Abstract
The liver X receptor (LXR) signaling pathway is an important modulator of atherosclerosis, but the relative importance of the two LXRs in atheroprotection is incompletely understood. We show here that LXRα, the dominant LXR isotype expressed in liver, plays a particularly important role in whole-body sterol homeostasis. In the context of the ApoE−/− background, deletion of LXRα, but not LXRβ, led to prominent increases in atherosclerosis and peripheral cholesterol accumulation. However, combined loss of LXRα and LXRβ on the ApoE−/− background led to an even more severe cholesterol accumulation phenotype compared to LXRα−/−ApoE−/− mice, indicating that LXRβ does contribute to reverse cholesterol transport (RCT) but that this contribution is quantitatively less important than that of LXRα. Unexpectedly, macrophages did not appear to underlie the differential phenotype of LXRα−/−ApoE−/− and LXRβ−/−ApoE−/− mice, as in vitro assays revealed no difference in the efficiency of cholesterol efflux from isolated macrophages. By contrast, in vivo assays of RCT using exogenously labeled macrophages revealed a marked defect in fecal sterol efflux in LXRα−/−ApoE−/− mice. Mechanistically, this defect was linked to a specific requirement for LXRα−/− in the expression of hepatic LXR target genes involved in sterol transport and metabolism. These studies reveal a previously unrecognized requirement for hepatic LXRα for optimal reverse cholesterol transport in mice.
Keywords: atherosclerosis, nuclear receptor, cholesterol metabolism, apoliporotein
High levels of plasma LDL cholesterol have long been recognized to be a primary driver of atherosclerotic lesion formation (1). More recently, the ability of an animal to remove excess cholesterol from tissues and the body through the process of reverse cholesterol transport (RCT) has begun to be appreciated as an important determinant of susceptibility (2, 3). During atherogenesis, macrophages infiltrate the artery wall and accumulate lipoprotein-derived cholesterol via scavenger receptors. Macrophages attempt to restore their cellular cholesterol homeostasis by effluxing cholesterol to lipid-poor acceptors such as apoA-1 and HDL, which in turn carry the excess cholesterol back to the liver and possibly intestine for excretion (2, 4). Mounting evidence indicates that disruption of the pathways for macrophage cholesterol efflux or RCT by HDL promotes atherogenesis in animal models.
The primary transcriptional regulators of the RTC pathway are liver X receptor (LXR)α and LXRβ, closely related members of the nuclear hormone receptor superfamily. These receptors function as “cholesterol sensors,” responding to elevated cellular concentrations of their oxysterol ligands by activating the expression of numerous genes linked to sterol transport and metabolism. Other studies have defined a role for the LXRs in repressing inflammatory genes and autoimmunity (5, 6). Although the two LXRs appear to regulate a similar set of target genes, their expression patterns differ. LXRβ is ubiquitously expressed, whereas LXRα is highly expressed liver, intestine, adipose, and macrophages. Interestingly, the liver is one of the few tissues that express predominantly LXRα rather than LXRβ (7–10). Prior studies have suggested that LXR isoforms may have differential impacts on atherosclerosis, but the physiological basis for these effects have remained unclear (7, 11, 12).
Considerable evidence indicates that macrophages contribute to the antiatherogenic effects of the LXR pathway (6). It is clear that complete loss of LXR activity in the macrophage predisposes the cell to cholesterol overload and foam-cell formation. Transplantation of LXRα−/−LXRβ−/− bone marrow into LDLR−/− mice leads to greatly increased atherosclerotic lesion formation (11). In addition, the ability of LXR ligands to reduce atherosclerotic lesion formation is reduced when both LXRα and LXRβ are deleted from the bone marrow compartment (13, 14). With the notable exception of the LXRα-specific target gene AIM/api6, prior gene expression analyses have suggested that most LXR target genes are comparably regulated by both LXRα and LXRβ and that combined deletion of the two isotypes is required to prevent target gene induction by LXR agonists (15–17).
We show here that ApoE−/− mice lacking LXRα but not LXRβ show enhanced atherosclerosis and peripheral cholesterol accumulation, indicating that LXRα plays a role in atherosclerosis susceptibility that is not redundant with LXRβ. Unexpectedly, this phenotype cannot be explained by differential function of the two LXR isotypes in macrophages. Rather, we show that LXRα is uniquely required for maximal postmacrophage RTC. Moreover, we show that the defect in LXRα-deficient mice is linked with a specific requirement for LXRα in the expression of hepatic LXR target genes involved in sterol transport.
METHODS
Animals and diets
LXRα−/−, LXRβ−/−, and LXRαβ−/− mice (C57Bl/6, greater than 10 generations backcrossed) were provided by David Mangelsdorf and bred with C57Bl/6 ApoE−/− mice from the Jackson Laboratory (18). Male mice were fed either standard chow, Western diet (21% fat, 0.21% cholesterol: D12079B; Research Diets, Inc.). For ligand treatment studies, mice were gavaged with vehicle or 40 mg/kg GW3965 once a day for 3 days. Tissues were harvested 4 h after the last gavage. Atherosclerotic lesion analysis was done as described (12). Animal experiments were approved by the UCLA Institutional Animal Care and Research Advisory Committee.
RNA analysis, cell culture, and reagents
Total RNA was isolated from tissues using TRIzol (Invitrogen) and analyzed by real-time PCR using an Applied Biosystems 7900HT sequence detector. Results show averages of duplicate experiments normalized to 36B4. The primer sequences are available upon request (12). LXR agonist GW3965 was provided by Tim Willson and Jon Collins (GlaxoSmithKline). RXR agonist LG268 was provided by Rich Heyman (Ligand Pharmaceuticals). Ligands were dissolved in dimethyl sulfoxide before use in cell culture. LXR ligands were used at 1 µmol/l, whereas retinoid X receptor (RXR) ligand was used at 100 nmol/l. 22(R)-hydroxycholesterol and 22(S)-hydroxycholesterol were purchased from Sigma and used at 2.5 μmol/l (12). Plasma and tissue lipid analysis was performed as described (12).
Cell culture
Primary peritoneal macrophages were obtained from thioglycollate-treated mice 4 days after injection. For gene expression studies, cells were placed in DMEM plus 0.5% FBS plus 5 μmol/l simvastatin plus 100 μmol/l mevalonic acid overnight. Cells were then treated with DMSO or ligand for LXR as indicated for 24 h. Total RNA was extracted and analyzed by real-time PCR. Peritoneal cells were placed in DMEM plus 0.5% FBS plus 5 μmol/l simvastatin plus 100 μmol/l mevalonic acid overnight. Cells were then stimulated with DMSO or ligand for LXR (1 μmol/l GW3965) for 24 h. Total RNA was extracted and analyzed by real-time PCR. Bodipy labeling of cellular lipids was done as previously described (19).
Tissue and plasmid lipid analysis
Lipids were extracted from tissues using the Folch method. Briefly, chloroform extracts were dried under nitrogen and resolubilized in water. Cholesterol content was determined using a commercially available enzymatic kit (Sigma-Aldrich). Data are expressed as milligrams of cholesterol per gram of tissue weight. For plasma lipid analysis, mice were fasted overnight and euthanized. Blood was collected from the abdominal vena cava. Aliquots of plasma were analyzed for cholesterol content and plasma lipoproteins were fractionated using an FPLC system.
Histological and lesion analysis
Immunohistochemistry of skin sections and preparation and staining of frozen and paraffin-embedded sections from tissues were performed as described previously. Atherosclerosis in the aortic roots and the descending aortas (en face) were quantified by computer-assisted image analysis. Atherosclerotic lesions at the aortic valve were analyzed as described. P < 0.05 was considered significant.
Cholesterol efflux
Peritoneal macrophages cells were labeled with [3H]cholesterol (1.0 μCi/ml) in the presence of acyl-CoA:cholestrol O-acyltransferase inhibitor (2 μg/ml) either with DMSO or with ligand for LXR and RXR (1 μM GW3965; 50 nM LG268). After equilibrating the cholesterol pools, cells were washed with PBS and incubated in DMEM containing 0.2% BSA in the absence or presence of ApoA-I (15 μg/ml) or HDL (50 μg/ml) for 6 h. Radioactivity in the medium and total cell-associated radioactivity was determined by scintillation counting (12).
RTC
J774 murine macrophages were cultured for 2 days in the presence of 5 μCi/ml [3H]cholesterol and 100 μg/ml Ac-LDL in 10% FBS, 1% Pen Strep, and DMEM for 48 h. The cells were collected and washed twice with cold 1× PBS and delivered to the mice by intraperitoneal injection. Two days after injection, fecal pellets were collected, lipid was extracted using the Folch METHOD, and radioactive counts were determined (3).
RESULTS
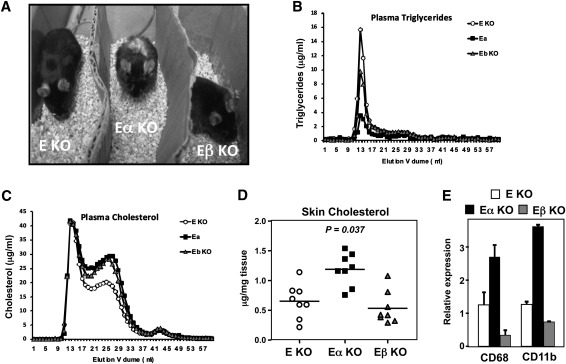
We previously reported that mice lacking both ApoE and LXRα exhibit a dramatic increase in susceptibility to atherosclerosis (12). However, the relative importance of LXRα and LXRβ expression in sterol homeostasis and atherogenesis in the context of the ApoE−/−background has not been addressed. To explore this, we generated ApoE−/− mice lacking LXRα, LXRβ, or both (C57Bl6 background; more than 10 generations backcrossed). ApoE−/−LXRα−/− (hereafter denoted E/α KO) mice and ApoE−/−LXRβ−/− (E/β KO) mice were obtained at the expected Mendelian ratios; however, LXRβ and E/β mice exhibited dramatically reduced fertility. At eight months of age, E/α mice developed an obvious external phenotype characterized by thickening of the skin and alopecia (Fig. 1A), consistent with prior results on a mixed C57BL6/SV129 background (12). Unexpectedly, however, E/β KO mice did not exhibit this phenotype, suggesting that loss of LXRα and LXRβ differentially affect whole body sterol homeostasis on the ApoE KO background.
Fig. 1.
Differential effect of LXRα or LXRβ deletion on the ApoE-null background. (A) Images of each genotype at 8 months of age. (B, C) FPLC analysis of plasma triglycerides and cholesterol for each genotype after 15 weeks of Western diet. Plasma from n = 17–22 per genotype was pooled for this analysis. (D, E) Tissue cholesterol and RNA was isolated from total skin tissue for cholesterol measurements and gene expression analysis (n = 8 per group). Statistical significance was determined using one-way ANOVA. Data represent mean ± SEM.
To explore this further, cohorts of each genotype were placed on Western diet for 15 weeks. FPLC analysis of plasma lipids revealed reduced plasma triglycerides and VLDL in E/α KO mice compared to E/β KO mice or ApoE KO controls (Fig. 1B). These findings suggest that LXRα expression is a particularly important determinant of plasma triglyceride levels in ApoE KO mice, and are consistent with earlier studies of mixed background LXR single KO mice (9, 11). Plasma cholesterol profiles of both E/α mice and E/β mice revealed elevated plasma cholesterol content compared to ApoE KO controls (Fig. 1C). Despite the similar plasma cholesterol levels of E/α mice and E/β mice, however, analysis of tissue lipid content revealed marked accumulation of cholesterol in the skin of only the E/α KO mice (Fig. 1D). Gene expression studies revealed increased CD68 and CD11b expression in the skin of E/α mice, indicative of macrophage infiltration into the skin, corresponding with the cholesterol deposition (Fig. 1E).
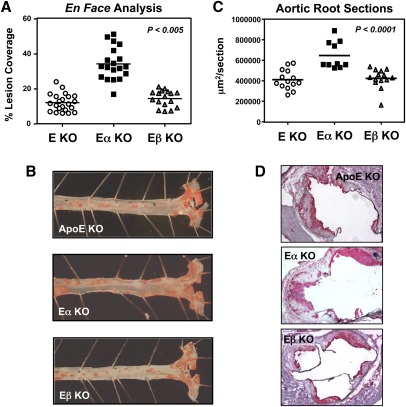
Next we analyzed atherosclerotic lesion formation after 15 weeks of Western diet. En face lesion analysis demonstrated a marked increase in lesion area in the aortas of E/α KO mice compared to E/β KO or ApoE KO controls (Fig. 2A, B). A similar degree of increase lesion area was also observed in of E/α KO mice compared to E/β KO or ApoE KO controls in analysis of aortic root sections (Fig. 2C, D).
Fig. 2.
LXRα-selective effect on atherogenesis in ApoE-null mice. (A, B) En face analysis quantified lesion coverage of the aorta. (C, D) Aortic root sections were stained with CD68 (red) and quantified. (n = 12–19 per group). Statistical significance was determined using one-way ANOVA.
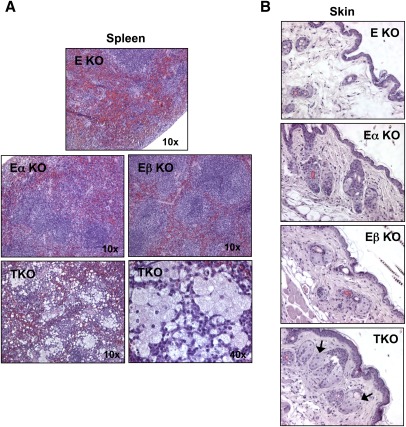
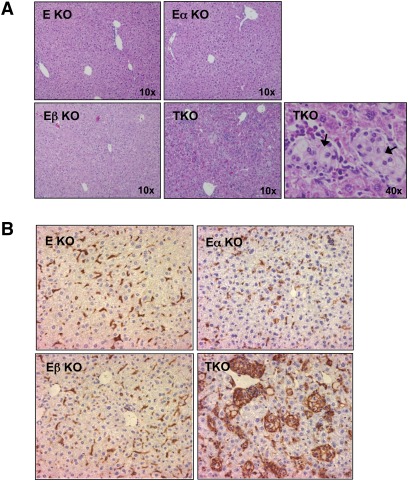
We also analyzed a limited number of ApoE−/−LXRα−/−LXRβ−/− (TKO) mice. Due to the infertility of LXRβ−/− mice on a C57Bl6 background, TKO mice were exceedingly difficult to generate and most did not survive past 10 weeks of age. Although we were unable to obtain sufficient numbers to perform atherosclerotic lesion analysis, histological analysis of tissues from young TKO mice revealed a dramatic macrophage foam-cell accumulation phenotype. Lipid-laden cells with the histological appearance of macrophages were readily identified in the spleen, dermis, and liver of 8-week-old TKO mice on a chow diet (Figs. 3A, B and 4A). Staining for the macrophage marker F4/80 confirmed the identify of these cells as macrophages (Fig. 4B). Few macrophage foam cells were identified in either E/α KO or E/β KO mice at this age. Furthermore, it was clear that peripheral cholesterol overload phenotype of the TKO mice was much more severe than that of the E/α KO mice. These data strongly support the conclusion that combined loss of LXRα and LXRβ has synergistic effect on lipid accumulation. Collectively, phenotypic analysis of compound ApoE/LXR-deficient mice suggests that both LXRα and LXRβ contribute to reverse cholesterol on the ApoE−/− background, but that LXRα plays a quantitatively more important role that becomes increasingly apparent as the mice age.
Fig. 3.
Synergistic effect of LXRα and LXRβ deletion on macrophage foam-cell formation in tissues. H and E stains of spleen (A) and skin (B) from 8-week-old female E KO, E/α KO, E/β KO and TKO mice.
Fig. 4.
Synergistic effect of LXRα and LXRβ deletion on macrophage foam-cell formation in liver. (A) H and E stains of livers from 8-week-old female E KO, E/α KO, E/β KO and TKO mice. (B) Immunostaining for the macrophage-specific marker F4/80.
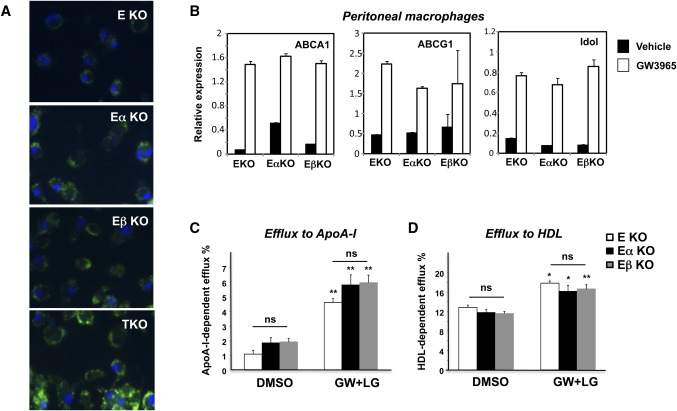
We initially suspected that the increased susceptibility of E/α KO mice to atherosclerosis compared with E/β KO mice might reflect a specific role for LXRα in the macrophage. However, we observed no difference in acetylated LDL loading after 24 h between isolated E/α KO and E/β KO macrophages (Fig. 5A). TKO macrophages loaded more readily than either E/α KO or E/β KO cells, again confirming that both LXR isotypes contribute to macrophage cholesterol efflux. Accordingly, we found that sterol transport-related genes such as ABCA1, ABCG1, and Idol were comparably expressed and responsive to LXR ligand (GW3965) in peritoneal macrophages derived from E/α KO and E/β KO mice (Fig. 5B). Similar results were obtained using naturally occurring oxysterol LXR agonists (supplementary Fig. I). In vitro analysis of LXR agonist-dependent cholesterol efflux revealed no difference in the efflux capacity between primary E/α KO and E/β KO peritoneal macrophages to either ApoA-I or HDL acceptors (Fig. 5C, D). Thus, the difference in lesion formation between E/α KO and E/β KO mice did not correlate with a differential ability of the isolated macrophages to efflux cholesterol.
Fig. 5.
LXRα and LXRβ exhibit comparable ability to control lipid metabolic gene expression and cholesterol efflux in macrophages. (A) Bone marrow-derived macrophages were loaded for 24 h with AcLDL and stained with DAPI (blue) and Bodipy (green). (B) Thioglycollate-elicited peritoneal macrophages from E KO, E/α KO, and E/β KO mice were treated for 24 h with 1 μM GW3965. Gene expression for known LXR targets, ABCA1, ABCG1, and IDOL was measured by real-time PCR. Thioglycollate-elicited peritoneal macrophages from E KO, E/α KO, and E/β KO mice were loaded with [3H]cholesterol (1.0 μCi/ml) in the presence of acyl-CoA:cholestrol O-acyltransferase inhibitor (2 μg/ml) either with DMSO or with ligand for LXR and RXR (1 μM GW3965, 50 nM LG268). Efflux was measured in the presence of (A) ApoA-I or (B) HDL. Experiments were conducted in triplicate. Data are expressed as mean ± SEM. DMSO versus GW+LG *P < 0.05, **P < 0.01; NS, non-significant.
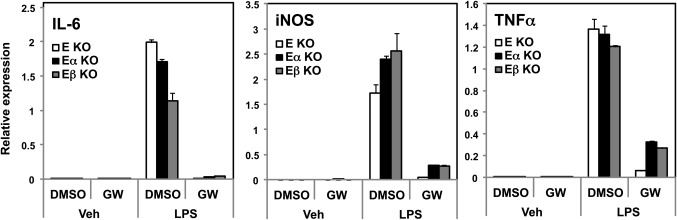
An alternative explanation was that loss of a single LXR isotype might have preferential effect on macrophage inflammatory responses. For example, it has been suggested based on transfection assays that LXRβ may be a stronger repressor of inflammatory gene expression than LXRα (11). We therefore considered the possibility that loss of ApoE expression might uncover unique inflammatory characteristics of each receptor. Peritoneal macrophages were collected, and then treated with ligand and/or stimulated with lipopolysaccharide (LPS). The expression of a panel of inflammatory genes was measured by real-time PCR. In all three genotypes, expression of inducible NO synthase (iNOS), interleukin (IL)-6, and tumor necrosis factor (TNF)α was equivalently stimulated in response to LPS (Fig. 6). Furthermore, the ability of LXR agonist to repress inflammatory gene expression was completely preserved in the absence of a single LXR isotype. These observations suggest that LXRα and LXRβ are functionally redundant in their ability to inhibit endogenous inflammatory gene expression.
Fig. 6.
LXRα and LXRβ exhibit comparable ability to control inflammatory gene expression in macrophages. Thioglycollate-elicited peritoneal macrophages from E KO, E/α KO and E/β KO mice were treated for 24 h with 1 μmol/L GW3965 then stimulate with LPS (100 nM) for 4 h. Gene expression of inflammatory genes was examined by real-time PCR.
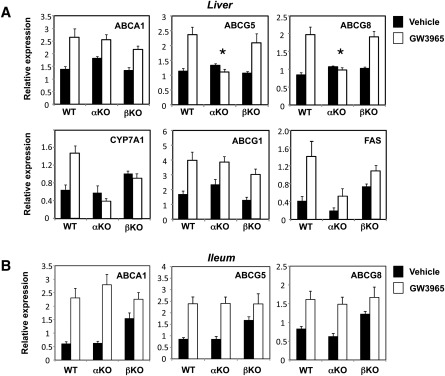
In addition to their function in effluxing cholesterol from peripheral cells, LXRs have been implicated in the later (postmacrophage) stages of the RCT pathway. We therefore proceeded to investigate pathways important for RCT in liver and intestine. Consistent with prior work, basal expression of most LXR target genes in E/α and E/β KO mice (with the exception of SREBP-1c and its targets) was not significantly different between control LXRα-deficient and LXRβ-deficient mice in the absence of agonist stimulation (supplementary Fig. II; data not shown) (12, 16, 20). We therefore employed agonist treatment to test the ability of LXR target genes to respond to LXR activation. Mice were treated with vehicle or GW3965 agonist (40 mpk) by gavage for 3 days. Remarkably, the liver was the only tissue in which deletion of LXRα but not LXRβ had a prominent effect on agonist-induced LXR target gene expression (Fig. 7; data not shown). In particular, the expression of ABCG5 and ABCG8, postulated to promote cholesterol secretion into bile, was preserved in LXRβ KO mice, but unresponsive to LXR agonist in LXRα KO mice. Consistent with the original work of Mangenlsdorf and colleagues (9), CYP7A1 expression was also primarily dependent on LXRα expression. Hepatic ABCA1 and ABCG1 expression was not different between genotypes. In the ileum, no significant difference in expression of ABCA1, ABCG5, or ABCG8 was observed between mice lacking LXRα or LXRβ (Fig. 7). Despite the lack of significant difference in basal ABCG5 and ABCG8 mRNA expression in E/α KO and E/β KO mice, compared to controls we did observe reduced basal expression of ABCG8 protein in E/α KO, as well as reduced response to LXR agonist administration (supplementary Fig. III).
Fig. 7.
LXRα-selective hepatic gene expression and postmacrophage RCT. (A, B) Control, LXRα-deficient, and LXRβ-deficient mice (n = 5 per group) were gavaged for 3 days with GW3965 (40 mpk). (A–C) Gene expression was analyzed by real-time PCR. Statistically significant (P < 0.05 by Student t-test) induction by ligand treatment was observed all genes with the exception of ABCG5 and ABCG8 in the LXRα KO livers, as noted by the asterisks. Data are expressed as mean ± SEM.
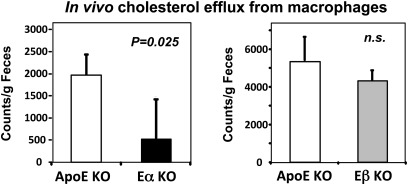
Our gene expression results strongly suggested that the difference in lesion susceptibility of E/α KO and E/β KO mice was linked to a postmacrophage hepatic defect in RCT in E/α KO mice. To provide direct support for this idea, we measured the rate of efflux of labeled cholesterol from lipid-loaded J774 macrophages injected into the peritoneum of ApoE KO mice. The appearance of labeled cholesterol in the feces was markedly reduced in E/α KO compared to ApoE KO mice (Fig. 8). By contrast, there was no difference between E/β KO and ApoE KO mice. Together, our results point to a specific role for hepatic LXRα expression in RTC and suggest that this may be due to its control of hepatic ABCG5 and ABCG8 expression.
Fig. 8.
In vivo cholesterol efflux from radiolabeled macrophages was determined as described in Methods (n = 3 per group). Significance was determined by Student t-test. Data are expressed as mean ± SEM.
DISCUSSION
Dysregulation of cholesterol homeostasis has important consequences for cardiovascular disease risk. Numerous studies have established an integral role for the LXRs in regulating cholesterol metabolism. Activation of LXR induces the transcription of genes involved in cholesterol uptake, excretion, and transport in a tissue-selective manner. Many LXR-dependent pathways are potentially relevant to atherosclerosis, but the relative importance of various processes, for example macrophage cholesterol efflux or hepatic sterol excretion, is unknown. In addition, the tissue-specific functions of the two LXR isotypes remain incompletely understood. Previous work by our laboratory revealed accelerated atherosclerosis and peripheral cholesterol overload in E/α KO mice (12). We hypothesized that this phenotype was most likely due to the fraction loss of LXR activity from the macrophage, given the well-documented importance of macrophage cholesterol efflux in atherogenesis. The observation that the ability of an LXR agonist to inhibit lesion development was preserved in the absence of LXRα provided further support for the idea that both LXRα and LXRβ contribute to maximal macrophage cholesterol efflux (13). Here, we report the unexpected observation that loss of either LXRα or LXRβ alone has differential consequences for peripheral cholesterol overload on the ApoE-null background, pointing to a previously unappreciated quantitative importance of the LXRα-dependent gene expression in atherosclerosis susceptibility in mice.
Our characterization of E/α and E/β mice provides strong support for the idea that LXRα and LXRβ play largely redundant roles in macrophage cholesterol homeostasis and inflammatory gene expression. Both LXRs are fully competent to stimulate cholesterol efflux and to suppress inflammatory mediator production in response to natural and synthetic LXR ligands. Furthermore, loss of both LXRα and LXRβ on the ApoE-null background leads to profound and synergistic effects on peripheral cholesterol accumulation. TKO mice die at a young age and show massive foam-cell accumulation in numerous tissues. At the same time, the fact that E/α KO but not E/β KO mice show a less dramatic but significant cholesterol overload phenotype is inconsistent with our original hypothesis that the macrophage was solely responsible for this phenotype. Loss of either LXRα or LXRβ alone leads to minimal functional deficit in isolated macrophages, suggesting that the phenotype of E/α KO mice must involve LXRα-dependent pathways in another tissue or cell type.
The fact that macrophage cholesterol efflux was not different between E/α KO and E/β KO mice suggested that the process of RCT downstream of macrophage efflux to ApoA-I was compromised in E/α KO mice. Indeed, when wild-type cholesterol-loaded macrophages were used as the source of labeled cholesterol in in vivo RCT assays, we observed a defect in label appearance in the feces in E/α KO but not in E/β KO mice. Collectively, our data suggest that LXRα and LXRβ play redundant roles in macrophage cholesterol efflux, but that LXRα is uniquely important for maximal postmacrophage RCT.
Analysis of LXR-dependent gene expression in a variety of cell types suggested that the liver was the principal tissue in which LXRα has a dominant effect on LXR target gene responses. Whereas loss of either LXRα or LXRβ alone has minimal effect on target gene expression in macrophages and intestine, prominent deficits were observed in liver of LXRα-deficient mice. These observations are consistent with the early characterization of LXRα-deficient mice by Mangelsdorf and colleagues (8, 9). Although LXRs are expressed in several cell types that contribute to peripheral cholesterol efflux and subsequent transport out of the body, including macrophages, hepatocytes, and intestinal epithelium, hepatocytes are the only cell type in which the two LXRs are expressed at dramatically different levels.
Mechanistically, the phenotype of E/α correlates with a specific requirement for LXRα for optimal sterol-dependent ABCG5 and ABCG8 transporter expression in the liver. The liver and intestinal compartments are key areas where these transporters return cholesterol from the enterocytes to luminal cavity for excretion, and ABCG5/8 have previously been shown to be critical for LXR-dependent sterol transport in these tissues (20–24). Perturbations in ABCG5/8 have been linked to excess cholesterol accumulation in mice and humans (22, 25, 26). Interestingly, Lazar and colleagues have previously reported that AAV-mediated expression of LXRα in the liver was sufficient to inhibit atherosclerotic lesion formation in mice (7). This observation is fits well with our analysis of E/α KO mice. Similarly, loss of LXRα and LXRβ on the LDLR background also has differential consequences for atherosclerosis lesion development (11).
Together these findings underscore the critical importance of LXR signaling for whole-body cholesterol homeostasis, as loss of both LXRs on the ApoE background leads to early lethality. Our results further expand our understanding of the contributions of the different LXR isotypes to atherosclerosis susceptibility and their tissue-selective functions in sterol homeostasis.
Acknowledgments
The authors are grateful to Dr. Helen Hobbs (USTW) for the ABCG8 antibody.
Footnotes
Abbreviations:
- E/α KO
- (knockout) mice, ApoE−/−LXRα−/− mice
- E/β KO
- (knockout) mice, ApoE−/−LXRβ−/− mice
- LXR
- liver X receptor
- RCT
- reverse cholesterol transport
- RXR
- retinoid X receptor
- TKO
- (total knockout) mice, ApoE−/−LXRα−/−LXRβ−/− mice
This work was supported in part by National Institutes of Health Grants HL-030568 and HL-066088. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. P. Tontonoz is an investigator of the Howard Hughes Medical Institute. A. M. Fogelman and S. T. Reddy are principals in Bruin Pharma, and A. M. Fogelman is an officer in Bruin Pharma.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of three figures.
REFERENCES
- 1.Glass C. K., Witztum J. L. 2001. Atherosclerosis. The road ahead. Cell. 104: 503–516 [DOI] [PubMed] [Google Scholar]
- 2.Cuchel M., Rader D. J. 2006. Macrophage reverse cholesterol transport: key to the regression of atherosclerosis? Circulation. 113: 2548–2555 [DOI] [PubMed] [Google Scholar]
- 3.Rader D. J., Alexander E. T., Weibel G. L., Billheimer J., Rothblat G. H. 2009. The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. J. Lipid Res. 50(Suppl.): S189–S194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang X., Collins H. L., Ranalletta M., Fuki I. V., Billheimer J. T., Rothblat G. H., Tall A. R., Rader D. J. 2007. Macrophage ABCA1 and ABCG1, but not SR-BI, promote macrophage reverse cholesterol transport in vivo. J. Clin. Invest. 117: 2216–2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.A-Gonzalez N., Bensinger S. J., Hong C., Beceiro S., Bradley M. N., Zelcer N., Deniz J., Ramirez C., Diaz M., Gallardo G., et al. 2009. Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity. 31: 245–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zelcer N., Tontonoz P. 2006. Liver X receptors as integrators of metabolic and inflammatory signaling. J. Clin. Invest. 116: 607–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lehrke M., Lebherz C., Millington S. C., Guan H. P., Millar J., Rader D. J., Wilson J. M., Lazar M. A. 2005. Diet-dependent cardiovascular lipid metabolism controlled by hepatic LXRalpha. Cell Metab. 1: 297–308 [DOI] [PubMed] [Google Scholar]
- 8.Peet D. J., Janowski B. A., Mangelsdorf D. J. 1998. The lxrs: A new class of oxysterol receptors. Curr. Opin. Genet. Dev. 8: 571–575 [DOI] [PubMed] [Google Scholar]
- 9.Peet D. J., Turley S. D., Ma W., Janowski B. A., Lobaccaro J. M., Hammer R. E., Mangelsdorf D. J. 1998. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXR alpha. Cell. 93: 693–704 [DOI] [PubMed] [Google Scholar]
- 10.Tontonoz P., Mangelsdorf D. J. 2003. Liver X receptor signaling pathways in cardiovascular disease. Mol. Endocrinol. 17: 985–993 [DOI] [PubMed] [Google Scholar]
- 11.Bischoff E. D., Daige C. L., Petrowski M., Dedman H., Pattison J., Juliano J., Li A. C., Schulman I. G. 2010. Non-redundant roles for LXRalpha and LXRbeta in atherosclerosis susceptibility in low density lipoprotein receptor knockout mice. J. Lipid Res. 51: 900–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bradley M. N., Hong C., Chen M., Joseph S. B., Wilpitz D. C., Wang X., Lusis A. J., Collins A., Hseuh W. A., Collins J. L., et al. 2007. Ligand activation of LXR beta reverses atherosclerosis and cellular cholesterol overload in mice lacking LXR alpha and apoE. J. Clin. Invest. 117: 2337–2346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tangirala R. K., Bischoff E. D., Joseph S. B., Wagner B. L., Walczak R., Laffitte B. A., Daige C. L., Thomas D., Heyman R. A., Mangelsdorf D. J., et al. 2002. Identification of macrophage liver X receptors as inhibitors of atherosclerosis. Proc. Natl. Acad. Sci. USA. 99: 11896–11901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joseph S. B., McKilligin E., Pei L., Watson M. A., Collins A. R., Laffitte B. A., Chen M., Noh G., Goodman J., Hagger G. N., et al. 2002. Synthetic LXR ligand inhibits the development of atherosclerosis in mice. Proc. Natl. Acad. Sci. USA. 99: 7604–7609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joseph S. B., Bradley M. N., Castrillo A., Bruhn K. W., Mak P. A., Pei L., Hogenesch J., O'Connell R. M., Cheng G., Saez E., et al. 2004. LXR-dependent gene expression is important for macrophage survival and the innate immune response. Cell. 119: 299–309 [DOI] [PubMed] [Google Scholar]
- 16.Hong C., Walczak R., Dhamko H., Bradley M. N., Marathe C., Boyadjian R., Salazar J. V., Tontonoz P. 2011. Constitutive activation of LXR in macrophages regulates metabolic and inflammatory gene expression: identification of ARL7 as a direct target. J. Lipid Res. 52: 531–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marathe C., Bradley M. N., Hong C., Lopez F., Ruiz de Galarreta C. M., Tontonoz P., Castrillo A. 2006. The arginase ii gene is an anti-inflammatory target of liver X receptor in macrophages. J. Biol. Chem. 281: 32197–32206 [DOI] [PubMed] [Google Scholar]
- 18.Zhang S. H., Reddick R. L., Piedrahita J. A., Maeda N. 1992. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 258: 468–471 [DOI] [PubMed] [Google Scholar]
- 19.Villanueva C. J., Waki H., Godio C., Nielsen R., Chou W. L., Vargas L., Wroblewski K., Schmedt C., Chao L. C., Boyadjian R., et al. 2011. TLE3 is a dual-function transcriptional coregulator of adipogenesis. Cell Metab. 13: 413–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Repa J. J., Berge K. E., Pomajzl C., Richardson J. A., Hobbs H., Mangelsdorf D. J. 2002. Regulation of ATP-binding cassette sterol transporters ABCG5 and ABCG8 by the liver X receptors alpha and beta. J. Biol. Chem. 277: 18793–18800 [DOI] [PubMed] [Google Scholar]
- 21.Yu L., Gupta S., Xu F., Liverman A. D., Moschetta A., Mangelsdorf D. J., Repa J. J., Hobbs H. H., Cohen J. C. 2005. Expression of ABCG5 and ABCG8 is required for regulation of biliary cholesterol secretion. J. Biol. Chem. 280: 8742–8747 [DOI] [PubMed] [Google Scholar]
- 22.Yu L., von Bergmann K., Lutjohann D., Hobbs H. H., Cohen J. C. 2004. Selective sterol accumulation in ABCG5/ABCG8-deficient mice. J. Lipid Res. 45: 301–307 [DOI] [PubMed] [Google Scholar]
- 23.Yu L., York J., von Bergmann K., Lutjohann D., Cohen J. C., Hobbs H. H. 2003. Stimulation of cholesterol excretion by the liver X receptor agonist requires ATP-binding cassette transporters G5 and G8. J. Biol. Chem. 278: 15565–15570 [DOI] [PubMed] [Google Scholar]
- 24.Pawar A., Botolin D., Mangelsdorf D. J., Jump D. B. 2003. The role of liver X receptor-alpha in the fatty acid regulation of hepatic gene expression. J. Biol. Chem. 278: 40736–40743 [DOI] [PubMed] [Google Scholar]
- 25.Hubacek J. A., Berge K. E., Cohen J. C., Hobbs H. H. 2001. Mutations in ATP-cassette binding proteins G5 (Abcg5) and G8 (Abcg8) causing sitosterolemia. Hum. Mutat. 18: 359–360 [DOI] [PubMed] [Google Scholar]
- 26.Yu L., Hammer R. E., Li-Hawkins J., Von Bergmann K., Lutjohann D., Cohen J. C., Hobbs H. H. 2002. Disruption of ABCG5 and ABCG8 in mice reveals their crucial role in biliary cholesterol secretion. Proc. Natl. Acad. Sci. USA. 99: 16237–16242 [DOI] [PMC free article] [PubMed] [Google Scholar]