SUMMARY
Diabetic ketoacidosis (DKA) has been considered a key clinical feature of Type 1 diabetes mellitus; however, increasing evidence indicates that DKA is also a common feature of Type 2 diabetes (T2DM). Many cases of DKA develop under stressful conditions such as trauma or infection but an increasing number of cases without precipitating cause have been reported in children and adults with T2DM. Such patients present with severe hyperglycemia and ketosis as in Type 1 diabetes mellitus but can discontinue insulin after a few months and maintain acceptable glycemic control on diet or oral agents. This subtype of diabetes has been referred to as ketosis-prone T2DM. In this article, we reviewed the literature on ketosis-prone T2DM and summarized the epidemiology, putative pathophysiology and approaches to management.
Diabetic ketoacidosis (DKA) is characterized by the triad of uncontrolled hyperglycemia, metabolic acidosis and increased total body ketone concentration. It is the most serious hyperglycemic emergency in patients with Type 1 diabetes mellitus (T1DM) and Type 2 diabetes mellitus (T2DM). The metabolic crisis is responsible for more than 130,000 hospital admissions and 500,000 hospital days per year in the USA [1,2]. For decades, DKA has been considered a key clinical feature of T1DM [3,4]; however, in recent years, an increasing number of ketoacidosis cases without precipitating cause have been reported in children and adults with T2DM [5–7]. At presentation, these patients have markedly impaired insulin secretion and insulin action [7,8], but more than half of patients with unprecipitated (no known secondary cause) DKA experience significant improvement in β-cell function and insulin sensitivity sufficient to allow discontinuation of insulin therapy within a few months of follow-up [9,10]. Upon discontinuation of insulin, the period of near-normoglycemic remission may last for a few months to several years [11–14]. This clinical presentation has been reported primarily in African–Americans (AA) and Latinos [6,7,9,15], but also in other minority ethnic groups [13,16–18]. This variant of T2DM has been referred to in the literature as idiopathic T1DM, atypical diabetes, Flatbush diabetes, diabetes Type 1½ and more recently as ketosis-prone Type 2 diabetes mellitus (KPDM) [8,10,19,20]. The aim of this article is to review current knowledge gained over the last five decades regarding the overall prevalence, clinical presentation, pathogenesis and management of KPDM.
Historical background
In the late 1960s, Dodu reported that a cohort of adults in the tropics with DKA were able to discontinue insulin therapy after a short period of time and remain in near-normoglycemic remission for several months to years [21]. In 1987, Winters et al. described this clinical presentation in 12 young AAs where nearly 50% of the cohort were obese, 70% were male, all lacked islet-cell autoantibodies (ICAs) and all patients had an insulin response to a mixed-meal test that was intermediate to secretion in nondiabetic subjects and those with T1DM [22]. In 1994, Banerji et al. described the occurrence of DKA in young, obese AAs of Caribbean descent who resided in the Flatbush area of Brooklyn (NY, USA) [9]. These patients had elevated serum C-peptide levels but negative ICAs or glutamic acid decarboxylase (GAD) antibodies and were labeled as having ‘Flatbush diabetes’. Our research group went on to demonstrate that the initial presentation of DKA in these patients is usually unprovoked and responds well to high-dose insulin administration, which can later be discontinued in the majority of patients [6]. Upon discontinuation of insulin, the period of near-normoglycemic remission may last for a few months to several years and many of these patients can be managed well with diet and oral hypoglycemic agents (OHAs) [6,8–10,23].
Prevalence
Recent data from the CDC show that from 1996 to 2006 there was a 35% increase in hospital admissions due to DKA, with a portion of the 136,510 visits representing admissions for DKA in patients with KPDM [101]. It was initially thought that KPDM was exclusively present among AAs; however, it is now reported across different ethnicities worldwide including Caucasians [24], Hispanics [25], Chinese [17], South Asians [26] and sub-Saharan Africans (Table 1) [27]. AAs and Hispanics still appear to have the highest risk and Caucasians [13] and Asians [16,28] have a much lower risk (<10%). Depending upon the population studied, several case series have reported that up to half of AAs and Hispanics hospitalized with DKA have a clinical presentation compatible with KPDM. The prevalence of KPDM is also growing in the pediatric population with one study reporting that 17% of obese adolescents have clinical characteristics of KPDM in that they present with DKA but are able to discontinue insulin and maintain good glycemic control [29].
Table 1.
Point prevalence of ketosis-prone Type 2 diabetes mellitus in different studies.
| Study (year) | Study Type |
Population type | n | Age (years) |
BMI | Ethnicity | Point prevalence (%) |
Ref. |
|---|---|---|---|---|---|---|---|---|
| Oli (1978) | PCS | Nonobese, newly diagnosed with DM and requiring insulin initially | 43 | - | - | Nigerian | 27.9 | [27] |
| Westphal (1996) | RCR | Patients with new-onset DKA | 228 | 32.2 ± 1.3 | 28.6 ± 1.2 | Caucasian (30%) Hispanic (36%) Native American (21%) AA (13%) | 24 | [32] |
| Pinhas-Hamiel et al. (1997) | RCR | ICA negative NIDDM Adolescents | 70 | 14.0 ± 0.7 | 40.7 ± 1.2 | AA | 17 | [29] |
| Umpierrez et al. (1997) | PCS | AA presenting with DKA | 144 | 37 ± 1 | 32 ± 0.3 | AA | 29 | [31] |
| Wilson et al. (1997) | RCR | Apache Indians with NIDDM who had been treated for an episode of DKA | 714 | 40.8 ± 13.9 | 24.9 ± 4.4 | Native American | 2.3 | [57] |
| Nagasaka et al. (1998) | RCT | Hospitalized T2DM patients of <30 years | 6000 | 18–23 | 36.4 ± 6.8 | Japanese | 10.3 | [28] |
| Umpierrez et al. (1999) | RCT | AA presenting with hyperglycemic crisis | 131 | 40 ± 2 | 37 ± 1 | AA | 59 | [6] |
| Balasubramanyam et al. (1999) | CC | Patients hospitalized with DKA | 141 | 47.3 ± 13.7 | 30.5 ± 7.5 | AA (37%) Hispanic (26%) Caucasian (6%) | 39 | [33] |
| Tan et al. (2000) | RCR | Outpatient diabetes clinic | >3000 | 34.8 ± 10.6 | 28.6 ± 2.3 | Chinese | <0.3 | [17] |
| Newton and Ruskin (2004) | CC | Patients hospitalized with DKA | 138 | 35.0 ± 12.5 | 30.5 ± 7.5 | AA (47%) Hispanic (35%) Caucasian (17%) Native American (1%) | 22 | [58] |
| Jabbar et al. (2004) | RCR | Patients hospitalized with DKA | 57 | 48 ± 7 | 25.5 ± 6.2 | Pakistani | 63 | [26] |
| Mauvais-Jarvis et al. (2004) | CC | Patients admitted for uncontrolled DM | 233 | 39.1 ± 9.5 | 24.9 ± 4.8 | French nationals of sub-Saharan African descent | 50 | [8] |
| Manrique et al. (2007) | RCR | Patients hospitalized with DKA | 54 | 46 ± 14 | 26.7 ± 6.3 | Peruvians | 46 | [59] |
| Sobngwi et al. (2008) | CC | All hospitalized diabetics who did not have T1DM | 187 | 38 ± 11 | 27.7 ± 4.3 | French nationals of sub-Saharan African descent | 43 | [14] |
AA: African–American; CC: Case–control study; DKA: Diabetic ketoacidosis; DM: Diabetes mellitus; ICA: Islet cell antibodies; NIDDM: Noninsulin dependent diabetes mellitus; PCS: Prospective case series; RCR: Retrospective chart review; RCT: Randomized controlled trial; T1DM: Type 1 diabetes mellitus; T2DM: Type 2 diabetes mellitus.
Clinical presentation
Most patients with new-onset KPDM present with <4 weeks of polyuria, polydipsia and weight loss (Table 2). In addition, patients can complain of nausea, vomiting and abdominal pain and are found to have severe hyperglycemia accompanied with urinary ketonuria or frank DKA. The majority of patients are overweight or obese and the unintentional weight loss reported at time of diagnosis varies between 4 and 12 kg [7,8,25]. The age of onset is usually in the fourth or fifth decade of life; however, KPDM has been increasingly reported in the pediatric population [29,30]. Men have a two- to three-fold higher prevalence of KPDM compared with women, the significance of which is unknown [25]. Up to 80% of KPDM patients have a family history of T2DM [31]. In addition, physical examination commonly reveals acanthosis nigricans and abdominal adiposity in both adults and children with KPDM [29].
Table 2.
Clinical and biochemical characteristics of Type 1 diabetes mellitus, Type 2 diabetes mellitus and ketosis-prone diabetes mellitus adult patients.
| Characteristics | T2DM | T1DM | KPDM |
|---|---|---|---|
| Clinical | |||
| Mean age at presentation (years) | Fifth decade | Third to fourth decade of life | Third to sixth decade of life |
| Ethnicity | Predominantly AA and Hispanics | Predominantly Caucasians and AA | Predominantly AA and Hispanics |
| Presentation | Moderate hyperglycemia | DKA or hyperglycemia | Usually as DKA or severe hyperglycemia |
| Duration of symptoms at presentation (weeks) | 9 | <4 | Acute, usually <6 |
| Acanthosis nigricans | Present | Absent | Present |
| Ability to achieve control with oral hypoglycemic | Variable | None | Initially, up to 75% after discontinuation of insulin; however, many require insulin again over time |
| Remission from insulin | Frequent | Rare | Frequent |
| Course | Loss of insulin secretion and worsening sensitivity | Progressive decline in insulin secretion | Remitting and relapsing course with overall progression similar to T2DM |
| BMI (kg/m2) | Overweight to obese | Lean | Overweight to obese |
| Male:female | 1:1 | 1:1 | 1.5:3 |
| Family history (%) | 30–80 | 30 | 80–100 |
| Biochemical | |||
| Primary defect | Insulin secretion and sensitivity | Lack of insulin | Acute defect in insulin secretion and sensitivity which improves with near-normoglycemic resolution |
| Markers of β-cell autoimmunity | Absent | Present | Absent |
| HLA genotype susceptibility | Absent | Present | Variable among study cohorts |
| Fasting C-peptide | Normal | Depressed | Normal |
| Insulin secretion (C-peptide response to mixed meal) upon presentation | Present | Absent | Present but lower than T2DM |
| Insulin secretion (C-peptide response to mixed meal or glucose) at follow-up | Similar to KPDM | Absent | Present |
| Insulin sensitivity at presentation | Decreased compared with obese nondiabetics | Decreased compared with obese nondiabetics | Decreased compared with obese nondiabetics |
| Insulin sensitivity at follow-up | Similar to obese nondiabetics | Increased as compared with T2DM/KPDM | Similar to obese nondiabetics |
| Development of ketosis | Resistant to ketosis | Associated with lack of insulin | Preceded by hyperglycemia and elevated FFA |
| Ref. | [7,8,33,60–64] | [7,8,33,58,60,65–69] | [7–12,17,24,25,31,33,56,60,70] |
AA: African–American; DKA: Diabetic ketoacidosis; FFA: Free fatty acid; KPDM: Ketosis-prone diabetes mellitus; T1DM: Type 1 diabetes mellitus; T2DM: Type 2 diabetes mellitus.
More than 75% of KPDM patients present with newly diagnosed diabetes and without a known precipitating cause for severe metabolic decompensation and ketoacidosis [8,24,32]. The biochemical profile and acid-base parameters at presentation are similar to that reported in T1DM [33]. Mean blood glucose (BG) is usually very high (>500 mg/dl) with a pH of <7.30 and with a mean hemoglobin A1c >12% [7,25,31,33].
Clinical course
By contrast to the chronic insulin dependence of T1DM patients with ketoacidosis and most obese patients with KPDM presenting with DKA frequently experience near-normoglycemic remission within the first few months of treatment (Table 2) [30,31]. In Brooklyn, McFarlane described the clinical course of newly-diagnosed AA patients admitted with DKA and followed them for at least 1 year [10]. Remission was defined as having an A1c ≤ 6.3% and a fasting plasma glucose <124 mg/dl (6.9 mmol/l) 3 months after discontinuing all pharmacological agents. A total of 42% of patients went into remission after a mean of 83 days and all of them remained in remission during 20 months of follow-up. In agreement with these studies, our group reported near-normoglycemic remission in 70% of obese DKA patients after 9 weeks of follow-up [6]. Mauvais-Jarvis et al. characterized a cohort of 223 newly-diagnosed KPDM patients from sub-Saharan Africa for a period of 10 years [8]. Only 23% were unable to ever discontinue insulin due to irreversible β-cell failure like that in T1DM. However, a total of 10 years after diabetes onset, less than 50% of patients with KPDM were still insulin independent. In fact, studies from our research group and other investigators have shown that most KPDM patients relapse within 2 years of initial presentation, especially if not treated with some sort of antidiabetic regimen [8,11,34].
Putative pathophysiology
β-cell function & insulin sensitivity
Our group compared indices of insulin secretion and insulin sensitivity in 57 AA, obese diabetics, 10 AA, lean diabetics and 10 AA, non diabetic subjects in Atlanta [7]. A total of 24 h after diagnosis, obese-DKA and obese-hyperglycemia patients had no detectable insulin response to intravenous dextrose infusion (0.3 mg/kg intravenous bolus). However, both groups at near-normoglycemic remission (10–12 weeks of follow-up), experienced a robust insulin response to repeated intravenous glucose challenge. Likewise, at time of diagnosis, obese-DKA and obese-hyperglycemia patients had C-peptide levels in response to 1 mg of intravenous glucagon stimulation that were between obese nondiabetic controls and lean-DKA patients; however at near-normoglycemic remission, both obese diabetic groups had stimulated C-peptide levels similar to the obese-control group. Using minimal model analysis, we also reported that insulin sensitivity was similarly reduced at presentation in obese subjects with DKA and hyperglycemia, but in both groups, insulin sensitivity improved to nondiabetic, control levels during near-normoglycemic remission. The improvement in both insulin sensitivity and more importantly, the improvement in β-cell function during follow-up allowed 25 of 35 (71%) obese-DKA patients and 16 of 22 (73%) obese-hyperglycemia patients to discontinue insulin therapy and maintain good metabolic control.
Mauvais-Jarvis and colleagues reported β-cell function in a 10-year follow-up study of sub-Saharan African patients with KPDM [8]. During follow-up, they showed that glucagon stimulated C-peptide levels in noninsulin dependent KPDM patients were similar to that of T2DM while insulin dependent KPDM patients had levels between that of T1DM and T2DM. They also reported that insulin sensitivity, which was similarly decreased in DKA and ketosis-resistant patients at diagnosis, significantly improved during follow-up.
Autoimmunity & genetic implications
Small case series of immunogenetic analyses in obese AA individuals presenting with DKA show mixed results. Winter et al. reported absence of ICAs, insulin autoantibodies and HLA associations [22]. Banerji et al. showed negative GAD antibodies but increased frequency of HLA-DR3 and HLA-DR4 (63%) in KPDM subjects [34]. Our group analyzed the immunological markers and HLA susceptibility genes in 131 obese and lean DKA patients and compared them to 51 obese subjects with non-ketotic hyperglycemia [6]. We reported similar prevalence of autoantibodies (insulin autoantibodies, ICA, GAD) in obese patients presenting with DKA (17%) or hyperglycemia (16%). However, the prevalence was significantly lower compared with lean DKA patients in whom 65% had at least one of the above antibodies present. Furthermore, there were no significant differences in HLA distribution between the obese DKA, lean DKA and obese control groups. In addition to the studies previously, Nalini et al., in their Houston cohort of KPDM patients, found that HLA class II alleles associated with susceptibility or resistance to T1DM correlated with the four antibody/β-cell function (Aβ) classifications of KPDM [35], suggested by Balasubramanyam et al. [36].
Several investigators have considered potential candidate genes that may be associated with the development of KPDM. Boutin et al. suggested that a point mutation in the HNF-1 gene could be a marker of KPDM in AA children and adolescents [37]. However, there was no similar association with HNF-1 mutation [38] in adults of sub-Saharan and Afro-Caribbean origin, respectively. The same authors instead reported high frequency of polymorphism in PAX4, a transcription factor essential for the development of insulin-producing pancreatic β-cells, in KPDM patients with phenotypes of A−β+ [39]. Finally, Sobngwi et al. found a higher prevalence of G6PD deficiency in the KPDM patients compared with controls and patients with T2DM [40]. Though there was no increased prevalence of G6PD mutation in KPDM patients, there was a proportional relationship between β-cell functional reserve and erythrocyte G6PD activity. To date, this finding has not been studied in other KPDM cohorts.
Glucotoxicity & lipotoxicity
Glucose toxicity is a metabolic phenomenon that happens when there is desensitization of β-cells and impaired insulin secretion in response to sustained elevations of plasma glucose. To determine if steady glucose exposure had any effect on stimulated insulin secretion, our research group studied the insulin secretory response to sequential 5 g arginine stimulation before (at baseline and after a 45-mindextrose infusion (200 mg/m2/min) and after a 20-h 10% dextrose infusion (200 mg/m2/min) in healthy, obese control subjects and in obese subjects with ketosis-resistant T2DM and KPDM following near-normoglycemic remission [41]. We reported a comparable response to sequential arginine stimulation at baseline and after the 20-h dextrose infusion. Infusion of dextrose was well tolerated without evidence of glucose toxicity in patients with a history of hyperglycemia and ketoacidosis. Thus, our studies failed to show glucotoxicity after short-term glucose infusion in recently diagnosed KPDM patients and suggest that a longer period of exposure may be n ecessary to induce β-cell exhaustion.
Some investigations have found that acute elevation of free fatty acid (FFA) levels in the blood can cause increased insulin resistance (lipotoxicity) and impair appropriate β-cell compensation [42–44]. To investigate the role of lipotoxicity in the development of metabolic decompensation in KPDM patients, we admitted patients with KPDM and history of hyperglycemia to receive 20% intralipid infusion for 48 h. FFAs increased fourfold with levels averaging 1.8 mmol/l during lipid infusion. We found that lipid infusion and increased FFAs were not associated with impaired insulin secretion or β-cell lipotoxicity in KPDM and ketosis-resistant obese subjects with hyperglycemia [45]. Some investigators have suggested that longer lipid infusion (>72 h) and exposure period to increased FFAs are needed to achieve significant lipid-induced impairment of insulin secretion [46,47].
Intracellular signaling
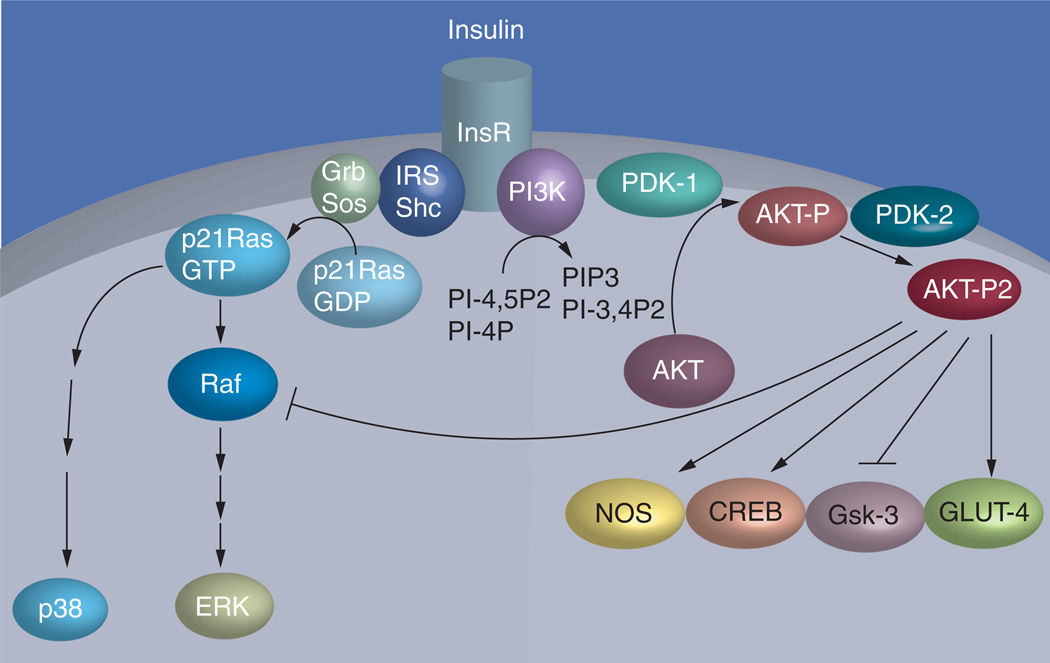
Insulin, by binding to its membranous receptors, causes activation of a cascade of intracellular signaling molecules via phosphorylation. One major end result is the activation of protein kinase enzyme AKT, which is responsible for the stimulation of glucose transport and glycogen accumulation in skeletal muscle cells (Figure 1) [48,49]. Lack of one of the isoforms, AKT-2, has in fact been shown to cause a diabetes-like condition in knockout mouse models [50]. Our research group and collaborators [51] studied the patterns of insulin-stimulated AKT phosphorylation and protein expression in muscle biopsy samples obtained from KPDM patients immediately after hyperglycemic crisis and again after near-normoglycemic remission. We found that AKT-2 expression and insulin stimulated phosphorylation was impaired during initial hyperglycemic crisis. However, at time of follow-up with near-normoglycemic resolution, both AKT-2 expression and phosphorylation improved. Thus, decreased AKT-2 response to insulin is one of the possible mechanisms of insulin resistance in KPDM patients presenting with hyperglycemia. In addition, defects in forkhead box transcription factors may play a role as they mediate adaptive responses of gene expression in many insulin target tissues [52] and mediate the action of insulin and leptin in the hypothalamus [53].
Figure 1. Mechanisms of insulin resistance in obese African–Americans with hyperglycemic crises.
Insulin exerts its biological effects by sequential activation of a cascade of upstream signaling molecules. Insulin binds to its receptor, which in turn leads to autophosphorylation of the receptor and activation of docking proteins including insulin receptor substrates. Tyrosine phosphorylation of IRS-1/2 allows the activation of PI3K. PIP3 production subsequently recruits the serine-threonine kinase AKT and its activating kinase 3-phosphoinositide-dependent PDK-1 to the membrane to initiate activation of AKT. Complete activation of AKT occurs only when it is dually phosphorylated by PDK-1 and PDK-2. AKT activation is required to stimulate GLUT-4 glucose transport and inhibit glycogen synthase kinase activity.
Adapted from [51].
Management
Acute management & treatment of DKA
Diabetic ketoacidosis is defined as a BG of >250 mg/dl, a pH ≤ 7.3, HCO3 <18 mEq/l and positive serum ketone bodies [2]. Treatment includes intravenous rehydration, continuous insulin drip administration, electrolyte repletion and the search for and treatment of an underlying precipitating cause (Box 1). DKA levels should be checked at least every 2 h in order to titrate the drip and monitor electrolyte trends. The response to treatment in KPDM patients is similar to those with T1DM and T2DM and the time to resolution is usually <14 h [7]. After resolution of DKA, a higher insulin dose is needed to control BG levels and prevent recurrence of ketoacidosis. Our experience indicates that a starting total daily dose of 0.7–0.8 units/kg of subcutaneous insulin is commonly needed to achieve glycemic control. A combination of a multidose regimen of neutral protamine hagedorn and regular insulin or basal bolus insulin are equally effective in achieving glycemic control. Subcutaneous insulin doses should be adjusted to achieve target fasting and premeal BG levels of <130 mg/dl.
Box 1. Management of diabetic ketoacidosis in patients with ketosis-prone Type 2 diabetes mellitus.
Laboratory assessment and electrolyte disturbances
-
▪
At admission: cell blood count with differential, complete metabolic profile, venous pH and serum β-hydroxybutyrate
-
▪
During treatment: glucose every 2 h and check basic metabolic profile, venous pH, phosphorus at 2 h, 4 h and every 4 h until resolution of DKA
K+ replacement
-
▪
If serum K+ 5.2 mmol/l, do not give K+ but check serum K+ every 2 h
-
▪
K+ = 3.3–5.2 mmol/l, add 20–30 mEq K+ to each liter of iv fluid
-
▪
K+ <3.3 mmol/l, hold insulin and give 20–30 mEq K+ until K+ >3.3 mEq/l
Initiate continuous insulin infusion
-
▪
Initial iv bolus of normal saline (0.9%): 0.1 unit/kg bodyweight, followed by continuous insulin infusion at 0.1 U/kg/h
-
▪
When BG <250 mg/dl, change iv fluids to D5W in half normal saline (0.45%) and reduce insulin infusion rate to 0.05 unit/kg/h to keep glucose ~200 mg/dl until resolution of ketoacidosis
Following the resolution of DKA
-
▪
Start multidose sc. insulin at a dose of 0.7–0.8 units/kg of bodyweight
-
▪
Give half of the total insulin dose as long-acting insulin (i.e., insulin glargine, NPH) and give the remaining half as prandial insulin divided three times/day
-
▪
Adjust insulin dose to achieve a target fasting and premeal glucose level of 70–130 mg/dl
Following discharge from the hospital
-
▪
Monitor patients every 2 weeks for the first 2 months to adjust insulin therapy, then every 2 or 3 months depending on glycemic control. Mean insulin requirement to achieve target BG is usually 1–1.2 units/kg of bodyweight
-
▪
Start tapering insulin once fasting BG levels reach <130 mg/dl for 2 weeks or if patient experiences hypoglycemia. Decrease total insulin dose by 25% at each visit
-
▪
Measure GAD antibodies and β-cell function (fasting C-peptide or 1 mg glucagon-stimulated C-peptide test at 0, 3 and 6 min)
After discontinuation of insulin therapy
-
▪
Subjects with negative GAD and with fasting or stimulated C-peptide levels >1.5 ng/dl and >2.25 ng/dl, respectively, start low-dose sulfonylurea (glyburide 1.25–2.5 mg/day), pioglitazone 30 mg/day or metformin (500 mg b.i.d.) therapy
-
▪
Patients with positive GAD or with inadequate insulin secretion are more likely to relapse and may be kept on insulin therapy, and/or should be carefully monitored for recurrence of hyperglycemia or ketosis
BG: Blood glucose; b.i.d.: Twice daily; DKA: Diabetic ketoacidosis; GAD: Glutamic acid decarboxylase; iv: Intravenous; K+: Potassium; NPH: Neutral protamine hagedorn; sc.: Subcutaneous.
Long-term treatment of ketosis-prone diabetes mellitus
Prior to insulin therapy, the patient should receive diabetes education that includes techniques on proper insulin administration and home BG monitoring. We recommend preprandial BG monitoring at least twice a day with subsequent reporting to the healthcare provider every 2–4 weeks for the first 3 months in order to adjust insulin therapy. The patient can then be assessed every 2–3 months depending on the degree of glycemic control and need for insulin. The home BG log or glucometer can be brought to follow-up appointments for review and adjustment of the regimen. The mean insulin requirement to achieve the target BG level is usually 1–1.2 U/kg of bodyweight/day. Insulin tapering can begin once fasting BG (FBG) levels are ≤ 130 mg/dl for 2 weeks or if the patient experiences hypoglycemia as defined as a BG <70 mg/dl. Over the course of 12 weeks, most patients can completely discontinue insulin by decreasing the total insulin dose by 25% at each follow-up visit. Balasubramanyam et al. suggested any attempt to withdraw insulin treatment should be based on a precise autoimmunity and β-cell function (Aβ) classification that has 99% sensitivity and 97% positive predictive value (Figure 2) [54]. Based on a subset of 138 newly-diagnosed KPDM subjects out of 294 in this study, 74% with a new diagnosis were classified as having the type 2B variant which signifies the absence of islet cell autoimmunity and preserved β-cell function (A−β+). The majority of KPDM patients with this classification will be able to discontinue insulin therapy. In addition, assessment of autoimmunity status (i.e., GAD, ICA antibodies) and β-cell secretory reserve via glucagon stimulation test [55] at 1–3 weeks of diagnosis have also been reported to predict the ability of the newly-diagnosed KPDM patient to discontinue insulin and remain in near-normoglycemic remission. Negative antibody status and positive β-cell reserve (classification A−β+), as determined by a fasting C-peptide level of >1.0 ng/dl (0.33 nmol/l) or a glucagon-stimulated C-peptide of >1.5 ng/dl (0.5 nmol/l), correlate with the ability to discontinue insulin therapy during follow-up. Continuation of insulin treatment is recommended in patients with low β-cell reserve and most of these patients require multiple daily insulin injections to avoid hyperglycemic relapse. Overall, C-peptide response to glucagon appears to be the best predictor of remission in KPDM [7,8]. Other potential predictive factors that support near-normoglycemic remission are AA or Hispanic ethnicity, newly-diagnosed diabetes, obesity, family history of T2DM and a lower insulin requirement [8,30,56].
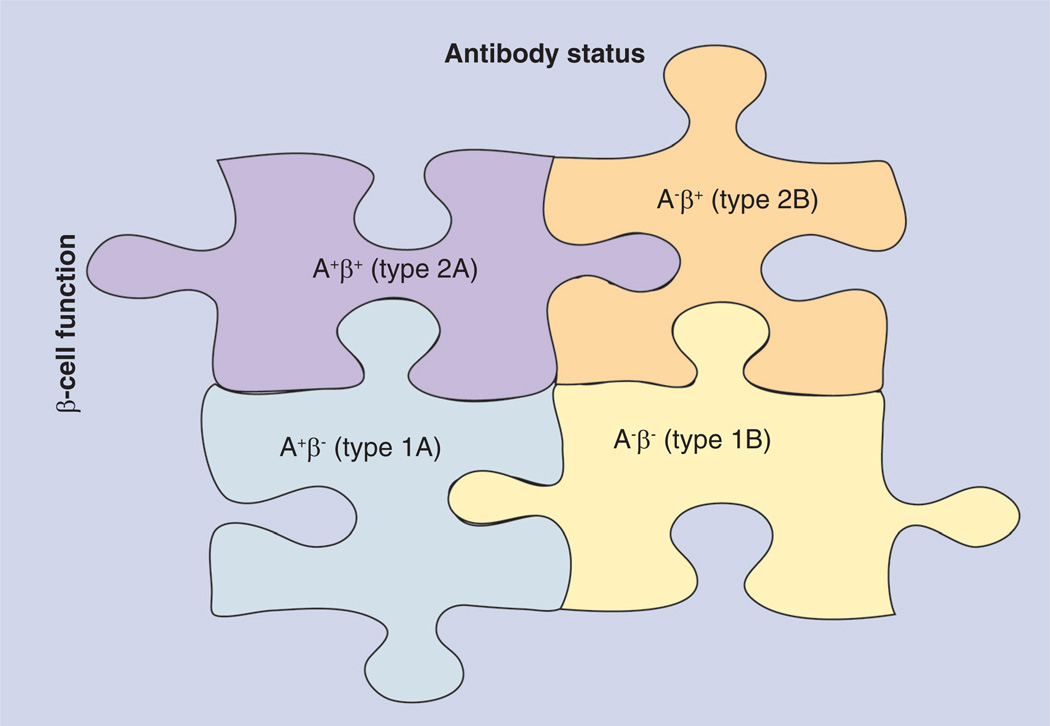
Figure 2. Aβ-classification system for ketosis-prone diabetes mellitus patients.
This diagram outlines the schema that can be used to identify the four variants of ketosis-prone Type 2 diabetes mellitus (KPDM) and predict clinical response to treatment based on the presence of β-cell function (b) and/or islet-cell autoimmunity (A). Those with KPDM type 2A (A−β+) have preserved β at diagnosis but have markers of islet cell A. Clinically, they can either regain β and discontinue insulin or have progressive β and require lifelong exogenous insulin therapy. Patients with KPDM type 1A (A+β−) have permanent β-cell failure with markers of islet cell A. They require insulin therapy for life. Those with KPDM type 1B (A−β−) also have permanent β-cell failure but lack islet cell A. They require insulin therapy for life. Patients with KPDM type 2B (A−β+) have preserved β and lack islet cell A. This KPDM subtype has the greatest chance of achieving near-normoglycemic remission and discontinuing insulin therapy.
Adapted from [54].
Prevention of recurrence of hyperglycemia
Near-normoglycemic remission in KPDM patients, typically achieved after 3 months or less of antidiabetic therapy, is defined as an A1c of ≤ 7% and a FBG of <130 mg/dl that is maintained after the discontinuation of treatment [7,8,13]. Although the majority of KPDM patients achieve remission, most studies have found these individuals have variable β-cell function and have an increasing insulin dependence over time if treated with diet and lifestyle modification alone. In the previously mentioned sub-Saharan cohort of Mauvais-Jarvis et al., only 40% of these patients were insulin-independent at 10 years and the authors reported that the gradual loss of β-cell function in KPDM patients was similar to that of T2DM patients [8]. From this, the authors inferred that KPDM was a subtype of T2DM. However, unlike T2DM patients, nearly 70% of KPDM patients presented with recurring episodes of relapse-remission within 2 years of diagnosis, and with each relapse, there was a progressive risk of becoming chronically insulin dependent. Although lifestyle and dietary modifications remain an important aspect of long-term KPDM management, recurrence of hyperglycemia or ketosis after the discontinuation of insulin occurs within 12–24 months of discontinuing insulin in nearly 60% of patients who are on diet alone [7,11]. These findings led to investigations evaluating the effects of OHAs on maintaining insulin-free remission and β-cell function. One of the first studies was from Banerji et al. in which 20 obese, black patients with new-onset KPDM presenting as severe hyperglycemia were blindly randomized to receive either 2.5 mg of glipizide or placebo daily and followed for a mean of 17.4 months [34]. Relapse to hyperglycemia was defined as a FBG level ≥140 mg/dl in this study and it was found that the use of glipizide compared with placebo significantly prolonged remission. Similarly, our research team followed the longitudinal clinical response of 35 obese AA patients with new-onset KPDM presenting with DKA or severe hyperglycemia. The subjects were placed on diet and low-dose glyburide (1.25–2.5 mg/day) versus diet alone. With a median follow-up of 16 months, hyperglycemia recurred in 72% treated with diet alone compared with only 20% in those treated with glyburide. Readmission with metabolic decompensation occurred in four patients treated with diet alone but in none of the patients treated with glyburide [11]. In a 3-year, randomized controlled trial recently completed in 44 overweight KPDM patients treated with either pioglitazone 30 mg daily or matching placebo, we found that pioglitazone treatment significantly reduced the number of patients with hyperglycemic relapse (68 vs 32%, respectively; p = 0.03) and allowed a longer period of remission (median: 809 vs 162 days; p = 0.01 [102]). The roles of other commonly used OHAs such as metformin, dipeptidyl peptidase 4 inhibitors and incretin mimetics have yet to be determined although ongoing investigations from our group will be able to p rovide more data in the near future [103].
Conclusion & future perspective
In summary, despite the acute presentation of severe insulin deficiency and ketoacidosis, most newly-diagnosed obese adult patients with spontaneous DKA have clinical and immunogenetic features of T2DM (e.g., lack of HLA association and islet-cell autoimmunity). The majority of such patients discontinue insulin therapy during follow-up and remain in near-normoglycemic remission off insulin for several months to years. During follow-up, the clinical features, insulin secretion, insulin action and immunologic markers are similar between KPDM and ketosis-resistant Type 2 diabetes. Based on the current body of literature and our clinical experience, KPDM represents a unique form of presentation of T2DM patients. More research needs to be aimed at determining the optimal pharmacological treatment approach in patients with KPDM and uncovering potential underlying genetic associations, particularly ones that relate to clinical response to treatment and long-term outcomes.
Practice Points.
-
■
Prevalence
-
-
Common in people of African–American and Hispanic ethnicity.
-
-
-
■
Clinical presentation
-
-
Patients typically present with polyuria, polydipsia, weight loss of less than 4 weeks duration and are found to have diabetic ketoacidosis (DKA), which is indistinguishable from Type 1 diabetes mellitus.
-
-
-
■
Pathogenesis
-
-
β-cell autoimmunity and HLA associations are uncommon.
-
-
Impaired insulin sensitivity at presentation that significantly improves at time of follow-up.
-
-
Impaired pancreatic β-cell reserve at presentation that significantly improves with follow-up.
-
-
Short-term glucotoxicity and lipotoxicity are not primary pathophysiologic factors in the development of β-cell decompensation.
-
-
-
■
Treatment
-
-
Acute management of DKA in ketosis-prone diabetes mellitus is the same as that of any DKA patient.
-
-
Insulin is required to achieve near-normoglycemic remission after presentation.
-
-
-
■
Disease course
-
-
Over the course of 10–12 weeks, most patients can completely discontinue insulin.
-
-
Apart from lifestyle and dietary modification, oral sulfonylureas and thiazolidinediones can prevent or delay hyperglycemic relapse after the discontinuation of insulin. Potential oral monotherapies that have been studied include low-dose glipizide (0.625–2.5 mg/day); low-dose glyburide (1.25–2.5 mg/day) and pioglitazone (30 mg/day).
-
-
Overall, C-peptide response to glucagon appears to be the best predictor of remission in ketosis-prone diabetes mellitus.
-
-
Acknowledgments
D Smiley receives research support from the NIH (K08 DK0830361). GE Umpierrez is supported clinical research grants from the American Diabetes Association (7–03-CR-35), Sanofi-Aventis and the NIH UL1 RR025008 (Atlanta Clinical and Translational Science Institute).
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
■ of interest
■■ of considerable interest
- 1.Levetan CS, Passaro MS, Jablonski KA, Ratner RE. Effect of physician specialty on outcomes in diabetic ketoacidosis. Diabetes Care. 1999;22(11):1790–1795. doi: 10.2337/diacare.22.11.1790. [DOI] [PubMed] [Google Scholar]
- 2.Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32(7):1335–1343. doi: 10.2337/dc09-9032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Umpierrez GE, Khajavi M, Kitabchi AE. Review: diabetic ketoacidosis and hyperglycemic hyperosmolar nonketotic syndrome. Am. J. Med. Sci. 1996;311(5):225–233. doi: 10.1097/00000441-199605000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Delaney MF, Zisman A, Kettyle WM. Diabetic ketoacidosis and hyperglycemic hyperosmolar nonketotic syndrome. Endocrinol. Metab. Clin. North Am. 2000;29(4):683–705. doi: 10.1016/s0889-8529(05)70159-6. V. [DOI] [PubMed] [Google Scholar]
- 5.American Diabetes Association Consensus Panel. Type 2 diabetes in children and adolescents. Pediatrics. 2000;105(3 Pt 1):671–680. doi: 10.1542/peds.105.3.671. [DOI] [PubMed] [Google Scholar]
- 6. Umpierrez GE, Woo W, Hagopian WA, et al. Immunogenetic analysis suggests different pathogenesis for obese and lean African–Americans with diabetic ketoacidosis. Diabetes Care. 1999;22(9):1517–1523. doi: 10.2337/diacare.22.9.1517. ■■ Indicates that most obese African–American patients with diabetic ketoacidosis (DKA) have Type 2 diabetes characterized by higher insulin secretion, the absence of autoimmune markers and a lack of HLA genetic association
- 7.Umpierrez GE, Casals MM, Gebhart SP, Mixon PS, Clark WS, Phillips LS. Diabetic ketoacidosis in obese African–Americans. Diabetes. 1995;44(7):790–795. doi: 10.2337/diab.44.7.790. [DOI] [PubMed] [Google Scholar]
- 8. Mauvais-Jarvis F, Sobngwi E, Porcher R, et al. Ketosis-prone Type 2 diabetes in patients of sub-Saharan African origin: clinical pathophysiology and natural history of β-cell dysfunction and insulin resistance. Diabetes. 2004;53(3):645–653. doi: 10.2337/diabetes.53.3.645. ■■ Reviews the ‘natural course’ of patients with ketosis-prone Type 2 diabetes mellitus (KPDM) and clearly distinguishes it from patients with Type 1 diabetes and nonketotic Type 2 diabetes
- 9.Banerji MA, Chaiken RL, Huey H, et al. GAD antibody negative NIDDM in adult black subjects with diabetic ketoacidosis and increased frequency of human leukocyte antigen DR3 and DR4. Flatbush diabetes. Diabetes. 1994;43(6):741–745. doi: 10.2337/diab.43.6.741. [DOI] [PubMed] [Google Scholar]
- 10. Mcfarlane SI, Chaiken RL, Hirsch S, Harrington P, Lebovitz HE, Banerji MA. Near-normoglycaemic remission in African–Americans with Type 2 diabetes mellitus is associated with recovery of β cell function. Diabet. Med. 2001;18(1):10–16. doi: 10.1046/j.1464-5491.2001.00395.x. ■ Demonstrates the benefits of sustained pancreatic β-cell function in KPDM patients in near-normoglycemic remission
- 11. Umpierrez GE, Clark WS, Steen MT. Sulfonylurea treatment prevents recurrence of hyperglycemia in obese African–American patients with a history of hyperglycemic crises. Diabetes Care. 1997;20(4):479–483. doi: 10.2337/diacare.20.4.479. ■ One of the first randomized controlled trials to evaluate the effects of an oral antidiabetic agent versus placebo on maintaining near-normoglycemic remission.
- 12.Banerji MA, Chaiken RL, Lebovitz HE. Long-term normoglycemic remission in black newly diagnosed NIDDM subjects. Diabetes. 1996;45(3):337–341. doi: 10.2337/diab.45.3.337. [DOI] [PubMed] [Google Scholar]
- 13.Maldonado MR, Otiniano ME, Lee R, Rodriguez L, Balasubramanyam A. Ethnic differences in β-cell functional reserve and clinical features in patients with ketosisprone diabetes. Diabetes Care. 2003;26(8):2469. doi: 10.2337/diacare.26.8.2469. [DOI] [PubMed] [Google Scholar]
- 14.Sobngwi E, Choukem SP, Agbalika F, et al. Ketosis-prone Type 2 diabetes mellitus and human herpesvirus 8 infection in sub-Saharan Africans. JAMA. 2008;299(23):2770–2776. doi: 10.1001/jama.299.23.2770. [DOI] [PubMed] [Google Scholar]
- 15.Balasubramanyam A, Yajnik C, Tandon N. Translational Endocrinology & Metabolism. Volume 2. The Endocrine Society; 2011. Non-traditional forms of diabetes worldwide. Implications for translational investigation (March) pp. 43–67. [Google Scholar]
- 16.Yamada K, Nonaka K. Diabetic ketoacidosis in young obese Japanese men. Diabetes Care. 1996;19(6):671. doi: 10.2337/diacare.19.6.671a. [DOI] [PubMed] [Google Scholar]
- 17.Tan KC, Mackay IR, Zimmet PZ, Hawkins BR, Lam KS. Metabolic and immunologic features of Chinese patients with atypical diabetes mellitus. Diabetes Care. 2000;23(3):335–338. doi: 10.2337/diacare.23.3.335. [DOI] [PubMed] [Google Scholar]
- 18.Likitmaskul S, Santiprabhob J, Sawathiparnich P, Numbenjapon N, Chaichanwatanakul K. Clinical pictures of Type 2 diabetes in Thai children and adolescents is highly related to features of metabolic syndrome. J. Med. Assoc. Thai. 2005;88(Suppl. 8):S169–S175. [PubMed] [Google Scholar]
- 19.Sobngwi E, Gautier JF. Adult-onset idiopathic Type I or ketosis-prone Type II diabetes. evidence to revisit diabetes classification. Diabetologia. 2002;45(2):283–285. doi: 10.1007/s00125-001-0739-8. [DOI] [PubMed] [Google Scholar]
- 20.Kitabchi AE. Ketosis-prone diabetes – a new subgroup of patients with atypical Type 1 and Type 2 diabetes? J. Clin. Endocrinol. Metab. 2003;88(11):5087–5089. doi: 10.1210/jc.2003-031656. [DOI] [PubMed] [Google Scholar]
- 21.Dodu SR. Diabetes in the tropics. Br. Med. J. 1967;2(5554):747–750. doi: 10.1136/bmj.2.5554.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winter WE, Maclaren NK, Riley WJ, Clarke DW, Kappy MS, Spillar RP. Maturity-onset diabetes of youth in black Americans. N. Engl. J. Med. 1987;316(6):285–291. doi: 10.1056/NEJM198702053160601. [DOI] [PubMed] [Google Scholar]
- 23.Sobngwi E, Vexiau P, Levy V, et al. Metabolic and immunogenetic prediction of long-term insulin remission in African patients with atypical diabetes. Diabet. Med. 2002;19(10):832–835. doi: 10.1046/j.1464-5491.2002.00802.x. [DOI] [PubMed] [Google Scholar]
- 24.Maldonado MR, Otiniano ME, Lee R, Rodriguez L, Balasubramanyam A. Characteristics of ketosis-prone diabetes in a multiethnic indigent community. Ethn. Dis. 2004;14(2):243–249. [PubMed] [Google Scholar]
- 25. Pinero-Pilona A, Litonjua P, Aviles-Santa L, Raskin P. Idiopathic Type 1 diabetes in Dallas, Texas: a 5-year experience. Diabetes Care. 2001;24(6):1014–1018. doi: 10.2337/diacare.24.6.1014. ■ Reviews the pathophysiological and clinical studies of patients with idiopathic Type 1 diabetes (now called KPDM).
- 26.Jabbar A, Farooqui K, Habib A, Islam N, Haque N, Akhter J. Clinical characteristics and outcomes of diabetic ketoacidosis in Pakistani adults with Type 2 diabetes mellitus. Diabet. Med. 2004;21(8):920–923. doi: 10.1111/j.1464-5491.2004.01249.x. [DOI] [PubMed] [Google Scholar]
- 27.Oli JM. Remittant diabetes mellitus in Nigeria. Trop. Geogr. Med. 1978;30(1):57–62. [PubMed] [Google Scholar]
- 28.Nagasaka S, Ishikawa S, Itabashi N, Rokkaku K, Saito T. Ketoacidosis-onset Type 2 diabetes in Japanese. Association with the widespread distribution of soft drinks and vending machines. Diabetes Care. 1998;21(8):1376–1378. doi: 10.2337/diacare.21.8.1376. [DOI] [PubMed] [Google Scholar]
- 29.Pinhas-Hamiel O, Dolan LM, Zeitler PS. Diabetic ketoacidosis among obese African–American adolescents with NIDDM. Diabetes Care. 1997;20(4):484–486. doi: 10.2337/diacare.20.4.484. [DOI] [PubMed] [Google Scholar]
- 30.Sellers EA, Dean HJ. Diabetic ketoacidosis: a complication of Type 2 diabetes in Canadian aboriginal youth. Diabetes Care. 2000;23(8):1202–1204. doi: 10.2337/diacare.23.8.1202. [DOI] [PubMed] [Google Scholar]
- 31.Umpierrez GE, Kelly JP, Navarrete JE, Casals MM, Kitabchi AE. Hyperglycemic crises in urban blacks. Arch. Intern. Med. 1997;157(6):669–675. [PubMed] [Google Scholar]
- 32.Westphal SA. The occurrence of diabetic ketoacidosis in non-insulin-dependent diabetes and newly diagnosed diabetic adults. Am J Med. 1996;101(1):19–24. doi: 10.1016/s0002-9343(96)00076-9. [DOI] [PubMed] [Google Scholar]
- 33.Balasubramanyam A, Zern JW, Hyman DJ, Pavlik V. New profiles of diabetic ketoacidosis. Type 1 vs Type 2 diabetes and the effect of ethnicity. Arch. Intern. Med. 1999;159(19):2317–2322. doi: 10.1001/archinte.159.19.2317. [DOI] [PubMed] [Google Scholar]
- 34. Banerji MA, Chaiken RL, Lebovitz HE. Prolongation of near-normoglycemic remission in black NIDDM subjects with chronic low-dose sulfonylurea treatment. Diabetes. 1995;44(44):466–470. doi: 10.2337/diab.44.4.466. ■ One of the first randomized controlled trials to evaluate the effects of an oral antidiabetic agent versus placebo on maintaining near-normoglycemic remission.
- 35.Nalini R, Gaur Lk, Maldonado M, et al. HLA class II alleles specify phenotypes of ketosis-prone diabetes. Diabetes Care. 2008;31(6):1195–1200. doi: 10.2337/dc07-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Balasubramanyam A, Garza G, Rodriguez L, et al. Accuracy and predictive value of classification schemes for ketosis-prone diabetes. Diabetes Care. 2006;29(12):2575–2579. doi: 10.2337/dc06-0749. ■■ In Introduces a novel classification scheme for patients with KPDM based on immunologic criteria and pancreatic β-cell function that can be used to determine clinical course and guide treatment
- 37.Boutin P, Gresh L, Cisse A, et al. Missense mutation Gly574Ser in the transcription factor HNF-1α is a marker of atypical diabetes mellitus in African–American children. Diabetologia. 1999;42(3):380–381. doi: 10.1007/s001250051166. [DOI] [PubMed] [Google Scholar]
- 38.Mauvais-Jarvis F, Boudou P, Sobngwi E, et al. The polymorphism Gly574Ser in the transcription factor HNF-1α is not a marker of adult-onset ketosis-prone atypical diabetes in Afro-Caribbean patients. Diabetologia. 2003;46(5):728–729. doi: 10.1007/s00125-003-1093-9. [DOI] [PubMed] [Google Scholar]
- 39.Mauvais-Jarvis F, Smith SB, Le May C, et al. PAX4 gene variations predispose to ketosis-prone diabetes. Hum. Mol. Genet. 2004;13(24):3151–3159. doi: 10.1093/hmg/ddh341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sobngwi E, Gautier JF, Kevorkian JP, et al. High prevalence of glucose-6-phosphate dehydrogenase deficiency without gene mutation suggests a novel genetic mechanism predisposing to ketosis-prone diabetes. J. Clin. Endocrinol. Metab. 2005;90(8):4446–4451. doi: 10.1210/jc.2004-2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gosmanov AR, Smiley D, Robalino G, et al. Effects of intravenous glucose load on insulin secretion in patients with ketosis-prone diabetes during near-normoglycemia remission. Diabetes Care. 2010;33(4):854–860. doi: 10.2337/dc09-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bevilacqua S, Bonadonna R, Buzzigoli G, et al. Acute elevation of free fatty acid levels leads to hepatic insulin resistance in obese subjects. Metabolism. 1987;36(5):502–506. doi: 10.1016/0026-0495(87)90051-5. [DOI] [PubMed] [Google Scholar]
- 43.Carpentier A, Mittelman SD, Lamarche B, Bergman RN, Giacca A, Lewis GF. Acute enhancement of insulin secretion by FFA in humans is lost with prolonged FFA elevation. Am. J. Physiol. 1999;276(6 Pt 1):E1055–E1066. doi: 10.1152/ajpendo.1999.276.6.E1055. [DOI] [PubMed] [Google Scholar]
- 44.Boden G, Chen X, Rosner J, Barton M. Effects of a 48-h fat infusion on insulin secretion and glucose utilization. Diabetes. 1995;44(10):1239–1242. doi: 10.2337/diab.44.10.1239. [DOI] [PubMed] [Google Scholar]
- 45.Umpierrez GE, Smiley D, Robalino G, Peng L, Gosmanov AR, Kitabchi AE. Lack of lipotoxicity effect on β-cell dysfunction in ketosis-prone Type 2 diabetes. Diabetes Care. 2010;33(3):626–631. doi: 10.2337/dc09-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kashyap S, Belfort R, Gastaldelli A, et al. A sustained increase in plasma free fatty acids impairs insulin secretion in nondiabetic subjects genetically predisposed to develop Type 2 diabetes. Diabetes. 2003;52(10):2461–2474. doi: 10.2337/diabetes.52.10.2461. [DOI] [PubMed] [Google Scholar]
- 47.Poitout V, Briaud I, Kelpe C, Hagman D. Gluco-lipotoxicity of the pancreatic β cell. Ann. Endocrinol. (Paris) 2004;65(1):37–41. doi: 10.1016/s0003-4266(04)95628-4. [DOI] [PubMed] [Google Scholar]
- 48.Brazil DP, Hemmings BA. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem. Sci. 2001;26(11):657–664. doi: 10.1016/s0968-0004(01)01958-2. [DOI] [PubMed] [Google Scholar]
- 49.Nikoulina SE, Ciaraldi TP, Mudaliar S, Carter L, Johnson K, Henry RR. Inhibition of glycogen synthase kinase 3 improves insulin action and glucose metabolism in human skeletal muscle. Diabetes. 2002;51(7):2190–2198. doi: 10.2337/diabetes.51.7.2190. [DOI] [PubMed] [Google Scholar]
- 50.Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. Akt1/PKBα is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J. Biol. Chem. 2001;276(42):38349–38352. doi: 10.1074/jbc.C100462200. [DOI] [PubMed] [Google Scholar]
- 51.Gosmanov AR, Umpierrez GE, Karabell AH, Cuervo R, Thomason DB. Impaired expression and insulin-stimulated phosphorylation of Akt-2 in muscle of obese patients with atypical diabetes. Am. J. Physiol. Endocrinol. Metab. 2004;287(1):E8–E15. doi: 10.1152/ajpendo.00485.2003. [DOI] [PubMed] [Google Scholar]
- 52.Barthel A, Schmoll D, Unterman TG. FoxO proteins in insulin action and metabolism. Trends Endocrinol. Metab. 2005;16(4):183–189. doi: 10.1016/j.tem.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 53.Kitamura T, Feng Y, Kitamura Yi, et al. Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Nat. Med. 2006;12(5):534–540. doi: 10.1038/nm1392. [DOI] [PubMed] [Google Scholar]
- 54.Balasubramanyam A, Nalini R, Hampe CS, Maldonado M. Syndromes of ketosis-prone diabetes mellitus. Endocr. Rev. 2008;29(3):292–302. doi: 10.1210/er.2007-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maldonado M, Hampe CS, Gaur LK, et al. Ketosis-prone diabetes. dissection of a heterogeneous syndrome using an immunogenetic and β-cell functional classification, prospective analysis, and clinical outcomes. J. Clin. Endocrinol. Metab. 2003;88(11):5090–5098. doi: 10.1210/jc.2003-030180. [DOI] [PubMed] [Google Scholar]
- 56. Umpierrez GE, Smiley D, Kitabchi AE. Narrative review. Ketosis-prone Type 2 diabetes mellitus. Ann. Intern. Med. 2006;144(5):350–357. doi: 10.7326/0003-4819-144-5-200603070-00011. ■■ Critically reviews the literature on KPDM from 1966 to 2005.
- 57.Wilson C, Krakoff J, Gohdes D. Ketoacidosis in Apache Indians with non-insulin-dependent diabetes mellitus. Arch. Intern. Med. 1997;157(18):2098–2100. [PubMed] [Google Scholar]
- 58.Newton CA, Raskin P. Diabetic ketoacidosis in Type 1 and Type 2 diabetes mellitus: clinical and biochemical differences. Arch. Intern. Med. 2004;164(17):1925–1931. doi: 10.1001/archinte.164.17.1925. [DOI] [PubMed] [Google Scholar]
- 59.Manrique HRE, Medina C, Talaverano A, Pinto M, Solis J. Epidemiological characteristics of the hyperglycemic crises. Revista de la Sociedad Peruana de Medicina Interna. 2007;20:21–25. [Google Scholar]
- 60.Ramos-Roman MA, Pinero-Pilona A, Adams-Huet B, Raskin P. Comparison of Type 1, Type 2, and atypical ketosis-prone diabetes at 4 years of diabetes duration. J. Diabetes Complications. 2006;20(3):137–144. doi: 10.1016/j.jdiacomp.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 61.Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with Type 2 diabetes mellitus. Progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA. 1999;281(21):2005–2012. doi: 10.1001/jama.281.21.2005. [DOI] [PubMed] [Google Scholar]
- 62.Del Prato S, Felton AM, Munro N, Nesto R, Zimmet P, Zinman B. Improving glucose management: ten steps to get more patients with Type 2 diabetes to glycaemic goal. Int. J. Clin. Pract. 2005;59(11):1345–1355. doi: 10.1111/j.1742-1241.2005.00674.x. [DOI] [PubMed] [Google Scholar]
- 63.Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in Type 2 diabetes. A consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32(1):193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Valdez R, Yoon Pw, Liu T, Khoury MJ. Family history and prevalence of diabetes in the U.S. population: the 6-year results from the National Health and Nutrition Examination Survey (1999–2004) Diabetes Care. 2007;30(10):2517–2522. doi: 10.2337/dc07-0720. [DOI] [PubMed] [Google Scholar]
- 65.Ellemann K, Soerensen JN, Pedersen L, Edsberg B, Andersen OO. Epidemiology and treatment of diabetic ketoacidosis in a community population. Diabetes Care. 1984;7(6):528–532. doi: 10.2337/diacare.7.6.528. [DOI] [PubMed] [Google Scholar]
- 66.Tuomilehto J, Zimmet P, Mackay IR, et al. Antibodies to glutamic acid decarboxylase as predictors of insulin-dependent diabetes mellitus before clinical onset of disease. Lancet. 1994;343(8910):1383–1385. doi: 10.1016/s0140-6736(94)92521-6. [DOI] [PubMed] [Google Scholar]
- 67.Hagopian WA, Sanjeevi CB, Kockum I, et al. Glutamate decarboxylase-, insulin-, and islet cell-antibodies and HLA typing to detect diabetes in a general population-based study of Swedish children. J. Clin. Invest. 1995;95(4):1505–1511. doi: 10.1172/JCI117822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Erlich H, Valdes AM, Noble J, et al. HLA DR-DQ haplotypes and genotypes and Type 1 diabetes risk. Analysis of the Type 1 diabetes genetics consortium families. Diabetes. 2008;57(4):1084–1092. doi: 10.2337/db07-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Greenbaum CJ. Insulin resistance in Type 1 diabetes. Diabetes Metab. Res. Rev. 2002;18(3):192–200. doi: 10.1002/dmrr.291. [DOI] [PubMed] [Google Scholar]
- 70.Tanaka K, Moriya T, Kanamori A, Yajima Y. Analysis and a long-term follow up of ketosis-onset Japanese NIDDM patients. Diabetes Res. Clin. Pract. 1999;44(2):137–146. doi: 10.1016/s0168-8227(99)00023-6. [DOI] [PubMed] [Google Scholar]
Websites
- 101.CDC National Hospital Discharge Survey. www.cdc.gov/nchs/about/major/hdasd/nhds.htm.
- 102.NIH clinical trial. Ketosis prone diabetes in African–Americans. http.//clinicaltrials.gov/ct2/show/NCT00426413.
- 103.NIH clinical trial: ketosis-prone diabetes mellitus (KPDM): metformin versus sitagliptin treatment. http://clinicaltrials.gov/ct2/show/NCT01099618.