Abstract
Gastric cancer is the fourth most common cancer worldwide, with a high rate of death and low 5-year survival rate. To date, there is a lack of efficient therapeutic protocols for gastric cancer. Recent studies suggest that cancer stem cells (CSCs) are responsible for tumor initiation, invasion, metastasis, and resistance to anticancer therapies. Thus, therapies that target gastric CSCs are attractive. However, CSCs in human gastric adenocarcinoma (GAC) have not been described. Here, we identify CSCs in tumor tissues and peripheral blood from GAC patients. CSCs of human GAC (GCSCs) that are isolated from tumor tissues and peripheral blood of patients carried CD44 and CD54 surface markers, generated tumors that highly resemble the original human tumors when injected into immunodeficient mice, differentiated into gastric epithelial cells in vitro, and self-renewed in vivo and in vitro. Our findings suggest that effective therapeutic protocols must target GCSCs. The capture of GCSCs from the circulation of GAC patients also shows great potential for identification of a critical cell population potentially responsible for tumor metastasis, and provides an effective protocol for early diagnosis and longitudinal monitoring of gastric cancer.
Keywords: cancer stem cells, gastric adenocarcinoma, CD44, CD54, circulating tumor cells
Introduction
Gastric adenocarcinoma (GAC) originates from malignant stomach mucosa. The disease incidence increases with age, and is highly associated with diet, bile reflux, and helicobacter pylori infection 1, 2. GAC is the fourth most common cancer worldwide and has a high number of deaths per year 3. The 5-year survival rates are very low (< 20%), and patients frequently die due to metastasis 3. To date, surgery remains a key curative therapy for gastric cancer, with chemotherapy serving as an important adjuvant therapy 4, 5. However, a lack of efficient therapeutic protocols persists, and as such, new strategies for GAC treatment are critically important.
The concept of cancer stem cells (CSCs) may provide a new approach for gastric cancer therapies. CSCs are a small subpopulation of cells that can give rise to tumor mass 6, 7. CSCs can be viewed as the result of mis-differentiation and possess self-renewal and differentiation potential 8. Recent studies demonstrated that CSCs are responsible for tumor initiation, invasion, distant metastasis, and resistance to anticancer drugs, so therapies that target CSCs are becoming increasingly appealing 9. Currently, CSCs have been found in many types of solid tumors, such as breast cancer 10, glioblastoma 11, and colon cancer 12, 13. However, CSCs of gastric adenocarcinoma (GCSCs) in primary human tumor tissues and in the peripheral blood of GAC patients have not yet been described. Here, we report the identification of CSCs in human GAC tumor tissues and the peripheral blood of GAC patients, and show that these cells can be captured and expanded for further study.
Results
Tumorigenic spheres formed from gastric adenocarcinoma patient tumor tissues
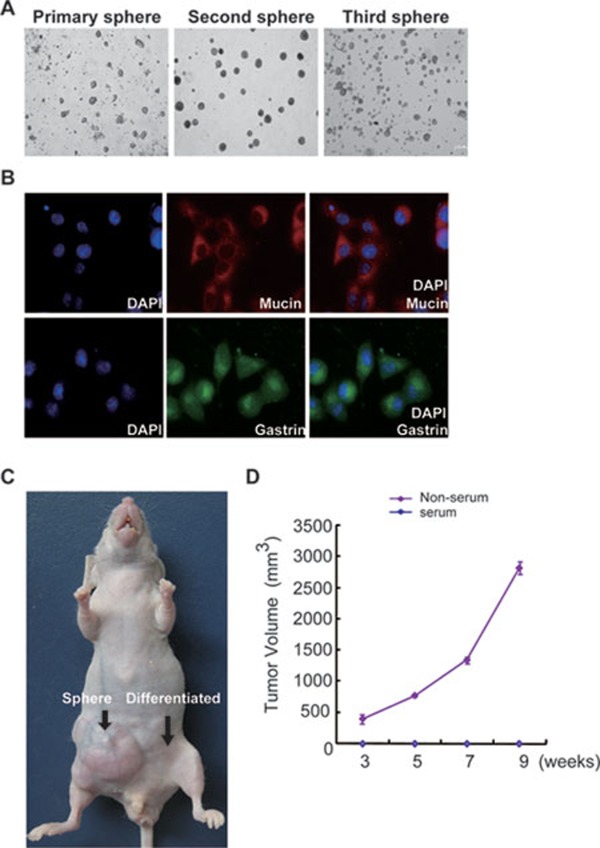
We first examined the immunophenotypes of tumor cells obtained from 18 GAC patients and found that they did not carry most known stem-cell-related markers. CSCs are believed to be able to form spheres in culture that possess extensive similarities to endogenous CSCs in human tumor tissues 13, 14, 15. Therefore, we cultured GAC cells to induce sphere formation and then examined the sphere cell surface proteins to determine the identity of GCSC markers. Isolated gastric cancer cells from human tumor tissues were cultured in a serum-free medium with EGF and FGF. After 3-4 weeks, some tumor cells grew to form spheres (Figure 1A). The tumor spheres were maintained in culture for at least 6 months and were passaged 12 times, indicating that the sphere cells were able to self-renew. The cells in the spheres at all passages were negative for gastric mucosa markers (Supplementary information, Figure S1). After addition of serum-containing media, these cells differentiated into gastric epithelial cells, as detected by specific antibodies (Figure 1B). Tumor spheres from the first three passages injected into immunodeficient mice formed tumors after 3 weeks, while sphere cells cultured in serum-containing media did not generate any detectable tumors in mice (Figure 1C and 1D). Thus, these data showed that cells in the tumor spheres are of gastric origin, have self-renewal capability in vitro, and are able to form tumors in mice, suggesting that GCSCs are enriched in tumor spheres.
Figure 1.
Tumor spheres derived from human gastric adenocarcinoma samples are of gastric origin, can self-renew, and can produce tumors in mice. (A) Example of tumor spheres generated from a human gastric adenocarcinoma sample that were passaged two times. Primary spheres: spheres directly generated from human tumor tissues. Second and third spheres: spheres represent the second and the third passages of primary spheres. (B) Spheres were cultured with serum and differentiated to gastric epithelial cells detected by specific antibodies against gastrin (green) and mucin (red) antigens. (C) Example showing that spheres cultured in serum-free media but not in serum-supplemented media produce tumors in mice. (D) Tumorigenic potential of tumor spheres after subcutaneous injection. Tumor volumes generated by spheres were derived from three independent experiments in duplicate, referring to those tumors in which 103 sphere cells were able to engraft.
Tumor spheres contain GAC stem cells
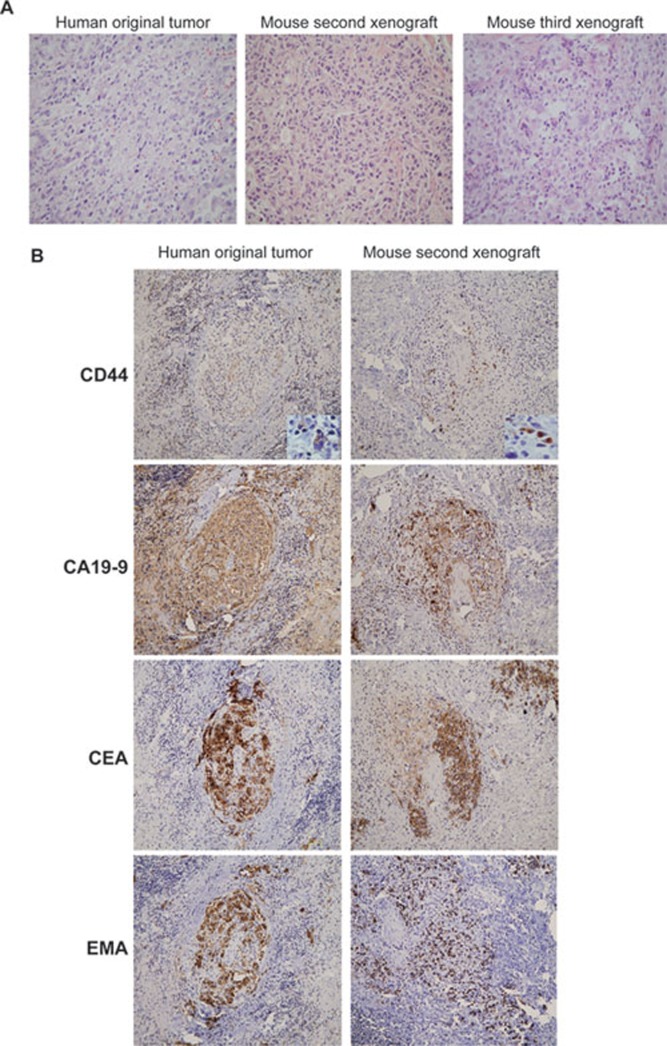
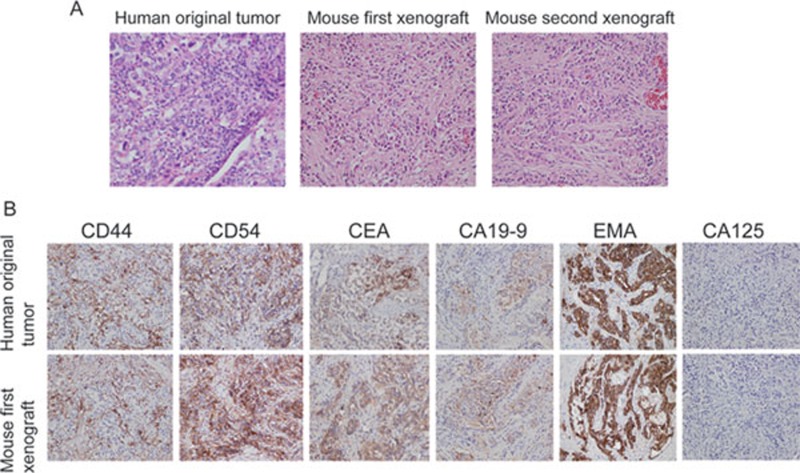
CSCs are characterized by their self-renewal in vivo 6, 7. Therefore, we performed serial transplantation in mice to test the self-renewal capacity of the sphere cells (Figure 2). The sphere cells were injected into immunodeficient mice subcutaneously. When the tumor diameter reached ∼1 cm, tumor mass was resected and dissociated to form tumor spheres in the culture for transplantation. Second and third transplantations were performed accordingly and showed that these cells also generated tumor mass in mice. These results demonstrate that the sphere cells possess self-renewal capability. Since another feature of CSCs is their ability to produce grafts in mice that are highly similar to the original human tumors 7, 9, we performed a pathology examination of murine xenografts. Hematoxylin & Eosin (H&E) and immunostaining analysis of original human GAC tumors and mouse xenografts showed that the first, second, and third xenografts were highly similar to the original human GAC tumors (Figure 2A and 2B, data not shown). Thus, our data demonstrate that sphere cells from GAC tissues are able to generate human GAC in mice and have self-renewal capability, indicating that the tumor cell population in the spheres contains GCSCs.
Figure 2.
Xenografts generated from tumor spheres highly resemble original human tumor tissues. (A) Hematoxylin & Eosin analysis with 40× original magnification of human gastric cancer sections from the original human tumor and corresponding xenografts obtained after injection of sphere cells. Second xenograft and third xenograft: tumors were obtained from the second and third transplantations, respectively. (B) Immunohistochemical analysis of the original human tumor and sphere-derived second xenografts. EMA: epithelial membrane antigen; CEA: carcinoma embryonic antigen. The inserts show the high-magnification images.
A single CD44+CD54+ cell can form gastric tumorigenic spheres
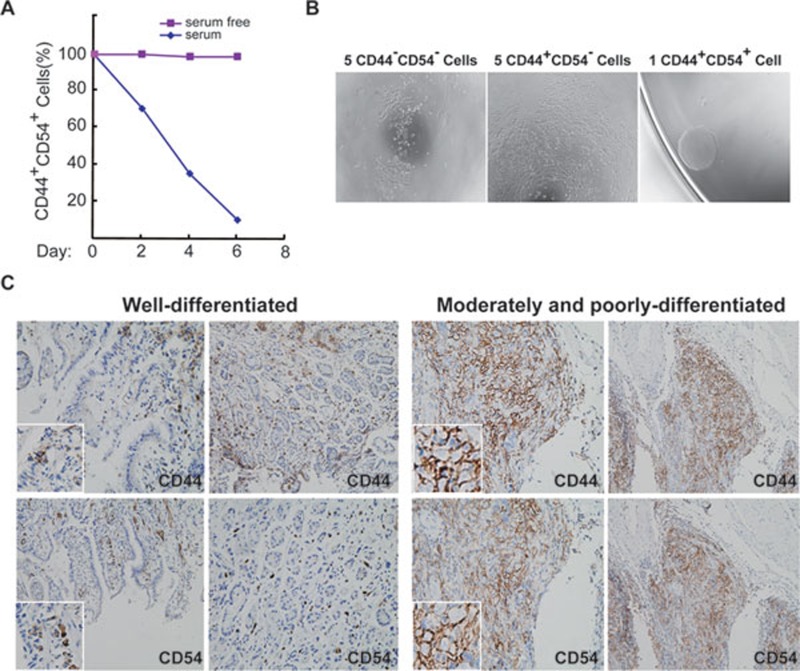
We next examined the cell surface markers of the tumor spheres. Two adhesion molecules, CD44 and CD54, were positively expressed by the majority of tumor sphere cells (Figure 3A, Supplementary information, Figure S2). Interestingly, the expression of both molecules decreased gradually after tumor spheres were cultured in a serum-containing medium (Figure 3A). Notably, high expression levels of CD10, CD29, and EGFR were also observed on the surface of many sphere cells (Supplementary information, Figure S2). Consistent with the result of the tumor tissues, most known stem-cell-related markers such as CD133, CD34, CD117, CD15, and especially Lgr5, which is associated with gastric stem cells in mice 16, were not detected. Next, we sorted the cell population by different surface markers expressed on the spheres and cultured the sorted cells. Five cells were seeded in each well of a 96-well plates and more than 300 wells were seeded for each cellular population. After 2 weeks, the CD44+CD54+ cells in each well formed spheres in a serum-free medium. These sphere cells were also able to generate tumors in mice. The other cellular populations, including CD44+CD54−, CD44−CD54+, and CD44−CD54− cells were unable to produce any spheres in culture and could not form tumors in mice (Figure 3B and data not shown). The same results were obtained from the tumor spheres derived from three tumor samples. Furthermore, a single CD44+CD54+ cell was sorted and formed spheres (Figure 3B) in serum-free media and subsequently generated tumors in mice. This single CD44+CD54+ tumor cell also had self-renewal ability in vivo, as tested by serial transplantation in mice. Taken together, these results indicate that the CD44+CD54+ subpopulation contains the GCSCs in the spheres derived from gastric cancers.
Figure 3.
CD44 and CD54 double-positive cells are detected in tumor spheres and original human tumor tissues. (A) Percentage of sphere cells carrying CD44 and CD54 antigen with and without serum in the culture media. (B) A single CD44 and CD54 double-positive cell generated one sphere in culture. (C) Immunohistochemical analysis revealed that CD44 and CD54 are expressed in original human tumors of different pathological grades. The inserts show the high-magnification images.
CD44+CD54+ gastric cancer stem cells in human GAC tissues
We next directly examined tumor cells from 18 human patients to confirm the presence of CD44+CD54+ gastric CSCs. CD44+CD54+ cells were identified and accounted for 0.1%-19.7% of the cells in the patient samples (Table 1). We also examined normal gastric mucosa and found that CD44+CD54+ cells were barely detectable. Immunohistochemical staining of normal tissues showed that CD44+ or CD54+ cells were sparingly distributed in the gastric mucosa. In contrast, poorly differentiated gastric cancer had a high frequency of CD44+ and CD54+ cells (Figure 3C). A few cells were CD44 or CD54 positive in a well-differentiated gastric cancer (Figure 3C). In these tumors, CD44 was also found to be expressed by mesenchymal cells of the tumor tissues. The CD44+CD54+ cells were sorted from the tumor tissues and injected into mice to test their capability to form xenografts or cultured to form tumor spheres in serum-free media (Table 1). Among the 18 samples, as few as 103 CD44+CD54+ cells derived from 12 samples were able to form tumors in mice and formed tumor spheres in vitro. The murine xenografts had self-renewal capacity, as tested by serial transplantation (Table 1). Up to 3 × 103 CD44+CD54− and CD44−CD54+ cells could also form tumor nodules in mice. In contrast, the injection of up to 1 × 104 CD44−CD54− cells failed to induce tumor formation (Supplementary information, Table S1). In addition, up to 2 × 106 of the total tumor cells from these samples were able to form tumors in mice (Table 1), suggesting that CD44+CD54+ cells are enriched by at least 103-fold for GCSCs from tumor tissues. Tumor cells sorted from the remaining six samples did not produce tumors in mice and did not form tumor spheres in culture (Table 1). Furthermore, 3 × 106 of the total tumor cells from these six samples were also unable to produce tumors in mice or spheres in culture. When the cell population expressing CD44 and CD54 in a single-cell suspensions of these six tumor samples was stained with DAPI, only a few living cells were observed (Supplementary information, Table S2), suggesting that tumor cells from these samples had no viable CD44+CD54+ GCSCs after in vitro procedures were performed to prepare the single-cell suspensions. In agreement with these findings, tissue slices from the same tumors transplanted subcutaneously were able to produce tumors in mice (Table 1). Thus, these data indicate that the CD44+CD54+ subpopulation from human GAC tissues contains GCSCs.
Table 1. Case description, sphere formation, and tumorigenic activity of CD44+ and CD54+ gastric cancer cells.
| Case | Sex/age | Site | Grade | CD44+CD54+ expression (%) | Sphere formation | Tumor formation |
Secondary tumor formation | ||
|---|---|---|---|---|---|---|---|---|---|
| CD44+CD54+ 1 | Slice (cm3) 2 | Total cell | |||||||
| 1 | F/71 | Pyloric | Moderate | 0.4 | No | 103 (0/3) | 0.05 (2/3) | 3 × 106 (0/3) | N/A |
| 2 | M/60 | Cardial | Moderate | 1.8 | Yes | 103 (3/3) | 0.06 (3/3) | 3 × 106 (2/3) | Yes |
| 3 | F/61 | Corpus | Moderate | 0.6 | No | 103 (0/3) | 0.07 (1/3) | 3 × 106 (0/3) | N/A |
| 4 | M/41 | Pyloric | Poor | 1.3 | Yes | 103 (3/3) | 0.05 (2/3) | 3 × 106 (2/3) | Yes |
| 5 | F/62 | Cardial | Moderate | 7.0 | Yes | 103 (3/3) | 0.05 (3/3) | 3 × 106 (3/3) | Yes |
| 6 | M/55 | Pyloric | Moderate | 0.2 | No | 103 (0/3) | 0.07 (2/3) | 3 × 106 (0/3) | N/A |
| 7 | M/53 | Cardial | Moderate with signet-ring cell | 0.8 | Yes | 103 (3/3) | 0.075 (2/3) | 3 × 106 (2/3) | Yes |
| 8 | M/62 | Corpus | Poor | 19.7 | Yes | 103 (3/3) | 0.06 (3/3) | 3 × 106 (1/3) | Yes |
| 9 | M/76 | Fundus | Moderate | 6.1 | Yes | 103 (3/3) | 0.07 (3/3) | 2 × 106 (3/3) | Yes |
| 10 | F/72 | Pyloric | Moderate | 1.2 | No | 103 (0/3) | 0.075 (3/3) | 3 × 106 (0/3) | N/A |
| 11 | F/59 | Pyloric | Moderate with signet-ring cell | 2.2 | Yes | 103 (3/3) | 0.068 (2/3) | 2 × 106 (2/3) | Yes |
| 12 | F/56 | Pyloric | Moderate with signet-ring cell | 1.5 | Yes | 103 (3/3) | 0.056 (3/3) | 2 × 106 (1/3) | Yes |
| 13 | M/58 | Cardial | Moderate | 0.1 | Yes | 103 (3/3) | 0.07 (3/3) | 2 × 106 (1/3) | Yes |
| 14 | M/55 | Cardial | Moderate | 0.1 | No | 103 (0/3) | 0.06 (2/3) | 3 × 106 (0/3) | N/A |
| 15 | M/77 | Angular notch | Moderate with signet-ring cell | 2.3 | Yes | 103 (3/3) | 0.05 (2/3) | 2 × 106 (2/3) | Yes |
| 16 | M/72 | Angular notch | Moderate | 0.2 | No | 103 (0/3) | 0.07 (3/3) | 3 × 106 (0/3) | N/A |
| 17 | M/41 | Pyloric | Moderate with signet-ring cell | 0.7 | Yes | 103 (3/3) | 0.075 (3/3) | 2 × 106 (2/3) | Yes |
| 18 | M/71 | Angular notch | Moderate with signet-ring cell | 0.6 | Yes | 103 (3/3) | 0.05 (2/3) | 2 × 106 (2/3) | Yes |
1Number of CD44+CD54+ cells injected into mice (number of mice that have tumor formation in three mice).
2Volume of tissue directly cut from tumor (number of mice that have tumor formation in three mice). N/A: not available.
Capture of cancer stem cells from peripheral blood of GAC patients
Tumor-derived cells have been reported in peripheral blood from cancer patients and are considered to be the origin of metastatic tumors 14. Current opinion also suggests that CSCs in the blood stream are the root of metastatic tumors 14. So far, strategies for isolating circulating tumor cells (CTCs) have been unable to identify CSCs in the circulation 17. Therefore, the identification of the GCSC markers prompted us to determine whether there are GCSCs in the peripheral blood of GAC patients. Analysis of CD44 and CD54 expression was performed in 10 normal humans, and FACS assays showed that CD44+ cells existed in all peripheral blood samples. In contrast, the CD44+CD54+ subpopulation was not detected in these blood samples (data not shown), indicating that the frequency of the CD44+CD54+ subpopulation, if present in normal peripheral blood, is too low to be detected. We then examined blood samples from 25 patients with gastric cancers and detected CD44+CD54+ epithelial-like cells in 24 samples (Tables 2 and 3, Supplementary information, Figure S3). The cells were sorted and verified by cytological examination (Figure 4A). Using the GCSCs markers, we performed serial dilution assays and were able to detect tumor cells in 107 blood cells (Supplementary information, Table S2).
Table 2. Case description and frequency of CD44+ and CD54+ gastric cancer cells in circulation.
| Case | Sex/age | Grade | Stage | Chemotherapy | Surgery | CD44+CD54+ expression (%)1 |
|---|---|---|---|---|---|---|
| 1 | M/38 | Poor | T3N1M0 | FOLFOX,CF | Yes | 3 |
| 2 | M/58 | Poor | T3N1M0 | Yes | 0.1 | |
| 3 | M/53 | Poor | T1N2M0 | FOLFOX | Yes | 12.9 |
| 4 | M/51 | Poor | T2N1M0 | Yes | 2.4 | |
| 5 | F/52 | Poor | T3N2M0 | FOLFOX | Yes | 1.7 |
| 6 | M/42 | Signet-ring cell | T2N1M0 | Yes | 1.3 | |
| 7 | M/56 | Moderate | T3N3M0 | Yes | 0.3 | |
| 8 | F/62 | Poor | T4aN1M0 | Yes | 0.2 | |
| 9 | F/36 | Poor | T1bN3M0 | Yes | 5 | |
| 10 | F/80 | Poor | T2N0M0 | Yes | 3.1 | |
| 11 | M/64 | Moderate to Poor | T4aN3M0 | Yes | 1.2 | |
| 12 | F/37 | N/A | IV | No | 0.4 | |
| 13 | M/79 | N/A | IV | No | 3.5 | |
| 14 | M/62 | Poor | T2N3M0 | Yes | 2.4 | |
| 15 | F/54 | Moderate | T2N0M0 | Yes | 0 | |
| 16 | F/33 | Poor | T4aN3M0 | Yes | 0.8 | |
| 17 | F/79 | Moderate | T4aN3M0 | Yes | 0.5 | |
| 18 | M/69 | Poor | T4aN1M0 | Yes | 4.2 |
1Ratio of CD44 and CD54 double-positive cells is derived from non-hematopoietic and non-endothelial cells in the peripheral blood of patients. Non-hematopoietic and non-endothelial cells were obtained by first depleting hematopoietic and endothelial cells by specific antibodies before FACS analysis and gating out the hematopoietic and endothelial-specific antibody labeling during FACS analysis.
Table 3. Case description and sphere formation and tumorigenic activity of CD44+ and CD54+ gastric cancer cells in circulation.
| Case | Sex/age | Grade | Stage | Metastasis | Chemotherapy/cycles | Surgery | CD44+CD54+ expression (%)1 | Sphere formation | Tumor formation2 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M/57 | Poor | TXNXM1 | Liver | Lapatinib (1) | No | 5.1 | Yes | 9 × 103 (3/3) |
| 2 | M/74 | Poor with signet-ring cell | T2N1M0 | No | FOLFOX (3) | Yes | 0.7 | Yes | 9 × 103 (3/3) |
| 3 | M/61 | Poor | TXNXM0 | No | FOLFOX (1) | Yes | 0.9 | Yes | 9 × 103 (3/3) |
| 4 | M/59 | Poor | T2N2M1 | Bone | DCF (2) | Yes | 2.1 | Yes | 9 × 103 (3/3) |
| 5 | M/66 | Poor | TXNXM1 | Liver | Mfolfox7 (4) | Yes | 0.9 | Yes | 9 × 103 (3/3) |
| 6 | M/59 | Poor with signet-ring cell | T2N1MO | No | FOLFOX (2) | Yes | 0.5 | No | No |
| 7 | M/62 | Poor with signet-ring cell | T2N1M0 | No | FOLFOX (2) | Yes | 0.4 | Yes | 9 × 103 (3/3) |
1Ratio of CD44 and CD54 double-positive cells is derived from non-hematopoietic and non-endothelial cells in the peripheral blood of patients. Non-hematopoietic and non-endothelial cells were obtained by first depleting hematopoietic and endothelial cells by specific antibodies before FACS analysis and gating out the hematopoietic and endothelial-specific antibody labeling during FACS analysis.
2Number of CD44+CD54+ cells from spheres injected into mice (number of mice which have tumor formation in three mice).
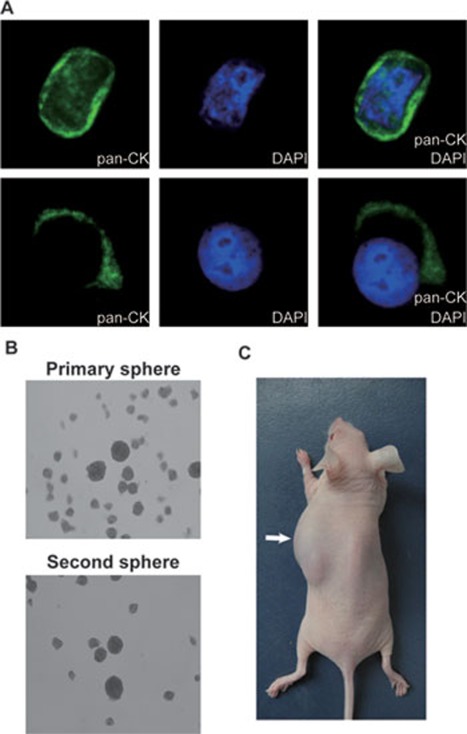
Figure 4.
Tumorigenic spheres are derived from the peripheral blood of gastric cancer patients. (A) CD44- and CD54-positive cells were sorted, stained with anti-pan-cytokeratin (PAN-CK) antibody and DAPI, and visualized by fluorescence microscopy. (B) Spheres were generated from fewer than 103 CD44-positive cells. Primary sphere: original spheres formed from blood cells. Second sphere: second passage of spheres. (C) An example of a mouse bearing a tumor (arrow) produced by blood-borne spheres.
CD44+CD54+ cells were sorted from the blood samples, but cell survival after sorting was low, possibly due to the low frequency of CD44+CD54+ epithelial-like cells and the long time needed for cell capture. Therefore, to maintain cell survival, a single CD44 antibody bound to magnetic beads was used to isolate CD44+ cells from non-hematopoietic and non-endothelial cells of the blood samples. Up to 103 CD44 positive non-hematopoietic and non-endothelial cells were sorted from 2-4 ml of blood from seven patients who were treated with one to four cycles of chemotherapy (Table 3). The cells obtained from six samples formed tumor spheres and were successfully passaged in the culture (Figure 4B, Table 3). The resulting CD44+CD54+ cells from the tumor spheres were injected into nude mice and quickly generated growing tumors that were highly similar to the original cancers from human stomach (Figures 4C, 5A and 5B, Table 3). Serial transplantation also demonstrated that the CD44+CD54+ cells carried self-renewal ability (Figure 5A and 5B). The remaining cellular population from the spheres had no ability to generate tumors in mice. These results indicate that the CD44+CD54+ subpopulation derived from the blood samples also displays CSC properties. After depleting CD44-positive cells, more than 106 non-hematopoietic and non-endothelial cells derived from patient blood samples were unable to form tumor spheres in culture and to generate tumors in mice. Taken together, our results indicate that the tumor cells in patient peripheral blood contain a GCSC population, and suggest that the GCSC markers are able to detect and capture GCSCs in the circulation of GAC patients.
Figure 5.
Xenografts generated from tumor spheres derived from blood samples highly resemble original human tumor tissues. (A) Hematoxylin & Eosin analysis with 40× original magnification of a human gastric cancer section from the original human tumor and corresponding xenografts obtained after injection of blood-borne sphere cells. First and second xenografts: tumors were obtained from the first and second transplantation, respectively. (B) Immunohistochemical analysis of original human tumor and sphere-derived first xenografts.
Discussion
In this study, we identified a CD44+CD54+ population from GAC tissues and the peripheral blood of GAC patients that possesses the capacity for sphere formation, self-renewal, gastric differentiation, and tumor initiation. The murine tumors formed by this CD44+CD54+ population were highly similar to the original human GAC tumors. All of these features are hallmarks of CSCs. In addition, we also showed that a single CD44+CD54+ tumor cell exhibits all of the CSC properties, whereas other cell populations in tumor tissues and the peripheral blood of patients did not have these properties. Thus, the CD44+CD54+ subpopulation in GAC tumor tissues and in peripheral blood from GAC patients contains GCSCs, and CD44 and CD54 are potential GCSC markers.
CD44 is a transmembrane glycoprotein that is well known as a CSC marker in several cancers, including breast, prostate, and colon cancer 18, 19. CD54 (also called intercellular adhesion molecule-1; ICAM-1) is a 90-kDa member of the immunoglobulin superfamily and is widely expressed in tumor, stromal, and immune cells 20. Both CD44 and CD54 are strongly associated with metastasis in gastric adenocarcinoma, and CD44 is positively and significantly associated with tumor recurrence and mortality in gastric cancer 21, 22. Here, we show that CD44+CD54+ cells exhibit CSC capabilities in GAC tissues, which is consistent with previous reports. In addition, we demonstrate that CD44+CD54+ cells from patient peripheral blood also display CSC features. CSCs are reportedly responsible for tumor initiation, invasion, and distant metastasis. Therefore, our data may explain previous observations that both CD44 and CD54 are associated with metastasis, tumor recurrence, and mortality in gastric cancer, and could be used as prognostic factors for gastric cancer and as cellular markers for disease surveillance and therapeutic response.
Cells expressing Lgr5 in the stomachs of mice have been reported to possess stem cell properties and the capacity for tumor initiation 16. However, we found no Lgr5 expression in gastric tumor cells from human samples. Instead, Lgr5 was stained in the stromal cells of tumor tissues. Therefore, differences in Lgr5 expression patterns may be species dependent.
Accumulated evidence suggests that CSCs may be a source for CTCs 23, 24, 25. For example, the subpopulation of CD45−CD90+ cells, which is a marker of liver CSCs, in peripheral blood from liver carcinoma patients showed tumor initiation capacity 17. In our study, we found that the CD44+CD54+ tumor cell subpopulation in peripheral blood from GAC patients is able to form tumor spheres in a serum-free medium, generates tumors where the histopathology resembles human GAC, and has self-renewal capability both in cell culture and in mouse models. These results indicate that the GCSCs are indeed in the circulation and support the hypothesis that the CTC population contains CSCs. Currently, CTCs are examined by RT-PCR, immunohistochemistry, immunofluorescence, and flow cytometry 14, 23, 26. However, these protocols have limitations in that they cannot directly confirm whether the examined CTCs are indeed cancer cells. Using our working protocol, CTCs with cancer characteristics can be defined. The identification of cancer-initiating cells in peripheral blood will allow for novel advances in therapeutic protocols for GAC, as well as prompt modifications of therapeutic strategies for patients according to their therapeutic response.
CSCs derived directly from human tumors by surface marker sorting and loading into NOD/SCID 7 or severe combined immune deficiency (SCID) mice 27, 28 have been used to establish tumor models reflecting the tumor features and development in the human body. However, the care of these immunocompromised mice is very difficult and expensive, which prevents the convenient development of models for tumor initiation by CSCs on a large scale. Traditionally, nude mice are used to establish tumor models for cell lines that are used on a large scale for cancer research and pharmaceutical screening. However, cell lines often do not accurately reflect the development and physiological properties of the original human tumors 15. Therefore, we tried to establish tumor models that do not require the care of SCID and nude mice to generate CSCs. We show here that our protocol is able to obtain sufficient CSCs from human cancers that can give rise to xenografts in both SCID and nude mice that reflect the original human tumors. This procedure can be used to obtain CSCs from original human tumor tissues and patient blood samples at a large scale for cancer research and pharmaceutical screening.
In this study, we demonstrated that tumorigenic stem cells of GACs are included in the rare CD44+- and CD54+-undifferentiated population that exists in tumor tissues and the peripheral blood of GAC patients. Our data indicate that a significant proportion of these cells is tumorigenic, which are in agreement with the CSC hypothesis that primary, invasive, and metastatic tumor mass are generated and maintained by a small subset of tumor cells that are able to self-renew and produce the bulk of cells in a tumor. In gastric cancer, the GCSCs seem to be a target of oncogenic transformation, invasion, and metastasis, similar to other types of cancer, such as leukemia 7, breast, colon, and brain cancer 29. Therefore, the molecular characterization of tumorigenic GCSCs is crucial in order to develop new therapeutic strategies.
Materials and Methods
Sample collection
Tumor tissues were obtained from patients who underwent gastrectomy for gastric tumors at the Department of Gastrointestinal Surgery, and blood samples were collected from patients who received chemotherapy at the Department of Oncology, West China Hospital, Sichuan University. Informed consent was obtained from all patients who provided samples and the relevant institutional Ethics Committees approved this study.
Cell culture
Tumor samples were subjected to mechanical and enzymatic dissociation. The resulting cancer cells were cultured in a serum-free medium supplemented with 20 ng/ml EGF and 10 ng/ml FGF-2. The tumor cells were subjected to DMEM medium containing 10% FBS for differentiation. To obtain primary tumor cell cultures, the cells were plated onto collagen-coated dishes in DMEM medium containing 10% FBS after enzymatic dissociation. Red blood cells were lysed from whole blood, to deplete the hematopoietic and endothelial cells, and the resulting cells were incubated with the following magnetic microbeads: CD31, CD45, CD19, CD56, CD64, CD4, CD8, and CD11b. Before flow-cytometry analysis, the PE-cy7-CD45 antibody was added to the samples again to deplete the hematopoietic cells.
Magnetic and cytofluorometric cell separation
For magnetic separation, cells were labeled 1-3 h after enzymatic dissociation with CD44 magnetic microbeads (Miltenyi Biotec) and separated according to the manufacturer's instructions. Alternatively, cells were labeled with CD44/CD54, CD45, CD31, CD19, CD56, CD65, CD4, CD8, CD11b, CD34, or CD117 antibodies (BD Biosciences) and sorted with a FACS Aria (BD Biosciences). After magnetic or cytofluorometric sorting, cell purity was evaluated by flow cytometry using CD44/CD54 antibodies (BD Biosciences).
Transplantation of cancer cells
Various unseparated and purified cell populations were injected subcutaneously into the flanks of SCID mice and nude mice as indicated. After 8-10 weeks, mice were sacrificed by cervical dislocation, tumors were removed, fixed in 10% neutral buffered formalin solution (Sigma), and were paraffin embedded.
Immunostaining
Immunostaining was performed on a formalin-fixed paraffin-embedded tissue, cell blocks, or frozen tissue. For immunohistochemistry, paraffin sections were subjected to antigen retrieval for 30 min at 95 °C and then dewaxed in xylene and rehydrated with distilled water. The slides were subsequently incubated with the following antibodies overnight at 4 °C: CD44, CEA, CA19-9, EMA, and CD54 (BD Transduction Laboratories). The reaction was performed using ABC systems (DakoCytomation) and DAB substrate chromogen (DakoCytomation) followed by hematoxylin counterstaining. For immunofluorescent staining, the cells were incubated with anti-gastrin and anti-mucin antibodies overnight at 4 °C. For sphere immunostaining, glass slides were coated with matrix gel, and then gastric tumor spheres were picked and cultured on the pre-coated glass slides with a serum-free medium containing EGF and FGF. After 1 day, the gastric tumor spheres were adhered to the glass slides and then examined by immunostaining.
Flow-cytometry analysis
Cells from tumor tissues, spheres, or blood samples were incubated on ice for 30 min with the following antibodies: anti-CD44, -EGFR, -CD31, -CD15, -beta-integrin, -CD184, -CD133, -CD29, -CD54, -CD34, -CD10, -CD117, -CD45, -CD19, -CD56, -CD65, -CD4, -CD8, and -CD11b. The cells were then washed with PBS and centrifuged for 5 min at 1 000 rpm. Samples were analyzed on a BD flow cytometer.
Acknowledgments
The relevant institutional Ethics Committees approved this study. We thank the participants and their families for their kind cooperation, generosity, and patience. This work was supported by the project of National Basic Research Program of China (2007CB947802, 2009CB941200).
Footnotes
(Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary Information
Spheres were cultured without serum and detected by specific antibodies against gastrin, mucin, CD44, or Ki67 antigens.
An example shows FACS analysis of immunopheotypes of the cells obtained from tumor tissues of human patients.
An example of FACS analysis of immunphenotypes of the cells obtained from tissue and blood samples of normal individuals and GAC patients.
Tumorigenic capacity of different cell subpopulations
Viability of both unsorted cells and sorted CD44+/CD54+ cells before injecting into mice
References
- Correa P, Piazuelo MB, Wilson KT. Pathology of gastric intestinal metaplasia: clinical implications. Am J Gastroenterol. 2010;105:493–498. doi: 10.1038/ajg.2009.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan FA, Shukla AN. Pathogenesis and treatment of gastric carcinoma: “an up-date with brief review”. J Cancer Res Ther. 2006;2:196–199. doi: 10.4103/0973-1482.29830. [DOI] [PubMed] [Google Scholar]
- Anderson WF, Camargo MC, Fraumeni JF, et al. Age-specific trends in incidence of noncardia gastric cancer in US adults. Jama-J Am Med Assoc. 2010;303:1723–1728. doi: 10.1001/jama.2010.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Hsu JS, Wu DC, et al. Gastric cancer: preoperative local staging with 3D multi-detector row CT – correlation with surgical and histopathologic results. Radiology. 2007;242:472–482. doi: 10.1148/radiol.2422051557. [DOI] [PubMed] [Google Scholar]
- House MG, Brennan MF. Neoadjuvant therapy for gastric cancer. Adv Surg. 2008;42:151–168. doi: 10.1016/j.yasu.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- Polyak K, Hahn WC. Roots and stems: stem cells in cancer. Nat Med. 2006;12:296–300. doi: 10.1038/nm1379. [DOI] [PubMed] [Google Scholar]
- Eaves CJ. Cancer stem cells: Here, there, everywhere. Nature. 2008;456:581–582. doi: 10.1038/456581a. [DOI] [PubMed] [Google Scholar]
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- Ricci-Vitiani L, Lombardi DG, Pilozzi E, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- Nagrath S, Sequist LV, Maheswaran S, et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450:1235–1239. doi: 10.1038/nature06385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Kotliarova S, Kotliarov Y, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Barker N, Huch M, Kujala P, et al. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6:25–36. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Yang ZF, Ho DW, Ng MN, et al. Significance of CD90(+) cancer stem cells in human liver cancer. Cancer Cell. 2008;13:153–166. doi: 10.1016/j.ccr.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Wielenga VJ, Smits R, Korinek V, et al. Expression of CD44 in Apc and Tcf mutant mice implies regulation by the WNT pathway. Am J Pathol. 1999;154:515–523. doi: 10.1016/S0002-9440(10)65297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrawala L, Calhoun T, Schneider-Broussard R, et al. Highly purified CD44(+) prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene. 2006;25:1696–1708. doi: 10.1038/sj.onc.1209327. [DOI] [PubMed] [Google Scholar]
- Lawson C, Wolf S. ICAM-1 signaling in endothelial cells. Pharmacol Rep. 2009;61:22–32. doi: 10.1016/s1734-1140(09)70004-0. [DOI] [PubMed] [Google Scholar]
- Mayer B, Jauch KW, Gunthert U, et al. De-novo expression of CD44 and survival in gastric cancer. Lancet. 1993;342:1019–1022. doi: 10.1016/0140-6736(93)92879-x. [DOI] [PubMed] [Google Scholar]
- Okayama H, Kumamoto K, Saitou K, et al. CD44v6, MMP-7 and nuclear Cdx2 are significant biomarkers for prediction of lymph node metastasis in primary gastric cancer. Oncol Rep. 2009;22:745–755. doi: 10.3892/or_00000496. [DOI] [PubMed] [Google Scholar]
- Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- Cristofanilli M. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. Semin Oncol. 2006;33(Suppl 9):S9–S14. doi: 10.1053/j.seminoncol.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Maheswaran S, Sequist LV, Nagrath S, et al. Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med. 2008;359:366–377. doi: 10.1056/NEJMoa0800668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stott SL, Lee RJ, Nagrath S, et al. Isolation and characterization of circulating tumor cells from patients with localized and metastatic prostate cancer. Sci Transl Med. 2010;2:25ra23. doi: 10.1126/scitranslmed.3000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana E, Shackleton M, Sabel MS, et al. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–598. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatton T, Murphy GF, Frank NY, et al. Identification of cells initiating human melanomas. Nature. 2008;451:345–349. doi: 10.1038/nature06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Spheres were cultured without serum and detected by specific antibodies against gastrin, mucin, CD44, or Ki67 antigens.
An example shows FACS analysis of immunopheotypes of the cells obtained from tumor tissues of human patients.
An example of FACS analysis of immunphenotypes of the cells obtained from tissue and blood samples of normal individuals and GAC patients.
Tumorigenic capacity of different cell subpopulations
Viability of both unsorted cells and sorted CD44+/CD54+ cells before injecting into mice