Abstract
Acyl-CoA:cholesterol acyltransferase 2 (ACAT2) generates cholesterol esters (CE) for packaging into newly synthesized lipoproteins and thus is a major determinant of blood cholesterol levels. ACAT2 is expressed exclusively in the small intestine and liver, but the relative contributions of ACAT2 expression in these tissues to systemic cholesterol metabolism is unknown. We investigated whether CE derived from the intestine or liver would differentially affect hepatic and plasma cholesterol homeostasis. We generated liver-specific (ACAT2L−/L−) and intestine-specific (ACAT2SI−/SI−) ACAT2 knockout mice and studied dietary cholesterol-induced hepatic lipid accumulation and hypercholesterolemia. ACAT2SI−/SI− mice, in contrast to ACAT2L−/L− mice, had blunted cholesterol absorption. However, specific deletion of ACAT2 in the intestine generated essentially a phenocopy of the conditional knockout of ACAT2 in the liver, with reduced levels of plasma very low-density lipoprotein and hepatic CE, yet hepatic-free cholesterol does not build up after high cholesterol intake. ACAT2L−/L− and ACAT2SI−/SI− mice were equally protected from diet-induced hepatic CE accumulation and hypercholesterolemia. These results suggest that inhibition of intestinal or hepatic ACAT2 improves atherogenic hyperlipidemia and limits hepatic CE accumulation in mice and that depletion of intestinal ACAT2 is sufficient for most of the beneficial effects on cholesterol metabolism. Inhibitors of ACAT2 targeting either tissue likely would be beneficial for atheroprotection.
Keywords: Acyl-CoA:cholesterol acyltransferase, cholesteryl ester, lipids and lipoproteins, atherosclerosis
Acyl-CoA:cholesterol acyltransferase (ACAT), also known as sterol O-acyltransferase, is a microsomal protein responsible for intracellular cholesterol ester (CE) synthesis. ACAT typically uses monounsaturated fatty acids, such as oleate from the acyl-CoA pool together with free cholesterol (FC) as substrates. Two subtypes of ACAT exist: ACAT1 is expressed in a variety of tissues, and ACAT2 is expressed exclusively in enterocyte of the intestine and hepatocyte of the liver, where one of its roles is to generate CE for packaging into chylomicrons and very low-density lipoproteins (VLDLs), respectively (1). Cholesterol ester, especially cholesterol oleate, is one of the major components of atherosclerotic plaques and, when found in apoB-containing lipoproteins, may play a critical role in the pathogenesis of atherosclerosis (2).
Deletion of ACAT2 in LDLr−/− mice led to a 78% decrease of aortic surface area as lesion and an 88% decrease in CE deposited in atherosclerotic plaques (3). The proatherogenic effect of ACAT2-derived CE also was found in APOE−/− mice after ACAT2 was deleted, and atherosclerosis was almost absent (4). These data indicate a critical role of CE as a sensitive marker in atherosclerotic lesion development. In the past few years, the role of hepatic ACAT2 on cholesterol homeostasis has been extensively studied using an antisense oligonucleotide (ASO)-mediated knockdown in mice (5–7). ASO-mediated hepatic ACAT2 depletion resulted in the lack of hepatic CE production, reduced plasma total cholesterol and VLDL-cholesterol, decreased plasma CE, and increased plasma triglyceride (TG) concentration. These studies indicate that the lack of hepatic cholesterol esterification and the reduced CE secretion into VLDL, as also observed in total body ACAT2−/− mice, is atheroprotective.
Intestinal ACAT2 determines how much cholesterol gets packaged into chylomicrons as CE and transported in lymph and plasma (8). Isotopic tracer measurements revealed that the cholesterol absorption rates varied from 20% to 80% of total cholesterol (around 1,500 mg/day in humans) present in the lumen (9). The majority of cholesterol delivered to the liver is esterified and resides in the core of chylomicron remnants. Nonspecific ACAT inhibitors have been shown to decrease cholesterol absorption by 20 to 80% in rats, hamsters, rabbits, pigs, and nonhuman primates (10–14). Whole body ACAT2−/− mice also have a 40% to 85% reduced level of cholesterol absorption (8, 15–18). Collectively, this implies that cholesterol esterification by intestinal ACAT2 plays an important role in regulating cholesterol sequestration for transport into the body.
Although the blunted cholesterol absorption via intestinal ACAT2 inhibition is anticipated to be hypocholesterolemic, the specific contribution made by intestine-derived CE to whole body cholesterol homeostasis is still unclear. A nonselective deletion or inhibition of ACAT2 would be expected to render the maximal cholesterol-lowering effect as described above and is apparently due to a combined inhibitory effect on hepatic and intestinal ACAT2 activities. To design effective therapeutic strategies targeting ACAT2-driven cholesterol esterification, we believe it is critically important to understand the function of ACAT2 in the small intestine and liver. This prompted us to create tissue-specific ACAT2 knockout (KO) mouse models. The hypothesis tested herein is whether blocking the influx of cholesterol only from intestine, as expected from intestine-specific ACAT2 KO mice, would protect animals from hypercholesterolemia and tissue cholesterol accumulation to a similar extent as seen in liver-specific ACAT2 KO mice.
MATERIALS AND METHODS
Generation of ACAT2 floxed mice
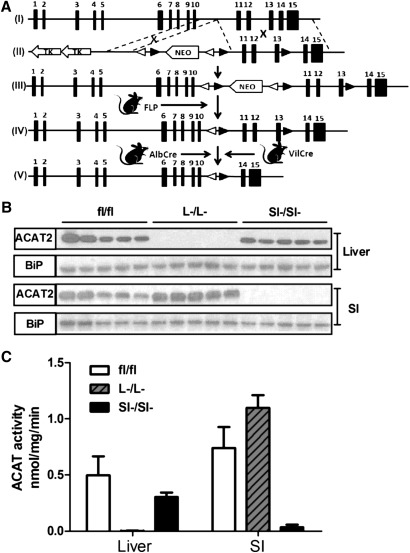
Conditional targeting of the mouse ACAT2 gene was achieved as depicted in Fig. 1A. An ACAT2 targeting vector was constructed to introduce loxP sites upstream and downstream of exons 11 to 13, which contain active site residues necessary for enzyme catalysis. To generate this targeting vector, regions of homology were generated by PCR using genomic DNA from mouse liver (129S6/SvEv strain) as a template. Both the short and long arm of homology were then inserted into the targeting vector pJB1 (kindly provided by Joachim Herz, University of Texas Southwestern Medical Center), which contained the NEO gene driven by the murine phosphoglycerate kinase promoter, flanked by loxP and FRT sites, and followed by two copies of the herpes simplex virus thymidine kinase genes. This vector also contains FRT sites flanking the neomycin resistance gene, which facilitates removal of the NEO cassette by Flp recombinase. This construct was linearized by digestion with PmeI and electroporated into SvEv mouse-derived embryonic stem cells. The cells were seeded onto STO feeder layers for 24 h before the addition of G418 (250 μg/ml). Forty-eight hours after electroporation the cells were selected with gancyclovir (2.5 μM) for 8 to 10 days. The embryonic stem cell colonies were expanded and screened for homologous recombination by PCR and Southern blotting. Two positive clones were injected into C57BL/6N blastocysts. Five chimeric male and two chimeric female mice with >70% agouti coat color were fertile. Two chimeric males were capable of germline transmission of the ACAT2 floxed allele. These mice were originally bred to homozygosity, but the floxed allele with NEO cassette intact was severely hypomorphic (<10% normal hepatic ACAT activity), necessitating removal of the NEO cassette. To accomplish this, we bred ACAT2 floxed mice with transgenic mice expressing FLP1 recombinase [129S4-Gt(ROSA)26Sortm1(FLP1)Dym/RainJ] maintained on a 129S/SvJae background (Jackson Laboratory, Bar Harbor, ME). Once FLP1-mediated excision of the NEO cassette was confirmed by PCR, the FLP1 transgene was bred out of resulting ACAT2 floxed mice. When the FLP1 recombined allele was bred to homozygosity, it was confirmed that the hypomorphic allele had been rescued, given that ACAT2 protein and ACAT2 enzymatic activity was not significantly different from that of ACAT2 wild-type mice (data not shown). To generate intestine-specific and liver-specific ACAT2 knockout, mice with two ACAT2 floxed alleles were bred to mice transgenically expressing Cre recombinase under the villin promoter (B6.SJL-Tg(Vil-cre)997Gum/J) or albumin promoter (B6.Cg-Tg(Alb-cre)21Mgn/J), respectively. The Cre trangenics were purchased from Jackson Laboratory (Bar Harbor, ME). Genotyping was confirmed by PCR of genomic DNA using primers flanking the loxP site in intron 13–14 using the following primers (forward: 5′ CTGACCACAGAAAAGCATTTG ATCA 3′ reverse: 5′ AATCCGTATTGCTCAGTTTACCCCT 3′) and PCR conditions (Step 1: 93°C for 3 min; Step 2: 93°C for 30 s; Step 3: 60°C for 30 s; Step 4: 65°C for 2 min; repeat step 2 to 4 for 40 cycles). Liver-specific ACAT2 KO (ACAT2fl/fl/AlbCre+) are designated as ACAT2L−/L−, intestine-specific ACAT2 KO (ACAT2fl/fl/VilCre+) are designated as ACAT2SI−/SI−, and appropriate littermate controls (ACAT2fl/flAlbCre— or VilCre—) are designated as ACAT2fl/fl.
Fig.1.
Conditional targeting of the ACAT2 allele allows for tissue-specific deletion. A: Targeting strategy of generating tissue-specific ACAT2 KO mice. (I) Wild-type allele, (II) targeting vector, (III) floxed allele showing hypomorphic phenotype, (IV) flippase (FLP) recombinase-mediated recombination of floxed allele, (V) knockout allele. NEO: neo cassette gene conferring neomycin selection marker; open triangle: FRT site (flippase recognition target); solid triangle: LoxP site. B: ACAT2 protein was measured by Western blot. A total of 25 μg (liver) or 15 μg (intestine) of microsomal proteins were loaded on each lane. Data represent five individual mice. Binding immunoglobulin protein (BiP) was probed as loading control. C: Microsomes from individual animals (n = 5) of each genotype shown in (B) were used to quantify the ACAT2 activity for liver or intestine.
Dietary studies
Only female mice were included in the study having been bred on a mixed background as described above. At the age of 7 to 12 weeks, subgroups of mice were fed with a semisynthetic diet containing 20% of energy as lard and added cholesterol at levels of 0.05, 0.1, or 0.5% (wt/wt) for a total of 6 weeks. Blood was drawn at baseline, 3 weeks, and 6 weeks. Necropsies were performed in mice fasted from 9:00 AM until 1:00 PM as previously described (3–7, 14). All mice used in the studies were housed in a pathogen-free barrier facility at Wake Forest University School of Medicine with the approval of the American Association for Accreditation of Laboratory Animal Care. The Institutional Animal Care and Use Committee approved all protocols before execution of the studies.
Plasma lipid and lipoprotein analysis
Total plasma cholesterol (TPC) and TG concentrations were measured using colorimetric assay as previously described (3–7, 14). Cholesterol distribution was quantified by FPLC as previously described (3–7, 14).
Immunoblotting
Microsomal proteins (25 μg/lane of liver and 15 μg/lane of intestine) were separated by 4 to 12% SDS-PAGE Tris-glycine gel, transferred to nitrocellulose membranes, and incubated with anti-ACAT2 rabbit polyclonal (19) or anti-BiP rabbit polyclonal antibodies (Stressgen Biotechnologies Corp., Canada) as described previously (3–7, 14).
Microsomal ACAT activity assay
ACAT activity was quantified by methods previously published (20, 21). Briefly, an aliquot of liver and small intestine segment 2 were homogenized in ACAT homogenization buffer in the presence of protease inhibitor cocktail. The microsomes were isolated, and the ACAT assays were performed in the absence and presence of 0.1 mM pyripyropene A, an ACAT2-specific inhibitor, so that ACAT1 and ACAT2 activities could be calculated from total ACAT activity. Relative ACAT enzyme activity was expressed as nmol CE synthesized per mg microsomal protein per min (nmol/mg/min).
Cholesterol absorption measurement by fecal dual-isotope
During the fourth week of diet treatment, all mice were gavaged with 50 μl soybean oil containing [14C]cholesterol and β-[3H]sitosterol. Fractional cholesterol absorption and fecal neutral sterol loss were quantified using previously published methods (6, 14).
Hepatic, intestinal, and biliary lipid analysis
Extraction of biliary, intestinal, and liver lipids for enzymatic quantification of total triglyceride, cholesteryl esters, FC, phospholipids, and bile acids were performed as previously described (3–7, 14). During necropsy, small intestine was flushed thoroughly with saline. The entire intestine was folded and cut into four equal length segments. Only segment 2 was used for cholesterol assay.
Quantitative real-time PCR
Tissue RNA extraction and quantitative PCR was conducted as previously described (3–7, 14) using the applied biosystems 7500 Real-Time PCR System. Primer sequences used for quantitative PCR are available on request.
Statistical analyses
All graphs were plotted by GraphPad Prism 5.05. Data were analyzed by ANOVA with Tukey post hoc test. Statistically significant differences were considered at P < 0.05.
RESULTS
Conditional targeting of the ACAT2 allele allows for tissue-specific deletion
Initial studies indicated that homozygous floxed mice (ACAT2fl/fl) that had been crossed with FLP-1 expressing mice had similar ACAT2 protein and activity as wild-type mice (ACAT2WT/WT; data not shown). For tissue-specific ACAT2 knockouts, intercrosses of heterozygotes (ACAT2WT/fl) yielded the expected 25% homozygotes. Both the ACAT2L−/L− and ACAT2SI−/SI− mice developed normally, were fertile, and exhibited normal body weight when fed rodent chow or a cholesterol-enriched diet. ACAT2 protein in ACAT2L−/L− mice was undetectable in the liver but was expressed at normal levels in the intestine compared with the ACAT2fl/fl mice (Fig. 1B). Hepatic ACAT2 activity was reduced by 99.7% in ACAT2L−/L− mice compared with ACAT2fl/fl mice (Fig. 1C). As anticipated, this pattern was opposite in ACAT2SI−/SI− mice, where ACAT2 protein was not detected in the small intestine, whereas hepatic ACAT2 was unaltered in ACAT2SI−/SI− mice (Fig. 1B). Intestinal ACAT2 activity was decreased by 95.6% compared with ACAT2fl/fl mice (Fig. 1C).
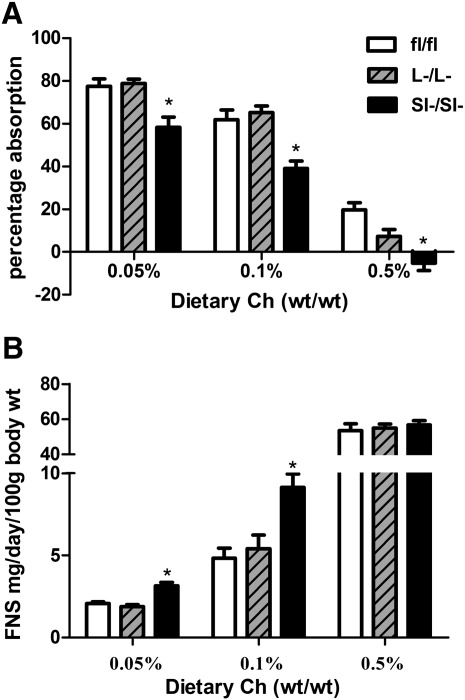
ACAT2SI−/SI− mice, but Not ACAT2L−/L− Mice, have diminished cholesterol absorption
ACAT2fl/fl mice had a stepwise decrease of percentage cholesterol absorption in response to increased dietary cholesterol (Fig. 2A). ACAT2L−/L− mice did not have significantly different percentage absorption values compared with control mice at any dietary cholesterol level (Fig. 2A). However, significantly decreased levels of cholesterol absorption were observed in ACAT2SI−/SI− mice for each different dietary cholesterol level when compared with control and ACAT2L−/L− mice (Fig. 2A). ACAT2fl/fl mice had a stepwise increase in fecal sterol loss that occurred to similar levels as that of ACAT2L−/L− mice (Fig. 2B). ACAT2SI−/SI− mice had significantly more fecal sterol loss at 0.05% and 0.1% dietary cholesterol levels, but values were comparable at the 0.5% cholesterol level when compared with ACAT2fl/fl control mice (Fig. 2B).
Fig.2.
Intestine-specific, but not liver-specific, deletion of ACAT2 reduces cholesterol absorption. A: Fractional cholesterol absorption was measured by the dual fecal isotope method. B: Fecal neutral sterol loss was measured by GC. Data represent the mean ± SEM from 7 to 9 mice per group. *Significantly different from fl/fl control mice within each dietary group with P < 0.05.
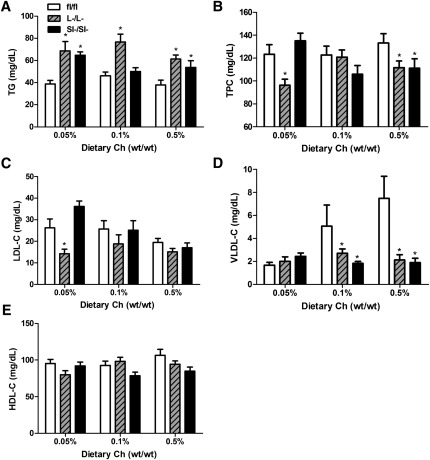
ACAT2L−/L− and ACAT2SI−/SI− mice have higher plasma concentrations of TG and lower concentrations of VLDL-C
After consuming the experimental diets for 6 weeks, ACAT2L−/L− mice had significantly higher concentrations of plasma TG at any given dietary cholesterol level compared with ACAT2fl/fl mice (Fig. 3A). An increased level of plasma TG also was observed in ACAT2SI−/SI− mice when fed with 0.05% and 0.5% (wt/wt) cholesterol diet (Fig. 3A). ACAT2L−/L− mice had a lower level of TPC on 0.05% cholesterol diet (Fig. 3B). The difference was presumably due to the reduction of LDL-C when compared with the response in ACAT2fl/fl mice (Fig. 3C). TPC was not different among three genotypes of mice when the mice were fed the 0.1% cholesterol diet (Fig. 3B). After being fed the 0.5% cholesterol diet for 6 weeks, conditional KO mice had decreased levels of TPC compared with control mice (Fig. 3B). Cholesterol distribution by FPLC indicated that VLDL-C was significantly reduced among ACAT2L−/L− and ACAT2SI−/SI− mice after consuming 0.1% and 0.5% cholesterol diet for 6 weeks (Fig. 3D). There was no effect of diet or genotype on HDL cholesterol levels (Fig. 3E).
Fig.3.
Tissue-specific deletion of ACAT2 alters plasma lipoprotein metabolism. Plasma TG (A), TPC (B), plasma LDL cholesterol (C), plasma VLDL cholesterol (D), and plasma HDL cholesterol (E). Each bar represents the average results of 7 to 8 animals. *Significantly different from fl/fl control mice within each dietary group with P < 0.05.
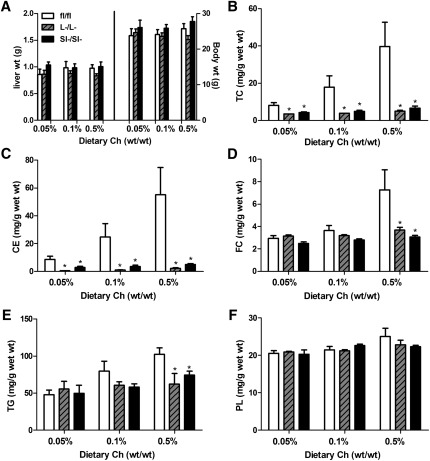
ACAT2L−/L− and ACAT2SI−/SI− mice are protected from diet-induced hepatic cholesterol accumulation
Liver weight and body weight were not different among experimental animals at the time of necropsy (Fig. 4A). ACAT2fl/fl mice had a stepwise increase of hepatic total cholesterol (TC) (Fig. 4B) and CE (Fig. 4C) in response to increased dietary cholesterol. In contrast, ACAT2L−/L− and ACAT2SI−/SI− mice had only modest levels of TC and CE at any given dietary cholesterol level (Fig. 4B, C). Interestingly, hepatic TC and CE levels were reduced to strikingly similar concentrations in ACAT2L−/L− and ACAT2SI−/SI− mice regardless of dietary cholesterol levels. Hepatic free cholesterol (FC) was not different among three genotypes when fed with 0.05% and 0.1% dietary cholesterol (Fig. 4D). However, ACAT2L−/L− and ACAT2SI−/SI− mice had significantly lower levels of FC in the liver on the 0.5% cholesterol diet, although it was the same as in other dietary groups (Fig. 4D). The average for hepatic TG was slightly lower in both groups of KO mice fed 0.1% dietary cholesterol compared with the level of TG in the ACAT2fl/fl mice. The TG concentration differences were statistically significant when ACAT2L−/L− and ACAT2SI−/SI− mice consumed 0.5% dietary cholesterol (Fig. 4E). Phospholipid concentrations were not significantly different at any level of dietary cholesterol feeding for any of the genotypes (Fig. 4F).
Fig.4.
Tissue-specific deletion of ACAT2 inhibits diet-induced cholesterol ester accumulation in the liver. A: Liver weight and body weight at necropsy. B: Hepatic total cholesterol (TC). C: cholesterol ester (CE). D: Free cholesterol (FC). E: Triglycerides (TG). G: Phospholipids (PL). Data represent the mean ± SEM from five mice per group. *Significantly different from fl/fl control mice within each dietary group with P < 0.05.
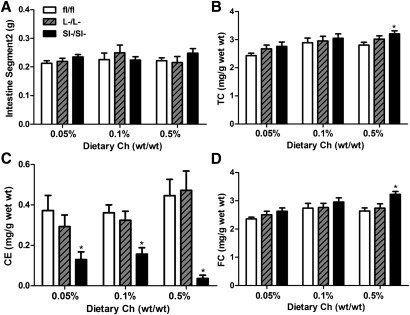
ACAT2SI−/SI− mice have less cholesteryl ester accumulation in the small intestine
After dietary cholesterol intake, all the experimental mice had similar intestine weights, represented by segment 2 in Fig. 5A. ACAT2SI−/SI− mice appeared to have slightly increased levels of TC in the intestine in response to increasing levels of dietary cholesterol (Fig. 5B), although only the intestine of ACAT2SI−/SI− mice had significantly higher concentrations of TC after 6 weeks of consumption of 0.5% dietary cholesterol compared with that of control mice (Fig. 5B). When the mice had no intestinal ACAT2, CE concentrations of intestine were significantly lower at all levels of dietary cholesterol intake compared with control and ACAT2L−/L− mice (Fig. 5C). Intestinal FC was significantly increased in ACAT2SI−/SI− mice fed 0.5% dietary cholesterol (Fig. 5D). However, no difference in FC was observed among animals of either genotype fed diets with 0.05% or 0.1% cholesterol.
Fig.5.
Knockout of intestinal ACAT2 reduces cholesterol ester accumulation in the enterocytes of the intestine. A: Weight of intestine segment 2. B: Intestinal TC. C: Intestinal CE. D: Intestinal FC. Data represent the mean ± SEM from five mice per group. *Significantly different from fl/fl control mice within each dietary group (P < 0.05).
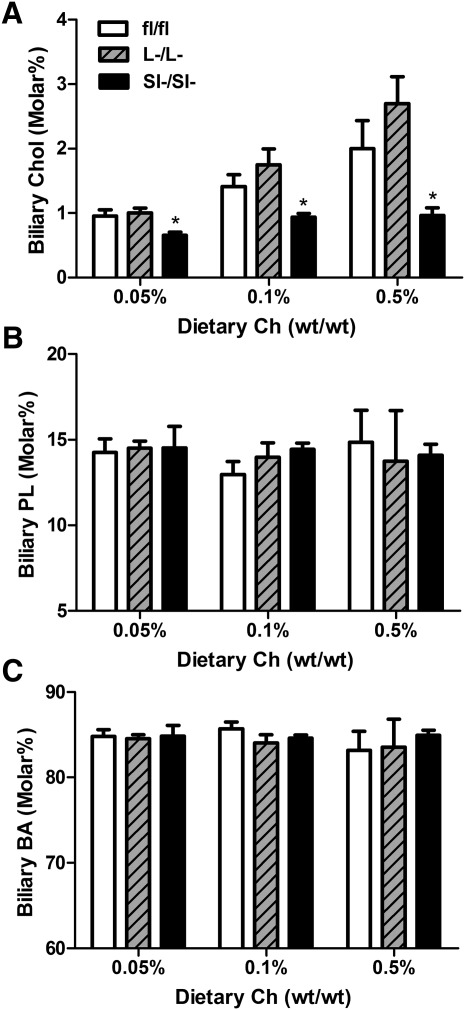
ACAT2SI−/SI− but not ACAT2L−/L− mice have lower levels of biliary cholesterol
In response to cholesterol feeding, ACAT2fl/fl mice tend to have a small increase of biliary cholesterol, although the dietary effect was not statistically significant (Fig. 6A). ACAT2L−/L− mice had similar level of biliary cholesterol compared with control mice (Fig. 6A). In contrast, ACAT2SI−/SI− mice had significantly lower levels of biliary cholesterol with no evidence of a response to increasing dietary cholesterol compared with ACAT2fl/fl and ACAT2L−/L− mice. There was no dietary or genotype difference in biliary phospholipids and total bile acid among the animals (Fig. 6B, C).
Fig.6.
Intestine-specific, but not liver-specific, deletion of ACAT2 reduces biliary cholesterol levels. Biliary cholesterol (A), biliary phospholipids (PL) (B), and biliary bile acids (BA) (C) were quantified in gall bladder bile samples. All values are expressed as percentage molar of total lipids (cholesterol, PL, and BA). Data represent the mean ± SEM from 6 to 8 mice per group. *Significantly different from fl/fl control mice within each dietary group (P < 0.05).
DISCUSSION
Previous ACAT2 ASO studies (5–7) have demonstrated that hepatic ACAT2 plays a central role in controlling whole body cholesterol homeostasis. This perspective is based on the fact that hepatic ACAT2 was knocked down by over 90% by ASO treatment, whereas intestinal ACAT2 generally remained intact among those ACAT2 ASO studies. However, low remaining levels of hepatic ACAT2 activity in the ASO studies made interpretation less than definitive. Through LoxP-Cre recombinase technologies, we have successfully generated two novel ACAT2 conditional KO models, the liver-specific (ACAT2L−/L−) and the intestine-specific (ACAT2SI−/SI−) KO mice. The reductions in tissue and plasma cholesterol and lipoprotein concentrations of ACAT2L−/L− mice as shown here generally agree with results from previous studies using mice with whole body ACAT2 KO or ASO-mediated ACAT2 knockdown (4–7, 15, 16). The consequences of increased dietary cholesterol in hepatic and plasma lipid changes of ACAT2SI−/SI− mice were essentially the same as occurred in ACAT2L−/L− mice and were minimal when compared with those of ACAT2 intact mice. The cholesterol changes (higher TC and FC but lower CE) of enterocytes of intestine among ACAT2SI−/SI− mice were consistent with the reports from Turley and colleagues (18). The present data suggest that blocking cholesterol esterification and absorption in the intestine alone is sufficient to alleviate most of the dietary cholesterol burden in the circulation and tissues. We have previously shown that, in the absence of ACAT2 in the intestine, the efficiency of absorption and transport of cholesterol in chylomicrons is greatly reduced because cholesterol esters cannot be formed for transport into the body in chylomicrons (8). Even though some unesterified cholesterol can be transported in chylomicrons in the absence of intestinal ACAT2 (8), the present data suggest it is not sufficient to stimulate the hepatic and plasma lipoprotein responses to dietary cholesterol that are typical in ACAT2 sufficient mice. The present study extends our current understanding of the important role of intestinal-derived CE in cholesterol trafficking in vivo.
In addition to the expected decreases in plasma VLDL-C seen in ACAT2 deficiency, one of the characteristic changes in plasma lipids was the significant increase of TG in the conditional ACAT2 knockouts, especially in ACAT2L−/L− mice. Similar findings were observed in several other studies using whole body ACAT2 KO or ASO-mediated hepatic ACAT2 knockdown models (4–7, 15, 16). Liver perfusion assay indicated that more TG appeared in the perfusate when ACAT2 was unable to provide CE (6, 7). These data led to the speculation that the higher availability of TG for secretion resulted from a higher rate of mobilization of hepatic TG (7). A suggestion was made that the lack of CE in the intracellular lipid droplets could have led to a more rapid fatty acid mobilization of preformed TG via intracellular triglyceride hydrolases, although the identity of the hydrolytic enzymes involved is unknown (7). ACAT2SI−/SI− mice also appeared to have higher plasma TG in response to increased amounts of dietary cholesterol. The exact mechanism is unknown. From lymph duct cannulation studies in our laboratory, chylomicrons from ACAT2−/− mice have more TG mass compared with wild-type mice (8). However, due to rapid and efficient clearance of chylomicron remnants from the circulation by liver (22, 23), this would not appear to be a major mechanism of TG increase in the plasma. It is possible that, as a result of chylomicrons delivering less CE to the liver in ACAT2SI−/SI− mice, a CE-poor lipid droplet may result in the liver, leading to a similar effect on TG mobilization as was observed in mice with the loss of hepatic ACAT2 (6, 7).
An interesting phenotype in the ACAT2 conditional KO mice is that free cholesterol (FC) does not accumulate in the liver in response to dietary cholesterol feeding. Especially among the ACAT2L−/L− mice, a large quantity of dietary FC is delivered to the liver, but it cannot be esterified. Logically one would anticipate that hepatic FC would be elevated. However, hepatic FC was not increased after dietary cholesterol feeding (Fig. 4D). Furthermore, no difference in FC level was detected in feces (Fig. 2B), bile (Fig. 6A), or total plasma cholesterol (Fig. 3B). Among previous studies in mice with hepatocyte-specific knockdown of ACAT2 using ASOs, we found that more FC appeared in the liver perfusate, whereas CE secretion persisted (6). In those studies we demonstrated that excessive hepatic FC was delivered to the proximal small intestine for fecal disposal. In pilot liver perfusion studies, we found that FC was not different between ACAT2L−/L− and ACAT2fl/fl control mice (J.M. Brown, J.K. Sawyer, M.A. Davis, and L.L. Rudel, unpublished observations). One major difference between the current studies and the work by Brown et al. (6) is that the latter was done in the mice on LDLr−/− apoB-100 only background. The absence of the LDL receptor dramatically alters hepatic lipoprotein metabolism. Additional studies are needed to clarify the mechanism by which excessive free cholesterol accumulation is avoided in ACAT2L−/L− mice fed high levels of dietary cholesterol.
Another possible mechanism of cholesterol trafficking out of the liver is through the membrane receptor-dependent pathway. Of particular interest is that ABCA1 is significantly increased in ACAT2L−/L− animals (Supplementary Table I). ABCA1 functions as a membrane transporter exporting free cholesterol out of the tissues and cells. Our group previously showed that ABCA1 was up-regulated in the liver when hepatic ACAT2 was specifically knocked down by ASO (6). Data presented herein suggested that ABCA1 mRNA expression tended to increase. However, plasma HDL was not significantly elevated, which would have been expected with increased levels of hepatic ABCA1. Future experiments with hepatocyte-specific deletion of ABCA1 and ACAT2 will allow us to address whether ABCA1 is necessary for export of hepatic cholesterol when ACAT2 is absent.
Our group recently showed in mice that cholesterol absorption and transport in chylomicrons as CE is significantly decreased due to the loss of intestinal ACAT2 (8). In the absence of ACAT2, chylomicrons collected via thoracic lymph duct cannulation were depleted in core CE, which had been replaced mainly with TG without any particle size alteration (8). When these CE-poor chylomicrons are delivered to liver as remnants, it presumably results in less cholesterol accumulation in the liver and subsequently less VLDL-C circulated into the blood. This implies possible pharmaceutical strategies for targeting intestinal ACAT2 to achieve a hypocholesterolemic effect. Furthermore, when probing for ACAT2 proteins from human liver and intestine biopsy samples, we have found that in many cases hepatic ACAT2 is expressed at a vanishingly low level, which is in contrast to the high expression level of ACAT2 in the human intestine (M.A. Davis and L.L. Rudel, unpublished observations). This also highlights what could be a potential approach to treat hypercholesterolemia in humans. Developing novel ACAT2 inhibitors could also benefit patients of lysosomal lipid storage diseases, such as lysozomal acid lipase deficiency or Niemann-Pick C diseases, where there is massive accumulation of cholesteryl esters or a build-up of unesterified cholesterol in a variety of tissues especially the liver (24–26). Another approach to lower intestinal cholesterol absorption is to inhibit the apical membrane transporter, the Niemann-Pick C1-like 1 (NPC1L1) protein, of the intestine. Ezetimibe, prescribed in clinics as Zetia, can effectively lower plasma LDL-C (27–29), presumably through its ability to inhibit NPC1L1 in enterocytes, which likely occurs without any defect in cholesterol esterification. Despite the reduced cholesterol absorption in both scenarios, the underlying mechanism would be distinctly different. This may warrant future studies to investigate whether there are advantages of developing intestinal ACAT2 inhibitors for reduction of CE transport during atherosclerosis progression versus the alternative approach of blocking cholesterol uptake from the intestinal lumen via NPC1L1.
In conclusion, our data demonstrate for the first time that CEs derived from liver and intestine promote potentially atherogenic hepatic CE accumulation and hyperlipidemia. Hepatic and intestinal ACAT2 could be potential pharmaceutical targets to prevent hypercholesterolemia and atherosclerosis progression. The present results suggest that inhibition of intestinal or hepatic ACAT2 can improve atherogenic hyperlipidemia, indicating that tissue-specific inhibitors for ACAT2 could be beneficial for atheroprotection.
Supplementary Material
Acknowledgments
We thank Heather Myers (Gladstone Institute of Cardiovascular Disease, UCSF) for her excellent technical assistance.
Footnotes
Abbreviations:
- ACAT2
- acyl-CoA:cholesterol acyltransferase 2
- ASO
- antisense oligonucleotide
- CE
- cholesterol ester
- FC
- free cholesterol
- KO
- knockout
- NPC1L1
- Niemann-Pick C1-like
- TG
- triglyceride
- TPC
- total plasma cholesterol
This work was supported by National Institutes of Health Grants P01-HL49373 (L.L.R.) and RO1-HL51710 (R.V.F.) and by American Heart Association Postdoctoral Fellowship 0625400U (J.M.B.). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or other granting agencies.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of a table.
REFERENCES
- 1.Joyce C., Skinner K., Anderson R. A., Rudel L. L. 1999. Acyl-coenzyme A:cholesteryl acyltransferase 2. Curr. Opin. Lipidol. 10: 89–95 [DOI] [PubMed] [Google Scholar]
- 2.Degirolamo C., Shelness G. S., Rudel L. L. 2009. LDL cholesteryl oleate as a predictor for atherosclerosis: evidence from human and animal studies on dietary fat. J. Lipid Res. 50(Suppl): S434–S439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee R. G., Kelley K. L., Sawyer J. K., Farese R. V., Jr, Parks J. S., Rudel L. L. 2004. Plasma cholesteryl esters provided by lecithin:cholesterol acyltransferase and acyl-coenzyme a:cholesterol acyltransferase 2 have opposite atherosclerotic potential. Circ. Res. 95: 998–1004 [DOI] [PubMed] [Google Scholar]
- 4.Willner E. L., Tow B., Buhman K. K., Wilson M., Sanan D. A., Rudel L. L., Farese R. V., Jr 2003. Deficiency of acyl CoA:cholesterol acyltransferase 2 prevents atherosclerosis in apolipoprotein E-deficient mice. Proc. Natl. Acad. Sci. USA. 100: 1262–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bell T. A., 3rd, Brown J. M., Graham M. J., Lemonidis K. M., Crooke R. M., Rudel L. L. 2006. Liver-specific inhibition of acyl-coenzyme a:cholesterol acyltransferase 2 with antisense oligonucleotides limits atherosclerosis development in apolipoprotein B100-only low-density lipoprotein receptor−/− mice. Arterioscler. Thromb. Vasc. Biol. 26: 1814–1820 [DOI] [PubMed] [Google Scholar]
- 6.Brown J. M., Bell T. A., 3rd, Alger H. M., Sawyer J. K., Smith T. L., Kelley K., Shah R., Wilson M. D., Davis M. A., Lee R. G., et al. 2008. Targeted depletion of hepatic ACAT2-driven cholesterol esterification reveals a non-biliary route for fecal neutral sterol loss. J. Biol. Chem. 283: 10522–10534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alger H. M., Brown J. M., Sawyer J. K., Kelley K. L., Shah R., Wilson M. D., Willingham M. C., Rudel L. L. 2010. Inhibition of acyl-coenzyme A:cholesterol acyltransferase 2 (ACAT2) prevents dietary cholesterol-associated steatosis by enhancing hepatic triglyceride mobilization. J. Biol. Chem. 285: 14267–14274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen T. M., Sawyer J. K., Kelley K. L., Davis M. A., Rudel L. L. 2012. Cholesterol esterification by ACAT2 is essential for efficient intestinal cholesterol absorption: evidence from thoracic lymph duct cannulation. J. Lipid Res. 53: 95–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grundy S. M. 1983. Absorption and metabolism of dietary cholesterol. Annu. Rev. Nutr. 3: 71–96 [DOI] [PubMed] [Google Scholar]
- 10.Heider J. G., Pickens C. E., Kelly L. A. 1983. Role of acyl CoA:cholesterol acyltransferase in cholesterol absorption and its inhibition by 57–118 in the rabbit. J. Lipid Res. 24: 1127–1134 [PubMed] [Google Scholar]
- 11.Krause B. R., Anderson M., Bisgaier C. L., Bocan T., Bousley R., DeHart P., Essenburg A., Hamelehle K., Homan R., Kieft K., et al. 1993. In vivo evidence that the lipid-regulating activity of the ACAT inhibitor CI-976 in rats is due to inhibition of both intestinal and liver ACAT. J. Lipid Res. 34: 279–294 [PubMed] [Google Scholar]
- 12.Lee H. T., Sliskovic D. R., Picard J. A., Roth B. D., Wierenga W., Hicks J. L., Bousley R. F., Hamelehle K. L., Homan R., Speyer C., et al. 1996. Inhibitors of acyl-CoA: cholesterol O-acyl transferase (ACAT) as hypocholesterolemic agents. CI-1011: an acyl sulfamate with unique cholesterol-lowering activity in animals fed noncholesterol-supplemented diets. J. Med. Chem. 39: 5031–5034 [DOI] [PubMed] [Google Scholar]
- 13.Ramharack R., Spahr M. A., Sekerke C. S., Stanfield R. L., Bousley R. F., Lee H. T., Krause B. K. 1998. CI-1011 lowers lipoprotein(a) and plasma cholesterol concentrations in chow-fed cynomolgus monkeys. Atherosclerosis. 136: 79–87 [DOI] [PubMed] [Google Scholar]
- 14.Suckling K. E., Stange E. F. 1985. Role of acyl-CoA: cholesterol acyltransferase in cellular cholesterol metabolism. J. Lipid Res. 26: 647–671 [PubMed] [Google Scholar]
- 15.Buhman K. K., Accad M., Novak S., Choi R. S., Wong J. S., Hamilton R. L., Turley S., Farese R. V., Jr 2000. Resistance to diet-induced hypercholesterolemia and gallstone formation in ACAT2-deficient mice. Nat. Med. 6: 1341–1347 [DOI] [PubMed] [Google Scholar]
- 16.Repa J. J., Buhman K. K., Farese R. V., Jr, Dietschy J. M., Turley S. D. 2004. ACAT2 deficiency limits cholesterol absorption in the cholesterol-fed mouse: impact on hepatic cholesterol homeostasis. Hepatology. 40: 1088–1097 [DOI] [PubMed] [Google Scholar]
- 17.Temel R. E., Lee R. G., Kelley K. L., Davis M. A., Shah R., Sawyer J. K., Wilson M. D., Rudel L. L. 2005. Intestinal cholesterol absorption is substantially reduced in mice deficient in both ABCA1 and ACAT2. J. Lipid Res. 46: 2423–2431 [DOI] [PubMed] [Google Scholar]
- 18.Turley S. D., Valasek M. A., Repa J. J., Dietschy J. M. 2010. Multiple mechanisms limit the accumulation of unesterified cholesterol in the small intestine of mice deficient in both ACAT2 and ABCA1. Am. J. Physiol. Gastrointest. Liver Physiol. 299: G1012–G1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee R. G., Willingham M. C., Davis M. A., Skinner K. A., Rudel L. L. 2000. Differential expression of ACAT1 and ACAT2 among cells within liver, intestine, kidney, and adrenal of nonhuman primates. J. Lipid Res. 41: 1991–2001 [PubMed] [Google Scholar]
- 20.Parini P., Davis M., Lada A. T., Erickson S. K., Wright T. L., Gustafsson U., Sahlin S., Einarsson C., Eriksson M., Angelin B., et al. 2004. ACAT2 is localized to hepatocytes and is the major cholesterol-esterifying enzyme in human liver. Circulation. 110: 2017–2023 [DOI] [PubMed] [Google Scholar]
- 21.Rudel L. L., Haines J., Sawyer J. K., Shah R., Wilson M. S., Carr T. P. 1997. Hepatic origin of cholesteryl oleate in coronary artery atherosclerosis in African green monkeys. Enrichment by dietary monounsaturated fat. J. Clin. Invest. 100: 74–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodman D. S. 1962. The metabolism of chylomicron cholesterol ester in the rat. J. Clin. Invest. 41: 1886–1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goodman D. S. 1965. Cholesterol ester metabolism. Physiol. Rev. 45: 747–839 [DOI] [PubMed] [Google Scholar]
- 24.Beltroy E. P., Liu B., Dietschy J. M., Turley S. D. 2007. Lysosomal unesterified cholesterol content correlates with liver cell death in murine Niemann-Pick type C disease. J. Lipid Res. 48: 869–881 [DOI] [PubMed] [Google Scholar]
- 25.Beltroy E. P., Richardson J. A., Horton J. D., Turley S. D., Dietschy J. M. 2005. Cholesterol accumulation and liver cell death in mice with Niemann-Pick type C disease. Hepatology. 42: 886–893 [DOI] [PubMed] [Google Scholar]
- 26.Ramirez C. M., Liu B., Aqul A., Taylor A. M., Repa J. J., Turley S. D., Dietschy J. M. 2011. Quantitative role of LAL, NPC2, and NPC1 in lysosomal cholesterol processing defined by genetic and pharmacological manipulations. J. Lipid Res. 52: 688–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ballantyne C. M., Abate N., Yuan Z., King T. R., Palmisano J. 2005. Dose-comparison study of the combination of ezetimibe and simvastatin (Vytorin) versus atorvastatin in patients with hypercholesterolemia: the Vytorin Versus Atorvastatin (VYVA) study. Am. Heart J. 149: 464–473 [DOI] [PubMed] [Google Scholar]
- 28.Bays H. E., Moore P. B., Drehobl M. A., Rosenblatt S., Toth P. D., Dujovne C. A., Knopp R. H., Lipka L. J., Lebeaut A. P., Yang B., et al. 2001. Effectiveness and tolerability of ezetimibe in patients with primary hypercholesterolemia: pooled analysis of two phase II studies. Clin. Ther. 23: 1209–1230 [DOI] [PubMed] [Google Scholar]
- 29.van Heek M., Farley C., Compton D. S., Hoos L., Alton K. B., Sybertz E. J., Davis H. R., Jr 2000. Comparison of the activity and disposition of the novel cholesterol absorption inhibitor, SCH58235, and its glucuronide, SCH60663. Br. J. Pharmacol. 129: 1748–1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.