Abstract
Colored fruits, particularly berries, are highly chemoprotective because of their antioxidant, anti-proliferative and anti-inflammatory activities. We report cancer chemoprotective potential of Syzygium cumini L., commonly known as ‘jamun’ or Indian blackberry. Anthocyanins and other polyphenolics were extracted with acidic ethanol, and enriched by amberlite XAD7/HP20 (1:1). The pulp powder was found to contain 0.54% anthocyanins, 0.17% ellagic acid/ellagitannins and 1.15% polyphenolics. Jamun seed contained no detectable anthocyanins, but had higher amounts of ellagic acid/ellagitannins (0.5%) and total polyphenolics (2.7%) than the pulp powder. Upon acid hydrolysis, the pulp extract yielded five anthocyanidins by HPLC: malvidin (44.4%), petunidin (24.2%), delphinidin (20.3%), cyanidin (6.6%), and peonidin (2.2%). Extracts of both jamun pulp (1,445±64 μmol of trolox equivalent (TE)/g) and seeds (3,379±151 μM of TE/g) showed high oxygen radical absorbance capacity (ORAC). Their high antioxidant potential was also reflected by 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid) (ABTS)- and 2,2-diphenyl-1-picrylhydrazyl (DPPH)-scavenging, and ferrous ion-chelating activities. We also analyzed anti-proliferative activity of jamun extracts against human lung cancer A549 cells. The hydrolyzed pulp and seed extracts showed significant antiproliferative activity. However, unhydrolyzed extracts showed much less activity. These data showed that in addition to five anthocyanidins, jamun contains appreciable amounts of ellagic acid/ellagitannins, with high antioxidant and antiproliferative activities.
Keywords: Anthocyanins, Syzygium cumini, ellagic acid and ellagitannins, antioxidant activity, anti-proliferative activity
Introduction
Reactive oxygen species (ROS) such as superoxide radicals (O−−2), hydroxyl radical (−OH), and peroxyl radicals (ROO−) have been associated with carcinogenesis, coronary heart disease, and many other health issues related to advanced age [1]. There are endogenous as well as exogenous systems to protect cells in animals. However, in many conditions such as with aging, the endogenous antioxidants are depleted, and therefore an exogenous supply of antioxidants is essential. Antioxidants play an important role in biological systems [2] by suppressing the formation of reactive oxygen species by reducing hydroperoxides (ROO−) and H2O2, and scavenging free radicals.
Since antioxidants terminate ROS-mediated oxidative reactions, they may be used in prevention of aging-associated diseases, including cancer. This has led to an accelerated search for pure compounds and extracts of natural origin with antioxidant principles. Antioxidants have been detected in many agricultural and food products, including fruits, vegetables and oil seeds [3, 4]. However, many studies have conclusively shown that the majority of the antioxidant activity may be due to compounds such as flavonoids, isoflavones, flavones and anthocyanins rather than the traditionally considered vitamins C, E and β-carotene [5, 6]. There is also a direct relationship between antioxidant activity and polyphenolic content of plant extracts [6, 7]. Antioxidant activity of phenolics is mainly due to their redox potential which allows them to act as reducing agents, hydrogen donors, singlet oxygen quenchers and metal chelators [8, 9].
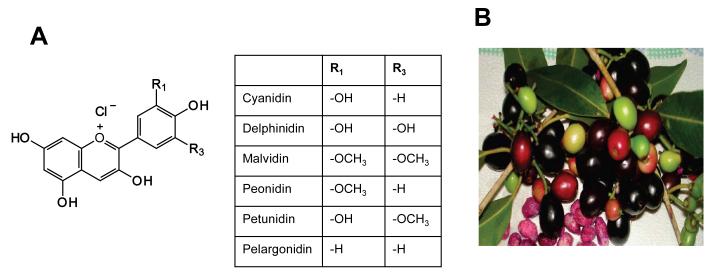
Anthocyanins are a group of water-soluble flavonoids that are responsible for the bright red, blue, and purple colors in fruits, vegetables, and ornamental crops. The anthocyanins found in foods are glycosides of six main aglycon anthocyanidins: cyanidin, delphinidin, pelargonidin, peonidin, malvidin, and petunidin (Figure 1A). Health benefits associated with the use of anthocyanin-enriched (colored) foods include reduced risk of coronary heart disease [10], protection against obesity and hypoglycemia [11], memory enhancement [12], and prevention of cancer [13-15].
Figure 1.
Structures of various anthocyanidins (A) in jamun fruit, the Indian blackberry (B).
Syzygium cumini L. (family, Myrtaceae), commonly known as jamun or Indian blackberry, is native to India; it thrives in a tropical climate. It is also found in some parts of America as well as Asian and East African countries. The fruits are oblong berries, deep purple or bluish in color with pinkish pulp (Figure 1B), and are widely consumed as a fruit and also used for the treatment of various diseases as an astringent, antiscorbutic, diuretic, antidiabetic, and in chronic diarrhea and enlargement of spleen [16-18]. Besides antidiabetic properties [19], jamun fruit has also been reported for strong antioxidant [20] and anti-genotoxic potential [21]. Though different parts of this plant are used in herbal formulations, chemical components of the fruit and seed are poorly defined. There have been three reports [18, 22, 23] on the anthocyanin composition of jamun fruit. The pulp of the fruit contains diglucoside of five anthocyanidins [22]. The other recent report revealed the high content of phenolics in the seeds [24]. Anthocyanins exhibit anticarcinogenic properties such as induction of cell-cycle arrest and apoptosis, as well as the inhibition of tumor formation and growth in animals [15]. The present study focused on extraction strategies and evaluating the chemical profile of both the jamun pulp and seed and associated antioxidant and anti-proliferative properties.
Material and methods
Chemicals
XAD-761 (Amberlite polymeric adsorbent of 20–50 mesh) and Diaion HP-20 were purchased from Supelco (Bellefonte, PA, USA). Ascorbic acid, 1,1-diphenyl-2-picryl hydrazyl (DPPH−), AAPH, fluorescein, and ABTS were procured from Sigma-Aldrich (St Louis, MO). Anthocyanin and anthocyanidin standards were obtained from ChromaDex Inc., Santa Ana, CA. All other chemicals used were of analytical grade.
Plant material and preparation of the extract
Syzygium cumini (jamun) fruits were collected from the garden of National Institute of Pharmaceutical Education and Research (NIPER), S.A.S. Nagar, India. The pulp with skin (dark purple color) of the fruits were manually separated from the seeds and immediately dried under vacuum at 40°C for two days. Dried pulp was powdered and stored at −20°C in vacuum-sealed packs until use. Seeds were also powdered and stored likewise.
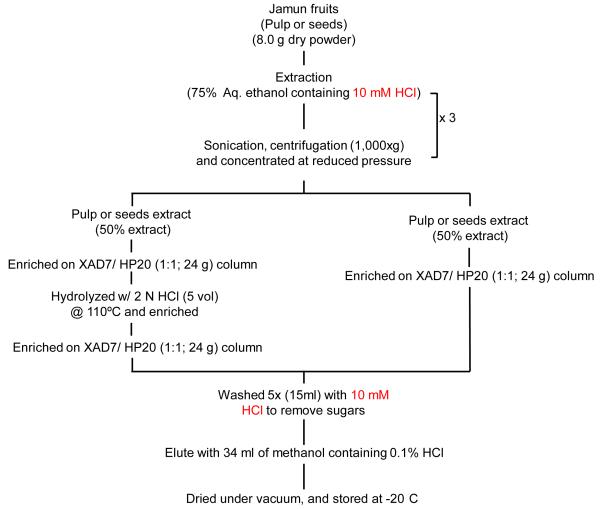
Jamun pulp and seed powders were extracted as described schematically (Figure 2). Briefly, 8 g each of pulp and seed powder were extracted with 3 vol (w/v; 24 ml) of 75% aqueous ethanol containing 10 mM HCl. To increase the extraction efficiency, samples were sonicated for 15 min in a bath-type sonicator. Samples were then centrifuged at 1000 x g for 10 min and decanted. Residues were extracted two more times in each case. The pooled supernatants were concentrated to about 12 ml volume under vacuum at 40°C using a rotary evaporator (Model R 215, Buchi, Switzerland). Enrichment of the anthocyanins, ellagitannins and other polyphenolics was achieved by loading the concentrated extracts (6 ml) on a XAD-761 and diaion HP-20 (1:1; 24 g total) column. XAD-761 has a high affinity for anthocyanins [25] while diaion HP-20 for ellagitannins and other polyphenolics [26]. Free sugars were eluted using five void-volumes of water. The polyphenolics, including anthocyanins and ellagitannins, were eluted under gravity using methanol (34 ml) containing 10 mM HCl. Pooled eluates were concentrated under vacuum at 40°C and one half of the concentrated eluates were hydrolyzed as described below.
Figure 2.
Scheme for the isolation of enriched anthocyanins/anthocyanidins and phenolics.
Acid hydrolysis of the enriched jamun pulp and seed extracts
The enriched extracts of jamun pulp and seeds (100 ml) were hydrolyzed with 2N HCl (~ 5 vol). Samples were refluxed at 110 °C for 1 h and then immediately cooled in an ice-bath. Hydrolysates were purified on a XAD-761 and diaion HP-20 column as described above. Polyphenolics, including anthocyanidins, were eluted with acidified (10 mM HCl) methanol. The enriched extracts were dried under vacuum using a Savant Speed-Vac (Thermo-Savant, Holbrook, NY).
HPLC analysis
HPLC analysis was carried out according to Li et al. [22] with minor modifications on a Shimadzu HPLC system (Kyoto, Japan) equipped with two LC-10ADvp pumps, autosampler, fluorescent and diode array detectors and operated by Class VP (ver 7.4 SP3). Jamun pulp and seed (hydrolyzed and unhydrolyzed) extracts (20 μL injection volume; 1 mg/ml) were analyzed on a ShimPack reverse phase column (Shimadzu; 250 × 4.6 mm, 2.2 μm). Two separate experiments were carried out using different gradients of 3.5% aqueous phosphoric acid (solvent A) and acetonitrile (solvent B). The first experiment was used to separate all anthocyanidins, and the second was used for the separation of anthocyanins, anthocyanidins, and other polyphenolics including ellagic acid and ellagitannins.
The first linear gradient contained solvent A initially 95% for 10 min; 90% at 20 min; 87% at 30 min; 82% at 35 min; 75% at 40 min; 40% for 41-61 min; and finally 95% in acetonitrile at a flow rate of 0.75 ml/min. The total run time was 61 min with 10 min intervals between the injections. Anthocyanidins were monitored at 520 nm. The second gradient contained solvent A initially - 90% for 0 - 5 min; 85% at 10 min; 80% at 15 min; 70% at 24 min; 62% at 35 min; 94% for 40-43 min; and finally 90% at 45 min. Ellagic acid/ellagitannins and polyphenolics were monitored at 366 and 280 nm respectively. Anthocyanidins and ellagic acid peaks were confirmed by co-chromatography with the reference materials.
Determination of total anthocyanins by pH-differential method
Anthocyanin content was determined using the pH-differential method [27, 28] and expressed as cyanidin-3-glucoside equivalent using molar extinction coefficient of 26,900 L cm−1 mol−1 and molecular mass of 449.2 g mol−1. Briefly, 0.2-ml samples containing different concentrations of extracts were mixed separately with 0.8 ml each of 0.025 M potassium chloride, pH 1.0 (adjusted with HCl) and 0.4 M sodium acetate, pH 4.5. The reaction mixtures were allowed to equilibrate at room temperature for 15 min and absorbance was measured at 510 and 700 nm. The difference in absorbance between the two samples was then calculated using the formula:
Finally, total anthocyanins/anthocyanidins in the samples were calculated by the following formula:
Determination of total soluble sugars
Total soluble sugars in the jamun pulp and seed extracts were determined according to the method of Yemm and Willis [29].
Determination of total phenolics
Total phenolic content in the jamun pulp and seed extracts was determined with the Folin-Ciocalteau reagent as described by Lister and Wilson [30] and expressed in mg of polyphenols as gallic acid equivalent per gram of dry weight (mg GAE/g dw).
Antioxidant assays
Total antioxidant potential by ORAC assay
ORAC capacity of the jamun pulp and seed extracts was determined at different concentrations [31] using fluorescein (FL) as the “fluorescent probe”. Briefly, 25 μl of phosphate buffer (75 mM, pH 7.0), trolox standard or jamun extracts were incubated with 150 μl of fluorescein (20 nM) at 37°C for 1 h. The reaction was started by thermal decomposition of AAPH (19 mM in 75 mM phosphate buffer, pH 7.0) and performed at 37°C in 96-well flat-bottom plates. Fluorescence was measured immediately after the addition of AAPH at excitation of 485 nm and an emission wavelength of 535 nm for 2 h at 37°C. The ORAC values, expressed as μM trolox equivalents (μM TE) were calculated by applying the following formula
Where CTrolox is the concentration (μM) of Trolox, k is the sample dilution factor, and AUC is the area under the fluorescence decay curve of the sample, blank, and trolox, respectively, calculated by Spectramax M2 software.
DPPH radical-scavenging assay
Free radical scavenging activity of jamun pulp and seed extracts against stable DPPH was determined spectrophotometrically [32]. Fifty μl of the pulp and seed extracts, yielding 12.5-100 μg/ml concentrations, were mixed with 0.5 ml of 0.1 mM DPPH in methanol and 450 μl of 50 mM Tris-HCl, pH 7.4. Methanol (50 μl) was used as a vehicle control in the experiment. After 30 min of incubation at room temperature the reduction of the DPPH free radical was measured at 517 nm. Inhibition percent was calculated from the following equation:
ABTS radical cation decolorization assay
The spectrophotometric analysis of ABTS−+ scavenging activity was determined according to the method of Re et al. [33]. ABTS−+ was produced by reacting 2 mM ABTS in H2O with 2.45 mM potassium persulfate, stored in the dark at room temperature overnight. The ABTS−+ solution was diluted to give an absorbance of 0.750 ± 0.025 at 734 nm in water. Then, 250 μl of ABTS−+ solution was added to 0.75 ml of jamun extracts containing different concentrations (1.56–50 μg/ml). The absorbance was recorded at 734 nm, 30 min after mixing, and the percentage of radical scavenging was calculated for each concentration against the blank [34] . The scavenging capability of test compounds was calculated using the following equation:
FRAP assay (Fe3+ reducing power assay)
The ferric reducing antioxidant power (FRAP) method [35] was employed with minor modification. Briefly, different concentrations of extracts (10-80 μg/ml) in 0.75 ml of distilled water were mixed with 1.25 ml of 0.2 M sodium phosphate, pH 6.6 and 1.25 ml of 1% potassium ferricyanide. After incubation at 50°C for 20 min, the reaction mixture was acidified with 1.25 ml trichloroacetic acid (10%). Finally, 0.5 ml of FeCl3 (0.1%) was added to the reaction mixture and the absorbance was measured at 700 nm. The increased absorbance of the reaction mixture indicates greater reduction capability.
Cell proliferation assay and measurements of cell viability
Inhibition of cell proliferation by jamun extracts was measured in the human non-small cell lung carcinoma cell line A549. Cells were maintained in DMEM supplemented with 10% fetal bovine serum, 100 units/ml penicillin and 100 μg/ml streptomycin. Cells were plated in 96-well culture plates at 5 × 103 cells/well. After 24 h incubation, cells were treated with extracts for 72 h, after which cell density was measured using MTT assay [36].
Statistical analysis
All calculations were performed with GraphPad Prizm statistical software (version 4.03; La Jolla, CA). Data represent average ± standard deviation of three replicates. For cell proliferation assay, IC50 values were calculated using Microsoft Excel, XL Fit version 5 software. Differences were considered a priori to be statistically significant if the P value was less than 0.05.
Results
Extraction and analysis of jamun pulp and seeds
Jamun fruit has been reported to contain a variety of phytochemicals. In this study we analyzed anthocyanins and ellagic acid/ellagitannins in the jamun pulp and seeds and compared their relative antioxidant and anti-proliferative potency. The jamun pulp and seeds were extracted separately in acidified aqueous ethanol (75% containing 10 mM HCl) and analyzed for i) total phenolics, ii) anthocyanins, and iii) free sugars. Jamun pulp powder yielded at least 33% extract after 3 extractions, whereas seed powder yielded only 11-12% (Table I). Based on multiple extractions we first determined that three repeat extractions can provide at least 80% of extract (data not shown).
Table I.
Composition and characterization of Syzygium cumini (Jamun) extracts.
| Dry powder | Dry extract (g) (% yield) |
Soluble sugar (g) (% yield) |
Total phenolics (g) (GAE) (% Yield) |
Total anthocyanins (g) (% Yield) |
|---|---|---|---|---|
| Pulp # 1 (8 g) | 2.85 (35.6) | 0.76 (9.5) | 0.094 (1.18) | 0.039 (0.49) |
| Pulp # 2 (8 g) | 2.63 (32.9) | 0.68 (8.5) | 0.089 (1.13) | 0.046 (0.58) |
| Seeds # 1 (8 g) | 0.98 (12.3) | 0.30 (3.8) | 0.203 (2.54) | ND |
| Seeds # 2 (8 g) | 0.87 (10.9) | 0.24 (3.0) | 0.227 (2.84) | ND |
ND, Not detected
Jamun pulp and seed extracts were applied onto the XAD-761/HP-20 column (1:1) (24 g) for the enrichment of anthocyanins and other polyphenolics, and one half of the enriched extracts was hydrolyzed to convert the glycones to aglycones (e.g., anthocyanins to anthocyanidins). The hydrolyzed pulp and seed extracts yielded nearly 5% and 15% extracts respectively (Table II). The yields for unhydrolyzed pulp and seed extracts were higher.
Table II.
Enrichment and analysis of anthocyanins and total phenolics in Syzygium cumini (Jamun) extracts by column chromatography.
| Dry extracta (weight) | Treatment | Enriched extract (g) (% yield) |
Soluble sugar (g) (% yield) |
Total anthocyanins (g) (% Yield) |
Total phenolics (g GAE) (% Yield) |
Free ellagic acid (mg) (% Yield) |
|---|---|---|---|---|---|---|
| Pulp extract 1 (2.63 g) | Unhydrolyzed | 0.33 (12.4) | 0.003 (0.11) | 0.015 (0.58) | 0.05 (1.77) | 5.40 (0.19) |
| Pulp extract 2 (2.85 g) | Hydrolyzed | 0.14 (4.9) | 0.001 (0.04) | 0.007 (0.23) | 0.04 (1.31) | 3.94 (0.15) |
| Seed extract 1 (0.87 g) | Unhydrolyzed | 0.26 (24.1) | 0.001 (0.10) | ND | 0.12 (14.13) | 36.34 (4.17) |
| Seed extract 2 (0.98 g) | Hydrolyzed | 0.15 (14.8) | 0.001 (0.10) | ND | 0.07 (7.14) | 44.25 (4.51) |
Extracts were derived from 8 g material as in Table I. ND, Not detected.
Soluble sugars
Anthocyanins as well as some other phenolics (e.g., ellagitannins) contain sugar moieties. We determined the sugar content in both pulp and seed extracts. As expected, the pulp powder had higher soluble sugars (9.0±0.5%) than the seed powder (3.4±0.4%); the enriched extracts were essentially free of soluble sugars (Tables I and II).
Total phenolics
Jamun pulp powder contained 1.15% total polyphenolics (w/w). Upon enrichment the polyphenolic content in the pulp was 1.77% that decreased to 1.31% upon hydrolysis and enrichment (Tables I and II). Jamun seed powder contained twice as much polyphenolics (2.69%). Polyphenol content in the seeds was increased to 14.13% after enrichment, which decreased by half upon hydrolysis.
Anthocyanin content
Jamun pulp and seeds were analyzed for total anthocyanin content using pH-differential method and expressed as cyanidin-3-glucoside equivalents. The jamun pulp powder contained 0.54% anthocyanins. Upon enrichment, anthocyanins content in the pulp was not changed. After hydrolysis, the enriched pulp extract yielded 0.23% of anthocyanidins. No anthocyanin was detected in the seed extracts (Table I and II).
HPLC analysis
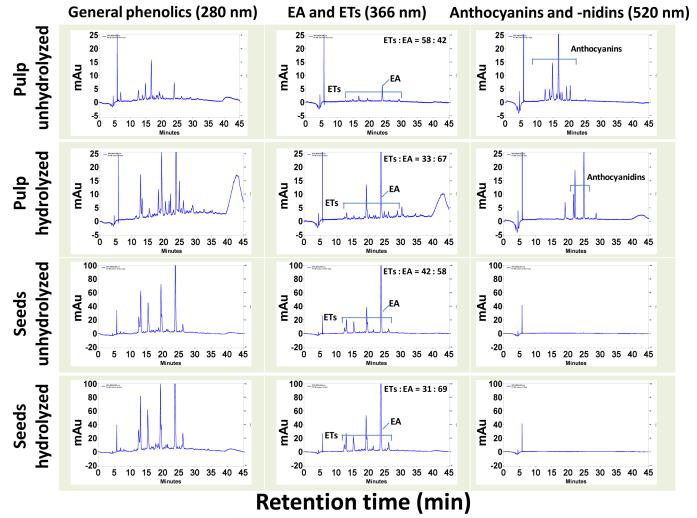
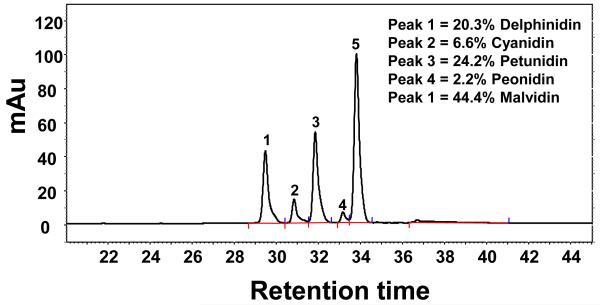
Extracts of the jamun pulp and seeds were analyzed by HPLC monitoring the chromatogram at 366 nm for ellagic acid/ellagitannins, 280 nm for other polyphenolics and 520 nm for anthocyanins/anthocyanidins. Anthocyanins, the glycones of delphinidin, cyanidin, petunidin, peonidin and malvidin, were detected in unhydrolyzed pulp extracts. No peaks corresponding to anthocyanins were detected in the jamun seed extracts (Figure 3). However, various peaks were detected at 366 and/or 280 nm, suggesting the presence of ellagitannins and other phenolic compounds. The presence of free ellagic acid and ellagitannins was also evident in jamun seeds. The identity of ellagic acid was established by co-chromatography with reference compound. The other peaks detected at 366 nm were presumably related to various ellagitannins as the hydrolyzed extracts of both jamun pulp and seeds showed reduction in most of these peaks (10-25% of relative area under curve) and enhancement in the ellagic acid peak (Figure 3), suggesting that the hydrolysis of the jamun pulp and seed extracts resulted in the conversion of some of the ellagitannins (ellagic acid conjugates) to ellagic acid. Due to commercial unavailability of standards, the ellagitannin peaks could not be confirmed directly. Further, upon hydrolysis, the jamun pulp extract revealed the presence of five anthocyanidins, identified by co-chromatography with standards, as delphinidin, cyanidin, petunidin, peonidin and malvidin (Figure 4).
Figure 3.
HPLC profile of jamun pulp and seeds (hydrolyzed and unhydrolyzed) extracts. Twenty μl of enriched extracts (1 mg/ml) were injected and analyzed using photo diodarray detector on a C18 (250×4.6 mm ID x 5 μm) reverse phase column in a gradient of 3.5% aqueous phosphoric acid and acetonitrile. Chromatograms in the first column represent various polyphenolics detected at 280 nm. Chromatograms in the second column represent ellagitannins (ETs) and free ellagic acid (EA) monitored at 366 nm. Chromatograms in the last column show anthocyanins and anthocyanidins. mAu, milliabsorbance units. For clarity of data y-axis scales are different.
Figure 4.
HPLC chromatogram of jamun anthocyanidins at 520 nm extracted from fruit pulp. Twenty μl of hydrolyzed pulp extract (1 mg/ml) was injected and analyzed using photo diodarray detector on a C18 reverse phase column in a gradient of 3.5% aqueous phosphoric acid and acetonitrile for 61 min. Inset represent relative (%) anthocyanidins content. Please note that solvent gradient used here was different than the gradient used in Figure 3 (see text).
Antioxidant Assays
ORAC Capacity
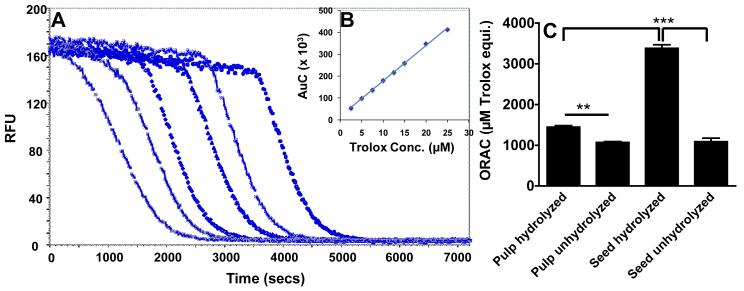
The pulp and seed extracts exhibited concentration-dependent total ORAC with respect to trolox equivalents. Figure 5 shows the results of AAPH-mediated fluorescein oxidation in the presence and absence of the extracts in phosphate buffer. In the absence of extracts or trolox, a progressive decrease of fluorescein fluorescence was observed, with almost total consumption after 30 min (Figure 5A). The decay curve of the fluorescein was concentration-dependent when tested with trolox and the jamun pulp and seed (Figure 5B) extracts. The area under curve of the kinetic profiles was linearly related to the amount of the extracts (Figure 5A and 5B). This behavior was observed for both pulp and seed extracts. At equal concentrations of hydrolyzed extracts, the jamun seeds showed a higher ORAC value (3,379±151 μM of TE/g) than the jamun pulp (1,445±64 μmol of TE/g) (Figure 5C).
Figure 5.
Oxygen radical absorbance capacity (ORAC) of jamun fruit pulp and seeds extracts. Fluorescence decay curve of fluorescein in the presence of either blank or different concentrations of trolox (from left to right, respectively, 0, 2.5, 5, 10, 20, and 25 μM) and AAPH (A). Inset represents the linear plot of area under curve (AUC) versus different concentrations of trolox (B). ORAC values are equivalent to trolox (μM/ g of extracts) (C). **p < 0.005; ***p <0.001.
DPPH Assay
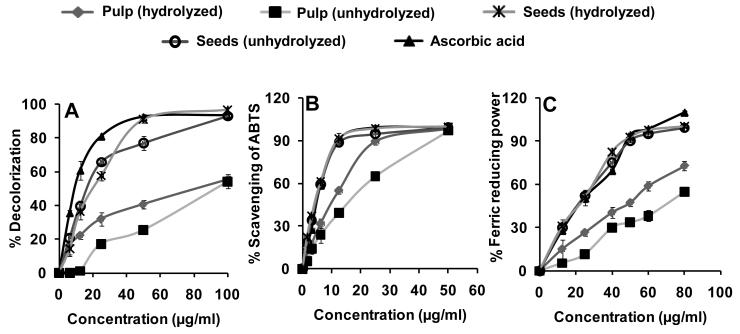
The free radical-scavenging activity of the jamun pulp and seed extracts was measured as decolorizing activity following the trapping of the unpaired electron of 1,1-diphenyl-2-picrylhydrazyl (DPPH) as shown in Figure 6A. A concentration-dependent response was observed with both the jamun pulp and seed extracts at the concentrations tested (10-80 μg/ml). The seed extracts almost completely inhibited DPPH absorption. Further, the unhydrolyzed seed showed somewhat better inhibition (~90% at 50 μg/ml) compared with hydrolyzed seed (76% inhibition at 50 μg/ml). Ascorbic acid, included as a positive control, inhibited DPPH absorption by 91% at 50 μg/ml. The activity was markedly lower in the unhydrolyzed (25%) and hydrolyzed jamun pulp extracts (46%). The concentrations of extracts that scavenged 50% of DPPH varied with, respectively, unhydrolyzed seeds, 16.3 μg/ml; hydrolyzed seeds, 19.8 μg/ml; hydrolyzed pulp, 79.0 μg/ml and unhydrolyzed pulp, 90.0 μg/ml.
Figure 6.
Antioxidant potential of Syzygium cumini fruit extracts (pulp and seed, hydrolyzed and unhydrolyzed in three different assays: (A) DPPH free radical scavenging activity; (B) ABTS scavenging activity; and (C) ferric ion reducing power. Each data point represents the mean of three experiments with standard deviation.
ABTS Assay
Figure 6B shows the scavenging of ABTS radicals by the jamun extracts. Both the seed and pulp extracts in unhydrolyzed and hydrolyzed forms at 50 μg/ml scavenged the ABTS radicals by greater than 99%. Even 20 μg/ml of the extracts contained at least 50% ABTS radical scavenging activity. The activity of the seed extract was comparable to that of the ascorbic acid at all concentrations. However, pulp extracts showed somewhat less activity. The concentrations of extracts that scavenged 50% of ABTS radicals during the 30-minute incubation, obtained by interpolation of plotted data, were, respectively: unhydrolyzed or hydrolyzed seed extracts, 4.6 μg/ml each; hydrolyzed pulp extract, 11.8 μg/ml; and unhydrolyzed pulp extract, 17.2 μg/ml (Figure 6B).
FRAP Assay
The ferric reducing/antioxidant power (FRAP assay) has been widely used in the evaluation of antioxidant component in dietary polyphenols. The reducing ability of the unhydrolyzed and hydrolyzed jamun pulp extracts were concentration-dependent (Figure 6C). The FRAP values for the seed extracts of the jamun fruits were significantly higher (p < .001) than those of pulp extracts. When compared with ascorbic acid as reference, the seed extract showed reducing ability either similar or higher. However, the jamun pulp demonstrated less activity than the ascorbic acid control. The concentrations of extracts that reduced 50% of ferric iron during the 20-minute incubation, obtained by interpolation of plotted data, were, respectively: unhydrolyzed seed extracts, 23.6 μg/ml; hydrolyzed seed extracts, 24.7 μg/ml; hydrolyzed pulp extract, 52 μg/ml; and unhydrolyzed pulp extract, 80 μg/ml (Figure 6C).
Anti-proliferative activity
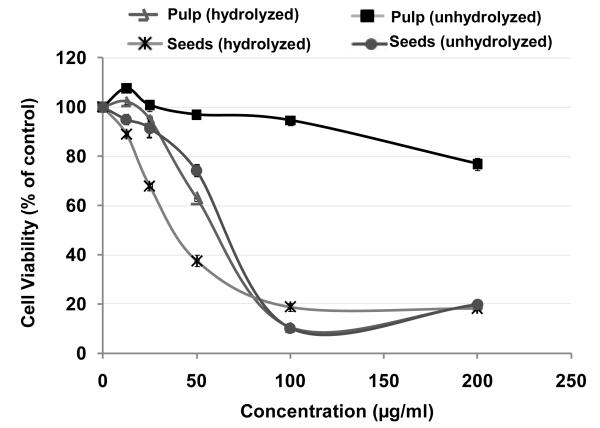
To evaluate the effect of jamun extracts on cell viability of human non-small lung cancer cells (A549), an MTT assay was performed. A concentration-dependent response was observed with both the jamun pulp and seed extracts at the concentrations tested (12.5-200 μg/ml) (Figure 7). Hydrolyzed jamun pulp showed strong antiproliferative activity with an IC50 value of 59 ± 4 μg/ml. However, unhydrolyzed pulp had a very weak effect at the tested concentrations. The hydrolyzed seed extract showed highest anti-proliferative activity (IC50 = 38 ± 3 μg/ml; p < 0.05). However, the activity of unhydrolyzed seed extract (IC50 = 64 ± 5 μg/ml) was similar to that of hydrolyzed pulp.
Figure 7.
Anti-proliferative activity of Syzygium cumini pulp and seeds extracts by MTT assay. Cells were treated with different jamun extracts at 12.5–200 μg/ml concentrations for 72 h. Data are expressed as percentage of untreated cells, mean ± SD (n =3). Each data point represents the mean of three experiments with standard deviation.
Discussion
Berries are an excellent source of various bioactive polyphenolics, including anthocyanins, proanthocyanidins, ellagitannins, flavonols and phenolic acids. In this study, Syzygium cumini (jamun, the Indian blackberry) fruit pulp and seed powder were extracted using 75% aqueous ethanol and tested for contents of anthocyanins, ellagic acid and other polyphenolics as well as their activities. . Thirty percent of jamun pulp powder was extractable, which decreased to 2-4% upon enrichment. Similarly, jamun seed powder yielded 2-3% enriched extract. This approach of using a mixture of XAD-761/diaion HP-20 served as an effective means to enrich both anthocyanins and ellagitannins along with other polyphenolics. The column was re-usable multiple times after regeneration and provided reproducible results.
Anthocyanins content in the jamun pulp powder used in this study (0.54) was more than the reported anthocyanins (0.23%) [18] where the pigments were extracted from the fresh pulp. The 3.5% anthocyanins reported in jamun extract is twice the amount found in our pulp extracts (1.5-1.8%) [22]. Different geographical regions and climatic conditions could potentially explain these differences although we cannot rule out if the lower content found in our preparation was due to the extraction procedure and drying method of the pulp. No anthocyanins were detected in the seed extracts either by pH differential method or HPLC analysis, consistent with the findings of others [22].
The conversion of essentially all anthocyanins into anthocyanidins confirms the complete hydrolysis. Irrespective of samples, various peaks were detected at 280 nm indicating the presence of unidentified polyphenolics (Figure 3). Jamun pulp extracts contained 0.17% free ellagic acid in our preparation. This EA was “free” because it was detected in addition to several peaks associated with ellagitannins without hydrolysis of the extract; upon hydrolysis ellagitannins were converted to ellagic acid, as discussed below. Ellagic acid/ellagitannin content in seed extract (4.17%) is in agreement with the reported level (4.2%) [24].
In this study, quantification of ellagitannins was not possible due to unavailability of authentic standards. Ellagic acid in the seed extracts was almost 20-fold higher than that in pulp extracts (Table II). In addition to ellagic acid, various compounds detected at 366 nm in the unhydrolyzed pulp and seeds are presumably related to ellagitannins. It is noteworthy that the level of ellagic acid increased upon hydrolysis of the extract. Since ellagitannins are complex derivatives of ellagic acid and yielded ellagic acid upon hydrolysis, these unidentified peaks detected at 366 nm may be related to ellagitannins. Jamun pulp powder used in this study contained 1.15% polyphenolics (inclusive of anthocyanins), and it is double the amount (0.56%) reported [18]. Enriched pulp contains 1.77% polyphenols that reduced to 1.31% upon hydrolysis. This decrease can be attributed to loss of sugar during the conversion of anthocyanins to anthocyanidins. Phenolic content in seed powder was twice higher (2.7%) than pulp and increased to 14.13% upon enrichment.
Jamun appears to be the only berry that contains the five anthocyanidins present in blueberry/bilberry along with significant amounts of ellagic acid/ellagitannins. In view of the known anticarcinogenicity potential of these five anthocyanidins in blueberry and cyanidin together with ellagic acid/ellagitannins in black raspberry against estrogen-mediated breast cancer [13, 37], this composition of anthocyanidins and ellagic acid/ellagitannins in jamun could potentially provide additive, if not synergistic, effects.
Antioxidants have attracted much interest with respect to their protective effects against free radical damage involved in many degenerative diseases, including cancer. The multiplicity of antioxidants present in whole fruits and vegetables is considered to elicit higher activity than any individual constituents [8]. Both pulp and seed of jamun had high antioxidant capacities relative to trolox. The higher ORAC value of the jamun seed extract (double that of pulp) may be attributed to the higher total polyphenolics compared to those in the pulp extract, given the linear relationship between ORAC and total phenolic content in fruit of Vaccinium species [38].
Jamun unhydrolyzed extract had maximum antioxidant potential both in DPPH and ABTS assays. The concentrations of extracts that scavenged 50% of DPPH varied with, respectively, unhydrolyzed seeds, 16.3 μg/ml; hydrolyzed seeds, 19.8 μg/ml; hydrolyzed pulp, 79.0 μg/ml and unhydrolyzed pulp, 90.0 μg/ml. The cyanidin- and delphinidin-derived pigments have a catechol (ortho-dihydroxyl) group and a pyrogallol (vicinal trihydroxyl) group, respectively. These structural features are likely to contribute a significant radical scavenging capacity of these compounds [8]. Likewise, the presence of cis-diols in ellagic acid/ellagitannins is known to scavenge free radicals induced by redox cycling of estrogen catechols [13, 39]. It is also suggested that aglycones have higher antioxidant activity over their glycone counterparts [15].
ABTS radicals are more reactive than DPPH radicals and unlike the reaction with DPPH radicals, which involve H-atom transfer, the reactions with ABTS−+ radicals involve an electron-transfer process. Jamun extracts are better scavengers of ABTS as compared to DPPH with much lower concentration that scavenged 50% of tested radicals.
Metal ion chelation is another important antioxidant property, and few compounds (e.g. resveratrol) have been shown for their ability to compete with ferrozine for ferrous ion in solution [40]. In this study, jamun showed a similar trend for antioxidant potential. Hydrolyzed seeds had activity comparable to ascorbic acid.
Compounds containing two or more of the functional groups like –OH, –SH, –COOH, –PO3H2, C=O, –NR2, –S– and –O– in a favorable structure-function configuration can show metal chelation activity [34]. Jamun pulp contained anthocyanins along with ellagic acid/ellagitannins, whereas seed contained higher content of ellagic acid/ellagitannins. These compounds have catechol and many –OH groups that might contribute to their antioxidant potential.
Endogenous oxidative DNA damage has been considered to be a significant factor in the initiation of human cancer. The cancer-protective effect of vegetables and fruits is attributed to the ability of the antioxidants in them to scavenge free radicals, preventing DNA damage and subsequent mutation. In the present study jamun pulp and seed extracts decreased the viable cell number of human lung cancer cell line. Hydrolyzed jamun pulp has strong anti-proliferative activity whereas unhydrolyzed extract is less effective. We have demonstrated that jamun pulp contained all five major anthocyanidins in different proportions. The antiproliferative activity of individual anthocyanidins has been shown in different cancer types [15]. The activity in the jamun hydrolyzed and unhydrolyzed seed extracts may be attributed to the high content of polyphenolics including various ellagitannins and EA. Ellagitannin-enriched fractions showed a similar effect against a breast cancer cell line [41].
Conclusion
XAD-7 and Diaion HP-20 resins provide an effective method for removing sugars as well as enriching a variety of polyphenolics, including anthocyanins and ellagic acid/ellagitannins. In addition to anthocyanins, jamun pulp is rich in ellagic acid/ellagitannins. On the other hand, jamun seeds are rich in ellagic acid/ellagitannins in addition to other unidentified polyphenolics. Jamun is the only berry that contains five anthocyanins and appreciable amounts of ellagic acid/ellagitannins. Phytochemicals in both jamun pulp and seed extracts were an effective antioxidant in different in vitro assays, including assays of free radical-scavenging (DPPH and ABTS), reducing power, and oxygen radical-absorbing capacity (ORAC), when compared with standard antioxidant compounds such as ascorbic acid and trolox. Together, our data show high antioxidant and antiproliferative activities in jamun, and this berry may be a viable candidate for chemoprevention of lung cancer.
Acknowledgements
This work was supported by Agnes Brown Duggan Endowment and from the USPHS grant CA-125152. Dr. Ramesh Gupta holds the Agnes Brown Duggan Chair in Oncological Research. Thanks to Technology Development Centre, NIPER, S.A.S. Nagar, India for vacuum drying of jamun pulp. Ms. Ellen Snell of the University of Louisville Writing Center is acknowledged for going through the manuscript.
Abbreviations
- DPPH
2,2-Diphenyl-1-Picrylhydrazyl
- ABTS
2,2′-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid)
- TE
trolox equivalent
- ROS
reactive oxygen species
- ORAC
Oxygen radical absorbance capacity
- Rt
retention time
References
- 1.Steer P, Millgard J, Sarabi DM, Basu S, Vessby B, et al. Cardiac and vascular structure and function are related to lipid peroxidation and metabolism. Lipids. 2002;37:231–236. doi: 10.1007/s11745-002-0885-3. [DOI] [PubMed] [Google Scholar]
- 2.Cheeseman KH, Slater TF. An introduction to free radical biochemistry. Br Med Bull. 1993;49:481–493. doi: 10.1093/oxfordjournals.bmb.a072625. [DOI] [PubMed] [Google Scholar]
- 3.Yu L, Haley S, Perret J, Harris M, Wilson J, et al. Free radical scavenging properties of wheat extracts. J Agric Food Chem. 2002;50:1619–1624. doi: 10.1021/jf010964p. [DOI] [PubMed] [Google Scholar]
- 4.Naczk M, Shahidi F. Phenolics in cereals, fruits and vegetables: occurrence, extraction and analysis. J Pharm Biomed Anal. 2006;41:1523–1542. doi: 10.1016/j.jpba.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Liu F, Ng TB. Antioxidative and free radical scavenging activities of selected medicinal herbs. Life Sci. 2000;66:725–735. doi: 10.1016/s0024-3205(99)00643-8. [DOI] [PubMed] [Google Scholar]
- 6.Savikin K, Zdunic G, Jankovic T, Tasic S, Menkovic N, et al. Phenolic content and radical scavenging capacity of berries and related jams from certificated area in Serbia. Plant Foods Hum Nutr. 2009;64:212–217. doi: 10.1007/s11130-009-0123-2. [DOI] [PubMed] [Google Scholar]
- 7.Ivanova D, Gerova D, Chervenkov T, Yankova T. Polyphenols and antioxidant capacity of Bulgarian medicinal plants. J Ethnopharmacol. 2005;96:145–150. doi: 10.1016/j.jep.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 8.Rice-Evans CA, Miller NJ. Antioxidant activities of flavonoids as bioactive components of food. Biochem Soc Trans. 1996;24:790–795. doi: 10.1042/bst0240790. [DOI] [PubMed] [Google Scholar]
- 9.Perron NR, Brumaghim JL. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem Biophys. 2009;53:75–100. doi: 10.1007/s12013-009-9043-x. [DOI] [PubMed] [Google Scholar]
- 10.Mazza GJ. Anthocyanins and heart health. Ann Ist Super Sanita. 2007;43:369–374. [PubMed] [Google Scholar]
- 11.Jayaprakasam B, Olson LK, Schutzki RE, Tai MH, Nair MG. Amelioration of obesity and glucose intolerance in high-fat-fed C57BL/6 mice by anthocyanins and ursolic acid in Cornelian cherry (Cornus mas) J Agric Food Chem. 2006;54:243–248. doi: 10.1021/jf0520342. [DOI] [PubMed] [Google Scholar]
- 12.Andres-Lacueva C, Shukitt-Hale B, Galli RL, Jauregui O, Lamuela-Raventos RM, et al. Anthocyanins in aged blueberry-fed rats are found centrally and may enhance memory. Nutr Neurosci. 2005;8:111–120. doi: 10.1080/10284150500078117. [DOI] [PubMed] [Google Scholar]
- 13.Aiyer HS, Srinivasan C, Gupta RC. Dietary berries and ellagic acid diminish estrogen-mediated mammary tumorigenesis in ACI rats. Nutr Cancer. 2008;60:227–234. doi: 10.1080/01635580701624712. [DOI] [PubMed] [Google Scholar]
- 14.Stoner GD, Wang LS, Zikri N, Chen T, Hecht SS, et al. Cancer prevention with freeze-dried berries and berry components. Semin Cancer Biol. 2007;17:403–410. doi: 10.1016/j.semcancer.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang LS, Stoner GD. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008;269:281–290. doi: 10.1016/j.canlet.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Achrekar S, Kaklij GS, Pote MS, Kelkar SM. Hypoglycemic activity of Eugenia jambolana and Ficus bengalensis: mechanism of action. In Vivo. 1991;5:143–147. [PubMed] [Google Scholar]
- 17.Chaturvedi A, Bhawani G, Agarwal PK, Goel S, Singh A, et al. Antidiabetic and antiulcer effects of extract of Eugenia jambolana seed in mild diabetic rats: study on gastric mucosal offensive acid-pepsin secretion. Indian J Physiol Pharmacol. 2009;53:137–146. [PubMed] [Google Scholar]
- 18.Veigas JM, Narayan MS, Laxman PM, Neelwarne B. Chemical nature, stability and bioefficacies of anthocyanins from fruit peel of syzygium cumini Skeels. Food Chem. 2007;105:619–627. [Google Scholar]
- 19.Prince PS, Menon VP, Pari L. Hypoglycaemic activity of Syzigium cumini seeds: effect on lipid peroxidation in alloxan diabetic rats. J Ethnopharmacol. 1998;61:1–7. doi: 10.1016/s0378-8741(98)00002-6. [DOI] [PubMed] [Google Scholar]
- 20.Kaur C, Kapoor HC. Acta Hort. (ISHS) 2005:563–565. [Google Scholar]
- 21.Arun R, Prakash MV, Abraham SK, Premkumar K. Role of Syzygium cumini seed extract in the chemoprevention of in vivo genomic damage and oxidative stress. J Ethnopharmacol. 2011;134:329–333. doi: 10.1016/j.jep.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 22.Li L, Adams LS, Chen S, Killian C, Ahmed A, et al. Eugenia jambolana Lam. berry extract inhibits growth and induces apoptosis of human breast cancer but not non-tumorigenic breast cells. J Agric Food Chem. 2009;57:826–831. doi: 10.1021/jf803407q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benherlal PS, Arumughan C. Chemical composition and in vitro antioxidant studies on Syzygium cumini fruit. J Sci Food Agric. 2007;87:2560–2569. doi: 10.1002/jsfa.2957. [DOI] [PubMed] [Google Scholar]
- 24.Sharma M, Li L, Celver J, Killian C, Kovoor A, et al. Effects of fruit ellagitannin extracts, ellagic acid, and their colonic metabolite, urolithin A, on Wnt signaling. J Agric Food Chem. 2010;58:3965–3969. doi: 10.1021/jf902857v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kähkönen MP, Heinämäki J, Ollilainen V, Heinonen M. Berry anthocyanins: isolation, identification and antioxidant activities. J. Sci. Food Agric. 1984;83:1403–1411. [Google Scholar]
- 26.Seeram NP, Lee R, Hardy ML, Heber D. Rapid large scale purification of ellagitannins from pomegranate husk, a by-product of the commercial juice industry. Separ. Purific. Technol. 2005;41 [Google Scholar]
- 27.Lee J, Durst RW, Wrolstad RE. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: collaborative study. J AOAC Int. 2005;88:1269–1278. [PubMed] [Google Scholar]
- 28.Guisti MM, Wrolstad RE. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy. In: Wrolstad RE, editor. Currents protocols in food analytical chemistry. John Wiley & Sons; New York, NY: 2001. pp. 1–13. [Google Scholar]
- 29.Yemm EW, Willis AJ. The estimation of carbohydrates in plant extracts by anthrone. Biochem J. 1954;57:508–514. doi: 10.1042/bj0570508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lister E, Wilson P. In: Measurement of total phenolics and ABTS assay for antioxidant activity. Institute CR, editor. Lincoln, New Zealand: 2001. [Google Scholar]
- 31.Ou B, Hampsch-Woodill M, Prior RL. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J Agric Food Chem. 2001;49:4619–4626. doi: 10.1021/jf010586o. [DOI] [PubMed] [Google Scholar]
- 32.Gyamfi MA, Yonamine M, Aniya Y. Free-radical scavenging action of medicinal herbs from Ghana: Thonningia sanguinea on experimentally-induced liver injuries. Gen Pharmacol. 1999;32:661–667. doi: 10.1016/s0306-3623(98)00238-9. [DOI] [PubMed] [Google Scholar]
- 33.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, et al. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 34.Ak T, Gulcin I. Antioxidant and radical scavenging properties of curcumin. Chemico-Biological Interac. 2008;174:27–37. doi: 10.1016/j.cbi.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 35.Oyaizu M. Studies on product of browning reaction prepared from glucose amine. Jap. J. Nutri. 1986;44:307–315. [Google Scholar]
- 36.Munagala R, Kausar H, Munjal C, Gupta RC. Withaferin A induces p53-dependent apoptosis by repression of HPV oncogenes and upregulation of tumor suppressor proteins in human cervical cancer cells. Carcinogenesis. 2011;32:1697–1705. doi: 10.1093/carcin/bgr192. [DOI] [PubMed] [Google Scholar]
- 37.Aiyer HS, Gupta RC. Berries and ellagic acid prevent estrogen-induced mammary tumorigenesis by modulating enzymes of estrogen metabolism. Cancer Prev Res (Phila) 2010;3:727–737. doi: 10.1158/1940-6207.CAPR-09-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prior RL, Cao GH, Martin A, Sofic E, McEwen J, et al. Antioxidant capacity as influenced by total phenolic and anthocyanin content, maturity, and variety of Vaccinium species. J. Agric. Food Chem. 1998;46:2686–2693. [Google Scholar]
- 39.Aiyer HS, Vadhanam MV, Stoyanova R, Caprio GD, Clapper ML, et al. Dietary berries and ellagic acid prevent oxidative DNA damage and modulate expression of DNA repair genes. Int. J. Mol. Sci. 2008;9:327–341. doi: 10.3390/ijms9030327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kehrer JP. The Haber-Weiss reaction and mechanisms of toxicity. Toxicology. 2000;149:43–50. doi: 10.1016/s0300-483x(00)00231-6. [DOI] [PubMed] [Google Scholar]
- 41.Adams LS, Zhang Y, Seeram NP, Heber D, Chen S. Pomegranate ellagitannin-derived compounds exhibit antiproliferative and antiaromatase activity in breast cancer cells in vitro. Cancer Prev Res (Phila) 2010;3:108–113. doi: 10.1158/1940-6207.CAPR-08-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]