Abstract
The Wnt/β-catenin pathway plays important roles in the differentiation of multiple cell types, including mesenchymal stem cells. Using a cell-based chemical screening assay with a synthetic chemical library of 270 000 compounds, we identified the compound SKL2001 as a novel agonist of the Wnt/β-catenin pathway and uncovered its molecular mechanism of action. SKL2001 upregulated β-catenin responsive transcription by increasing the intracellular β-catenin protein level and inhibited the phosphorylation of β-catenin at residues Ser33/37/Thr41 and Ser45, which would mark it for proteasomal degradation, without affecting CK1 and GSK-3β enzyme activities. Biochemical analysis revealed that SKL2001 disrupted the Axin/β-catenin interaction, which is a critical step for CK1- and GSK-3β-mediated phosphorylation of β-catenin at Ser33/37/Thr41 and Ser45. The treatment of mesenchymal stem cells with SKL2001 promoted osteoblastogenesis and suppressed adipocyte differentiation, both of which were accompanied by the activation of Wnt/β-catenin pathway. Our findings provide a new strategy to regulate mesenchymal stem cell differentiation by modulation of the Wnt/β-catenin pathway.
Keywords: Wnt pathway, Axin/β-catenin complex, small molecule, mesenchymal stem cell
Introduction
The Wnt/β-catenin pathway plays an important role in cell proliferation, morphology, motility, fate determination, axis formation, and organ development 1, 2, 3. The level of intracellular β-catenin, which is a key regulator of the Wnt/β-catenin pathway, is controlled by the proteasomal degradation pathway in a phosphorylation-dependent manner. Casein kinase 1 (CK1) and glycogen synthase kinase-3β (GSK-3β) sequentially catalyze β-catenin phosphorylation at residues Ser45, Thr 41, Ser37, and Ser33 in a complex with adenomatous polyposis coli (APC) and Axin 4, 5. Phosphorylated β-catenin is then recognized by the F-box β-transducin repeat-containing protein (β-TrCP), a component of the ubiquitin ligase complex, leading to its ubiquitin-dependent degradation by the proteasome 6, 7. Upon Wnt stimulation, Wnt, the Frizzled (Fz) receptor, and the low-density lipoprotein receptor-related protein5/6 (LRP5/6) co-receptor form a complex. The recruitment of Dishevelled (Dvl) to Fz leads to LRP5/6 phosphorylation, which induces the association of the Axin complex with phosphorylated LRP5/6, thereby inhibiting Axin-mediated β-catenin phosphorylation and stabilizing intracellular β-catenin 8.
Multipotent mesenchymal stem cells are able to differentiate into osteoblasts, adipocytes, chondrocytes, and myoblasts 9, 10. The differentiation of mesenchymal stem cells is regulated by interaction with specific extracellular mediators. Recent studies have implicated Wnt signaling in the commitment of mesenchymal stem cells to their various lineages. For example, the Wnt/β-catenin pathway promotes osteogenesis by stimulating Runx2 gene expression 11 and represses adipogenic differentiation of mesenchymal stem cells through the downregulation of adipogenic factors 12, 13. Wnt signaling also suppresses the induction of Sox9, a key regulator of chondrogenesis, which results in the inhibition of mesenchymal stem cell differentiation to chondrocytes 14, 15. In addition, Wnt signaling upregulates two key regulators of myogenesis, MyoD and Myf 5, in the induction of myogenic differentiation 16.
In this study, we used a forward chemical genetic approach to identify a small molecule capable of activating the Wnt/β-catenin pathway. SKL2001 stabilizes intracellular β-catenin via disruption of the Axin/β-catenin interaction, and can be applied to regulate mesenchymal stem cell differentiation.
Results
Identification of a small-molecule activator of the Wnt/β-catenin pathway
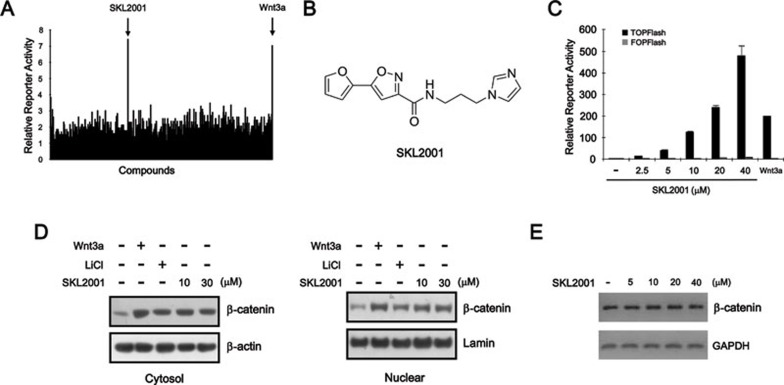
To screen for small molecules that modulate the Wnt/β-catenin pathway, we generated HEK293 reporter cells that were stably transfected with hFZ-1 expression plasmid and TOPflash, a synthetic β-catenin/Tcf-dependent luciferase reporter 17. Screening a small molecule library containing ∼270 000 compounds using these HEK293 reporter cells revealed that SKL2001 (5-furan-2yl-isoxazole-3-carboxylic acid (3-imidazol-1yl-propyl)-amide) strongly activated β-catenin responsive transcription (CRT) (Figure 1A and 1B). SKL2001 upregulated TOPflash reporter activity in a dose-dependent manner, whereas it did not affect the activity of FOPFlash, a negative control reporter with mutated β-catenin/Tcf-binding elements, in HEK293 control cells (Figure 1C). SKL2001 did not affect either NF-κB or p53 reporter activity (Supplementary information, Figure S1). In addition, SKL2001 upregulated the expression of Axin2, which is a downstream target of the Wnt/β-catenin pathway (Supplementary information, Figure S2). These results indicate that SKL2001 is a specific activator of the Wnt/β-catenin pathway.
Figure 1.
Identification of SKL2001 as a small-molecule activator of Wnt/β-catenin signaling. (A) Screening of compounds that inhibit Wnt/β-catenin signaling. (B) Chemical structure of SKL2001. (C) Dose-dependent response for CRT activation with increasing concentrations of SKL2001. HEK293 reporter and control cells were incubated with the indicated concentrations of SKL2001 for 15 h and luciferase activity was determined. The results are shown as the average of three experiments; the bars indicate standard deviations. (D) Cytosolic and nuclear proteins were prepared from HEK293 reporter cells treated with either vehicle (DMSO), Wnt3a CM, LiCl (20 mM) or SKL2001 (10 and 30 μM) and were then subjected to western blotting with anti-β-catenin antibody. The blots were reprobed with anti-actin antibody as a loading control. (E) Semi-quantitative RT-PCR for β-catenin, and GAPDH was performed with total RNA prepared from HEK293 reporter cells treated with the vehicle (DMSO) or indicated concentrations of SKL2001 for 15 h.
We next investigated the effect of SKL2001 on the level of intracellular β-catenin by western blotting using anti-β-catenin antibodies. Consistent with previous data 18, 19, the β-catenin level was increased by incubation with Wnt3a-conditioned medium (Wnt3a-CM) or LiCl, a GSK-3β inhibitor (Figure 1D). Interestingly, treatment with SKL2001 resulted in an increase in β-catenin in both the cytosolic and nuclear fractions, consistent with its effect on CRT (Figure 1D). However, the mRNA level of β-catenin was unchanged in response to various concentrations of SKL2001 (Figure 1E). These results suggest that SKL2001 activates Wnt/β-catenin signaling by increasing the stability of the β-catenin protein.
SKL2001 inhibits β-catenin phosphorylation without affecting GSK-3β activity
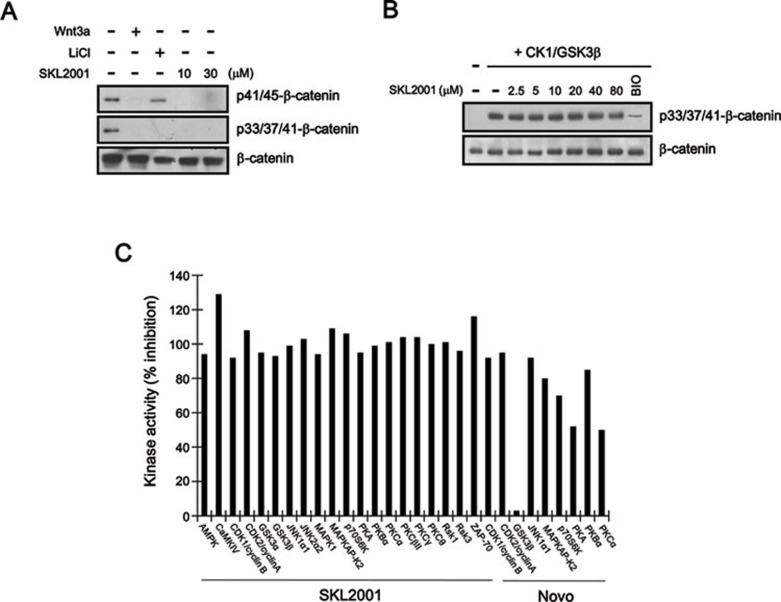
Previous studies have suggested that the phosphorylation of β-catenin at specific N-terminal Ser/Thr residues and its subsequent interaction with β-TrCP result in the degradation of β-catenin 20. Therefore, we examined the change in the phosphorylation status at these residues in response to SKL2001 treatment in HEK293 cells. In agreement with previous data 21, western blot analysis with phospho-specific anti-β-catenin antibodies showed that Wnt3a-CM and LiCl inhibited the phosphorylation of β-catenin at the Ser33/37/Thr41 residues (Figure 2A). Treatment with SKL2001 also inhibited Ser33/37/Thr41 phosphorylation (Figure 2A) without affecting the phosphorylation of GSK-3β at Ser9 (Supplementary information, Figure S3), indicating that this inhibition of β-catenin phosphorylation may be involved in the SKL2001-mediated β-catenin stabilization. Interestingly, Wnt3a-CM and SKL2001 prevented the phosphorylation of β-catenin at residues Thr41 and Ser45, whereas LiCl did not affect the phosphorylation of these residues (Figure 2A). Because the N-terminal Ser/Thr residues of β-catenin are phosphorylated by GSK-3β, we examined the in vitro effect of SKL2001 on β-catenin phosphorylation by this kinase, using purified β-catenin, CK1, and GSK-3β. As shown in Figure 2B, SKL2001 did not affect GSK-3β-mediated β-catenin phosphorylation in vitro; in contrast, 6-bromoindirubin-3′-oxim (BIO), an inhibitor of GSK-3β 22, potently prevented the phosphorylation of β-catenin by GSK-3β. In addition, when SKL2001 was assayed at 10 μM against a panel of recombinant kinases, it did not inhibit the activity of any tested kinase, including GSK-3β (Figure 2C). Under these conditions, 1-(4-aminofurazan-3-yl)-5-dialkylaminomethyl-1H-[1,2,3]triazole-4-carboxylic acid (Novo), which is an ATP-competitive inhibitor of GSK-3β 23, selectively inhibited GSK-3β (Figure 2C). Together, these results indicate that SKL2001 inhibits β-catenin phosphorylation without affecting GSK-3β activity.
Figure 2.
SKL2001 inhibits N-terminal β-catenin phosphorylation without affecting GSK-3β activity. (A) HEK293 reporter cells were incubated with either vehicle (DMSO), Wnt3a CM, LiCl (20 mM) or SKL2001 (10 and 30 μM). Cytosolic fractions were prepared and subjected to western blot analysis with anti-phospho-p45/41-β-catenin, anti-phospho-p33/37/41-β-catenin or anti-β-catenin antibody. The same amount of β-catenin was loaded in each lane. (B) SKL2001 does not affect GSK-3β-mediated β-catenin phosphorylation. GST-β-catenin (100 ng) was incubated with purified GSK3β and CK1 at the indicated concentrations of SKL2001 or BIO (5 μM). The samples were analyzed by western blotting with anti-phospho-p33/37/41-β-catenin antibody. The blot was reprobed with anti-β-catenin antibody as a loading control. (C) Recombinant kinase activity at 10 μM of SKL2001 or Novo. Kinase assays were performed as described in Materials and Methods.
SKL2001 disrupts the Axin/β-catenin interaction
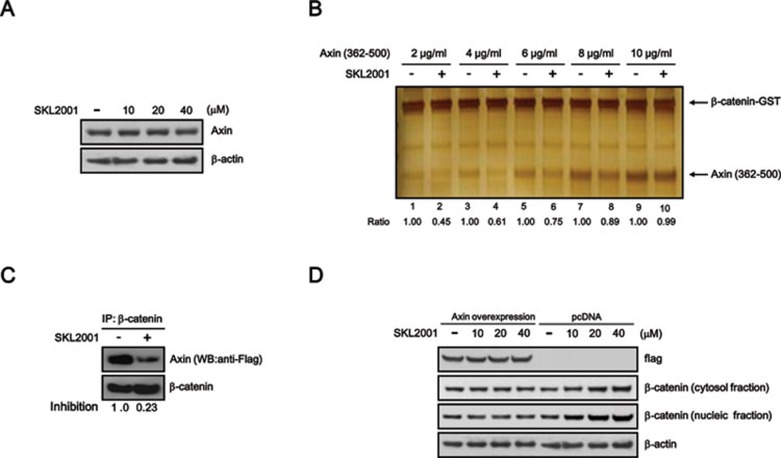
Axin regulates GSK-3β-mediated β-catenin phosphorylation through the interaction with CK1/GSK-3β and β-catenin 24. To further investigate the mechanism of activation of the Wnt/β-catenin pathway by SKL2001, we first tested the effect of SKL2001 on the level of Axin. As shown in Figure 3A, the expression of Axin was unchanged by any of the concentrations of SKL2001 used (Figure 3A). We next examined whether SKL2001 inhibits the interaction of β-catenin with Axin. Using purified fragment of Axin (362-500) and β-catenin, we demonstrated that the Axin/β-catenin interaction was disrupted by treatment with SKL2001 (Figure 3B, lanes 1 and 2); however, this effect was abolished with increased amounts of Axin (Figure 3B, lanes 3 to 10). We also performed immunoprecipitation assays in HEK293 cells to further confirm that SKL2001 inhibits the interaction of Axin and β-catenin. As shown in Figure 3C, the coimmunoprecipitation of β-catenin with Axin was prevented by SKL2001. In accordance with our in vitro data, SKL2001-mediated CRT activation and β-catenin stabilization were abrogated by overexpression of Axin in HEK293 reporter cells (Figure 3D and Supplementary information, Figure S4A). Notably, SKL2001 was not able to activate CRT in Axin null SNU475 cells (Supplementary information, Figure S4B). Moreover, when we depleted the endogenous β-catenin using siRNA, SKL2001 was unable to activate the Wnt/β-catenin pathway (Supplementary information, Figure S5).
Figure 3.
SKL2001 disrupts the Axin/β-catenin interaction. (A) The effect of SKL2001 on Axin expression. Cytosolic proteins were prepared from HEK293 reporter cells treated with either vehicle (DMSO) or the indicated concentrations of SKL2001 and were then subjected to western blotting with anti-Axin and anti-β-catenin antibodies. (B) SKL2001 competes with Axin for binding to β-catenin. The indicated amounts of Axin fragment (362-500) were added to GST-β-catenin (200 ng) with a constant amount of SKL2001 (20 μM) and then pull-down with glutathione-sepharose bead. Unbound proteins were washed away, and the complexes were visualized by silver staining. (C) HEK293 cells were co-transfected with Axin-flag (6 μg) and wild-type β-catenin (18 μg) and then incubated with SKL2001 (40 μM) for 15 h. Whole-cell extracts were immunoprecipitated with the anti-β-catenin antibody. Normal IgG was used as a negative control. The proteins in the β-catenin complex were analyzed by western blotting with anti-β-catenin and anti-flag antibodies. (D) HEK293 cells were transfected with Axin-flag (24 μg) and then incubated with the indicated concentration of SKL2001. Cytosolic proteins were subjected to western blotting with anti-flag and anti-β-catenin antibodies. In A and D, the blots were reprobed with anti-actin antibody as a loading control.
To examine whether intracellular β-catenin is specifically stabilized by SKL2001, we synthesized an inactive derivative of SKL2001 called SKL11324 (Supplementary information, Figure S6A). As shown in the Supplementary information (Figure S6B and S6C), SKL11324 neither disrupted Axin/β-catenin interaction nor activated the Wnt/β-catenin pathway. Taken together, these results suggest that the disruption of the Axin/β-catenin interaction is the molecular basis for SKL2001-mediated β-catenin stabilization.
SKL2001 induces osteoblast differentiation
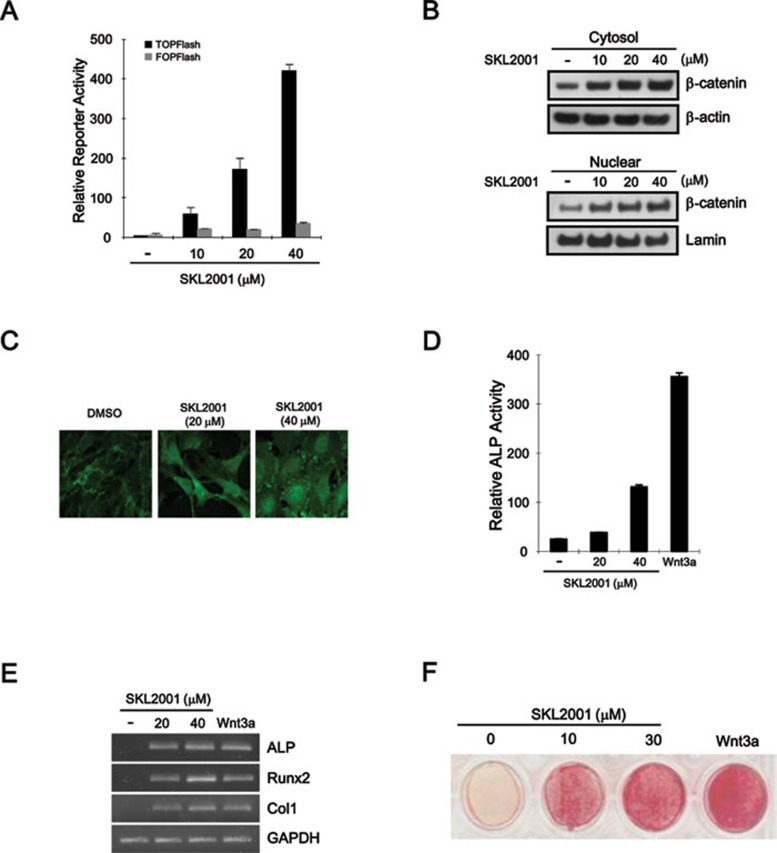
Activation of the Wnt/β-catenin pathway, followed by an accumulation of intracellular β-catenin, increases bone mass via the stimulation of osteoblastogenesis 25, 26. Given that SKL2001 stimulates the Wnt/β-catenin pathway, we hypothesized that SKL2001 would also promote the differentiation of multipotent mesenchymal stem cells to osteoblasts. To explore this hypothesis, we first examined whether multipotent mesenchymal ST2 cells are capable of activating the Wnt/β-catenin pathway when treated with SKL2001. As expected, ST2 cells treated with SKL2001 showed a robust, concentration-dependent increase in TOPflash reporter activity, without a substantial change in FOPflash reporter activity (Figure 4A). In addition, the intracellular β-catenin level, which is an indicator of the activation status of the Wnt/β-catenin pathway, also increased after incubation with SKL2001 in ST2 cells (Figure 4B and 4C). These results suggest that Wnt/β-catenin signaling is stimulated in response to SKL2001 in ST2 cells. We next examined whether SKL2001 promotes osteoblast differentiation. The incubation of ST2 and human primary mesenchymal stem cells with SKL2001 led to a dose-dependent rise in alkaline phosphatase (ALP) activity, which is an indicator of osteoblastogenesis (Figure 4D and Supplementary information, Figure S7). We also analyzed the mRNA expression of established osteoblast markers in ST2 cells treated with SKL2001 by semi-quantitative reverse transcriptase-PCR. Consistent with the increase in ALP activity, the mRNA expression of ALP was markedly elevated in response to SKL2001 (Figure 4E). Moreover, SKL2001 induced the mRNA expression of other osteoblast markers such as type I collagen and Runx2 (Figure 4E), suggesting that SKL2001 induces osteoblastogenesis by activating the Wnt/β-catenin pathway. The stimulatory effect of SKL2001 on osteoblastogenesis was further supported by the observation that mineralization, as assessed by Alizarin red staining, was increased in ST2 cells treated with SKL2001 (Figure 4F).
Figure 4.
SKL2001 induces osteoblast differentiation through the activation of the Wnt/β-catenin pathway. (A) Dose-dependent response of CRT activation with increasing concentrations of SKL2001. ST2 cells were co-transfected with TOPFlash or FOPflash and pCMV-RL plasmids and incubated with the indicated concentrations of SKL2001 for 15 h. Luciferase activity was measured 39 h after transfection. (B) Cytosolic and nuclear proteins were prepared from ST2 cells treated with either vehicle (DMSO) or indicated concentrations of SKL2001 and were then subjected to western blotting with anti-β-catenin antibody. (C) Immunofluorescence analysis of ST2 cells incubated with either vehicle (DMSO) or SKL2001 (20 and 40 μM). After fixation, the cells were stained with anti-β-catenin antibody and observed at 400× magnification. (D) ST2 cells were treated with either vehicle (DMSO) or the indicated concentrations of SKL2001. ALP activity was measured in cell lysates 72 h after treatment and normalized to the protein content. (E) SKL2001 upregulates the expression of osteoblast marker genes. Semi-quantitative RT-PCR for ALP, Runx2, type1 collagen (Coll1), and GAPDH was performed with total RNA prepared from ST2 cells treated with the vehicle (DMSO) or the indicated concentrations of SKL2001 for 72 h. (F) ST2 cells were treated with SKL2001 at the indicated concentrations for 10 days, and then cells were stained with Alizarin red.
SKL2001 suppresses preadipocyte differentiation
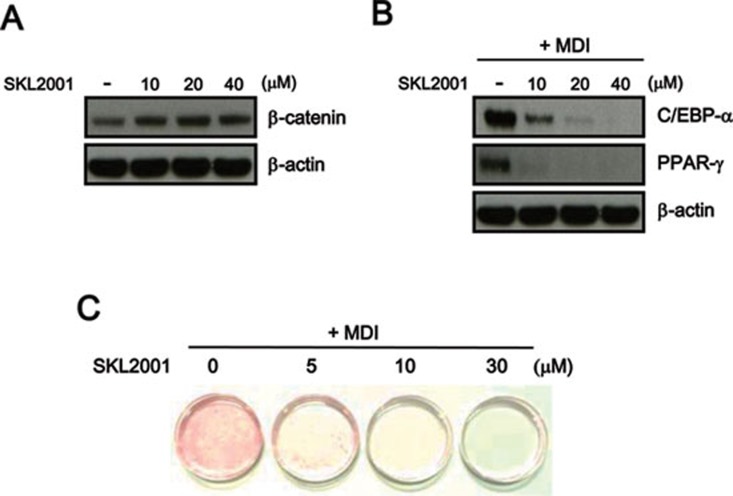
Wnt signaling represses preadipocyte differentiation, the alternative mesenchymal differentiation pathway, which is accompanied by the downregulation of master adipogenic transcription factors, such as C/EBPα and PPARγ 12, 13. Therefore, we examined whether the activation of Wnt/β-catenin signaling by SKL2001 suppressed adipogenic differentiation. SKL2001 consistently induced the accumulation of intracellular β-catenin (Figure 5A) and inhibited the expression of C/EBPα and PPARγ, which was upregulated by incubation with dexamethasone and insulin (MDI) in 3T3-L1 preadipocytes (Figure 5B). The exposure of 3T3-L1 and ST2 cells to SKL2001 resulted in a concentration-dependent decrease in lipid droplet accumulation in response to MDI as visualized by Oil Red O staining (Figure 5C and Supplementary information, Figure S8); these results are similar to the cellular response to the activation of Wnt/β-catenin signaling.
Figure 5.
SKL2001 stabilizes intracellular β-catenin in 3T3-L1 cells. (A, B) SKL2001 (5, 10, and 30 μM) was administered to cells during adipocyte differentiation for 3 days. The total proteins were prepared and subjected to western blotting with anti-β-catenin, anti-PPARγ and anti-C/EBPα antibodies. The blots were re-probed with anti-actin antibody as a loading control. (C) Increasing amounts of SKL2001 (5, 10, 30 μM) were administered to cells during adipocyte differentiation. One week after the induction of differentiation, adipocytes were stained with Oil Red O and photographed.
Discussion
The Wnt/β-catenin pathway is involved in the development and maintenance of many organs and tissues, including the bone 27, 28. The function of β-catenin, a key effector of the Wnt/β-catenin pathway, is determined by its N-terminal phosphorylation state, specifically residues Ser33/37. The phosphorylation of these residues is a prerequisite for the subsequent ubiquitination and degradation of β-catenin. In this study, a screen of 270 000 compounds produced one novel compound, SKL2001, which specifically activated β-CRT in a dose-dependent manner. SKL2001 inhibited the phosphorylation of β-catenin at residues Ser33/37/Thr41/Ser45, leading to an increased level of intracellular β-catenin. Several small molecules have been reported to induce the accumulation of intracellular β-catenin by inhibiting GSK-3β activity 22, 29, 30. Because GSK-3β is involved in multiple signaling pathways other than Wnt/β-catenin signaling, GSK-3β inhibitors are less than ideal drugs, as they could have unexpected effects on the cell and/or model organisms. In addition, although pyrimidine analogs have been identified as agonist of the Wnt/β-catenin pathway, their molecular targets and mechanisms of action are still unknown 31. In our study, SKL2001 did not affect GSK-3β activity in regard to β-catenin phosphorylation at Ser33/37/Thr41 residues in vitro, suggesting that a novel mechanism, not dependent on the inhibition of GSK-3β activity, may mediate the inhibition of β-catenin phosphorylation by SKL2001 in cells.
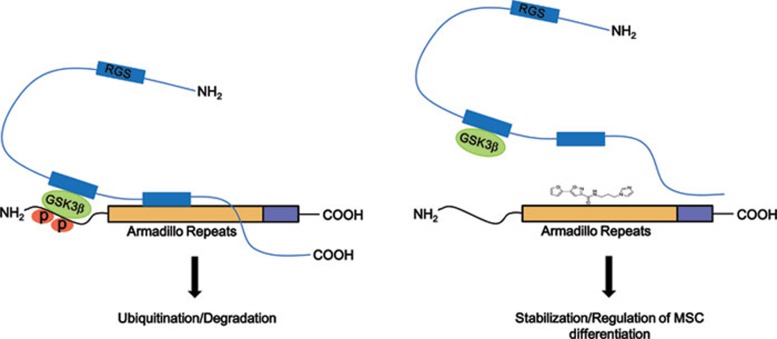
The scaffolding protein Axin interacts with GSK-3β, CK1, and β-catenin through separate domains and coordinates sequential phosphorylation of β-catenin at Ser45 by CK1 and then at Ser33/37/Thr41 by GSK-3β 32. The interaction of various Wnts (Wnt1, Wnt3a, and Wnt8) with their receptors (Fz) and co-receptors (LRP/5/6) triggers the recruitment of Axin to LRP6, promoting the dissociation of β-catenin from Axin and thereby inhibiting β-catenin phosphorylation and its subsequent degradation 33, 34. Biochemical and structural studies have demonstrated that Axin binds to β-catenin at a site on armadillo repeats 3-5 and that Phe253 and Lys292 of β-catenin contribute to this interaction 35, 36, 37. In our study, just as in the case of Wnt-mediated Axin inactivation, SKL2001 inhibited both the CK1- and GSK-3β-mediated phosphorylation of β-catenin at Ser45 and Ser33/37/Thr41, respectively, in HEK293 cells. Biochemical studies demonstrated that SKL2001 blocked the Axin/β-catenin interaction and competed with Axin for binding to β-catenin. Interestingly, molecular modeling predicted that SKL2001 binds to β-catenin at the same site as Axin via interaction with β-catenin residues Phe253 and Lys292 (Supplementary information, Figure S9 and Supplementary information method). Based on these results, we propose that SKL2001 selectively disrupts the Axin/β-catenin interaction, thereby inhibiting the CK1/GSK-3β-mediated phosphorylation of β-catenin at Ser33/37/Thr41 and Ser45. This unphosphorylated β-catenin is not targeted for degradation and accumulates in the cytoplasm, finally resulting in the activation of its downstream target genes (Figure 6).
Figure 6.
Proposed model for the disruption of the Axin/β-catenin interaction by SKL2001. β-catenin is phosphorylated by CK1/GSK-3β when these proteins are in a complex with Axin. It is then degraded by the ubiquitin-dependent proteasomal pathway (left panel). SKL2001 promotes the dissociation of β-catenin from Axin, thereby stabilizing the protein through prevention of its phosphorylation with the end result of altering the regulation of mesenchymal stem cell differentiation.
Previous studies implicate the Wnt/β-catenin pathway in the regulation of mesenchymal stem cell fate 38. Transgenic mice expressing Wnt10b under the control of the adipose-specific fatty acid binding protein-4 (FABP4) promoter (FABP-Wnt10b) show a 50% decline in total body fat and are resistant to high-fat-diet-induced white adipose tissue accumulation 39. In addition to reduced adiposity, FABP-Wnt10b mice exhibit an increase in bone mass 40. Other evidence for a role of the Wnt/β-catenin pathway in regulating mesenchymal stem cell differentiation is illustrated by the genetic deletion of the Wnt antagonist Sfrp1, which leads to a 20% reduction in body fat concomitant with increased bone mass 41. Furthermore, treatments with pharmacological inhibitors of GSK-3β stabilize intracellular β-catenin, thereby stimulating osteoblastogenesis and blocking adipocyte differentiation 42, 43. In the present study, disruption of the interaction of Axin and β-catenin by a small molecule, rather than inhibition of GSK-3β activity, promoted mesenchymal precursors to differentiate into osteoblasts and restrained adipocyte differentiation, mimicking the activation of the Wnt/β-catenin pathway.
In summary, we used high-throughput screening to identify a novel synthetic agonist of the Wnt/β-catenin pathway, which regulates the differentiation of mesenchymal stem cells. SKL2001 prevented the formation of the Axin/β-catenin complex, leading to inhibition of β-catenin phosphorylation/degradation. Because the Axin/β-catenin interaction is a key control point of the Wnt/β-catenin pathway, which plays important roles in cellular differentiation and development 27, 28, SKL2001 can be used as a controllable reagent for investigating biological processes that involve this pathway, such as cell differentiation, stem cell renewal, and tissue regeneration. In addition, SKL2001 represents a promising candidate in the development of therapeutics for diseases associated with the abnormal regulation of the Wnt/β-catenin pathway.
Materials and Methods
Cell Culture, transfection, luciferase assay, alkaline phosphatase assay, alizarin red staining and oil-red O staining
HEK293, ST2, 3T3L1 and Wnt3a-secreting L cells were obtained from the American Type Culture Collection and human mesenchymal stem cells were purchased from Cambrex Bio Science. The cells were maintained in Dulbecco's modified eagle medium supplemented with 10% fetal bovine serum, 120 μg/ml penicillin, and 200 μg/ml streptomycin. Wnt3a CM was prepared as previously described 44. Transfection was carried out with Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Luciferase assays were performed using the Dual luciferase assay kit (Promega). ALP activity was determined in cell lysates with the Alkaline Phosphatase Opt Kit (Roche Meolecular Biochemicals). Mineralization of ST-2 cells was carried out by staining with 1% Alizarin red after fixation in 50% ethanol as previously described 45. 3T3L1 preadipocytes were rinsed in PBS and fixed in 3.7% paraformaldehyde for 1 h at 4 °C. Briefly, plates were stained with 1% Oil Red O in 60% isopropanol for 10 min. The stain was then differentiated with 60% isopropanol and plates were washed several times in distilled water prior to visualization under a phase-contrast microscope.
Screening for small-molecule activators of Wnt/β-catenin signaling
The HEK293 reporter and control cell lines were established as previously described 17. The HEK293 reporter cells were inoculated into 384-well plates at 10 000 cells per well and grown for 24 h. Next, each compound in the chemical library (∼270 000) was added to at a final concentration of 20 μM. After 15 h, the plates were assayed for firefly luciferase activity.
Plasmid and recombinant proteins
The pTOPflash and pFOPflash reporter plasmid was obtained from Upstate Biotechnology. pGEX-4T-1-β-catenin plasmid was a gift from Dr Woo Keun Song (GIST, Korea). Recombinant β-catenin, GSK-3β, CK1, and Axin (362-500) proteins were prepared as previously described 46, 47.
Western blot analysis
The cytosolic and nuclear fraction was prepared as previously described 48. Proteins were separated using 4-12% gradient SDS-PAGE (Invitrogen) and transferred to nitrocellulose membranes (Bio-Rad). The membranes were blocked with 5% nonfat milk and probed with anti-β-catenin (BD Transduction Laboratories), anti-phospho-β-catenin (Ser33/37/Thr41) (Cell Signaling Technology), anti-phospho-β-catenin (Thr41/Ser45) (Cell Signaling Technology), anti-Axin (BD Transduction Laboratories) and anti-actin antibodies (Cell Signaling Technology). The membranes were then incubated with horseradish peroxidase-conjugated anti-mouse IgG or anti-rabbit IgG (Santa Cruz Biotechnology) and visualized using the ECL system (Santa Cruz Biotechnology).
RNA extraction and semi-quantitative RT-PCR
Total RNA was isolated with Trizol reagent (Invitrogen) in accordance with the manufacturer's instructions. cDNA synthesis, reverse transcription, and PCR were performed as previously described 49. The amplified DNA was separated on 2% agarose gels and stained with ethidium bromide.
In vitro kinase assay
Kinase assays were performed with purified CK1 and GSK-3 using purified GST-β-catenin (100 ng) as a substrate as previously described 46. The proteins were subjected to SDS-PAGE and transferred onto nitrocellulose membranes. The transferred proteins were analyzed using western blotting with anti-phospho-β-catenin antibody (Cell Signaling Technology), and the membrane was exposed to X-ray film. In addition, kinase assays were conducted by Milipore's KinaseProfiler according to the manufacturer's protocols.
GST pull-down assay
Purified β-catenin-GST was mixed with 40 μM of SKL2001 in 500 μl of ADBII (20 mM MOPS, pH 7.2, 25 mM β-glycerol phosphate, 1 mM Na3VO4, 1 mM DTT, 1 mM CaCl2) containing pre-equilibrated glutathione-Sepharose 4B beads, followed by incubation at 4 °C with gentle rotation. After 1 h, Axin (362-500) were added to the mixture and incubated 2h at 4 °C. Beads were then centrifuged at 600× g for 1 min and washed five times with 1 ml of PBS. Proteins bound to the beads were resuspended in 50 μl 2× LDS loading buffer and analyzed by western blotting for β-catenin and silver staining for Axin (362-500).
Silver staining
SDS-PAGE gel was visualized by silver staining. The gel was placed in the fixing solution (50% methanol, 12% acetic acid) for 90min with gentle shaking. The gel is washed by 50% ethanol for 2 times (2 × 20 min) and then incubated in the sensitization solution containing 0.02% Na2S2O3 for 1 min. Rinse the gel three times (1 min each) in water, incubate the gel in silver nitrate solution (1% AgNO3 and 0.75 ml/l formalin (37%)) for 20 min. Discard silver nitrate solution and rinse twice in distilled water for 1 min each time. Develop the silver stain by soaking the gel in develop solution (Na2CO3, 0.0004% Na2S2O3, 0.5 ml/l formalin (37%)) until bands appear. Development was stopped with 1% acetic acid.
Immunoprecipitation analysis
For immunoprecipitation experiments, HEK293 cells were transiently transfected with β-catenin and Axin and then treated with SKL2001. The final reaction volume was 500 μl, containing ∼20 μl of protein A-agarose (Roche Diagnostics, Meylan, France), 200 μg of cell lysate, 1 μg of antibody and buffer A (10mM HEPES (pH 7.4 at 4 °C), 1.5 mM MgCl2, 10 mM KCl and 0.5 mM DTT) at 4 °C for overnight. Immunocomplexes were washed five times with PBS and boiled in the SDS-PAGE loading buffer, and the proteins were detected by western blotting.
Immunofluorescence analysis
ST2 cells were cultured on glass chamber slides and then treated with DMSO or SKL2001 for 15 h. After treatment, the cells were washed with PBS, fixed with 4% formaldehyde, permeabilized in 0.3% Triton X-100, and blocked in 4% bovine serum albumin for 1 h. The cells were stained with anti-β-catenin antibody and then analyzed by confocal microscopy using a Zeiss LSM510 Meta microscope.
Acknowledgments
This work was supported by SK Biopharmaceuticals Co., Ltd, by the new faculty research program 2011 of the Kookmin University in Korea, and by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2009-0072221).
Footnotes
(Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary Material
SKL2001 does not affect p53 and NF-κB signalings.
SKL2001 up-regulates the expression of the β-catenin-dependent gene.
SKL2001 activates Wnt/β-catenin signaling without affecting GSK-3β activity.
The effect of Axinon SKL2001-mediated activation of the Wnt/β-catenin pathway.
Knock-down of β-catenin abrogates SKL2001-mediated activation of the Wnt/β-catenin pathway.
The specificity of SKL2001.
The effect of SKL2001 on human mesenchymal stem cell differentiation.
SKL2001 inhibits adipocyte differentiation in ST2 cells.
SKL2001 binds to β-catenin through armadillo repeats 3-5.
Material and Methods
References
- Miller JR. The Wnts. Genome Biol. 2002;3:reviews3001.1–reviews3001.15. doi: 10.1186/gb-2001-3-1-reviews3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodarz A, Nusse R. Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol. 1998;14:59–88. doi: 10.1146/annurev.cellbio.14.1.59. [DOI] [PubMed] [Google Scholar]
- Huelsken J, Birchmeier W. New aspects of Wnt signaling pathways in higher vertebrates. Curr Opin Gene Dev. 2001;11:547–553. doi: 10.1016/s0959-437x(00)00231-8. [DOI] [PubMed] [Google Scholar]
- Hart MJ, de los Santos R, Albert IN, Rubinfeld B, Polakis P. Downregulation of β-catenin by human Axin and its association with the APC tumor suppressor, β-catenin and GSK3 β. Curr Biol. 1998;8:573–581. doi: 10.1016/s0960-9822(98)70226-x. [DOI] [PubMed] [Google Scholar]
- Liu C, Li Y, Semenov M, et al. Control of β-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–847. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- Latres E, Chiaur DS, Pagano M. The human F box protein β-Trcp associates with the Cul1/Skp1 complex and regulates the stability of β-catenin. Oncogene. 1999;18:849–854. doi: 10.1038/sj.onc.1202653. [DOI] [PubMed] [Google Scholar]
- Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. β-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16:3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald BT, Tamai K, He X. Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- Gaur T, Lengner CJ, Hovhannisyan H, et al. Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J Biol Chem. 2005;280:33132–33140. doi: 10.1074/jbc.M500608200. [DOI] [PubMed] [Google Scholar]
- Bennett CN, Hodge CL, MacDougald OA, Schwartz J. Role of Wnt10b and C/EBPα in spontaneous adipogenesis of 243 cells. Biochem Biophys Res Commun. 2003;302:12–16. doi: 10.1016/s0006-291x(03)00092-5. [DOI] [PubMed] [Google Scholar]
- Christodoulides C, Laudes M, Cawthorn WP, et al. The Wnt antagonist Dickkopf-1 and its receptors are coordinately regulated during early human adipogenesis. J Cell Sci. 2006;119:2613–2620. doi: 10.1242/jcs.02975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/β-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005;8:739–750. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Hu H, Hilton MJ, Tu X, Yu K, Ornitz DM, Long F. Sequential roles of Hedgehog and Wnt signaling in osteoblast development. Development. 2005;132:49–60. doi: 10.1242/dev.01564. [DOI] [PubMed] [Google Scholar]
- Shang YC, Wang SH, Xiong F, et al. Wnt3a signaling promotes proliferation, myogenic differentiation, and migration of rat bone marrow mesenchymal stem cells. Acta Pharmacol Sin. 2007;28:1761–1774. doi: 10.1111/j.1745-7254.2007.00671.x. [DOI] [PubMed] [Google Scholar]
- Cho M, Gwak J, Park S, et al. Diclofenac attenuates Wnt/β-catenin signaling in colon cancer cells by activation of NF-κB. FEBS Lett. 2005;579:4213–4218. doi: 10.1016/j.febslet.2005.06.049. [DOI] [PubMed] [Google Scholar]
- Shibamoto S, Higano K, Takada R, Ito F, Takeichi M, Takada S. Cytoskeletal reorganization by soluble Wnt-3a protein signalling. Genes Cells. 1998;3:659–670. doi: 10.1046/j.1365-2443.1998.00221.x. [DOI] [PubMed] [Google Scholar]
- Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci USA. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston JT, Strack P, Beer-Romero P, Chu CY, Elledge SJ, Harper JW. The SCFβ-TRCP-ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IκBα and β-catenin and stimulates IκBα ubiquitination in vitro. Genes Dev. 1999;13:270–283. doi: 10.1101/gad.13.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amit S, Hatzubai A, Birman Y, et al. Axin-mediated CKI phosphorylation of β-catenin at Ser 45, a molecular switch for the Wnt pathway. Genes Dev. 2002;16:1066–1076. doi: 10.1101/gad.230302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer L, Skaltsounis AL, Magiatis P, et al. GSK-3-selective inhibitors derived from Tyrian purple indirubins. Chem Biol. 2003;10:1255–1266. doi: 10.1016/j.chembiol.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Olesen PH, Sorensen AR, Urso B, et al. Synthesis and in vitro characterization of 1-(4-Aminofurazan-3-yl)-5-dialkylaminomethyl-1H-[1,2,3]triazole-4-carboxylic acid derivatives. A new class of selective GSK-3 inhibitors. J Med Chem. 2003;46:3333–3341. doi: 10.1021/jm021095d. [DOI] [PubMed] [Google Scholar]
- Kimelman D, Xu W. β-Catenin destruction complex: insights and questions from a structural perspective. Oncogene. 2006;25:7482–7491. doi: 10.1038/sj.onc.1210055. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Bryant HU, Macdougald OA. Regulation of bone mass by Wnt signaling. J Clin Invest. 2006;116:1202–1209. doi: 10.1172/JCI28551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron R, Rawadi G, Roman-Roman S. Wnt signaling: a key regulator of bone mass. Curr Top Dev Biol. 2006;76:103–127. doi: 10.1016/S0070-2153(06)76004-5. [DOI] [PubMed] [Google Scholar]
- Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1997;11:3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- Zhang C, Cho K, Huang Y, et al. Inhibition of Wnt signaling by the osteoblast-specific transcription factor Osterix. Proc Natl Acad Sci USA. 2008;105:6936–6941. doi: 10.1073/pnas.0710831105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S, Wu TY, Brinker A, et al. Synthetic small molecules that control stem cell fate. Proc Natl Acad Sci USA. 2003;100:7632–7637. doi: 10.1073/pnas.0732087100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand JA, Thieffine S, Vulpetti A, et al. Structural characterization of the GSK-3β active site using selective and non-selective ATP-mimetic inhibitors. J Mol Biol. 2003;333:393–407. doi: 10.1016/j.jmb.2003.08.031. [DOI] [PubMed] [Google Scholar]
- Wang W, Walker JR, Wang X, et al. Identification of small-molecule inducers of pancreatic β-cell expansion. Proc Natl Acad Sci USA. 2009;106:1427–1432. doi: 10.1073/pnas.0811848106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelman D, Xu W. β-catenin destruction complex: insights and questions from a structural perspective. Oncogene. 2006;25:7482–7491. doi: 10.1038/sj.onc.1210055. [DOI] [PubMed] [Google Scholar]
- Liu X, Rubin JS, Kimmel AR. Rapid, Wnt-induced changes in GSK3β associations that regulate β-catenin stabilization are mediated by Gα proteins. Curr Biol. 2005;15:1989–1997. doi: 10.1016/j.cub.2005.10.050. [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Graham TA, Weaver C, Mao F, Kimelman D, Xu W. Crystal structure of a β-catenin/Tcf complex. Cell. 2000;103:885–896. doi: 10.1016/s0092-8674(00)00192-6. [DOI] [PubMed] [Google Scholar]
- von Kries JP, Winbeck G, Asbrand C, et al. Hot spots in β-catenin for interactions with LEF-1, conductin and APC. Nat Struct Biol. 2000;7:800–807. doi: 10.1038/79039. [DOI] [PubMed] [Google Scholar]
- Xing Y, Clements WK, Kimelman D, Xu W. Crystal structure of a β-catenin/axin complex suggests a mechanism for the β-catenin destruction complex. Genes Dev. 2003;17:2753–2764. doi: 10.1101/gad.1142603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christodoulides C, Lagathu C, Sethi JK, Vidal-Puig A. Adipogenesis and WNT signalling. Trends Endocrinol Metab. 2009;20:16–24. doi: 10.1016/j.tem.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo KA, Wright WS, Kang S, et al. Wnt10b inhibits development of white and brown adipose tissues. J Biol Chem. 2004;279:35503–35509. doi: 10.1074/jbc.M402937200. [DOI] [PubMed] [Google Scholar]
- Wright WS, Longo KA, Dolinsky VW, et al. Wnt10b inhibits obesity in ob/ob and agouti mice. Diabetes. 2007;56:295–303. doi: 10.2337/db06-1339. [DOI] [PubMed] [Google Scholar]
- Bodine PV, Zhao W, Kharode YP, et al. The Wnt antagonist secreted frizzled-related protein-1 is a negative regulator of trabecular bone formation in adult mice. Mol Endocrinol. 2004;18:1222–1237. doi: 10.1210/me.2003-0498. [DOI] [PubMed] [Google Scholar]
- Bennett CN, Ross SE, Longo KA, et al. Regulation of Wnt signaling during adipogenesis. J Biol Chem. 2002;277:30998–31004. doi: 10.1074/jbc.M204527200. [DOI] [PubMed] [Google Scholar]
- Ross SE, Hemati N, Longo KA, et al. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- Park S, Gwak J, Cho M, et al. Hexachlorophene inhibits Wnt/β-catenin pathway by promoting Siah-Mediated β-catenin degradation. Mol Pharmacol. 2006;70:960–966. doi: 10.1124/mol.106.024729. [DOI] [PubMed] [Google Scholar]
- Lai CF, Chaudhary L, Fausto A, et al. Erk is essential for growth, differentiation, integrin expression, and cell function in human osteoblastic cells. J Biol Chem. 2001;276:14443–14450. doi: 10.1074/jbc.M010021200. [DOI] [PubMed] [Google Scholar]
- Park CS, Kim SI, Lee MS, et al. Modulation of β-catenin phosphorylation/degradation by cyclin-dependent kinase 2. J Biol Chem. 2004;279:19592–19599. doi: 10.1074/jbc.M314208200. [DOI] [PubMed] [Google Scholar]
- Yum S, Lee SJ, Piao S, et al. The role of the Ser/Thr cluster in the phosphorylation of PPPSP motifs in Wnt coreceptors. Biochem Biophys Res Commun. 2009;381:345–349. doi: 10.1016/j.bbrc.2009.02.044. [DOI] [PubMed] [Google Scholar]
- Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaji H, Naito J, Sowa H, Sugimoto T, Chihara K. Smad3 differently affects osteoblastdifferentiation depending upon its differentiation stage. Horm Metab Res. 2006;38:740–745. doi: 10.1055/s-2006-955085. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SKL2001 does not affect p53 and NF-κB signalings.
SKL2001 up-regulates the expression of the β-catenin-dependent gene.
SKL2001 activates Wnt/β-catenin signaling without affecting GSK-3β activity.
The effect of Axinon SKL2001-mediated activation of the Wnt/β-catenin pathway.
Knock-down of β-catenin abrogates SKL2001-mediated activation of the Wnt/β-catenin pathway.
The specificity of SKL2001.
The effect of SKL2001 on human mesenchymal stem cell differentiation.
SKL2001 inhibits adipocyte differentiation in ST2 cells.
SKL2001 binds to β-catenin through armadillo repeats 3-5.
Material and Methods