the first locus, but shortly before the identification of AGPAT2, a second locus was discovered via linkage analysis on CGL families in Norway and Lebanon. This newly identified gene of unknown function was named BSCL2, or seipin (16), and is the subject of this review. Loss-of-function mutations in seipin cause the most-severe cases of CGL (described below), and yet seipin remains the most mysterious lipodystrophic protein in terms of function.
While most CGL patients have documented mutations in either AGPAT2 or seipin, more recent studies using a candidate gene approach found CGL patients with mutations in the genes encoding either caveolin-1 (CAV1) or cavin-1 (PTRF), both of which are crucially involved in caveolae formation (24, 25). Caveolae appear to be particularly important in adipocyte function; they are extremely dense on the adipocyte plasma membrane, comprising approximately 20% of the cell surface, and the lack of caveolae in these patients is likely to disrupt adipocyte signaling and lipid uptake (26, 27). Although genetic studies indicate that more genetic loci exist for CGL, the four described here, AGPAT2, seipin, CAV1, and cavin-1, currently remain the only genes identified (2).
PHENOTYPIC DIFFERENCES AMONG CGL PATIENTS
While all CGL patients share the characteristic clinical picture of lipodystrophy, hypertriglyceridemia, and insulin resistance, important phenotypic differences have been observed among the known CGL genotypes. Although AGPAT2-deficient patients display typical poor fat accumulation in the so-called “metabolically active” adipose tissues (subcutaneous, visceral, and bone marrow fat), the “mechanical” adipose tissues (retro-orbital, buccal, tongue, palmar, plantar, crista galli, scalp, perineum, vulvar, peri-articular, epidural, and pericalyceal fat) remain unaffected (28). These patients also display lytic bone lesions that are absent in other forms of CGL (28, 29).
In the single known caveolin-1-deficient patient, all adipose depots except bone marrow are diminished. This patient also exhibits the unique manifestation of short stature, hypocalcemia, and apparent vitamin D resistance, presumably due to a role of caveolae in renal function (25). Cavin-1-deficient patients have a secondary deficiency of caveolin-1 in adipocytes and therefore present a similar pattern of generalized lipodystrophy with bone marrow preservation, although they retain some mechanical adipose tissue and have vestigial dorsal subcutaneous fat. In addition, cavin-1-deficient patients manifest features of muscular dystrophy, impaired bone formation, smooth-muscle hypertrophy, and occasional cardiac arrhythmia; this phenotype is probably due to secondary deficiency of muscle-specific caveolin-3 and a more global defect in caveolae (24, 30–32).
Seipin-deficient patients present with the most extreme lipodystrophic phenotype, since none of the adipose tissue depots described above appear to be significantly preserved in these patients (28). This often leads to an earlier onset of diabetes (33, 34). While seipin deficiency represents the only truly whole-body form of congenital lipodystrophy, it is important to note that even these patients are not entirely deficient in adipocytes. Examination of subcutaneous biopsies from several of Seip's patients found scattered groups of small adipocytes with low but detectable lipid content (35).
These residual adipocytes may even retain some degree of endocrine function. Leptin levels are extremely low in CGL patients, lower than in AGL patients, but are not undetectable (36, 37). Reports conflict as to whether leptin levels are higher in AGPAT2- or seipin-deficient patients, but the differences observed in circulating leptin between these two groups are relatively small (33, 34, 38). Intriguingly, while adiponectin levels are nearly undetectable in AGPAT2-deficient patients, circulating adiponectin in seipin-deficient patients is approximately 40% of that in healthy controls, a level comparable to that observed in familial partial lipodystrophy (38). The remaining adiponectin may come from three possible sources: 1) compensatory upregulation of adiponectin secretion in a few small, residual adipocytes, 2) unexpected secretion from nonadipocytes, or 3) secretion from “quasi-adipocytes” although normal mature adipocytes are scarce, CGL patients could have abundant cells that cannot carry out certain mature adipocytic functions, such as lipid accumulation, but remain capable of other adipocyte functions, such as adiponectin secretion. Further work will be required to determine the source of adiponectin in seipin-deficient patients and the mechanism of this difference between seipin and AGPAT2.
Two additional phenotypes have been noted in seipin-deficient patients that so far show no obvious relation to the lipodystrophic and metabolic manifestations of this disease. First, seipin-deficient patients are at much higher risk for hypertrophic cardiomyopathy, a significant cause of mortality that may contribute to the higher rates of premature death observed in seipin-deficient patients compared with other CGL patients (29, 33). This may represent a cell-autonomous defect in cardiomyocytes, because these patients do not display obvious features of cardiac disease secondary to hyperlipidemia: cardiac histology shows no signs of lipid accumulation within cardiomyocytes, and coronary arteries show no clinically significant atherosclerotic placques (39).
In addition, seipin-deficient patients exhibit much higher rates of mild mental retardation than do patients with other CGL genotypes. These cognitive effects are speculated to relate to the high expression of seipin in the brain (see below), rather than the metabolic phenotype (16, 21). Although the pathogenesis of mental retardation in these patients has not yet been explained, this may point to a role for seipin in neuronal function.
NEUROLOGICAL SEIPINOPATHIES
The potential neuronal role for seipin described above is supported by findings in patients with an entirely different class of seipin mutations. While loss of seipin function results in lipodystrophy, dominant gain-of-function or gain-of-toxic mutations in the same seipin gene result in neurological disorders, now termed seipinopathies (40). Symptoms are heterogenous and represent both upper and motor neuron disruption, because patients can present with muscle weakness and spasticity in their lower limbs (Silver Syndrome), weakness of the distal muscles of their upper limbs (distal hereditary motor neuropathy type V), and wasting of the hand muscles (seen in both classifications). Complex forms of the disease can include deafness, dementia, or mental retardation. The seipin gene is one of several loci responsible for these symptoms. Penetrance and severity of disease vary widely for seipinopathies, even within a given pedigree, although the interaction with other genetic loci or epigenetic phenomena is unknown. These patients generally do not have a reduced life span, unlike patients with seipin-deficient lipodystrophy. Only two mutations are known to produce these seipinopathies; both of these missense mutations reside in a glycosylation site on seipin and are believed to have an effect through endoplasmic reticulum (ER) stress (see below).
Recently, a Taiwanese man with lipodystrophy and dystonia was demonstrated to have two distinct mutant seipin alleles; his deceased sisters had reportedly experienced similar symptoms but could not be genotyped. Both mutations were outside the glycosylation site (Table 1 and Fig. 1), and each has been elsewhere identified in homozygous CGL patients lacking dystonia or other neurological symptoms (41, 42). It is still unclear whether the dystonia observed in this single patient (and possibly his siblings) represents a unique effect of this combination of mutations, a rare effect of either or both alleles, the presence of a modifier gene, or an unrelated disease. If validated, the association of these alleles with dystonia would further suggest a role for seipin in neuronal function (43).
TABLE 1.
Pathogenic seipin mutations in humans
| Isoform 1 | Isoform 2 | OMIM # | First Report | ||
| Truncations | |||||
| a | S128fs | S64fs | 606158.0001 | Magré et al. 2001 | |
| b | M165fs | M101fs | 606158.0002 | ||
| c | Y170fs | Y106fs | 606158.0003 | ||
| d | Y170fs | Y106fs | 606158.0005 | ||
| e | T173fs | T109fs | 606158.0006 | ||
| f | R202X | R138X | 606158.0007 | ||
| g | L278fs | L214fs | 606158.0010 | ||
| h | Y289fs | Y225fs | 606158.0011 | ||
| i | ΔY289-Q335fs | ΔY255-Q271fs | 606158.0012 | ||
| j | R339X | R275X | 606158.0015 | Ebihara et al. 2004 | |
| kb | E228X | E189X | 606158.0016 | Jin et al. 2007 | |
| lb | I326fs | I262fs | — | Wu et al. 2008 | |
| m | A282fs | A218fs | — | Shirwallar et al. 2008 | |
| n | Q455X | Q391X | — | Miranda et al. 2009 | |
| Large deletions | |||||
| o | del/ins exons 5–6 | 606158.0004 | Magré et al. 2001 | ||
| p | del exon 4 | 606158.0008 | |||
| q | del exons 4-6 | — | |||
| r | del exon 5 | — | Miranda et al. 2009 | ||
| Missense mutations | |||||
| s | A276P | A212P | 606158.0009 | Magré et al. 2001 | |
| ta | N152S | N88S | 606158.0013 | Windpassinger et al. 2004 | |
| ua | S154L | S90L | 606158.0014 | ||
| v | T142A | T78A | — | Miranda et al. 2009 | |
| w | L155P | L91P | — | ||
| x | Y251C | Y187C | — | Nishiyama et al. 2009 |
Frameshift mutations are listed by the first affected amino acid.
Mutations causing neuronal seipinopathy without lipodystrophy.
Mutations found in one compound heterozygous lipodystrophic patient with dystonia; known homozygotes display no neuronal symptoms.
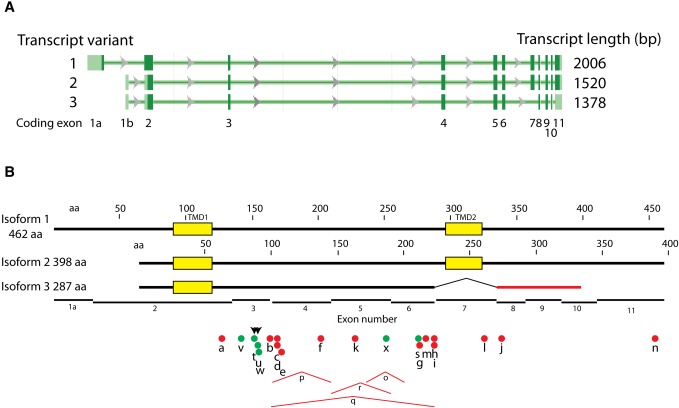
Fig. 1.
The human seipin gene, major transcripts, and mutations. A: Three coding transcripts, modified from NCBI Gene webpage for BSCL2. Exons are shown as rectangles, introns as lines with direction of transcription indicated. Open reading frames in dark green, untranslated regions in light green. Exon numbers are indicated. B: Seipin protein isoforms and mutations. Yellow boxes are the two consensus TMDs. Amino acids are numbered separately, corresponding to isoforms 1 and 2. Black lines, normal sequence; red line, aberrant sequence of isoform 3 due to out-of-register exon splicing. Dots and tents underneath indicate mutations. Red dots are truncation mutations, green dots are missense mutations, and red tents correspond to large deletions. Arrowheads indicate the two mutations in the glycosylation site. Mutation lettering corresponds to those listed in Table 1.
SEIPIN STRUCTURE
The seipin gene was originally identified in mammals and the fruit fly (16) and later extended to fungi and plants (44, 45). The orthologs share a central region of about 230 amino acids, conserved in secondary structure, that consists of two probable transmembrane domains (TMDs) and an intervening sequence referred to as the loop (Fig. 1). Outside of the central region, the protein sequences diverge. The N-terminal extension varies from 4 amino acids in Caenorhabditis elegans to 102 amino acids in rice. In humans, there are two major splice forms yielding amino terminal extensions of 25 or 90 amino acids (see below). Similarly, the carboxy-terminal extension beyond the second TMD ranges from 4 amino acids in Kluyveromyce lactis to 467 in Dictyostelium discoidium (138 in humans). The mammalian proteins terminate in a CAAX box motif (CSSS), the physiological significance of which is not known.
The human seipin gene (OMIM #606158) is on chromosome 11q13, with protein coding on the Crick strand. There are three validated coding transcripts in GenBank (Fig. 1A). The primary transcript originally described (16) contained 11 exons with protein coding beginning on exon 2 and ending in exon 11 (transcript variant 2), resulting in a 398 amino acid protein with two strongly predicted TMDs, coded in exons 2 and 7 (isoform 2) (Fig. 1). However, a longer transcript (variant 1) is generated with an alternative first exon containing a translational start site that results in an additional 64 amino acids at the N terminus, 462 amino acids in total (isoform 1). A third coding transcript (variant 3) splices out exon 7 and produces a shortened and altered carboxy terminus in exon 10, generating a protein of 287 amino acids (isoform 3). Additionally, several coding transcripts are listed in the Consensus Coding Sequence (CCDS) database (www.ncbi.nlm.nihlgov/CCDS): two that encode isoform 1 (2,038 and 1,683 bp), three that code for isoform 2 (1,693, 1,516, and 1,440 bp), one that encodes isoform 3 (1,364 bp), and one that encodes a 464 amino acid protein containing a 6 base insertion in exon 9 (1,984 bp). No differences in function have been reported between variants 1 and 2; there is no information regarding variant 3 or the 464 amino acid product predicted from the CCDS database.
The majority of the known CGL-causing seipin mutations are frameshift or nonsense mutations that result in truncated proteins. The truncation that preserves the most primary sequence is caused by the nonsense mutation Q391X, in which only seven amino acids are deleted from the carboxy terminus. (Note that we number amino acids based on isoform 2 to conform with nearly all reports in the literature; the corresponding isoform 1 numbering of mutant sites is included in Table 1.) Both of the two unrelated patients identified with this mutation also have a missense mutation, T78A (described below), in their other allele (46). It is surprising that this 7 amino acid deletion could generate lipodystrophy, since the C-terminal cytosolic tail is not required for fat body lipid accumulation in flies (see below) (47). The loss of activity of Q391X might be manifest only in combination with T78A coded on the other allele; no Q391X homozygotes have been reported. However, two additional truncations that delete nearly all of the C-terminal tail (I262fs and R275X) cause lipodystrophy as homozygotes (41, 48), indicating that the C terminus of seipin is likely to be more functionally important in humans than in flies. These three alleles are the only known human mutations distal to TMD2.
The remaining mutations all occur within seipin's loop domain. These include four large deletions that indicate that, minimally, exons 4 and 5 are required for seipin function in humans (Table 1 and Fig. 1) (16). In addition, six missense mutations have been identified in the loop domain. Several of these cluster at the single asparagine-linked glycosylation site (NVS) in seipin (49). The two mutations that cause neuronal seipinopathy, N88S and S90L, are located directly within this site (50). In addition to disrupting glycosylation, these mutations result in aggregation of seipin and initiation of the ER stress response (see below). Two additional missense mutations that cause lipodystrophy, not seipinopathy, are also located close to this glycosylation site; these are T78A and L91P (46). The L91 residue is particularly interesting in that it is well conserved among species that contain the NVS glycosylation site (45), indicating that this region of the protein may be critical for both protein stability and seipin function. Additional missense mutations include Y187C (50), a residue that is not conserved but is usually hydrophobic (45), and A212P (17), a site that is well conserved among the known seipin orthologs (45). Analysis of a mutation corresponding to A212P in the yeast ortholog of seipin indicates that this missense mutation affects stability of the protein (51). To our knowledge, no studies have yet noted any significant phenotypic variability among seipin's many CGL-causing mutations, but such variability cannot yet be ruled out.
SEIPIN EXPRESSION
Humans
Magre et al. (16) initially reported three main seipin mRNA isoforms in humans by Northern blotting at ∼1.8, ∼2.0, and ∼2.4 kb, considered in subsequent studies (and for this discussion) to be 1.6, 1.8, and 2.2 kb, respectively. Testes displayed the most intense signal, which appeared to consist of all three bands and perhaps others. Brain also showed strong expression, and was unique in expressing only the 1.8 kb band. Most other tissues tested yielded detectible but weaker expression of the 1.6 and 2.2 kb forms, with kidney and pancreas high and adipose tissue relatively low. These results were largely confirmed by Windpassinger et al. (50). Using a 5′-terminal probe, they detected a brain-specific 1.8 kb form and a ubiquitously expressed 2.2 kb form. Their probe, from the 5′ untranslated region, apparently did not allow detection of the lower 1.6 kb band. This group did not measure expression in adipose tissue.
Both the 1.8 and 2.2 kb RNAs can encode both the 398 amino acid and 462 amino acid protein (isoforms 2 and 1, respectively), while the 1.6 kb form can only encode the 398 amino acid form, due to alternative splicing (49). When both start sites are present in DNA transfected into cells, isoform 1 is produced in greater abundance (49). Therefore, it appears that both isoforms 1 and 2 are potentially expressed in most tissues that express both 1.6 kb and 2.2 kb mRNAs. The ratio of long to short forms may be greater in brain, which mainly expresses the 1.8 kb form. Northern blots do not reveal significant amounts of a 1.4 kb form, suggesting that expression of isoform 3 is low in all tissues thus far examined.
Particularly intriguing are the high levels of mRNA in human testes and brain, which point to roles for seipin in unexpected tissues. There has been no report of infertility in seipin deficiency, and at least one male BSCL2 patient has been reported with multiple healthy children (10), indicating that the protein is not likely to be essential for viable sperm development, although whether they are as robust as sperm from healthy individuals has not been examined. An important role for seipin in nervous tissue is suggested by the mental retardation that affects many seipin lipodystrophy patients and the neural pathology of dominant seipin mutations.
While several studies have compared mRNA expression patterns for seipin, there has been limited information regarding protein expression. However, an antibody developed against a C-terminal peptide of human seipin (termed for this discussion “the seipin antibody” use outside the originating lab has not been reported) strongly reacted with motor neurons in the human spinal cord and cortical neurons in the human frontal lobe (52). Significantly, the group found that staining was much weaker in a patient with amyotropic lateral sclerosis (ALS), indicating that seipin can be specifically observed in motor neurons (the cell type which is degenerated in ALS patients) (52, 53).
Fly and mouse
In situ hybridization in fruit fly larvae revealed expression in the fat body (an organ with adipose and hepatic functions), midgut, and salivary gland (47). Quantitative real-time PCR showed highest expression in the fat body and intermediate expression in midgut, muscle, and salivary gland; expression in the brain was detectible but low.
Expression of seipin mRNA (measured by real-time PCR) in the mouse was similar to the original study in humans in that highest expression was found in the testes (54). However, unlike in humans, expression in fat depots was strong, especially in brown adipose tissue. Intermediate levels were seen in skeletal muscle and adrenal gland. Other tissues, including brain, had detectible but lower expression. Considering the role of seipin in adipogenesis, lipid metabolism, and cytoplasmic lipid droplet (LD) function (discussed below), it is somewhat surprising that levels of seipin correlate imperfectly with the mass of neutral lipid in tissues. Perhaps they correlate better with lipid dynamics rather than steady-state levels.
The seipin antibody has also been used to detect seipin protein expression in mice. This antibody reacted with neuronal cell bodies (marked with an anti-NeuN antibody) in the anterior horn of mouse spinal cord in an intracellular pattern similar to that of anti-KDEL antibody marking the ER (53). The group also found strong expression in ACTH-positive mouse cells from the anterior lobe of the mouse pituitary (52). Additionally, staining also was strong in a subset of spermatids in mouse testes. The signal was detected in both Step 6 and 7 spermatids, where protein synthesis and glycolytic activity are relatively low, and in Step 12 spermatids, where protein synthesis and glycolytic enzymes are upregulated (52). Oil Red O staining of testes revealed a similar staining pattern (Hamra and Goodman, unpublished observations), suggesting that testicular seipin may mark cells with significant neutral lipid accumulation or metabolism.
Surprisingly, human brain is considerably higher in seipin mRNA expression compared with adipose tissue, whereas the ratio is reversed in the fly and mouse. Whether these differences are absolute, are dependent on stage of development or age, or are due to technical differences in assay methods must await more careful comparative analysis. In summary, there appears to be some correlation of seipin expression with lipid metabolism, perhaps more so than with steady-state lipid concentrations.
During adipogenesis
While results differ between humans and mice regarding seipin expression in adipose tissue, it is clear from studies in cultured cells from both organisms that seipin expression is connected to adipogenesis, the process of adipocyte differentiation that is driven by several factors in a complex temporal pathway (55, 56). Adipogenesis can be divided into two major phases: 1) determination, during which mesenchymal stem cells are converted to preadipocytes, and 2) terminal differentation, in which preadipocytes induce the enzymes required for neutral lipid synthesis and storage. This latter stage is driven by expression of PPARγ and C/EBPα. Adipogenesis can be recapitulated in several lines of cultured mammalian cells by treatment with a hormone cocktail. Seipin mRNA expression has been shown to increase several-fold during hormone-induced adipogenesis in C3H10T1/2 and 3T3-L1 cells, as well as in mouse embryonic stem cells and primary mouse and human preadipocytes (54, 57), suggesting an important role of seipin in this developmental process. Seipin is probably not important for the earlier determination phase, because its expression was not changed during treatment with BMP4, a factor known to be important during this phase (54). The kinetics of induction of seipin appeared to parallel or slightly precede those of PPARγ and C/EBPα, suggesting a role for seipin in terminal differentiation, possibly in controlling activation of PPARγ. Results from knockdown experiments (see below) are also consistent with a role later on in the pathway.
DOMAINS, TOPOLOGY, AND OLIGOMERIZATION
The topology of seipin was first predicted in a survey of 37 likely yeast membrane proteins of unknown function. Based on charge distribution and hydrophobicity, the protein was predicted to span a membrane twice, with both termini facing the cytosolic side (58). This topology was later supported in an elegant study in which the glycosylation of wild-type and mutated seipin proteins was probed after in vitro insertion into dog pancreas microsomes (49). The native glycosylation site (NVS at amino acids 88–90 in isoform 2, 152–154 in isoform 1) within the loop was utilized, indicating insertion was accurate. No glycosylation occurred if this site was inactivated. Conversely, glycosylation sites engineered into the amino- or carboxy-termini outside of the TMDs were not utilized, consistent with their facing the cytosol.
The aqueous solubility of the loop region, which faces the lumen, is unknown. It contains a patch of hydrophobic amino acids, suggesting it may be anchored to the membrane through hydrophobic interactions. A construct containing the loop preceded by a signal sequence to drive it into the ER lumen and followed by an HDEL ER-retention signal is tightly bound to the membrane under conditions in which luminal proteins are released (unpublished data), suggesting an intimate association.
While physiological binding partners of seipin have yet to be identified, it is clear that seipin can self-associate (51, 59). When extracted from yeast membranes with Triton X-100, seipin behaves as a discrete and stable oligomer. Accounting for detergent binding by using H2O and D2O sucrose gradients, seipin, tagged with either mCherry or 13 copies of the myc epitope (to allow detection of protein driven by its native chromosomal promoter), appears to be a homo-oligomer of about nine subunits. There does not seem to be any other protein associated in stoichiometric amounts (51).
LOCALIZATION
Seipin is a membrane protein in the ER. Overexpressed green fluorescent protein-tagged human seipin was first shown to colocalize with an ER marker (calreticulin) in EA.hy926 umbilical-derived cells (50). Ectopic bright spots were also observed in these cells and were interpreted as aggregates (described in more detail below). Other groups have had similar results in several mammalian cells lines with tagged seipins (54, 59). Consistent with immuno- or live cell-fluorescence experiments, seipin expressed in Neuro 2a neuroblastoma cells partially cofractionated with an ER marker on sucrose gradients (52). Importantly, native seipin in these neurons also colocalizes with KDEL, an ER marker (53).
Overexpressed fluorescently tagged yeast seipin sorts to the ER, where it forms large patches (45). When expressed from its endogenous promoter, it is punctate, comprising several dots in the perinuclear ER adjacent to cytoplasmic LDs. Droplets in these cells always have an associated seipin punctum, but when cultured in oleic acid, there can be seipin puncta with no apparent adjacent droplet (Adeyo and Goodman, unpublished data). Electron microscopy with immunogold-stained tagged seipin confirms an ER localization with densities adjacent to droplets [(44) and Hilton and Goodman, unpublished data].
Ito et al. (52) dissected human seipin to identify targeting signals. The protein (fluorescently tagged at the carboxy terminus) displayed a clear ER pattern, colocalizing with BiP, and this localization could occur without its cytosolic N terminus, its cytosolic carboxy terminus, the second TMD, or the luminal loop (when each was deleted separately). Localization to the ER was diminished in the absence of the first TMD, suggesting an important role for TMD1 in this process. Surprisingly, however, there was still partial colocalization in the absence of this first TMD, suggesting redundant ER targeting signals. These results are similar to our laboratory's findings for the yeast protein (unpublished data).
PROBING THE FUNCTION OF SEIPIN
The function of seipin is unknown; there are no motifs contained in its sequence that provide clues to function, and no heterologous binding partners have yet been identified. Seipin is clearly involved in adipocyte function and/or adipogenesis, because its mRNA expression increases during this process (54, 57), the protein is largely located in the ER at junctions with LDs (44, 45), and null mutants in seipin are responsible for human lipodystrophy (16). To gain clues to seipin function, the effects of gene knockdown in cultured cells and of knockout in yeast, flies, and mice have been studied (summarized in Table 2). The results suggest that seipin functions in lipid homeostasis and/or LD function, and that preadipocytes may be exquisitely sensitive to perturbations in these functions. Moreover, transgenic mice have yielded additional information regarding a potentially important role of seipin in the nervous system.
TABLE 2.
Phenotypes of altered seipin expression
| Organism/cell manipulation | Phenotypes | Reference(s) |
| Saccharomyces cerevisiae FLD1 disruption | Shorter and more-saturated acyl chains on phospholipids | Fei et al. 2008 |
| Increase in PA (isolated microsomes), TAG, and SE levels | Fei et al. 2008Fei et al. 2011 PLoS Gen | |
| No increase in oleate incorporation into TAG and SE; presumed decrease in lipid utilization | Fei et al. 2008 | |
| Upregulation of INO1 and OPI3 | Fei et al. 2011 PLoS Gen | |
| Small clustered LDs wrapped in ER | Szymanski et al. 2007 | |
| Enhanced LD fusion and supersized droplets (rescued by inositol supplementation) | Fei et al. 2008Fei et al. 2011 PLoS Gen | |
| Tgl3p mistargeting | Wolinski et al. 2011 | |
| LD segregation defect | Wolinski et al. 2011 | |
| Drosophila melanogaster seipin knockout | Fat body: less neutral lipid; small droplets; decreased lipogenesis | Tian et al. 2011 |
| Salivary gland and midgut: larger and more-numerous LDs; PA and DGAT elevated; phenotype suppressed by DGAT knockdown | ||
| Organism: hypersensitive to starvation; lower glucose tolerance | ||
| Mus musculusseipin knockout | Adipose tissue: decrease in WAT and BAT mass; small unilocular droplets | Cui et al. 2011 |
| Liver: enlarged; large increase in lipid storage | ||
| Organism: lower body weight at birth; higher temperature; decreased adipokines; diabetic; impaired insulin signaling | ||
| HeLa seipin knockdown | Increase in TAG, LD proliferation and clustering (with exogenous oleate) | Fei et al. 2011 JLR |
| HaLa seipinoverexpression | Reduction in TAG with no change in FA uptake or lipolysis (with exogenous oleate) | |
| Mouse 3T3-L1seipin knockdown | Block in terminal differentiation | Chen et al. 2009 |
| Increased TAG (with exogenous oleate) | Fei et al. 2011 JLR | |
| Mouse C3H 10T1/2 seipin knockdown | Block in terminal differentiation | Payne et al. 2008 |
| BSCL2 patient-derived cells | Fibroblasts: small numerous LDs | Szymanski et al. 2007 |
| Transformed lymphocytes: small numerous LDs; more-saturated FAs (presumed due to less SCD1 activity) | Boutet et al. 2009 |
Adipogenesis
Consistent with a role for seipin in adipogenesis, knockdown of seipin prior to induction of the adipogenic pathway in cultured cells attenuates this program, thus recapitulating the situation found in BSCL2 patients. The determination phase (in which preadipocytes are generated) was not affected, since expression of markers of that phase (C/EBPβ, C/EBPγ, ETO, and Pref-1) was unchanged in the presence of seipin shRNAs, and generation of preadipocytes by the classical hormone cocktail or by BMP4 was not affected (54, 57). In contrast, expression of transcription factors involved in the conversion of preadipocytes to adipocytes during terminal differentiation (C/EBPα, PPARγ1, PPARγ2, and SREBP1c), as well as several of their downstream lipogenic enzyme targets, was drastically attentuated with seipin shRNAs (54, 57). While both of these reports showed blunted PPARγ expression in 3T3L-1 cells during the adipogenic program, results were not consistent in C3H10T1/2 pluripotent stem cells. Nevertheless, the downstream effects indicated lack of activation of this transcription factor. Significantly, addition of the PPARγ agonist pioglitazone could rescue cells from seipin shRNA treatment, allowing downstream lipogenic enzymes to be expressed (54). The authors speculated that seipin acted on an unidentified factor that was necessary for PPARγ activation. Similarly, fibroblasts from a seipin CGL patient also responded to pioglitazone to upregulate lipogenic enzymes (60).
Fat body formation was also curtailed in recently reported seipin knockout flies (47). LDs in the fat body in larvae and young adults were much smaller, and flies were hypersensitive to starvation. Importantly, the lipid phenotype could be reversed by overexpression of diacylglycerol acyltransferase (DGAT), suggesting that the seipin lesion was at the level of or proximal to TAG synthesis by DGAT. The fat body phenotype could be rescued by expression of seipin lacking the carboxy-terminal region after TMD2, suggesting that the core of the protein was sufficient for normal fat body differentiation.
Yang and colleagues (61), who generated the fly seipin knockout, also recently made a seipin knockout mouse. Similar to results in flies and human patients, adipogenesis in the knockout mice was severely curtailed. Fat accumulation in all major fat depots was reduced, although not as drastically as in lipodystrophic humans, and there was almost no gonadal fat. Moreover, there was a 60% decrease in brown fat, a tissue that has a lineage different from that of white fat but is still dependent on PPARγ for differentiation (62). White adipose fat from both epididymal and subcutaneous tissues showed immature adipocytes with small unilocular droplets. Consistent with the dearth of this tissue, plasma adiponectin and leptin were decreased. The animals showed symptoms of diabetes: delayed glucose clearance during a glucose tolerance test and impaired insulin sensitivity.
In summary, seipin is required for normal adipogenesis in cultured cells as well as in fly, mouse, and human. Compared with the fly and the mouse, humans suffer a more-severe phenotype in the absence of seipin, inasmuch as the absence of normal adipose stores in patients is almost absolute. Experiments with cultured cells indicate that the lesion is probably at the level of PPARγ activation, which is essential for terminal differentiation. It is unknown whether PPARγ activation is a direct or an indirect function of seipin. Due to the exclusive ER localization of seipin, an indirect function seems likely; such indirect effects may be due to an endogenous ligand that fails to be produced or delivered to the transcription factor, some toxic factor that interferes with PPARγ action, or a compensatory pathway that inhibits PPARγ-induced adipogenesis. PA, elevated in both seipin and AGPAT2 deficiency, has been suggested as a candidate toxic ligand for PPARγ (63).
Lipid homeostasis
While the absence of seipin can dramatically attenuate normal differentiation and lipid accumulation in adipocytes, the response of nonadipose tissue to seipin deficiency is quite different. Both subtle and dramatic changes have been described. In yeast, knockout of FLD1 (encoding seipin) had pleiotropic effects on lipid metabolism, impacting FA composition of phospholipids, acylglycerol intermediates, and neutral lipids. There is a shift in whole-cell FAs derived from phospholipids (especially from phosphatidyl serine and phosphatidyl inositol) in cells in the stationary phase, in which long-chain monounsaturated FAs (principally 18:1) were decreased as short-chain saturated FAs (16:0, 14:0, and 12:0) increased (44). PA in seipin knockout yeast cells is slightly increased; the difference becomes significant in isolated microsomes (64). Finally, the knockout strain accumulated twice as much TAG and about 70% as much steryl ester as did wild-type cells. However, there was little difference in the rate of incorporation of radioactive oleate into TAG or steryl ester, suggesting that utilization of FAs from neutral lipids, rather than synthesis of TAG and steryl ester, was affected (64).
Accumulation of neutral lipid was also observed in nonadipose tissues in the knockout flies. While there was a decrease in neutral lipid in their fat bodies, the animals accumulated large LDs in the proventriculus (midgut) and salivary glands. The authors showed that this paradoxical effect was cell autonomous and not a result of lipid release from the fat body (47). While the higher level of TAG in the yeast mutant was attributable to a decrease in FA utilization, in flies, it appears to be due to an increase in DGAT activity. Indeed, suppression of DGAT by knockdown reversed the phenotype in salivary glands. Similar to the yeast knockout, the absence of seipin resulted in elevated PA levels in salivary glands. Interestingly, while seipin missing its carboxy terminus could complement the fat body phenotype in flies, this region was essential for reversing the ectopic lipid accumulation in salivary and gut tissues.
An increase in ectopic fat accumulation was also seen in the knockout mice (61). Livers were pale and enlarged two-fold, mirroring the phenotype in human lipodystrophy. There was an increase in Oil Red O staining of liver tissue, and a 200% increase in TAG concentration in the organ. Concurrent with increased lipogenesis, some adipogenic proteins were elevated in liver tissue [FAS, PPARγ, and stearoyl-CoA desaturatase-1 (SCD1)], while others (SREBP-1c, DGAT, and ACC1) did not change. The level of microsomal TAG transfer protein, which facilitates the loading of neutral lipid onto apoB in lipoprotein production, was decreased. Unlike the fly, the contribution of tissue-autonomous effects in the mouse is not clear.
In fibroblasts from a seipin lipodystrophy patient, an increase in droplets and lipid storage was apparent (45). An increase in droplet number was also seen in Epstein-Barr virus-transformed lymphocytes from 20 seipin patients (65). Similar to the yeast knockout, there was an increase in saturated FAs at the expense of unsaturated species in these patients’ cells; there were more saturated FAs (palmitic and stearic acids) and less unsaturated FAs (palmitoleic, vaccenic, and oleic acids). This suggested to the authors that the absence of seipin leads to a decrease in SCD1 activity. Whether this is reflected in a decrease in SCD1 protein or is an issue with substrate availability is not known.
Although the panoply of effects on lipid metabolism in seipin-deficient nonadipogenic cells might suggest a complex mechanism, several of these data point to a simpler role for seipin in regulating TAG levels. This is supported by more detailed experiments on the effects of seipin knockdown in cultured cells. When subjected to seipin siRNA, HeLa cells and 3T3-L1 preadipocytes (not terminally differentiated) synthesized up to 80% more TAG upon exposure to exogenous oleic acid. The levels of TAG from endogenous FAs were not tested. Conversely, overexpression of seipin had a dramatic effect in reducing TAG levels from exogenous oleate while enhancing steryl ester formation. There were no changes in lipolysis or FA import; this suggests that seipin controls TAG synthesis, possibly through controlling DGAT activity. However, DGAT activity measured in microsomal fractions was not altered by manipulation of seipin expression, nor were DGAT mRNA levels changed (59). This could indicate that seipin regulates a TAG synthesis step proximal to DGAT, or that seipin controls access of DGAT to substrate, rather than amount or intrinsic activity of the enzyme itself.
Thus far, it is clear that the attenuation or overexpression of seipin can have dramatic effects on lipid metabolites and products in cells and tissues, and that preadipogenic tissue responds uniquely to an absence of the protein. The upregulation of PA might partially explain the increase in TAG stores and other changes (64). Conversely, changes in neutral lipid or LD morphology (described below) might give rise to a secondary increase in PA by disrupting ER or LD function (Fig. 2). Assuming a similar mechanism of seipin in yeast, flies, and mice, it is difficult yet, despite significant effort, to separate direct from indirect effects in explaining ectopic lipid formation and lipid interconversions in the knockdown/knockout animals and cells.
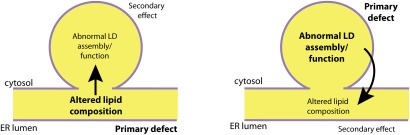
Fig. 2.
The seipin conundrum. Several abnormalities of lipid composition have been observed upon manipulating seipin expression, affecting PA, TAG, and a shift in balance of unsaturated and saturated FAs. Abnormalities in LD morphology have also been observed in seipin-deficient cells, including heterogenous LD size, LD clustering, LD wrapping in ER tangles, supersized/fusogenic LDs, and inefficient localization of LD surface proteins. Seipin may function directly in the metabolism of certain lipids, and this altered lipid composition in the absence of seipin may secondarily give rise to morphologically abnormal LDs (left). Alternatively, the direct function of seipin may be in organizing or maintaining LDs, and loss of seipin may affect lipid metabolic pathways by disrupting enzymes on the LD surface or in the ER bilayer (right). It may even be possible for these two hypothetical causal pathways to feed into each other.
LDs
In addition to changes in lipids that occur upon manipulating seipin expression, dramatic changes in the morphology and function of LDs are also seen. Aberrant liver LDs, mitochondria, and peroxisomes were observed in a CGL (probably seipin-related) lipodystrophy patient (66); important physical and functional interactions between LDs and mitochondria and peroxisomes are now well known (67). The morphology of droplets in the yeast fld1Δ strain has been studied extensively. Of note is the apparent disorganization of fld1Δ droplets; when cultured in the presence of oleic acid, droplets are extremely heterogenous in size and are often misshapen, compared with wild-type. Two strikingly distinct morphologic phenotypes are evident, however: clustered small droplets and “supersized” ones (44, 45, 68). The small clustered droplets, many of which have electron-dense inclusions, are wrapped in layers of the ER, suggesting both a local ER proliferation and a defect in droplet formation. These clusters are unable to segregate efficiently into buds during cytokinesis, probably because they are impeded by the ER tangles (68). While clusters predominate in cells growing in rich medium, supersized droplets are more frequently seen in cells growing in minimal medium (44, 68). Supersized droplets are greatly reduced in minimal medium containing inositol, while the addition of choline or ethanolamine hardly affects their frequency, suggesting that a deficiency in certain phospholipids is responsible for their large size (64). Droplets derived from seipin-null yeast have been shown to be more fusogenic than wild-type (44), suggesting that the supersized droplets are a result of fusion of smaller droplets, exacerbated by a shortage of phospholipids.
A proliferation of small droplets of abnormal morphology is also observed in cells from seipin patients: both fibroblasts and lymphoblasts show this phenotype (45, 65). Most droplets in these cells, when observed by fluorescence microscopy, appear as very fine and indistinct puncta, hundreds per cell; electron micrographs confirm a proliferation of small droplets.
The aberrant LDs in seipin-deficient yeast and BSCL2 patient cells gives the impression that seipin may control the loading of lipid and possibly protein into droplets, consistent with its localization at ER-LD junctions. In keeping with a role in protein loading, a sorting defect of Tgl3p, a TAG lipase normally localized to LDs, has been observed in some but not all droplets [(68) and our unpublished results]. It is unclear, however, whether this defect is secondary to the development of ER tangles.
The increase in droplet number in seipin-knockout cells could be a result of ER stress, because agents that cause stress result in an increase in droplet number (69). However, the morphology of droplets under the two conditions is very different. Moreover, an increase in HAC1 RNA splicing, a direct measure of ER stress in yeast, was not observed in fld1Δ cells (Hilton, Lee, and Goodman, unpublished data).
Potential neural function
While ER stress appears to play no role in the development of aberrant droplets in seipin-deficient cells, expression of the dominant, unglycosylated N88L and S90L mutants, which cause seipinopathies in patients, results in a strong stress response and in segregation of seipin from the bulk ER (50).
Overexpression of these mutants (fluorescently tagged) results in the accumulation of large aggregates within cells, much more so than with overexpression of the wild-type protein (50, 52, 59). These seipin accumulations were initially thought to cause the neurological disease seen in patients. However, there is a negative correlation between these seipin aggregates and cell death: cells with an ER reticular staining of N88S seipin have a higher frequency of apoptotic markers than cells with these seipin inclusion bodies, regardless of level of seipin expression (70), suggesting that segregation of seipin is actually protective. The nature of the seipin aggregates is unclear; the particles do not colocalize with typical aggresomal markers such as ubiquitin, pericentrin, vimentin, or chaperone proteins (52), even though seipin N88S has been shown to be polyubiquitinated and partially degraded by the proteasome (53). Interestingly, these unusual aggregations colocalize with mutated α1-antitrypsin, which also causes neurological disease and is sequestered into a so-called ER-derived protective organelle (70). An ultrastructural study suggested to the authors that aggregated seipin is associated with membrane vesicles (70), although these structures also resembled LDs.
In addition to aggregation, overexpression of N88S and S90L results in an ER unfolded protein response. There is an increase in several markers of ER stress, including BiP, Grp94, and PERK-ATF4, and detectible interaction of these seipin mutants with calnexin (53). These results led to the hypothesis that ER stress in neurons was responsible for the neurological pathology in patients (40). However, considering the protective effect of aggregated seipin, it also seems plausible that this ER stress protects against rather than instigates cell damage.
To gain more insight into the etiology of N88S seipin neuropathology, a transgenic mouse was recently established in which N88S seipin was driven by the neuronal-specific Thy-1 promoter (71). The animals recapitulated the neurological symptoms of human seipinopathy, displaying a spastic motor neuron defect in the limbs as well as muscular atrophy. Axonal transport was found to be lower in these animals. Neurons also showed an upregulation of the ER stress response, inasmuch as BiP, protein disulfide isomerase, and XBP1 were induced, as well as aggregated seipin, but no neuronal death was observed. This supports the notion that ER stress protects cells from more serious damage. It is also striking that neuronal death appears not to be necessary for disease.
Assuming that ER stress and seipin aggregation are protective rather than pathological mechanisms, the etiology of seipinopathy remains elusive. The effect on axonal transport could be key. Perhaps unglycosylated seipin is interfering with tubulin dynamics directly, or it may act indirectly by titrating out an important factor in this process. Regardless, the disease may indicate an important but yet unknown role of seipin in neuronal communication.
WHAT IS THE FUNCTION OF SEIPIN?
The disease states associated with seipin have provided us with a number of phenotypes and hints at function, but a cohesive mechanism to explain all these data has remained elusive. While the gain-of-function seipin mutants suggest a potential role in axonal transport and neural function (71), the loss-of-function mutants indicate a role for the protein in adipogenesis (probably at the level of PPARγ activation), lipid metabolism (probably via control of terminal steps in TAG formation), and LD biogenesis and maintenance (44, 45, 47, 61). Are these observations similar to blind men feeling a different part of the same elephant, or is there more than one distinct function of seipin?
Some evidence does indeed suggest the existence of multiple seipin functions, depending on tissue context. The ability of the core domain (i.e., excluding the cytosolic carboxy terminus) to complement the fat body phenotype, but not the salivary gland phenotype in the fly knockout, suggests two distinct, even opposing roles for seipin: an adipose role, where seipin promotes lipid accumulation, and a nonadipose role, where seipin limits lipid accumulation (47). Alternatively, this fly data may be a result of quantitative versus qualitative differences (differential stabilities, folding, or threshold effects of the seipins involved). Evidence, however, is available for tissue-specific seipin functions in mammals: high expression in the testes may indicate a nonadipogenic or nonlipogenic role of the protein, and a brain-specific role is suggested by its elevated expression in nervous tissue and the sensitivity of this tissue to the glycosylation-defective alleles. However, it is still possible that expression in testis is correlated with the presence of LDs, and expression in nerves may indicate robust lipid metabolism and sensitivity to perturbation of these pathways.
Based on what is known now, it seems likely to us that seipin must link neutral lipid synthesis with LD assembly. A scenario is presented in Fig. 3. We assume that LDs remain in intimate communication with the ER; in yeast, at least, droplets remain associated with the ER, although they can move laterally (45, 72). We posit that seipin is a part of a membrane protein complex that coordinates this communication. It allows normal filling of droplets with neutral lipid and perhaps also facilitates trafficking of phospholipids and proteins. If its role were limited to LD biogenesis, seipin should not remain adjacent to all LDs in the cell, as it does (45); therefore, it must provide function to droplets throughout their lifetime. The localization of seipin suggests that it could couple TAG synthesis with the funneling of neutral lipid into droplets. Evidence from the knockout, knockdown, and overexpression studies suggests a potential functional tie with DGAT enzymes, since seipin expression and TAG levels are inversely correlated and DGAT overexpression can rescue the lipid phenotype in flies (44, 47, 59, 61). While DGAT protein is associated with both the ER and LDs (73, 74), regulation of activity may depend on interaction with seipin, although no direct interaction between DGAT and seipin has been reported. We further posit that through its contributions to neutral lipid and droplet homeostasis, seipin plays a role in maintaining the ER bilayer. In its presence, ER function, in terms of membrane enzyme activity and metabolic flux, is normal and may include the generation of molecules important for cellular function, such as ligand(s) for PPARγ.
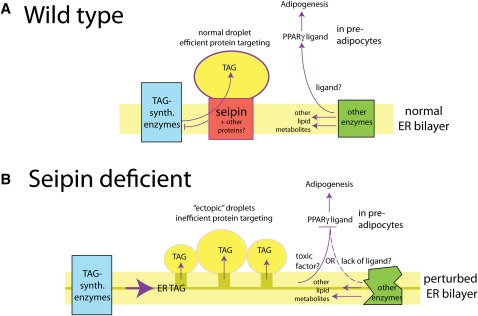
Fig. 3.
Scenario for seipin function. A: Wild-type cells. Seipin regulates droplet formation by coupling TAG synthesis to droplet filling. This may involve interaction with DGAT or upstream enzymes in the pathway. In preadipocytes, the ER probably generates a ligand that activates adipogenesis through PPARγ. B: Seipin-deficient cells. In the absence of seipin, neutral lipid accumulates in the ER and has pleotropic effects on ER membrane function: lipid biosynthetic enzymes are dysregulated and the PPARγ ligand fails to be produced in sufficient amounts, or a receptor toxin such as PA is produced. Overproduced TAG leads to ectopic LDs that are functionally defective.
In the absence of seipin, we suggest that the inability to channel TAG into LDs (possibly through collaboration with DGAT), combined with the increase in TAG production, destabilizes the ER bilayer. As shown by the dark yellow rectangle in Fig. 3B, this may involve infusion of neutral lipids into the bilayer itself. A model lipid bilayer can accommodate up to 3% TAG without losing its integrity (75), and increased membrane neutral lipid has been recently documented in a yeast lipin knockout (72). The sequellae of an increase in neutral lipid within the ER may be multiple: ectopic droplets may be generated as a result of high local concentration of neutral lipids, causing membrane blebbing; because their synthesis is unregulated, these droplets would be less uniform in size, shape, and protein composition; they may be more prone to nonspecific fusion; and they may not be fully functional. Increased plasticity of the membrane might lead to localized membrane proliferation, generating the ER-LD tangles seen in the yeast seipin knockout strain. Instability of the ER bilayer or surface disruptions on the LD itself may also affect the activity of other lipid metabolic enzymes, leading to changes in lipid profiles that may not be reflected in corresponding protein levels. As discussed above, such changes in the ER could be detected in the nucleus via the generation or depletion of lipids that bind PPARγ.
While this is one possible scenario, there are variations on the theme: as low-quality droplets are generated and the ER becomes destabilized in the absence of seipin, sensors in either compartment may report back to the nucleus to elicit compensatory pathways that could evoke the various downstream effects. For example, enzymes in phospholipid biogenesis are induced in seipin-deficient yeast (64). It also seems possible that the normal LD biosynthetic protein complex in the ER could partially function without seipin, analogous to the activity of a DNA polymerase without proofreading capability, such that droplet formation in the absence of seipin could be more than a stochastic function of physicochemical properties of the altered ER.
Despite the myriad of questions that remain, it seems clear that insights into seipin's function will be extremely beneficial in understanding LD biology and lipid homeostasis. Interest in seipin has increased in the past few years, and the pace of progress is likely to follow suit. We should not have to wait too long before the primary target, or targets, of this fascinating protein, be it LD assembly, a neutral lipid biosynthetic enzyme, both, or neither, become uncovered.
Acknowledgments
The authors thank Kent Hamra for sharing his unpublished data on ORO staining of testes.
Footnotes
Abbreviations:
- AGL
- acquired generalized lipodystrophy
- ALS
- amyotropic lateral sclerosis
- BSCL
- Berardinelli-Seip congenital lipodystrophy
- CGL
- congenital generalized lipodystrophy
- DGAT
- diacylglycerol acyltransferase
- ER
- endoplasmic reticulum
- LD
- lipid droplet
- PA
- phosphatidic acid
- PPARγ
- peroxisome proliferator-activated receptor γ
- TAG
- triacylglycerol
- TMD
- transmembrane domain
- TZD
- thiazolidinedione
This work was supported by National Institutes of Health Cell and Molecular Biology Training Program Grant 5T32-GM-008203 (B.R.C.), and by National Institutes of Health Grant R01 GM-084210 (J.M.G.). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Mitchell S. W. 1885. Singular case of absence of adipose matter in the upper half of the body. Am. J. Med. Sci. 179: 105–106 [Google Scholar]
- 2.Garg A. 2011. Lipodystrophies: genetic and acquired body fat disorders. J. Clin. Endocrinol. Metab. 96: 3313–3325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawrence R. D. 1946. Lipodystrophy and hepatomegaly, with diabetes, lipaemia, and other metabolic disturbances: a case throwing new light on the action of insulin. Lancet. 1: 724–731, 773–775 [PubMed] [Google Scholar]
- 4.Berardinelli W. 1954. An undiagnosed endocrinometabolic syndrome: report of 2 cases. J. Clin. Endocrinol. Metab. 14: 193–204 [DOI] [PubMed] [Google Scholar]
- 5.Seip M. 1959. Lipodystrophy and gigantism with associated endocrine manifestations. A new diencephalic syndrome? Acta Paediatr. 48: 555–574 [PubMed] [Google Scholar]
- 6.Moller D. E., O'Rahilly S.1993. Syndromes of severe insulin resistance: clinical and pathophysiological features. In Insulin Resistance. D. E. Moller, editor. Wiley and Sons, New York. 49–81.
- 7.Agarwal A. K., Garg A. 2006. Genetic basis of lipodystrophies and management of metabolic complications. Annu. Rev. Med. 57: 297–311 [DOI] [PubMed] [Google Scholar]
- 8.Arioglu E., Duncan-Morin J., Sebring N., Rother K. I., Gottlieb N., Lieberman J., Herion D., Kleiner D. E., Reynolds J., Premkumar A., et al. 2000. Efficacy and safety of troglitazone in the treatment of lipodystrophy syndromes. Ann. Intern. Med. 133: 263–274 [DOI] [PubMed] [Google Scholar]
- 9.Oral E. A., Simha V., Ruiz E., Andewelt A., Premkumar A., Snell P., Wagner A. J., DePaoli A. M., Reitman M. L., Taylor S. I., et al. 2002. Leptin-replacement therapy for lipodystrophy. N. Engl. J. Med. 346: 570–578 [DOI] [PubMed] [Google Scholar]
- 10.Seip M., Trygstad O. 1996. Generalized lipodystrophy, congenital and acquired (lipoatrophy). Acta Paediatr. Suppl. 413: 2–28 [DOI] [PubMed] [Google Scholar]
- 11.Trygstad O., Seip M., Oseid S. 1977. Lipodystrophic diabetes treated with fenfluramine. Int. J. Obes. 1: 287–292 [PubMed] [Google Scholar]
- 12.Ebihara K., Kusakabe T., Hirata M., Masuzaki H., Miyanaga F., Kobayashi N., Tanaka T., Chusho H., Miyazawa T., Hayashi T., et al. 2007. Efficacy and safety of leptin-replacement therapy and possible mechanisms of leptin actions in patients with generalized lipodystrophy. J. Clin. Endocrinol. Metab. 92: 532–541 [DOI] [PubMed] [Google Scholar]
- 13.Tontonoz P., Hu E., Spiegelman B. M. 1994. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 79: 1147–1156 [DOI] [PubMed] [Google Scholar]
- 14.Garg A. 2004. Acquired and inherited lipodystrophies. N. Engl. J. Med. 350: 1220–1234 [DOI] [PubMed] [Google Scholar]
- 15.Gomes K. B., Fernandes A. P., Ferreira A. C., Pardini H., Garg A., Magre J., Pardini V. C. 2004. Mutations in the seipin and AGPAT2 genes clustering in consanguineous families with Berardinelli-Seip congenital lipodystrophy from two separate geographical regions of Brazil. J. Clin. Endocrinol. Metab. 89: 357–361 [DOI] [PubMed] [Google Scholar]
- 16.Magré J., Delepine M., Khallouf E., Gedde-Dahl T., Jr, Van Maldergem L., Sobel E., Papp J., Meier M., Megarbane A., Bachy A., et al. 2001. Identification of the gene altered in Berardinelli-Seip congenital lipodystrophy on chromosome 11q13. Nat. Genet. 28: 365–370 [DOI] [PubMed] [Google Scholar]
- 17.Agarwal A. K., Arioglu E., De Almeida S., Akkoc N., Taylor S. I., Bowcock A. M., Barnes R. I., Garg A. 2002. AGPAT2 is mutated in congenital generalized lipodystrophy linked to chromosome 9q34. Nat. Genet. 31: 21–23 [DOI] [PubMed] [Google Scholar]
- 18.Garg A., Wilson R., Barnes R., Arioglu E., Zaidi Z., Gurakan F., Kocak N., O'Rahilly S., Taylor S. I., Patel S. B., et al. 1999. A gene for congenital generalized lipodystrophy maps to human chromosome 9q34. J. Clin. Endocrinol. Metab. 84: 3390–3394 [DOI] [PubMed] [Google Scholar]
- 19.Leung D. W. 2001. The structure and functions of human lysophosphatidic acid acyltransferases. Front. Biosci. 6: D944–953 [DOI] [PubMed] [Google Scholar]
- 20.Takeuchi K., Reue K. 2009. Biochemistry, physiology, and genetics of GPAT, AGPAT, and lipin enzymes in triglyceride synthesis. Am. J. Physiol. Endocrinol. Metab. 296: E1195–E1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agarwal A. K., Barnes R. I., Garg A. 2004. Genetic basis of congenital generalized lipodystrophy. Int. J. Obes. Relat. Metab. Dis. 28: 336–339 [DOI] [PubMed] [Google Scholar]
- 22.Gale S. E., Frolov A., Han X., Bickel P. E., Cao L., Bowcock A., Schaffer J. E., Ory D. S. 2006. A regulatory role for 1-acylglycerol-3-phosphate-O-acyltransferase 2 in adipocyte differentiation. J. Biol. Chem. 281: 11082–11089 [DOI] [PubMed] [Google Scholar]
- 23.Cortés V. A., Curtis D. E., Sukumaran S., Shao X., Parameswara V., Rashid S., Smith A. R., Ren J., Esser V., Hammer R. E., et al. 2009. Molecular mechanisms of hepatic steatosis and insulin resistance in the AGPAT2-deficient mouse model of congenital generalized lipodystrophy. Cell Metab. 9: 165–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayashi Y. K., Matsuda C., Ogawa M., Goto K., Tominaga K., Mitsuhashi S., Park Y. E., Nonaka I., Hino-Fukuyo N., Haginoya K., et al. 2009. Human PTRF mutations cause secondary deficiency of caveolins resulting in muscular dystrophy with generalized lipodystrophy. J. Clin. Invest. 119: 2623–2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim C. A., Delepine M., Boutet E., El Mourabit H., Le Lay S., Meier M., Nemani M., Bridel E., Leite C. C., Bertola D. R., et al. 2008. Association of a homozygous nonsense caveolin-1 mutation with Berardinelli-Seip congenital lipodystrophy. J. Clin. Endocrinol. Metab. 93: 1129–1134 [DOI] [PubMed] [Google Scholar]
- 26.Fan J. Y., Carpentier J. L., van Obberghen E., Grunfeld C., Gorden P., Orci L. 1983. Morphological changes of the 3T3-L1 fibroblast plasma membrane upon differentiation to the adipocyte form. J. Cell Sci. 61: 219–230 [DOI] [PubMed] [Google Scholar]
- 27.Parton R. G., Simons K. 2007. The multiple faces of caveolae. Nat. Rev. Mol. Cell Biol. 8: 185–194 [DOI] [PubMed] [Google Scholar]
- 28.Simha V., Garg A. 2003. Phenotypic heterogeneity in body fat distribution in patients with congenital generalized lipodystrophy caused by mutations in the AGPAT2 or seipin genes. J. Clin. Endocrinol. Metab. 88: 5433–5437 [DOI] [PubMed] [Google Scholar]
- 29.Van Maldergem L., Magre J., Khallouf T. E., Gedde-Dahl T., Jr, Delepine M., Trygstad O., Seemanova E., Stephenson T., Albott C. S., Bonnici F., et al. 2002. Genotype-phenotype relationships in Berardinelli-Seip congenital lipodystrophy. J. Med. Genet. 39: 722–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dwianingsih E. K., Takeshima Y., Itoh K., Yamauchi Y., Awano H., Malueka R. G., Nishida A., Ota M., Yagi M., Matsuo M. 2010. A Japanese child with asymptomatic elevation of serum creatine kinase shows PTRF-CAVIN mutation matching with congenital generalized lipodystrophy type 4. Mol. Genet. Metab. 101: 233–237 [DOI] [PubMed] [Google Scholar]
- 31.Rajab A., Straub V., McCann L. J., Seelow D., Varon R., Barresi R., Schulze A., Lucke B., Lutzkendorf S., Karbasiyan M., et al. 2010. Fatal cardiac arrhythmia and long-QT syndrome in a new form of congenital generalized lipodystrophy with muscle rippling (CGL4) due to PTRF-CAVIN mutations. PLoS Genet. 6: e1000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shastry S., Delgado M. R., Dirik E., Turkmen M., Agarwal A. K., Garg A. 2010. Congenital generalized lipodystrophy, type 4 (CGL4) associated with myopathy due to novel PTRF mutations. Am. J. Med. Genet. A. 152A: 2245–2253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agarwal A. K., Simha V., Oral E. A., Moran S. A., Gorden P., O'Rahilly S., Zaidi Z., Gurakan F., Arslanian S. A., Klar A., et al. 2003. Phenotypic and genetic heterogeneity in congenital generalized lipodystrophy. J. Clin. Endocrinol. Metab. 88: 4840–4847 [DOI] [PubMed] [Google Scholar]
- 34.Gomes K. B., Pardini V. C., Ferreira A. C., Fernandes A. P. 2005. Phenotypic heterogeneity in biochemical parameters correlates with mutations in AGPAT2 or Seipin genes among Berardinelli-Seip congenital lipodystrophy patients. J. Inherit. Metab. Dis. 28: 1123–1131 [DOI] [PubMed] [Google Scholar]
- 35.Rognum T. O., Bjerve K. S., Seip M., Trygstad O., Oseid S. 1978. Fat cell size and lipid content of subcutaneous tissue in congenital generalized lipodystrophy. Acta Endocrinol. (Copenh.). 88: 182–189 [DOI] [PubMed] [Google Scholar]
- 36.Haque W. A., Shimomura I., Matsuzawa Y., Garg A. 2002. Serum adiponectin and leptin levels in patients with lipodystrophies. J. Clin. Endocrinol. Metab. 87: 2395. [DOI] [PubMed] [Google Scholar]
- 37.Pardini V. C., Victoria I. M., Rocha S. M., Andrade D. G., Rocha A. M., Pieroni F. B., Milagres G., Purisch S., Velho G. 1998. Leptin levels, beta-cell function, and insulin sensitivity in families with congenital and acquired generalized lipoatropic diabetes. J. Clin. Endocrinol. Metab. 83: 503–508 [DOI] [PubMed] [Google Scholar]
- 38.Antuna-Puente B., Boutet E., Vigouroux C., Lascols O., Slama L., Caron-Debarle M., Khallouf E., Levy-Marchal C., Capeau J., Bastard J. P., et al. 2010. Higher adiponectin levels in patients with Berardinelli-Seip congenital lipodystrophy due to seipin as compared with 1-acylglycerol-3-phosphate-o-acyltransferase-2 deficiency. J. Clin. Endocrinol. Metab. 95: 1463–1468 [DOI] [PubMed] [Google Scholar]
- 39.Bjørnstad P. G., Foerster A., Ihlen H. 1996. Cardiac findings in generalized lipodystrophy. Acta Paediatr. Suppl. 413: 39–43 [DOI] [PubMed] [Google Scholar]
- 40.Ito D., Suzuki N. 2009. Seipinopathy: a novel endoplasmic reticulum stress-associated disease. Brain. 132: 8–15 [DOI] [PubMed] [Google Scholar]
- 41.Huang H. H., Chen T. H., Hsiao H. P., Huang C. T., Wang C. C., Shiau Y. H., Chao M. C. 2010. A Taiwanese boy with congenital generalized lipodystrophy caused by homozygous Ile262fs mutation in the BSCL2 gene. Kaohsiung J. Med. Sci. 26: 615–620 [DOI] [PubMed] [Google Scholar]
- 42.Jin J., Cao L., Zhao Z., Shen S., Kiess W., Zhi D., Ye R., Cheng R., Chen L., Yang Y., et al. 2007. Novel BSCL2 gene mutation E189X in Chinese congenital generalized lipodystrophy child with early onset diabetes mellitus. Eur. J. Endocrinol. 157: 783–787 [DOI] [PubMed] [Google Scholar]
- 43.Wu Y. R., Hung S. I., Chang Y. C., Chen S. T., Lin Y. L., Chung W. H. 2009. Complementary mutations in seipin gene in a patient with Berardinelli-Seip congenital lipodystrophy and dystonia: phenotype variability suggests multiple roles of seipin gene. J. Neurol. Neurosurg. Psychiatry. 80: 1180–1181 [DOI] [PubMed] [Google Scholar]
- 44.Fei W., Shui G., Gaeta B., Du X., Kuerschner L., Li P., Brown A. J., Wenk M. R., Parton R. G., Yang H. 2008. Fld1p, a functional homologue of human seipin, regulates the size of lipid droplets in yeast. J. Cell Biol. 180: 473–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Szymanski K. M., Binns D., Bartz R., Grishin N. V., Li W. P., Agarwal A. K., Garg A., Anderson R. G., Goodman J. M. 2007. The lipodystrophy protein seipin is found at endoplasmic reticulum lipid droplet junctions and is important for droplet morphology. Proc. Natl. Acad. Sci. USA. 104: 20890–20895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miranda D. M., Wajchenberg B. L., Calsolari M. R., Aguiar M. J., Silva J. M., Ribeiro M. G., Fonseca C., Amaral D., Boson W. L., Resende B. A., et al. 2009. Novel mutations of the BSCL2 and AGPAT2 genes in 10 families with Berardinelli-Seip congenital generalized lipodystrophy syndrome. Clin. Endocrinol. (Oxf.). 71: 512–517 [DOI] [PubMed] [Google Scholar]
- 47.Tian Y., Bi J., Shui G., Liu Z., Xiang Y., Liu Y., Wenk M. R., Yang H., Huang X. 2011. Tissue-autonomous function of Drosophila seipin in preventing ectopic lipid droplet formation. PLoS Genet. 7: e1001364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ebihara K., Kusakabe T., Masuzaki H., Kobayashi N., Tanaka T., Chusho H., Miyanaga F., Miyazawa T., Hayashi T., Hosoda K., et al. 2004. Gene and phenotype analysis of congenital generalized lipodystrophy in Japanese: a novel homozygous nonsense mutation in seipin gene. J. Clin. Endocrinol. Metab. 89: 2360–2364 [DOI] [PubMed] [Google Scholar]
- 49.Lundin C., Nordstrom R., Wagner K., Windpassinger C., Andersson H., von Heijne G., Nilsson I. 2006. Membrane topology of the human seipin protein. FEBS Lett. 580: 2281–2284 [DOI] [PubMed] [Google Scholar]
- 50.Windpassinger C., Auer-Grumbach M., Irobi J., Patel H., Petek E., Horl G., Malli R., Reed J. A., Dierick I., Verpoorten N., et al. 2004. Heterozygous missense mutations in BSCL2 are associated with distal hereditary motor neuropathy and Silver syndrome. Nat. Genet. 36: 271–276 [DOI] [PubMed] [Google Scholar]
- 51.Binns D., Lee S., Hilton C. L., Jiang Q. X., Goodman J. M. 2010. Seipin is a discrete homooligomer. Biochemistry. 49: 10747–10755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ito D., Fujisawa T., Iida H., Suzuki N. 2008. Characterization of seipin/BSCL2, a protein associated with spastic paraplegia 17. Neurobiol. Dis. 31: 266–277 [DOI] [PubMed] [Google Scholar]
- 53.Ito D., Suzuki N. 2007. Molecular pathogenesis of seipin/BSCL2-related motor neuron diseases. Ann. Neurol. 61: 237–250 [DOI] [PubMed] [Google Scholar]
- 54.Chen W., Yechoor V. K., Chang B. H., Li M. V., March K. L., Chan L. 2009. The human lipodystrophy gene product Berardinelli-Seip congenital lipodystrophy 2/seipin plays a key role in adipocyte differentiation. Endocrinology. 150: 4552–4561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lefterova M. I., Lazar M. A. 2009. New developments in adipogenesis. Trends Endocrinol. Metab. 20: 107–114 [DOI] [PubMed] [Google Scholar]
- 56.Rosen E. D., MacDougald O. A. 2006. Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 7: 885–896 [DOI] [PubMed] [Google Scholar]
- 57.Payne V. A., Grimsey N., Tuthill A., Virtue S., Gray S. L., Dalla Nora E., Semple R. K., O'Rahilly S., Rochford J. J. 2008. The human lipodystrophy gene BSCL2/seipin may be essential for normal adipocyte differentiation. Diabetes. 57: 2055–2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim H., Melen K., von Heijne G. 2003. Topology models for 37 Saccharomyces cerevisiae membrane proteins based on C-terminal reporter fusions and predictions. J. Biol. Chem. 278: 10208–10213 [DOI] [PubMed] [Google Scholar]
- 59.Fei W., Li H., Shui G., Kapterian T. S., Bielby C., Du X., Brown A. J., Li P., Wenk M. R., Liu P., et al. 2011. Molecular characterization of seipin and its mutants: implications for seipin in triacylglycerol synthesis. J. Lipid Res. 52: 2136–2147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Victoria B., Cabezas-Agricola J. M., Gonzalez-Mendez B., Lattanzi G., Del Coco R., Loidi L., Barreiro F., Calvo C., Lado-Abeal J., Araujo-Vilar D. 2010. Reduced adipogenic gene expression in fibroblasts from a patient with type 2 congenital generalized lipodystrophy. Diabet. Med. 27: 1178–1187 [DOI] [PubMed] [Google Scholar]
- 61.Cui X., Wang Y., Tang Y., Liu Y., Zhao L., Deng J., Xu G., Peng X., Ju S., Liu G., et al. 2011. Seipin ablation in mice results in severe generalized lipodystrophy. Hum. Mol. Genet. 20: 3022–3030 [DOI] [PubMed] [Google Scholar]
- 62.Seale P., Bjork B., Yang W., Kajimura S., Chin S., Kuang S., Scime A., Devarakonda S., Conroe H. M., Erdjument-Bromage H., et al. 2008. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 454: 961–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fei W., Du X., Yang H. 2011. Seipin, adipogenesis and lipid droplets. Trends Endocrinol. Metab. 22: 204–210 [DOI] [PubMed] [Google Scholar]
- 64.Fei W., Shui G., Zhang Y., Krahmer N., Ferguson C., Kapterian T. S., Lin R. C., Dawes I. W., Brown A. J., Li P., et al. 2011. A role for phosphatidic acid in the formation of “supersized” lipid droplets. PLoS Genet. 7: e1002201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boutet E., El Mourabit H., Prot M., Nemani M., Khallouf E., Colard O., Maurice M., Durand-Schneider A. M., Chretien Y., Gres S., et al. 2009. Seipin deficiency alters fatty acid Delta9 desaturation and lipid droplet formation in Berardinelli-Seip congenital lipodystrophy. Biochimie. 91: 796–803 [DOI] [PubMed] [Google Scholar]
- 66.de Craemer D., Van Maldergem L., Roels F. 1992. Hepatic ultrastructure in congenital total lipodystrophy with special reference to peroxisomes. Ultrastruct. Pathol. 16: 307–316 [DOI] [PubMed] [Google Scholar]
- 67.Goodman J. M. 2008. The gregarious lipid droplet. J. Biol. Chem. 283: 28005–28009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wolinski H., Kolb D., Hermann S., Koning R. I., Kohlwein S. D. 2011. A role for seipin in lipid droplet dynamics and inheritance in yeast. J. Cell Sci. 124: 3894–3904 [DOI] [PubMed] [Google Scholar]
- 69.Fei W., Wang H., Fu X., Bielby C., Yang H. 2009. Conditions of endoplasmic reticulum stress stimulate lipid droplet formation in Saccharomyces cerevisiae. Biochem. J. 424: 61–67 [DOI] [PubMed] [Google Scholar]
- 70.Ito D., Yagi T., Ikawa M., Suzuki N. 2012. Characterization of inclusion bodies with cytoprotective properties formed by seipinopathy-linked mutant seipin. Hum. Mol. Genet. 21: 635–646 [DOI] [PubMed] [Google Scholar]
- 71.Yagi T., Ito D., Nihei Y., Ishihara T., Suzuki N. 2011. N88S seipin mutant transgenic mice develop features of seipinopathy/BSCL2-related motor neuron disease via endoplasmic reticulum stress. Hum. Mol. Genet. 20: 3831–3840 [DOI] [PubMed] [Google Scholar]
- 72.Adeyo O., Horn P. J., Lee S., Binns D. D., Chandrahas A., Chapman K. D., Goodman J. M. 2011. The yeast lipin orthologue Pah1p is important for biogenesis of lipid droplets. J. Cell Biol. 192: 1043–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McFie P. J., Banman S. L., Kary S., Stone S. J. 2011. Murine diacylglycerol acyltransferase-2 (DGAT2) can catalyze triacylglycerol synthesis and promote lipid droplet formation independent of its localization to the endoplasmic reticulum. J. Biol. Chem. 286: 28235–28246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jacquier N., Choudhary V., Mari M., Toulmay A., Reggiori F., Schneiter R. 2011. Lipid droplets are functionally connected to the endoplasmic reticulum in Saccharomyces cerevisiae. J. Cell Sci. 124: 2424–2437 [DOI] [PubMed] [Google Scholar]
- 75.Hamilton J. A. 1989. Interactions of triglycerides with phospholipids: incorporation into the bilayer structure and formation of emulsions. Biochemistry. 28: 2514–2520 [DOI] [PubMed] [Google Scholar]