Abstract
Pulmonary artery endothelial plexiform lesion is responsible for pulmonary vascular remodeling (PVR), a basic pathological change of pulmonary arterial hypertension (PAH). Recent evidence suggests that epoxyeicosatrienoic acid (EET), which is derived from arachidonic acid by cytochrome p450 (CYP) epoxygenase, has an essential role in PAH. However, until now, most research has focused on pulmonary vasoconstriction; it is unclear whether EET produces mitogenic and angiogenic effects in pulmonary artery endothelial cells (PAEC). Here we found that 500 nM/l 8,9-EET, 11,12-EET, and 14,15-EET markedly augmented JNK and c-Jun activation in PAECs and that the activation of c-Jun was mediated by JNK, but not the ERK or p38 MPAK pathway. Moreover, treatment with 8,9-EET, 11,12-EET, and 14,15-EET promoted cell proliferation and cell-cycle transition from the G0/G1 phase to S phase and stimulated tube formation in vitro. All these effects were reversed after blocking JNK with Sp600125 (a JNK inhibitor) or JNK1/2 siRNA. In addition, the apoptotic process was alleviated by three EET region isomers through the JNK/c-Jun pathway. These observations suggest that 8,9-EET, 11,12-EET, and 14,15-EET stimulate PAEC proliferation and angiogenesis, as well as protect the cells from apoptosis, via the JNK/c-Jun pathway, an important underlying mechanism that may promote PAEC growth and angiogenesis during PAH.
Keywords: epoxyeicosatrienoic acid, cell cycle, apoptosis, c-Jun N-terminal kinase
Pulmonary arterial hypertension (PAH), of which the etiology and pathogenesis of the vascular alteration are still largely unknown, is characterized histologically by the tremendous changes that are associated with functional impairment of the endothelium in increasing vascular resistance and pressure as well as flow that occurs in the pulmonary circulation, ultimately overloading the right ventricle (1, 2). Endothelial dysfunction, including disordered endothelial cell (EC) proliferation, along with concurrent neoangiogenesis leads to the formation of plexiform lesions as a common pathological feature of PAH (3, 4). Endothelial cell proliferation in PAH is of fundamental importance with major implications regarding its pathogenesis and diagnosis. Many other recent reports provide evidence that the growth of endothelial cells and the tumorlets and plexiform lesions composed of proliferated endothelial cells, which obliterate medium-sized arteries, are observed in the pulmonary artery of patients with PAH (1).
Many arachidonic acid products, such as eicosanoids and leukotrienes, serve as markers and mediators of lung damage and are generated during acute lung injury (2). But there is no evidence about role of epoxyeicosatrienoic (EET) acids, metabolized by the CYP epoxygenase pathway of arachidonic acid, in lung injury and plexiform lesions of vascular endothelial cells. Intracellular EETs (5,6-EET, 8,9-EET, 11,12-EET, and 14,15-EET) are regulated by the activity of the CYP epoxygenases and by the soluble epoxide hydrolase (sEH) (5). So far, most research has focused on the anti-inflammatory properties of EETs or vasodilatation in the systemic circulation and pulmonary circulation (5), but less is known regarding the proliferative and angiogenic properties of EETs in pulmonary artery endothelial cells (PAEC) and the mechanisms of EETs on pulmonary vascular remodeling (6).
c-Jun N-terminal kinase (JNK), a member of the mitogen-associated protein kinase (MAPK) family, is encoded by three genes: JNK 1and JNK 2, which are ubiquitously expressed, and JNK3, which is limited to the testis, heart, and brain (7). Broad varieties of stress stimulate JNK activation and in turn phosphorylate several transcription factors, such as c-Jun, JunB, and ATF-2, required for cell survival, proliferation, transformation, and death (8, 9). Previous observations suggest a potential role for JNK in allergic airway diseases and fibrosis and show that the phosphorylation of JNK is elevated in the airway epithelium and pulmonary endothelium of patients with idiopathic pulmonary fibrosis (10). However, the linkage among JNK, pulmonary hypertension, and endothelium growth requires more study.
On the basis of the possible roles of EET and JNK/c-Jun pathway in pulmonary hypertension, we hypothesized that pulmonary artery endothelial plexiform lesions and lumen narrow were mediated by EETs through JNK/c-Jun pathway. We demonstrated that EETs (8,9-EET, 11,12-EET, and 14,15-EET) promoted cell proliferation and angiogenesis in a JNK-dependent manner. Moreover, EETs promoted the cell transition from G0/G1 phase to S phase by regulating the cell-cycle regulatory proteins and attenuated a series of apoptotic events, which were reversed by the JNK inhibitor or silencing of JNK 1/2 genes. Protective and angiogenic effects of EETs on PAECs would be considered an important underlying mechanism for improving the therapy of PAH and obstructive pulmonary disease.
MATERIALS AND METHODS
5,6-EET, 8,9-EET, 11,12-EET, and 14,15-EET were purchased from Cayman Chemical Co. (Ann Arbor, MI). Growth factor-reduced Matrigel and the CycleTEST™ PLUS DNA Reagent Kit were obtained from BD Biosciences (Bedford, MA). Bromodeoxyuridine (BrdU) proliferation assay kit was from Roche Applied Science (Indianapolis, IN). KinaseSTARTM JNK Activity Assay Kit was purchased from BioVision Inc. (Mountain View, CA). Antibodies against PCNA, Cyclin A, P21, p27, c-Jun, β-actin, JNK1, and JNK2 were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Cyclin D1 antibody was obtained from Cell Signal Corporation. SP600125, SB203580, U0126, JC-1 probe, and Caspase-3 activity kit were obtained from Beyotime Institute of Biotechnology (Haimen, China). All other reagents were from common commercial sources.
Cell culture
Fresh bovine PAECs were isolated as previously described (11). The pulmonary tissues were obtained from a local slaughterhouse with all protocols reviewed and approved by the Ethical Committee of Laboratory Animals at Harbin Medical University. The identity was confirmed by typical endothelial cell morphology and by positive anti-factor-VIII staining.
JNK activity assay
We used KinaseSTARTM JNK Activity Assay Kit (catalog #K431-40, BioVision) to detect JNK activity according to the manufacturer's instruction. Briefly, after treatment with EETs (8,9-EET, 11,12-EET and 14,15-EET), we prepared the cell lysate of each group and then used JNK-specific antibody to immunoprecipitate JNK, followed by Western immunoblotting with anti-phospho-c-Jun (Ser 73)-specific antibody to detect kinase activity.
Bromodeoxyuridine incorporation
PAECs were plated in 96-well plates at the density of 5,000 cells/well, and then subjected to growth arrest for 24 h before treatment with different agents in 1% FBS DMEM. We measured BrdU incorporation according to the Roche BrdU proliferation assay kit instructions. Briefly, after treatment, 10 µM BrdU labeling solution was added per well for 24 h at 37°C, and then incubated for 30 min in FixDenat solution at room temperature. Flicking off the FixDenat solution thoroughly, we added anti-BrdU-POD working solution 200 μl/well for 90 min at room temperature. Each well was rinsed three times with 200 μl washing solution and incubated for 10 min in 100 μl/well substrate solution. The absorbance of the samples was recorded in an ELISA reader at 370 nm.
MTT assay
PAECs at passage 2 were cultured at a density of 5,000 cells/well in a 96-well culture plate and then treated with 5,6-EET (500 nmol/l); 8,9-EET (500 nmol/l); 11,12-EET (500 nmol/l); 14,15-EET (500 nmol/l); Sp600125 (5 μmol/l); and EETs plus Sp600125 in DMEM with 1% FBS. Ethanol and DMSO were added at the indicated concentrations. At the end of incubation at 37°C, the cells were incubated for 4 h in a medium containing 0.5% 3-[4, 5-dimethylthiazol-2-yl]2, 5-diphenyl-tetrazolium bromide (MTT), the yellow mitochondrial dye. The amount of blue formazan dye formed from MTT is proportional to the number of survival cells. The reaction was terminated after adding 150 µl DMSO and incubating the cells for 10 min. Absorbance at 540 nm was recorded by an ELISA plate reader.
Cell cycle and DNA analysis
The CycleTEST PLUS DNA Reagent Kit was used for examining whether the cell cycle was regulated by EETs. PAECs were treated in groups as indicated and then harvested with trypsin and fixed using 70% ethanol. The ethanol was removed, and the cells were incubated in 200 μl PBS. The cells were stained according to the kit protocol. DNA fluorescence was measured and flow cytometry was performed using BD FACSCalibur Flow Cytometer (Bedford, MA). For each sample, 2 × 104 events were accumulated in a histogram. The proportions of cells in the different phases of the cell cycle were calculated from each histogram.
Immunocytochemistry
PAECs were cultured on a cover glass (15 mm diameter). After treatment, cells were fixed with 4% paraformaldehyde in PBS at room temperature for 15 min, permeabilized with 0.5% Triton X-100 for 10 min, blocked with 3% normal bovine serum in PBS at 37°C for 30 min, and incubated with anti-phospho-JNK primary antibody (1:50) in PBS at 4°C overnight. After washing three times with PBS, the cells were incubated with FITC-conjugated secondary antibody (1:100) diluted with PBS at 37°C for 2 h away from light. Slides were then examined with a microscope (Nikon, Japan), and images were recorded by digital photomicrography (Olympus, Japan).
Tube formation assay
For the tube formation assay, 96-six-well culture plates (Costar, Corning) were coated with 30 μl growth factor-reduced Matrigel (BD Biosciences) and solidified for 30 min at 37°C. Then PAECs were trypsinized and resuspended at 5 × 104 per milliliter, followed by adding 200 μl of this cell suspension into each well. Vehicle or reagents at the indicated concentrations were added to the appropriate well. Tube formation was observed under an inverted microscope (Nikon, Japan). Tube length was measured using the Image-Pro Plus 6.0.
Measurement of caspase-3 activity
Cleavage of Ac-DEVD-pNA (acetyl-Asp-Glu-Val-Asp p-nitroanilide), a caspase-3 substrate, was examined for caspase-3 activity according to the manufacturer's protocol (12). The specific caspase-3 activity was normalized for total protein and then expressed as the fold of the baseline caspase-3 activity of control cells cultured in DMEM with 20% FBS.
siRNA and CYP 2J2 plasmid design and transfections
PAECs were transfected with JNK 1/2 small interfering RNA (siRNA), which was designed and synthesized by GenePharma. Nontargeted control siRNA (siNC) was used as negative control. The sequences of siRNA against JNK 1, JNK 2, and nontargeted control sequences were JNK 1 (sense: 5′-GGCAUGGGCUAUAAAGAAATT-3′, anti-sense: 5′-UUUCUUUGUAGCCCAUGCCTT-3′); JNK 2 (sense: 5′-GCCAGAGACUUAUUAUCAATT-3′, anti-sense: 5′-UUGAUAAUAAGUCUCUGGCTT-3′); and NC control (sense: 5′-UUCUCCGAACGUGUCACGUTT-3′, anti-sense: 5′-ACGUGACACGUUCGGAGAATT-3′). The CYP 2J2 plasmid was kindly provided by Dr. Darryl Zeldin (National Institute of Environmental Health Sciences, Research Triangle Park, NC). PAECs were cultured until 30–50% confluence, and then 1.5 μg siRNA or plasmid and 7.5 μl X-treme Gene Transfection Reagent were diluted in serum-free Opti-MEM-1 medium, respectively, and then mixed them together gently. The mixture was incubated at room temperature for 20 min and added directly to the cells. After transfection, cells were quiescent for 24 h and then used as required.
Western blot analysis
The proteins were extracted from PAECs as previously reported (12). The protein samples (20–50 µg) were fractionated by SDS-PAGE (12% polyacrylamide gels) and transferred onto nitrocellulose membrane. After incubation in a blocking buffer (Tris 20 mM, pH 7.6, NaCl 150 mM, and Tween 20 0.1%) containing 5% nonfat dry milk powder, the appropriate antibodies at suitable concentrations were incubated overnight at 4°C, followed by reaction with horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence reagents, with β-actin as an internal control.
RT-PCR
PAEC RNA was extracted using Trizol reagent and then reverse-transcribed using SuperScriptTM First-Strand Synthesis System followed by amplification in a DNA thermal cycler (PxG20809, Thermo Electron Co.), applying Taq polymerase. The RT-PCR products were visualized by ethidium bromide-stained agarose gel electrophoresis. The images were recorded and the band intensities were analyzed using the gel imaging analysis system (Gene Genius, Syngene, United Kingdom). The gene-specific primers were designed from coding regions and the nucleotide sequences of primers were as follows: JNK 1 (GenBank accession number NM_001192974.1, sense: 5′ -AAGAATGTCCTACCTGCTC-3′, anti-sense: 5′-TTCCCTTTCATCTAACTGC-3′, 661 bp); JNK 2 (GenBank accession number NM_001046369.1, sense: 5′-TTTGGTATGACCCTGCTGA-3′, antisense: 5′-CATTGATGGACGACGACTG-3′, 216 bp); Cyclin A (GenBank accession number NM_001075123.1, sense: 5′ -AAATGTAAGCCTAAAGTGG-3′, anti-sense: 5′- GATAACTGACGGCAAATAC-3′, 492 bp); Cyclin D (GenBank accession number NM_001046273.1, sense: 5′ -GTCGCTGGAGCCCGTGAAA-3′, anti-sense: 5′-GGGCGGGTTGGAAATGAA-3′, 334 bp); P21 (GenBank accession number NM_001098958.1, sense: 5′- TGCCGCTGCCTCTTTGGT-3′, antisense: 5′-TGCTGGTCTGCCGCCGTTT-3′, 379 bp); P27 (GenBank accession number NM_001100346.1, sense: 5′-CTAACGGGAGTCCGAGCCT-3′, antisense: 5′- ATGCGTGTCCTCTGGGTTT-3′, 368 bp); and β-actin (GenBank accession number NM_173979.3, sense: 5′-AACTGGGACGACATGGAGAA-3′, antisense: 5′-TCCTGCTTGCTGATCCACAT-3′, 851 bp). β-actin was used as an internal control.
Mitochondrial depolarization assay
We monitored the mitochondrial membrane potentials by determining the relative amounts or dual emissions from both mitochondrial 5,5′,6,6′-tetrachloro-1,1′3,3′-tetraethylbenzimidazolcarbocyanine iodide (JC-1) monomers (green) and aggregates (red) by using an Olympus fluorescent microscope under Argon-ion 488 nm laser excitation. Images obtained by a fluorescent microscope of PAECs reacted with JC-1 were analyzed for green and red fluorescence. Mitochondrial depolarization was expressed by an increase in the intensity ratio of green/red fluorescence.
Statistical analysis
The composite data are expressed as means ± SEM. Statistical analysis was performed with chi-square test, Student t-test or one-way ANOVA followed by Dunnett's test where appropriate. P < 0.05 was considered statistically significant.
RESULTS
EETs induced the activation of JNK and nuclear translocation of phospho-JNK in PAECs
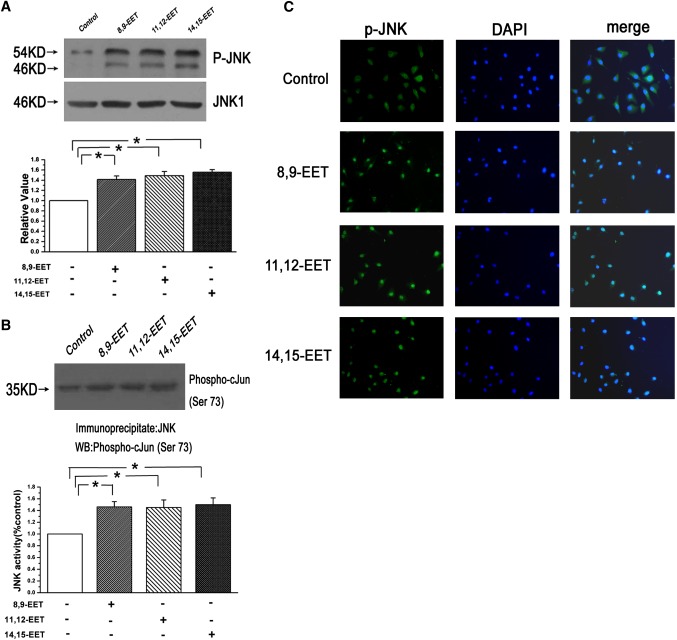
To test whether EETs (8,9-EET, 11,12-EET, and 14,15-EET) are capable of activating JNK pathway in cultured PAECs, we first examined the phosphorylation of JNK and JNK activity. We found that 500 nM/l EETs greatly induced the expression of phospho-JNK and increased JNK activity (n = 3, P < 0.05; Fig. 1A, B). As shown in Fig. 1C, although phospho-JNK was distributed in both cytosol and nucleus in the normal group, treatment with EETs could render the phospho-JNK redistribution and accumulation in the cellular nucleus. These results showed that activation of JNK by EET stimulation was associated with phospho-JNK translocation into the cellular nucleus.
Fig. 1.
Activation of JNK and nuclear translocation of phospho-JNK were induced by EETs in PAECs. A: Exogenous EETs increased the protein expression of phospho-JNK (n = 3, *P < 0.05). B: The JNK activity was increased after treatment with EETs as determined by the JNK activity assay kit (n = 3, *P < 0.05). C: EETs stimulated the phospho-JNK translocation into the cellular nucleus as determined by immunocytochemistry (n = 6).
Activation of c-Jun by EET is mediated by JNK but not by ERK or p38 MAPK
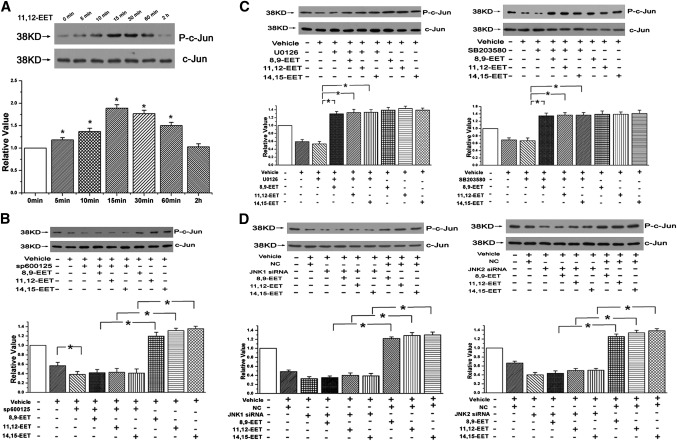
c-Jun, a major substrate of JNK, was also determined in our study. We first treated PAECs with 11,12-EET at different time points, and we found that phosphorylation of c-Jun was increased after stimulating with 11,12-EET for 5 min, and it arrived at the peak at 15 min, indicating that the phosphorylation of c-Jun by EET was time-dependent (n = 3, P < 0.05; Fig. 2A). And as shown in Fig. 2B, there was an increase of the c-Jun phosphorylation in the presence of EETs, but the promotive effect of EETs on phospho-c-Jun was weakened after depressing the JNK activation with Sp600125. However, no notable reduction of the c-Jun phosphorylation stimulated by EETs was observed in the presence of ERK pathway inhibitor (U0126) or p38 MAPK pathway inhibitor (SB203580) (n = 3, P < 0.05; Fig. 2C).
Fig. 2.
JNK, but not the ERK or p38 MAPK pathway, mediated the activation of c-Jun induced by EET. A: The phosphorylation of c-Jun was increased by 11,12-EET in a time-dependent manner. B: EETs promoted the phosphorylation of c-Jun in PAECs through the JNK pathway. C: c-Jun phosphorylation after stimulation with EETs in the presence of U0126 or SB203580. D: Phosphorylation of c-Jun after transient transfection of JNK 1 or JNK 2 siRNA into PAECs. “Vehicle” means the cells cultured in 1% serum medium. All values are denoted as means ± SEM from three or more independent batches of cells (for each group n = 3; *P < 0.05).
To exclude the possible nonspecific inhibition caused by the chemical inhibitor, we used specific siRNA to silence the JNK1 or JNK2 gene expression in PAECs. RT-PCR and Western blot analyses were performed to ensure the adequate knocking down of JNK1 or JNK 2 (n = 3, P < 0.05; supplementary Fig. I-A). As shown in Fig. 2D, the effects of EETs on c-Jun phosphorylation were significantly attenuated in PAECs treated with transient transfection of JNK1/2 siRNA. These results certify that c-Jun is phosphorylated by JNK at the N-terminal site to promote the transcriptional activity in PAECs and that the ERK and p38 MAPK pathways are not involved in this process.
EETs promote PAECs proliferation through JNK/c-Jun pathway
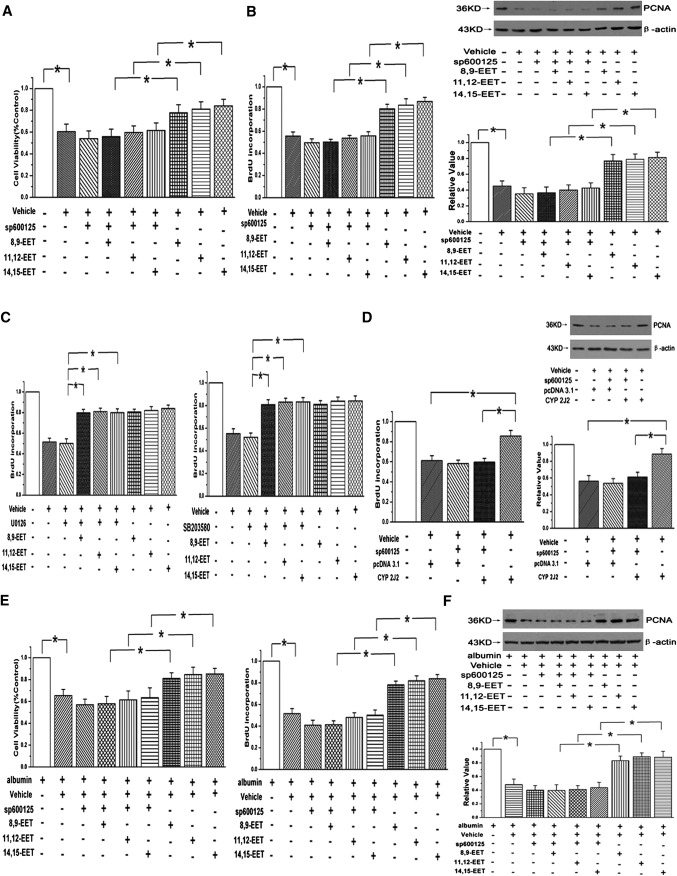
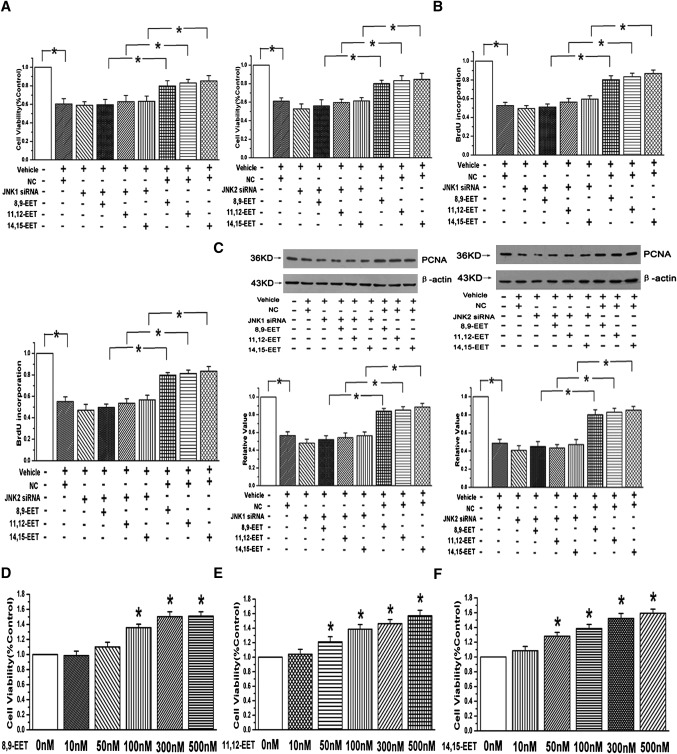
To examine whether the effects of EETs on PAEC proliferation are dependent on the JNK/c-Jun pathway, cell viability was determined by MTT assay. Our results showed that although three region-isomeric epoxides (8,9-EET, 11,12-EET, and 14,15-EET) could reverse the decrease of cell viability caused by 1% serum, the cell viability of incubating with EETs in 1% serum medium were slightly weaker than that of the control group (containing 20% serum). Moreover, the protective effects of EETs were partially weakened by the usage of 5 μM/l Sp600125 (n = 6, P < 0.05; Fig. 3A) or knocking down the JNK 1/2 gene with siRNAs (n = 6, P < 0.05; Fig. 4A). To ascertain the role of EETs and the JNK pathway in PAEC proliferation, BrdU incorporation assay and expression of proliferating cell nuclear antigen (PCNA) were examined in our study. The results showed that EETs enhanced the BrdU incorporation and induced the expression of PCNA in the medium containing 1% serum and that the effects were markedly abolished in the presence of the JNK pathway inhibitor (n = 3, P < 0.05; Fig. 3B) or siRNA sequences of JNK1/2 (n = 3, P < 0.05; Fig. 4B, C). Furthermore, PAEC proliferation induced by EETs was not attenuated by ERK and p38 MAPK pathway inhibitors as determined by the BrdU incorporation (n = 3, P < 0.05; Fig. 3C).
Fig. 3.
JNK/c-Jun pathway is involved in PAEC proliferation induced by EETs, but not ERK or p38 MPAK. A: The protective effect of EETs on cell viability was weakened by adding Sp600125 in PAECs (n = 6, *P < 0.05). B: BrdU incorporation assay and PCNA protein expression were examined after using a JNK inhibitor in PAECs (n = 3, *P < 0.05). C: EET-induced proliferation was not mediated by the ERK or p38 MAPK pathway (n = 3, *P < 0.05). D: BrdU incorporation and PCNA expression were studied after overexpression of CYP2J2 in PAECs (n = 3, *P < 0.05). E: EETs increased the cell viability and BrdU incorporation in the presence of 100 μM albumin in PAECs, which was attenuated by Sp600125 (*P < 0.05). F: EETs induced the PCNA expression through JNK pathway even after incubation with 100 μM albumin (n = 3, *P < 0.05).
Fig. 4.
The proliferative effects of EETs were abolished after silencing the JNK1/2 gene. A: EETs increased the cell viability in a JNK-dependent manner determined by silencing JNK 1/2 gene with siRNA (n = 6, *P < 0.05). B: BrdU incorporation assay was detected after blocking JNK pathway with JNK 1/2 siRNAs in PAECs (n = 3, *P < 0.05). C: The promotive effects of EETs on PCNA expression were attenuated after the silence of JNK1/2 gene (n = 3, *P < 0.05). D: The promotive effects of 8,9-EET on cell viability at different concentrations in PAECs. E: 11,12-EET improved the cell viability in a concentration-dependent manner. F: Responses provoked by 14,15-EET on cell viability were concentration-dependent. *P < 0.05 compared with 0 nM in panels D and F.
In addition, CYP2J2, which is expressed in the vessel endothelial cell and metabolized arachidonic acid to EET, was overexpressed by stable transfection of the pcDNA3.1 CYP2J2 plasmid into PAECs (13). The protein expression of CYP2J2 was studied to confirm the plasmid of CYP 2J2 had been transfected into PAECs (n = 3, P < 0.05; supplementary Fig. I-B). We found that CYP2J2 overexpression enhanced the BrdU incorporation and PCNA expression and that the proliferative effects were eliminated by administration of the JNK inhibitor, consistent with applying exogenous EETs (n = 3, P < 0.05; Fig. 3D).
As BSA (BSA) in the medium served as the lipid carrier, we repeated the experiments in the presence of 100 μM albumin to make sure the promotive effects and mechanism of EETs on PAEC proliferation presented above were not confounded by cell toxicity. The data showed that EETs promoted the cell growth, BrdU incorporation, and PCNA expression through the JNK pathway even in the presence of a high concentration of BSA, which was similar to the experiments carried out in the medium containing 1% serum (n = 3, P < 0.05; Fig. 3E, F). All of these results indicate that EETs indeed induce PAEC proliferation through the activation of the JNK/c-Jun pathway.
EETs promote the cell growth in a concentration-dependent manner
MTT assay was applied to determine the prosurvival effects of EETs at different concentrations in PAECs. We observed that the cell viability was apparently increased after the incubation with 100 nM 8,9-EET and that the peak response concentration of 11,12-EET or 14,15-EET was 500 nM (n = 3, P < 0.05; Fig. 4DndashF). The results suggest that the responses provoked by EETs on cell viability are concentration-dependent.
EETs regulate cell-cycle-related proteins and promote the cell transition from G0/G1 phase to S phase via the JNK/c-Jun pathway
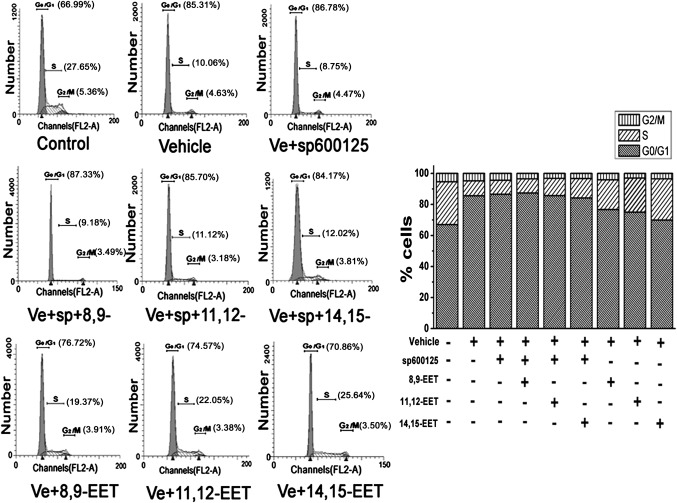
As shown in Fig. 5, the amount of S-phase cells became significantly increased and the percentage of G0/G1-phase cells was reduced in EET-treated PAECs. Compared with untreated vehicle cells, the percentage of cells in S phase was increased by 17.08%, accompanied with a concomitant reduction of cells in the G0/G1 phase from 85.6% to 69.86% after treatment with 14,15-EET for 24 h. The effects of other two EETs on cell-cycle progression were slightly weaker than that of 14,15-EET. However, the promotive role of EETs in cell-cycle progression was relieved by the JNK inhibitor.
Fig. 5.
EETs promoted the cell transition from G0/G1 phase to S phase and regulated cell-cycle-related proteins via the JNK/c-Jun pathway in PAECs. The number of cells in each phase of the cell cycles was examined by FACS analysis among groups (n = 2). The results showed that EETs increased the percentage of cells in S phase through the JNK/c-Jun pathway, which was accompanied with a concomitant reduction of cells at G0/G1 phase. “Vehicle” means the cells cultured in 1% serum medium.
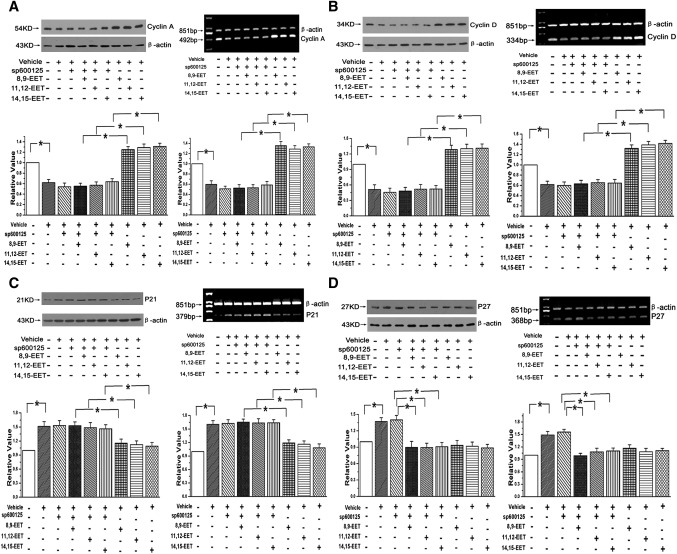
The expression of cell-cycle regulatory proteins was also detected in both transcriptional and translational levels. We found EETs increased both the protein and mRNA of cyclin A and cyclin D1 and coincided with a decrease in the expression of p21; these effects of EETs were significantly attenuated after inhibiting the JNK pathway with Sp600125 (n = 3, P < 0.05; Fig. 6AndashC). With regard to p27, although its protein and mRNA expressions were deceased by EETs, no detectable upregulation was observed in the group treated with Sp600125+EETs compared with the group treated with EETs alone (n = 3, P < 0.05; Fig. 6D). The data reveal that p21 is critical to the cell-cycle transition by EETs in a manner that depends on activation of JNK/c-Jun, but that p27 is not important to this process.
Fig. 6.
EETs regulated the cell-cycle regulatory proteins to promote the cell-cycle transition through the JNK/c-Jun pathway. A: Protein and mRNA expressions of cyclin A after EET treatment (n = 3, *P < 0.05). B: Protein and mRNA expressions of cyclin D1 after treating PAECs with EETs (n = 3, *P < 0.05). C: Protein and mRNA expressions of p21 after adding exogenous EETs to PAECs (n = 3, *P < 0.05). D: Protein and mRNA expressions of p27 with treatment of exogenous EETs (n = 3, *P < 0.05).
EETs inhibit apoptosis of pulmonary artery smooth muscle cells via the JNK/c-Jun pathway
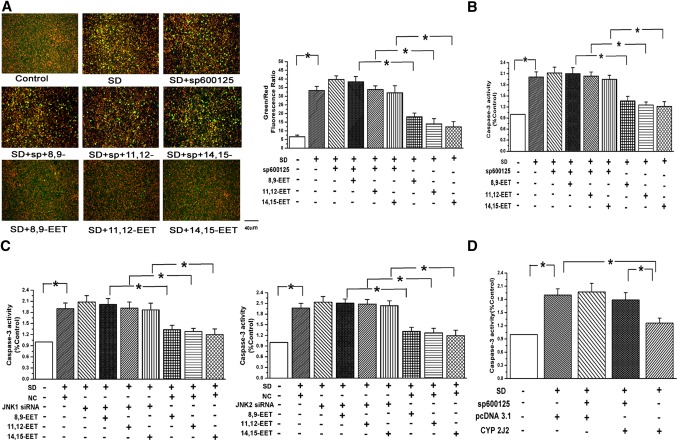
Now that JNK and c-Jun activation by EETs lead to PAEC proliferation, we further studied whether EETs also inhibit PAEC apoptosis. We applied serum deprivation (SD) for making a cell apoptotic model (12). The mitochondrial membrane potential and caspase-3 activity were examined in the starved PAECs. We used JC-1 probe to assess the changes of mitochondrial potential among groups. A significant increase in the green (low ΔΨm) to the red (high ΔΨm) ratio was observed in SD-treated cells compared with untreated cell (grown in the medium containing 20% serum), and the green fluorescence was decreased when treating the starved cells with EETs. However, the inhibitory effects of EETs on mitochondrial membrane potential reduction were blocked in the presence of Sp600125 (n = 6, P < 0.05; Fig. 7A). The results show that EETs protect against mitochondrial dysfunction induced by SD and maintain mitochondrial integrity via the JNK/c-Jun pathway.
Fig. 7.
EETs inhibited PAECs apoptosis via the JNK/c-Jun signaling pathway. A: EETs protected against the loss of mitochondrial membrane potentials evoked by SD in a JNK-dependent manner. PAECs were stained with JC-1 probe and imaged by the fluorescent microscope and quantitative analysis of the shift of mitochondrial green fluorescence to red fluorescence from six randomly selected fields obtained from each group (n = 6; *P < 0.05). B: Activation of Caspase-3 induced by starvation was repressed by adding EETs in bovine PAECs, which was relieved by a JNK inhibitor (n = 3, *P < 0.05). C: Inhibitory effects of EETs on caspase-3 activation were reversed by silencing the JNK 1/2 gene (n = 3, *P < 0.05). D: CYP2J2 overexpression repressed the activation of caspase-3 by SD through the JNK pathway (n = 3, *P < 0.05).
As shown in Fig. 7B, we examined the activity of caspase-3, which is considered an important regulator of the cell apoptosis. We found that SD enhanced the activation of caspase-3 and that a treatment of starved cells with EETs significantly decreased caspase-3 activity, but EETs did not further inhibit the activation of caspase-3 after blocking the JNK pathway with Sp600125 or silencing the JNK gene with JNK1/2 siRNA in PAECs (n = 3, P < 0.05; Fig. 7B, C). Furthermore, the activation of caspase-3 was eliminated after transfecting the CYP2J2 plasmid into starved PAECs via the JNK pathway (n = 3, P < 0.05; Fig. 7D). These results show that EETs protect PAECs against apoptosis via the JNK/c-Jun pathway.
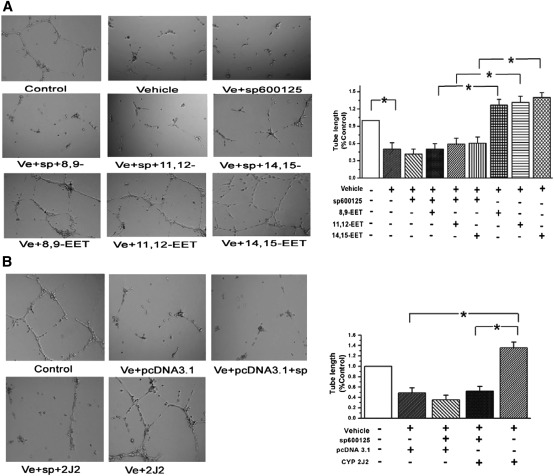
EET-induced tube formations in vitro are mediated by JNK/c-Jun pathway in PAECs
To elucidate whether JNK/c-Jun pathway is involved in EET-induced angiogenic events, we performed the tube formation assay. We found exogenous EETs stimulated the tube formation of PAECs in vitro, which was reversed by the JNK pathway inhibitor (n = 3, P < 0.05; Fig. 8A). Furthermore, the tube formation induced by CYP2J2 overexpression was mediated by the JNK pathway in PAECs (n = 3, P < 0.05; Fig. 8B). These results demonstrate that CYP2J2 or EETs stimulate matrigel plug angiogenesis by a mechanism involving the activation of the JNK/c-Jun pathway.
Fig. 8.
EETs induced the tube formation in vitro through the JNK/c-Jun pathway. A: Tube formation of PAECs after treatment of EETs was depressed by Sp600125 (a JNK inhibitor). B: Tube formation of PAECs after CYP2J2 overexpression. All values are denoted as means ± SEM from three or more independent batches of cells (for each group n = 3, *P < 0.05).
DISCUSSION
Previous studies have indicated that hypoxia, one major reason of PAH, induces the expression of CYP and EET formation (14), and EET plays an important role in pulmonary vasoconstriction as a vasoconstriction factor (15–17). Trigger of EC proliferation and plexiform lesion formation by proliferating ECs are important pathological changes in PAH (18). However, whether EETs are responsible for the tumorlets of endothelial cells that obliterate pulmonary arteries, as well as the relative mechanisms, remains unclear. New evidence is provided by present study that the activation of the JNK/c-Jun pathway is involved in the processes by which EETs (8,9-EET, 11,12-EET, and 14,15-EET) stimulate cell proliferation, inhibit cell apoptosis, and motivate angiogenesis in PAECs.
PAH is a disease of the small pulmonary arteries characterized by sustained vasoconstriction and vascular remodeling (19). Accumulating evidence suggests that EETs and sEH are determinants of PAH (20). The effects of EETs on vasoconstriction have been widely studied. There are reports that EETs constrict isolated rabbit pulmonary arteries and that pulmonary vasoconstriction increased by sEH inhibition is abolished by pretreatment with CYP epoxygenase inhibitors or EET antagonists (15), but direct evidence that EETs affect PVR was lacking until now. By using JNK inhibitor (Sp600125) or specific siRNA of JNK1/2 in this study, we observed that exogenous EETs or overexpression of CYP2J2 motivates the proliferation and survival of PAECs by activating the JNK/c-Jun pathway. To the best of our knowledge, this is the first systematic study to associate JNK and c-Jun activation with plexiform lesions composed of endothelial cells and PVR in EET stimulation.
In addition, an interesting phenomenon is found that EETs have no notable effects on PASMC proliferation and survival, the major components of the pulmonary vascular media (supplementary Fig. III-A, B). Because EETs are mainly hydrolyzed by sEH and become inactive, there is a higher expression of sEH in PASMCs than that in PAECs (supplementary Fig. III-C). It is possible that the inconsistent results are due to different ratios of EET metabolism between PASMCs and PAECs, which is proved by our results that EETs promoted the proliferation of PASMCs after inhibiting the activity of sEH with an sEH inhibitor (AUDA; supplementary Fig. III-D). These results show that EETs mainly influence the growth of PAECs, but not of PASMCs, in the progression of PVR.
Different signaling cascade systems and mediators act on the angiogenic and mitogenic effects of EETs in vascular endothelial cells, such as induction of growth factor expression [epidermal growth factor (EGF), vascular EGF (VEGF), etc.], activation of PI3K/AKT, endothelial nitric-oxide synthase (eNOS) and sphingosine kinase-1, upregulation of EphB4 expression, and stimulation of tyrosine phosphorylation (21–27). Intriguingly, although the JNK signal pathway plays an important role in regulating angiogenesis, the effects of JNK on cell proliferation and survival are complex and not always consistent in previous studies. The activation of the JNK pathway contributes to the growth inhibitory effect of glucose in ECs, and the proliferation of human ECs is enhanced by decreasing the JNK activity (28, 29). However, in other studies, dominant negative JNK1 or preventing JNK translocation inhibited VEGF-induced bovine aortic EC proliferation (30). Furthermore, induced JNK/c-Jun mediated by growth factor activates prosurvival signaling in ECs (31), and activation of JNK is a critical molecule in PASMC proliferation and tumor cell growth (32, 33). In the present study, we observed that EETs promote the nuclear translocation of JNK and activate the JNK/c-Jun pathway, leading to the increase of PAEC growth and angiogenesis. Possible explanations for these controversial responses might be different stimului, species, or cell types used in the research. Now that we have shown that the JNK pathway is involved in growth factor-induced endothelium proliferation, EETs are likely to activate the JNK/c-Jun pathway via modulating some growth factors in PAECs; this conclusion requires further study.
As c-Jun phosphorylation and transcriptional activation have been shown to be mediated by members of the MAPK family, including JNKs, ERKs, and p38 MAPK (34, 35), it should be identified whether c-Jun activation and proliferation induced by EETs are mediated by all or only some of them. We find that only the JNK inhibitor attenuates the phosphorylation of c-Jun and proliferation induced by EETs. These data demonstrate that ERK and p38 MAPK are not necessary for c-Jun activation by EETs in bovine PAECs and that JNK plays a particularly unique role in mediating EET-induced PAECs proliferation.
Cell-cycle progression and cell proliferation, controlled by cyclins, cyclin-dependent kinases (CDK), and cyclin-dependent kinase inhibitors (CDKI), are carefully regulated to ensure that the appropriate numbers of cells are produced (36, 37). We find that EETs promote the cell transition from G0/G1 phase to S phase in a JNK/c-Jun-dependent manner. Cell-cycle progression from the first gap phase (G1 phase) to the DNA replication phase (S phase) in mammalian cells is regulated by the accumulation of cyclin A and cyclin D1. Moreover, p21CIP1 and p27KIP1, two important CDKIs, almost inhibit transition of the cell cycle at all stages. Therefore, their protein and mRNA expressions were examined in our study. The results indicate that EETs increase the expression of cyclin A and cyclin D1 and decrease the expression of p21 to advance cell-cycle progression and make more cells enter into DNA endoreduplication through the JNK/c-Jun pathway. Our data also show that p27 seems not to be critical for EET-induced proliferation because the inhibition of EET-induced proliferation by the JNK inhibitor is not associated with the upregulation of p27 mRNA and protein expression. And p21, a key transcriptional target of c-Jun, may be directly regulated and repressed by c-Jun, consistent with published data describing a role for c-Jun in negative regulation of the p21 promoter (38, 39).
The balance between cell apoptosis and proliferation is responsible for maintaining normal function of tissues and organs (40). Enhanced endothelial cells growth is likely to be a major reason leading to endothelial hypertrophy and plexiform lesions (5). In our experiments, we find EETs or CYP2J2 overexpression promotes proliferation and affords protection from starvation-induced apoptosis in PAECs in a JNK-dependent manner. These four EETs belong to a series of region- and stereospecific epoxides, but there is a difference among them in promoting PAEC proliferation. We find that 11,12-EET and 14,15-EET are slightly more efficacious than 8,9-EET in promoting PAEC proliferation, whereas 5,6-EET has no detectable effect; the results suggest that the role of EETs in PAEC proliferation and survival depends on the EET's chemical structure (supplementary Fig. II).
Although we have demonstrated that only JNK in the MAPK pathway participates in EET-induced proliferation in PAECs, further studies are needed to evaluate whether other parallel signaling pathways, such as ROCK and PI3K/Akt, are also involved. Furthermore, as our study was carried out only in cultured cells in vitro, relevant in vivo experiments should be addressed in future studies.
In summary, our data imply that the JNK/c-Jun signaling pathway mediates the proliferation and survival of pulmonary arterial endothelial cell by EETs, contributing to tumorlet formation and lumen obliteration. These observations raise a novel therapeutic potential target to treat pulmonary artery hypertension and improve therapeutic efficacy in the future.
Supplementary Material
Acknowledgments
The authors are grateful to Dr. Darryl Zeldin (National Institute of Environmental Health Sciences, Research Triangle Park, NC), who has kindly provided us the CYP 2J2 plasmid.
Footnotes
Abbreviations:
- BrdU
- bromodeoxyuridine
- CYP
- cytochrome p450
- EC
- endothelial cell
- EET
- epoxyeicosatrienoic acid
- sEH
- soluble epoxide hydrolase
- JNK
- c-Jun N-terminal kinase
- PAEC
- pulmonary artery endothelial cell
- PAH
- pulmonary arterial hypertension
- PASMC
- pulmonary artery smooth muscle cell
- PVR
- pulmonary vascular remodeling
- SD
- serum deprivation
This work was supported by the National Natural Science Foundation of China (No. 31071007).
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of three figures.
REFERENCES
- 1.Lee S. D., Shroyer K. R., Markham N. E., Cool C. D., Voelkel N. F., Tuder R. M. 1998. Monoclonal endothelial cell proliferation is present in primary but not secondary pulmonary hypertension. J. Clin. Invest. 101: 927–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stiebellehner L., Belknap J. K., Ensley B., Tucker A., Orton E. C., Reeves J. T., Stenmark K. R. 1998. Lung endothelial cell proliferation in normal and pulmonary hypertensive neonatal calves. Am. J. Physiol. 275: L593–L600 [DOI] [PubMed] [Google Scholar]
- 3.Budhiraja R., Tuder R. M., Hassoun P. M. 2004. Endothelial dysfunction in pulmonary hypertension. Circulation. 109: 159–165 [DOI] [PubMed] [Google Scholar]
- 4.Taraseviciene-Stewart L., Kasahara Y., Alger L., Hirth P., Mc Mahon G., Waltenberger J., Voelkel N. F., Tuder R. M. 2001. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB J. 15: 427–438 [DOI] [PubMed] [Google Scholar]
- 5.Xu X., Zhang X. A., Wang D. W. 2011. The roles of CYP450 epoxygenases and metabolites, epoxyeicosatrienoic acids, in cardiovascular and malignant diseases. Adv. Drug Deliv. Rev. 63: 597–609 [DOI] [PubMed] [Google Scholar]
- 6.Pozzi A., Macias-Perez I., Abair T., Wei S., Su Y., Zent R., Falck J. R., Capdevila J. H. 2005. Characterization of 5,6- and 8,9-epoxyeicosatrienoic acids (5,6- and 8,9-EET) as potent in vivo angiogenic lipids. J. Biol. Chem. 280: 27138–27146 [DOI] [PubMed] [Google Scholar]
- 7.Johnson G. L., Nakamura K. 2007. The c-jun kinase/stress-activated pathway: regulation, function and role in human disease. Biochim. Biophys. Acta. 1773: 1341–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smeal T., Binetruy B., Mercola D. A., Birrer M., Karin M. 1991. Oncogenic and transcriptional cooperation with Ha-Ras requires phosphorylation of c-Jun on serines 63 and 73. Nature. 354: 494–496 [DOI] [PubMed] [Google Scholar]
- 9.Dong C., Yang D. D., Wysk M., Whitmarsh A. J., Davis R. J., Flavell R. A. 1998. Defective T cell differentiation in the absence of Jnk1. Science. 282: 2092–2095 [DOI] [PubMed] [Google Scholar]
- 10.Alcorn J. F., van der Velden J., Brown A. L., McElhinney B., Irvin C. G., Janssen-Heininger Y. M. 2009. c-Jun N-terminal kinase 1 is required for the development of pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 40: 422–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Medhora M., Chen Y., Gruenloh S., Harland D., Bodiga S., Zielonka J., Gebremedhin D., Gao Y., Falck J. R., Anjaiah S., et al. 2008. 20-HETE increases superoxide production and activates NAPDH oxidase in pulmonary artery endothelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 294: L902–L911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma J., Liang S., Wang Z., Zhang L., Jiang J., Zheng J., Yu L., Zheng X., Wang R., Zhu D. 2010. ROCK pathway participates in the processes that 15-hydroxyeicosatetraenoic acid (15-HETE) mediated the pulmonary vascular remodeling induced by hypoxia in rat. J. Cell. Physiol. 222: 82–94 [DOI] [PubMed] [Google Scholar]
- 13.Deng Y., Edin M. L., Theken K. N., Schuck R. N., Flake G. P., Kannon M. A., DeGraff L. M., Lih F. B., Foley J., Bradbury J. A., et al. 2011. Endothelial CYP epoxygenase overexpression and soluble epoxide hydrolase disruption attenuate acute vascular inflammatory responses in mice. FASEB J. 25: 703–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michaelis U. R., Xia N., Barbosa-Sicard E., Falck J. R., Fleming I. 2008. Role of cytochrome P450 2C epoxygenases in hypoxia-induced cell migration and angiogenesis in retinal endothelial cells. Invest. Ophthalmol. Vis. Sci. 49: 1242–1247 [DOI] [PubMed] [Google Scholar]
- 15.Keserü B., Barbosa-Sicard E., Popp R., Fisslthaler B., Dietrich A., Gudermann T., Hammock B. D., Falck J. R., Weissmann N., Busse R., et al. 2008. Epoxyeicosatrienoic acids and the soluble epoxide hydrolase are determinants of pulmonary artery pressure and the acute hypoxic pulmonary vasoconstrictor response. FASEB J. 22: 4306–4315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keserü B., Barbosa-Sicard E., Schermuly R. T., Tanaka H., Hammock B. D., Weissmann N., Fisslthaler B., Fleming I. 2010. Hypoxia-induced pulmonary hypertension: comparison of soluble epoxide hydrolase deletion vs. inhibition. Cardiovasc. Res. 85: 232–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mandegar M., Fung Y. C., Huang W., Remillard C. V., Rubin L. J., Yuan J. X. 2004. Cellular and molecular mechanisms of pulmonary vascular remodeling: role in the development of pulmonary hypertension. Microvasc. Res. 68: 75–103 [DOI] [PubMed] [Google Scholar]
- 18.Archer S. L., Michelakis E. D. 2006. An evidence-based approach to the management of pulmonary arterial hypertension. Curr. Opin. Cardiol. 21: 385–392 [DOI] [PubMed] [Google Scholar]
- 19.Farkas L., Gauldie J., Voelkel N. F., Kolb M. 2011. Pulmonary hypertension and idiopathic pulmonary fibrosis: a tale of angiogenesis, apoptosis, and growth factors. Am. J. Respir. Cell Mol. Biol. 45: 1–15 [DOI] [PubMed] [Google Scholar]
- 20.Revermann M., Barbosa-Sicard E., Dony E., Schermuly R. T., Morisseau C., Geisslinger G., Fleming I., Hammock B. D., Brandes R. P. 2009. Inhibition of the soluble epoxide hydrolase attenuates monocrotaline-induced pulmonaryhypertension in rats. J. Hypertens. 27: 322–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spector A. A. 2009. Arachidonic acid cytochrome P450 epoxygenase pathway. J. Lipid Res. 50: S52–S56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheranov S. Y., Karpurapu M., Wang D., Zhang B., Venema R. C., Rao G. N. 2008. An essential role for SRC-activated STAT-3 in 14,15-EET-induced VEGF expression and angiogenesis. Blood. 111: 5581–5591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan G., Chen S., You B., Sun J. 2008. Activation of sphingosine kinase-1 mediates induction of endothelial cell proliferation and angiogenesis by epoxyeicosatrienoic acids. Cardiovasc. Res. 78: 308–314 [DOI] [PubMed] [Google Scholar]
- 24.Michaelis U. R., Fisslthaler B., Medhora M., Harder D., Fleming I., Busse R. 2003. Cytochrome P450 2C9-derived epoxyeicosatrienoic acids induce angiogenesis via cross-talk with the epidermal growth factor receptor (EGFR). FASEB J. 17: 770–772 [DOI] [PubMed] [Google Scholar]
- 25.Zhang B., Cao H., Rao G. N. 2006. Fibroblast growth factor-2 is a downstream mediator of phosphatidylinositol 3-kinase-Akt signaling in 14,15-epoxyeicosatrienoic acid-induced angiogenesis. J. Biol. Chem. 281: 905–914 [DOI] [PubMed] [Google Scholar]
- 26.Webler A. C., Popp R., Korff T., Michaelis U. R., Urbich C., Busse R., Fleming I. 2008. Cytochrome P450 2C9-induced angiogenesis is dependent on EphB4. Arterioscler. Thromb. Vasc. Biol. 28: 1123–1129 [DOI] [PubMed] [Google Scholar]
- 27.Hoebel B. G., Graier W. F. 1998. 11,12-Epoxyeicosatrienoic acid stimulates tyrosine kinase activity in porcine aortic endothelial cells. Eur. J. Pharmacol. 346: 115–117 [DOI] [PubMed] [Google Scholar]
- 28.Liu W., Schoenkerman A., Jr. Lowe W. L. 2000. Activation of members of the mitogen-activated protein kinase family by glucose in endothelial cells. Am. J. Physiol. Endocrinol. Metab. 279: E782–E790 [DOI] [PubMed] [Google Scholar]
- 29.Potente M., Michaelis U. R., Fisslthaler B., Busse R., Fleming I. 2002. Cytochrome P450 2C9-induced endothelial cell proliferation involves induction of mitogen-activated protein (MAP) kinase phosphatase-1, inhibition of the c-Jun N-terminal kinase, and up-regulation of cyclin D1. J. Biol. Chem. 277: 15671–15676 [DOI] [PubMed] [Google Scholar]
- 30.Pedram A., Razandi M., Levin E. R. 1998. Extracellular signal-regulated protein kinase/Jun kinase cross-talk underlies vascular endothelial cell growth factor-induced endothelial cell proliferation. J. Biol. Chem. 273: 26722–26728 [DOI] [PubMed] [Google Scholar]
- 31.Salameh A., Galvagni F., Bardelli M., Bussolino F., Oliviero S. 2005. Direct recruitment of CRK and GRB2 to VEGFR-3 induces proliferation, migration, and survival of endothelial cells through the activation of ERK, AKT, and JNK pathways. Blood. 106: 3423–3431 [DOI] [PubMed] [Google Scholar]
- 32.Wei L., Liu Y., Kaneto H., Fanburg B. L. 2010. JNK regulates serotonin-mediated proliferation and migration of pulmonary artery smooth muscle cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 298: L863–L869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen C., Wei X., Rao X., Wu J., Yang S., Chen F., Ma D., Zhou J., Dackor R. T., Zeldin D. C., et al. 2011. Cytochrome P450 2J2 is highly expressed in hematologic malignant diseases and promotes tumor cell growth. J. Pharmacol. Exp. Ther. 336: 344–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marinissen M. J., Chiariello M., Pallante M., Gutkind J. S. 1999. A network of mitogen-activated protein kinases links G protein-coupled receptors to the c-jun promoter: a role for c-Jun NH2-terminal kinase, p38s, and extracellular signal-regulated kinase 5. Mol. Cell. Biol. 19: 4289–4301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lam K. K., Chiu P. C., Lee C. L., Pang R. T., Leung C. O., Koistinen H., Seppala M., Ho P. C., Yeung W. S. 2011. Glycodelin-A protein interacts with Siglec-6 protein to suppress trophoblast invasiveness by down-regulating extracellular signal-regulated kinase (ERK)/c-Jun signaling pathway. J. Biol. Chem. 286: 37118–37127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mani S., Wang C., Wu K., Francis R., Pestell R. 2000. Cyclin-dependent kinase inhibitors: novel anticancer agents. Expert Opin. Investig. Drugs. 9: 1849–1870 [DOI] [PubMed] [Google Scholar]
- 37.Shah M. A., Schwartz G. K. 2003. Cyclin-dependent kinases as targets for cancer therapy. Cancer Chemother. Biol. Response Modif. 21: 145–170 [DOI] [PubMed] [Google Scholar]
- 38.Kardassis D., Papakosta P., Pardali K., Moustakas A. 1999. c-Jun transactivates the promoter of the human p21(WAF1/Cip1) gene by acting as a superactivator of the ubiquitous transcription factor Sp1. J. Biol. Chem. 274: 29572–29581 [DOI] [PubMed] [Google Scholar]
- 39.Kolomeichuk S. N., Bene A., Upreti M., Dennis R. A., Lyle C. S., Rajasekaran M., Chambers T. C. 2008. Induction of apoptosis by vinblastine via c-Jun autoamplification and p53-independent down-regulation of p21WAF1/CIP1. Mol. Pharmacol. 73: 128–136 [DOI] [PubMed] [Google Scholar]
- 40.Orlandi A., Francesconi A., Cocchia D., Corsini A., Spagnoli L. G. 2001. Phenotypic heterogeneity influences apoptotic susceptibility to retinoic acid and cis-platinum of rat arterial smooth muscle cells in vitro: Implications for the evolution of experimental intimal thickening. Arterioscler. Thromb. Vasc. Biol. 21: 1118–1123 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.