Abstract
Cholesterol regulates the signaling of µ-opioid receptor in cell models, but it has not been demonstrated in mice or humans. Whether cholesterol regulates the signaling by mechanisms other than supporting the entirety of lipid raft microdomains is still unknown. By modulating cholesterol-enriched lipid raft microdomains and/or total cellular cholesterol contents in human embryonic kidney cells stably expressing µ-opioid receptor, we concluded that cholesterol stabilized opioid signaling both by supporting the lipid raft's entirety and by facilitating G protein coupling. Similar phenomena were observed in the primary rat hippocampal neurons. In addition, reducing the brain cholesterol level with simvastatin impaired the analgesic effect of opioids in mice, whereas the opioid analgesic effect was enhanced in mice fed a high-cholesterol diet. Furthermore, when the records of patients were analyzed, an inverse correlation between cholesterol levels and fentanyl doses used for anesthesia was identified, which suggested the mechanisms above could also be applicable to humans. Our results identified the interaction between opioids and cholesterol, which should be considered in clinics as a probable route for drug-drug interaction. Our studies also suggested that a low cholesterol level could lead to clinical issues, such as the observed impairment in opioid functions.
Keywords: anesthesia, cAMP signaling, G protein coupling, simvastatin
As an essential structural component of cell membrane, cholesterol is required to establish proper membrane permeability and fluidity. The contributions of cholesterol to the signaling of G protein-coupled receptors (GPCR) have been explained by the critical role of cholesterol in lipid raft microdomains (1). Lipid raft microdomains are dynamic plasma membrane microdomains containing high levels of cholesterol and sphingolipids (2). They are enriched with a variety of signaling factors, such as G proteins, GPCRs, and adenylyl cyclases (AC) (3–5). Because lipid raft microdomains may serve as an environment for the interaction between GPCRs and signaling molecules (1), the localization inside lipid raft microdomains might be essential for the signaling of GPCRs. Extracting cholesterol with methyl-β-cyclodextrin disrupts the lipid raft microdomains and attenuates the signaling of several GPCRs (4, 6, 7).
A cholesterol-palmitoyl interaction has been reported in the crystallographic dimeric interface of a β2-adrenergic receptor crystal structure (8). As GPCRs and G proteins are usually highly palmitoylated (9), it is possible to observe similar cholesterol-palmitoyl interaction with other GPCRs. If this cholesterol-palmitoyl interaction did facilitate the dimerization of GPCRs, it might be another route for cholesterol to regulate receptor signaling, as the interaction surface appears to be too small for GPCR monomer to interact with G protein (10).
To determine whether cholesterol regulates receptor signaling by mechanisms other than supporting the entirety of lipid raft microdomains, two inhibitors were used in our studies. Simvastatin, a HMG-CoA reductase inhibitor, blocks the initial synthesis step of cholesterol, from HMG-CoA to mevalonate (11). Thus simvastatin could decrease the cellular content of cholesterol and subsequently disrupt the cholesterol-rich lipid raft microdomains. D-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol (DPDMP), a glucosylceramide synthase inhibitor, blocks the synthesis of glycospingolipids, which are also important components of lipid raft microdomains (12). Thus DPDMP should disrupt lipid raft microdomains without affecting cholesterol content. By monitoring the effects of these two inhibitors on receptor signaling, how cholesterol modulates receptor signaling could be partially explored.
As a typical Gi/o coupled receptor, µ-opioid receptor (OPRM1) locates in the lipid raft microdomains (13). Reducing cholesterol level not only disrupts the lipid raft microdomains but also attenuates the downstream signaling. Thus OPRM1 is a suitable model to study the mechanisms used by cholesterol to regulate receptor signaling.
Similar to other GPCRs, OPRM1 also exhibits agonist-biased signaling (14). Cholesterol has different influences on the signaling induced by these two types of agonists (13). Extracting the cholesterol attenuates the AC inhibition induced by either morphine or etorphine. Reducing the cholesterol level inhibits only morphine-induced, not etorphine-induced, extracellular signal-regulated kinase (ERK) phosphorylation. To avoid the possible influences from agonist-biased signaling, AC inhibition (but not ERK phosphorylation) was monitored in the current studies.
In addition, the relationship between cholesterol level and opioid signaling was tested in the primary cultures of rat hippocampal neurons that have OPRM1 expressed endogenously (15). Correlation between cholesterol level and in vivo opioid functions was also tested by manipulating the cholesterol level in mice and by analyzing the clinical records of patients.
MATERIALS AND METHODS
Cell models and OPRM1 signaling
HEK293 cells that stably express OPRM1 and primary cultures of rat hippocampal neurons were prepared as described previously (15, 16). Opioid-induced AC inhibition was quantified by measuring the intracellular cAMP level according to the previous reports (17). The data was analyzed by using GraphPad Prism 5.0.
Animal studies
The research was conducted in conformity with Public Health Service Policy on Humane Care and Use of Laboratory Animals, incorporated in the Institute for Laboratory Animal Research Guide for Care and Use of Laboratory Animals. The studies were also approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Minnesota.
Six-week-old CD1 (ICR) male mice were obtained from Charles River Laboratories two weeks before experiments. Light intensity was adjusted for 3–5 s baseline latency. Cutoff time of 12 s was used to minimize tail tissue damage. Tail-flick responses were recorded 30 min after subcutaneous morphine injection. Percentage of maximum possible effect (%MPE) was calculated by the following formula: (measured latency − baseline latency) 100 / (cutoff time − baseline latency). Each dose involved 8–12 mice. ED50 values were generated by analyzing the dose response curves by nonlinear regression.
For the simvastatin experiment, one group of mice was given simvastatin subcutaneously at 10 mg/kg, three times a day for 14 days. Another group was given a vehicle in the same paradigm. For the cholesterol supplement experiment, one group of mice was fed with control diet (58R4), while another group was fed with high-cholesterol diet (5S8M) for 48 days. The ED50 values of opioids were measured before and after the diet manipulations. The doses of simvastatin and cholesterol were much higher than those under normal conditions. These high doses were used to modulate brain cholesterol level and to assess the possible consequences.
Continuous sucrose gradient
Cells or tissues were homogenized in 0.32 M sucrose and 10 mM HEPES (pH 7.7). Crude lysate was then centrifuged at 1,000 g for 10 min at 4°C, the supernatant was collected, and the pellet was rehomogenized. These processes were repeated until the pellet appeared translucent. The collected supernatant was centrifuged at 100,000 g for 60 min at 4°C. The resulted pellet was dissolved in 0.5 M sodium carbonate and then underlaid in a 5–30% continuous sucrose gradient generated with the Gradient Station (BioComp, Fredericton, Canada) and centrifuged for 16 h at 32,000 rpm in a SW41 rotor (Beckman, Brea, CA) as reported previously (13). The gradient was then separated into 12 fractions (1 ml each from low to high density). Cholesterol concentrations were determined in the first 10 fractions using an Amplex Red Cholesterol Assay Kit (Invitrogen, Carlsbad, CA). The amount of OPRM1 in the same fractions was determined by using [3H] diprenorphine binding as described previously (18).
Membrane purification and cholesterol assay
Cholesterol concentrations were determined by using the Amplex Red Cholesterol Assay Kit (Invitrogen) on the cell membrane preparation and whole-cell lysate.
Fluorescence resonance energy transfer
CFP was fused to the C terminus of OPRM1. YFPGαi2 has YFP inserted between residues 91 and 92 of Gαi2 (19). Throughout the studies, all fluorescence resonance energy transfer (FRET) values are expressed as the normalized net FRET by the following formula: [IFRET – (ICFP × CoA) – (IYFP × CoB)] / [the square root of (ICFP × IYFP)]. IFRET is the fluorescence intensity when a CFP-YFP (excitation-emission) filter set is used, ICFP is the fluorescence intensity when a CFP-CFP filter set is used, and IYFP is the fluorescence intensity when a YFP-YFP filter set is used. CoA was determined in the cells transfected with only CFP constructs by the following formula: CoA = IFRET / ICFP. CoB was determined similarly. Including “square root” in the formula eliminates the influence from the differential expression of CFP- and YFP-conjugated protein. Briefly, more than 20 individual regions on the cell membrane of a single cell were analyzed, and more than 12 individual cells were analyzed for each sample.
Human studies
The human studies did not include any analysis of human samples. All the studies were based on the patients’ clinical records. The investigation was conducted according to Declaration of Helsinki principles and approved by the Institutional Review Board. The written informed consents were received from participants or their representatives prior to inclusion in the study. Participants were identified by number, not by name.
Transient transfection
The pCMV-shuttle vector (Stratagene) was used in these studies. cDNA of receptor Gαi2 and their fluorescence-conjugated constructs were controlled by the CMV promoter. The transient transfection was performed with Lipofectamine 2000 (Invitrogen), following instructions provided by the company. Cells were allowed to rest for 24 h before further treatment.
RESULTS
Simvastatin and DPDMP modulate membrane composition
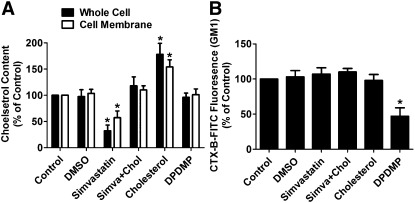
Human embryonic kidney (HEK) cells stably expressing OPRM1 (named HEKOPRM1) were used in this study. These cells have OPRM1 expressed exogenously at about 6 pmol/mg protein. To decrease the cholesterol level in the HEKOPRM1 cells, simvastatin, a HMG-CoA reductase inhibitor, was used. As indicated in Fig. 1A, 12 h treatment with 0.5 µM simvastatin induced a 43 ± 13% (n = 4) decrease in cholesterol level on cell membrane and a 68 ± 11% (n = 4) decrease in whole-cell lysate. These decreases were not due to the DMSO that was used to dissolve the simvastatin (stock concentration of simvastatin: 1 mM) (Fig. 1A). In addition, when the cells were cultured in medium supplemented with 40 ng/ml cholesterol for 12 h, the cholesterol levels were increased. When both simvastatin and cholesterol were included in the culture medium, no significant change in cholesterol level was observed (Fig. 1A).
Fig. 1.
Simvastatin and DPDMP affect membrane composition. HEKOPRM1 cells were treated with PBS (Control), DMSO (DMSO), 0.5 μM simvastatin (Simvastatin), 0.5 μM simvastatin with 40 ng/ml cholesterol (Simva+Chol), 40 ng/ml cholesterol (Cholesterol), or 20 μM DPDMP (DPDMP) for 12 h. The cholesterol levels were determined in whole-cell lysis and on cell membrane in (A). The amounts of membrane GM1 were determined by FACS with CTX-B-FITC in (B). Data were analyzed by one-way ANOVA with Dunnett's posthoc test. Error bars and asterisks represent SD (n ≥ 4) and significant difference from Control, respectively.
DPDMP was used to inhibit glucosylceramide synthase and to block the synthesis of glycospingolipids. The effect of DPDMP was verified by using cholera toxin B conjugated with FITC (CTX-B-FITC) to stain the monosialotetrahexosylganglioside (GM1) on the cell membrane. As indicated in Fig. 1B, 12 h treatment with 20 µM DPDMP decreased membrane GM1 to 47 ± 12% (n = 4) of the basal level. But simvastatin and cholesterol did not affect the amount of membrane GM1 (Fig. 1B). DPDMP treatment had no effect on the cellular cholesterol levels (Fig. 1A).
Both simvastatin and DPDMP disrupt lipid raft microdomains
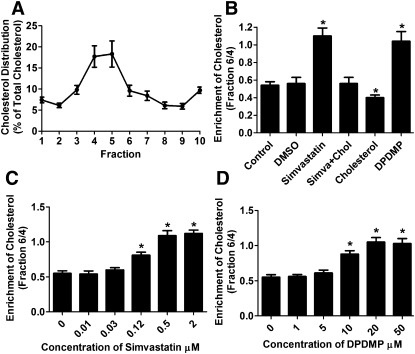
Continuous sucrose gradient was used to separate the purified cell membrane of HEKOPRM1 cells into 12 fractions, and the cholesterol levels in first 10 fractions were then determined. More cholesterol was identified in fractions 4 and 5 than in other fractions (Fig. 2A). To quantify this enrichment, the cholesterol amount in fraction 6 was compared with that in fraction 4; the ratio was 0.54 ± 0.04 and 0.56 ± 0.07 (n = 4) in control and DMSO-treated cells, respectively (Fig. 2B). The 12 h simvastatin treatment disrupted this enrichment of cholesterol and increased the ratio to 1.10 ± 0.09 (n = 4) (Fig. 2B). Cholesterol antagonized the effect of simvastatin and decreased the ratio to the basal level when it was added to the medium at the same time as simvastatin. Therefore, modulating cholesterol level with simvastatin affected the organization of cell membrane by inhibiting cholesterol synthesis.
Fig. 2.
Simvastatin and DPDMP affect cholesterol distribution on membrane. (A) Cell membrane of HEKOPRM1 cells were subjected for the continuous sucrose gradient as described in Materials and Methods. The cholesterol amounts were determined in each fraction and normalized against the whole cholesterol amount in the first 10 fractions. (B) Cells were treated as in Fig. 1A. The ratios of cholesterol amounts in fraction 6 to those in fraction 4 were summarized. (C, D) HEKOPRM1 cells were treated with indicated concentration of simvastatin (C) or DPDMP (D) for 12 h. The ratios of cholesterol amounts in fraction 6 to those in fraction 4 were summarized. Data were analyzed by one-way ANOVA with Dunnett's posthoc test. Error bars and asterisks represent SD (n ≥ 4) and significant difference from Control, respectively.
Although DPDMP treatment did not alter the total cholesterol level either in whole-cell homogenates or in cell membrane preparations (Fig. 1A), DPDMP treatment disrupted the lipid raft microdomains in HEKOPRM1 cells as reported with other cell lines (20–22). The ratio of cholesterol level in fraction 6 to that in fraction 4 was increased to 1.04 ± 0.11 (n = 4) (Fig. 2B). Thus inhibiting glucosylceramide synthase with DPDMP also affected the organization of cell membrane.
To compare the functions of simvastatin and DPDMP, the dose dependency of the two inhibitors to affect cholesterol enrichment was determined (Fig. 2C, D). The 0.5 μM simvastatin and 20 μM DPDMP induced similar and close to maximum impairment on cholesterol enrichment. These concentrations of simvastatin and DPDMP were used in subsequent studies to investigate the probable mechanism of cholesterol effects on OPRM1 signaling.
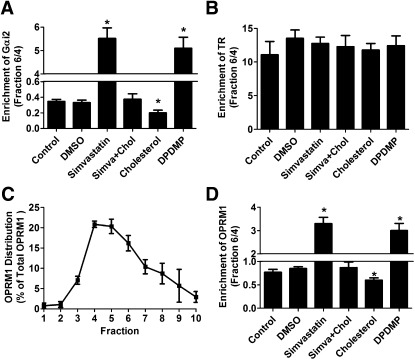
If simvastatin and DPDMP both disrupted the lipid raft microdomains, consistent membrane distributions of Gαi2 (marker of lipid raft microdomains) and transferrin receptor (TR; marker of nonraft microdomains) should be observed after these treatments (23). In control untreated HEKOPRM1 cells, the ratio of Gαi2 amount in faction 6 to that in fraction 4 was 0.35 ± 0.03 (n = 4). This ratio was increased to 5.52 ± 0.55 and 5.10 ± 0.47 (n = 4) after treatment with simvastatin and DPDMP, respectively (Fig. 3A). Cholesterol treatment decreased this ratio to 0.20 ± 0.03 when used alone, and it decreased the ratio to basal level (0.38 ± 0.07, n = 4) when used with simvastatin (Fig. 3A). The distribution of TR was not affected by these treatments, as majority of TR locates in the nonraft microdomains (Fig. 3B). As many other GPCRs (1, 13), OPRM1 locates within the lipid raft in the absence of agonist (Fig. 3C). To further verify the entirety of the lipid raft, the amounts of receptor in fraction 6 were compared with those in faction 4. In control and DMSO-treated cells, OPRM1 located in the lipid raft microdomains (Fig. 3D). OPRM1 translocated into nonraft microdomains after treatment with either simvastatin or DPDMP. Thus, both simvastatin and DPDMP treatment disrupted the lipid raft microdomains.
Fig. 3.
Simvastatin and DPDMP disrupt lipid raft microdomains. HEKOPRM1 cells were treated with PBS (Control), DMSO (DMSO), 0.5 μM simvastatin (Simvastatin), 0.5 μM simvastatin with 40 ng/ml cholesterol (Simva+Chol), 40 ng/ml cholesterol (Cholesterol), or 20 μM DPDMP (DPDMP) for 12 h. Cell membrane was subjected for the continuous sucrose gradient. The ratios of protein amounts in fraction 6 to those in fraction 4 were calculated. Lipid raft marker of Gαi2 was summarized in (A) and nonraft marker TR was summarized in (B). The distribution of OPRM1 on cell membrane was illustrated by the percentage amounts of OPRM1 in the first 10 fractions in the continuous sucrose gradient under “Control” condition (C). The ratios of OPRM1 amounts in fraction 6 to those in fraction 4 were summarized in (D). Data were analyzed by one-way ANOVA with Dunnett's posthoc test. Error bars and asterisks represent SD (n ≥ 4) and significant difference from Control, respectively.
Simvastatin and DPDMP treatments alter OPRM1 signaling differentially
Decreasing the cholesterol level leads to the attenuation of morphine-induced signaling in HEKOPRM1 cells (13). The intracellular level of cAMP was monitored to determine how cholesterol affects the abilities of opioids to induce AC inhibition. Morphine induced AC inhibition with a KI value of 11.5 ± 2.0 nM and a maximum inhibition at 85 ± 3.1% (n = 4) in control untreated cells (Table 1). AC inhibition was attenuated in simvastatin-treated HEKOPRM1 cells. This attenuation could be prevented when 40 ng/ml cholesterol was included in the medium. DPDMP treatment also decreased the ability of morphine to induce AC inhibition.
TABLE 1.
Cholesterol level affects opioid signaling in HEKOPRM1 cells
| AC Inhibition by Morphine |
AC Inhibition by Fentanyl |
|||
| KI (nM) | Maximum inhibition (%) | KI (pM) | Maximum inhibition (%) | |
| Control | 11.5 ± 2.0 | 85 ± 3.1 | 103 ± 20 | 83 ± 1.7 |
| DMSO | 10.8 ± 2.4 | 83 ± 2.7 | 108 ± 19 | 85 ± 2.6 |
| Simvastatin | 33.7 ± 3.1* | 43 ± 3.4* | 378 ± 41* | 40 ± 3.9* |
| Simva+Chol | 12.8 ± 2.4 | 80 ± 2.7 | 112 ± 31 | 80 ± 3.9 |
| Cholesterol | 8.3 ± 1.6* | 93 ± 1.1* | 78 ± 26* | 91 ± 1.4* |
| DPDMP | 23.9 ± 1.9* | 62 ± 2.9* | 247 ± 36* | 55 ± 4.0* |
The HEKOPRM1 cells were treated with PBS (Control), DMSO (DMSO), 0.5 μM simvastatin (Simvastatin), 0.5 μM simvastatin with 40 ng/ml cholesterol (Simva+Chol), 40 ng/ml cholesterol (Cholesterol), or 20 μM DPDMP (DPDMP) for 12 h. Serial concentrations of morphine (10 μM∼0.01 nM) or fentanyl (100 nM∼0.1 pM) were used to determine the concentration-dependent AC inhibition as described in Materials and Methods. AC inhibition induced by agonists was indicated by averaging the IC50 (KI) and the in individual experiments. No difference in the basal levels of OPRM1 signaling was observed in different groups. Data were analyzed by one-way ANOVA with Dunnett's posthoc test. ± and asterisks represent SD (n ≥ 4) and significant difference from Control, respectively. There were also significant differences between the Simvastatin and DPDMP groups.
When the effects of simvastatin on morphine-induced AC inhibition were compared with those of DPDMP, significant differences were identified (Table 1). Simvastatin impaired the maximal AC inhibition by about 49%, whereas DPDMP impaired it only by about 27%. As simvastatin and DPDMP disrupted lipid raft microdomains similarly and near totally at these concentrations (Fig. 2C, D), the difference in modulating receptor signaling should be due to their different abilities to affect cholesterol content, thereby leading to lipid raft disruption.
When fentanyl was used as the agonist, similar observations were obtained (Table 1). Simvastatin had higher ability than DPDMP to impair fentanyl-induced AC inhibition. Thus, cellular cholesterol content probably affects receptor signaling by mechanisms other than supporting the entirety of lipid raft microdomains.
Dual functions of cholesterol in regulating receptor signaling
Simvastatin, but not DPDMP, decreased the cellular content of cholesterol (Fig. 1). In addition, the two inhibitors exhibited differences in their abilities to affect opioid-induced AC inhibition. Thus cholesterol might contribute to receptor signaling via two mechanisms: supporting the entirety of lipid raft microdomains and facilitating receptor signaling directly. The higher ability of simvastatin to impair receptor signaling than DPDMP could be due to the ability of simvastatin to impair both mechanisms.
Opioid-induced AC inhibition has been suggested to be mediated by Gαi2, and OPRM1 is anchored to the lipid raft microdomain by Gαi2 in the absence of an agonist (13). Thus cholesterol may regulate receptor signaling by directly influencing receptor-G protein coupling.
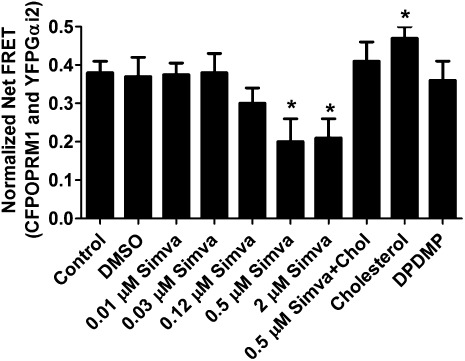
Receptor-Gαi2 coupling was determined by testing the FRET between CFPOPRM1 and YFPGαi2 in HEK cells. The amounts of transfected CFPOPRM1 and YFPGαi2 were tightly controlled to achieve similar expression levels of fluorescence-conjugated proteins in different experimental groups. As indicated in Fig. 4, the normalized net FRET value under control condition was 0.38 ± 0.03 (n = 12), which could be decreased to 0.20 ± 0.06 (n = 12) by 0.5 µM simvastatin. The dose dependency of simvastatin to affect receptor-G protein coupling was also determined. However, DPDMP did not affect Gαi2 coupling, as indicated by the normalized net FRET value after this inhibitor treatment (0.39 ± 0.05, n = 12) (Fig. 4A).
Fig. 4.
Simvastatin and DPDMP affect Gαi2 coupling differentially. HEKOPRM1 cells were transfected with CFPOPRM1 and YFPGαi2. One day after the transfection, the cells were treated PBS (Control), DMSO (DMSO), 0.01 μM simvastatin (0.01 µM Simva), 0.03 μM simvastatin (0.03 µM Simva), 0.12 μM simvastatin (0.12 µM Simva), 0.5 μM simvastatin (0.5 µM Simva), 2 μM simvastatin (2 µM Simva), 0.5 μM simvastatin with 40 ng/ml cholesterol (Simva+Chol), 40 ng/ml cholesterol (Cholesterol), or 20 μM DPDMP (DPDMP) for 12 h. The normalized net FRET was determined on the cell membrane as described in Materials and Methods. Data were analyzed by one-way ANOVA with Dunnett's posthoc test. Error bars and asterisks represent SD (n ≥ 12) and significant difference from Control, respectively.
In summary, DPDMP disrupted lipid raft microdomains, but it did not affect Gαi2 coupling with the receptor. Thus lipid raft microdomains contributed to receptor signaling by a mechanism other than the protein partitioning into microdomains facilitating receptor-G protein coupling due to their proximity. In addition, the higher ability of simvastatin than DPDMP to impair AC inhibition could be explained by the fact that simvastatin not only disrupted lipid raft microdomains but also decreased the cholesterol content. Thus cholesterol contributes to receptor signaling both by supporting the entirety of lipid raft microdomains and by directly stabilizing the interaction between receptor and Gαi2.
Dual functions of cholesterol in primary neuronal cultures
The HEKOPRM1 cell used above heterologously expressed a high level of OPRM1. To establish a similar cholesterol effect on endogenously expressed OPRM1, the AC inhibition was also monitored in the primary cultures of rat hippocampal neurons. The AC inhibition induced by opioids was much lower in primary cultures of rat hippocampal neurons than in HEKOPRM1 cells. Morphine induced AC inhibition with KI and maximum inhibition at 20.3 ± 2.3 nM and 35 ± 2.9% (n = 4), respectively (Table 2). The ability of morphine to induce AC inhibition in primary cultures was only about 41% of that in HEKOPRM1 cells. However, similar results were observed with the two inhibitors.
TABLE 2.
Cholesterol level affects opioid signaling in primary hippocampal cultures
| AC Inhibition by Morphine |
AC Inhibition by Fentanyl |
|||
| KI (nM) | Maximum inhibition (%) | KI (pM) | Maximum inhibition (%) | |
| Control | 20.3 ± 2.3 | 35 ± 2.9 | 175 ± 26 | 29 ± 2.7 |
| DMSO | 24.0 ± 3.4 | 31 ± 3.3 | 198 ± 22 | 31 ± 2.4 |
| Simvastatin | 55.7 ± 4.1* | 14 ± 2.7* | 432 ± 37* | 11 ± 1.9* |
| Simva+Chol | 21.8 ± 2.8 | 30 ± 2.5 | 212 ± 21 | 27 ± 2.9 |
| Cholesterol | 16.3 ± 2.1* | 47 ± 3.1* | 122 ± 18* | 41 ± 3.4* |
| DPDMP | 34.6 ± 3.2* | 22 ± 2.6* | 317 ± 27* | 21 ± 3.0* |
The primary neuron cultures were treated with PBS (Control), DMSO (DMSO), 0.5 μM simvastatin (Simvastatin), 0.5 μM simvastatin with 40 ng/ml cholesterol (Simva+Chol), 40 ng/ml cholesterol (Cholesterol), or 20 μM DPDMP (DPDMP) for 24 h. AC inhibition was carried out and analyzed as in Table 1. There were also significant differences between the Simvastatin and DPDMP groups. Asterisks represent the significant difference from Control.
Simvastatin attenuated opioid-induced AC inhibition more significantly (maximum inhibition from 35 ± 2.9% to 14 ± 2.7%, n = 4) than did DPDMP (maximum inhibition from 35 ± 2.9% to 22 ± 2.6%, n = 4). Thus the dual functions of cholesterol in regulating receptor signaling also exist in endogenously expressed OPRM1.
Simvastatin decreases the antinociceptive effects of opioids in mice
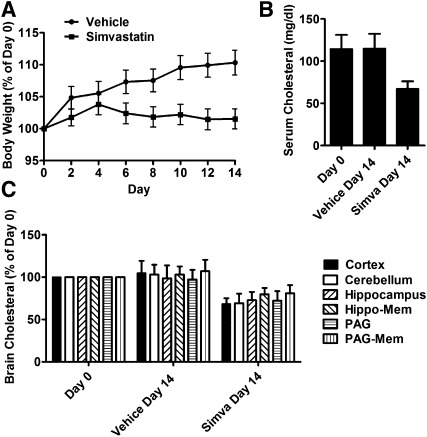
Cholesterol content influenced the signaling of OPRM1 in both HEKOPRM1 cells and primary neuron cultures. Whether cholesterol can affect the in vivo functions of opioids remains to be demonstrated. We decided to lower the in vivo cholesterol level in mice by the injection of simvastatin. Two-month-old CD-1 mice were divided into two groups. One group of mice was injected with 10 mg/kg simvastatin subcutaneously three times a day for 14 days. The other group of mice was given a vehicle on the same schedule. The mice injected with vehicle gained weight more quickly (10 ± 2%, n = 32) than the mice injected with simvastatin (2 ± 2%, n = 32) (Fig. 5A). However, there was no significant difference between the amounts of food or water consumed.
Fig. 5.
Simvastatin modulates opioid analgesia. Two groups were injected with 10 mg/kg simvastatin or vehicle twice per day for 14 days. (A) Body weights of each mouse were measured every two days and normalized to those in day 0. (B) Cholesterol levels were determined on day 0 and day 14 in serum. (C) Cholesterol levels were determined on day 0 and day 14 in cortex, cerebellum, hippocampus, the cell membrane of neurons in hippocampal (Hippo-Mem), PAG, and the cell membrane of neurons in PAG (PAG-Mem). Data were analyzed by one-way ANOVA with Dunnett's posthoc test. Error bars represent SD (n ≥ 10) and significant difference from day 0, respectively.
Fourteen-day injection of simvastatin decreased the serum cholesterol from 114 ± 16 mg/dl (n = 64) to 67 ± 9 mg/dl (n = 32) (Fig. 5B). In addition, the cholesterol contents in different regions of mice brains were determined. Although brain cholesterol level is tightly regulated (24), statins, including simvastatin and lovastatin, can modulate the brain cholesterol level (25, 26). The cholesterol contents in the cortex, cerebellum, hippocampus and periaqueductal gray (PAG) in mice injected with simvastatin were decreased. It has been reported that OPRM1 has a high expression level in hippocampus and PAG (27); therefore, the cell membrane of these two regions were extracted. As indicated in Fig. 5C, there was a 20 ± 8% decrease in the membrane cholesterol in hippocampal region, and a 19 ± 10% decrease in PAG region. Simvastatin injection, therefore, decreased the cholesterol level in mouse brain.
The antinociceptive effects of opioids were measured both before and after the simvastatin or vehicle administration. On day 0, the ED50 value of morphine was determined to be 2.7 (1.9∼3.9) mg/kg (Table 3). When morphine antinociceptive effect was tested in the mice injected with simvastatin for 14 days, a rightward shift of the dose-response curve was observed, and the morphine ED50 value increased to 5.6 (4.2∼9.1) mg/kg. The increase in morphine ED50 was not due to the change in the baseline of tail-flick response (Table 3).
TABLE 3.
Cholesterol level influences the antinociceptive effects of opioids in mice
| Tail-flick Baseline | ED50 of Morphine | ED50 of Fentanyl | |
| mg/kg sc | µg/kg sc | ||
| Low cholesterol | |||
| Day 0 | 3.7 ± 0.5 | 2.7 (1.9∼3.9) | 27 (20∼35) |
| Vehicle day 14 | 4.2 ± 0.5 | 2.2 (1.7∼2.9) | 25 (16∼36) |
| Simvastatin day 14 | 3.6 ± 0.6 | 5.6 (4.2∼9.1)* | 50 (36∼79)* |
| High cholesterol | |||
| Day 0 | 3.9 ± 0.4 | 2.9 (2.0∼4.4) | 31 (26∼40) |
| Vehicle day 48 | 4.4 ± 0.6 | 2.9 (2.4∼3.9) | 30 (25∼37) |
| High-cholesterol day 48 | 4.0 ± 0.4 | 1.6 (1.2∼2.1)* | 21 (15∼27)* |
Two groups of mice (n = 32) were injected with 10 mg/kg simvastatin or vehicle twice per day for 14 days. The baseline latencies of tail flick test on day 0 and day 14 were summarized. ED50 of opioids were determined on day 0 and in two groups of mice separately on day 14. ED50 were calculated from the dose-response curves with GraphPad Prism 5.0. The 95% confidence areas of ED50 were listed and used to do statistical analysis with GraphPad Prism 5.0. Simvastatin injection led to the significant changes of the ED50 of opioids. Another two groups of mice (n = 32) were fed with control diet and high-cholesterol diet for 48 days. The ED50 of opioids were determined on day 0 and day 48 and further analyzed as above. Asterisks represent the significant difference from Day 0.
The antinociceptive effect of fentanyl was determined under the same paradigm: 14-day simvastatin treatment increased the ED50 value of fentanyl from 27 (20∼35) µg/kg to 50 (36∼79) µg/kg (Table 3). Thus, similar to morphine, the antinociceptive effect of fentanyl can be influenced by serum, brain, and membrane cholesterol level.
High-cholesterol diet increases the antinociceptive effect of opioids
To further test the correlation between cholesterol content and opioid antinociceptive effect in mice, a special designed diet from TestDiet (Richmond, IN) was given to the mice. The control diet (58R4) was not supplemented with cholesterol, and only 10.3% of the whole energy (3.78 kcal/g) was provided by fat. The high-cholesterol diet (5S8M) was supplemented with 5% cholesterol by weight, and 42.9% of the whole energy (4.63 kcal/g) was provided by fat. The detailed ingredients of the two diets are presented in Table 4.
TABLE 4.
Ingredients of control and high-cholesterol diets
| Ingredients (%) | 58R4 | 5S8M |
| Casein-vitamin free | 18.9555 | 22.1766 |
| Corn starch | 35.5417 | 18.2536 |
| Cocoa butter | 1.8956 | 17.1869 |
| Sucrose | 18.9555 | 12.5298 |
| Maltodextrin | 11.8472 | 7.8727 |
| Powdered cellulose | 4.7389 | 5.5442 |
| Cholesterol | 0.0000 | 5.0000 |
| Soybean oil | 2.3694 | 2.7721 |
| Potassium citrate | 1.5638 | 2.0000 |
| Calcium phosphate | 1.2321 | 1.8296 |
| AIN-76A vitamin mix | 0.9478 | 1.4415 |
| Clinton salt mix | 0.9478 | 1.1088 |
| Calcium carbonate | 0.5213 | 0.6099 |
| L-cystine | 0.2843 | 0.3327 |
| Choline bitartrate | 0.1896 | 0.2218 |
| Blue dye | 0.0000 | 0.0055 |
| Red dye | 0.0000 | 0.0055 |
| Yellow dye | 0.0095 | 0.0000 |
| Energy (kcal/g) | 3.78 | 4.63 |
| Protein (%) | 18.7 | 19.1 |
| Fat (%) | 10.3 | 42.9 |
| Carbohydrate (%) | 71.0 | 38.0 |
Ingredients of the control diet (58R4) and high-cholesterol diet (5S8M), as well as the energy provided by the different compositions of the diets.
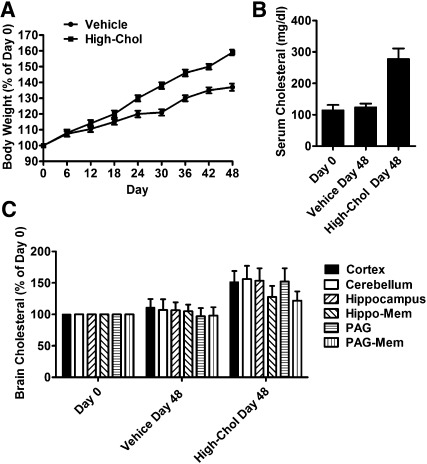
Two-month-old CD-1 mice were divided into two groups. One group of mice was fed with control diet for 48 days. The other group of mice was fed with high-cholesterol diet. The weight of the mice fed the control diet increased by 37 ± 2%, whereas the weight of the mice fed the high-cholesterol diet increased by 59 ± 2% after 48 days (n = 32) (Fig. 6A). However, there was no significant difference in food or water consumption.
Fig. 6.
High-cholesterol diet modulates opioid analgesia. Two groups were fed with control diet and high-cholesterol diet for 48 days. (A) Body weights of each mouse were measured every six days and normalized to those in day 0. (B) Cholesterol levels were determined on day 0 and day 48 in serum. (C) Cholesterol levels were determined on day 0 and day 48 in different regions in brain as in Fig. 3C. Data were analyzed by one-way ANOVA with Dunnett's posthoc test. Error bars represent SD (n ≥ 10) and significant difference from day 0, respectively.
The high-cholesterol diet increased the serum cholesterol from 115 ± 17 mg/dl (n = 64) to 278 ± 33 mg/dl (n = 32), whereas the control diet did not influence the serum cholesterol level significantly (Fig. 6B). The cholesterol contents in cortex, cerebellum, hippocampus, and PAG were increased by 51 ± 18%, 56 ± 21, 53 ± 20%, and 52 ± 21%, respectively. As indicated in Fig. 6C, there was a 28 ± 17% increase in the membrane cholesterol in hippocampal region, and a 22 ± 15% increase in PAG region. Thus the high-cholesterol diet efficiently increased the cholesterol level in mouse brain.
When morphine antinociceptive effect was tested in the mice fed the high-cholesterol diet for 48 days, a leftward shift of the dose-response curve was observed and the ED50 value decreased to 1.6 (1.2∼2.1) mg/kg. Similar observations were obtained when fentanyl was used. Therefore, the cholesterol level affects the opioid agonist antinociceptive effects (Table 3).
Cholesterol level correlates with the fentanyl usage in humans
An analysis of the clinical records in the China-Japan Friendship Hospital (Beijing, China) was carried out to determine whether a relationship exists between serum cholesterol level and opioid functions in humans.
From more than 2,000 orthopedic surgery records, 151 records of patients who had orthopedic surgery were selected. These patients had either decompression of the thoracic canal (with the fusion of bone graft and the pedicle screw fixation) or bilateral total hip arthroplasty. Patients had their serum cholesterol levels recorded before the surgery. The patients did not have severe diseases or receive intensive treatment for the previous five years. Because these patients were administered fentanyl to achieve similar levels of general endotracheal anesthesia at the beginning of surgery, the amounts of fentanyl were used to reflect the efficacy of fentanyl in these patients.
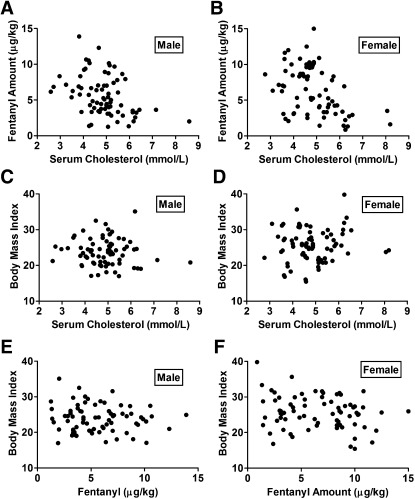
The 151 patients were separated into two groups according to gender, and their total serum cholesterol levels (mmol/l) were plotted against the fentanyl amounts (microgram per kilogram of weight) used before surgery (Fig. 7A, B). The correlation between cholesterol levels and fentanyl usage was tested with two-tailed Pearson's rank test (parametric). In male patients, Pearson's rank correlation coefficient was −0.3864 (P = 0.0006, Gaussian approximation, n = 75) (Fig. 7A). In female patients, Pearson's rank correlation coefficient was −0.4371 (P < 0.0001, Gaussian approximation, n = 76) (Fig. 7B).
Fig. 7.
Serum cholesterol levels inversely correlate with the amounts of fentanyl used for anesthesia. The total serum cholesterol levels were plotted against the fentanyl amounts used before the surgery, Male in (A) and Female in (B). The BMIs were plotted against the total serum cholesterol levels, Male in (C) and Female in (D). The BMIs were plotted against the fentanyl amounts used before the surgery, Male in (E) and Female in (F). The correlation was tested with two-tailed Pearson rank test. The Pearson's rank correlation coefficients and P values are listed in the text.
The two males and the two females with the highest cholesterol levels were then removed from our analysis to exclude any possible influence from these extreme cases. In male patients, Pearson's rank correlation coefficient was −0.3517 (P = 0.0023, Gaussian approximation, n = 73). In female patients, Pearson's rank correlation coefficient was −0.3998 (P = 0.0004; Gaussian approximation, n = 74). We also tested the correlation between cholesterol levels and fentanyl usages in patients whose serum cholesterol levels were between 3.2 and 6.2 mmol/l. In male patients, Pearson's rank correlation coefficient was −0.3062 (P = 0.0117, Gaussian approximation, n = 67). In female patients, Pearson's rank correlation coefficient was −0.2722 (P = 0.0258, Gaussian approximation, n = 67).
No correlation between body mass index (BMI) and cholesterol level was identified in the current study. The P values of Pearson's test were 0.5444 (n = 75) in male patients and 0.3769 (n = 76) in female patients (Fig. 7C, D). In addition, there was no correlation between BMI and fentanyl level. The P values of Pearson test were 0.3966 (n = 75) in male patients and 0.0785 (n = 76) in female patients (Fig. 7E, F).
We then selected patient pairs from the clinical records to carry out further analysis. The two patients in one pair had similar ages (the age difference was less than five years) and similar BMIs (the smaller BMI was more than 90% of the larger BMI) but significant differences in serum cholesterol (cholesterol difference was larger than 2 mmol/l). Only 27 patient pairs fulfilled these requirements. In 23 of these 27 pairs, the amount of fentanyl used by the patients with higher serum cholesterol level was at least 30% less than that used by the patient with lower serum cholesterol.
Therefore, a negative correlation between the serum cholesterol level and opioid efficacy exists in human subjects observed with in vitro cell models and in in vivo antinociceptive response in mice.
DISCUSSION
Disadvantages of low cholesterol levels
It has been widely accepted that high total cholesterol and high low-density lipoprotein cholesterol levels can lead to the high risk of cardiovascular diseases (28). It has also been suggested that lowering cholesterol can help the patients with cardiovascular diseases (29). Statins are a class of drugs used to lower cholesterol levels by inhibiting HMG-CoA reductase; there are several statins available on the market, including atorvastatin, lovastatin, pravastatin, and simvastatin. Using statins to lower cholesterol levels might also have debatable benefit for the patients with Alzheimer's disease (30, 31). Because the advantages of lowering cholesterol have been reported extensively, whether low cholesterol can lead to unexpected clinical issues has not been addressed.
In the current studies, we demonstrated a positive correlation between cholesterol level and the functions of opioids. Decreasing the cholesterol level leads to the impaired signaling of opioids in cell models and reduced analgesia effects in vivo, whereas increasing the cholesterol level results in the opposite effects. The clinical records studied also confirmed that subjects with lower cholesterol required more fentanyl to achieve the similar in vivo general endotracheal anesthesia effect. Thus, lowering cholesterol to a level below normal may result in decreased drug efficacy, as in the case of opioid analgesics.
Because cholesterol contributes to the signaling of many other GPCRs by maintaining the entirety of lipid raft microdomains (1), it is reasonable to deduce that lowering cholesterol may also impair the functions of other GPCRs. As over 40% marketed drugs target GPCRs, lowering the cholesterol to a level below normal may impair the efficacies of these drugs.
For opioids to function, they must cross the blood-brain barrier. Hence, subjects with larger fat tissues should have reduced, but sustained, opioid effects. Because our study was carried out to correlate cholesterol level and the usage of fentanyl at the beginning of surgery, subjects with a higher cholesterol level or more fat tissue should use more fentanyl to achieve the similar anesthesia stage if the pharmacokinetic was the major determinant. However, the actual observation was just the opposite, which suggests that the pharmacokinetics or the drug distribution was not the critical determinant under the current paradigm.
Opioid function is more sensitive to the decrease than to the increase of cellular cholesterol level
Because the blood-brain barrier is impermeable to circulating lipoprotein-bound cholesterol, it is considered difficult to modulate the cholesterol level in the central nervous system under normal conditions. However, we managed to control the brain cholesterol content in the current studies similar to other studies (25, 26, 32). As predicted, the modulations in serum cholesterol were much greater than those in brain regions (Figs. 5 and 6). The extreme conditions of high doses of simvastatin (10 mg/kg per injection, three injections per day for 14 days) and elevated cholesterol in the diet (5% in diet and ∼43% energy from fat) might be the reason we were able to the control brain cholesterol level successfully. Although these high doses of simvastatin and cholesterol would not be used clinically under normal conditions, they enabled us to investigate the possible consequences when the brain cholesterol level was modulated and they might have clinical implications when patients with abnormal cholesterol level are considered.
The signaling capability of OPRM1 was more sensitive to the decrease than to the increase in cholesterol content. On the one hand, the high-cholesterol diet increased the cholesterol content in the membrane from PAG by 22 ± 15%, whereas it decreased the ED50 value only by ∼45%. On the other hand, simvastatin administration decreased the cholesterol content by 19 ± 10%, but it increased the ED50 value of morphine by ∼107%. Similar phenomena were observed with the HEKOPRM1 cells.
These phenomena suggest that the cholesterol content in the cell membrane under normal conditions was sufficient to support the function of OPRM1. Thus, the increase in cholesterol content may be difficult to enhance the signaling of receptor, as the receptor level is the limiting factor. It is also reasonable to suggest that the influence on drug efficacy might not be observed when the cholesterol level could not be increased by a large amount in vivo.
Dual functions of cholesterol in regulating receptor signaling
Cholesterol-lowering chemicals disrupt lipid raft microdomains and subsequently affect OPRM1 signaling. This observation has been explained by lipid raft microdomains that provide the environment for signaling molecules to interact with the receptor (33). In the current studies, we demonstrated that maintaining lipid raft microdomains is not the only mechanism in which cholesterol controls receptor signaling. The concentrations of the two inhibitors that could disrupt lipid rafts were titrated to achieve similar effects on lipid raft microdomains. However, the two inhibitors exhibited differential effects when the AC inhibition and G-protein coupling were monitored. Because the effects induced by simvastatin could be restored by cholesterol supplementation and only simvastatin reduced cellular cholesterol level, it is suggested that cholesterol could regulate receptor signaling via mechanisms other than maintaining lipid raft microdomains.
As simvastatin, but not DPDMP, affected the receptor-Gαi2 coupling, the lipid raft integrity should not contribute to the receptor-Gαi2 coupling. Thus the decreased receptor-Gαi2 coupling after simvastatin treatment might be due to the reduction in cholesterol content within the receptor signaling complex. On the basis of these observations, we concluded that cholesterol regulates OPRM1 signaling via two mechanisms: i) facilitating the receptor-Gαi2 coupling and ii) maintaining the entirety of lipid raft microdomains, which might provide the environment for the interaction between the receptor and signaling molecules.
Although cholesterol did not selectively affect AC inhibition and analgesia effects induced by different opioids, the possible influence of interaction between cholesterol and agonist-biased signaling could not be eliminated.
In summary, the current studies demonstrated the mechanisms used by cholesterol to regulated OPRM1 signaling in HEKOPRM1 cells. The correlation between cholesterol content and opioid function was illustrated both in vitro (HEKOPRM1 cells and primary cultures) and in vivo (mice and humans). These results not only extend the understanding on GPCR signaling but also provide valuable information on the probable clinical interactions between opioids and cholesterol-lowering drugs.
Footnotes
Abbreviations:
- AC
- adenylyl cyclase
- BMI
- body mass index
- DPDMP
- d-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol
- FRET
- fluorescence resonance energy transfer
- GM1
- monosialotetrahexosylganglioside
- GPCR
- G protein-coupled receptor
- HEK
- human embryonic kidney
- OPRM1
- μ-opioid receptor
- PAG
- periaqueductal gray
- TR
- transferrin receptor
This work was supported by the National Natural Science Foundation of China (Grant 31100773), the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant XDA01020302), the Major New Drugs Innovation of the Major National Scientific and Technological Project (Grant 2011ZX09102-010-01), and the National Institutes of Health (Grants DA-023905 and DA-011806). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Chini B., Parenti M. 2004. G-protein coupled receptors in lipid rafts and caveolae: how, when and why do they go there? J. Mol. Endocrinol. 32: 325–338 [DOI] [PubMed] [Google Scholar]
- 2.Helms J. B., Zurzolo C. 2004. Lipids as targeting signals: lipid rafts and intracellular trafficking. Traffic. 5: 247–254 [DOI] [PubMed] [Google Scholar]
- 3.Ostrom R. S., Gregorian C., Drenan R. M., Xiang Y., Regan J. W., Insel P. A. 2001. Receptor number and caveolar co-localization determine receptor coupling efficiency to adenylyl cyclase. J. Biol. Chem. 276: 42063–42069 [DOI] [PubMed] [Google Scholar]
- 4.Navratil A. M., Bliss S. P., Berghorn K. A., Haughian J. M., Farmerie T. A., Graham J. K., Clay C. M., Roberson M. S. 2003. Constitutive localization of the gonadotropin-releasing hormone (GnRH) receptor to low density membrane microdomains is necessary for GnRH signaling to ERK. J. Biol. Chem. 278: 31593–31602 [DOI] [PubMed] [Google Scholar]
- 5.Li S., Couet J., Lisanti M. P. 1996. Src tyrosine kinases, Galpha subunits, and H-Ras share a common membrane-anchored scaffolding protein, caveolin. Caveolin binding negatively regulates the auto-activation of Src tyrosine kinases. J. Biol. Chem. 271: 29182–29190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monastyrskaya K., Hostettler A., Buergi S., Draeger A. 2005. The NK1 receptor localizes to the plasma membrane microdomains, and its activation is dependent on lipid raft integrity. J. Biol. Chem. 280: 7135–7146 [DOI] [PubMed] [Google Scholar]
- 7.Zhang L., Tetrault J., Wang W., Loh H. H., Law P. Y. 2006. Short- and long-term regulation of adenylyl cyclase activity by delta-opioid receptor are mediated by Galphai2 in neuroblastoma N2A cells. Mol. Pharmacol. 69: 1810–1819 [DOI] [PubMed] [Google Scholar]
- 8.Cherezov V., Rosenbaum D. M., Hanson M. A., Rasmussen S. G., Thian F. S., Kobilka T. S., Choi H. J., Kuhn P., Weis W. I., Kobilka B. K., et al. 2007. High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science. 318: 1258–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Probst W. C., Snyder L. A., Schuster D. I., Brosius J., Sealfon S. C. 1992. Sequence alignment of the G-protein coupled receptor superfamily. DNA Cell Biol. 11: 1–20 [DOI] [PubMed] [Google Scholar]
- 10.Milligan G., Canals M., Pediani J. D., Ellis J., Lopez-Gimenez J. F. 2006. The role of GPCR dimerisation/oligomerisation in receptor signalling. Ernst Schering Found. Symp. Proc. 145–161 [DOI] [PubMed] [Google Scholar]
- 11.Duane W. C., Hunninghake D. B., Freeman M. L., Pooler P. A., Schlasner L. A., Gebhard R. L. 1988. Simvastatin, a competitive inhibitor of HMG-CoA reductase, lowers cholesterol saturation index of gallbladder bile. Hepatology. 8: 1147–1150 [DOI] [PubMed] [Google Scholar]
- 12.Inokuchi J., Mason I., Radin N. S. 1987. Antitumor activity via inhibition of glycosphingolipid biosynthesis. Cancer Lett. 38: 23–30 [DOI] [PubMed] [Google Scholar]
- 13.Zheng H., Chu J., Qiu Y., Loh H. H., Law P. Y. 2008. Agonist-selective signaling is determined by the receptor location within the membrane domains. Proc. Natl. Acad. Sci. USA. 105: 9421–9426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng H., Loh H. H., Law P. Y. 2010. Agonist-selective signaling of G protein-coupled receptor: mechanisms and implications. IUBMB Life. 62: 112–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liao D., Lin H., Law P. Y., Loh H. H. 2005. Mu-opioid receptors modulate the stability of dendritic spines. Proc. Natl. Acad. Sci. USA. 102: 1725–1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng H., Loh H. H., Law P. Y. 2008. Beta-arrestin-dependent mu-opioid receptor-activated extracellular signal-regulated kinases (ERKs) translocate to nucleus in contrast to G protein-dependent ERK activation. Mol. Pharmacol. 73: 178–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chakrabarti S., Law P. Y., Loh H. H. 1998. Distinct differences between morphine- and [D-Ala2,N-MePhe4,Gly-ol5]-enkephalin-mu-opioid receptor complexes demonstrated by cyclic AMP-dependent protein kinase phosphorylation. J. Neurochem. 71: 231–239 [DOI] [PubMed] [Google Scholar]
- 18.Chu J., Zheng H., Loh H. H., Law P. Y. 2008. Morphine-induced mu-opioid receptor rapid desensitization is independent of receptor phosphorylation and beta-arrestins. Cell. Signal. 20: 1616–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frank M., Thümer L., Lohse M. J., Bünemann M. 2005. G Protein activation without subunit dissociation depends on a G{alpha}(i)-specific region. J. Biol. Chem. 280: 24584–24590 [DOI] [PubMed] [Google Scholar]
- 20.Nagafuku M., Kabayama K., Oka D., Kato A., Tani-ichi S., Shimada Y., Ohno-Iwashita Y., Yamasaki S., Saito T., Iwabuchi K., et al. 2003. Reduction of glycosphingolipid levels in lipid rafts affects the expression state and function of glycosylphosphatidylinositol-anchored proteins but does not impair signal transduction via the T cell receptor. J. Biol. Chem. 278: 51920–51927 [DOI] [PubMed] [Google Scholar]
- 21.Zhu D., Xiong W. C., Mei L. 2006. Lipid rafts serve as a signaling platform for nicotinic acetylcholine receptor clustering. J. Neurosci. 26: 4841–4851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Szoke E., Borzsei R., Toth D. M., Lengl O., Helyes Z., Sandor Z., Szolcsanyi J. 2010. Effect of lipid raft disruption on TRPV1 receptor activation of trigeminal sensory neurons and transfected cell line. Eur. J. Pharmacol. 628: 67–74 [DOI] [PubMed] [Google Scholar]
- 23.Macdonald J. L., Pike L. J. 2005. A simplified method for the preparation of detergent-free lipid rafts. J. Lipid Res. 46: 1061–1067 [DOI] [PubMed] [Google Scholar]
- 24.Dietschy J. M., Turley S. D. 2004. Thematic review series: brain lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J. Lipid Res. 45: 1375–1397 [DOI] [PubMed] [Google Scholar]
- 25.Eckert G. P., Kirsch C., Mueller W. E. 2001. Differential effects of lovastatin treatment on brain cholesterol levels in normal and apoE-deficient mice. Neuroreport. 12: 883–887 [DOI] [PubMed] [Google Scholar]
- 26.Kirsch C., Eckert G. P., Mueller W. E. 2003. Statin effects on cholesterol micro-domains in brain plasma membranes. Biochem. Pharmacol. 65: 843–856 [DOI] [PubMed] [Google Scholar]
- 27.Arvidsson U., Riedl M., Chakrabarti S., Lee J. H., Nakano A. H., Dado R. J., Loh H. H., Law P. Y., Wessendorf M. W., Elde R. 1995. Distribution and targeting of a mu-opioid receptor (MOR1) in brain and spinal cord. J. Neurosci. 15: 3328–3341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lewington S., Whitlock G., Clarke R., Sherliker P., Emberson J., Halsey J., Qizilbash N., Peto R., Collins R. 2007. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet. 370: 1829–1839 [DOI] [PubMed] [Google Scholar]
- 29.Robson J. 2008. Lipid modification: cardiovascular risk assessment and the modification of blood lipids for the primary and secondary prevention of cardiovascular disease. Heart. 94: 1331–1332 [DOI] [PubMed] [Google Scholar]
- 30.Reid P. C., Urano Y., Kodama T., Hamakubo T. 2007. Alzheimer's disease: cholesterol, membrane rafts, isoprenoids and statins. J. Cell. Mol. Med. 11: 383–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stefani M., Liguri G. 2009. Cholesterol in Alzheimer's disease: unresolved questions. Curr. Alzheimer Res. 6: 15–29 [DOI] [PubMed] [Google Scholar]
- 32.Thelen K. M., Rentsch K. M., Gutteck U., Heverin M., Olin M., Andersson U., von Eckardstein A., Bjorkhem I., Lutjohann D. 2006. Brain cholesterol synthesis in mice is affected by high dose of simvastatin but not of pravastatin. J. Pharmacol. Exp. Ther. 316: 1146–1152 [DOI] [PubMed] [Google Scholar]
- 33.Chini B., Parenti M. 2009. G-protein coupled receptors, cholesterol and palmitoylation: facts about fats. J. Mol. Endocrinol. 42: 371–379 [DOI] [PubMed] [Google Scholar]