Abstract
Normally, cell proliferation and death are carefully balanced in higher eukaryotes, but one of the most important regulatory mechanisms, apoptosis, is upset in many malignancies, including hepatocellular-derived ones. Therefore, reinforcing cell death often is mandatory in anticancer therapy. We previously reported that a combination of tumor necrosis factor-α (TNF) and cycloheximide (CHX) efficiently kill HTC cells, a rat hepatoma line, in an apoptosis-like mode. Death is actively mediated by the lysosomal compartment, although lysosomal ceramide was previously shown not to be directly implicated in this process. In the present study, we show that TNF/CHX increase lysosomal ceramide that is subsequently converted into sphingosine. Although ceramide accumulation does not significantly alter the acidic compartment, the sphingosine therein generated causes lysosomal membrane permeabilization (LMP) followed by relocation of lysosomal cathepsins to the cytoplasm. TNF/CHX-induced LMP is effectively abrogated by siRNAs targeting acid sphingomyelinase or acid ceramidase, which prevent both LMP and death induced by TNF/CHX. Taken together, our results demonstrate that lysosomal accumulation of ceramide is not detrimental per se, whereas its degradation product sphingosine, which has the capacity to induce LMP, appears responsible for the observed apoptotic-like death.
Keywords: ceramide, hepatocellular carcinoma, lysosomes, sphingolipids, tumor necrosis factor-α
A tightly controlled balance between cellular proliferation and death is the basis for tissue homeostasis in multicellular organisms. Dysregulation of this poise results in abnormal production or loss of cells, accounting for common diseases such as neurodegenerative disorders and malignancies. Malignant transformation would not occur in the presence of intact cell suicide mechanisms. However, due to mutations in oncogenes and tumor suppressor genes, malignancies are often characterized by increased resistance to apoptogenic agents rather than by enhanced proliferation (reviewed in Ref. 1). Liver malignancies are not exceptions (2), and hence, resistance to programmed cell death (PCD) supports their substantial lack of sensitivity to irradiation and anticancer drugs (3). Therefore, many efforts have been devoted to develop new strategies for the treatment of malignant hepatomas that cannot be surgically removed. The cytotoxic cytokine tumor necrosis factor-α (TNF), a member of a family of ligands for a number of death receptors that also includes FASL and TRAIL, is of interest for its partial selectivity for malignant cells (4, 5). Ligation of death receptors results in a cascade of events that is eventually followed by PCD (6). For some cytokines, e.g., FASL, the molecular events spanning from receptor engagement to cell death have been detailed, whereas for TNF, the precise signaling still remains elusive. TNF effects differ depending on variations in metabolism and content of pro- and antiapoptotic proteins among cell types (7, 8). Besides classical caspase-dependent apoptosis and other forms of death, TNF-induced PCD can take place through caspase-independent, apoptosis-like processes that exploit alternative mechanisms involving lysosomal membrane permeabilization (LMP). Although already hinted at long ago (9), the involvement of LMP in apoptosis was long overlooked, but nowadays it is generally acknowledged (10–18).
TNF-induced cytotoxicity was also found to involve formation of ceramide, achieved by involvement of acid or neutral sphingomyelinases (aSMase, nSMase) or activation of the de novo pathway, yet the role of these enzymes is debated (19, 20). Recently, the role played by ceramide in TNF cytotoxicity was reevaluated because during TNF-induced PCD, sphingolipids other than ceramide, such as sphingosine, are also affected (21–25). As the latter is a split product of ceramide, it is often not easy to discriminate between the effects of these two sphingolipids.
In a previous article, we described that, in rat hepatoma-derived HTC cells, TNF in association with cycloheximide (CHX) induces an apoptosis-like death that involves the lysosomal compartment (26). As this death was prevented by a putative aSMase inhibitor, desipramine (Dpm), we next investigated whether a ceramide-related mechanism is involved in it (23). The results indicated that ceramide-induced death mechanistically differs from that triggered by TNF/CHX, thus ruling out a direct role of ceramide in the latter. However, the results from these studies (23, 26) did not satisfactorily answer the question as to whether TNF treatment alters cellular levels of ceramide or other sphingolipids that may be involved in TNF-induced death. This issue is addressed in the present work, showing that ligation of the TNF receptor triggers the generation of ceramide and sphingosine, and that the lysosomotropic and detergent properties of the latter induce LMP, thereby mediating HTC cell death.
MATERIALS AND METHODS
Reagents
Unless otherwise indicated, chemicals were from Sigma-Aldrich (Milan, Italy). Human recombinant TNF-α was from R & D Systems (Minneapolis, MN). CHX and Dpm were dissolved in sterile water, C2-ceramide (C2-cer) and sphingosine (Sph) in DMSO. Vehicles alone were added in control cultures. The anti-neutral sphingomyelinase 2 (nSMase; H-195, sc-67305) and anti-cathepsin B antibodies (CB; C-19, sc-6490) were from Santa Cruz Biotechnology (Heidelberg, Germany); the anti-acid sphingomyelinase (aSMase; AP12227b) from Abgent (Oxfordshire, UK); the anti-acid ceramidase (aCDase; 612302, clone 23) from BD Biosciences (Buccinasco, Milan, Italy); and the anti-β-actin (clone AC-15) from Sigma-Aldrich (Milan, Italy).
Cell cultures
The rat hepatoma cell line HTC was routinely grown in a 1:1 mixture of DMEM/Ham's F12 medium (Sigma-Aldrich), containing 10% FBS (Gibco, Milan, Italy), 2 mM glutamine, 100 U/ml penicillin, and 100 µg/ml streptomycin in humidified air with added 5% CO2. The human hepatoma cell lines Hep G2 and Huh7 were grown in DMEM (Sigma-Aldrich), the SK-HEP-1 cells in DMEM/Ham's F12 medium. Culture media were supplemented with 10% FBS (Gibco), 2 mM glutamine, 100 U/ml penicillin, and 100 µg/ml streptomycin. For experiments, HTC cells were seeded at 104 cells/cm2, Hep G2 and SK-HEP-1 at 1.5 × 104 cells/cm2, Huh7 at 2 × 104 cells/cm2 and exposed 24 h later to 20 ng/ml TNF/10 µg/ml CHX for different times.
Annexin V/propidium iodide staining and FACS analysis
The percentage of apoptotic cells was estimated by counting the phosphatidylserine (PS)-positive/propidium iodide (PI)-negative fraction using the Annexin V/PI kit (ImmunoStep, Salamanca, Spain). Adherent cells were detached with 0.25% trypsin and collected together with floating ones, centrifuged, and resuspended in 190 µl of binding buffer (10 mM HEPES-NaOH pH 7.4, 140 mM NaCl, 2.5 mM CaCl2). Annexin V and PI were then added. At least 5,000 cells were analyzed in a FACScan equipped with a 488 nm argon laser (Becton-Dickinson, Milan, Italy) using the CellQuest software (Becton-Dickinson).
Sphingolipid LC/MS
Extracts for sphingolipid determination were prepared from samples containing about 2 mg protein as described elsewhere (27). Before extraction, lysates were probed with internal standards (0.2 nmol each of C17-sphinganine, N-dodecanoylsphingosine, N-dodecanoylglucosylsphingosine, and N-dodecanoyl sphingosylphosphorylcholine) to normalize the lipid extraction processes. The analyses were performed on a Waters Aquity UPLC system linked to a Waters LCT Premier orthogonal accelerated-TOF mass spectrometer (Waters, Millford, MA). The analytical LC column was a 100 × 2.1 mm, 1.7 µm C8 Aquity UPLC BEH (Waters), operated with the mobile phases and the gradient conditions as specified in Ref. 27. For MS-TOF, mass accuracy and reproducibility were ensured using an independent reference spray via LockSpray. The range of linearity was determined by sampling standard sphingolipid mixtures. Identification of the sphingolipid species was achieved by comparison of the mass and LC retention times of significant peaks with relevant standards.
Acridine orange uptake test
Lysosomal membrane permeabilization was assayed with the acridine orange (AO) uptake technique (28). Following treatments, cells were exposed to AO (5 µg/ml) for 15 min at 37°C in complete growth medium. Both attached and floating cells were collected and resuspended in 200 µl PBS. Red AO-induced fluorescence (from highly concentrated AO in intact lysosomes) was measured by FACS as described above. When appropriate, AO-stained cells were observed under a fluorescence microscope (see below). Digital images were recorded with an AxioCam CCD camera and composed with Photoshop 7.0 (Adobe Systems Inc., San Jose, CA).
Cathepsin B immunofluorescence
Immunofluorescent detection of subcellular localization of cathepsin B was performed essentially as described (29, 30) on cells seeded at 8 × 103 cells/cm2 on six-channel µ-Slides VI (Ibidi GmbH, Planegg/Martinsried, Germany). Following treatments, cells were fixed in each growth channel for 20 min at −20°C with methanol:acetone (1:1), incubated overnight at 4°C with the anti-CB antibody (1:100 in TBS-0.5% Tween) and, following three washes with PBS +Triton 0.5%, incubated for 1 h with a Cy3-conjugated anti-goat IgG (1:500 in TBS-0.5% Tween). After three additional washes as above, slides were observed under a fluorescence microscope (see below). Representative images were acquired and composed with Photoshop 7.0.
RNA interference
Gene expression silencing was achieved by RNA interference technology. For HTC cells, 100 nM of the indicated MISSION predesigned siRNAs (Sigma-Aldrich) were transfected for 24 h using Metafectene Pro (Biontex, Martinsried/Planegg, Germany) in antibiotic-free medium: aSMase, 5′-GAACAUAGCGCCACUAAAU-3′ nSMase, 5′-CUUUGGCUAUGUGAUGAUU-3′ aCDase, 5′-CCAAGAAUAUAUUGACCAA-3′ and nCDase, 5′-GAUAGAGCACCAAUAGGCA-3′. For human hepatoma cells, the following sequences were employed: aSMase, 5′-CCCAAAUGCCUGUGGUU-3′ nSMase, 5′-CGCAUUGACUACGUGCUUU-3′ aCDase, 5′-CUGUUCCAGUCUUACACUGA-3′ and nCDase, 5′-CAGUGUUUGUCAGCAUCGA-3′. Scrambled siRNAs (Eurofins MWG Operons, Ebersberg, Germany) were used as controls. Target-specific and control siRNAs were transfected at the final concentration of 100 nM for 18 h (Huh7 cells) and 200 nM for 24 h (SK-HEP-1 and Hep G2 cells) with Metafectene Pro in antibiotic-free medium. Then cells were treated with TNF/CHX for 6 or 24 h for HTC/Huh7 and Hep G2/SK-HEP-1 cells, respectively, and either assayed for cell death and LMP by FACS or stained with 5 µg/ml AO for fluorescence microscopy analysis. Effectiveness of silencing was verified by Real-Time PCR using the iQ SYBR Green Supermix (Bio-Rad Laboratories, Milan, Italy); the relative change of the mRNA levels of each target was calculated using the 2−ΔΔCT equation with respect to GAPDH levels for both rat and human cells.
RNA extraction and cDNA synthesis
Total RNA was extracted with the TriPure Isolation Reagent (Roche, Milan, Italy), dissolved in sterile water, and quantified spectrophotometrically. cDNA was synthesized from 1 µg of total RNA with the Reverse Transcription System kit (Promega Italia, Milan, Italy) in a final volume of 20 µl. A volume corresponding to 50 ng of total RNA was used for each amplification reaction.
Transfection of cathepsin B-GFP
The cathepsin B-GFP-encoding plasmid (15) was kindly provided by Prof. G. Gores (Mayo Clinic College of Medicine, Rochester, MN). For fluorescence microscopy, the HTC cells were seeded at 8 × 103 cells/cm2 on six-channel µ-Slides VI and transfected by Lipofectamine 2000 (Invitrogen, San Giuliano Milanese, Italy) 18 h later. For each slide, 2 µg pEGFP-CatB was mixed with 3 µl Lipofectamine 2000 in a final volume of 200 µl Opti-MEM I (Gibco) and incubated for 20 min at room temperature. Subsequently, 30 µl of the complexes were added to each channel of the µ-Slides VI in antibiotic-free medium. Next day, cells were returned to standard culture conditions and grown for another 24 h before further treatments.
Western blotting
Cell pellets were sonicated in PBS containing 1% Igepal CA-630, 0.5% sodium deoxycholate, and 0.1% SDS; 20–70 µg of total lysates, as determined with the Bio-Rad Protein Assay Dye Reagent Concentrate (Bio-Rad Laboratories), were incubated for 10 min at 37°C with 20 µg/ml DNase and RNase and resolved on a 10% (a- and nSMase) or 15% (aCDase) PAGE. For aCDase only, resolved proteins were transferred to 0.2 µm Trans-Blot Transfer Medium (Bio-Rad Laboratories) in a buffer containing 25 mM Tris-Cl, 193 mM glycine, 20% methanol, pH 8.3, supplemented with 2 mM CaCl2. The membrane was fixed for 1 h at room temperature with 2.5% glutaraldehyde, washed twice with PBS, and once with 50 mM ethanolamine in PBS. All the membranes were then saturated for 1 h with TBS 0.05% Tween containing 5% milk and incubated overnight at 4°C with the primary antibodies. Blots were washed three times with TBS-Tween and incubated with the appropriate secondary antibody (1:10000, Bio-Rad Laboratories). The total amount of protein loaded in each lane was revealed by probing the same blots with an anti-β-actin antibody. Chemiluminescent detection of bands was performed with the Western Blotting Luminol Reagent (Santa Cruz Biotechnology). For densitometric analysis, the TotalLab TL100 Analytical software (Nonlinear Dynamics, Ver. 2006c, Newcastle upon Tine, UK) was used.
Microscopy
Cells were observed and photographed in the phase contrast mode under a Nikon Eclipse TS 1000 inverted microscope equipped with a Nikon Coolpix 4500 digital camera. Fluorescence images were captured with an Axiovert 35 inverted fluorescence microscope equipped with a 40× long distance objective (Carl Zeiss MicroImaging GmbH, Munich, Germany) connected to an AxioCam camera using AxioVision software (Carl Zeiss MicroImaging GmbH) and composed with Photoshop 7.0.
Statistical analysis
If not otherwise indicated, data represent means ± SD from at least three independent experiments, each assayed in triplicate. Differences between groups were analyzed by one-way ANOVA followed by Student-Newman-Keuls post test (Instat, GraphPad, San Diego, CA).
RESULTS
TNF/CHX alter ceramide and sphingosine levels in HTC cells
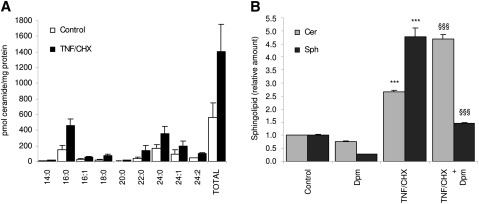
We previously reported that TNF/CHX-induced death requires a properly functioning lysosomal compartment (26). As TNF toxicity and the lysosomal compartment are linked to each other by the production of ceramide (16), the amount of this lipid messenger was screened. LC/MS confirmed that TNF/CHX increased the cellular concentration of various forms of ceramide, the most abundant being the 16:0 and the 24:0 species (Fig. 1A). In addition to ceramides, TNF/CHX also elevated sphingosine (Fig. 1B), another sphingolipid known to be increased by TNF to induce cell death (21, 22). These results confirm that TNF modulates the metabolism of sphingolipids, markedly increasing both ceramides and sphingosine levels. In a previous study, we demonstrated that the toxic action of TNF and ceramide rely on different pathways (23). We also evidenced that Dpm significantly protects from TNF/CHX toxicity. Because this drug is a known functional inhibitor of the acid sphingomyelinase (aSMase), we investigated whether this protection was secondary to a change in sphingolipid levels. LC/MS evidenced that Dpm, whose specificity for aSMase is questionable (31), further enhanced TNF/CHX-induced ceramide accumulation (Fig. 1B), thus strengthening the finding that protection afforded by this drug does not depend upon ceramide decrease. In contrast, Dpm almost totally abrogated the TNF/CHX-induced generation of sphingosine, the concentration of which fell to control levels. Therefore, the sphingolipid fluctuations confirm that protection by Dpm reflects its interference with the formation and accumulation of sphingosine, rather than of ceramide, in the acidic compartment.
Fig. 1.
TNF/CHX increase ceramide and sphingosine. HTC cells were exposed (or not) for 6 h to TNF/CHX. All cells, including floating ones, were collected, washed twice with PBS, and then centrifuged. The pellets were lyophilized overnight and then stored at 4°C until LC/MS sphingolipid profiling was performed. (A) Cellular amount of ceramides with different acyl chains. (B) Relative changes in total ceramide (Cer) and sphingosine (Sph); where indicated, cells were treated with TNF/CHX in the presence or not of 50 µM desipramine (Dpm). Data represent the means ± SD from three independent measurements. ***P < 0.001 versus control cells; §§§P < 0.001 versus TNF/CHX.
In conclusion, the results presented demonstrate that TNF/CHX increase both ceramide and sphingosine in the acidic compartment and confirm our previous findings that ceramide does not mediate TNF/CHX toxicity in HTC cells.
Sphingosine mediates TNF/CHX-induced cytotoxicity by triggering lysosomal membrane permeabilization
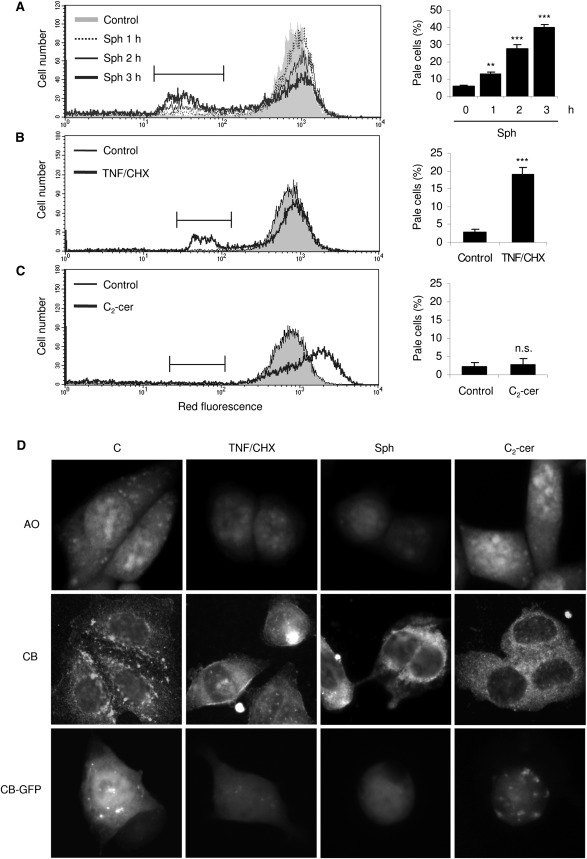
The mechanisms by which sphingosine mediates TNF/CHX-induced toxicity were subsequently analyzed. As sphingosine toxicity has been reported to rely on induction of LMP (22), occurrence of the latter was next investigated. HTC cells were found sensitive to sphingosine, which determined the onset of the classical signs of LMP, namely, the appearance of a population of cells with a reduced number of intact lysosomes, accounting for the lower intracellular red fluorescence following exposure to AO (Fig. 2A); such cells, whose number increases time-dependently, are referred to as “pale” cells (28). It was then observed that TNF/CHX treatment also raised a clearly defined population of pale cells, indicating that LMP occurs in HTC cells treated with the cytokine (Fig. 2B). However, no evidence of LMP was found in cells treated with C2-cer (Fig. 2C), demonstrating that TNF/CHX-induced LMP does not result from intralysosomal ceramide accumulation and thus supporting a role for sphingosine. As a further confirmation, the integrity of lysosomal compartment of HTC cells treated with TNF/CHX, sphingosine, or C2-cer was evaluated by AO staining and visualization under a fluorescence microscope (Fig. 2D, top panels). AO-stained lysosomes were abundant and intact in controls, but they almost vanished in both TNF/CHX- and sphingosine-treated cells, which is suggestive of low lysosomal dye accumulation due to occurrence of LMP. By contrast, C2-cer-treated cells showed extensive AO uptake (also see Fig. 2C) and no LMP. We subsequently verified whether, in addition to increasing the permeability to small molecules like AO, TNF/CHX- or sphingosine-induced LMP would grant relocation to the cytoplasm of higher molecular weight lysosomal contents. LMP was thus evaluated also by immunofluorescent detection of subcellular localization of cathepsin B (CB; Fig. 2D, middle panels). In control cells, CB was detected in discrete fluorescent dots representing intact lysosomes. Both TNF/CHX and Sph, but not C2-cer, shifted the punctate fluorescence of controls to diffuse, rather indicative of CB release from permeabilized lysosomes and of its diffusion to the cytoplasm. In C2-cer-treated cells, however, CB immunofluorescence displayed again as a fine cytoplasmic granularity similar to that of control cells. To further support the above findings, HTC cells were transiently transfected with a cathepsin B-GFP (CB-GFP) construct (Fig. 2D, bottom panels). In control cells, the resulting green fluorescence predominantly clustered in a number of granules, conferring a punctate pattern characteristic of cells with intact lysosomes. Exposure to TNF/CHX or sphingosine, but not to C2-cer, changed this pattern to a diffuse one, indicative of lysosomal-cytoplasmic relocation of CB-GFP. The results thus demonstrate that sphingosine, but not ceramide, induces a LMP that allows relocation of lysosomal contents to the cytoplasm.
Fig. 2.
TNF/CHX and sphingosine, not C2-ceramide, induce both LMP and relocation of lysosomal contents in HTC cells. Cells were exposed to 25 µM sphingosine for the indicated times (A), TNF/CHX for 6 h (B), or 50 µM C2-cer for 24 h (C), stained for 15 min with 5 µg/ml AO, and analyzed by flow cytometry. Cytograms are representative of at least three independent experiments. The fluorescence peak of control cells was set approximately at 103 and retained for all measurements. “Pale” cells, representing cells with fewer than normal intact lysosomes, accumulate to the left of the main peak, just below intensity 102. Quantification of pale cells is shown as bar graphs on the right of each cytogram. (D) For morphological evaluation of lysosomal integrity, HTC cells were exposed to TNF/CHX for 6 h, 25 µM sphingosine for 3 h, or 50 µM C2-cer for 24 h as indicated, either loaded with AO (top panels) or fixed with methanol-acetone, stained with an anti-CB antibody (middle panels), and observed under a fluorescence microscope. Intact lysosomes appear as a network of fluorescent discrete dots both in AO and anti-CB-stained cells. LMP abrogates AO punctation of cells and brings about diffusion of CB immunofluorescence to the whole cell, suggestive of its release from lysosomes to the cytoplasm. Alternatively, cells were transfected with a vector encoding cathepsin B-GFP (bottom panels), which localizes clearly, though not exclusively, to the lysosomes in control cells. TNF/CHX and Sph, but not C2-cer, induce LMP that turns CB-GFP fluorescence from punctate to diffuse, also indicating its lysosomal-cytoplasmic release. Numerical results represent the mean ± SD of at least three independent experiments. **P < 0.01 and ***P < 0.001, respectively, versus controls; n.s. not significantly different versus control cells. Sph: 25 µM sphingosine ; C2-cer: 50 µM C2-cer.
Interference with generation of sphingosine protects from TNF/CHX toxicity
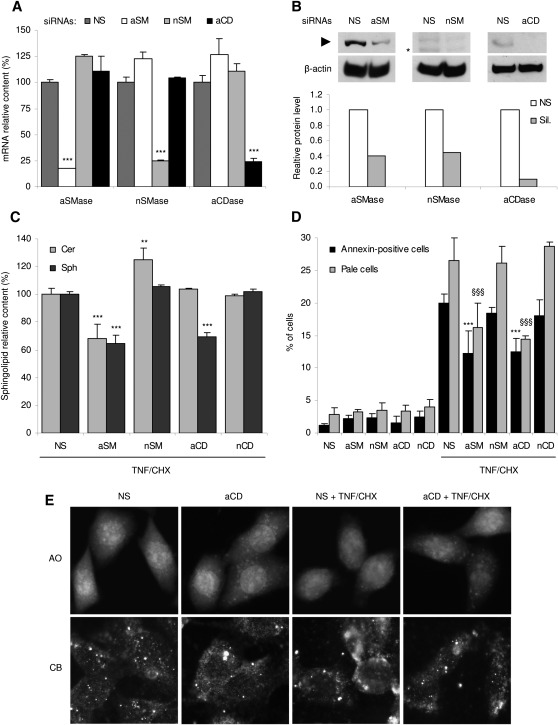
Next it was investigated whether decreasing lysosomal sphingosine or its precursor ceramide protects from TNF/CHX. To this aim, siRNAs targeting acid and neutral sphingomyelinase (a- and nSMase) and ceramidase (a- and nCDase) were transfected into HTC cells for 24 h before TNF/CHX treatment. siRNAs reduced the amount of their specific mRNA targets by about 75–80% without affecting other transcripts (Fig. 3A) and the content of their related proteins by about 60–80% (Fig. 3B). Remarkably, in these cells, the nCDase mRNA was undetectable, whereas it was abundantly present in a total rat liver RNA preparation used as a control (not shown). This finding thus reveals that nCDase is not present in these hepatoma cells. As expected, silencing changed the sphingolipid levels: both ceramide and sphingosine were markedly reduced by knockdown of aSMase, but they were almost unaffected by silencing nSMase (Fig. 3C). aCDase silencing selectively depressed sphingosine, whereas in agreement with the lack of nCDase expression in HTC cells, siRNAs against the latter enzyme did not affect the levels of ceramide or sphingosine, ruling out any activation of the neutral pathway of ceramide breakdown following TNF/CHX treatment.
Fig. 3.
Effect of modulation of the sphingolipid content by RNA interference on TNF/CHX-induced LMP and death of HTC cells. (A) HTC cells were transfected for 24 h with 100 nM siRNAs targeting the neutral and acidic forms of rat SMase and CDase. The effectiveness of silencing of each target was determined with respect to cells transfected with nonspecific siRNAs, following normalization with the GAPDH mRNA. (B) The effect of silencing on the various target proteins was detected by Western blotting (upper panels, arrowhead), normalized with respect to β-actin (lower panels). The asterisk represents a nonspecific band detected by the anti-nSMase antibody. Histograms show the relative amount of each protein in silenced samples (gray bars) with respect to cells transfected with control siRNAs (open bars). (C) Levels of ceramide and sphingosine were measured by LC/MS in cells silenced as in (A) and treated with TNF/CHX for 6 h. Relative changes in the sphingolipid amount brought about by gene silencing are expressed with respect to cells transfected with control siRNAs and treated with TNF/CHX for 6 h; for these samples, the levels of ceramide and sphingosine were 880.7 and 21.4 pmoles/mg protein, respectively. (D) HTC were transfected as indicated, left untreated or exposed to TNF/CHX for 6 h. Cells from supernatants and monolayers were then collected, pooled, and analyzed for annexin V exposure (dark bars) or pale cells detection (light bars) by FACS. (E) Cells transfected with control or aCDase siRNAs were treated or not with TNF/CHX for 6 h, then stained with either AO (top panels) or anti-CB antibody (bottom panels) as in Fig. 2 and observed under a fluorescence microscope. Intact lysosomes appear as intensely fluorescent cytoplasmic or perinuclear dots, whereas those permeabilized by TNF/CHX in mock-silenced cells (NS plus TNF/CHX) are barely detectable on a more intensely fluorescent cytoplasmic background due to both acridine orange diffuse in the cytoplasm and immunofluorescence of CB released from lysosomes. Of interest, aCDase silencing restores the punctate pattern of intact lysosomes as evidenced by both AO staining and CB immunofluorescence. Results are means ± SD of three independent experiments, each assayed in triplicate (A, C, D) or as means of two independent experiments (B). NS: control siRNAs; aSM, nSM, aCD, nCD: acid and neutral sphingomyelinase and ceramidase siRNAs. aSMase, nSMase, aCDase: acid or neutral sphingomyelinase and acid ceramidase. ***P < 0.001 versus nonspecific siRNAs (A) or versus cells transfected with nonspecific siRNAs and exposed to TNF/CHX (C); **P < 0.01 versus TNF/CHX plus controls siRNAs (C); ***P < 0.001 versus TNF/CHX plus controls siRNAs for annexin V/PI assay and §§§P < 0.001 versus TNF/CHX plus controls siRNAs for pale cells assay (D).
To verify the effects of the sphingolipid modulation on TNF/CHX toxicity, silenced cells were exposed for 6 h to the cytokine and then analyzed for annexin V-binding and LMP. Although exposure to neither control nor specific siRNAs was intrinsically toxic, knockdown of the acidic SMase and CDase significantly reduced both PS externalization and the appearance of pale cells (Fig. 3D). aCDase silencing also increased the morphological integrity of lysosomal compartment, as demonstrated by both the restored AO uptake (Fig. 3E, upper panels) and the reversion to a punctate pattern of CB immunofluorescence (Fig. 3E, lower panels), as a consequence of attenuation of TNF/CHX-induced LMP. These results thus demonstrate that preventing the formation of sphingosine in the lysosomal compartment markedly attenuates LMP and TNF/CHX-induced death.
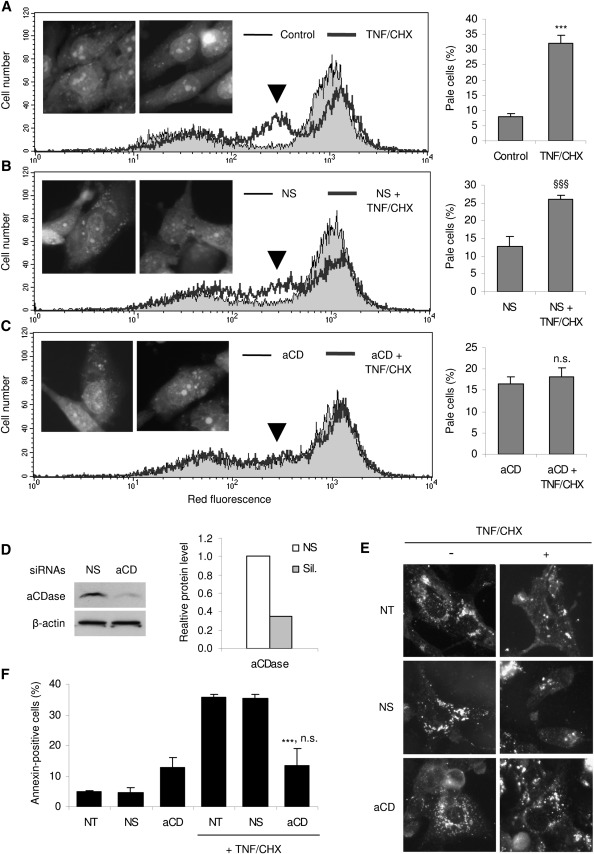
We subsequently investigated whether these observed lysosomal mechanisms are peculiar to HTC cells or may also occur in other hepatoma cells of human origin, such as the SK-HEP-1, Hep G2, and Huh7 lines. SK-HEP-1 cells, in particular, exhibited pronounced LMP (about 30% of total cell population; see histograms in Fig. 4A, right) and lysosomal alterations following TNF/CHX treatment for 24 h (Fig. 4A, cytogram and inserts). This effect, although not significantly modified by control siRNAs (Fig. 4B, plus inserts and histograms), was prevented by siRNAs targeting aCDase (Fig. 4C, plus inserts and histograms), which reduced the aCDase protein by about 60% (Fig. 4D). As a further confirmation, aCDase-specific siRNAs, yet not the scrambled ones, effectively reverted the cytoplasmic CB relocation brought about by TNF/CHX, as evidenced by immunofluorescence analysis of its subcellular localization (Fig. 4E). Eventually, aCDase silencing strikingly attenuated death of SK-HEP-1 cells, as demonstrated by the reduced number of annexin V-positive cells determined by TNF/CHX. This finding, in particular, demonstrates that LMP and ensuing PCD brought about by TNF-induced sphingosine generation in the acidic compartment is of physiological relevance, rather than a general response to amphiphilic compounds. LMP was also observed in both Hep G2 and Huh7 cell lines, yet it was not significantly modified by aCDase silencing (not shown), thus reflecting the heterogeneity of hepatoma cells responses to death-inducing agents.
Fig. 4.
aCDase silencing abrogates TNF/CHX-induced LMP in SK-HEP-1 cells. SK-HEP-1 cells that were nontransfected (A), or transfected with control (B), or aCDase siRNAs (C) were left untreated (gray area) or exposed to TNF/CHX for 24 h (heavy dark line). Following staining with AO as in Fig. 2, samples were analyzed for detection of pale cells (arrowhead) by FACS. A representative cytogram of at least three replicates for each condition is shown; quantification of pale cells is given in the graph at the right of each cytogram. The inserts depict the effect of TNF/CHX on lysosome integrity of nontransfected controls (A) and cells transfected with nonspecific (B, NS) or aCDase (C, aCD) siRNAs as observed under a fluorescence microscope following staining with AO; for each series, the left and the right panels show cells treated as indicated, in the absence and presence of TNF/CHX, respectively. (D) SK-HEP-1 cells were transfected with control (NS) and aCD siRNAs; the amount of aCDase was evaluated by Western blotting (top left), normalized with respect to the β-actin content (bottom left) and shown (right) as the relative amount of protein compared with cells transfected with control siRNAs. (E) Control cells and cells transfected with scrambled or aCD siRNAs were treated or not with TNF/CHX as indicated, fixed, stained with an anti-CB antibody, and observed under a fluorescence microscope as in Fig. 2. aCD-specific siRNAs revert the TNF/CHX-induced cytoplasmic diffusion of CB immunofluorescence and restore the punctate lysosomal pattern. (F) Cells were transfected or not as indicated, left untreated or exposed to TNF/CHX for 6 h; cells from media and monolayers were then collected, pooled, and analyzed by FACS for quantification of annexin V-positive/PI-negative cells (early apoptotic); 10,000 cells were run per each sample. NT: non transfected cells; NS, aCD: nonspecific and acid CDase-specific siRNAs. aCDase: acid ceramidase. Results are means ± SD of three (A–C, F) or as the mean of two independent experiments (D). ***P < 0.001 versus control cells (A) or versus TNF/CHX plus control siRNAs (F); §§§P < 0.001 versus cells transfected with control siRNAs (B); n.s.: not significantly different versus cells transfected with siRNAs specific for aCDase (C and F).
DISCUSSION
TNF is one of the most powerful known inducers of apoptosis and active on a variety of malignant cells. Its cytotoxicity depends on binding to a cell surface death receptor (TNFRI), which triggers a plethora of intracellular events culminating into canonical caspase-dependent apoptosis (8) or even caspase-independent, but still apoptotic-like, death (32, 33). As reported previously (26), in HTC hepatoma cells, TNF/CHX trigger an apoptotic-like death that requires the participation of lysosomes. Lysosomes have been postulated to partake in TNF-induced PCD by a variety of mechanisms, including production of ceramide and/or other sphingolipids (16). The present results show that exposure of HTC cells to TNF/CHX markedly increases levels of ceramide and sphingosine, the latter mainly by activation of the acidic pathway of ceramide breakdown, in agreement with results from other research groups (25, 34–36). We previously demonstrated that, in these cells, ceramide-induced death takes place with characteristics totally different from those typical of TNF/CHX (23), thus raising concern about the actual role of ceramide in cellular death. It has been proposed, in fact, that intralysosomally generated ceramide could promote the relocation to the cytoplasm of cathepsins, B (14) and D (16) in particular, eventually resulting in Bid cleavage and activation of the mitochondrial death pathway. In addition, ceramide has been reported to activate caspase-independent death pathways (37). By contrast, other evidences strongly argue against these views (21, 22, 38) and demonstrate that ceramide per se neither is intrinsically cytotoxic nor sensitizes cells to death. Cells genetically deficient of aCDase accumulate high levels of ceramide, yet are neither more prone to spontaneous death nor more susceptible to various stress-inducing agents than normal cells (39). These cells, however, display a higher sensitivity toward receptor-mediated death, a finding so far ascribed to plasma membrane ceramide that would affect raft formation (40), thereby altering death receptor clustering and signal generation or transduction (41). Hence, the present observation that TNF/CHX-induced ceramide does not directly account for death in our system is consistent with previous results from our as well as other research groups. In addition, the finding that Dpm further increases ceramide above the levels attained with TNF/CHX, yet protects against its toxicity, demonstrates that even a large increase of ceramide has no direct damaging effects. Our results point out that C2-cer does not elicit LMP, which is, however, triggered by TNF/CHX or exogenous sphingosine, and totally agree with previous reports in which the lysosome-permeabilizing and death-inducing capability of sphingosine was evidenced (22). Further supporting the privileged role of sphingosine in mediating TNF/CHX toxicity is an earlier finding that only TNF/actinomycin D or sphingosine, although not C6-ceramide, induces LMP in hepatocytes or purified lysosomes obtained from normal, but not from cathepsin B−/−, mice (21). Hence, in our hands, neither treatment with exogenous analogs nor intralysosomal accumulation of endogenous ceramide directly promotes LMP. Importantly, we provide, to the best of our knowledge, the first direct demonstration that TNF/CHX-induced LMP is accounted for by sphingosine generated inside the lysosomal compartment following exposure to TNF/CHX. The present observation that TNF/CHX elicit LMP in both rat and human hepatoma cell lines indicates that LMP is not an uncommon response to TNF toxicity and may potentially represent a unifying effector mechanism for TNF and possibly other cytotoxic agents (21, 42). On the other hand, a role for ceramide cannot be totally ruled out when it comes to other death models, such as those based on antiblastic agents, such as daunorubicin, vinblastine, and doxorubicin, in hepatoma cells of various origin (43). Death triggered by these cytostatics was shown to be mediated by mitochondria, in which a damaging effect of both endogenous and synthetic analogs of ceramide(s) has been described (44). In the present article, silencing-dependent reduction of the enhanced levels of sphingosine induced by TNF/CHX abrogates the occurrence of LMP and, partly, of cell death. RNA interference confirmed that the acidic pathway of ceramide breakdown accounts for lysosomal sphingosine accumulation, LMP, and TNF toxicity in both HTC and SK-HEP-1, although not in Hep G2 and Huh7 cells. However, the precise roles of the sphingolipids in determining death or survival in normal and cancer cells are far from being fully elucidated; in particular, information is lacking about the fine mechanisms by which TNF affects sphingolipid metabolism. Although beyond the aims of the present investigation, it is worth noting that phosphorylation inhibitors such as genistein suppress the IL-1β-induced activation of aCDase (45). As TNF is known to alter the phosphorylation status of many proteins, the possibility that aCDase as well as other enzymes of this metabolic pathway are similarly regulated by the cytokine cannot be excluded.
In agreement with old observations (19), our data give prominence to the fact that the acidic compartment is one of the main targets of TNF in hepatoma cells and demonstrate that TNF-induced sphingosine, although not ceramide, accounts for LMP and toxicity in rat and human hepatoma cells (19, 21). Here we show that LMP allows the relocation of lysosomal cathepsins, both endogenous CB and a transfected CB-GFP chimera, to the cytoplasm of TNF/CHX-treated cells, thus outlining a potential role for these acidic proteases in TNF-induced death. In fact, it has been evidenced that cathepsins S and H also retain some activity at nearly neutral pH (46) and are thus capable of degrading substrates in the cytoplasm. However, additional studies are required for a full understanding of the role of LMP in TNF/CHX toxicity on hepatoma cells.
In conclusion, here we show that exposure of HTC cells to TNF/CHX subverts sphingolipid metabolism and brings about the generation of large amounts of ceramide and sphingosine inside the lysosomes. Ceramide, even at high concentrations, does not account for the lethal action and any of the relevant effects of TNF/CHX. The latter, by contrast, can be fully ascribed to the intralysosomal conversion of ceramide into sphingosine, which in turn is responsible for LMP and ensuing cellular death.
Acknowledgments
The authors are grateful to Professor Gregory Gores for the kind gift of the cathepsin B-GFP construct, to Professor Thierry Levade for critical reading of the manuscript, and to Mrs. Eva Dalmau for technical assistance.
Footnotes
Abbreviations:
- AO
- acridine orange
- C2-cer
- C2-ceramide
- CB-GFP
- cathepsin B-GFP
- CDase
- ceramidase
- CHX
- cycloheximide
- Dpm
- desipramine
- LMP
- lysosomal membrane permeabilization
- PCD
- programmed cell death
- PI
- propidium iodide
- PS
- phosphatidylserine
- SMase
- sphingomyelinase
- Sph
- sphingosine
- TNF
- tumor necrosis factor-α
This work was supported by grant SAF2008-00706 from Ministero dell'Università e della Ricerca, Regione Piemonte and by grant SAF2011-22444 from Ministerio de Ciencia e Innovación.
REFERENCES
- 1.Debatin K. M. 2004. Apoptosis pathways in cancer and cancer therapy. Cancer Immunol. Immunother. 53: 153–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fabregat I., Roncero C., Fernández M. 2007. Survival and apoptosis: a dysregulated balance in liver cancer. Liver Int. 27: 155–162 [DOI] [PubMed] [Google Scholar]
- 3.Farazi P. A., DePinho R. A. 2006. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat. Rev. Cancer. 6: 674–687 [DOI] [PubMed] [Google Scholar]
- 4.Rath P. C., Aggarwal B. B. 1999. TNF-induced signaling in apoptosis. J. Clin. Immunol. 19: 350–364 [DOI] [PubMed] [Google Scholar]
- 5.Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. 1975. An endotoxin-induced serum factor that causes necrosis of tumors. Proc. Natl. Acad. Sci. USA. 72: 3666–3670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wajant H., Pfizenmaier K., Scheurich P. 2003. Tumor necrosis factor signaling. Cell Death Differ. 10: 45–65 [DOI] [PubMed] [Google Scholar]
- 7.Xu Y., Bialik S., Jones B. E., Iimuro Y., Kitsis R. N., Srinivasan A., Brenner D. A., Czaja M. J. 1998. NF-kappaB inactivation converts a hepatocyte cell line TNF-alpha response from proliferation to apoptosis. Am. J. Physiol. 275: C1058–C1066 [DOI] [PubMed] [Google Scholar]
- 8.Scaffidi C., Fulda S., Srinivasan A., Friesen C., Li F., Tomaselli K. J., Debatin K. M., Krammer P. H., Peter M. E. 1998. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 17: 1675–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Duve C. 1966. The significance of lysosomes in pathology and medicine. Proc. Inst. Med. Chic. 26: 73–76 [PubMed] [Google Scholar]
- 10.Turk B., Stoka V., Rozman-Pungercar J., Cirman T., Droga-Mazovec G., Orešic K., Turk V. 2002. Apoptotic pathways: involvement of lysosomal proteases. Biol. Chem. 383: 1035–1044 [DOI] [PubMed] [Google Scholar]
- 11.Turk B., Turk V. 2009. Lysosomes as “suicide bags” in cell death: myth or reality? J. Biol. Chem. 284: 21783–21787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stoka V., Turk V., Turk B. 2007. Lysosomal cysteine cathepsins: signaling pathways in apoptosis. Biol. Chem. 388: 555–560 [DOI] [PubMed] [Google Scholar]
- 13.Tardy C., Codogno P., Autefage H., Levade T., Andrieu-Abadie N. 2006. Lysosomes and lysosomal proteins in cancer cell death (new players of an old struggle). Biochim. Biophys. Acta. 1765: 101–125 [DOI] [PubMed] [Google Scholar]
- 14.Guicciardi M. E., Deussing J., Miyoshi H., Bronk S. F., Svingen P. A., Peters C., Kaufmann S. H., Gores G. J. 2000. Cathepsin B contributes to TNF-alpha-mediated hepatocyte apoptosis by promoting mitochondrial release of cytochrome c. J. Clin. Invest. 106: 1127–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roberts L. R., Kurosawa H., Bronk S. F., Fesmier P. J., Agellon L. B., Leung W. Y., Mao F., Gores G. J. 1997. Cathepsin B contributes to bile salt-induced apoptosis of rat hepatocytes. Gastroenterology. 113: 1714–1726 [DOI] [PubMed] [Google Scholar]
- 16.Schütze S., Tchikov V., Schneider-Brachert W. 2008. Regulation of TNFR1 and CD95 signalling by receptor compartmentalization. Nat. Rev. Mol. Cell Biol. 9: 655–662 [DOI] [PubMed] [Google Scholar]
- 17.Kurz T., Terman A., Gustafsson B., Brunk U. T. 2008. Lysosomes in iron metabolism, ageing and apoptosis. Histochem. Cell Biol. 129: 389–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brunk U. T., Neuzil J., Eaton J. W. 2001. Lysosomal involvement in apoptosis. Redox Rep. 6: 91–97 [DOI] [PubMed] [Google Scholar]
- 19.Monney L., Olivier R., Otter I., Jansen B., Poirier G. G., Borner C. 1998. Role of an acidic compartment in tumor-necrosis-factor-alpha-induced production of ceramide, activation of caspase-3 and apoptosis. Eur. J. Biochem. 251: 295–303 [DOI] [PubMed] [Google Scholar]
- 20.Xu J., Yeh C. H., Chen S., He L., Sensi S. L., Canzoniero L. M., Choi D. W., Hsu C. Y. 1998. Involvement of de novo ceramide biosynthesis in tumor necrosis factor-alpha/cycloheximide-induced cerebral endothelial cell death. J. Biol. Chem. 273: 16521–16526 [DOI] [PubMed] [Google Scholar]
- 21.Werneburg N. W., Guicciardi M. E., Bronk S. F., Gores G. J. 2002. Tumor necrosis factor-alpha-associated lysosomal permeabilization is cathepsin B dependent. Am. J. Physiol. Gastrointest. Liver Physiol. 283: G947–G956 [DOI] [PubMed] [Google Scholar]
- 22.Kågedal K., Zhao M., Svensson I., Brunk U. T. 2001. Sphingosine-induced apoptosis is dependent on lysosomal proteases. Biochem. J. 359: 335–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Autelli R., Ullio C., Prigione E., Crepaldi S., Schiavone N., Brunk U. T., Capaccioli S., Baccino F. M., Bonelli G. 2009. Divergent pathways for TNF and C(2)-ceramide toxicity in HTC hepatoma cells. Biochim. Biophys. Acta. 1793: 1182–1190 [DOI] [PubMed] [Google Scholar]
- 24.Cuvillier O. 2002. Sphingosine in apoptosis signaling. Biochim. Biophys. Acta. 1585: 153–162 [DOI] [PubMed] [Google Scholar]
- 25.Woodcock J. 2006. Sphingosine and ceramide signalling in apoptosis. IUBMB Life. 58: 462–466 [DOI] [PubMed] [Google Scholar]
- 26.Autelli R., Crepaldi S., De Stefanis D., Parola M., Bonelli G., Baccino F. M. 2005. Intracellular free iron and acidic pathways mediate TNF-induced death of rat hepatoma cells. Apoptosis. 10: 777–786 [DOI] [PubMed] [Google Scholar]
- 27.Canals D., Mormeneo D., Fabriàs G., Llebaria A., Casas J., Delgado A. 2009. Synthesis and biological properties of Pachastrissamine (jaspine B) and diastereoisomeric jaspines. Bioorg. Med. Chem. 17: 235–241 [DOI] [PubMed] [Google Scholar]
- 28.Zhao M., Eaton J. W., Brunk U. T. 2000. Protection against oxidant-mediated lysosomal rupture: a new anti-apoptotic activity of Bcl-2? FEBS Lett. 485: 104–108 [DOI] [PubMed] [Google Scholar]
- 29.Guicciardi M. E., Bronk S. F., Werneburg N. W., Gores G. J. 2007. cFLIPL prevents TRAIL-induced apoptosis of hepatocellular carcinoma cells by inhibiting the lysosomal pathway of apoptosis. Am. J. Physiol. Gastrointest. Liver Physiol. 292: G1337–G1346 [DOI] [PubMed] [Google Scholar]
- 30.Boya P., Andreau K., Poncet D., Zamzami N., Perfettini J. L., Metivier D., Ojcius D. M., Jäättelä M., Kroemer G. 2003. Lysosomal membrane permeabilization induces cell death in a mitochondrion-dependent fashion. J. Exp. Med. 197: 1323–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeidan Y. H., Pettus B. J., Elojeimy S., Taha T., Obeid L. M., Kawamori T., Norris J. S., Hannun Y. A. 2006. Acid ceramidase but not acid sphingomyelinase is required for tumor necrosis factor-{alpha}-induced PGE2 production. J. Biol. Chem. 281: 24695–24703 [DOI] [PubMed] [Google Scholar]
- 32.Leist M., Jäättelä M. 2001. Four deaths and a funeral: from caspases to alternative mechanisms. Nat. Rev. Mol. Cell Biol. 2: 589–598 [DOI] [PubMed] [Google Scholar]
- 33.Chipuk J. E., Green D. R. 2005. Do inducers of apoptosis trigger caspase-independent cell death? Nat. Rev. Mol. Cell Biol. 6: 268–275 [DOI] [PubMed] [Google Scholar]
- 34.Malagarie-Cazenave S., Andrieu-Abadie N., Ségui B., Gouazé V., Tardy C., Cuvillier O., Levade T. 2002. Sphingolipid signalling: molecular basis and role in TNF-alpha-induced cell death. Expert Rev. Mol. Med. 4: 1–15 [DOI] [PubMed] [Google Scholar]
- 35.Kim M. Y., Linardic C., Obeid L., Hannun Y. 1991. Identification of sphingomyelin turnover as an effector mechanism for the action of tumor necrosis factor alpha and gamma-interferon. Specific role in cell differentiation. J. Biol. Chem. 266: 484–489 [PubMed] [Google Scholar]
- 36.Dbaibo G. S., El-Assaad W., Krikorian A., Liu B., Diab K., Idriss N. Z., El-Sabban M., Driscoll T. A., Perry D. K., Hannun Y. A. 2001. Ceramide generation by two distinct pathways in tumor necrosis factor alpha-induced cell death. FEBS Lett. 503: 7–12 [DOI] [PubMed] [Google Scholar]
- 37.Thon L., Möhlig H., Mathieu S., Lange A., Bulanova E., Winoto-Morbach S., Schutze S., Bulfone-Paus S., Adam D. 2005. Ceramide mediates caspase-independent programmed cell death. FASEB J. 19: 1945–1956 [DOI] [PubMed] [Google Scholar]
- 38.Ségui B., Bezombes C., Uro-Coste E., Medin J. A., Andrieu-Abadie N., Augé N., Brouchet A., Laurent G., Salvayre R., Jaffrezou J. P., et al. 2000. Stress-induced apoptosis is not mediated by endolysosomal ceramide. FASEB J. 14: 36–47 [DOI] [PubMed] [Google Scholar]
- 39.Burek C., Roth J., Koch H. G., Harzer K., Los M., Schulze-Osthoff K. 2001. The role of ceramide in receptor- and stress-induced apoptosis studied in acidic ceramidase-deficient Farber disease cells. Oncogene. 20: 6493–6502 [DOI] [PubMed] [Google Scholar]
- 40.Gulbins E., Dreschers S., Wilker B., Grassmé H. 2004. Ceramide, membrane rafts and infections. J. Mol. Med. 82: 357–363 [DOI] [PubMed] [Google Scholar]
- 41.Gulbins E., Grassmé H. 2002. Ceramide and cell death receptor clustering. Biochim. Biophys. Acta. 1585: 139–145 [DOI] [PubMed] [Google Scholar]
- 42.Werneburg N., Guicciardi M. E., Yin X. M., Gores G. J. 2004. TNF-alpha-mediated lysosomal permeabilization is FAN and caspase 8/Bid dependent. Am. J. Physiol. Gastrointest. Liver Physiol. 287: G436–G443 [DOI] [PubMed] [Google Scholar]
- 43.Morales A., París R., Villanueva A., Llacuna L., García-Ruiz C., Fernández-Checa J. C. 2007. Pharmacological inhibition or small interfering RNA targeting acid ceramidase sensitizes hepatoma cells to chemotherapy and reduces tumor growth in vivo. Oncogene. 26: 905–916 [DOI] [PubMed] [Google Scholar]
- 44.Siskind L. J. 2005. Mitochondrial ceramide and the induction of apoptosis. J. Bioenerg. Biomembr. 37: 143–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coroneos E., Martinez M., McKenna S., Kester M. 1995. Differential regulation of sphingomyelinase and ceramidase activities by growth factors and cytokines. Implications for cellular proliferation and differentiation. J. Biol. Chem. 270: 23305–23309 [DOI] [PubMed] [Google Scholar]
- 46.Droga-Mazovec G., Bojic L., Petelin A., Ivanova S., Romih R., Repnik U., Salvesen G. S., Stoka V., Turk V., Turk B. 2008. Cysteine cathepsins trigger caspase-dependent cell death through cleavage of bid and antiapoptotic Bcl-2 homologues. J. Biol. Chem. 283: 19140–19150 [DOI] [PubMed] [Google Scholar]