Abstract
Biosimilars are here, but there’s no clear pathway yet for their approval. Difficult realities about their effect on drug prices, innovation, and competition need to be faced.
The Patient Protection and Affordable Care Act of 2010 (PPACA) includes the Biologics Price Competition and Innovation Act of 2009, which provides for the U.S. Food and Drug Administration’s development of a pathway for the expedited approval of follow-on biologics — or biosimilars, as they are more commonly known. The issues the FDA must confront are many and exist in a climate fraught with uncertainty for makers of biosimilars. At stake are continued drug innovation and market competition.
Biologics are becoming increasingly important in terms of new drug products and sales. In 2000, only 1 of the top 10 drugs was a biological product. In 2008, half were biologics. Biologics are large-molecule drugs produced in living organisms and are used to treat some of the most serious life-threatening diseases. They are more costly to manufacture than chemical drugs — research and development can cost $1.2 billion vs. $500 million to $800 million for chemical drugs. Biologic development takes, on average, between 10 and 15 years, and biopharmaceutical firms spend about 30 percent of their revenues on R&D, among the highest of any U. S. industry. Biologics are also expensive — for example, adalimumab (Humira) to treat rheumatoid arthritis or Crohn’s disease costs $50,000 a year and imiglucerase (Cerezyme) to treat Gaucher disease costs $200,000 a year.
Biosimilars are seen as a cost-effective alternative to the high-priced branded biologics, offering cost advantages to both payers and patients. Joseph Miletich, MD, PhD, Amgen’s senior vice president of R&D, has stated, “The great economic advantage of biosimilars is that a manufacturer only needs to recreate the idea that has already been shown to work.”

Erwin A. Blackstone, PhD, [top] is professor of economics at Temple University, in Philadelphia.
Joseph P. Fuhr Jr., PhD, is professor of economics at Widener University, in Chester, Pa.
Biosimilar experience
Biosimilars are available in the European Union (EU) and Canada because regulations permit their approval. In the EU, which first approved a biosimilar in 2006, there are 16 biosimilars for three reference (brand) products: erythropoietin (EPO), granulocyte-colony stimulating factor, and somatropin (human growth hormone).
In the United States, four so-called biosimilars have been approved under 505(b)(2) of the Hatch-Waxman Amendments to the Federal Food, Drug and Cosmetic Act with a hybrid Abbreviated New Drug Application, generally used for small-molecule drugs. Most biologics go through a Biologics License Application (BLA) process. The FDA is developing a pathway for approving biosimilars through an abbreviated BLA (aBLA) process, and the first applications are expected to be for biosimilars already approved in the EU (Table).
TABLE.
Biosimilars pipeline
| Product name | Proposed indication | Company | Phase of FDA study | Comments |
|---|---|---|---|---|
| MK-2578 (pegylated erythropoietin) | Anemia, chronic kidney disease | Merck | N/A | Merck has discontinued development of this biosimilar product. |
| Neutroval | Reduction in the duration of severe neutropenia and the incidence of febrile neutropenia in patients treated with established myelosuppressive chemotherapy for cancer | Teva | Phase 3 | Teva entered into a settlement with Amgen that will prohibit Teva from launching Neutroval until November 2013. |
| Lipegfilgrastim | Reduction in the duration of severe neutropenia and the incidence of febrile neutropenia in patients treated with established myelosuppressive chemotherapy for cancer | Teva | Phase 3 | Teva entered into a settlement with Amgen that will prohibit Teva from launching lipegfilgrastim until November 2013. |
| Erythropoietin (EPO) | Anemia caused by chronic kidney disease | Hospira | Phase 2 | Hospira already sells Retacrit, a biosimilar EPO, in Europe. |
| Rituximab | Rheumatoid arthritis | Sandoz | Phase 2 | Sandoz initiated a phase 2 trial of biosimilar rituximab in January 2011. |
Source: 2011 Medical Pharmacy & Oncology Trend Report, ICORE Healthcare
Biosimilars in the EU are usually priced 15 percent to 30 percent below their referenced products, but they have achieved little market share because of their lack of interchangeability. A major exception is in Germany, where EPO biosimilars garnered 52 percent of the market in 2009. Germany’s Federal Healthcare Committee has encouraged the use of biosimilars and is able to bargain for rebates. Sandoz, for example, increased its Binocrit discount in 2007 from 15 percent to 33 percent and obtained 30 percent of the market. On the other hand, in the United States, Sandoz’s biosimilar somatropin (Omnitrope), priced at least 25 percent less than the branded drug, garnered only $4 million in sales and a 1 percent market share after three years on the market.
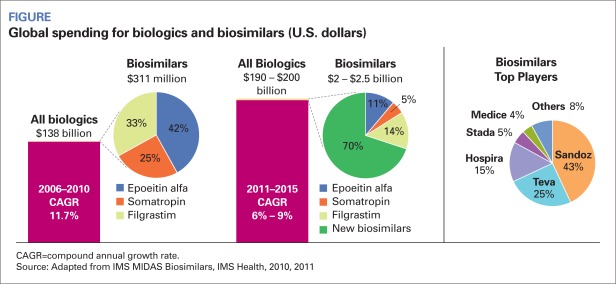
In 2010, biosimilar sales were around $235 million in the EU, Japan, Canada, and Australia compared to total biopharmaceutical sales of $134 billion. One study posits that brand erosion will be greater in the United States, with half of physicians prescribing the EPO biosimilar within 6 months and 88 percent within a year. The Figure (page 26) shows the expected market penetration of biosimilars.
FIGURE.
Global spending for biologics and biosimilars (U.S. dollars)
CAGR=compound annual growth rate.
Source: Adapted from IMS MIDAS Biosimilars, IMS Health, 2010, 2011
What the FDA faces
In February 2012, the FDA issued preliminary guidelines for biosimilar entry, which will be available for comment for 60 days. The FDA will then implement the actual guidelines, most likely by the end of 2012. A biosimilar manufacturer can then apply for biosimilar status for its drug under an aBLA. The regulatory approval process may take at least two years, so the first biosimilar would not enter the U.S. market until 2015.
As in the EU, each biosimilar will have its own unique set of guidelines. For example, in November 2010, the EU set out broad guidelines for the approval of biosimilar monoclonal antibodies (mAbs), allowing for the possibility of different diseases being addressed by the same copy mAb — this was more than four years after a biosimilar process was approved for less-complex biologics. The European Medicines Agency (EMA) has received requests for scientific advice for six biosimilar mAbs. In the United States, it may take four to five years to create a mAb pathway and another two years for the first approval, which puts the earliest a biosimilar mAb could hit the market at 2017.
The FDA can decide whether clinical trials are necessary to determine similarity and it’s likely that the agency will require such trials, albeit shortened ones. Human testing won’t be an across-the-board requirement — Janet Woodcock, MD, head of the FDA’s Center for Drug Evaluation and Research, has said it “depends on how confident you can be of the absolute sameness to the innovator product.”
Other issues that must be resolved include the basis for testing whether a product is biosimilar to the branded product. Unlike chemical drugs, a biologic constantly evolves and can change as the manufacturing process changes, a phenomenon known as drift. Which product will be the benchmark — the original branded biologic or the one currently being manufactured? In some cases, a biosimilar could be more similar to the branded product than the branded product is from batch to batch.
Will the FDA’s biosimilar policy differ by product? Generally, efficacy and safety have to be demonstrated separately for each indication and for postmarketing. Unlike chemical generics, biosimilars probably will have to go through postmarketing surveillance, or pharmacovigilance, which helps determine whether the biosimilar is similar to the branded product and what side effects occur in the biosimilar that are not present in the branded product. The biosimilar product each patient is taking will be tracked. Tracking may require that reference biologics and biosimilars be named differently and that their drug codes be different. Still, if the original drug is no longer produced, can it still be used as a benchmark? Will the FDA allow clinical trials done outside the country, such as those done in the EU, to be used for the approval process?
Immunogenicity and interchangeability
The most serious safety concern, unique to biologics, is immunogenicity — a patient's antibody reaction to a drug that the body perceives to be a foreign microorganism or virus. The science does not exist for the precise duplication of a branded biopharmaceutical. Thus, biosimilars are similar but not identical to the reference product.
To be considered interchangeable, the safety and efficacy risk of the biosimilar must not be “greater than the risk of using the reference product without such alteration or switch.” This means that there will not be automatic substitution at the pharmacy level. If physicians prescribe a branded biologic, pharmacists will have to dispense it and not the biosimilar. Interchangeability probably will not be allowed until the biosimilar has a track record and is shown through postmarketing studies to produce results identical to that of the branded product. Also, to allow for interchangeability, expensive crossover studies may be necessary for which recruiting could be difficult. The EU mandates preapproval clinical testing and post-approval monitoring for biosimilars, and the EMA requires all drugs to have postmarketing pharmacovigilance to ensure drug safety.
But the question remains: Because both the branded drug and the biosimilar will probably change over time (drift), will they always be considered interchangeable or will they have to be reassessed?
Cost and reimbursement
As the biopharmaceutical share of the total pharmaceutical market increases, the high cost and efficacy data of biologics will come under greater scrutiny, which will place additional burdens on payers. The average annual cost of a branded biopharmaceutical is estimated to be $34,550, and some payers require co-payments of $500 per prescription or co-insurance rates of 33 percent. Moreover, the rate of price increases for biopharmaceuticals far exceeds the overall rate of inflation. Through 2010, biopharmaceuticals experienced a 9.2 percent price increase compared to a 0.3 percent increase in the Consumer Price Index. Further, under the PPACA, Medicaid rebates have increased from 15 percent to 23 percent — which is essentially a greater price reduction.
The high and rising prices of bio-pharmaceuticals, along with tight payer budgets, explains the push for risk sharing. The single-payer systems in Europe have served as an impetus for risk-sharing agreements. For example, some biopharmaceutical companies have negotiated risk-sharing arrangements to have their branded product reimbursed by the United Kingdom’s National Health Service. In Germany, Roche will refund payments to hospitals and public insurers if patients with certain cancers do not respond to bevacizumab (Avastin).
Medicare expenditures for biologics total billions of dollars and are increasing each year. Because Medicare must cover substantially all drugs that are unique, there is little incentive for Medicare providers to reduce prices for biologics. In addition, Medicare covers most drug expenditures under Part D once catastrophic coverage begins, which also reduces the incentives for drug plans to encourage the use of lower-cost alternatives. If biosimilars are available and are considered interchangeable, the average selling price would include both the lower-priced biosimilar and the higher-priced branded drug, which would provide an incentive under Part B to encourage the use of biosimilars. The reimbursement markup for biosimilars would be 6 percent of the selling price of the branded product. Thus, physicians would receive the same monetary reward for both branded drugs and biosimilars.
Will biosimilars be less costly?
Chemical generics lowered drug prices by as much as 90 percent and captured a large share of the branded drug market in a short period of time, often within a few months. Biosimilars, however, are probably going to be only 20 percent to 30 percent less than the branded biologics. Therefore, a brand product manufacturer could reduce its price by a fairly small amount, perhaps 10 percent to 20 percent, to discourage buyers from switching to the biosimilar. The penetration rate for biosimilars is expected to be higher in patient populations that have high turnover than in chronic disease populations. Patients and physicians may be reluctant to switch to a biosimilar for small cost savings if the branded product is working. On the other hand, new patients may be more willing to try a biosimilar.
Conclusion
The PPACA has given the FDA the authority to create a pathway for approving biosimilars — copies of brand biologics that have the potential to keep healthcare costs down. In early February, the FDA issued draft guidelines for the approval of biosimilars. However, issues such as efficacy and safety must be resolved, and clinical trials may be required as well as pharmacovigilance.
We expect to see considerable controversy as various interests either support or oppose a biosimilars approval pathway. We also expect that the FDA will ensure that the biosimilars market develops. It is critical that the FDA attain a balance that encourages both innovation and competition in the biopharmaceutical industry.
Footnotes
Disclosure: Erwin A. Blackstone, PhD, and Joseph P. Fuhr Jr., PhD, report they have no financial arrangements or affiliations with any manufacturers or products mentioned in this article.
References
- Alazraki M. How to approve ‘biosimilar’ drugs? The FDA has to figure that out. Daily Finance. http://www.daily-finance.com/2010/11/03/biosimilar-drugs-fda-approval-process. Accessed Feb. 15, 2012.
- Blackstone EA, Fuhr JP. Biosimilars and innovation: an analysis of the possibility of increased competition in biopharmaceuticals. Future Med Chem. 2010;2:1641–1649. doi: 10.4155/fmc.10.248. [DOI] [PubMed] [Google Scholar]
- Brookings Institution Biosimilars in the United States: implementation challenges and lessons learned from the European Union [transcript] Dec. 2010. http://www.brookings.edu/events/2010/1201_biosimilars.aspx. Accessed Feb. 15, 2012.
- Carlson B. Satisfaction guaranteed or your money back. Biotechnol Healthcare. 2009;6(4):14–22. [PMC free article] [PubMed] [Google Scholar]
- Carroll J. FDA outlines steep hurdles, early fees for first-gen biosimilars. FierceBiotech. http://www.fiercebiotech.com/print/node/108932. May 10, 2011. Accessed Feb. 15, 2012.
- Decision Resources Group . Biosimilar erosion of branded ESA market share will be more rapid in the U.S. than in Europe [news release] Burlington, MA.: Decision Resources Group; Nov. 2010. http://decisionresources.com/News-and-Events/Press-Releases/BAS-11410-(1). Accessed Feb. 15, 2012. [Google Scholar]
- FDA (U.S. Food and Drug Administration) Biologics Price Competition and Innovation Act of 2009; options for a user fee program for biosimilar and interchangeable biological product applications for fiscal years 2013 through 2017 [notice and request for comments] Fed. Regist. 2011;76(90):27062–27067. [Google Scholar]
- FDA (U.S. Food and Drug Administration) http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm291232.htm. Accessed Feb. 9, 2012.
- Harris J. Promise of biosimilars tempered by complexity, caution. HemOnc Today. http://www.hemonctoday.com/article.aspx?rid=79243. Jan. 10, 2011. Accessed Feb. 15, 2012.
- Kozlowski S, Woodcock J, Midthun K, Berman Sherman R. Developing the nation’s biosimilars program. N Engl J Med. 2011;365:385–388. doi: 10.1056/NEJMp1107285. [DOI] [PubMed] [Google Scholar]
- Ransohoff T, Levine H. Biosimilars: Europe 14, US 4. BioProcessBlog. http://www.bioprocessblog.com/archives/92. Mar. 8, 2011. Accessed Feb. 15, 2012.
- Sandle P. EU guidelines clear way for biosimilar antibodies. Reuters. Nov. 2010. http://www.reuters.com/article/2010/11/26/us-eu-biosimilars-idUSTRE6AP3RQ20101126. Accessed Feb. 15, 2012.
- Sarma BG. Manufacturing challenges for biosimilars — the process defines the product. EJHP Practice. 2007;13(5):54–6. [Google Scholar]
- Senior M. European biosimilars market performance mirrors US legislative progress: slow but steady. Biopharma Today. http://www.biopharmatoday.com/2009/06/european-biosimilars-market-performance-mirrors-us-legislative-progress-slow-but-steady.html. May 19, 2009. Accessed Feb. 15, 2012.
- Sheppard A. The biosimilars market today and tomorrow. Pharmaceutical Technology Europe. http://pharmtech.findpharma.com/pharmtech/Manufacturing/The-Biosimilars-Market-Today-And-Tomorrow/ArticleStandard/Article/detail/685049. Sep. 1, 2010. Accessed Feb. 15, 2012.
- Snyder G, Ruchin K, Hoffman T, Deloitte Life Sciences Showdown over drug pricing – get ready for another round. http://www.deloitte.com/us/drugpricing. Nov. 2010. Accessed Feb. 15, 2012.
- Standard & Poor’s . Industry Surveys: Biotechnology. New York, NY: Standard & Poor’s; Feb. 2011. [Google Scholar]
- Staton T. Roche offers a money-back deal on Avastin in Germany. Fierce Pharma. http://www.fiercepharma.com/print/node/66544. Oct. 23, 2011. Accessed Feb. 15, 2012.