Abstract
Dendritic cells (DC) are the initiators and modulators of the immune responses. Some species of pathogenic microorganisms have developed immune evasion strategies by controlling antigen presentation function of DC. Simian virus 40 (SV40) is a DNA tumor virus of rhesus monkey origin. It can induce cell transformation and tumorigenesis in many vertebrate species, but often causes no visible effects and persists as a latent infection in rhesus monkeys under natural conditions. To investigate the interaction between SV40 and rhesus monkey DC, rhesus monkey peripheral blood monocyte-derived DC were induced using recombinant human Interleukin-4 (rhIL-4) and infective SV40, the phenotype and function of DC-specific intracellular adhesion molecule-3 grabbing nonintegrin (DC-SIGN)+ DC were analyzed by flow cytometry (FCM) and mixed lymphocyte reaction (MLR). Results showed that SV40 can down-regulate the expression of CD83 and CD86 on DC and impair DC-induced activation of T cell proliferation. These findings suggest that SV40 might also cause immune suppression by influencing differentiation and maturation of DC.
Keywords: Dendritic cell, Simian virus 40, Rhesus monkey
1. Introduction
Dendritic cells (DC) are the most prominent antigen presenting cells which play a pivotal role in the orchestration of the various forms of immunity and tolerance [1,2]. The DC-SIGN+ dendritic cells (DDC), which were found in the interstitium of heart, liver, lung, kidney, skin, etc., are usually thought to play a pivotal role in the immunosuppressive induction of a number of pathogens [3-6]. These pathogens include measles virus [7-9], human immunodeficiency virus [10-12], human hepatitis B virus [13,14], Hepatitis C Virus [15], tubercle bacillus [16,17], typhoid bacillus [18,19], anthrax bacillus [20,21], etc. Details of immune evasion of these pathogens are still unclear at present [22,23]. Simian virus 40 (SV40) is a double-stranded DNA tumor virus that was first identified by Sweet in 1960 [24]. SV40 can transform many types of cells and cause carcinogenesis in vitro, including those of human origin [25-27]. The virus is usually dormant and asymptomatic in rhesus monkeys under natural conditions. Whether SV40 subvert dendritic cell function remains unclear. The present study was designed to examine the effect of SV40 infection on DC-SIGN+ dendritic cells.
2. Materials and methods
2.1. Animal
Five- to ten-year-old female rhesus macaques were obtained from the Kunming National Primate Research Center of China. The SV40 large T antigen (LT-AG) gene fragment in rhesus monkey peripheral blood was tested using the forward primer (5'-AACAGCCC AGCCACTATAAGTACC-3', 3892~3869) and the reverse primer (5'-AGCAACTCCAGCCATCCATTC-3', 3642~3662). The expected PCR product is 251 bp. The SV40 major capsid protein VP1 gene fragment in rhesus monkey peripheral blood was detected using the following forward primer (5'-CTCAAATGG GCAATCCTGATG-3', 1665~1685) and the reverse pri mer (5'-CATAGCAGTTACCCCAATAACCTC-3', 1882~1859). The expected PCR product is 218 bp. Those rhesus monkeys that tested SV40-DNA fragment negative were selected to participate in this study.
2.2. Virus and cell
The reference strain SV40-776 was proliferated in Vero cells (ATCC, CCL-81) which were cultured in Minimum Essential Medium (MEM, GIBCO) containing 2% fetal bovine serum (FBS, GIBCO), 2 mM L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin at 37°C, 5% CO2, 95% air and 100% relative humidity. Repetitive freeze-thaw cycles were used to lyse the cells and release intracellular virus particles when more than 75 per cent of the infected Vero cells show evident cytopathic effect (CPE). The supernatant of infected cells suspension, which was obtained by centrifuging the freshly thawed virus suspension at 1000 × g for 30 min at room temperature to remove any cell debris, was precipitated with 10% (wt/vol) polyethylene glycol 6000 in the presence of 0.5 M NaCl overnight at 4°C and centrifugation at 12,000 × g for 20 min at 4°C. The pellet was resuspended in 4 ml phosphate buffered saline (PBS, pH 7.2), and virus particles were purified by ultracentrifugation in a cesium chloride (CsCl) density gradient at 120,000 × g for 24 h at 20°C. The purified SV40 were divided into two parts, half of them was inactivated by β-propiolactone (BPL) with a final concentration of 0.01 M for 72 h at 4°C and then was settled at 37°C for 2 h to decompose BPL completely. The inactivated SV40 was stored at -20°C after demonstration of no infectivity. The other half was assayed for plaque forming units (pfu) of the purified SV40 and stored at -80°C until use.
2.3. Induction of rhesus macaque DC
Mononuclear cells were isolated from 3~5 ml SV40-negative rhesus monkey peripheral blood by Ficoll-Hypaque density gradient centrifugation. Monocytes were separated from lymphocyte by glass adherence, and proliferated by using RPMI 1640 medium (GIBCO) containing 10% FBS, 20 mM HEPES, 1 mM sodium pyruvate, 2 mM L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 500 ng/ml recombinant human granulocyte-macrophage colony stimulating factor (rhGM-CSF, R&D Systems) at 37°C, 5% CO2, 100% relative humidity, and induced to differentiate toward IDC by using 10 ng/ml recombinant human interleukin-4 (rhIL-4, R&D Systems).
2.4. Isolation of T-Lymphocytes
T-lymphocytes were isolated from cell suspensions consisting of T cells and B cells with Nylon Fiber Column T (Wako) and cultured in RPMI 1640 medium supplemented with 1 ng/ml recombinant human interleukin-2 (rhIL-2, R&D Systems).
2.5. Characterization of rhesus macaque DC
The morphological changes of DC were examined under ordinary light microscope, phase contrast microscope and scanning electron microscope. The phenotype of the rhesus monkey peripheral blood monocyte-derived dendritic cells in vitro was analyzed by flow cytometry (FCM) using the following fluorescence-labeled mouse anti-non-human primate monoclonal antibodies: HLA-DR, DP, DQ-PE(clone Tu39, BD), CD83-PE(clone HB15e, BD), CD86-FITC (clone FUN-1, BD), CD1a-PE(clone BL 6, Beckman Coulter) and PE-conjugated mouse antihuman DC-SIGN monoclonal antibodies, CD209-PE (Clone 120507, R&D Systems), which exhibited the highest affinity to both human DC-SIGN and rhesus macaque DC-SIGN. Cell proliferation effect of auto T cell activated by DC infected with SV40 at a multiplicity of infection (MOI) of 20 in mixed lymphocyte reaction (MLR) were mensurated by 3Hthymidine (3H-TdR) incorporation assay. The control dendritic cells were pulsed with 50 ng/ml inactivated SV40.
2.6. Elispot assay
The interleukin 12 (IL-12) enzyme linked immunospot (ELISPOT) assay was carried out in rhesus macaque peripheral blood monocyte-derived DC-SIGN+ dendritic cells using a commercial kit (U-CyTech biosciences, Monkey ELISPOT kit, UH-CT135-PR2). Infective SV40 and inactivated SV40 were used as specific antigen stimulators of the treatment group and the control group respectively. The spots were counted with an automated reader. The number of IL-12 spot forming cells (SFC) was calculated by subtracting the non-specific SFC in the presence of antigen-unloaded DC.
2.7. Statistical analysis
Differences between groups were analyzed by using the statistical software package SPSS 15.0 for Windows. Statistical significance was set at P < 0.05.
3. Results
3.1. Morphology of rhesus macaque DC
Rhesus macaque monocytes were proliferated by using RPMI 1640 medium supplemented with 500 ng/ml rhGM-CSF. IL-4 which induces monocytes to differentiate toward DDC was added to culture medium after 7 days of culture, and then infective SV40 and inactivated SV40 were added to culture medium after 3 days of IL-4 inducement culture respectively. After additional 9 days of culture, a great number of spiny, crab-like, pompon-like, stellate cells were seen floating in the culture medium (Figure 1B, E). At day 12 of culture, these monocyte-derived dendritic cells, which mainly exhibit squamous, petallike, veillike, sheet-like processes, could be observed under scanning electron microscope (Figure 1C, F). Morphocytological changes of experiment group (infective SV40-treated group) are similar to that of negative control group (inactivated SV40-treated group). The morphology of blank control group (antigen-untreated group), in contrast, remained unchanged all the time (Figure 1A, D).
Figure 1.
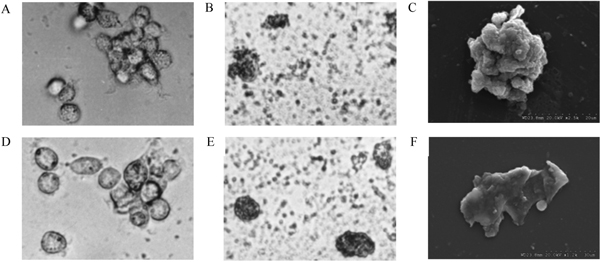
Morphology of rhesus macaque monocyte-derived DC. (A) Cell colonies of dendritic cell precursors of antigen-untreated group on day 3 of culture (magnification, × 400). (B) Morphology of DC of inactivated SV40-treated group on day 9 of culture (magnification, × 400). (C) Morphology of DC of inactivated SV40-treated group on day 12 of culture (magnification, × 2500). (D) Cell colonies of dendritic cell precursors of antigen-untreated group on day 9 of culture (magnification, × 400). (E) Morphology of DC of infective SV40-treated group on day 9 of culture (magnification, × 400). (F) Morphology of DC of infective SV40-treated group on day 12 of culture (magnification, × 1200).
3.2. Phenotype of antigen-unloaded DC
The expression of CD83, CD209 on rhesus macaque DC cell surface of antigen-untreated group was analyzed by flow cytometric analysis. The results showed that peripheral blood monocyte-derived DC cultured with GM-CSF and IL-4 express CD83 and CD209 on cell surface. At day 9 of cell culture, the percentage of DC expressing CD83 and CD209 is 10.8 ± 2.3% and 69.7 ± 5.2%, respectively (Figure 2).
Figure 2.
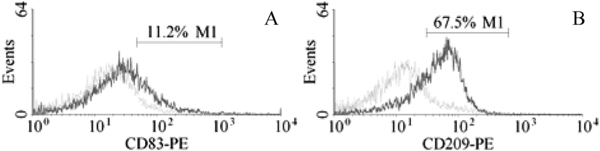
Expression of CD83, CD209 on DC cell surface of antigen-untreated group. (A) Expression of CD83 on DC cell surface of antigen-untreated group at day 9 culture (the dotted line, isotype control; the solid line, CD83 labeling). (B) Expression of CD209 on DC cell surface of antigen-untreated group at day 9 culture (the dotted line, isotype control; the solid line, CD209 labeling).
3.3. Phenotype of Antigen-Treated DC
The expression of HLA-DR, CD1a, CD86, CD83 on cell surface of DC that had been released from CFU in antigen-untreated group, inactivated SV40-treated group, infective SV40-treated group at day 3, 6, 9 of culture were analyzed by flow cytometry. The results showed that, after three days of culture, rhIL-4 cannot significantly elevate the expression of the above molecules except for HLA-DR (Figure 3), and the inactivated SV40 antigen can obviously increase the expression of HLA-DR, CD86 and CD83 (Figure 3A, C, D), while infective SV40, in contrast, can suppress the expression of HLA-DR, CD86 and CD83. The expression of CD1a on DC remained unchanged all the time during the entire study (Figure 3B)
Figure 3.
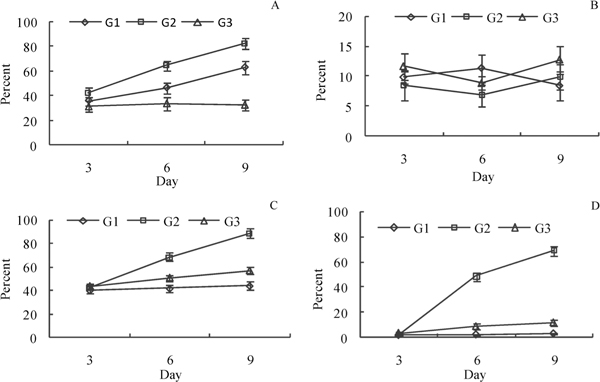
Expression of HLA-DR, CD1a, CD86, CD83 on cell surface of antigen-treated DC. G1, antigen-untreated group; G2, inactivated SV40-treated group; G3, infective SV40-treated group. (A) Expression of HLADR on rhesus macaque DC. (B) Expression of CD1a on rhesus ma caque DC. (C) Expression of CD86 on rhesus macaque DC. (D) Expression of CD83 on rhesus macaque DC.
3.4. Function Analysis of Antigen-Treated DC
Auto-T cells proliferation effect in MLR activated by rhesus macaque DC on day 3, 6, 9 of culture was analyzed with 3H-TdR incorporation assay. The results demonstrated that the proliferation of T cells activated by DC treated with inactivated SV40 was more prominent than that activated by DC treated with infective SV40 (P < 0.05).
3.4. Interleukin 12 Secreting activity of antigen-treated DC
Interleukin 12, which is naturally produced by dendritic cells in response to antigen stimulation, is involved in the differentiation of naive T cells. In order to evaluate the function of DC in response to different antigen stimulation, the relative number of IL-12 secreting DC on day 3, 6, 9 of culture following stimulation with inactivated SV40 and infective SV40 was analyzed by an ELISPOT assay. The results showed that the frequency of IL-12 producing cells of inactivated SV40-treated group was higher than that of infective SV40-treated group (P < 0.05).
4. Discussion
Dendritic cells can be divided into at least three distinct subsets, two myeloid lineages and one lymphoid lineage. Langerhans cells and interstitial DC constitute two types of immature myeloid DC in vivo [28-30]. Langerhans cells can be generated from bone marrow, cord blood, or adult blood CD34+ hema topoietic progenitors cultured with GM-CSF, transforming growth factor (TGF)-β, and tumor necrosis factor (TNF)-α in vitro [31-33]. DC-SIGN-expressing immature dendritic cells can arise from peripheral blood monocytes by culturing with GM-CSF and IL-4 in vitro. These interstitial DC display striking similarities to dermal DC [34,35].
Recent studies have demonstrated that several pathogens can manipulate DC functions to facilitate their survival by employing DC-SIGN/CD209 which is expressed by a subset of immature DC. This receptor is associated with receptor-mediated cellular endocytosis and involved in the capture of various patho gens. However, it has been demonstrated that DCSIGN+ immature dendritic cells are related to immunosuppressive inducement of many pathogenic microorganism [5,6].
Cutaneous dendritic cells mainly comprise epidermal Langerin+ dendritic cells, namely, Langerhans cells (LC) and dermal DC-SIGN+ dendritic cells (DDC), namely, interstitial dendritic cells (IDC) [36]. Although both LC and IDC share equally common bone marrow origin, accumulating evidence suggests that their immunological orchestration function appear different in antiviral immunity [37,38]. DDC located in mucosal tissues of the genital tract are considered to play a central role in the early steps of HIV transfer from DC to T cells through sexual transmission route [39]. DC-SIGN-expressing immature DC exposed to a small amount of HIV particles can promote more efficient infection of T cells than that by free viruses. DC-SIGN-expressing cells can evidently improve the stability and infectivity of HIV. Research shows that HIV particles captured by DC-SIGN-expressing DC remain infectious after 4 days of culture, whereas cell-free virus rapidly loses its infectivity [5,40]. Langerin+ DC residing in the epidermal or epithelial cells in the skin and mucosa are the first DC subset to encounter HIV-1 and has generally been thought to mediate the spread of HIV-1 to T cells through the C-type lectin Langerin, similarly to transmission by DC-SIGN on dendritic cells. Instead, recent research suggests that in contrast to DC-SIGN, Langerin on LC can protect against HIV-1 transmission by internalization and degradation of the virus [37,38].
Measles virus (MV) is a high contagious pathogen which can induce profound immunosuppression, resulting in a high mortality rate. It has been demonstrated that DC play a crucial role in the pathogenesis of MV infection [41,42]. Various immunosuppression mechanisms of MV infection elicited by DC have been described. Although DC-SIGN is not an entry receptor of MV, yet it functions as an attachment receptor to enhance dendritic cell infection [11].
In this study, the phenotype and function of infective SV40-treated peripheral blood monocytes derived DC were analyzed. The results showed that the phenotype and function of inactivated SV40-treated DC and infective SV40-treated DC are rather different. The expression levels of major histocompatibility complex (MHC) class II molecules, CD86 and CD83 of infective SV40-treated DC on day 6, 9 of culture were lower than those of inactivated SV40-treated DC (Figure 3A, C, D). T cell proliferation activity stimulated by infective SV40-treated DC was also lower than that stimulated by inactivated SV40-treated DC (Figure 4). MHC class II molecule of DC is related to antigen processing and presentation of exogenous antigen. CD86 is an important costimulatory molecule for the priming of naive T cells. CD83 is one of the best markers for mature dendritic cells. Recent studies showed that CD83 expressed on DC plays a costimulatory role in T cell initiation [43]. Although CD209+ DC can uptake infective SV40, the virus infection is not productive. Down-regulation of the expression of MHC class II molecules, CD86 and CD83 on infective SV40-treated DC suggests that infective SV40 can inhibit the differentiation and maturation of rhesus macaque DCSIGN+ dendritic cells and subvert antigen presentation function of DC. Compare with inactivated SV40 antigen, infective SV40 can significantly down-regulate the expression of dendritic cell-derived interleukin-12 and facilitates immunosuppression (Figure 4). These preliminary data suggest that SV40 can manipulate antigen presentation function of DC-SIGN+ DC and initiate immune tolerance.
Figure 4.
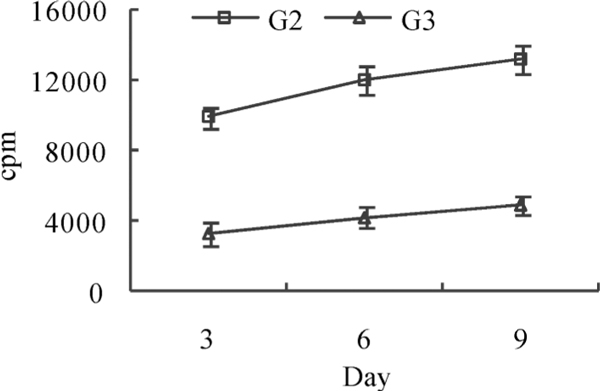
Cell proliferation effect of auto T cell activated by DC in mixed lymphocyte reaction. cpm, counts per minute; G2, inactivated SV40-treated group; G3, infective SV40-treated group.
Figure 5.
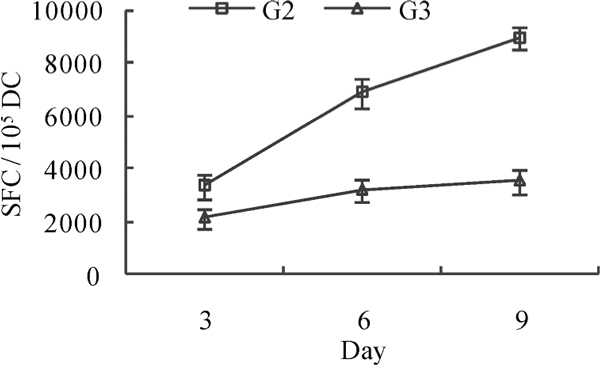
Il-12 secreting spot forming cell (SFC) frequency in inactivated and infective SV40-treated group. G2, inactivated SV40-treated group; G3, infective SV40-treated group.
Acknowledgements
This work was supported by the National Mega-projects of Science Research for the 11th Five-Year of China (No.2008ZX10001-006) and the opening foundation of the State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, The First Affiliated Hospital of Medical College, Zhejiang University (No.2008A06).
References
- Steinman RM. Dendritic cells: versatile controllers of the immune system. Nat Med. 2007;13:1155–1159. doi: 10.1038/nm1643. [DOI] [PubMed] [Google Scholar]
- Muriel M. Dendritic cells in immunity and tolerance--do they display opposite functions? Immunity. 2003;19:5–8. doi: 10.1016/S1074-7613(03)00182-1. [DOI] [PubMed] [Google Scholar]
- Curtis BM, Scharnowske S, Watson AJ. Sequence and expression of a membrane-associated C-type lectin that exhibits CD4-independent binding of human immunodeficiency virus envelope glycoprotein gp120. Proc Natl Acad Sci USA. 1992;89:8356–8360. doi: 10.1073/pnas.89.17.8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geijtenbeek TB, Torensma R, van Vliet SJ, van Duijnhoven GC, Adema GJ, van Kooyk Y, Figdor CG. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 2000;100:575–585. doi: 10.1016/S0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- van Kooyk Y, Geijtenbeek TB. DC-SIGN: escape mechanism for pathogens. Nat Rev Immunol. 2003;3:697–709. doi: 10.1038/nri1182. [DOI] [PubMed] [Google Scholar]
- Zhou T, Chen Y, Hao L, Zhang Y. DC-SIGN and immunoregulation. Cell Mol Immunol. 2006;3:279–283. [PubMed] [Google Scholar]
- Marie JC, Kehren J, Trescol-Biémont MC, Evlashev A, Valentin H, Walzer T, Tedone R, Loveland B, Nicolas JF, Rabourdin-Combe C, Horvat B. Mechanism of measles virus-induced suppression of inflammatory immune responses. Immunity. 2001;14:69–79. doi: 10.1016/S1074-7613(01)00090-5. [DOI] [PubMed] [Google Scholar]
- Slifka MK, Homann D, Tishon A, Pagarigan R, Oldstone MB. Measles virus infection results in suppression of both innate and adaptive immune responses to secondary bacterial infection. J Clin Invest. 2003;111:805–810. doi: 10.1172/JCI13603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Witte L, Abt M, Schneider-Schaulies S, van Kooyk Y, Geijtenbeek TB. Measles virus targets DC-SIGN to enhance dendritic cell infection. J Virol. 2006;80:3477–3486. doi: 10.1128/JVI.80.7.3477-3486.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geijtenbeek TB, Kwon DS, Torensma R, van Vliet SJ, van Duijnhoven GC, Middel J, Cornelissen IL, Nottet HS, KewalRamani VN, Littman DR, Figdor CG, van Kooyk Y. DC-SIGN, a dendritic cell-specific HIV-1binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–597. doi: 10.1016/S0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- Engering A, Van Vliet SJ, Geijtenbeek TB, Van Kooyk Y. Subset of DC-SIGN(+) dendritic cells in human blood transmits HIV-1 to T lymphocytes. Blood. 2002;100:1780–1786. doi: 10.1182/blood-2001-12-0179. [DOI] [PubMed] [Google Scholar]
- Piguet V, Steinman RM. The interaction of HIV with dendritic cells: outcomes and pathways. Trends Immunol. 2007;28:503–510. doi: 10.1016/j.it.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Op den Brouw ML, Binda RS, van Roosmalen MH, Protzer U, Janssen HL, van der Molen RG, Woltman AM. Hepatitis B virus surface antigen impairs myeloid dendritic cell function: a possible immune escape mechanism of hepatitis B virus. Immunology. 2009;126:280–289. doi: 10.1111/j.1365-2567.2008.02896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Zou ZS, Fu JL, Cai L, Jin L, Liu YJ, Wang FS. Severe dendritic cell perturbation is actively involved in the pathogenesis of acute-on-chronic hepatitis B liver failure. J Hepatol. 2008;49:396–406. doi: 10.1016/j.jhep.2008.05.017. [DOI] [PubMed] [Google Scholar]
- Ludwig IS, Lekkerkerker AN, Depla E, Bosman F, Musters RJ, Depraetere S, van Kooyk Y, Geijtenbeek TB. Hepatitis C virus targets DC-SIGN and L-SIGN to escape lysosomal degradation. J Virol. 2004;78:8322–8332. doi: 10.1128/JVI.78.15.8322-8332.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geijtenbeek TB, Van Vliet SJ, Koppel EA, Sanchez-Hernandez M, Vandenbroucke-Grauls CM, Appelmelk B, Van Kooyk Y. Mycobacteria target DC-SIGN to suppress dendritic cell function. J Exp Med. 2003;197:7–17. doi: 10.1084/jem.20021229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha A, Salam N, Gupta S, Natarajan K. Mycobacterium tuberculosis and dendritic cells: recognition, activation and functional implications. Indian J Biochem Biophys. 2007;44:279–288. [PubMed] [Google Scholar]
- van der Velden AW, Velasquez M, Starnbach MN. Salmonella rapidly kill dendritic cells via a caspase-1-dependent mechanism. J Immunol. 2003;171:6742–6749. doi: 10.4049/jimmunol.171.12.6742. [DOI] [PubMed] [Google Scholar]
- Cheminay C, Möhlenbrink A, Hensel M. Intracellular Salmonella inhibit antigen presentation by dendritic cells. J Immunol. 2005;174:2892–9289. doi: 10.4049/jimmunol.174.5.2892. [DOI] [PubMed] [Google Scholar]
- Chou PJ, Newton CA, Perkins I, Friedman H, Klein TW. Suppression of dendritic cell activation by anthrax lethal toxin and edema toxin depends on multiple factors including cell source, stimulus used, and function tested. DNA Cell Biol. 2008;27:637–648. doi: 10.1089/dna.2008.0760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A, Lingappa J, Leppla SH, Agrawal S, Jabbar A, Quinn C. et al. Impairment of dendritic cells and adaptive immunity by anthrax lethal toxin. Nature. 2003;424:329–334. doi: 10.1038/nature01794. [DOI] [PubMed] [Google Scholar]
- Mitchell DA, Fadden AJ, Drickamer K. A novel mechanism of carbohydrate recognition by the C-type lectins DC-SIGN and DC-SIGNR. Subunit organization and binding to multivalent ligands. J Biol Chem. 2001;276:28939–28945. doi: 10.1074/jbc.M104565200. [DOI] [PubMed] [Google Scholar]
- van Liempt E, Bank CM, Mehta P, Garciá-Vallejo JJ, Kawar ZS, Geyer R, Alvarez RA, Cummings RD, Kooyk Y, van Die I. Specificity of DC-SIGN for mannose- and fucose-containing glycans. FEBS Lett. 2006;580:6123–6131. doi: 10.1016/j.febslet.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Sweet BH, Hilleman MR. The vacuolating virus, SV40. Proc Soc Exp Biol Med. 1960;105:420–427. doi: 10.3181/00379727-105-26128. [DOI] [PubMed] [Google Scholar]
- Sáenz-Robles MT, Sullivan CS, Pipas JM. Transforming functions of Simian Virus 40. Oncogene. 2001;20:7899–7907. doi: 10.1038/sj.onc.1204936. [DOI] [PubMed] [Google Scholar]
- Vilchez RA, Kozinetz CA, Arrington AS, Madden CR, Butel JS. Simian virus 40 in human cancers. Am J Med. 2003;114:675–684. doi: 10.1016/S0002-9343(03)00087-1. [DOI] [PubMed] [Google Scholar]
- Martini F, Corallini A, Balatti V, Sabbioni S, Pancaldi C, Tognon M. Simian virus 40 in humans. Infect Agent Cancer. 2007;2:13. doi: 10.1186/1750-9378-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortman K, Caux C. Dendritic cell development: multiple pathways to nature's adjuvants. Stem Cells. 1997;15:409–419. doi: 10.1002/stem.150409. [DOI] [PubMed] [Google Scholar]
- Anchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Ueno H, Klechevsky E, Morita R, Aspord C, Cao T, Mat-sui T, Di Pucchio T, Connolly J, Fay JW, Pascual V, Palucka AK, Banchereau J. Dendritic cell subsets in health and disease. Immunol Rev. 2007;219:118–142. doi: 10.1111/j.1600-065X.2007.00551.x. [DOI] [PubMed] [Google Scholar]
- Caux C, Dezutter-Dambuyant C, Schmitt D, Banchereau J. GM-CSF and TNF-a cooperate in the generation of dendritic Langerhans cells. Nature. 1992;360:258–261. doi: 10.1038/360258a0. [DOI] [PubMed] [Google Scholar]
- Strunk D, Rappersberger K, Egger C, Strobl H, Krömer E, Elbe A, Maurer D, Stingl G. Generation of human dendritic cells/Langerhans cells from circulating CD34+ hematopoietic progenitor cells. Blood. 1996;87:1292–1302. [PubMed] [Google Scholar]
- Arrighi JF, Soulas C, Hauser C, Saeland S, Chapuis B, Zubler RH, Kindler V. TNF-alpha induces the generation of Langerin/(CD207)+ immature Langerhans-type dendritic cells from both CD14-CD1a and CD14+CD1aprecursors derived from CD34+ cord blood cells. Eur J Immunol. 2003;33:2053–2063. doi: 10.1002/eji.200323714. [DOI] [PubMed] [Google Scholar]
- Caux C, Massacrier C, Dubois B, Valladeau J, Dezutter-Dambuyant C, Durand I, Schmitt D, Saeland S. Respective involvement of TGF-beta and IL-4 in the development of Langerhans cells and non-Langerhans dendritic cells from CD34+ progenitors. J Leukoc Biol. 1999;66:781–791. doi: 10.1002/jlb.66.5.781. [DOI] [PubMed] [Google Scholar]
- Guironnet G, Dezutter-Dambuyant C, Vincent C, Bechetoille N, Schmitt D, Peguet-Navarro J. Antagonistic effects of IL-4 and TGF-beta1 on Langerhans cell-related antigen expression by human monocytes. J Leukocyte Biol. 2002;71:845–853. [PubMed] [Google Scholar]
- Valladeau J, Saeland S. Cutaneous dendritic cells. Semin Immunol. 2005;17:273–283. doi: 10.1016/j.smim.2005.05.009. [DOI] [PubMed] [Google Scholar]
- de Witte L, Nabatov A, Pion M, Fluitsma D, de Jong MA, de Gruijl T, Piguet V, van Kooyk Y, Geijtenbeek TB. Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nat Med. 2007;13:367–371. doi: 10.1038/nm1541. [DOI] [PubMed] [Google Scholar]
- de Witte L, Nabatov A, Geijtenbeek TB. Distinct roles for DC-SIGN+-dendritic cells and Langerhans cells in HIV-1 transmission. Trends Mol Med. 2008;14:12–19. doi: 10.1016/j.molmed.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Hu J, Gardner MB, Miller CJ. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J Virol. 2000;74:6087–6095. doi: 10.1128/JVI.74.13.6087-6095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geijtenbeek TB, Kwon DS, Torensma R, van Vliet SJ, van Duijnhoven GC, Middel J, Cornelissen IL, Nottet HS, KewalRamani VN, Littman DR, Figdor CG, van Kooyk Y. DC-SIGN, a dendritic cell-specific HIV-1binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–597. doi: 10.1016/S0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- Schneider-Schaulies S, Klagge IM, ter Meulen V. Dendritic cells and measles virus infection. Curr Top Microbiol Immunol. 2003;276:77–101. doi: 10.1007/978-3-662-06508-2_4. [DOI] [PubMed] [Google Scholar]
- Servet-Delprat C, Vidalain PO, Valentin H, Rabourdin-Combe C. Measles virus and dendritic cell functions: how specific response cohabits with immunosuppression. Curr Top Microbiol Immunol. 2003;276:103–123. doi: 10.1007/978-3-662-06508-2_5. [DOI] [PubMed] [Google Scholar]
- Lechmann M, Berchtold S, Hauber J, Steinkasserer A. CD83 on dendritic cells: more than just a marker for matu ration. Trends Immunol. 2002;23:273–275. doi: 10.1016/S1471-4906(02)02214-7. [DOI] [PubMed] [Google Scholar]


