Abstract
Plasma cholesteryl ester transfer protein (CETP) promotes the cholesterol enrichment of apoB-containing lipoproteins (VLDL and LDL) at the expense of HDL. Recent studies demonstrated that apoC1 is a potent CETP inhibitor in plasma of healthy, normolipidemic subjects. Our goal was to establish whether the modulation of CETP activity by apoC1 is influenced by dyslipidemia in patients with documented coronary artery disease (CAD). In the total CAD population studied (n = 240), apoC1 levels correlated negatively with CETP activity, independently of apoE-epsilon, CETP-Taq1B, and apoC1-Hpa1 genotypes. In multivariate analysis, the negative relationship was observed only in normolipidemic patients, not in those with hypercholesterolemia, hypertriglyceridemia, or combined hyperlipidemia. In the normolipidemic subjects, apoC1 levels were positively associated with higher HDL- to LDL-cholesterol ratio (r = 0.359, P < 0.001). It is concluded that apoC1 as a CETP inhibitor no longer operates on cholesterol redistribution in high-risk patients with dyslipidemia, probably due to increasing amounts of VLDL-bound apoC1, which is inactive as a CETP inhibitor. Patients with dyslipidemia could experience major benefits from treatment with pharmacological CETP inhibitors, which might compensate for blunted endogenous inhibition.
Keywords: cholesteryl ester transfer protein, dyslipidemia, hypercholesterolemia, hypertriglyceridemia, combined hyperlipidemia, lipoproteins, metabolism
Large cohort studies have indicated that the risk for cardiovascular disease correlates positively with the cholesterol content of apoB-containing lipoproteins (VLDL and LDL) but negatively with the cholesterol content of HDL (1). Thus, interest in intervention strategies that lead not only to a significant reduction in LDL-cholesterol (LDL-C) but also to a concomitant increase in HDL-cholesterol (HDL-C) has grown in recent years. In this context, particular attention has been paid to the inhibition of cholesteryl ester transfer protein (CETP) because this factor promotes the cholesterol enrichment of apoB-containing lipoproteins at the expense of HDL (2). High CETP activity was found to accelerate the progression of atherosclerosis and to increase the incidence of coronary artery disease (3–6). Recent intervention trials of secondary prevention in high-risk patients confirmed that pharmacological inhibition of CETP leads to a marked, unrivalled rise in the HDL-C to LDL-C ratio (7–9). Although the outcome of the ILLUMINATE study was negative, probably because of an off-site target effect of the CETP inhibitor torcetrapib, treatments with anacetrapib in the DEFINE study (9) and dalcetrapib in the dal-PLAQUE study (10) did not result in adverse cardiovascular effects. Thus, prevention of cardiovascular disease with pharmacological CETP inhibitors is still worthwhile to pursue, and whether CETP inhibition might be equally relevant in subgroups of high-risk patients with distinct lipoprotein profiles remains an unanswered question.
Unlike apoB-containing lipoproteins, HDL was found in earlier studies to inhibit CETP activity in a concentration-dependent manner (11). In subsequent studies, and among all the apolipoprotein components of HDL, apoC1 arose as a potent inhibitor of CETP in vitro (12). Besides apoC1, apoF was described as a potent CETP inhibitor in vitro (13). However, unlike the lipid transfer inhibitory activity of apoC1 that is associated with the plasma HDL fraction (12), apoF exerts its effect almost exclusively when localized in LDL (13). The human apoC1 inhibitor operates through the modification of the HDL electrostatic charge, which is recognized as a major determinant of the CETP-lipoprotein interaction and, consequently, of the uptake and exchange of core neutral lipids (14, 15). Studies in apoC1-knocked out/human CETP transgenic (apoC1KO/HuCETPTg) and human apoC1 transgenic/human CETP transgenic (HuapoC1Tg/HuCETPTg) mice provide direct support to earlier in vitro observations with purified human apoC1 (16, 17).
In humans, no cases of isolated apoC1 deficiency have been reported. In normolipidemic, healthy subjects, plasma apoC1 concentration was reported to correlate negatively with cholesteryl ester transfer activity on the one hand (15), and positively with HDL-C concentration on the other hand (18, 19). However, the impact of apoC1 on both CETP activity and HDL levels was not addressed simultaneously in one given population, and thus evidence for increased HDL-C levels, as well as increased HDL-C to LDL-C ratio because of the apoC1-mediated blockade of CETP activity remains to be brought. Most importantly, whether apoC1 still exerts a potent inhibitory effect on CETP in dyslipidemic patients is unknown.
The aim of the present study was to address the functionality of plasma apoC1 as a CETP inhibitor in normo- and dyslipidemic patients with documented coronary artery disease (CAD) and to determine the consequences in terms of cholesterol distribution between HDL and apoB-containing lipoproteins.
METHODS
Study design and patients
Two hundred and forty consecutive patients of Caucasian origin underwent a diagnostic cardiac catheterization from September 2006 to January 2008 at the Cardiology Unit in Bordeaux University Hospital for the indication of acute myocardial infarction or stable coronary artery disease. Included patients were found to have at least one significant coronary lesion ≥ 50% on angiography. Myocardial infarction was defined on the basis of a troponin I level ≥ 0.02 ng/ml and ST segment elevation myocardial infarction (STEMI) or non-STEMI (NSTEMI) electrocardiographic changes. The severity of the CAD was recorded on computerized angiographic data forms. Patients with documented diabetes were excluded. Included patients were of unrestricted age and gender and gave written informed consent for blood samples to be used in a confidential “deoxyribonucleic bank,” complying with the Declaration of Helsinki and approved by the local Research Ethics Committee. Baseline characteristics, including age, gender, clinical presentation, and initial cardiac catheterization results, were collected prospectively. Fasting blood samples and clinical parameters were obtained 3 months after inclusion. All of the CAD patients were under statin therapy for at least 3 months. Some patients were treated for hypertension, and all of them received lifestyle recommendations (including smoking and alcohol cessation, lipid-poor diet, and increase in walking activity). Lipoprotein profile remained stable after the initial, first month of treatment.
Hypertension was defined on the basis of a systolic blood pressure of 140 mmHg or higher, and/or a diastolic blood pressure of 90 mmHg or higher, or a history of hypertension requiring treatment (20). Body mass index was calculated by body weight in kilograms divided by the square of height in meters. At 3 months of follow-up, some patients were normolipidemic because of their initial normal lipid profile or the statin treatment. Definition of dyslipidemia was determined 3 months after initiation of statin treatment. Hypercholesterolemia was defined as plasma LDL-C level higher than 2.6 mmol/l; hypertriglyceridemia was defined as a plasma triglyceride (TG) level higher than 1.7 mmol/l; and combined hyperlipidemia was defined as a plasma LDL-C level higher than 2.6 mmol/l and a plasma TG level higher than 1.7 mmol/l (21).
ApoC1 distribution studies in a population of healthy subjects
For mechanistic insights and apoC1 distribution studies, an independent group of 23 healthy subjects was recruited (main characteristics are shown in supplementary Table I). VLDL were isolated by a 90 min, 100,000 rpm ultracentrifugation at d = 1.006 in a TLA.100 rotor in a TLX ultracentrifuge (Beckman, Palo Alto, CA). Supernatants contained VLDL and infranatants contained IDL, LDL, and HDL. Both fractions were subsequently subjected to apoC1 quantitation (see below).
Plasma and DNA preparation
EDTA anticoagulated blood samples were centrifuged for serum separation within the hour of collection. Plasmas were aliquoted and stored at −80°C until their analysis. DNA was extracted from 200 µl buffy coat of a centrifuged EDTA anticoagulated blood sample, with a QiaAmp®DNA minikit (Qiagen S.A., Courtaboeuf, France) according to the manufacturer's protocol.
Lipids, lipoproteins, and apolipoprotein C1
Total serum cholesterol (TC), TG, and HDL-C levels were determined using enzymatic assay kits (Roche modular, Roche Diagnostics SA, France). LDL-C was calculated by the Friedewald equation when TG were below 5.7 mmol/l, and it was quantitated by a direct assay from Beckman coulter (OSR6183 kit) when TG were above 5.7 mmol/l. ApoC1 was quantitated in total plasma and in lipoprotein fractions by a specific ELISA using an anti-human apoC1 antiserum from rabbit (22).
Gene polymorphism genotyping
CETP, apoE, and apoCI genotyping was performed by a restriction fragment length polymorphism (RFLP)-based method as previously described (23–26). Briefly, polymerase chain reaction (PCR) amplification was performed using the following primers: i) surrounding the TaqIB polymorphism in intron 1 of the CETP gene: 5′-CAC ACC ACT GCC TGA TAA CC-3′ (forward) and 5′-GTG ACC CCC AAC ACC AAA TA-3′ (reverse); ii) surrounding the HpaI polymorphism in the promoter of the ApoC1 gene: 5′-ATC GAT CAC GAC CCT CTC- 3′ (forward) and 5′-TCC CCC ACT CAG AAT GTA- 3′ (reverse); and iii) in the exon 4 of the ApoE gene: 5′-TAA GCT TGG CAC GGC TGT CCA AGG A- 3′ (forward) and 5′-ACA GAA TTC GCC CCG GCC TGG TAC AC-3′ (reverse). The amplification mixture included 25 pmol of each primer, 100 ng genomic DNA, 1× GoTaq® reaction buffer, 1.5 mmol/l MgCl2, 0.2 mmol/l of each dNTP, and 0.5 U of GoTaq® DNA Polymerase in a total volume of 25 µl (Promega Corporation, Madison, WI). Amplification was performed for 40 cycles of 30 s at 95°C, 15 s at 60°C, and 15 s at 72°C with an initial denaturation period of 5 min at 95°C. About 20 µl of PCR product was digested with 2 U of the restriction enzyme TaqI for CETP gene polymorphism, HpaI for ApoC1 gene polymorphism, and HhaI for ApoE gene polymorphism according to the recommendations of the supplier (Fermentas, Life Sciences). Fragments obtained for CETP and ApoC1 genes polymorphism, were separated on a 2% MP agarose gel (Boehringer Mannheim, Germany) and stained with ethidium bromide. For ApoE gene polymorphism analysis, fragments were separated on an 8% polyacrylamide gel and stained with ethidium bromide. One fragment of 505 bp indicated the absence of the TaqI restriction site in the CETP gene fragment (B2B2 genotype), two fragments of 415 and 90 bp indicated the presence of the restriction site (B1B1), and three fragments of 505, 415, and 90 bp indicated heterozygosity for the restriction site (B1B2). Presence of site restriction for HpaI enzyme corresponds to H2 allele and results in two migratory species (58 and 137 bp) in homozygous form (H2H2) or in three fragments (58, 137, and 195 bp) in heterozygous form (H1H2). Absence on the two alleles of the restriction site results in one migratory fragments of 195 bp (H1H1). Six potent restriction sites are present on the ApoE gene fragments, of which two are polymorphic, resulting in three distinct alleles. Presence of a migratory fragment of 83 bp corresponds to the E2 allele; a fragment of 72 bp corresponds to the E4 allele, and copresence of the two migratory species of 48 and 91 bp corresponds to the E3 allele.
CETP activity
The cholesteryl ester transfer rate in individual plasma samples was the measured transfer from [3H]cholesteryl ester-containing HDL toward endogenous apoB-containing lipoproteins (6). CETP mass concentration was measured by a specific ELISA with TP1 anti-CETP antibodies (27). CETP activity values were calculated as the ratio of the plasma cholesteryl ester transfer rate to the plasma CETP mass concentration, and it was expressed as nanomoles of 3H-CE transferred per milligram of CETP per hour.
Statistics
Data are shown as mean ± SD or percentage as indicated. Differences between groups were evaluated by the unpaired t-test for continuous variables and by the chi-square test for categorical variables. Relationships between CETP activity and apoC1 concentration were analyzed by linear or multivariate linear regression analysis, with body mass index (BMI) as covariate. P < 0.05 was considered statistically significant.
RESULTS
Plasma apoC1 concentration correlated with CETP activity among patients with coronary stenosis
As indicated in Table 1, the mean age of the study population was 53.1 ± 10.8 years, and 87% were male patients. Twenty-six percent of the patients presented with hypercholesterolemia, 14% with hypertriglyceridemia, 17% with combined hyperlipidemia, and 30% with hypertension; 65% were smokers. One hundred and thirty nine patients (58%) were considered dyslipidemic, i.e., presented with hypercholesterolemia, combined hyperlipidemia, or hypertriglyceridemia. Two hundred and twenty-one patients (92%) presented with myocardial infarction.
TABLE 1.
Characteristics of total population (n = 240) and of normolipidemic patients and dyslipidemic subgroups
| Total |
Normolipidemia |
Hypercholesterolemia |
Hypertriglyceridemia |
Combined Hyperlipidemia |
|
| (n = 240) | (n = 101) | (n = 63) | (n = 34) | (n = 42) | |
| Age (yr) | 53.13 ± 10.79 | 54.17 ± 10.73 | 53.78 ± 9.05 | 51.91 ± 13.11 | 50.61 ± 11.17 |
| Gender M [n (%)] | 209 (87) | 91 (90) | 50 (79) | 30 (88) | 39 (93) |
| BMI | 27.38 ± 4.4 | 26.59 ± 3.74 | 27.47 ± 4.82 | 29.01 ± 5.11c | 27.9 ± 4.38 |
| HypoHDLemia [n (%)] | 69 (29) | 26 (26) | 14 (22) | 17 (50)be | 12 (29) |
| Hypertension [n (%)] | 72 (30) | 35 (35) | 19 (30) | 12 (35) | 6 (14)ch |
| Current smoker [n (%)] | 157 (65) | 63 (62) | 41 (65) | 24 (71) | 29(69) |
| MI [n(%)] | 221 (92) | 95 (94) | 58 (92) | 31 (91) | 37 (88)c |
| Aspirin [n (%)] | 161 (67) | 69 (68) | 41 (65) | 22 (65) | 29 (69) |
| β-blocker [n (%)] | 77 (32) | 29 (29) | 21 (33) | 12 (35) | 15 (36) |
| ACE inhibitor or ARAII [n (%)] | 58 (24) | 26 (26) | 15 (24) | 8 (24) | 9 (21) |
| Statin [n (%)] | 240 (100) | 101 (100) | 63 (100) | 34 (100) | 42 (100) |
| Glycemia (mmol/l) | 5.45 ± 0.73 | 5.36 ± 0.63 | 5.34 ± 0.69a | 5.69 ± 1.01bd | 5.63 ± 0.71adg |
| Total cholesterol (mmol/l) | 4.49 ± 1.07 | 3.75 ± 0.67 | 5.06 ± 0.91a | 4.16 ± 0.63ae | 5.68 ± 0.83ad |
| Triglycerides (mmol/l) | 1.83 ± 2.06 | 1.09 ± 0.32 | 1.27 ± 0.32 | 2.95 ± 2.79ae | 3.58 ± 3.53h |
| HDL-C (mmol/l) | 1.29 ± 0.37 | 1.36 ± 0.44 | 1.31 ± 0.3a | 1.11 ± 0.27d | 1.24 ± 0.25ag |
| LDL-C (mmol/l) | 2.59 ± 0.85 | 1.98 ± 0.41 | 3.31 ± 0.62a | 2.09 ± 0.4ad | 3.42 ± 0.67ag |
| HDL-C/LDL-C ratio | 0.55 ± 0.25 | 0.71 ± 0.27 | 0.4 ± 0.1a | 0.54 ± 0.15e | 0.37 ± 0.1cf |
| CETP mass (mg/l) | 2.76 ± 0.76 | 2.55 ± 0.63 | 3.15 ± 0.84a | 2.61 ± 0.71b | 2.82 ± 0.75a |
| CETP activity (nmol3 H-CE/mg/h) | 0.7 ± 0.33 | 0.61 ± 0.32 | 0.72 ± 0.32 | 0.8 ± 0.32 | 0.82 ± 0.33c |
| ApoC1 mass (mg/l) | 51.92 ± 14.64 | 49.84 ± 13.1 | 50.85 ± 16.69 | 55.09 ± 13.49 | 56.07 ± 15.01 |
| CETP TaqIb [n (%)] | |||||
| B1B1 | 89 (37) | 44 (44) | 19 (30) | 11 (32) | 15 (36) |
| B1B2 | 121 (50) | 48 (48) | 33 (52) | 18 (53) | 22 (52) |
| B2B2 | 30 (13) | 9 (9) | 11 (17) | 5 (15) | 5 (12) |
| ApoCI HpaI [n (%)] | |||||
| H1H1 | 170 (71) | 71 (70) | 45 (71) | 23 (68) | 31 (74) |
| H1H2 | 66 (28) | 29 (29) | 17 (27) | 10 (29) | 10 (24) |
| H2H2 | 4 (2) | 1 (1) | 1 (2) | 1 (3) | 1 (2) |
| ApoE [n (%)] | |||||
| E3E3 | 155 (65) | 57 (57) | 45 (73)c | 23 (70) | 30 (71) |
| E4E3 + E4E4 | 48 (20) | 26 (26) | 10 (16) | 6 (18) | 6 (14) |
| E2E3 + E2E2 | 34 (14) | 17 (17) | 7 (11) | 4 (12) | 6 (14) |
Otherwise specified, values are mean ± SD.
Signifigantly different from normolipidemia, P < 0.05, P < 0.01, and P < 0.001, respectively.
Significantly different from hypercholesterolemia, P < 0.05, P < 0.01, and P < 0.001, respectively.
Significantly different from hypertriglyceridemia, P < 0.05, and P < 0.001, respectively.
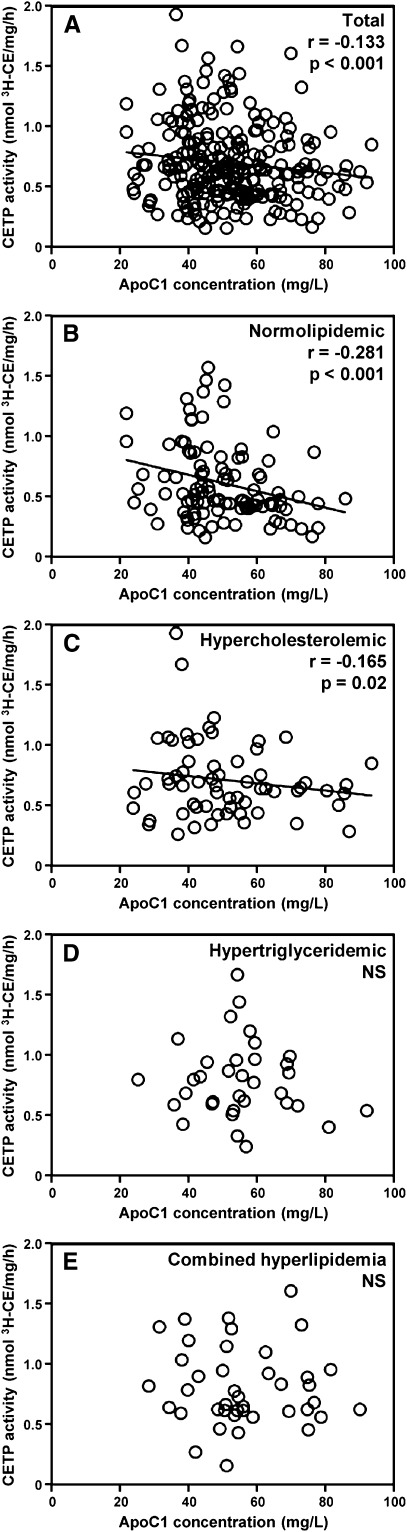
In a first attempt to assess the relationship between apoC1 and CETP activity, apoC1 concentration and CETP activity levels were determined in plasma from 240 consecutive patients with documented CAD. As shown in Fig. 1A, a significant, negative correlation was observed between plasma apoC1 concentration and CETP activity in CAD patients (r = −0.133, P < 0.001; n = 240), although the relationship was weaker than that reported earlier by the same authors using the same experimental design in healthy, normolipidemic controls (15). In multivariate regression analysis with BMI and common variants that may have influenced CETP activity, plasma lipid levels, and/or apoC1 concentration (including Epsilon gene polymorphism for apoE, TaqIB for CETP, and HpaI for apoC1), again apoC1 concentration was found to contribute significantly to CETP activity in the whole CAD population studied (P = 0.031; Table 2).
Fig. 1.
Relationship between apolipoprotein C1 concentration and CETP activity in (A) total patients with coronary stenosis (n = 240) and in (B) normolipidemic patients (n = 101), and in patients presenting with (C) hypercholesterolemia (LDL-C levels above 2.6 mmol/l; n = 63), (D) hypertriglyceridemia (TG levels above 1.7 mmol/l; n = 34), or (E) combined hyperlipidemia (LDL-C and TG levels above 2.6 mmol/l and 1.7 mmol/l, respectively; n = 42). Correlation coefficients were calculated by linear regression analysis.
TABLE 2.
Analyses of contributing factors for CETP activity and HDL-cholesterol level
| CETP Activity (nmol3 H-CE/mg/h) |
HDL-C (mmol/l) |
|||
| β ± SE | P | β ± SE | P | |
| Total (n = 240) | ||||
| ApoE-epsilon | 0.0804 ± 0.0419 | 0.056 | −0.0221 ± 0.0413 | 0.593 |
| CETP-Taq1b | −0.0237 ± 0.0317 | 0.455 | −0.0039 ± 0.0348 | 0.910 |
| ApoC1-Hpa1 | −0.0458 ± 0.0426 | 0.284 | −0.0405 ± 0.0468 | 0.388 |
| BMI | 0.0055 ± 0.0048 | 0.250 | −0.018 ± 0.0053 | 0.001 |
| ApoC1 mass | −0.0031 ± 0.0014 | 0.031 | 0.0037 ± 0.0016 | 0.020 |
| Normolipidemia (n = 101) | ||||
| ApoE-epsilon | 0.0927 ± 0.0509 | 0.070 | −0.035 ± 0.0684 | 0.605 |
| CETP-Taq1b | −0.0154 ± 0.0493 | 0.754 | −0.0008 ± 0.0663 | 0.990 |
| ApoC1-Hpa1 | −0.04 ± 0.0684 | 0.555 | −0.1325 ± 0.092 | 0.151 |
| BMI | −0.0093 ± 0.0086 | 0.281 | −0.0225 ± 0.0116 | 0.053 |
| ApoC1 mass | −0.0078 ± 0.0024 | 0.002 | 0.009 ± 0.0033 | 0.007 |
| Hypercholesterolemia (n = 63) | ||||
| ApoE-epsilon | 0.0764 ± 0.0744 | 0.307 | 0.0321 ± 0.0713 | 0.653 |
| CETP-Taq1b | −0.0146 ± 0.0577 | 0.801 | −0.068 ± 0.0553 | 0.222 |
| ApoC1-Hpa1 | −0.0969 ± 0.0787 | 0.221 | 0.0233 ± 0.0754 | 0.758 |
| BMI | 0.0114 ± 0.0082 | 0.170 | −0.0097 ± 0.0079 | 0.223 |
| ApoC1 mass | −0.0029 ± 0.0023 | 0.214 | 0.0037 ± 0.0022 | 0.102 |
| Hypertriglyceridemia (n = 34) | ||||
| ApoE-epsilon | −0.02 ± 0.1348 | 0.883 | 0.0549 ± 0.1117 | 0.624 |
| CETP-Taq1b | −0.0221 ± 0.0936 | 0.814 | −0.1162 ± 0.0776 | 0.138 |
| ApoC1-Hpa1 | 0.0243 ± 0.1101 | 0.826 | 0.0687 ± 0.0913 | 0.453 |
| BMI | −0.004 ± 0.0119 | 0.740 | −0.0073 ± 0.0099 | 0.461 |
| ApoC1 mass | −0.0023 ± 0.0045 | 0.604 | 0.0023 ± 0.0037 | 0.539 |
| Combined hyperlipidemia (n = 42) | ||||
| ApoE-epsilon | 0.0181 ± 0.1051 | 0.864 | −0.0466 ± 0.0805 | 0.564 |
| CETP-Taq1b | −0.0359 ± 0.0856 | 0.676 | 0.0579 ± 0.0656 | 0.380 |
| ApoC1-Hpa1 | 0.0617 ± 0.1129 | 0.586 | −0.0038 ± 0.0865 | 0.965 |
| BMI | 0.0108 ± 0.0142 | 0.449 | −0.0158 ± 0.0108 | 0.149 |
| ApoC1 mass | 0.0002 ± 0.0041 | 0.955 | −0.0022 ± 0.0031 | 0.481 |
Multiple linear regression analysis was performed. The dependent variable was CETP activity or HDL-C level. Genotypes were encoded as ordinal variables: for apoE-epsilon genotypes, E2E2 + E2E3 = 3, E3E3 = 2, E4E4 + E4E3 = 1; for ApoC1 HpaI genotypes, H1H1 = 3, H1H2 = 2, H2H2 = 1; and for CETP TaqIB genotypes, B1B1 = 3, B1B2 = 2, B2B2 = 1. Statistically significant relationships are presented with bold values.
Dyslipidemia abrogates the ability of plasma apoC1 to modulate the CETP-mediated lipid transfer reaction in patients with CAD
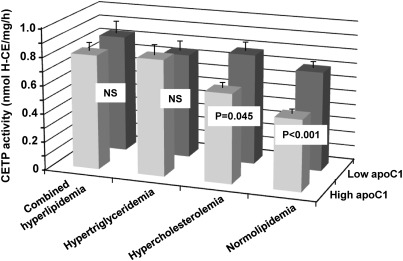
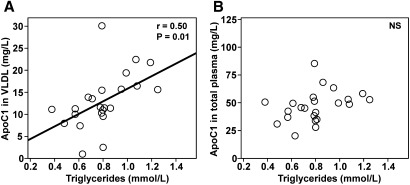
Patients were classified into four groups according to their LDL-C and TG levels. Patients of the normolipidemic group had LDL-C levels and TG levels below 2.6 mmol/l and 1.7 mmol/l, respectively. Other patients presented with hypercholesterolemia, hypertriglyceridemia, or combined hyperlipidemia. As shown in Table 1, no significant differences in medication use were observed between the normolipidemic and dyslipidemic subgroups, in particular with all patients being treated with statins for at least 3 months. As shown in Fig. 1B, correlation analysis revealed a strong, significant, negative correlation between plasma apoC1 and CETP activity in the normolipidemic group (r = −0.281, P < 0.001). A weaker, however significant, relationship was also observed in the hypercholesterolemic group (r = −0.165, P = 0.02; Fig. 1C), whereas no significant relationship between apoC1 and CETP activity was observed in the groups with hypertriglyceridemia or combined hyperlipidemia (Fig. 1D, E). A significant impact of hypertriglyceridemia in blunting the inhibitory property of apoC1 on plasma CETP activity was evidenced by diagonal stratification according to apoC1 levels on the two sides of the 50.6 mg/l median value. Indeed, and as shown in Fig. 2, high apoC1 concentration was associated with a significantly reduced CETP activity in the normolipidemic subgroup (−28.6% in “high apoC1” versus “low apoC1” subjects; P < 0.001) and, to a lesser extent, in the hypercholesterolemic subgroup (−19.2% in “high apoC1” versus “low apoC1” patients; P = 0.045). In subgroups of patients with isolated hypertriglyceridemia or combined hyperlipidemia, similarly elevated plasma cholesteryl ester transfer rates were observed whether the apoC1 level was in the high or the low range. In fact, a blunting effect on the inhibitory property of plasma apoC1 was observed with all the dyslipidemic states when common variants of apoE, CETP, and apoC1 genes were included in the analysis (Table 2). Overall, these observations support the ability of apoC1 to modulate CETP activity in plasma, however, only in normolipidemic, not in dyslipidemic, patients. In a complementary investigation of healthy subjects (supplementary Table I), plasma TG levels were positively correlated with apoCI-associated VLDL [inactive as CETP inhibitor (22)] levels, but not with total apoCI levels, suggesting that entrapment of apoCI in VLDL may contribute to the blunting of the constitutive inhibition of CETP activity in dyslipidemia (Fig. 3).
Fig. 2.
Diagonal stratification of CETP activity according to apoC1 and the presence or the absence of dyslipidemia. Subgroups were constituted by distinguishing between patients with normolipidemia (LDL-C and TG levels below 2.6 mmol/l and 1.7 mmol/l, respectively; n = 101), hypercholesterolemia (LDL-C levels above 2.6 mmol/l; n = 63), hypertriglyceridemia (TG levels above 1.7 mmol/l; n = 34), or combined hyperlipidemia (LDL-C and TG levels respectively above 2.6 mmol/l and 1.7 mmol/l; n = 42). Low and high levels of apoC1 mass were on both sides of the median value (50.6 mg/l). Vertical bars show mean ± SEM. Mean differences between low and high apoC1 groups were tested by unpaired t-test.
Fig. 3.
Relationship between apoC1 levels in VLDL (A) and in total plasma (B) and plasma TG levels in a group of healthy subjects. VLDL was isolated by sequential ultracentrifugation as described in Materials and Methods. Apolipoprotein C1 concentrations in total plasma and in VLDL fractions were determined by a specific ELISA as described in Materials and Methods. Characteristics of healthy subjects are shown in supplementary Table I. Correlation coefficients were calculated by linear regression analysis. Correlation between total apoC1 and VLDL-apoC1: r = 0.78, P < 0.0001.
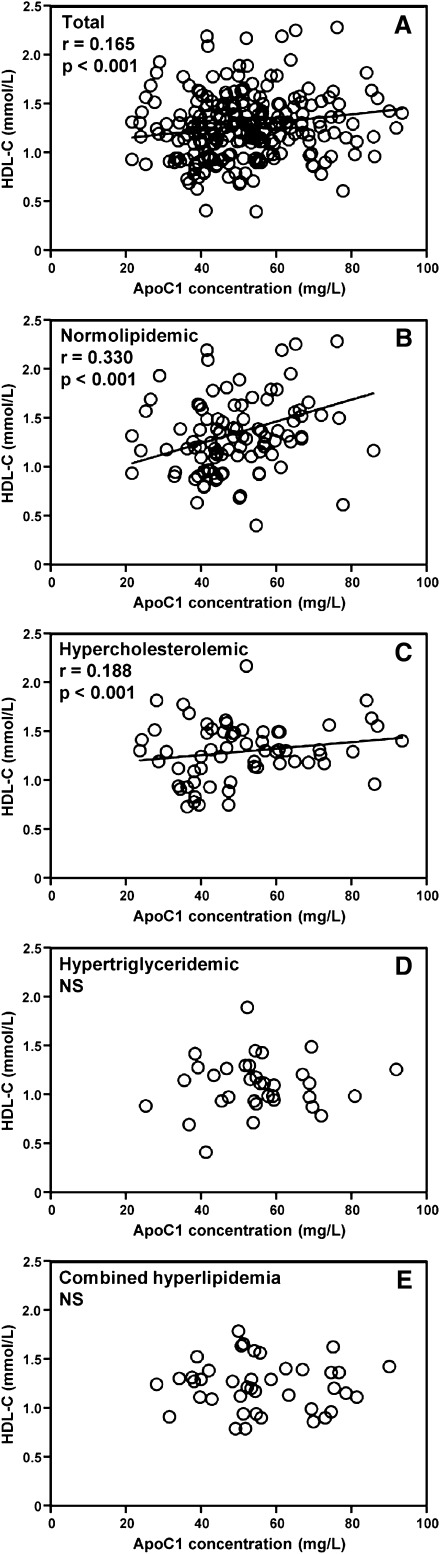
A beneficial impact of plasma apoC1 levels on the cholesterol distribution between HDL and LDL was found only in the subgroup of normolipidemic patients
As shown in Fig. 4A, a significant positive correlation between apoC1 and HDL-C levels was observed in the total population (r = 0.165, P < 0.001). This correlation was even stronger in the normolipidemic patients (r = 0.330, P < 0.001; Fig. 4B). A weaker, but still significant, correlation was observed in hypercholesterolemic patients (r = 0.188, P < 0.001; Fig. 4C); however in comparison with the normolipidemic individuals, no correlation was observed between plasma apoC1 levels and the HDL-C to LDL-C ratio (Table 2). Plasma apoC1 levels were associated neither with HDL-C levels nor with the HDL-C to LDL-C ratio in patients with hypertriglyceridemia or combined hyperlipidemia, and it was associated positively, not negatively, with LDL-C in all dyslipidemic subgroups (Fig. 4C, D and Table 3). This finding might reflect, in part, the previously reported liver X receptor (LXR)-dependent upregulation of apoC1 gene expression in the presence of increasing amounts of cholesterol in apoB-containing lipoproteins (28).
Fig. 4.
Relationship between apolipoprotein CI concentration and HDL-C levels in total patients (A) and in subgroups of patients presenting with normolipidemia (B), hypercholesterolemia (C), hypertriglyceridemia (D), or combined hyperlipidemia (E) (patient subgroups and statistics as in legend to Fig. 1).
TABLE 3.
Correlations between plasma apoC1 concentration and total plasma cholesterol, LDL-C, HDL-C, and HDL-C/LDL–C ratio in normolipidemic and in dyslipidemic subgroups
| ApoC1 Mass (mg/l) |
||
| r | P | |
| Normolipidemia (n = 101) | ||
| Total cholesterol (mmol/l) | 0.107 | 0.255 |
| LDL-C (mmol/l) | −0.131 | 0.004 |
| HDL-C (mmol/l) | 0.330 | <0.001 |
| HDL-C/LDL-C ratio | 0.359 | <0.001 |
| Hypercholesterolemia (n = 63) | ||
| Total cholesterol (mmol/l) | 0.291 | <0.001 |
| LDL-C (mmol/l) | 0.201 | <0.001 |
| HDL-C (mmol/l) | 0.188 | <0.001 |
| HDL-C/LDL-C ratio | 0.051 | 1.000 |
| Hypertriglyceridemia (n = 34) | ||
| Total cholesterol (mmol/l) | 0.204 | 0.182 |
| LDL-C (mmol/l) | 0.248 | 0.020 |
| HDL-C (mmol/l) | 0.078 | 1.000 |
| HDL-C/LDL-C ratio | −0.144 | 0.864 |
| Combined hyperlipidemia (n = 42) | ||
| Total cholesterol (mmol/l) | 0.203 | 0.054 |
| LDL-C (mmol/l) | 0.249 | 0.002 |
| HDL-C (mmol/l) | −0.027 | 1.000 |
| HDL-C/LDL-C ratio | −0.187 | 0.145 |
Statistically significant relationships are presented with bold values.
In multivariate analysis, including Epsilon gene polymorphism for apoE, TaqIB for CETP, and HpaI for apoC1, a significant contribution of apoC1 to HDL-C levels was observed in the normolipidemic subgroup only (Table 2).
Plasma lipid values and CETP parameters were compared in subgroups of patients with low versus high apoC1 concentrations on the two sides of the 50.6 mg/l median value (Table 4). In the total population, there was no difference between the high and low apoC1 concentration subgroups with regard to CETP mass concentration, but CETP activity was lower in patients with supramedian (“above”) apoC1 concentration compared with patients with inframedian (“below”) apoC1 concentration (0.66 ± 0.30 versus 0.74 ± 0.35 nmol 3H-CE/mg/h, respectively; P < 0.05; Table 4). Higher HDL-C levels mostly accounted for the higher total cholesterol levels in patients with high apoC1 levels. Among normolipidemic patients, lower CETP activity (−28.1%; P < 0.001), higher HDL-C (+20.7%; P < 0.05), and higher HDL-C to LDL-C ratio (+26.3%; P < 0.05) were observed with apoC1 concentration above the median value. These differences in plasma lipoprotein parameters and cholesterol distribution in normolipidemic patients occurred in the absence of differences in total plasma cholesterol, triglycerides, and CETP mass concentration (Table 4). Among hypercholesterolemic patients, higher total cholesterol levels (+13.0%; P < 0.05), lower CETP activity (−19.0%; P < 0.05), and no significant difference in HDL-C and in the HDL-C to LDL-C ratio were observed in the high apoC1 subgroup. Among patients with hypertriglyceridemia or combined hyperlipidemia, no significant differences in CETP activity or in the lipoprotein distribution of cholesterol were observed when comparing patients with supramedian versus inframedian plasma apoC1 concentration (Table 4).
TABLE 4.
Distribution of blood lipid parameters according to the median of apoC1 concentration in either total population or subgroups of normolipidemic and dyslipidemic patients
| Total |
Normolipidemia |
Hypercholesterolemia |
Hypertriglyceridemia |
Combined Hyperlipidemia |
|||||||||||
| ApoC1 Median |
ApoC1 Median |
ApoC1 Median |
ApoC1 Median |
ApoC1 Median |
|||||||||||
| Below | Above | Difference | Below | Above | Difference | Below | Above | Difference | Below | Above | Difference | Below | Above | Difference | |
| (n = 119) | (n = 121) | (%) | (n = 59) | (n = 42) | (%) | (n = 36) | (n = 27) | (%) | (n = 10) | (n = 24) | (%) | (n = 15) | (n = 27) | (%) | |
| Glycemia (mmol/l) | 5.42 ± 0.57 | 5.48 ± 0.87 | 1.1 | 5.44 ± 0.56 | 5.26 ± 0.72 | −3.4 | 5.37 ± 0.6 | 5.3 ± 0.81 | −1.3 | 5.21 ± 0.34 | 5.9 ± 1.14b | 13.3 | 5.62 ± 0.63 | 5.64 ± 0.75 | 0.3 |
| Total cholesterol (mmol/l) | 4.25 ± 1.01 | 4.72 ± 1.09a | 10.9 | 3.68 ± 0.58 | 3.85 ± 0.78 | 4.6 | 4.8 ± 0.93 | 5.42 ± 0.77b | 13.0 | 3.84 ± 0.94 | 4.3 ± 0.39 | 11.8 | 5.55 ± 0.7 | 5.74 ± 0.9 | 3.4 |
| Triglycerides (mmol/l) | 1.89 ± 2.75 | 1.77 ± 1 | −6.1 | 1.09 ± 0.34 | 1.1 ± 0.31 | 1.1 | 1.26 ± 0.36 | 1.29 ± 0.25 | 1.9 | 4.5 ± 4.92 | 2.3 ± 0.5 | −48.8 | 5 ± 5.61 | 2.83 ± 1.33 | −43.3 |
| HDL-C (mmol/l) | 1.23 ± 0.33 | 1.35 ± 0.39b | 9.3 | 1.25 ± 0.36 | 1.51 ± 0.51b | 20.7 | 1.25 ± 0.32 | 1.39 ± 0.25 | 11.0 | 1.03 ± 0.3 | 1.14 ± 0.26 | 10.9 | 1.25 ± 0.25 | 1.24 ± 0.26 | −1.5 |
| LDL-C (mmol/l) | 2.52 ± 0.78 | 2.66 ± 0.9 | 5.6 | 2.01 ± 0.37 | 1.94 ± 0.46 | −3.4 | 3.2 ± 0.58 | 3.45 ± 0.65 | 8.0 | 1.91 ± 0.54 | 2.17 ± 0.31 | 13.5 | 3.38 ± 0.57 | 3.44 ± 0.72 | 1.8 |
| HDL-C/ LDL-C ratio | 0.53 ± 0.21 | 0.57 ± 0.28 | 7.2 | 0.64 ± 0.22 | 0.81 ± 0.31b | 26.3 | 0.4 ± 0.11 | 0.41 ± 0.09 | 3.9 | 0.55 ± 0.16 | 0.54 ± 0.15 | −3.3 | 0.38 ± 0.08 | 0.37 ± 0.11 | −1.3 |
| CETP mass (mg/l) | 2.76 ± 0.78 | 2.77 ± 0.75 | 0.6 | 2.53 ± 0.67 | 2.56 ± 0.59 | 1.1 | 3.13 ± 0.89 | 3.19 ± 0.79 | 2.0 | 2.73 ± 0.89 | 2.56 ± 0.64 | −6.2 | 2.75 ± 0.52 | 2.86 ± 0.85 | 4.2 |
| CETP activity (nmol H-CE/ mg/h) | 0.74 ± 0.35 | 0.66 ± 0.3b | -10.6 | 0.7 ± 0.36 | 0.5 ± 0.2a | −28.1 | 0.78 ± 0.37 | 0.63 ± 0.2b | −19.0 | 0.74 ± 0.2 | 0.82 ± 0.35 | 10.4 | 0.83 ± 0.32 | 0.81 ± 0.33 | −3.2 |
| ApoC1 mass (mg/l) | 40.3 ± 7 | 63.45 ± 10.54a | 57.4 | 40.9 ± 7.09 | 62.39 ± 8.44a | 52.6 | 39.22 ± 7.14 | 66.37 ± 12.58a | 69.2 | 39.96 ± 6.6 | 61.4 ± 10.15a | 53.7 | 40.79 ± 6.91 | 63.99 ± 11.48a | 56.9 |
The median value for apoC1 was 50.6 mg/l. Otherwise specified, values are mean ± SD. Statistically significant differences are presented with bold values.
P < 0.001 for apoC1 “above” versus apoC1 “below” the median value.
P < 0.05 for apoC1 “above” versus apoC1 “below” the median value.
DISCUSSION
Apolipoprotein C1 is a small, basic apolipoprotein that is mainly produced by the liver. It is known to enter the bloodstream as a component of TG-rich lipoproteins (TRL) (29). TRL-bound apoC1 was found to inhibit lipoprotein lipase-mediated triglyceride hydrolysis and to impair recognition of apoC1-containing TRL by cellular receptors (30, 31). In vivo studies in animal models, which showed delayed catabolism of TRL in transgenic mice overexpressing human apoC1 (32, 33), have supported earlier in vitro observations. In the present study involving patients with documented CAD, no significant differences in plasma TG levels were observed whether apoC1 concentration was in the low range (below the 50.6 mg/l median value) or in the high range (above the 50.6 mg/l median value; Table 3). Whereas the blockade of TRL clearance by apoC1 was found to have pathophysiological relevance in hypertriglyceridemic mouse models (30, 33) and in some human populations (34), we found no direct support of the latter in the high-risk patients studied here. Interestingly, in a previous study (35), the apoC1 content of TRL was not a predictor of postprandial concentration of triglycerides in middle-aged men with early atherosclerosis.
Importantly, apoC1, a highly exchangeable apolipoprotein, is known to dissociate from TRL during it intravascular transport to rapidly associate with HDL, which is the main carrier of apoC1 in normolipidemic plasma (29). HDL-bound apoC1 is a potent inhibitor of CETP activity (22), which has been shown to be a strong, negative predictor of HDL-C levels in several human cohorts (18, 19). The ability of apoC1 to decrease specific CETP activity and, as a consequence, to reduce the net mass transfer of cholesteryl esters from HDL toward VLDL and LDL has been documented earlier in HuCETPTg/apoC1-KO mice [with higher specific CETP activity (16)] and in HuCETPTg/HuapoC1Tg mice [with lower specific CETP activity (17)] when compared with HuCETPTg counterparts. More recently, a negative correlation between plasma concentrations of apoC1 and levels of CETP activity was reported in healthy, normolipidemic subjects (15). Again, the resulting apoC1-mediated blockade of the CETP-mediated flux of cholesteryl esters out of HDL might actually provide an explanation for the positive correlation between plasma apoC1 and HDL-C in normolipidemic CAD patients of the present study as well as in 85-year-old participants of the prospective population-based Leiden 85-Plus Study (18). The CETP-dependent mechanism comes in addition to the effect of apoC1 on LCAT (36, 37), HL (38, 39), and SRB1 (40). In fact, the CETP modulatory effect of apoC1 might normally contribute to its effect on plasma cholesterol distribution. Indeed, higher HDL-C to LDL-C ratio (i.e., the hallmark of an effective CETP activity blockade) was measured in the present study in normolipidemic patients with high versus low plasma apoC1 concentration.
Whereas the present study conducted in patients with CAD brings new support to the physiological relevance of the CETP-inhibitory property of human apoC1, the apoC1-dependent modulation of both CETP activity and plasma cholesterol distribution appeared to operate mostly in normolipidemic patients. All the patients were under statin therapy. Thus, the preservation of the CETP inhibitory property of apoC1 in patients with normalized lipoprotein profile might have made a significant contribution to the reduction in cholesteryl ester transfer rates after statin treatment. It comes in addition to the previously reported decrease in CETP mass and apoB-containing lipoprotein acceptors after statin treatment (41). Two main reasons might account for the partial or complete loss of CETP inhibitory potential of apoC1 in subgroups of patients with hypercholesterolemia, hypertriglyceridemia, or combined hyperlipidemia, who all displayed significantly higher plasma TG levels compared with patients in the normolipidemic subgroup. First, and in accordance with earlier observations in type 2 diabetic patients (4), abundant TRL, as preferential acceptors of HDL cholesteryl esters, probably drives the CETP-mediated cholesteryl ester transfer reaction. As a consequence, it is conceivable that variations in the level of the apoC1 inhibitor would have a weaker impact as triglyceridemia increases. Second, because HDL-bound apoC1 [i.e., the only one to be able to inhibit CETP activity (22)] is known to come largely from the dissociation of VLDL-associated apoC1 during the course of lipolysis (29), accumulation of circulating apoB-containing lipoproteins might result in the sequestration of apoC1 in these fractions and finally loose control of the CETP-mediated redistribution of cholesterol between circulating lipoproteins. In support of this hypothesis, the CETP inhibitory potential of apoC1 was found to decrease as lipoprotein size increases (14), and VLDL-bound apoC1, unlike HDL-bound apoC1, was found to be ineffective as a CETP inhibitor (22). In new support of the blunted inhibitory potential of apoC1 when apoB-containing lipoproteins accumulate, we report here that plasma triglycerides correlate positively with nonactive, VLDL-bound apoC1 in a group of healthy subjects. It suggests further that the concomitant enrichment of TG-rich remnant lipoproteins with cholesterol and apoC1, which was reported to occur earlier in men with either early carotid atherosclerosis (35) or CAD (34) could actually relate, at least in part, to unrestrained plasma CETP activity, because apoC1 no longer operates as a CETP inhibitor when bound to apoB-containing lipoproteins. It might also account for the recent observation that apoC1 is an independent risk factor, and not a protective one, in patients with metabolic syndrome and systemic inflammation (42).
We conclude that apoC1 has the ability to inhibit CETP activity in normolipidemic subjects, thus contributing to the modulation of cholesterol distribution between HDL and apoB-containing lipoproteins. Importantly, and unlike in normolipidemic individuals, apoC1 does not operate properly as a CETP inhibitor in dyslipidemic patients, in particular those with hypertriglyceridemia or combined hyperlipidemia. Whether in this case treatment with pharmacological CETP inhibitors might compensate for blunted endogenous CETP inhibition is unknown.
Supplementary Material
Acknowledgments
The authors thank Philip Bastable for manuscript editing.
Footnotes
Abbreviations:
- BMI
- body mass index
- CAD
- coronary artery disease
- CETP
- cholesteryl ester transfer protein
- HDL-C
- HDL-cholesterol
- LDL-C
- LDL-cholesterol
- MI
- myocardial infarction
- NSTEMI
- non-STEMI
- STEMI
- ST segment elevation myocardial infarction
- TC
- total cholesterol
- TG
- triglyceride
- TRL
- TG-rich lipoprotein
This work was supported by grants from the Université de Bourgogne, the Centre Hospitalier Universitaire de Dijon, the Conseil Régional de Bourgogne, the Institut National de la Santé et de la Recherche Médicale (INSERM), the Agence Nationale de la Recherche (ANR)-Atherolip Project, and the Fondation de France.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of one table.
REFERENCES
- 1.Gotto A. M., Jr, Brinton E. A. 2004. Assessing low levels of high-density lipoprotein cholesterol as a risk factor in coronary heart disease: a working group report and update. J. Am. Coll. Cardiol. 43: 717–724 [DOI] [PubMed] [Google Scholar]
- 2.Barter P. J., Brewer H. B., Jr, Chapman M. J., Hennekens C. H., Rader D. J., Tall A. R. 2003. Cholesteryl ester transfer protein: a novel target for raising HDL and inhibiting atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 23: 160–167 [DOI] [PubMed] [Google Scholar]
- 3.Boekholdt S. M., Kuivenhoven J. A., Wareham N. J., Peters R. J., Jukema J. W., Luben R., Bingham S. A., Day N. E., Kastelein J. J., Khaw K. T. 2004. Plasma levels of cholesteryl ester transfer protein and the risk of future coronary artery disease in apparently healthy men and women: the prospective EPIC (European Prospective Investigation into Cancer and nutrition)-Norfolk population study. Circulation. 110: 1418–1423 [DOI] [PubMed] [Google Scholar]
- 4.de Vries R., Perton F. G., Dallinga-Thie G. M., van Roon A. M., Wolffenbuttel B. H., van Tol A., Dullaart R. P. 2005. Plasma cholesteryl ester transfer is a determinant of intima-media thickness in type 2 diabetic and nondiabetic subjects: role of CETP and triglycerides. Diabetes. 54: 3554–3559 [DOI] [PubMed] [Google Scholar]
- 5.Klerkx A. H., de Grooth G. J., Zwinderman A. H., Jukema J. W., Kuivenhoven J. A., Kastelein J. J. 2004. Cholesteryl ester transfer protein concentration is associated with progression of atherosclerosis and response to pravastatin in men with coronary artery disease (REGRESS). Eur. J. Clin. Invest. 34: 21–28 [DOI] [PubMed] [Google Scholar]
- 6.Zeller M., Masson D., Farnier M., Lorgis L., Deckert V., Pais de Barros J. P., Desrumaux C., Sicard P., Grober J., Blache D., et al. 2007. High serum cholesteryl ester transfer rates and small high-density lipoproteins are associated with young age in patients with acute myocardial infarction. J. Am. Coll. Cardiol. 50: 1948–1955 [DOI] [PubMed] [Google Scholar]
- 7.Barter P. J., Caulfield M., Eriksson M., Grundy S. M., Kastelein J. J., Komajda M., Lopez-Sendon J., Mosca L., Tardif J. C., Waters D. D., et al. 2007. Effects of torcetrapib in patients at high risk for coronary events. N. Engl. J. Med. 357: 2109–2122 [DOI] [PubMed] [Google Scholar]
- 8.Cannon C. P., Dansky H. M., Davidson M., Gotto A. M., Jr, Brinton E. A., Gould A. L., Stepanavage M., Liu S. X., Shah S., Rubino J., et al. 2009. Design of the DEFINE trial: determining the efficacy and tolerability of CETP inhibition with anacetrapib. Am. Heart J. 158: 513–519.e3 [DOI] [PubMed] [Google Scholar]
- 9.Cannon C. P., Shah S., Dansky H. M., Davidson M., Brinton E. A., Gotto A. M., Stepanavage M., Liu S. X., Gibbons P., Ashraf T. B., et al. 2010. Safety of anacetrapib in patients with or at high risk for coronary heart disease. N. Engl. J. Med. 363: 2406–2415 [DOI] [PubMed] [Google Scholar]
- 10.Fayad Z. A., Mani V., Woodward M., Kallend D., Abt M., Burgess T., Fuster V., Ballantyne C. M., Stein E. A., Tardif J. C., et al. 2011. Safety and efficacy of dalcetrapib on atherosclerotic disease using novel non-invasive multimodality imaging (dal-PLAQUE): a randomised clinical trial. Lancet. 378: 1547–1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barter P. J., Jones M. E. 1980. Kinetic studies of the transfer of esterified cholesterol between human plasma low and high density lipoproteins. J. Lipid Res. 21: 238–249 [PubMed] [Google Scholar]
- 12.Gautier T., Masson D., de Barros J. P., Athias A., Gambert P., Aunis D., Metz-Boutigue M. H., Lagrost L. 2000. Human apolipoprotein C–I accounts for the ability of plasma high density lipoproteins to inhibit the cholesteryl ester transfer protein activity. J. Biol. Chem. 275: 37504–37509 [DOI] [PubMed] [Google Scholar]
- 13.Wang X., Driscoll D. M., Morton R. E. 1999. Molecular cloning and expression of lipid transfer inhibitor protein reveals its identity with apolipoprotein F. J. Biol. Chem. 274: 1814–1820 [DOI] [PubMed] [Google Scholar]
- 14.Dumont L., Gautier T., de Barros J. P., Laplanche H., Blache D., Ducoroy P., Fruchart J., Fruchart J. C., Gambert P., Masson D., et al. 2005. Molecular mechanism of the blockade of plasma cholesteryl ester transfer protein by its physiological inhibitor apolipoprotein CI. J. Biol. Chem. 280: 38108–38116 [DOI] [PubMed] [Google Scholar]
- 15.de Barros J. P., Boualam A., Gautier T., Dumont L., Vergès B., Masson D., Lagrost L. 2009. Apolipoprotein CI is a physiological regulator of cholesteryl ester transfer protein activity in human plasma, but not in rabbit plasma. J. Lipid Res. 50: 1842–1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gautier T., Masson D., Jong M. C., Duverneuil L., Le Guern N., Deckert V., Pais de Barros J. P., Dumont L., Bataille A., Zak Z., et al. 2002. Apolipoprotein CI deficiency markedly augments plasma lipoprotein changes mediated by human cholesteryl ester transfer protein (CETP) in CETP transgenic/ApoCI-knocked out mice. J. Biol. Chem. 277: 31354–31363 [DOI] [PubMed] [Google Scholar]
- 17.Gautier T., Masson D., Jong M. C., Pais de Barros J. P., Duverneuil L., Le Guern N., Deckert V., Dumont L., Bataille A., Zak Z., et al. 2005. Apolipoprotein CI overexpression is not a relevant strategy to block cholesteryl ester transfer protein (CETP) activity in CETP transgenic mice. Biochem. J. 385: 189–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berbée J. F., Mooijaart S. P., de Craen A. J., Havekes L. M., van Heemst D., Rensen P. C., Westendorp R. G. 2008. Plasma apolipoprotein CI protects against mortality from infection in old age. J. Gerontol. A Biol. Sci. Med. Sci. 63: 122–126 [DOI] [PubMed] [Google Scholar]
- 19.Carlson L. A., Holmquist L. 1982. Concentrations of apolipoproteins B, C–I, C–II, C–III and E in sera from normal men and their relation to serum lipoprotein levels. Clin. Chim. Acta. 124: 163–178 [DOI] [PubMed] [Google Scholar]
- 20.Mancia G., De Backer G., Dominiczak A., Cifkova R., Fagard R., Germano G., Grassi G., Heagerty A. M., Kjeldsen S. E., Laurent S., et al. 2007. 2007 Guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur. Heart J. 28: 1462–1536 [DOI] [PubMed] [Google Scholar]
- 21.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) 2002. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 106: 3143–3421. [PubMed] [Google Scholar]
- 22.Dautin G., Soltani Z., Ducloux D., Gautier T., Pais de Barros J. P., Gambert P., Lagrost L., Masson D. 2007. Hemodialysis reduces plasma apolipoprotein C-I concentration making VLDL a better substrate for lipoprotein lipase. Kidney Int. 72: 871–878 [DOI] [PubMed] [Google Scholar]
- 23.Freeman D. J., Griffin B. A., Holmes A. P., Lindsay G. M., Gaffney D., Packard C. J., Shepherd J. 1994. Regulation of plasma HDL cholesterol and subfraction distribution by genetic and environmental factors. Associations between the TaqI B RFLP in the CETP gene and smoking and obesity. Arterioscler. Thromb. 14: 336–344 [DOI] [PubMed] [Google Scholar]
- 24.Nillesen W. M., Smeets H. J., van Oost B. A. 1990. Human ApoC1 HpaI restriction site polymorphism revealed by the polymerase chain reaction. Nucleic Acids Res. 18: 3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu Y., Berglund L., Ramakrishnan R., Mayeux R., Ngai C., Holleran S., Tycko B., Leff T., Shachter N. S. 1999. A common Hpa I RFLP of apolipoprotein C–I increases gene transcription and exhibits an ethnically distinct pattern of linkage disequilibrium with the alleles of apolipoprotein E. J. Lipid Res. 40: 50–58 [PubMed] [Google Scholar]
- 26.Hixson J. E., Vernier D. T. 1990. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J. Lipid Res. 31: 545–548 [PubMed] [Google Scholar]
- 27.Guyard-Dangremont V., Lagrost L., Gambert P., Lallemant C. 1994. Competitive enzyme-linked immunosorbent assay of the human cholesteryl ester transfer protein (CETP). Clin. Chim. Acta. 231: 147–160 [DOI] [PubMed] [Google Scholar]
- 28.Mak P. A., Laffitte B. A., Desrumaux C., Joseph S. B., Curtiss L. K., Mangelsdorf D. J., Tontonoz P., Edwards P. A. 2002. Regulated expression of the apolipoprotein E/C-I/C-IV/C-II gene cluster in murine and human macrophages. A critical role for nuclear liver X receptors alpha and beta. J. Biol. Chem. 277: 31900–31908 [DOI] [PubMed] [Google Scholar]
- 29.Cohn J. S., Tremblay M., Batal R., Jacques H., Veilleux L., Rodriguez C., Bernier L., Mamer O., Davignon J. 2002. Plasma kinetics of VLDL and HDL apoC-I in normolipidemic and hypertriglyceridemic subjects. J. Lipid Res. 43: 1680–1687 [DOI] [PubMed] [Google Scholar]
- 30.Westerterp M., de Haan W., Berbée J. F., Havekes L. M., Rensen P. C. 2006. Endogenous apoC-I increases hyperlipidemia in apoE-knockout mice by stimulating VLDL production and inhibiting LPL. J. Lipid Res. 47: 1203–1211 [DOI] [PubMed] [Google Scholar]
- 31.Weisgraber K. H., Mahley R. W., Kowal R. C., Herz J., Goldstein J. L., Brown M. S. 1990. Apolipoprotein C–I modulates the interaction of apolipoprotein E with beta-migrating very low density lipoproteins (beta-VLDL) and inhibits binding of beta-VLDL to low density lipoprotein receptor-related protein. J. Biol. Chem. 265: 22453–22459 [PubMed] [Google Scholar]
- 32.Shachter N. S., Ebara T., Ramakrishnan R., Steiner G., Breslow J. L., Ginsberg H. N., Smith J. D. 1996. Combined hyperlipidemia in transgenic mice overexpressing human apolipoprotein Cl. J. Clin. Invest. 98: 846–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jong M. C., Dahlmans V. E., van Gorp P. J., van Dijk K. W., Breuer M. L., Hofker M. H., Havekes L. M. 1996. In the absence of the low density lipoprotein receptor, human apolipoprotein C1 overexpression in transgenic mice inhibits the hepatic uptake of very low density lipoproteins via a receptor-associated protein-sensitive pathway. J. Clin. Invest. 98: 2259–2267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Björkegren J., Boquist S., Samnegârd A., Lundman P., Tornvall P., Ericsson C. G., Hamsten A. 2000. Accumulation of apolipoprotein C–I-rich and cholesterol-rich VLDL remnants during exaggerated postprandial triglyceridemia in normolipidemic patients with coronary artery disease. Circulation. 101: 227–230 [DOI] [PubMed] [Google Scholar]
- 35.Hamsten A., Silveira A., Boquist S., Tang R., Bond M. G., de Faire U., Björkegren J. 2005. The apolipoprotein CI content of triglyceride-rich lipoproteins independently predicts early atherosclerosis in healthy middle-aged men. J. Am. Coll. Cardiol. 45: 1013–1017 [DOI] [PubMed] [Google Scholar]
- 36.Soutar A. K., Garner C. W., Baker H. N., Sparrow J. T., Jackson R. L., Gotto A. M., Smith L. C. 1975. Effect of the human plasma apolipoproteins and phosphatidylcholine acyl donor on the activity of lecithin: cholesterol acyltransferase. Biochemistry. 14: 3057–3064 [DOI] [PubMed] [Google Scholar]
- 37.Jonas A., Sweeny S. A., Herbert P. N. 1984. Discoidal complexes of A and C apolipoproteins with lipids and their reactions with lecithin: cholesterol acyltransferase. J. Biol. Chem. 259: 6369–6375 [PubMed] [Google Scholar]
- 38.Kinnunen P. K., Ehnolm C. 1976. Effect of serum and C-apoproteins from very low density lipoproteins on human postheparin plasma hepatic lipase. FEBS Lett. 65: 354–357 [DOI] [PubMed] [Google Scholar]
- 39.Conde-Knape K., Bensadoun A., Sobel J. H., Cohn J. S., Shachter N. S. 2002. Overexpression of apoC-I in apoE-null mice: severe hypertriglyceridemia due to inhibition of hepatic lipase. J. Lipid Res. 43: 2136–2145 [DOI] [PubMed] [Google Scholar]
- 40.de Haan W., Out R., Berbée J. F., van der Hoogt C. C., van Dijk K. W., van Berkel T. J., Romijn J. A., Jukema J. W., Havekes L. M., Rensen P. C. 2008. Apolipoprotein CI inhibits scavenger receptor BI and increases plasma HDL levels in vivo. Biochem. Biophys. Res. Commun. 377: 1294–1298 [DOI] [PubMed] [Google Scholar]
- 41.Chapman M. J., Le Goff W., Guerin M., Kontush A. 2010. Cholesteryl ester transfer protein: at the heart of the action of lipid-modulating therapy with statins, fibrates, niacin, and cholesteryl ester transfer protein inhibitors. Eur. Heart J. 31: 149–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van der Ham R. L., Dehnavi R. A., van den Berg G. A., Putter H., de Roos A., Berbée J. F., Romijn J. A., Rensen P. C., Tamsma J. T. 2009. Apolipoprotein CI levels are associated with atherosclerosis in men with the metabolic syndrome and systemic inflammation. Atherosclerosis. 203: 355–357 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.