Abstract
Objective
The influcence of cytomechanical forces in cellular migration, proliferation and differentation of mesenchymal stem cells (MSCs) is still poorly understood in detail.
Methods
Human MSCs were isolated and cultivated onto the surface of a 3 × 3 mm porcine collagen I/III carrier. After incubation, cell cultures were transfered to the different cutures systems: regular static tissue flasks (group I), spinner flasks (group II) and rotating wall vessels (group III). Following standard protocols cells were stimulated lineage specific towards the osteogenic and chondrogenic lines. To evaluate the effects of applied cytomechanical forces towards cellular differentiation distinct parameters were measured (morphology, antigen and antigen expression) after a total cultivation period of 21 days in vitro.
Results
Depending on the cultivation technique we found significant differences in both gen and protein expression.
Conclusion
Cytomechanical forces with rotational components strongly influence the osteogenic and chondrogenic differentiation.
Keywords: mesenchymal stem cells, cytomechanical forces, differentiation, osteoblast, chondroblast
Introduction
Bone grafting is a common procedure in orthopaedic surgery and the implantation of autologous bone grafts supplying osteoinductive growth factors, osteogenic cells, and a structural scaffold, has become the gold standard for the surgical treatment of bone defects caused by trauma, tumor, infection or congenital abnormalities. In addition, bone grafts are frequently used for spinal fusion, joint revision surgery, corrective osteotomy and bone reconstruction. The amount of bone available for autografting is limited and bone graft harvesting procedures are associated with a multitude of risks, such as pain, neurovasculare injury, persisting haematoma or infection at the donor site [1-3]. The application of allograft bone as an alternative treatment option carries the potential risk of infection and graft failure as a consequence of the reduced osteoinducitvity of allograft bone [4]. Several biomaterials such as metal alloys, ceramics or bone cements have been used for decades as permanent implants to overbridge or stabilize bone defects. Although those bone substitutes have proven utility, they have often resulted in complications such as stress shielding-induced resorption of the surrounding bone and fatigue failure of the implant.
During the last years tissue engineering based treatment concepts and cell therapeutics showed promising results in vitro. Mesenchymal stem cells (MSCs) can easily be isolated and expanded from bone marrow (BM) aspirates. Because of their capacity for ex vivo proliferation and differentiation they provide a good source of osteoprogenitor cells within custom-shaped scaffolds for implantable autologous bone tissue thus allowing the generation of a large transplantable cell population from a small biopsy [5-11].
However, the influcence of sheer stress in cellular migration, proliferation and differentation of MSCs is still poorly understood in detail. Most experimental designs consider laminar or rotation flow, dynamic or hydrostatic pressure, and bending or compressive strain devices to evaluate cytomechanical in vitro-effects. One limitation of the static cultivating technique is the inhomogenous oxygen and nutrient concentration and transport within the cellular carrier (scaffold), resulting in a decrease of differentiation and proliferation an thus restricting the size of the scaffolds [9,12]. Different bioreactor systems have been used to overcome such limitations, mimicking certain aspects of the native cell environment of functional tissues and providing physiologically relevant physical signals [13-15]. Recent investigations have shown that spinner flasks applied in cell culture to regenerate cartilage and bone tissue can improve cellular distribution and differentiation in scaffolds [16-19].
For the quantification of cellular differentiation at the molecular level, osteogenic differentiation of MSCs is controlled by the interaction of hormones and transcription factors: runt-related transcription factor-2 (RUNX2) effectuates the expression of bone-specific genes, e.g. osterix (OSX), collagen type 1 alpha-1 (COL1A1), osteocalcin (OC), and bone sialoprotein (BSP) by binding to the promoters of these genes. Generally, alkaline phosphatase (ALP), COL1A1, BSP, RUNX2, transforming growth factor-beta 1 (TGFB1), osteonectin (ON), and bone morphogenetic protein-2 (BMP2) are known to be early markers of osteoblastic differentiation, whereas OC and osteopontin (OPN) are expressed later in the differentiation process [20].
In the presented study, the MSC cells were cultured in either osteogenic or chondrogenic induction medium and then incubated for 21 days into three culture system designs, including static culture (group I, STAT), spinner flask bioreactor (group II, SPUN) and rotating wall vessel reactor (group III, RWV). The aim of our study was to investigate and compare gene and protein expression after different cytomechanical forces were applied.
Materials and methods
Bioreactors
The investigation included three different systems. In a spinner flask device (Figure 1), scaffolds are placed in a tissue culture cassette hanging from the lid of the flask with convective forces generated by a magnetic stirrer bar allowing continuous mixing of the media surrounding the scaffolds [21]. The rotating wall vessel bioreactor (Figure 2) (Cellon S.A, Bereldange, Luxemburg) is made of two concentric cylinders, with the cell bearing scaffolds placed in the annular space [22,23]. Gas exchange occurs through the stationary inner cylinder whereas the outer cylinder is impermeable and rotates at a controlled rate. The free falling of the constructs inside the bioreactor as a result of gravity can be balanced by the centrifugal forces due to the rotation of the outercylinder, thus establishing microgravity-like culturing conditions [24,25]. A conventional non-cytomechnically stimulated (static) cell culture served as control.
Figure 1.

Spinner Flasc.
Figure 2.

Rotating Wall Vessel Incubator.
Cell culture
Human MSCs were isolated via density gradient centrifugation after bone marrow aspiration from a healthy, 39-year old female donor volunteer after informed consent was obtained according to the Declaration of Helsinki in its present form. Cell culture conditions were DMEM-low glucose media, 20% fetal calf serum (FCS)-gold (all agents PAA Laboratories, Cölbe, Germany), L-glutamin with 1% penicillin/streptomycin (PAA) in tissue culture polystyrene flasks in 5 Vol. % CO2 at 37°C. Medium was exchanged twice a week. Adherent cells, judged 80% - 90% confluent by phase contrast microscopy, were detached mechanically and passaged supported by 0.05% trypsin/0.02% EDTA solution (PAA). Cells from 3rd passage were re-suspended and a total number of 3 × 106 cells were cultivated onto the surface of a 3 × 3 mm porcine collagen I/III carrier (BioGide®, Geistlich Pharma AG, Wolhusen, Switzerland) which consists of a porous, porcine-derived, semipermeable, resorbable, non-crosslinked porous collagen I/III membrane (height 1000 μm). According to the producer's information, telomer peptides were removed during manufacture [26].
After an incubation time of 30 minutes to allow cellular adherence, cell cultures were transfered to the different cultures systems: regular tissue flasks (group I, 50 ml), spinner flasks (group II, 100 ml, 93 UpM) and rotating wall vessels (group III, 50 ml, 25 UpM). Following standard protocols [27] cells were stimulated lineage specific towards the osteogenic [(DMEM-low glucose (PAA), dexamethasone (Sigma Taufkirchen, Germany), L-ascorbic-2-phosphate (Sigma), β-glycerolphosphatate (Sigma)] and chondrogenic [(DMEM-high glucose PAA), insulin/trans ferrin/selenic acid (ITS) (Sigma), dexamethasone (Sigma), Lascorbic-2-phosphate (Sigma), TGF-β1 (Sigma), pyruvate (Sigma)] lines. After a total cultivation period of 21 days in vitro the following techniques were applied and parameters were measured for evaluation.
Immuncytochemical stainings
For immunocytochemical evaluation, we used 10 μm frozen sections (Cryostat, Zeiss, Göttingen, Germany) that were fixed onto slides (Superfrost plus, Menzel, Braunschweig, Germany) after an in vitro follow-up of 21 days. To detect osteogenic differentiation we used anti-collagen I, anti-collagen III (Chemicon Int., Hampshire, UK), anti-osteocalcin (OC) (Santa Cruz Europe, Heidelberg, Germany) alkaline phosphatase (ALP; Blue Alkaline Phosphatase Substrate Kit III, #SK-5300, Vector Laboratories, Burlingame, CA), anti-RANKL (Santa Cruz), CD34, CD105 (CD34 and CD105 antibodies; DAKO-Cytomation, Hamburg, Germany). For the detection of chondrogenic differentiation we used anti-collagen II (Chemicon Int., Hampshire, UK), chondrogenic oligometric matrix protein (COMP) (AbD Serotec, Düsseldorf Germany) cartilage proteoglycan (CP) (Chemicon), anti-Runx2 (R&D Systmes, Wiesbaden, Germany). In addition, the mesenchymal marker CD105 and the hematopoietic antigen CD34 were analyzed.
The polymer samples were fixed in 4% paraformaldehyde (Roth, Karlsruhe, Germany) at 20°C for 30 min and rinsed in PBS. Endogenous peroxidases of the specimen were blocked by 0.3% perhydrol-PBS solution. After rinsing in PBS, the cell culture dishes were incubated with primary antibodies against different antigens for chondrogenic (CD34, CD105, collagen II, COMP, CP, Runx2) und for osteogenic (CD 34, CD 105, collagen I, collagen III, OC, Rankl) with further incubation at 4°C for 12 h. A second antibody system (Anti-mouse-IgG biotinylated, anti-rat-IgG biotinylated, anti-Rabbit-IgG biot., anti-goat-IgG biot. and avidin-biotin complex (all Vector) and 3,3-diaminobenzidine (Sigma) was used for optical visualization. ALP activity was measured after direct substrate incubation (SK-5.200; Vector) for 30 min at RT in the dark. The cell cultures were analyzed and blinded by an independent observer using episcopic light microscopy (Axiovert 200; Zeiss) in combination with a computer-supported imaging picture analysis system (Axiovision; Zeiss). Feulgen staining (Feulgen-Kit; VWR, Darmstadt, Germany) served for semiquantitative DNA evaluation. Specimens were air-dried for 1 h, fixed in buffered 4% paraformaldehyde (pH 7.0), rinsed in water for 10 min, and incubated in 5 M HCl at 22°C for 50 min. Specimens were then washed twice in aqua dest., transferred to Schiff 's reagent for 60 min at RT, rinsed in bisulfite solution twice (3 min) and in aqua dest. 2 min at RT, followed by dehydration in graded alcohols.
At follow-up, bone marrow cells were morphologically analyzed using phase-contrast microscopy (Axiovert 200, Zeiss) supported by a computer picture analysis system (Axiovision, Zeiss). For semiquantitative analysis of the antigen expression by polystyrene adherent cells, the following score was used: no cells: 0; single cells (< 10% of the surface): 1; sub-confluent monolayer of + cells (10%-59% of the surface): 2; confluent monolayer of + cells (> 60% of the surface): 3.
RT-PCR
For mRNA analysis, the adherent cells were removed from culture dishes supported by 0.05% trypsin/0.02% EDTA solution (PAA)and resuspended in each 350 ml RLT buffer (Quiagen, Hilden, Germany) supplemented by 1: 100 14.3 M beta-mercaptoethanol (VWR, Darmstadt, Germany). One-step RT-PCR was performed using a thermal cycler (Mastercycler Gradient, Eppendorf AG, Hamburg, Germany). The reaction mixture (25 μL) contains 2 μl of human RNA, 1 μL 10 mM dNTP-Mix (Qiagen)), 1 μL recombinant RnasinTM/ribonuclease inhibitor 1:4 (Promega, Madi son, WI), 1 μl of each primer, 1 μl enzyme mix, 5 μl RT-buffer, 5 μl Q-solution, and 8 μl RNAse free aqua (Quiagen). For transcription and amplification, we used an enzyme mix containing OmnicriptTM Reverse transcriptase, SensiciriptTM Reverse Transcriptase, and HotStarTaq DNA polymerase (OneStep RT-PCR Kit 100, Quiagen). The thermal cycle conditions used were as follows: 30 min at 50°C (reverse transcription), 15 min at 95°C (denaturation) followed by RT-PCR cycling of 95°C for 30 s (denaturation), 55°C for 30 s (annealing), and 72°C for 60 s (extension), and lastly a final extension for 60 s at 72°C. RT-PCR was performed 35 cycles. GAPDH used as housekeeping gene. RT-PCR products were combined and resolved in a 2% agarose gel stained with ethidium bromide. Documentation and semiquantitative evaluation was performed using a gel documentation system and software (including a real-time camera with ethidium bromide filter combined with Alpha-DigiDoc TM RT software, Alpha Innotec, San Leonardo, CA).
Oligonucleotide primers: (Thermo Fisher Scientific, Schwerte, Germany)
Osteocalcin (OC): 50-agt cca gca aag gtg cag c-30/30-ggc cgt aga gcg ccg at- 50 (NM_199173),
RANKL: 50-cag agc gca gat gga tcc t-30/30-gta cca aga gga cag act ca- 50 (NM_003701),
Runx2: 50-cct tca agg tgg tag ccc t-30/30-gcc tgg ggt ctg taa tct g-50 (NM_004348),
Collagen 1A1: 50-gat ggc tgc acg agt cac a-30/30- gcc aga tgg caa ggc ttc t-50 (NM_000088),
Collagen 2A1: 50-ttt ccc agg tca aga tgg tc-30/30-ctg cag cac ctg tct cac ca-50 (NM_001844),
Collagen 3A1: 50- gga cat cga gga ttc cct g-30/30- cac tct tga gtt cag gat gg-50 (NM_000090),
GAPDH: 50-ctc aag atc atc agc aat gcc-30/30-gat ggt aca tga caa ggt gc- 50 (NM_002046),
COMP: 50- ctg gct gtg ggt tac act g-30/30- gat gat gtt ctc ctg gga g-50 (NM_000095),
CD 34: 50- cat cac aga aac gac agt caa-30/30- ctg cct tga tgt cac tta gg-50 (NM_001773.1),
CD 105: 50- ggc cgc acg ctc gag tg-30/30- aca tga gca gct ccg ggc-50 (NM 000118).
Results
Depending on the cultivation technique we found significant differences in both, gen and protein expression. The semiquantitative antigen pattern before lineage stimulation was:
ALP (+++), col I (+++), col II (-), col III (-), COMP (+++), CP (-), RANKL (++), Runx2 (+), OC (+), CD34 (-), CD105 (+++).
The antigen pattern after follow up showed a persisting COMP expression in all three systems whereas CP was only expressed in cell cultures which were exposed to the RWV system significantly. In contrast to the spinning cultures (group II), RWV and regular static tissue flasks allowed for CD105 and Runx2 expression. Col II and CD34 were negative in all cultures. Osteogenic stimulated cultures expressed higher levels of ALP in non-spinning cultures whereas col I, OC, RANKL and CD105-expression was increased in group II and III (Figures 3 and 4).
Figure 3.
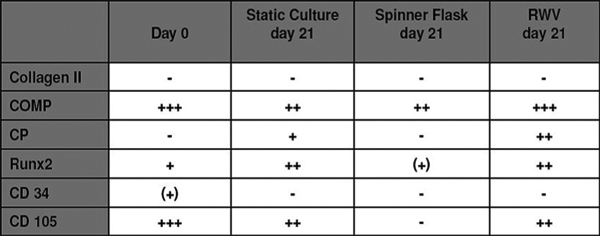
Chondrogenic differentiaion: no specific antigen detectable (-), < 10% positive adherent cells (+), 10-59% positive adherent cells (++), 60-100% positive adherent cels (+++); COMP: chondrogenic oligometric matrix protein; CP: cartilage proteoglycan; Runx2: runt-related transcription factor-2; CD34: hematopoietic antigen CD34; CD105: mesenchymal marker CD105.
Figure 4.
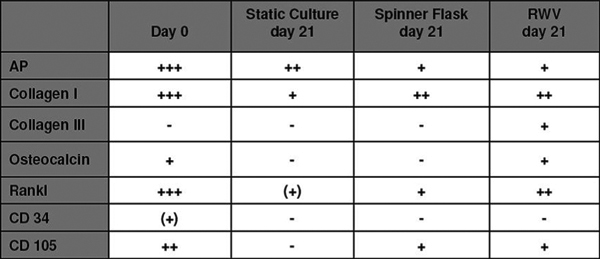
Osteotgenic differentiation: no specific antigen detectable (-), < 10% positive adherent cells (+), 10-59% positive adherent cells (++), 60-100% positive adherent cels (+++); AP: alkaline phosphatase; Rankl: Receptor Activator of NF-_B Ligand; CD34: hematopoietic antigen CD34; CD105: mesenchymal marker CD105.
RT-PCR
There were no differences in gene expression after chondrogenic stimulation within the different groups (Figure 5). After osteogenic stimulation, there were no differences between the three different groups for the expression of collagen I, Osteocalcin and Trap. The expression of RANKL, however, was documented only in the static group while it was not expressed in the spinner or in the RWV group (Figure 6). Notable, the expression of TRAP after osteogenic stimulation might be interpreted as a sign of osteoclast activation.
Figure 5.
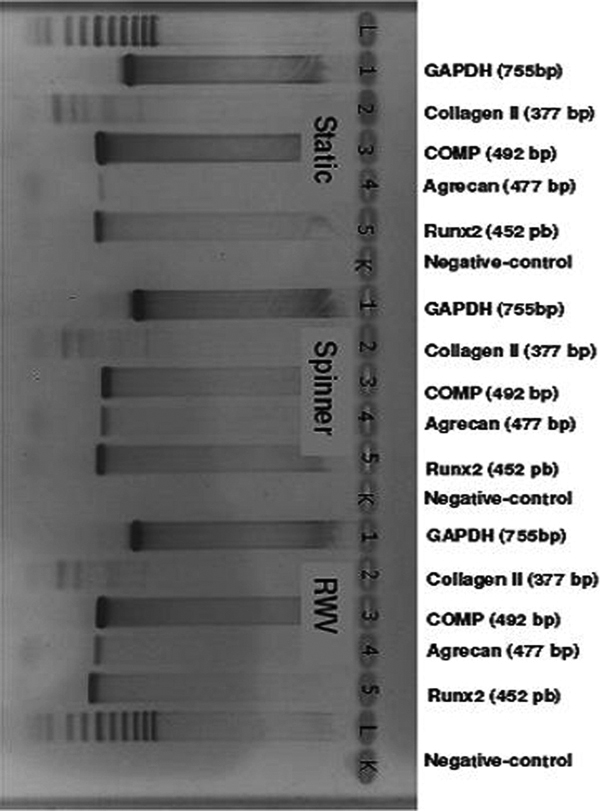
Rt-PCR after chondrogenic differentiation (bp: base pairs; gAdPH: d-glyceraldehyde-3-phosphate dehydrogenase; RAnkl: Receptor Activator of nF-_b ligand; tRAP: tartrate resistant acid phosphatase Static: static culture; Spinner: spinning culture; RWV: rotating wall vessel).
Figure 6.
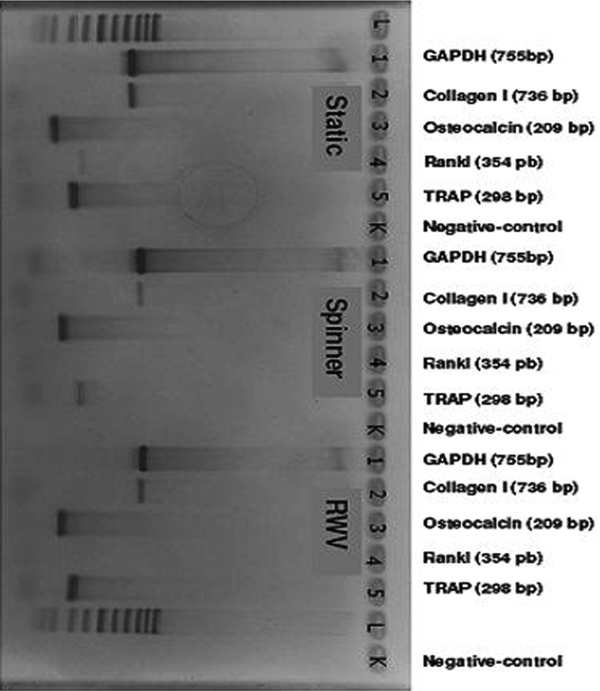
RT-PCR after osteogenic differentiation (STAT: bp: base pairs; GADPH: d-glyceraldehyde-3-phosphate dehydrogenase; COMP: cartilage oligomeric matrix protein; Runx 2: runt-related transcription factor-2; Static: static culture; Spinner: spinning culture; RWV: rotating wall vessel).
Immuncytochemical Stainings
Chondrogenic stimulation (Figure 7): The semipermeable collagen membrane allows for cellular attachment, adherence and proliferation. In STAT and RWV we found significant amounts of CP expression in the superficial cell-layers (up to 150 μm depth within the collagen texture). Also COMP was expressed in all three systems. In contrast to these findings, CD 34 and Col II were not detected. However, there were also signs of osteogenic differentiation as demonstrated fot the osteoblasts typical antigens Runx2 and CD 105 in STAT and RWV.
Figure 7.
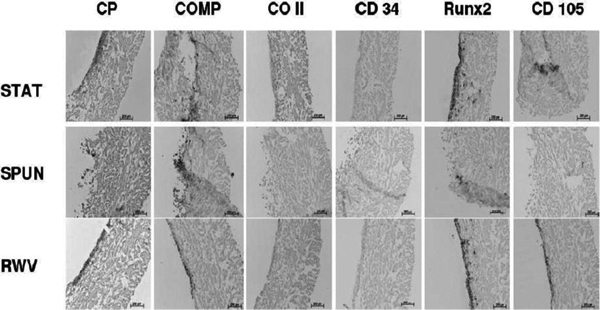
Antigen expression of MSC cultured onto a collagen I/II scaffold 21 days after chondrogenic and cytomechanical stimulation (CP: cartilage proteoglycan, COMP: chondrogenic oligometric matrix protein; CO II: collagen II; CD 34: hematopoietic antigen CD34; Runx 2: runt-related transcription factor-2; CD 105: mesenchymal marker CD105; STAT: static culture; SPUN: spinning culture; RWV: rotating wall vessel).
Under osteogenic stimulation (Figure 8) ALP was expressed in relevant amounts only in the STAT culture, whereas RWV allowed only ALP expression in the superficial layers and SPUN cultures only scattered over all layers within the scaffold. These results are confirmed by the expression of the osteoblastic marker RANKL and Col I.
Figure 8.
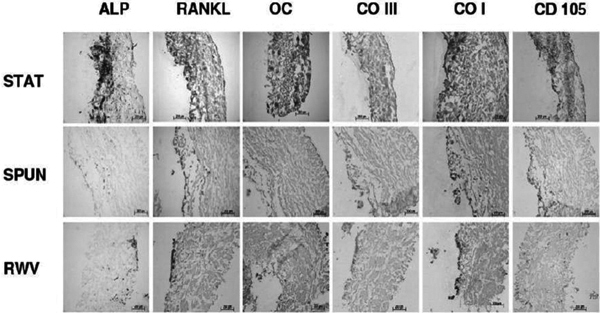
Antigenexpression of MSC cultured onto a collagen I/II scaffold 21 days after osteogenic and cytomechanical stimulation (ALP: alkaline phosphatase, RANKL: Receptor Activator of NF-κB Ligand; OC: osteocalcin; CO III: collagen III; CO I: collagen I; CD105: mesenchymal marker CD105; STAT: static culture; SPUN: spinning culture; RWV: rotating wall vessel).
Discussion
In our study we showed that cytomechanical forces with rotational components influence the osteogenic and chondrogenic in vitro-differentiation of MSCs. In contrast to the static forces or convential tissue flasks, spinning incubators are characterized by sheer forces, which may have different effects on cellular proliferation, differentiation and protein expression on bone marrow cells. The finding that sheer forces promote osteoblastic differentiation is corresponding to the current hypothesis suggesting that interstitial fluid flow is an important component to stimulate putative mechanosensors such as integrins in bone cells. For example, it was shown that activated integrins transduce their signal to the actin cytoskeleton and induce changes in the gene and protein expression such as cyclooxytgenase 2, cfos or NO [28]. In addition, there is evidence that also connexin 43 hemichannels represent another distinct subgroup of mechanosensors which are activated by stretch forces but also by fluid flow allowing paracine factors (small signalling molecules) to induce intracellular signalling pathways [29-33]. It was demonstrated by McAllister et al. [34] that fluid shear stress is the determining stimulus in the response of osteoblasts. Our results that spinning cultures promote osteoblast differentiation stronger than a rotating wall vessel is also corresponding to other authors who showed that the formation of actin stress fibers in osteoblasts lasts 1 h of steay fluid flow compared to 5 h of oscillatory fluid flow [35]. As demonstrated by Li, boths oscillatory and also steady fluid flow enhance expression of osteoblastic markers such as osteopontin and osteoclacin and are related to PGE2 release [36].
Also the osteoblast promoting effects induced by dynamic lodings and oscillating fluid flow are reproduced by the tubulences within the scaffold in a spinning incubator.
Our results indicate that cytomechanical sheer forces and medium flow induce cellular proliferation. The spinning culture system and static forces (control) seem to promote osteoblast differentiation. In contrast, both chondrogenic markers (COMP, CP) were expressed only by those cell cultures which were exposed to the RWV system. However the different number of antigen positive cells on the collagen I/III carrier can also be based on different cellular proliferation rates in the defined systems.
It has been demonstrated on MSC that fluid flow can induce a significant increase in proliferation within one hour via the activation of MAP and calcium signalling cascades [37].
Besides direct cytomechanical effects on cellular gene and protein expression the application of bioreactor systems also promotes the supply and local concentration of nutrients into porous scaffolds.
The transport of nutrients and waste products within a cell-seeded matrix derives primarily from diffusion [38]. In the absence of fluid flow, the primary mechanism allowing transport of nutrients to the center of scaffolds cultured under static conditions is diffusion, which may not meet the significant metabolic requirements of cells seeded on three dimensional scaffolds and cultured for extended time periods [39]. This may explain the cell proliferation rate in static culture was not comparable to dynamic spinner flask and rotating wall vessel culture conditions. Two different bioreactor culture systems were performed in parallel to the static culture conditions. These bioreactor culture systems were used to compare our dynamic cultures with established static culture approaches.
The static culture represents one established methodology for the culture of BM-derived adhesion-dependent cells. In static culture, MSCs were cultured without exposure to hydrodynamic shear forces, and with mass transfer by molecular diffusion only. In spinner flask culture, flow and mixing of culture medium was associated with turbulent shear at constant surfaces. Mass transport between the constructs and culture medium was enhanced by convection, whereas mass transport within the constructs remained controlled by diffusion. In rotating wall vessel culture, the utility provides a microgravity environment with low fluid shear and dynamic intercellular interactions [14,40]. It is suggested that shear stress is an important biomechanical parameter in regulating human MSC construct development [41].
These MSCs are cultivated under different culture systems with same osteogenic induction medium. We found that some proteins as ALP mentioned earlier were expressed in spinner dynamic culture when compared with static culture condition, which means that mechanical stress had effects during osteogenesis process. Some studies indicate that intermittent mechanical loading could promote chondrogenesis of BM-derived MSCs through responsive gene regulating pathway [42,43]. On the other hand, the mechanical stimulation was minimized because of the microgravitational environment in the rotating reactor culture system. The expression of osteoblastic and chondrocytic lineage proteins appeared to be obviously inhibited.
A significant difference in gene expression between the different culture conditions was observed. The better mixing provided in the spinner flasks may explain the accelerated proliferation and differentiation of MSCs, and the localization of the enhanced mineralization on the external surface of the scaffolds. The results were in agreement with the previous research studies reported by Sikavitsas et al [18] and Wang et al [14]. Wang et at concluded, that the mechanically active environment present in the spinner flask bioreactors mediates the effectiveness of adult human MSCs in 3D scaffolds for osteogenic and chondrogenic differentiation [14].
The trends observed in the cell/polymer constructs cultured in the spinner flask bioreactor and the static culture are in agreement with the temporal expression and cell growth of osteoblastic cells described. Similar cellular behavior was seen by Goldstein et al. [17] for the early osteoblastic differentiation.
MSCs represent a promising, readily available autologous cell source for bone tissue-engineering applications [26,27,44-48]. Several preclinical studies directed at de novo osteogenesis have been conducted using rat marrow stromal cell culture [17,18,49-53] or osteoblastic cell lines [24,54,55] as a surrogate for primary human cells. However, primary human MSCs are the idal cell type for such a study because of interspecies variation.
To investigate the efficiency of bone tissue-engineering protocols using primary human MSCs, researchers would ideally include replicates of primary cell cultures from a sufficient number of donors to account for biological variation. The data obtained should then be stratified by relevant factors, e.g. sex, age, and comorbidity. However, such a setup seems far from realistic due to limitations in experimental replicates and restricted availability of primary human MSCs cultures. Another problem is that primary MSCs gradually lose both their proliferation and differentiation potential during ex vivo expansion [46]. Thus early-passage cultures are needed for preclinical studies, further limiting the amounts of biological material available. MSC cultures from young patients are of particular interest because especially young patients may have musculoskeletal diseases and would benefit from the implantation of durable, tissue-engineered, autologous bone graft composites. In the present study, we used a well-characterized immortalized human BM-derived MSC population.
Several studies show that fluid shear stress upregulates anabolic factors relating to proliferation and differentiation of adherent bone cells [56-58]. The effect of fluid flow on cellular response is known to involve typical mechanotransduction pathways, e.g. integrin activation, influx of extracellular Ca2+, and activation of transcription factors via second messenger systems resulting in the upregulation of bone-related genes and the expression of signaling molecules [59]. Fluid flow stimulation of ex vivo 3-D cultured osteoprogenitor cells therefore represents a promising approach to mimic the native environment of these cells in vivo.
To combine fluid flow stimulation and mitigation of external mass transport limitation, a number of dynamic culture strategies have been developed. Among those systems, bioreactors based on axial perfusion of the cell-loaded scaffold [17,19,49,52,53,60] and spinner flask bioreactors [17-19] have been tested for bone tissue engineering. The spinner flask is recognized as a controllable system for minimizing diffusional gradients within cell/scaffold constructs and exposing 3-D cultured cells to fluid convection by mixing of culture medium [16]. Here, we investigated the efficiency of this type of bioreactor system in relation to proliferation, distribution, and osteogenic differentiation of 3-D cultured human MSCs.
Cellularity of the scaffolds was assessed by total DNA assay. The results generally indicated a continued proliferation of human MSCs within the scaffolds for up to 3 weeks of culture, regardless of the culture method. Sikavitsas et al. reported that continuous fluid convection significantly stimulated proliferation of rat marrow stromal cells after 7 and 14 days, but not after 21 days of 3-D spinner flask culture compared with static culture [18]. In a similar study, Goldstein et al. found the same tendency for 7 and 14 days periods of culture [17].
To come back to the initial question wheter osteoblasts prefer spinning around or stagnation we assume that stagnation (static load, gravity) but also spinning allow for osteoblast in vitro differentiation. In contrast chondroblasts prefer continuus sheer forces as simulated by the rotation vessel. However, a different situation was found in scaffold-associated cultures. Here boths, the STAT and in less amounts the RWV promote osteogenic differentiation. The same tendency was found for chondroblast differentiation under chondrogenic mixture. The absence of col II can be interpretated as a wash-out effect promoted by short molecule length compared the relatively large collagen I triple-helix [61]. The polarity in antigen-expression dependent on the semipermeable or the permeable (highly porous) site of the scaffold reflects the strong influence of surface parameters on cell differentiation. The dynamic medium flow with turbulences does not allow any conclusion of the effects of further surface geometry paramters such as convexity or concavity in our investigation.
Conclusion
We used MSCs derived from adult human BM and applied three different culture environments (static culture, spinner flask, rotating wall vessel) to study osteogenesis and chondrogenesis under controlled in vitro conditions. Our results are in agreement with other studies and suggest that osteogenesis and chondrogenesis in cultured MSCs can be modulated by fluid flow and cytomechanical forces. Here, spinner flask bioreactor systems can mimic some aspects of the native biophysical environment and could be utilized in controlled studies on cell function and tissue engineering.
References
- Jäger M, Westhoff B, Wild A, Krauspe R. Bone harvesting from the iliac crest. Orthopade. 2005;34(10):976–982. doi: 10.1007/s00132-005-0839-0. 84, 86-90, 92-94. [DOI] [PubMed] [Google Scholar]
- Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA. Complications of iliac crest bone graft harvesting. Clin Orthop Relat Res. 1996;329:300–309. doi: 10.1097/00003086-199608000-00037. [DOI] [PubMed] [Google Scholar]
- Younger EM, Chapman MW. Morbidity at bone graft donor sites. J Orthop Trauma. 1989;3(3):192–195. doi: 10.1097/00005131-198909000-00002. [DOI] [PubMed] [Google Scholar]
- Muschler GF, Huber B, Ullman T, Barth R, Easley K, Otis JO, Lane JM. Evaluation of bone-grafting materials in a new canine segmental spinal fusion model. J Orthop Res. 1993;11(4):514–524. doi: 10.1002/jor.1100110406. [DOI] [PubMed] [Google Scholar]
- Gronthos S, Simmons PJ. The biology and application of human bone marrow stromal cell precursors. J Hematother. 1996;5(1):15–23. doi: 10.1089/scd.1.1996.5.15. [DOI] [PubMed] [Google Scholar]
- Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997;64(2):295–312. doi: 10.1002/(SICI)1097-4644(199702)64:2<295::AID-JCB12>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Aubin JE. Osteoprogenitor cell frequency in rat bone marrow stromal populations: role for heterotypic cell-cell interactions in osteoblast differentiation. J Cell Biochem. 1999;72(3):396–410. doi: 10.1002/(SICI)1097-4644(19990301)72:3<396::AID-JCB9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Haynesworth SE, Goshima J, Goldberg VM, Caplan AI. Characterization of cells with osteogenic potential from human marrow. Bone. 1992;13(1):81–88. doi: 10.1016/8756-3282(92)90364-3. [DOI] [PubMed] [Google Scholar]
- Ishaug SL, Crane GM, Miller MJ, Yasko AW, Yaszemski MJ, Mikos AG. Bone formation by three-dimensional stromal osteoblast culture in biodegradable polymer scaffolds. J Biomed Mater Res. 1997;36(1):17–28. doi: 10.1002/(SICI)1097-4636(199707)36:1<17::AID-JBM3>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- van den Dolder J, Farber E, Spauwen PH, Jansen JA. Bone tissue reconstruction using titanium fiber mesh combined with rat bone marrow stromal cells. Biomaterials. 2003;24(10):1745–1750. doi: 10.1016/S0142-9612(02)00537-9. [DOI] [PubMed] [Google Scholar]
- Ohgushi H, Caplan AI. Stem cell technology and bioceramics: from cell to gene engineering. J Biomed Mater Res. 1999;48(6):913–927. doi: 10.1002/(SICI)1097-4636(1999)48:6<913::AID-JBM22>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Ishaug-Riley SL, Crane-Kruger GM, Yaszemski MJ, Mikos AG. Three-dimensional culture of rat calvarial osteoblasts in porous biodegradable polymers. Biomaterials. 1998;19(15):1405–1412. doi: 10.1016/S0142-9612(98)00021-0. [DOI] [PubMed] [Google Scholar]
- Freed L, Vunjak-Novacovic G. In: Principles of Tissue Engineering. 2. Lanza R, Langer R, Vacanti J, editor. San Diego, CA: Academic Press; 2000. Tissue engineering bioreactors; pp. 143–56. [Google Scholar]
- Wang TW, Wu HC, Wang HY, Lin FH, Sun JS. Regulation of adult human mesenchymal stem cells into osteogenic and chondrogenic lineages by different bioreactor systems. J Biomed Mater Res A. 2008. [DOI] [PubMed]
- Kale S, Biermann S, Edwards C, Tarnowski C, Morris M, Long MW. Three-dimensional cellular development is essential for ex vivo formation of human bone. Nat Biotechnol. 2000;18(9):954–958. doi: 10.1038/79439. [DOI] [PubMed] [Google Scholar]
- Vunjak-Novakovic G, Martin I, Obradovic B, Treppo S, Grodzinsky AJ, Langer R, Freed LE. Bioreactor cultivation conditions modulate the composition and mechanical properties of tissue-engineered cartilage. J Orthop Res. 1999;17(1):130–138. doi: 10.1002/jor.1100170119. [DOI] [PubMed] [Google Scholar]
- Goldstein AS, Juarez TM, Helmke CD, Gustin MC, Mikos AG. Effect of convection on osteoblastic cell growth and function in biodegradable polymer foam scaffolds. Biomaterials. 2001;22(11):1279–1288. doi: 10.1016/S0142-9612(00)00280-5. [DOI] [PubMed] [Google Scholar]
- Sikavitsas VI, Bancroft GN, Mikos AG. Formation of three-dimensional cell/polymer constructs for bone tissue engineering in a spinner flask and a rotating wall vessel bioreactor. J Biomed Mater Res. 2002;62(1):136–148. doi: 10.1002/jbm.10150. [DOI] [PubMed] [Google Scholar]
- Meinel L, Karageorgiou V, Fajardo R, Snyder B, Shinde-Patil V, Zichner L, Kaplan D, Langer R, Vunjak-Novakovic G. Bone tissue engineering using human mesenchymal stem cells: effects of scaffold material and medium flow. Ann Biomed Eng. 2004;32(1):112–122. doi: 10.1023/b:abme.0000007796.48329.b4. [DOI] [PubMed] [Google Scholar]
- Long MW. Osteogenesis and bone-marrow-derived cells. Blood Cells Mol Dis. 2001;27(3):677–690. doi: 10.1006/bcmd.2001.0431. [DOI] [PubMed] [Google Scholar]
- Wang TW, Wu HC, Huang YC, Sun JS, Lin FH. Biomimetic bilayered gelatin-chondroitin 6 sulfate-hyaluronic acid biopolymer as a scaffold for skin equivalent tissue engineering. Artif Organs. 2006;30(3):141–149. doi: 10.1111/j.1525-1594.2006.00200.x. [DOI] [PubMed] [Google Scholar]
- Schwarz RP, Goodwin TJ, Wolf DA. Cell culture for three-dimensional modeling in rotating-wall vessels: an application of simulated microgravity. J Tissue Cult Methods. 1992;14(2):51–57. doi: 10.1007/BF01404744. [DOI] [PubMed] [Google Scholar]
- Qiu QQ, Ducheyne P, Ayyaswamy PS. Fabrication, characterization and evaluation of bioceramic hollow microspheres used as microcarriers for 3-D bone tissue formation in rotating bioreactors. Biomaterials. 1999;20(11):989–1001. doi: 10.1016/S0142-9612(98)00183-5. [DOI] [PubMed] [Google Scholar]
- Botchwey EA, Pollack SR, Levine EM, Laurencin CT. Bone tissue engineering in a rotating bioreactor using a microcarrier matrix system. J Biomed Mater Res. 2001;55(2):242–253. doi: 10.1002/1097-4636(200105)55:2<242::AID-JBM1011>3.0.CO;2-D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vunjak-Novakovic G, Obradovic B, Martin I, Bursac PM, Langer R, Freed LE. Dynamic cell seeding of polymer scaffolds for cartilage tissue engineering. Biotechnol Prog. 1998;14(2):193–202. doi: 10.1021/bp970120j. [DOI] [PubMed] [Google Scholar]
- Jager M, Feser T, Denck H, Krauspe R. Proliferation and osteogenic differentiation of mesenchymal stem cells cultured onto three different polymers in vitro. Ann Biomed Eng. 2005;33(10):1319–1332. doi: 10.1007/s10439-005-5889-2. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Mar shak DR. Multilineage potential of adult human mesen chymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Riddle RC, Donahue HJ. From streaming-potentials to shear stress: 25 years of bone cell mechanotransduction. J Orthop Res. 2009;27(2):143–149. doi: 10.1002/jor.20723. [DOI] [PubMed] [Google Scholar]
- Spray DC, Ye ZC, Ransom BR. Functional connexin "hemichannels": a critical appraisal. Glia. 2006;54(7):758–773. doi: 10.1002/glia.20429. [DOI] [PubMed] [Google Scholar]
- Genetos DC, Kephart CJ, Zhang Y, Yellowley CE, Donahue HJ. Oscillating fluid flow activation of gap junction hemichannels induces ATP release from MLO-Y4 osteocytes. J Cell Physiol. 2007;212(1):207–214. doi: 10.1002/jcp.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang JX, Cherian PP. Hemichannels formed by connexin 43 play an important role in the release of prostaglandin E(2) by osteocytes in response to mechanical strain. Cell Commun Adhes. 2003;10(4-6):259–264. doi: 10.1080/cac.10.4-6.259.264. [DOI] [PubMed] [Google Scholar]
- Cherian PP, Siller-Jackson AJ, Gu S, Wang X, Bonewald LF, Sprague E, Jiang JX. Mechanical strain opens connexin 43 hemichannels in osteocytes: a novel mechanism for the release of prostaglandin. Mol Biol Cell. 2005;16(7):3100–3106. doi: 10.1091/mbc.E04-10-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziambaras K, Lecanda F, Steinberg TH, Civitelli R. Cyclic stretch enhances gap junctional communication between osteoblastic cells. J Bone Miner Res. 1998;13(2):218–228. doi: 10.1359/jbmr.1998.13.2.218. [DOI] [PubMed] [Google Scholar]
- McAllister TN, Frangos JA. Steady and transient fluid shear stress stimulate NO release in osteoblasts through distinct biochemical pathways. J Bone Miner Res. 1999;14(6):930–936. doi: 10.1359/jbmr.1999.14.6.930. [DOI] [PubMed] [Google Scholar]
- Ponik SM, Triplett JW, Pavalko FM. Osteoblasts and osteocytes respond differently to oscillatory and unidirectional fluid flow profiles. J Cell Biochem. 2007;100(3):794–807. doi: 10.1002/jcb.21089. [DOI] [PubMed] [Google Scholar]
- Li YJ, Batra NN, You L, Meier SC, Coe IA, Yellowley CE, Jacobs CR. Oscillatory fluid flow affects human marrow stromal cell proliferation and differentiation. J Orthop Res. 2004;22(6):1283–1289. doi: 10.1016/j.orthres.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Riddle RC, Taylor AF, Genetos DC, Donahue HJ. MAP kinase and calcium signaling mediate fluid flow-induced human mesenchymal stem cell proliferation. Am J Physiol Cell Physiol. 2006;290(3):C776–C784. doi: 10.1152/ajpcell.00082.2005. [DOI] [PubMed] [Google Scholar]
- Grodzinsky A, Kamm R, Lauffenburger D. Principles of Tissue Engineering. San Diego: Academic Press; 2000. Quantitative aspects of tissue engineering: Basic issues in kinetics, transport, and mechanics; pp. 195–206. [Google Scholar]
- Mueller SM, Mizuno S, Gerstenfeld LC, Glowacki J. Medium perfusion enhances osteogenesis by murine osteosarcoma cells in three-dimensional collagen sponges. J Bone Miner Res. 1999;14(12):2118–2126. doi: 10.1359/jbmr.1999.14.12.2118. [DOI] [PubMed] [Google Scholar]
- Chen X, Xu H, Wan C, McCaigue M, Li G. Bioreactor expansion of human adult bone marrow-derived mesenchymal stem cells. Stem Cells. 2006;24(9):2052–2059. doi: 10.1634/stemcells.2005-0591. [DOI] [PubMed] [Google Scholar]
- Zhao F, Chella R, Ma T. Effects of shear stress on 3-D human mesenchymal stem cell construct development in a perfusion bioreactor system: Experiments and hydrodynamic modeling. Biotechnol Bioeng. 2007;96(3):584–595. doi: 10.1002/bit.21184. [DOI] [PubMed] [Google Scholar]
- Huang CY, Reuben PM, Cheung HS. Temporal expression patterns and corresponding protein inductions of early responsive genes in rabbit bone marrow-derived mesenchymal stem cells under cyclic compressive loading. Stem Cells. 2005;23(8):1113–1121. doi: 10.1634/stemcells.2004-0202. [DOI] [PubMed] [Google Scholar]
- Mouw JK, Connelly JT, Wilson CG, Michael KE, Levenston ME. Dynamic compression regulates the expression and synthesis of chondrocyte-specific matrix molecules in bone marrow stromal cells. Stem Cells. 2007;25(3):655–663. doi: 10.1634/stemcells.2006-0435. [DOI] [PubMed] [Google Scholar]
- Caplan AI. Review: mesenchymal stem cells: cell-based reconstructive therapy in orthopedics. Tissue Eng. 2005;11(7-8):1198–1211. doi: 10.1089/ten.2005.11.1198. [DOI] [PubMed] [Google Scholar]
- Bruder SP, Jaiswal N, Ricalton NS, Mosca JD, Kraus KH, Kadiyala S. Mesenchymal stem cells in osteobiology and applied bone regeneration. Clin Orthop Relat Res. 1998;355(Suppl):S247–S256. doi: 10.1097/00003086-199810001-00025. [DOI] [PubMed] [Google Scholar]
- Mauney JR, Volloch V, Kaplan DL. Role of adult mesenchymal stem cells in bone tissue engineering applications: current status and future prospects. Tissue Eng. 2005;11(5-6):787–802. doi: 10.1089/ten.2005.11.787. [DOI] [PubMed] [Google Scholar]
- Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36(4):568–584. doi: 10.1016/j.biocel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesen chymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119(Pt 11):2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- Holtorf HL, Jansen JA, Mikos AG. Flow perfusion culture induces the osteoblastic differentiation of marrow stroma cell-scaffold constructs in the absence of dexa methasone. J Biomed Mater Res A. 2005;72(3):326–334. doi: 10.1002/jbm.a.30251. [DOI] [PubMed] [Google Scholar]
- Abukawa H, Terai H, Hannouche D, Vacanti JP, Kaban LB, Troulis MJ. Formation of a mandibular condyle in vitro by tissue engineering. J Oral Maxillofac Surg. 2003;61(1):94–100. doi: 10.1053/joms.2003.50015. [DOI] [PubMed] [Google Scholar]
- Qiu Q, Ducheyne P, Gao H, Ayyaswamy P. Formation and differentiation of three-dimensional rat marrow stromal cell culture on microcarriers in a rotating-wall vessel. Tissue Eng. 1998;4(1):19–34. doi: 10.1089/ten.1998.4.19. [DOI] [PubMed] [Google Scholar]
- Bancroft GN, Sikavitsas VI, van den Dolder J, Sheffield TL, Ambrose CG, Jansen JA, Mikos AG. Fluid flow increases mineralized matrix deposition in 3D perfusion culture of marrow stromal osteoblasts in a dose-dependent manner. Proc Natl Acad Sci USA. 2002;99(20):12600–12605. doi: 10.1073/pnas.202296599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikavitsas VI, Bancroft GN, Holtorf HL, Jansen JA, Mikos AG. Mineralized matrix deposition by marrow stromal osteoblasts in 3D perfusion culture increases with increasing fluid shear forces. Proc Natl Acad Sci USA. 2003;100(25):14683–14688. doi: 10.1073/pnas.2434367100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou YF, Dunn JC, Wu BM. In vitro response of MC3T3-E1 pre-osteoblasts within three-dimensional apatite-coated PLGA scaffolds. J Biomed Mater Res B Appl Biomater. 2005;75(1):81–90. doi: 10.1002/jbm.b.30261. [DOI] [PubMed] [Google Scholar]
- Rucci N, Migliaccio S, Zani BM, Taranta A, Teti A. Characterization of the osteoblast-like cell phenotype under microgravity conditions in the NASA-approved Rotating Wall Vessel bioreactor (RWV) J Cell Biochem. 2002;85(1):167–179. doi: 10.1002/jcb.10120. [DOI] [PubMed] [Google Scholar]
- Reich KM, McAllister TN, Gudi S, Frangos JA. Activation of G proteins mediates flow-induced prostaglandin E2 production in osteoblasts. Endocrinology. 1997;138(3):1014–1018. doi: 10.1210/en.138.3.1014. [DOI] [PubMed] [Google Scholar]
- Nauman EA, Satcher RL, Keaveny TM, Halloran BP, Bikle DD. Osteoblasts respond to pulsatile fluid flow with short-term increases in PGE(2) but no change in mineralization. J Appl Physiol. 2001;90(5):1849–1854. doi: 10.1152/jappl.2001.90.5.1849. [DOI] [PubMed] [Google Scholar]
- Bakker AD, Soejima K, Klein-Nulend J, Burger EH. The production of nitric oxide and prostaglandin E(2) by primary bone cells is shear stress dependent. J Biomech. 2001;34(5):671–677. doi: 10.1016/S0021-9290(00)00231-1. [DOI] [PubMed] [Google Scholar]
- Iqbal J, Zaidi M. Molecular regulation of mechanotransduction. Biochem Biophys Res Commun. 2005;328(3):751–755. doi: 10.1016/j.bbrc.2004.12.087. [DOI] [PubMed] [Google Scholar]
- Vance J, Galley S, Liu DF, Donahue SW. Mechanical stimulation of MC3T3 osteoblastic cells in a bone tissue-engineering bioreactor enhances prostaglandin E2 release. Tissue Eng. 2005;11(11-12):1832–1839. doi: 10.1089/ten.2005.11.1832. [DOI] [PubMed] [Google Scholar]
- Roughley PJ, Lee ER. Cartilage proteoglycans: structure and potential functions. Microsc Res Tech. 1994;28(5):385–397. doi: 10.1002/jemt.1070280505. [DOI] [PubMed] [Google Scholar]


