INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is characterized by a gradual and progressive loss of lung function. The consensus guidelines adopted by the American Thoracic Society and the European Respiratory Society define COPD as a “preventable and treatable disease state characterized by airflow limitation that is not fully reversible.”1 COPD is caused by an abnormal inflammatory response of the lungs to the inhalation of noxious gases or suspended particles in the air. Cigarette smoke is the most common source of exposure. Extended exposure to these irritants may lead to emphysema or obstructive bronchitis, the two hallmark conditions associated with COPD.
The pathophysiology of COPD involves a complex series of chronic inflammatory processes that progressively destroy the pulmonary vasculature and lung parenchyma. Two main pathophysiological processes occur in COPD: inflammation and unopposed oxidation.
The inflammatory process is believed to be mediated by chemical factors, such as tumor necrosis factor–alpha (TNF-α), interleukin-8 (IL-8), and leukotriene B4.2,3 When noxious gases or particles have been introduced into the lungs and irritation has occurred, the chemical “messengers” propagate the inflammatory process and recruit neutrophils, macrophages, and lymphocytes to the site of injury.
The second pathophysiological process involves a shift in the balance of normal defense mechanisms, resulting in unopposed oxidation. Guidelines from the Global Initiative for Chronic Obstructive Lung Disease (GOLD) identify disruption of the oxidant/antioxidant or trypsin/antitrypsin balance as a major determinant of damage to the lung parenchyma.4 Tobacco has been implicated in the disruption of both processes by (1) increasing oxidation, thereby overwhelming antioxidant protective factors, and (2) inducing proteases from macrophages and neutrophils. Tobacco smoke has thus been identified as the single greatest risk factor for COPD because of the processes of cellular damage and because of the high incidence of tobacco use worldwide.
The socioeconomic impact of COPD is substantial. Quality of life, morbidity, and mortality are all negatively affected by the disease. In the National Health and Nutrition Examination Sur vey (NHANES), conducted from 1988 to 1994, the estimated prevalence of mild COPD in Americans 25 to 75 years of age was 6.9% and the prevalence of moderate COPD was 6.6%.5 This was equivalent to approximately 35 million individuals living with COPD in the U.S. Moreover, morbidity data suggest that emergency department visits and hospitalization are significantly more common in persons with COPD than in the general population; this trend is expected to increase as the population ages.6,7 COPD is currently the fourth major cause of death in the U.S. and is expected to become the third major cause of death by 2020.8
With these high morbidity and mortality rates, the economic burden of the disease is significant. Sullivan et al. estimated that the combined direct and indirect cost of COPD in 1993 was $23.9 billion.9
Because COPD is a heterogeneous disease state with increasing degrees of severity, pharmacotherapy is multimodal. According to the GOLD guidelines,4 COPD is stratified by severity based on two spirometry parameters: forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC). A summary of the COPD types and their basic treatment is shown in Table 1 (see page 150).4
Table 1.
GOLD Guideline Criteria and Recommended Drug Therapy Options
| GOLD Category | Symptoms | Spirometry | Drug Therapy | |
|---|---|---|---|---|
| FEV1:FVC | FEV1 | |||
| Class 0 (at risk) | Risk factors; no symptoms | Normal | Normal | Avoidance of risk factors (e.g., smoking); influenza and pneumococcal vaccines |
| Class I (mild) | With or without symptoms | <70% | ≥80% | Short-acting bronchodilator as needed |
| Class II (moderate) | With or without symptoms | <70% | 50%–80% | Therapy for Class I COPD plus long-acting bronchodilator |
| Class III (severe) | With or without symptoms | <70% | 30%–50% | Therapy for Class II COPD plus inhaled glucocorticoids |
| Class IV (very severe) | Chronic respiratory failure or right-sided heart failure | <70% | <30% | Therapy for Class III COPD plus long-term oxygen treatment |
FEV = forced expiratory volume; FVC = forced vital capacity.
Modified from data by Global Initiative for Chronic Obstructive Lung Disease (GOLD).4
The GOLD guidelines focus primarily on two classes of pharmacotherapeutic agents for COPD: bronchodilators and inhaled corticosteroids. Other drugs are generally used as adjunctive or second-line agents. One adjunctive agent, roflumilast (Daliresp, Forest), is the first new therapy for COPD in nearly 20 years. Early clinical trials sought to obtain indications for roflumilast in asthma and allergic rhinitis because of its anti-inflammatory properties. However, in March 2011, the FDA approved roflumilast only for patients with COPD.10
INDICATION
Roflumilast is indicated as a treatment to reduce the risk of COPD exacerbations in patients with severe COPD associated with chronic bronchitis and a history of exacerbations. This medication is not a bronchodilator, and it is not indicated for the treatment of acute bronchospasm.11
PHARMACOLOGY
The precise mechanism of action of roflumilast has not been determined (Figure 1). Roflumilast and its active metabolite, roflumilast N-oxide, are selective inhibitors of phosphodiesterase type 4 (PDE4), a major cyclic-3,5-adenosine monophosphate (cAMP)–metabolizing enzyme found primarily in lung tissue.11
Figure 1.
Chemical structure of roflumilast. (From Daliresp prescribing information.11)
On a cellular level, PDE4 converts cAMP to adenosine monophosphate (AMP), terminating the cellular messaging initiated by cAMP.12 Roflumilast blocks the effect of PDE4, leading to an accumulation of cAMP within target cells and a corresponding increase in cAMP messaging. The clinical relevance of blocking PDE4 is unknown. However, it is thought that the accumulation of cAMP within localized immune cells and lung tissue is important in preventing the pathogenesis of COPD, particularly inflammation.11,12
In a randomized clinical study by Hohlfeld et al., 37 healthy adults received oral roflumilast (500 mg once daily) or placebo for 4 weeks. They were then subjected to segmental endotoxin challenge, which induced a response similar to the inhalation of tobacco smoke.13 After the endotoxin challenge, the influx of neutrophils and eosinophils was decreased by 39% (P < 0.02) and 74% (P < 0.01), respectively, in the roflumilast-treated subjects, compared with those who were given placebo; macrophages and lymphocytes were not affected. The investigators concluded that the anti-inflammatory effects of roflumilast were related to its ability to block the recruitment of neutrophils and eosinophils.
The results of this trial, along with those of other studies,14,15 suggest that the PDE4 inhibition achieved with roflumilast reduces inflammation by limiting the localization of immune cells.
Pharmacokinetics
Roflumilast is a lipophilic, highly permeable molecule that exhibits rapid and nearly complete absorption after oral administration.16 In pharmacokinetic studies, oral roflumilast reached its maximum plasma concentration (Cmax) in approximately 1 hour (range, 0.5–2 hours) after administration. In comparison, the Cmax of roflumilast N-oxide was reached in approximately 8 hours (range, 4–13 hours).11 However, N-oxide plasma concentrations plateau at between 5 and 15 hours after administration.16
The absolute bioavailability of roflumilast after a 500-mcg oral dose is approximately 80%, and it appears to be affected primarily by gut and hepatic first-pass metabolism.11,16 Total drug absorption is unaffected by the presence of food; however, a fed state decreases the Cmax by approximately 40% and delays the time to maximum plasma concentration (tmax) by approximately 1 hour.11,17
In vivo, roflumilast undergoes extensive hepatic metabolism by both phase 1 (cytochrome P450 [CYP]) and phase 2 (conjugation) reactions.11 The primary metabolic pathway is mediated by several CYP enzymes (CYP1A1/2, CYP2C19, and CYP3A4), which are responsible for the N-oxidation of roflumilast to the less potent roflumilast N-oxide. CYP1A2 and CYP3A4 appear to be the most active CYP enzymes in the metabolism of roflumilast. Phase 2 conjugation produces several glucuronidated metabolites, including roflumilast N-oxide glucuronide and 4-amino-3,5-dichloro pyridine N-oxide glucuronide.
Roflumilast and its N-oxide metabolite exhibit high serum protein binding, although the doses tested in clinical trials were non-saturable. Protein binding is approximately 99% for roflumilast and 97% for roflumilast N-oxide.11,18 The volume of distribution after a single 500-mcg dose of roflumilast is 2.9 L/kg.11
After intravenous (IV) or oral administration of roflumilast, about 70% of the drug is eliminated by the kidneys, primarily as conjugated metabolites. In one study, roflumilast was not detected in urine; however, certain metabolites, such as roflumilast N-oxide, roflumilast N-oxide glucuronide, and 4-amino-3,5-dichloropyridine N-oxide, were present. The median plasma half-lives of roflumilast and its N-oxide metabolite are approximately 17 and 30 hours, respectively.11
In vitro data indicate that roflumilast is two to three times more potent than roflumilast N-oxide at inhibiting the PDE4 enzyme.19 However, the area-under-the-curve (AUC) concentration of roflumilast N-oxide was approximately 10 times greater than that of roflumilast in pharmacokinetic trials, suggesting that most of its pharmacological effects (possibly up to 90%) can be attributed to its N-oxide metabolite.16,18
It is unclear whether other metabolites of roflumilast are pharmacologically active.
Pharmacodynamics
Four weeks of treatment with roflumilast (500 mcg once daily) reduced sputum neutrophils and eosinophils by 31%, and 42%, respectively.11 Moreover, after segmental pulmonary lipopolysaccharide challenge in healthy volunteers, this regimen reduced the number of total cells, neutrophils, and eosinophils in bronchoalveolar lavage fluid by 35%, 38%, and 73%, respectively.11
The clinical significance of these findings is unknown.
DRUG INTERACTIONS
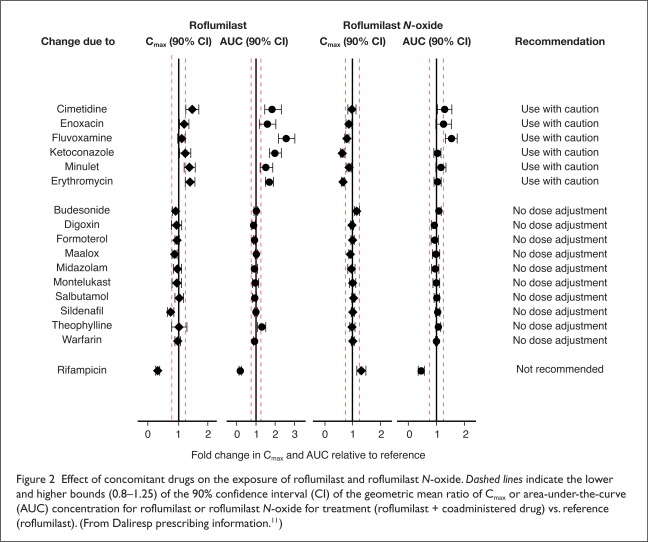
Because the conversion of roflumilast to roflumilast N-oxide depends on several hepatic CYP enzymes, the drug’s known interactions are associated with the inhibition or induction of these enzymes by other medications. The effects of various concomitant drugs on the exposure of roflumilast and roflumilast N-oxide are shown in Figure 2.
Figure 2.
Effect of concomitant drugs on the exposure of roflumilast and roflumilast N-oxide. Dashed lines indicate the lower and higher bounds (0.8–1.25) of the 90% confidence interval (CI) of the geometric mean ratio of Cmax or area-under-the-curve (AUC) concentration for roflumilast or roflumilast N-oxide for treatment (roflumilast + coadministered drug) vs. reference (roflumilast). (From Daliresp prescribing information.11)
In one pharmacokinetic study involving 16 healthy men, steady-state rifampin (Rifadin, Sanofi), a potent CYP3A4 inducer, caused profound changes in the AUC concentration and tmax of both roflumilast and roflumilast N-oxide and reduced the total PDE4 inhibitory activity of roflumilast by 58%.20 Conversely, several studies have reported the inhibition of CYP enzymes involved in roflumilast metabolism. Studies of roflumilast use with the CYP3A4 inhibitors erythromycin and ketoconazole (Nizoral, Janssen) found a nearly two-fold increase in the AUC of roflumilast, whereas the AUC concentration of the N-oxide metabolite was largely unchanged.21,22
In a drug–drug interaction study of roflumilast plus fluvoxamine (Luvox, Solvay), an inhibitor of both CYP1A2 and CYP2C19, serum roflumilast and roflumilast N-oxide levels were increased by 2.6-fold and 1.5-fold, respectively.23 A 59% increase in the total PDE4 inhibitory activity of roflumilast was attributed primarily to the CYP2C19 isoform.
The manufacturer does not recommend the concurrent administration of roflumilast with CYP3A4 inducers, such as rifampin, phenobarbital, carbamazepine (Carbatrol, Shire; Tegretol, Novartis), or phenytoin (Dilantin, Pfizer).11 The labeling also warns of increased systemic exposure of roflumilast and potential adverse reactions when roflumilast is given concurrently with strong CYP3A4 inhibitors (e.g., triazoles and protease inhibitors) or with inhibitors of both CYP3A4 and CYP1A2 (e.g., erythromycin, ketoconazole, fluvoxamine, and cimetidine (Tagamet, GlaxoSmithKline).
Although the labeling for roflumilast does not address co-administration with strong CYP2C19 inhibitors, roflumilast should not be used with these agents.
Caution is also required when roflumilast is used with oral contraceptives that contain ethinyl estradiol, as this drug interaction increases systemic levels of roflumilast.
In pharmacokinetic studies, no interactions were found between roflumilast and the bronchodilator albuterol (e.g., Ventolin, GlaxoSmithKline; Proventil, Schering),24 the glucocorticoid budesonide (Pulmicort, Entocort, Astra-Zeneca),25 the benzodiazepine midazolam (Versed, Roche),26 or the leukotriene receptor antagonist montelukast (Singulair, Merck).27
EFFICACY IN CLINICAL TRIALS
To date, 12 randomized, controlled clinical trials of roflumilast have been conducted: one study analyzed the drug’s efficacy and safety in patients with allergic rhinitis; six studies evaluated its effect in patients with asthma; and five studies assessed roflumilast in patients with COPD. The following studies focus mainly on the use of roflumilast for patients with COPD, the drug’s only indication.
Early Trial Data in Asthma
The early phase 2 and dose-ranging studies of roflumilast were conducted in patients with asthma. In a double-blind, parallel-group trial, 693 asthma patients were treated with roflumilast (100, 250, or 500 mcg once daily) for 12 weeks.28 The 500-mcg dose was found to be superior to the 100-mcg dose in improving FEV1 (P = 0.002). Moreover, a nonsignificant improvement in asthma symptoms was noted with 500 mcg compared with 250 mcg, without a significant increase in adverse drug events (AEs). Based on these findings, the authors recommended the 500-mcg dose of roflumilast for the treatment of asthma.
Phase 3 Trials in Chronic Obstructive Pulmonary Disease
Rabe et al.15
Roflumilast (250 and 500 mcg) was evaluated in a randomized, double-blind study of 1,411 patients with COPD. The patients received roflumilast (250 mcg once daily; n = 576), roflumilast (500 mcg once daily; n = 555), or placebo (n = 280) for 24 weeks. The study’s primary outcomes—post-bronchodilator FEV1 and health-related quality of life—were significantly improved by both doses of roflumilast compared with placebo (P < 0.0001). This study provided sufficient evidence to support use of the once-daily 500-mg dose in patients with COPD.
Calverley et al. (2007)29
Although preliminary data suggested that roflumilast was effective for the short-term treatment of mild-to-moderate COPD (for less than 4 months), information about long-term outcomes in severe COPD was lacking. Calverley and coworkers therefore conducted a randomized, double-blind study of 1,513 patients with COPD who were given roflumilast (500 mcg once daily) or placebo for 1 year. The primary outcomes included post-bronchodilator FEV1, exacerbation rates, and AEs.
The roflumilast group showed improved post-bronchodilator FEV1 from baseline, whereas FEV1 in the placebo group worsened. The FEV1 increased by 39 mL with roflumilast compared with placebo (P = 0.001). Exacerbation rates and AEs did not differ significantly between the two treatment groups. In a post hoc analysis, however, patients with moderate (stage II) or very severe (stage IV) COPD requiring systemic corticosteroids had significantly fewer exacerbations with roflumilast than with placebo. There was a modest but statistically significant improvement in spirometry outcomes with roflumilast. This study suggested that extended treatment with roflumilast might help prevent the deterioration of lung function associated with severe or very severe COPD.
A concern regarding the study was the heterogeneity of the treatments. Patients were permitted to continue with short-acting anticholinergics, short-acting beta-agonists, or inhaled corticosteroids if they had started these drugs before enrollment. The study protocol also excluded an important class of COPD medications, the long-acting beta-agonists (LABAs).
Fabbri et al.30
Fabbri and colleagues reported the results of two randomized, multicenter studies that compared roflumilast (500 mcg once daily) with placebo in patients with moderate-to-severe COPD who were also receiving the LABA salmeterol (Advair, GlaxoSmithKline) or tiotropium (Spiriva, Pfizer/Boehringer Ingelheim), a long-acting anticholinergic. In these studies, 1,580 patients, 40 years of age or older, were treated for 6 months.
Compared with placebo, roflumilast improved mean pre-bronchodilator FEV1 by 49 mL in patients receiving salmeterol and by 80 mL (P < 0.0001) in those treated with tiotropium (P < 0.0001). Similar improvements in post-bronchodilator FEV1 were noted in both groups. The frequency of exacerbations, the time to first exacerbation, and the proportion of patients with exacerbations generally improved with the active-treatment combinations compared with placebo. However, roflumilast resulted in the best outcomes and the most significant improvements when it was administered with salmeterol.
Treatment-related AEs occurred in approximately 3% to 18% of patients, with the highest rate of AEs in the roflumilast/salmeterol group. Diarrhea, nausea, and weight loss (approximately 2 kg) were the most common AEs. The tendency for treatment discontinuation was higher in patients who received roflumilast. This observation was consistent with that in previous studies.
In summary, the Fabbri report demonstrated that roflumilast could provide additional clinical benefits for patients with severe COPD who were receiving LABA therapy.
Calverley et al. (2009)31
Two additional studies were conducted to address the scarcity of data regarding the efficacy of roflumilast in preventing COPD exacerbations. In these double-blind, multicenter trials, a total of 3,091 patients with severe COPD and a history of exacerbations received roflumilast (500 mcg once daily; n = 1,537) or placebo (n = 1,554) for 1 year. The study populations were sufficiently similar to allow composite data analysis. The rate of moderate or severe COPD exacerbations was significantly lower with roflumilast than with placebo (1.14 per patient per year vs. 1.37 per patient per year, respectively; P < 0.0003). Moreover, pre-bronchodilator and post-bronchodilator FEV1 and FVC were significantly improved with roflumilast when compared with placebo.
Rates of AEs were higher with roflumilast (67%) than with placebo (62%), as were rates of discontinuation (14% vs. 12%, respectively). The most common AEs associated with roflumilast included diarrhea, nausea, vomiting, and headache. Cardiac dysrhythmias were less common in the roflumilast group; the authors did not offer a possible explanation for this occurrence.
As in the study by Fabbri et al., the roflumilast group lost more weight (2 kg more) than the placebo patients.
Chong et al.32
After the approval of roflumilast in March 2011, a Cochrane meta-analysis was conducted to review the efficacy and safety of PDE4 inhibitors in patients with stable COPD. Nine randomized controlled trials of roflumilast (N = 9,211) met the criteria for inclusion in the analysis.
The authors found a statistically significant improvement in FEV1 with roflumilast regardless of disease severity (P < 0.00001) and in FVC (P < 0.00001). Similarly, patient questionnaire responses and rates of exacerbations were statistically significant, although the absolute difference was small. Diarrhea, headache, dyspepsia, vomiting, and abdominal pain were more common in the roflumilast group, as were the rates of trial discontinuations resulting from AEs. Rates of nonfatal serious AEs and death were not statistically significant.
SAFETY
Many of the placebo-controlled clinical trials of roflumilast included analyses of AEs. A summary of common treatment-related AEs is presented in Table 2. The most common AEs associated with roflumilast included diarrhea (8%–9%), weight loss (6%–12%), and nausea (5%). These AEs occurred at considerably higher rates with treatment than with placebo. Nasopharyngitis (5%–8%) and upper respiratory tract infections (4%) were also reported, but these AEs occurred at roughly equal rates in both the treatment and placebo groups. In general, cumulative AE rates were higher for roflumilast than for placebo.
Table 2.
Adverse Events in Placebo-Controlled Trials of Roflumilast In Patients With Chronic Obstructive Pulmonary Disease*
| Roflumilast 500 mcg Once Daily | Placebo | |
|---|---|---|
| Rabe et al. (2005)15 | n = 555 | n = 280 |
| Diarrhea | 9% | 2% |
| Nasopharyngitis | 8% | 7% |
| Nausea | 5% | 1% |
| Upper respiratory tract infection | 4% | 5% |
| Calverley et al. (2007)29 | n = 760 | N = 753 |
| Diarrhea | 9% | 3% |
| Nasopharyngitis | 7% | 7% |
| Headache | 6% | 2% |
| Influenza | 5% | 5% |
| Nausea | 5% | 1% |
| Fabbri et al. (2009)30 | ||
| Trial M2-127 | n = 466 | n = 467 |
| Weight loss | 9% | 1% |
| Diarrhea | 8% | 3% |
| Nasopharyngitis | 7% | 7% |
| Nausea | 5% | < 1% |
| Trial M2-128 | ||
| Diarrhea | 9% | < 1% |
| Nasopharyngitis | 6% | 5% |
| Weight loss | 6% | < 1% |
| Caverley et al. (2009)31 | ||
| Trial M2-124 | n = 769 | n = 755 |
| Weight loss | 12% | 3% |
| Diarrhea | 8% | 3% |
| Nasopharyngitis | 7% | 7% |
| Acute bronchitis | 5% | 5% |
| Nausea | 5% | 2% |
| Trial M2-125 | n = 778 | n = 790 |
| Diarrhea | 9% | 3% |
| Weight loss | 8% | 3% |
| Nasopharyngitis | 5% | 6% |
| Upper respiratory tract infection | 4% | 5% |
The 2011 Cochrane meta-analysis reported that AEs occurred significantly more often in COPD patients receiving roflumilast (500 mcg once daily) than in placebo-treated patients (P = 0.00019).32 Moreover, the roflumilast patients were more likely to withdraw from the study (15%) than those receiving placebo (9%).
The labeling for roflumilast includes safety data from eight clinical studies: four 1-year placebo-controlled trials, two 6-month placebo-controlled trials, and two 6-month drug add-on trials.11 A total of 8,630 COPD patients were enrolled; 3,136 and 1,232 patients were exposed to roflumilast 500 mcg once daily for 6 months and 1 year, respectively, and 4,192 patients received placebo.
Diarrhea, weight loss, nausea, and headache were the most common AEs associated with roflumilast. Serious AEs that occurred more frequently in roflumilast-treated patients than in placebo-treated patients included diarrhea, atrial fibrillation, acute pancreatitis, and acute renal failure. The most common AEs that led to discontinuation of roflumilast were diarrhea (2.4%) and nausea (1.6%).11
Roflumilast is associated with an increase in psychiatric AEs.11 In clinical studies, 5.9% of patients treated with roflumilast (500 mcg) once daily experienced psychiatric AEs compared with 3.3% of placebo-treated patients. The most frequently reported psychiatric AEs were insomnia, anxiety, and depression. Instances of suicidal ideation and behavior, including completed suicide, have been observed in clinical trials of roflumilast. Prescribers should carefully weigh the risks and benefits of treatment with roflumilast in prospective patients with a history of depression, psychiatric illness, or suicidal thoughts.
Weight loss was also a common AE in patients receiving roflumilast (500 mcg once daily), affecting 7.5%, compared with 2.1% of placebo subjects.11 In two placebo-controlled clinical trials of 1 year’s duration in which weight was prospectively assessed, 20% of patients treated with roflumilast experienced moderate weight loss (between 5% and 10% of body weight) compared with 7% of patients who received placebo. In addition, 7% of patients who received roflumilast experienced severe weight loss (more than 10% of body weight) compared with 2% of placebo-treated patients. For patients receiving roflumilast, their weight should be monitored regularly.
Roflumilast is a Pregnancy Category C drug. No adequate or well-controlled studies of the medication have been conducted in pregnant women.
Roflumilast and its metabolites are excreted into the milk of lactating rats, and this may also occur in humans. Therefore, this drug should not be prescribed to nursing women. No human studies have investigated the effects of roflumilast on breast-fed infants.11
DOSAGE AND ADMINISTRATION
The recommended dosage of roflumilast is one 500-mcg tablet per day, with or without food.11 Because the drug undergoes extensive hepatic metabolism, serum concentrations of roflumilast and roflumilast N-oxide may be increased by up to 92% and 41%, respectively, in patients with moderate hepatic impairment (Child–Pugh class B).11 The clinical significance of these increased serum levels is unknown. Roflumilast is contraindicated in patients with moderate or severe hepatic impairment (Child–Pugh class B or C).11,33
Although approximately 70% of roflumilast and its metabolites is eliminated via the kidneys,11 an open-label study found only minor increases in the AUC concentration, tmax, and elimination half-life of roflumilast in 12 subjects with a creatinine clearance (CrCl) of less than 30 mL/minute.34 Therefore, dosage adjustments in patients with renal impairment are not currently required.
P&T COMMITTEE CONSIDERATIONS
The clinical trials of roflumilast provide ample evidence to support the drug’s indication for the treatment of severe COPD. It is reasonable, therefore, to reserve roflumilast for patients with severe COPD whose disease remains uncontrolled despite optimal therapy. P&T committees interested in adding roflumilast to their formularies should plan to use the drug in a manner that is consistent with the evidence-based data. Criteria for use or formulary restrictions should require severe (stage III) COPD or very severe (stage IV) COPD and the concurrent use of a short-acting bronchodilator, a long-acting bronchodilator, and an inhaled glucocorticoid unless the use of a glucocorticoid is contraindicated. Because the efficacy data for roflumilast in COPD are derived entirely from combination therapies, monotherapy should be avoided.
P&T committees must also weigh the perceived benefits of roflumilast against the potential for drug-related AEs. Despite a relatively low overall incidence of AEs in clinical studies, questions about the safety and tolerability of roflumilast persist. The most common AEs (e.g., diarrhea, nausea, and headache) are generally mild, tolerable, and reversible after discontinuation. However, the more serious sequelae (psychiatric events and weight loss) are a cause for concern.
The long-term safety of roflumilast has not been evaluated beyond 52 weeks, and rare AEs might not have been fully identified because of the limited numbers of patients in clinical trials. Sentinel monitoring programs, such as the FDA’s Med-Watch, will be beneficial in addressing concerns about the long-term safety of roflumilast. Until additional postmarketing surveillance data are available, health care professionals are advised to prescribe roflumilast with a certain degree of reservation.
COST
In addition to conducting a risk–benefit analysis, P&T committees will be expected to address the additional costs of therapy with roflumilast. Because roflumilast is an adjunctive agent and does not replace any of the first-line therapies, its inclusion in drug regimens will add significant cost to the treatment of severe COPD. Based on the drug’s current average wholesale price (AWP), the cost of one 500-mcg tablet per day is approximately $7.00, or about $210 per month.35
High drug costs can often be justified by compelling indications or strong outcomes data. However, the published clinical studies of roflumilast lack epidemiological outcomes information, such as rehospitalization rates, morbidity and mortality rates, office and physician visits, and the use of medical services. The high cost of roflumilast, coupled with insufficient epidemiological information, may provide an incentive for health systems and managed care organizations to restrict the use of this medication.
CONCLUSION
Roflumilast is the first PDE4 inhibitor to be approved for the prevention of COPD exacerbations and the first novel therapy for COPD in nearly 20 years. PDE4 inhibitors appear to act independently of other COPD drugs, and early studies have suggested that the mechanism of action of these agents is based on anti-inflammatory effects within pulmonary tissues.
Several randomized controlled clinical trials provided consistently strong evidence that roflumilast, in addition to standard therapy, improves severe COPD, both symptomatically and subjectively. There are no known negative trial results with roflumilast in COPD, and the strength of the evidence supporting the drug’s efficacy is favorable.
Concerns remain about the agent’s short-term and long-term safety. The most common non-serious AEs in clinical trials included diarrhea, nausea, and headache. Psychiatric events and weight loss have also been reported, and the product labeling recommends caution on patients who are prone to these conditions. Nevertheless, daily roflumilast, in addition to standard therapy with bronchodilators and inhaled corticosteroids, provides a new option for severe COPD.
Footnotes
Disclosure: The author reports that he has no financial or commercial/industrial relationships to disclose in regard to this article.
REFERENCES
- 1.Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: A summary of the ATS/ERS [American Thoracic Society/European Respiratory Society] position paper. Eur Respir J. 2004;23(6):932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 2.Williams DM, Bourdet SV. Chronic obstructive pulmonary disease. In: DiPiro JT, Talbert RL, Yee GC, et al., editors. Pharmacotherapy: A Pathophysiologic Approach. 7th ed. New York: McGraw-Hill Medical; p. 2008. [Google Scholar]
- 3.Hill AT, Bayley D, Stockley RA. The interrelationship of sputum inflammatory markers in patients with chronic bronchitis. Am J Respir Crit Care Med. 1999;160(3):893–898. doi: 10.1164/ajrccm.160.3.9901091. [DOI] [PubMed] [Google Scholar]
- 4.Global Initiative for Chronic Obstructive Lung Disease Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. (Updated 2011). Available at: www.gold-copd.org/uploads/users/files/gold_Re-port_2011_Jan21.pdf. Accessed February 2, 2012.
- 5.Mannino DM, Gagnon RC, Petty TL, Lydick E. Obstructive lung disease and low lung function in adults in the United States: Data from the National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med. 2000;160(11):1683–1689. doi: 10.1001/archinte.160.11.1683. [DOI] [PubMed] [Google Scholar]
- 6.Vestbo J, Prescott E, Lange P. Association of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pulmonary disease morbidity. Am J Respir Crit Care Med. 1996;153(5):1530–1535. doi: 10.1164/ajrccm.153.5.8630597. [DOI] [PubMed] [Google Scholar]
- 7.Feenstra TL, van Genugten ML, Hoogen-veen RT, et al. The impact of aging and smoking on the future burden of chronic obstructive pulmonary disease: A model analysis in the Netherlands. Am J Respir Crit Care Med. 2001;164(4):590–596. doi: 10.1164/ajrccm.164.4.2003167. [DOI] [PubMed] [Google Scholar]
- 8.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet. 1997;349(9064):1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 9.Sullivan SD, Ramsey SD, Lee TA. The economic burden of COPD. Chest. 2000;117(2 Suppl):5S–9S. doi: 10.1378/chest.117.2_suppl.5s. [DOI] [PubMed] [Google Scholar]
- 10.FDA approves new drug to treat chronic obstructive pulmonary disease, March 1, 2011. Available at: www.fda.gov. Accessed November 21, 2011.
- 11.Daliresp (roflumilast) Tablets, prescribing information. New York: Forest; February 2011. Available at: www.frx.com/pi/Daliresp_pi.pdf. Accessed November 1, 2011.
- 12.Blumenthal DK, Garrison JC. Pharmacodynamics: Molecular mechanisms of drug action. In: Brunton LL, Chabner BA, Knollmann BC, editors. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 12th ed. New York: McGraw-Hill; 2011. [Google Scholar]
- 13.Hohlfeld JM, Schoenfeld K, Lavae-Mokhtari M, et al. Roflumilast attenuates pulmonary inflammation upon segmental endotoxin challenge in healthy subjects: A randomized placebo-controlled trial. Pulm Pharmacol Ther. 2008;21(4):616–623. doi: 10.1016/j.pupt.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Grootendorst DC, Gauw SA, Verhoosel RM, et al. Reduction in sputum neutrophil and eosinophil numbers by the PDE4 inhibitor roflumilast in patients with COPD. Thorax. 2007;62(12):1081–1087. doi: 10.1136/thx.2006.075937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rabe KF, Bateman ED, O’Donnell D, et al. Roflumilast—an oral anti-inflammatory treatment for chronic obstructive pulmonary disease: A randomised controlled trial. Lancet. 2005;366(9485):563–571. doi: 10.1016/S0140-6736(05)67100-0. [DOI] [PubMed] [Google Scholar]
- 16.Bethke TD, Lahu G. High absolute bioavailability of the new oral phosphodiesterase-4 inhibitor roflumilast. Int J Clin Pharmacol Ther. 2011;49(1):51–57. doi: 10.5414/cpp49051. [DOI] [PubMed] [Google Scholar]
- 17.Hauns B, Hermann R, Hunnemeyer A, et al. Investigation of a potential food effect on the pharmacokinetics of roflumilast, an oral, once-daily phosphodiesterase 4 inhibitor, in healthy subjects. J Clin Pharmacol. 2006;46(10):1146–1153. doi: 10.1177/0091270006291621. [DOI] [PubMed] [Google Scholar]
- 18.Bethke TD, Bohmer GM, Hermann R, et al. Dose-proportional intraindividual single- and repeated-dose pharmacokinetics of roflumilast, an oral, once-daily phosphodiesterase 4 inhibitor. J Clin Pharmacol. 2007;47(1):26–36. doi: 10.1177/0091270006294529. [DOI] [PubMed] [Google Scholar]
- 19.Hatzelmann A, Schudt C. Anti-inflammatory and immunomodulatory potential of the novel PDE4 inhibitor roflumilast in vitro. J Pharmacol Exp Ther. 2001;297(1):267–279. [PubMed] [Google Scholar]
- 20.Nassr N, Hünnemeyer A, Herzog R, et al. Effects of rifampicin on the pharmacokinetics of roflumilast and roflumilast N-oxide in healthy subjects. Br J Clin Pharmacol. 2009;68(4):580–587. doi: 10.1111/j.1365-2125.2009.03478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lahu G, Hünnemeyer A, Herzog R, et al. Effect of repeated doses of erythromycin on the pharmacokinetics of roflumilast and roflumilast N-oxide. Int J Clin Pharmacol Ther. 2009;47(4):236–245. doi: 10.5414/cpp47236. [DOI] [PubMed] [Google Scholar]
- 22.Lahu G, Hünnemeyer A, von Richter O, et al. Effect of single and repeated doses of ketoconazole on the pharmacokinetics of roflumilast and roflumilast N-oxide. J Clin Pharmacol. 2008;48(11):1339–1349. doi: 10.1177/0091270008321941. [DOI] [PubMed] [Google Scholar]
- 23.von Richter O, Lahu G, Hünnemeyer A, et al. Effect of fluvoxamine on the pharmacokinetics of roflumilast and roflumilast N-oxide. Clin Pharmacokinet. 2007;46(7):613–622. doi: 10.2165/00003088-200746070-00006. [DOI] [PubMed] [Google Scholar]
- 24.Bethke TD, Giessmann T, Westphal K, et al. Roflumilast, a once-daily oral phosphodiesterase 4 inhibitor, lacks relevant pharmacokinetic interactions with inhaled salbutamol when co-administered in healthy subjects. Int J Clin Pharmacol Ther. 2006;44(11):572–579. doi: 10.5414/cpp44572. [DOI] [PubMed] [Google Scholar]
- 25.Hermann R, Siegmund W, Giessmann T, et al. The oral, once-daily phosphodiesterase 4 inhibitor roflumilast lacks relevant pharmacokinetic interactions with inhaled budesonide. J Clin Pharmacol. 2007;47(8):1005–1013. doi: 10.1177/0091270007300950. [DOI] [PubMed] [Google Scholar]
- 26.Nassr N, Lahu G, von Richter O, et al. Lack of a pharmacokinetic interaction between steady-state roflumilast and single-dose midazolam in healthy subjects. Br J Clin Pharmacol. 2007;63(3):365–370. doi: 10.1111/j.1365-2125.2006.02762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bohmer GM, Nassr N, Wenger M, et al. The targeted oral, once-daily phosphodiesterase 4 inhibitor roflumilast and the leukotriene receptor antagonist montelukast do not exhibit significant pharmacokinetic interactions. J Clin Pharmacol. 2009;49(4):389–397. doi: 10.1177/0091270008330980. [DOI] [PubMed] [Google Scholar]
- 28.Bateman ED, Izquierdo JL, Harnest U, et al. Efficacy and safety of roflumilast in the treatment of asthma. Ann Allergy Asthma Immunol. 2006;96(5):679–686. doi: 10.1016/S1081-1206(10)61065-4. [DOI] [PubMed] [Google Scholar]
- 29.Calverley PM, Sanchez-Toril F, McIvor A, et al. Effect of 1-year treatment with roflumilast in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;176(2):154–161. doi: 10.1164/rccm.200610-1563OC. [DOI] [PubMed] [Google Scholar]
- 30.Fabbri LM, Calverley PM, Izquierdo-Alonso JL, et al. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with long-acting bronchodilators: Two randomised clinical trials. Lancet. 2009;374(9691):695–703. doi: 10.1016/S0140-6736(09)61252-6. [DOI] [PubMed] [Google Scholar]
- 31.Calverley PM, Rabe KF, Goehring UM, et al. Roflumilast in symptomatic chronic obstructive pulmonary disease: Two randomised clinical trials. Lancet. 2009;374(9691):685–694. doi: 10.1016/S0140-6736(09)61255-1. [DOI] [PubMed] [Google Scholar]
- 32.Chong J, Poole P, Leung B, Black PN. Phosphodiesterase 4 inhibitors for chronic obstructive pulmonary disease (review) Cochrane Database Syst Rev. 2011;5 doi: 10.1002/14651858.CD002309.pub3. No. CD002309. [DOI] [PubMed] [Google Scholar]
- 33.Hermann R, Nassr N, Lahu G, et al. Steady-state pharmacokinetics of roflumilast and roflumilast N-oxide in patients with mild and moderate liver cirrhosis. Clin Pharmacokinet. 2007;46(5):403–416. doi: 10.2165/00003088-200746050-00003. [DOI] [PubMed] [Google Scholar]
- 34.Bethke TD, Hartmann M, Hünnemeyer A, et al. Influence of renal impairment on the pharmacokinetics of oral roflumilast: An open-label, parallel-group, single-center study. Int J Clin Pharmacol Ther. 2011;49(8):491–499. doi: 10.5414/cp201556. [DOI] [PubMed] [Google Scholar]
- 35.McKesson Connect. Available at: http://connect.mckesson.com. Accessed November 1, 2011.