Abstract
The amyloid precursor protein (APP) has been under intensive study in recent years, mainly due to its critical role in the pathogenesis of Alzheimer's disease (AD). β-Amyloid (Aβ) peptides generated from APP proteolytic cleavage can aggregate, leading to plaque formation in human AD brains. Point mutations of APP affecting Aβ production are found to be causal for hereditary early onset familial AD. It is very likely that elucidating the physiological properties of APP will greatly facilitate the understanding of its role in AD pathogenesis. A number of APP loss- and gain-of-function models have been established in model organisms including Caenorhabditis elegans, Drosophila, zebrafish and mouse. These in vivo models provide us valuable insights into APP physiological functions. In addition, several knock-in mouse models expressing mutant APP at a physiological level are available to allow us to study AD pathogenesis without APP overexpression. This article will review the current physiological and pathophysiological animal models of APP.
Keywords: Alzheimer's disease, APP, Aβ, knock-in, animal models
Introduction
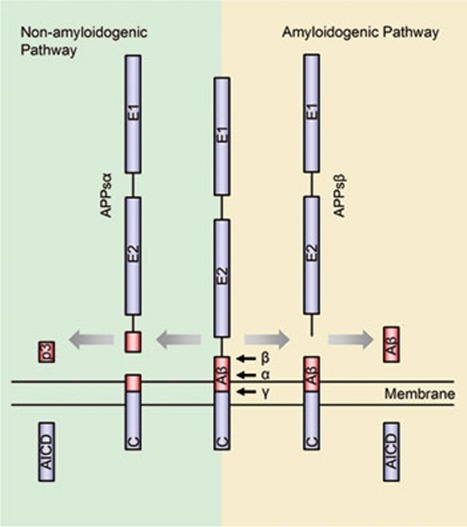
The amyloid precursor protein (APP) is one of the most intensively studied molecules since it was first cloned more than 20 years ago 1, 2, 3. A notable feature of APP is that it undergoes finely regulated secretase cleavage processing (Figure 1). In general, the α-secretase or β-secretase cleaves the APP extracellular domain generating the soluble N-terminal fragments APPsα or APPsβ and the membrane-associated C-terminal fragments CTFα and CTFβ, respectively. CTFα or CTFβ will further be cleaved by the γ-secretase within the transmembrane domain releasing the p3 peptide or β-amyloid (Aβ) peptide. Following γ-secretase cleavage, both CTFs will also release the APP intracellular domain (AICD) into the cytoplasm. α-Secretase-mediated APP cleavage prevents Aβ production, and thus is called the non-amyloidogenic pathway, whereas the β-secretase-mediated APP cleavage generates Aβ peptides and is called the amyloidogenic pathway. However, recent studies showed that APP proteolytic cleavage could be more complicated than above (reviewed by Chow et al. 4). For instance, besides the γ-site cleavage of the CTFs by γ-secretase, CTFs may also undergo 'ɛ-site' cleavage near the cytoplasmic membrane boundary of APP 5.
Figure 1.
APP amyloidogenic and non-amyloidogenic processing pathways (not drawn to scale). The Aβ domain is highlighted in red. For simplicity, only one cleavage site is shown for each enzyme.
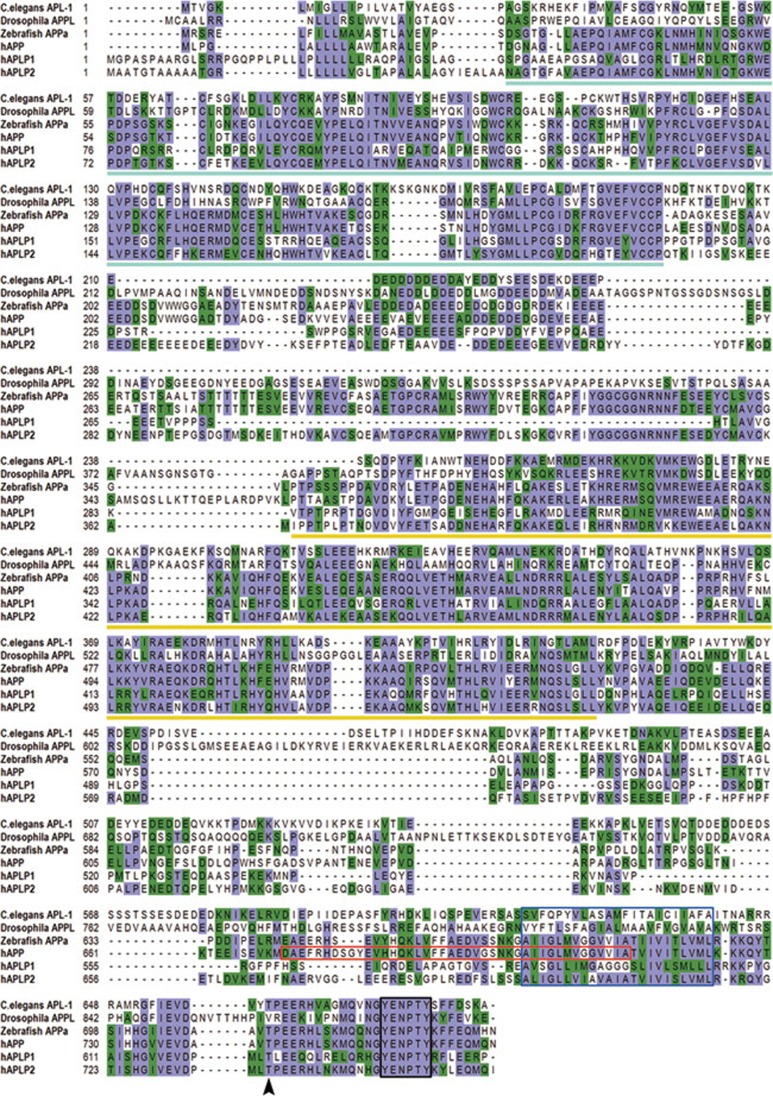
The normal physiological functions of APP have raised curiosity, since it belongs to an evolutionarily conserved protein family (Figure 2) and combined deficiency of APP and its homologs leads to early postnatal lethality in mammals 6, 7. A number of APP loss-of-function and gain-of-function in vivo models have been established and characterized in different model organisms, including Caenorhabditis elegans, Drosophila, zebrafish and mouse. Studies from different species indicate conservation of function between APP family members, including roles in cell adhesion, neuron migration and synaptogenesis.
Figure 2.
Homology between APP family members. Comparison of protein sequences of C. elegans APL-1, Drosophila APPL, zebrafish APPa and the human APP family members reveals distinct areas of homology. Purple sequences indicate identical homology while green references similar amino acids. Homologous regions include the E1 domain (pale blue line), E2 domain (yellow line) and sequences within the C-terminus such as the conserved Thr site (arrow head) and YENPTY motif (black box). The transmembrane domain and Aβ sequence are noted by the blue and red boxes, respectively.
Enormous scientific efforts have been put into APP-related studies mainly because of its vital pathophysiological functions in Alzheimer's disease (AD). AD is the leading cause of dementia in the aged population. There are three pathological hallmarks of AD: extracellular amyloid plaques, intracellular neurofibrillary tangles and neuronal degeneration including synapse loss. Amyloid plaques are extracellular deposits primarily composed of Aβ peptides that are derived from proteolytic cleavage of APP and associated with other components (reviewed by Armstrong et al. 8). The pathogenic mechanism of AD is very controversial, however, the Aβ cascade hypothesis is currently most favored. This hypothesis states that the excessive Aβ peptides generated from mis-regulated cleavage of APP or impaired clearance play a central role in AD 9. While the vast majority of AD cases occur after 65 years of age, a small percentage of patients (<5%) develop clinical symptoms before the age of 65 years due to autosomal dominant familial AD (FAD) mutations 10. These mutations have been discovered in three different genes: APP, Presenilin 1 and 2 (PSEN1 and PSEN2). Most mutations in the APP and PSEN (or PS) genes alter the production of Aβ cleavage profiles, leading to accelerated amyloid pathology. Several lines of knock-in mice (KI) with APP FAD mutations have been generated in recent years, either by mutation of APP alone or in combination with PS1 mutations. Compared to traditional AD transgenic mice, which have been used extensively in the field, these KI models do not suffer from the confounding effects of APP overexpression and offer us a more physiologically relevant platform to investigate AD pathogenesis. We believe a review of these in vivo APP animal models will facilitate the understanding of AD pathogenesis in the context of APP physiology.
Non-mammalian APP animal models
C. elegans
The C. elegans homolog of APP, APL-1, is structurally similar to its mammalian counterpart and shares sequence homology in the N-terminal E1 and E2 domains and the intracellular C-terminal domain, which has the highest sequence conservation (Figure 2) 11. There are no known coding region splice variants detected in APL-1, which most closely resembles the neuronal isoform of APP, APP695 11. However, APL-1 does not contain the amyloid-β sequence, similar to the functionally redundant mammalian APP homologs APLP1 and APLP2 11, 12, 13, 14.
Nematode development includes four larval stages after each of which is a molt where a new, larger exoskeleton is formed to accommodate the growth of the larvae. In worms, loss of function of the single apl-1 gene leads to developmental arrest and lethality during the first larval stage (L1), likely due to a molting defect 15, 16. Many of the apl-1-null worms arrest at the L1/L2 transition, unable to shed old cuticle and exhibiting massive internal degradation 15, 16. RNAi knockdown of apl-1 also leads to a molting defect, although less severe than the null mutant, as the worms exhibit loose cuticle primarily around the head beginning at the L3/L4 molt 16, 17. In addition to the molting defect, apl-1 knockdown leads to hypersensitivity to the acetylcholinesterase inhibitor aldicarb, signifying a defect in cholinergic neurotransmission 16. The neuronal phenotype and the molting defect were found to be independent of one another, suggesting apl-1 contributes to multiple functions within the worm. However, more studies are needed to determine the mechanism of apl-1 regulation in each of these functions.
Structure-function studies have been performed in an attempt to identify functional domains of apl-1 through rescue of the apl-1-null lethality and molting defect. Surprisingly, both phenotypes were found to be rescued by either a C-terminal truncation of apl-1 or the soluble N-terminus, showing that the highly conserved C-terminus is not required for apl-1 function in the worm 15, 16. This differs from the mammalian system in which the APP C-terminus is essential for viability on a non-redundant background (see discussion under 'KI models expressing distinct APP fragments') 18, 19. Hornsten et al. 15 were unable to accomplish rescue using the C-terminus alone, indicating, together with the other rescue experiments, that the N-terminus is the primary functional domain of APL-1 in the worm.
Drosophila
APPL is the APP homolog in Drosophila, and, like the worm homolog, does not contain the Aβ sequence (Figure 2). Appl-deficient flies are viable and fertile with no obvious phenotypes; however, these flies do have subtle behavioral defects, such as fast phototaxis impairment. This defect can be partially rescued by transgenic expression of either wild-type fly APPL or human APP, which demonstrates the functional conservation of APP between different species 20.
Subsequent loss and gain-of-function studies revealed more specific functions of APPL. APPL plays an important role in axonal transport, since either Appl deletion or its overexpression will cause axonal transport defects similar to kinesin and dynein mutants 21. APPL is required for the development of neuromuscular junctions (NMJs), since Appl deletion leads to decreased NMJ bouton number, whereas Appl overexpression dramatically increases bouton number 22. This activity can be explained by the formation of a potential complex including APPL, the APPL-binding protein dX11/Mint, and the cell adhesion molecule FasII, which together can regulate synapse formation 23. APPL also plays a role in the development of the Drosophila peripheral nervous system (PNS) as both Appl deletion and Appl RNAi leads to the loss of scutellar mechano-sensory organs (MSOs). Overexpression of human APP homologs in the fly leads to MSO Notch gain-of-function phenotypes, possibly through the interaction of the APP YENPTY motif with Numb/Pon and Dab 24.
APPL promotes post-developmental neurite arborization, which may be involved in brain injury response and repair. This function was revealed in a study showing that overexpression of human APP and APPL can induce post-developmental axonal arborization. This phenotype is dependent on the APP C-terminal domain and its interaction with the Abelson tyrosine kinase. Interestingly, similar to mammals, APPL is strongly upregulated after traumatic brain injury (TBI) in flies upon which APPL-deficient flies suffer a higher mortality rate compared to controls 25.
Overall, Appl loss and gain-of-function studies in Drosophila models offer us important insights into the physiological functions of APP and some of these findings have already been validated in mouse models.
Zebrafish
Zebrafish have two APP homologs, APPa and APPb. APPa is a 738-aa protein and contains the Kunitz protease inhibitor domain in the N-terminus, which resembles the human APP770 isoform. APPb is a 694-aa protein, which is more similar to the human APP695 isoform 26. The study of a transgenic zebrafish model expressing GFP under the appb promoter control reveals that the GFP is mainly expressed in subregions of brain, spinal cord and the developing vasculature of zebrafish embryos. The GFP expression level increases during development, and in adult transgenic zebrafish, GFP was abundantly expressed in the brain 27. Though appa knockdown in zebrafish does not show any significant defects in development, appb knockdown animals show several early developmental deficits, including a shortened body axis, a short and curly tail and mild synopthalmia, which can be explained by defective convergent-extension movements 26. These defects can be largely rescued by human APP695 mRNA, partially rescued by human APPsα mRNA, but cannot be rescued by APP bearing the Swedish mutation, which leads to FAD in humans. It is interesting that this study indicates APP FAD mutations may not only contribute to AD pathogenesis during aging but also affect early development due to APP loss-of-function.
Though there are not many APP physiological function studies employing zebrafish, as a well-established model organism for vertebrate development, we expect to see more novel findings from the zebrafish system in the near future.
APP models in mice
To understand the physiological functions of APP and its family members, individual and combined mouse mutants of APP, APLP1 and APLP2 have been generated. For APP, there are two knockout (KO) alleles 28, 29, one hypomorphic allele 30, two conditional alleles 31, 32, four defined truncation KI alleles 18, 19, 33 and two C-terminal point mutation KI alleles reported 34, 35.
APP, APLP1 and APLP2 single KO mice
APP KO mice are viable and fertile but suffer from various defects and abnormalities, such as reduced body and brain weight, decreased size of forebrain commissures, increased frequency and severity of corpus callosum agenesis 28, 30, reactive gliosis 28, increased sensitivity to kainate-induced seizures 36, increased copper and iron levels in the cerebral cortex and liver 37, 38, upregulated cholesterol and sphingomyelin levels in the brain 39 and decreased plasma glucose levels as well as hyperinsulinemia 40. Behavioral studies revealed that APP KO mice have decreased locomotor activity, reduced forelimb grip strength and deficits in the Morris water maze task as well as passive avoidance learning 41, 42, 43. Electrophysiology studies show that these mice are also defective in long-term potentiation (LTP), which is associated with attenuated paired-pulse depression of GABA-mediated inhibitory postsynaptic currents 44. Recently, APP was found to be involved in the regulation of L-type calcium channel function 45. Loss of APP leads to increased levels of the L-type calcium channel subunit Cav1.2 in the striatum, which is associated with reduced GABAergic paired pulse inhibition and increased GABAergic post-tetanic potentiation 45. However, these behavioral and electrophysiological impairments may not be caused by a gross loss of neurons or synapses because unbiased stereological quantification failed to detect any significant neuronal or synapse loss in aged APP KO mice 42. Also, attempts to examine dendritic spine density in APP KO mice have revealed mixed results. Bittner et al. 46 reported that APP deletion leads to a twofold higher density of spines in apical dendrites of layer III and layer V neurons of the somatosensory cortex at 4-6 months of age, whereas Lee et al. 47 found a significant decrease in spine density in cortical layers II/III and hippocampal CA1 pyramidal neurons in 1-year-old APP KO mice compared with wild-type controls. This discrepancy may be due to a region-specific effect, a contribution of aging and/or an adaptive mechanism. Nevertheless, additional study is warranted in this area.
The phenotypes of APLP2 and APLP1 single KO mice are also mild 6, 7. APLP2 KO mice are viable, fertile and normal in size. No abnormalities were detected in tissues examined by histochemical analysis. APLP2 KO mice showed no difference from controls in forelimb strength as well as learning and memory performance in the Morris water maze and conditioned avoidance tasks. Similarly, APLP1 single KO mice are viable, fertile, but have a postnatal growth deficit. However, these mice show normal locomotor activity and forelimb grip strength 6, 7.
Mice with combined KO of APP family members
Though the phenotypes of APP family member single KOs are all relatively subtle, APP/APLP2 or APLP1/APLP2 double KO (dKO) mice exhibit early postnatal lethality. This strongly indicates that functional redundancy exists among APP family members. Intriguingly, the APP/APLP1 dKO mice are viable 7, suggesting a unique property specific to APLP2 that is required when APP or APLP1 is absent.
In the PNS, APP and APLP2 play an essential yet redundant role in the formation and function of neuromuscular synapses. APP/APLP2 dKO mice have poorly formed NMJs, with excessive nerve growth and reduced apposition of pre- and postsynaptic elements. The number of synaptic vesicles at the presynaptic terminals is also reduced in parallel with impaired neurotransmitter release 48. Moreover, study of the ultrastructure of the submandibular synapse also revealed significant reductions in synaptic vesicle density, active zone size and docked vesicle number per active zone in APP/APLP2 dKO mice 49.
Mice lacking all three APP family members survive through embryonic development but die shortly after birth 50. Cranial abnormalities were seen in 81% of the triple KO mice while 68% showed cortical dysplasia resembling human type II lissencephaly. Additionally, the mutant mice showed partial loss of cortical Cajal Retzius cells at E18.5, suggesting a role for APP family members in neuronal migration and/or adhesion 50. A very similar cortical dysplasia phenotype was also observed in mice that are double deficient for Fe65 and Fe65L1, a family of proteins that have been well known to interact with the APP cytoplasmic domain 51, suggesting an APP/Fe65 signaling complex mediating neuronal migration. The role of APP in neuronal migration/adhesion was further supported by the finding that acute knockdown of APP in rats using shRNA electroporation resulted in defective cortical plate entry and migration of neuronal precursor cells in the embryonic cortex 52.
APP conditional KO
To counter postnatal lethality and further dissect APP physiological functions, two conditional alleles of APP have been generated 31, 32. Tissue-specific deletion of APP in either neurons or muscle in the APLP2 KO background resulted in neuromuscular defects similar to phenotypes seen in APP/APLP2 dKO mice, suggesting that APP expression is required in both motor neurons and muscle cells for the proper formation and function of neuromuscular synapses 31. The authors propose that the trans-synaptic homotypic interaction of APP promotes neuromuscular synapse development. This was further supported by the synaptogenic properties of APP revealed by hippocampal and HEK293 mixed culture experiments 31. Interestingly, muscle APP expression is required for the proper pre-synaptic localization of CHT (high-affinity choline transporter) and synaptic transmission, suggesting that trans-synaptic APP interaction is necessary for recruiting the presynaptic APP/CHT complex. Consistent with the role of APP in synaptic plasticity and synaptic function, a recent study that attempted to identify APP-interacting proteins using a tagged APP transgenic approach revealed a list of synaptic proteins including bassoon and neurexin 53. Whether the trans-synaptic interaction of APP is involved in the recruitment of these presynaptic molecules and how it coordinates with other synaptic adhesion complexes such as neuroligin/neurexin are exciting questions awaiting further investigation.
Knock-in models expressing distinct APP fragments and single amino acid mutations
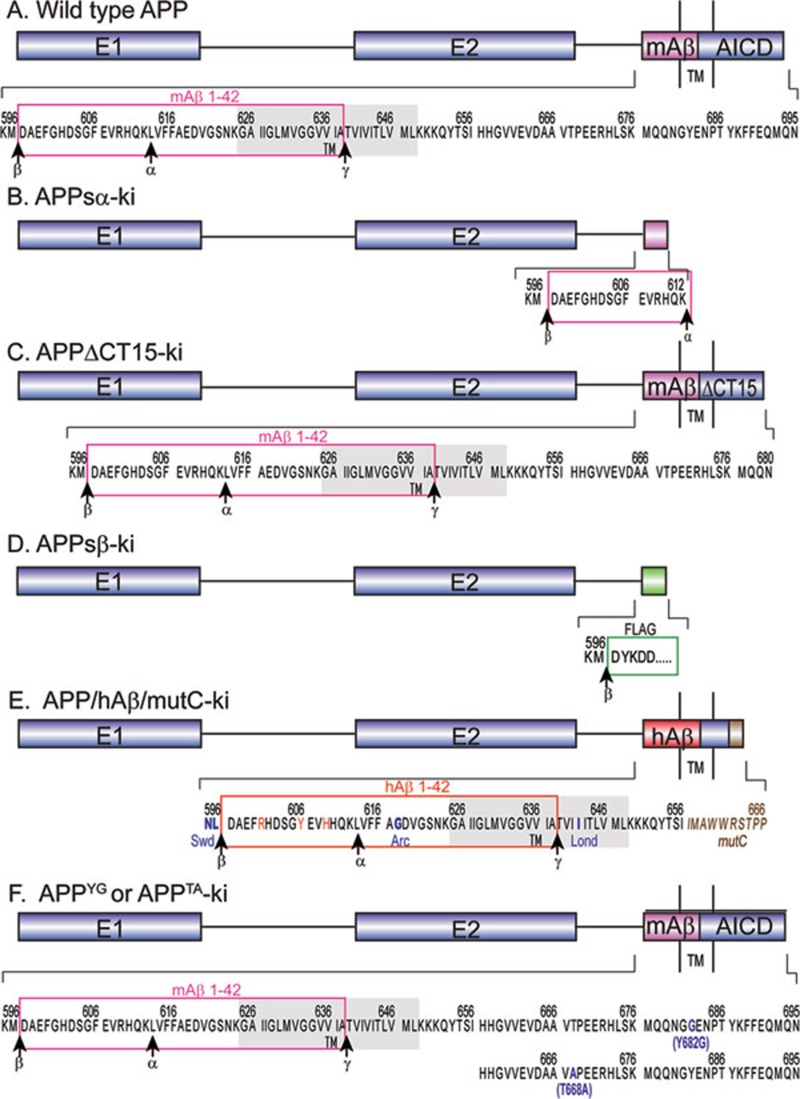
As mentioned earlier, sequential proteolytic processing of APP generates three fragments: a large soluble ectodomain (APPsα or APPsβ), a short peptide such as P3 or Aβ and the AICD. To investigate physiological functions exerted by these different APP fragments, two research groups have generated four different gene-targeted APP mutant KI alleles, in which Aβ and AICD regions, are missing or mutated (Figure 3).
Figure 3.
APP knock-in alleles expressing distinct APP fragments. (A) Wild-type APP695 with the mouse Aβ region (mAβ) framed in pink, and transmembrane area (TM) shaded in grey. AICD, APP intracellular domain. α, β and γ are cleavage sites of the α-, β, and γ-secretases, respectively. (B and C) APPsα and APPΔCT15 knock-in alleles, respectively, reported by Ring et al, 33. (D) APPsβ knock-in reported by Li et al, 18. FLAG-tag sequence is framed in green. (E) APP/hAβ/mutC knock-in by Li et al. 19. Humanized Aβ region (hAβ) is framed in red, and the different residues between human and mouse sequence are also shown in red. Additional FAD mutations are bolded in blue. Swd, Arc and Lond, represent Swedish, Arctic and London mutations, respectively. The sequence resulting from a frame-shift mutation at C-terminus is illustrated in brown. (F) APPYG and APPTA knock-ins harbor APP Y682G and T668A mutations, respectively 34, 35.
In order to determine the role of APPsα and APP C-terminal domain, the Müller group created two KI mouse lines 33. The first expresses APPsα (APPsα-ki) and the second expresses a C-terminal truncation of APP removing the highly conserved YENPTY motif (APPΔCT15-ki) by deletion of the last 15 aa residues (Figure 3B and 3C). Both of the lines have an overall reduction of mutant APP expression in both mRNA and protein levels, which is attributed to an effect of SV40 polyA cassette. In APPΔCT15-ki brains, even with a reduction of mutant APP expression of more than 50%, there is a relatively higher percentage of cell surface APP and less Aβ production compared to controls. This is consistent with the previous in vitro studies showing that the C-terminal region was involved in APP endocytosis and processing 54, 55.
In comparison with APP KO mice, the APPsα-ki and APPΔCT15-ki mice appear to rescue a variety of phenotypes observed in APP KO mice. For instance, the body and brain weight loss phenotype of APP KO animals at different ages is largely rescued. Behaviorally, these KI mice do not exhibit any defects in grip strength test and water maze test. Electrophysiology experiments showed that LTP deficits observed in APP KO mice at 9-12 months were also corrected in both KI lines. Taken together, these results suggest that, similar to what was observed with APL-1 in the C. elegans model 15, the C-terminal region of APP may not be required for certain physiological functions of APP.
To further dissect the function of different APP domains, our research group has also generated two distinct KI lines. The first model expresses APPsβ with a FLAG tag at the C-terminus named APPsβ-ki18 (Figure 3D). The second model, named APP/hAβ/mutC 19, expresses APP with a humanized Aβ region, including three FAD mutations (Swedish, Arctic and London) to facilitate Aβ production, and a frameshift mutation in the C-terminus that deletes the last 39 aa residues of APP (Figure 3E). Similar to the APPsα-ki and APPΔCT15-ki lines, APPsβ-ki and APP/hAβ/mutC-ki mice do not show any obvious growth or anatomical deficits. As mentioned above, when APP KO mice are bred to the APLP2 KO background, APP/APLP2 dKO pups exhibit postnatal lethality. Similarly, when crossed with APLP2 KO mice, neither the APPsβ-ki nor APP/hAβ/mutC-ki allele is able to rescue the lethality. Moreover, the deficits of NMJs observed in APP/APLP2 dKO pups, including aberrant apposition of presynaptic proteins with postsynaptic endplates and diffuse synaptic band width, also exist in APPsβki/ki/APLP2−/− and APP/hAβ/mutCki/ki/APLP2−/− pups. Considering that neither of the KI alleles contains a functional APP C-terminal domain, our data clearly demonstrate a critical and indispensable role of the conserved C-terminal region of APP in early development.
Though our KI alleles are not able to rescue lethality, they do rescue certain gene expression changes caused by APP KO. In APP/APLP2 dKO mice, the mRNA level of transthyretin (TTR) and Klotho is reduced 18. However, the expression of TTR and Klotho is normal in the APPsβki/−/APLP2−/− pups. This finding indicates that soluble APP can modulate the expression of certain genes through a currently unknown pathway. TTR has been shown to have Aβ binding ability 56, 57, 58 and Klotho has been extensively implicated in the aging process 59, 60, 61. The enhancement of TTR and Klotho expression by APPsβ offers the fascinating possibility of a self-protective mechanism in the APP processing pathway to counter the production and toxicity of Aβ during aging.
When we crossed the APP/hAβ/mutC KI line onto the PS1 M146V (a PS1 FAD mutation) KI background to accelerate Aβ deposition, we found massive plaques in the hippocampus and cortex in mice over 1 year of age. This implies that amyloid pathogenesis could progress independently without the conserved C-terminal sequence 19.
The essential role of the highly conserved C-terminal region of APP in early development revealed by APPsβki/ki/APLP2−/− and APP/hAβ/mutCki/ki/APLP2−/− mice was further confirmed by a recent study of Barbagallo et al. 34. They generated a KI mouse with a single amino acid mutation at Tyr682 (Y682G), and showed that, in the absence of APLP2 functional redundancy, the Y682G mutation resulted in postnatal lethality and neuromuscular synapse defects similar to APP/APLP2-double-deficient mice 34, 62. The importance of this Tyr682 site is strengthened by a parallel study, which found that a distinct mutation at a different Thr site, Thr668 (T668A), had no effect on both animal survival and formation of neuromuscular synapses 34, 35. Tyr682 is an essential residue of the Y682ENPTY687 motif, which is the docking site for numerous cytosolic proteins 63. Some APP interactors require phosphorylated Tyr682 64, 65, 66, 67, whereas others require non-phosphorylated Tyr682 68. This leads to questions in regards to how the selective phosphorylation state of Tyr682 is regulated and their corresponding functional consequences.
In summary, these different KI lines expressing different segments of APP have revealed that APP is involved in multiple functions. Some of these functions are carried out by APP N-terminal ectodomain alone without the APP C-terminal intracellular domain. However, the AICD, especially the YENPTY motif, which is well conserved from worms to humans, plays a critical role in developmental regulation in mice. Finally, Aβ aggregation and amyloid deposition can proceed without the APP C-terminus, implying that APP physiological functions could be genetically uncoupled with AD pathogenesis.
APP FAD mutant KI models
Many APP transgenic models expressing APP with FAD mutations under the control of a robust exogenous promoter have been generated and have provided us with valuable knowledge about AD pathogenesis, especially Aβ plaque deposition. However, in order to accelerate plaque development, transgenic mice usually overexpress mutant APP at a very high level. For instance, a commonly used transgenic line, Tg2576, overexpresses APP to almost sixfold using the hamster prion promoter 69. Considering the crucial and various functions of APP in nervous system development, this provokes the question of whether the memory and behavioral defects observed in AD-transgenic mice are due to Aβ pathology or simply APP overexpression.
To avoid the complications of overexpression and build a more physiologically relevant model, several labs generated different FAD APP KI lines by introducing human FAD mutations and/or humanized Aβ to the endogenous mouse APP gene. Compared to traditional transgenic models, APP KI mice exhibit a number of advantages. Since the KI allele is under the native APP promoter control, the amount of APP expression remains at physiological level. For the same reason, the temporal and spatial expression patterns of mutant APP are not affected. In contrast to the transgenic lines in which the wild-type mouse APP gene is still intact, where mouse APP and Aβ may complicate the phenotype, the KI models have mouse Aβ replaced with the human Aβ sequence.
One FAD KI model was developed by Reaume et al., 70 who generated APPNLh/NLh mice bearing the Swedish FAD mutation (K670N/M671L) with a humanized Aβ sequence expressed at endogenous levels. Amyloidogenic β-secretase cleavage of this mutant APP is accurate and also enhanced, while the non-amyloidogenic processing is repressed. Human Aβ production is significantly increased in these mice, a ninefold increase compared to normal human brain tissue 70. Though this APP KI model overproduces human Aβ40 and Aβ42, Aβ plaque deposition was not detectable 71. Later, the APPNLh/NLh strain was crossed with the PS-1P264L/P264L line (a PS1 FAD mutant KI line) to generate the APPNLh/NLh/PS-1P264L/P264L double KI (DKI) model 71. APPNLh/NLh/PS-1P264L/P264L DKI animals exhibit elevated levels of Aβ42 and initial Aβ deposition begins at 6 months of age. Aβ deposition at 6 months was predominately seen in the cerebral cortex with the majority of deposits found in the frontal cortex, followed by the parietal cortex 71. Microarray analysis was performed to compare the gene expression profile changes between APPNLh/NLh/PS-1P264L/P264L animals and two transgenic models, Tg2576/PS-1P264L/+ and Tg2576/PS-1P264L/P264L, both before and after detectable Aβ deposition 72. Gene expression difference was analyzed in APPNLh/NLh/PS-1P264L/P264L DKI animals and their wild-type littermates at 2 months of age, when plaques in DKI brains are absent, and at 18 months of age, when the DKI cortical plaque load is 3.2%. Similar experiments were also performed to compare expression changes in Tg2576/PS-1P264L/+, Tg2576/PS-1P264L/P264L and non-transgenic controls (non-Tg/PS-1+/+) at 2 months of age, when Tg2576/PS-1P264L/+ had no plaques and the cortical plaque load of Tg2576/PS-1P264L/P264L is 0.09%, and at 12 months of age, when the plaque load of Tg2576/PS-1P264L/+ and Tg2576/PS-1P264L/P264Lwas 10.6% and 25.8%, respectively. In young animals before Aβ deposition, the DKI animals do not share any altered gene expression when compared with the two transgenic models, which is possibly due to APP overexpression in transgenic models and/or a genetic background difference. However, there are a number of common changes found in the three models with Aβ deposition and many of these changes are also found in human AD brains 72. This finding strongly supports this DKI animal model as a valuable AD research tool. Chang et al. 73 performed electrophysiological and behavioral studies with APPNLh/NLh/PS-1P264L/P264L animals. DKI animals showed an age-related decrease of AMPA receptor-mediated evoked currents and spontaneous miniature currents. The downregulation of synaptic AMPA receptor is confirmed by electron microscopic study. DKI animals also exhibit age-related deficits in bidirectional plasticity seen during analysis of LTP and LTD in these mice as well as memory flexibility when examined by Morris water maze. Additionally, long-lasting and selective impairment in adult hippocampal neurogenesis is found in 9-18 months old APPNLh/NLh/PS-1P264L/P264L DKI animals, while the olfactory bulb neurogenic system is not affected. The number of MCM2-positive neural stem and progenitor cells is decreased by 3-fold compared to controls and doublecortin-positive neuroblasts are decreased by twofold as well. This neurogenesis impairment phenotype may be explained by Aβ deposition-induced microglia activation and neuroinflammation 74.
Another similar FAD mutant APP KI line was generated by Kohler et al. 75, which includes the humanized Aβ sequence containing both Swedish (K670N/M671L) and London (V717F) mutations. APPSL/SL single KI animals do not show any Aβ deposits. Once this strain was crossed to an FAD PS1 transgenic strain (PS1M146L-tg), then Aβ deposition occurred in a similar pattern to APP transgenic animals. However, the Aβ deposits in APPSL/SL/PS1M146L-tg mice began much later and developed more slowly than APP transgenic animals, with no apparent neurodegeneration 75. Further characterization revealed that APPSL/SL/PS1M146L-tg mice have increased levels of Aβ peptides at 10 months of age and amyloid plaques at 14 months of age. Microdialysis showed that the hippocampal extracellular acetylcholine (ACh) levels of 15–27 months old APPSL/SL/PS1M146L-tg mice are slightly but significantly reduced. However, there were no major changes found in stimulated ACh release and overall central cholinergic function was not affected 76.
The KI model attempting to genetically mimic human FAD to the greatest extent was generated by Kawasumi et al. 77. This strain contains an APP V642I mutant KI allele without modifying the mouse Aβ sequence, which was then studied in a heterozygous state. APP V642I/+ animals appear normal compared to their wild-type littermates up to 2.5 years of age. However, their long-term spatial memory is significantly impaired and acquisition of spatial memory is slightly affected. Histologically, the ratio of Aβ42 (43)/Aβ40 is greatly increased in mutants, but there are no plaques or neurofibrillary tangles in the mutant brains.
All the mouse models mentioned above are summarized in Table 1.
Table 1. Summary and comparison of APP knock-in mouse models.
| APP allele | APP knock-in mutation(s) | Mouse model | Aβ Plaque | Major findings |
|---|---|---|---|---|
| APPNLh | K670N/M671L (Swedish mutation) Humanized Aβ | APPNLh/NLh | No | The human Aβ production is 9-fold higher than normal human brain tissue 70. |
| APPNLh | K670N/M671L (Swedish mutation) Humanized Aβ | APPNLh/NLh/PS-1P264L/P264L | Yes | 1. Aβ deposition begins at 6 m/o 71. 2. The altered gene expression profile of APPNLh/NLh/PS-1P264L/P264L animals after plaque deposition is shared by two transgenic models as well as human AD brains 72. 3. APPNLh/NLh/PS-1P264L/P264L animals show age-related decreasing of AMPA receptor number and activity. They also exhibit age-related deficits in LTP and LTD studies as well as memory impairment in water maze test 73. 4. APPNLh/NLh/PS-1P264L/P264L mice have long-lasting and selective impairment in adult hippocampal neurogenesis at 9-18 m/o 74. |
| APPSL | K670N/M671L; (Swedish mutation) V717F (London mutation) Humanized Aβ | APPSL/SL/PS1M146L-tg | Yes | 1. APPSL/SL single knock-in animals do not show any Aβ deposits 75. 2. APPSL/SL/PS1M146L-tg mice have increased levels of Aβ peptides at 10 m/o and amyloid plaques at 14 m/o 75. 3. Microdialysis study indicates that the hippocampal extracellular acetylcholine (ACh) levels of 15-27 m/o APPSL/SL/PS1M146L-tg mice are slightly but significantly reduced 76. |
| APPV642I | V642I | APPV642I/+ | No | Long-term spatial memory is significantly impaired and acquisition of spatial memory is slightly affected 77. |
Concluding Remarks
In vivo models of APP family members provide us with a considerable amount of insight into the physiological function of APP. It is now well known that APP and its homologs are important for early developmental events, such as mediating cell adhesion, cell migration and synaptogenesis. These models also highlight several intriguing directions for future study. First, full-length APP is vital for neuromuscular synapse assembly and possibly central synaptogenesis. Second, APPsβ, independent of full-length APP, can regulate the expression of certain genes like TTR and Klotho. The receptor mediating such an event remains to be identified. Third, APP/APLP2 germline dKO animals are early postnatal lethal, whereas animals with a conditional deletion of APP only in the central nervous system (CNS) on an APLP2-null background are viable. This finding strongly suggests that APP has vital, yet undiscovered, functions outside the CNS. Finally, it is known that APP is strongly upregulated in brains that have experienced TBI in both human patients and in mice. TBI-induced APP upregulation may contribute to higher Aβ production and increased risk of AD in later life. Since studies in Drosophila demonstrated that the mortality rate of Appl-deficient flies is higher than controls after TBI treatment, it will be extremely interesting to know how APP/APLP2 conditional KO animals survive TBI and to further dissect the functional role of APP in post-TBI brain repair.
All of the FAD APP KI AD mouse models share mild phenotypes and pathological manifestations. To generate plaque pathology, both an APP KI mutation and an FAD PS1 allele are needed. Considering that a heterozygous APP FAD mutation is able to trigger early onset AD in humans, the effects of these mutations in mice are considerably weaker. This difference may be due to the short-life span of mice and the intrinsic physiological differences between humans and mice. Nevertheless, these APP KI models provide us with an alternative platform and a more physiologically relevant disease model to study human AD. More comprehensive and sensitive assays will be required for better characterizing APP KI mice and consequently AD pathogenesis in the future.
Acknowledgments
The authors' work cited in this review was supported by grants from NIH (AG020670, AG032051 and AG033467) and the American Health and Assistance Foundation (A2008-052).
References
- Goldgaber D, Lerman MI, McBride OW, Saffiotti U, Gajdusek DC. Characterization and chromosomal localization of a cDNA encoding brain amyloid of Alzheimer's disease. Science. 1987;235:877–880. doi: 10.1126/science.3810169. [DOI] [PubMed] [Google Scholar]
- Kang J, Lemaire HG, Unterbeck A, et al. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987;325:733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Tanzi RE, Gusella JF, Watkins PC, et al. Amyloid beta protein gene: cDNA, mRNA distribution, and genetic linkage near the Alzheimer locus. Science. 1987;235:880–884. doi: 10.1126/science.2949367. [DOI] [PubMed] [Google Scholar]
- Chow VW, Mattson MP, Wong PC, Gleichmann M. An overview of APP processing enzymes and products. Neuromolecular Med. 2010;12:1–12. doi: 10.1007/s12017-009-8104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidemann A, Eggert S, Reinhard FB, et al. A novel epsilon-cleavage within the transmembrane domain of the Alzheimer amyloid precursor protein demonstrates homology with Notch processing. Biochemistry. 2002;41:2825–2835. doi: 10.1021/bi015794o. [DOI] [PubMed] [Google Scholar]
- von Koch CS, Zheng H, Chen H, et al. Generation of APLP2 KO mice and early postnatal lethality in APLP2/APP double KO mice. Neurobiol Aging. 1997;18:661–669. doi: 10.1016/s0197-4580(97)00151-6. [DOI] [PubMed] [Google Scholar]
- Heber S, Herms J, Gajic V, et al. Mice with combined gene knock-outs reveal essential and partially redundant functions of amyloid precursor protein family members. J Neurosci. 2000;20:7951–7963. doi: 10.1523/JNEUROSCI.20-21-07951.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong RA, Lantos PL, Cairns NJ. What determines the molecular composition of abnormal protein aggregates in neurodegenerative disease. Neuropathology. 2008;28:351–365. doi: 10.1111/j.1440-1789.2008.00916.x. [DOI] [PubMed] [Google Scholar]
- Korczyn AD. The amyloid cascade hypothesis. Alzheimers Dement. 2008;4:176–178. doi: 10.1016/j.jalz.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Zheng H, Koo EH. The amyloid precursor protein: beyond amyloid. Mol Neurodegener. 2006;1:5. doi: 10.1186/1750-1326-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daigle I, Li C. apl-1, a Caenorhabditis elegans gene encoding a protein related to the human beta-amyloid protein precursor. Proc Natl Acad Sci USA. 1993;90:12045–12049. doi: 10.1073/pnas.90.24.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slunt HH, Thinakaran G, Von Koch C, Lo AC, Tanzi RE, Sisodia SS. Expression of a ubiquitous, cross-reactive homologue of the mouse beta-amyloid precursor protein (APP) J Biol Chem. 1994;269:2637–2644. [PubMed] [Google Scholar]
- Wasco W, Bupp K, Magendantz M, Gusella JF, Tanzi RE, Solomon F. Identification of a mouse brain cDNA that encodes a protein related to the Alzheimer disease-associated amyloid beta protein precursor. Proc Natl Acad Sci USA. 1992;89:10758–10762. doi: 10.1073/pnas.89.22.10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasco W, Gurubhagavatula S, Paradis MD, et al. Isolation and characterization of APLP2 encoding a homologue of the Alzheimer's associated amyloid beta protein precursor. Nat Genet. 1993;5:95–100. doi: 10.1038/ng0993-95. [DOI] [PubMed] [Google Scholar]
- Hornsten A, Lieberthal J, Fadia S, et al. APL-1, a Caenorhabditis elegans protein related to the human beta-amyloid precursor protein, is essential for viability Proc Natl Acad Sci USA 20071041971–1976.Epub 2007 Jan 1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese M, Antebi A, Zheng H. Intracellular trafficking and synaptic function of APL-1 in Caenorhabditis elegans. PLoS ONE. 2010;5:e 12790. doi: 10.1371/journal.pone.0012790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hada K, Asahina M, Hasegawa H, Kanaho Y, Slack FJ, Niwa R. The nuclear receptor gene nhr-25 plays multiple roles in the Caenorhabditis elegans heterochronic gene network to control the larva-to-adult transition. Dev Biol. 2010;344:1100–1109. doi: 10.1016/j.ydbio.2010.05.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Wang B, Wang Z, et al. Soluble amyloid precursor protein (APP) regulates transthyretin and Klotho gene expression without rescuing the essential function of APP. Proc Natl Acad Sci USA. 2010;107:17362–17367. doi: 10.1073/pnas.1012568107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Wang Z, Wang B, et al. Genetic dissection of the amyloid precursor protein in developmental function and amyloid pathogenesis. J Biol Chem. 2010;285:30598–30605. doi: 10.1074/jbc.M110.137729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, Tully T, White K. Human amyloid precursor protein ameliorates behavioral deficit of flies deleted for Appl gene. Neuron. 1992;9:595–605. doi: 10.1016/0896-6273(92)90024-8. [DOI] [PubMed] [Google Scholar]
- Gunawardena S, Goldstein LS. Disruption of axonal transport and neuronal viability by amyloid precursor protein mutations in Drosophila. Neuron. 2001;32:389–401. doi: 10.1016/s0896-6273(01)00496-2. [DOI] [PubMed] [Google Scholar]
- Torroja L, Packard M, Gorczyca M, White K, Budnik V. The Drosophila beta-amyloid precursor protein homolog promotes synapse differentiation at the neuromuscular junction. J Neurosci. 1999;19:7793–7803. doi: 10.1523/JNEUROSCI.19-18-07793.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley J, Packard M, Ataman B, Budnik V. Fasciclin II signals new synapse formation through amyloid precursor protein and the scaffolding protein dX11/Mint. J Neurosci. 2005;25:5943–5955. doi: 10.1523/JNEUROSCI.1144-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merdes G, Soba P, Loewer A, Bilic MV, Beyreuther K, Paro R. Interference of human and Drosophila APP and APP-like proteins with PNS development in Drosophila. EMBO J. 2004;23:4082–4095. doi: 10.1038/sj.emboj.7600413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyssen M, Ayaz D, Hebert SS, Reeve S, De Strooper B, Hassan BA. Amyloid precursor protein promotes post-developmental neurite arborization in the Drosophila brain. EMBO J. 2005;24:2944–2955. doi: 10.1038/sj.emboj.7600757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi P, Liang JO, DiMonte K, Sullivan J, Pimplikar SW. Amyloid precursor protein is required for convergent-extension movements during Zebrafish development. Dev Biol. 2009;335:1–11. doi: 10.1016/j.ydbio.2009.07.041. [DOI] [PubMed] [Google Scholar]
- Lee JA, Cole GJ. Generation of transgenic zebrafish expressing green fluorescent protein under control of zebrafish amyloid precursor protein gene regulatory elements. Zebrafish. 2007;4:277–286. doi: 10.1089/zeb.2007.0516. [DOI] [PubMed] [Google Scholar]
- Zheng H, Jiang M, Trumbauer ME, et al. beta-Amyloid precursor protein-deficient mice show reactive gliosis and decreased locomotor activity. Cell. 1995;81:525–531. doi: 10.1016/0092-8674(95)90073-x. [DOI] [PubMed] [Google Scholar]
- Li ZW, Stark G, Gotz J, et al. Generation of mice with a 200-kb amyloid precursor protein gene deletion by Cre recombinase-mediated site-specific recombination in embryonic stem cells. Proc Natl Acad Sci USA. 1996;93:6158–6162. doi: 10.1073/pnas.93.12.6158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller U, Cristina N, Li ZW, et al. Behavioral and anatomical deficits in mice homozygous for a modified beta-amyloid precursor protein gene. Cell. 1994;79:755–765. doi: 10.1016/0092-8674(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Wang Z, Wang B, Yang L, et al. Presynaptic and postsynaptic interaction of the amyloid precursor protein promotes peripheral and central synaptogenesis. J Neurosci. 2009;29:10788–10801. doi: 10.1523/JNEUROSCI.2132-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallm JP, Tschape JA, Hick M, Filippov MA, Muller UC. Generation of conditional null alleles for APP and APLP2. Genesis. 2010;48:200–206. doi: 10.1002/dvg.20601. [DOI] [PubMed] [Google Scholar]
- Ring S, Weyer SW, Kilian SB, et al. The secreted beta-amyloid precursor protein ectodomain APPs alpha is sufficient to rescue the anatomical, behavioral, and electrophysiological abnormalities of APP-deficient mice. J Neurosci. 2007;27:7817–7826. doi: 10.1523/JNEUROSCI.1026-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbagallo AP, Weldon R, Tamayev R, et al. Tyr(682) in the intracellular domain of APP regulates amyloidogenic APP processing in vivo. PLoS One. 2010;5:e 15503. doi: 10.1371/journal.pone.0015503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbagallo AP, Wang Z, Zheng H, D'Adamio L. The intracellular threonine of amyloid precursor protein that is essential for docking of pin1 is despensable for developmental function. PLoS One. 2011;6:e 18006. doi: 10.1371/journal.pone.0018006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach JP, Muller U, Leist M, Li ZW, Nicotera P, Aguzzi A. Hypersensitivity to seizures in beta-amyloid precursor protein deficient mice. Cell Death Differ. 1998;5:858–866. doi: 10.1038/sj.cdd.4400391. [DOI] [PubMed] [Google Scholar]
- White AR, Multhaup G, Maher F, et al. The Alzheimer's disease amyloid precursor protein modulates copper-induced toxicity and oxidative stress in primary neuronal cultures. J Neurosci. 1999;19:9170–9179. doi: 10.1523/JNEUROSCI.19-21-09170.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duce JA, Tsatsanis A, Cater MA, et al. Iron-export ferroxidase activity of beta-amyloid precursor protein is inhibited by zinc in Alzheimer's disease. Cell. 2010;142:857–867. doi: 10.1016/j.cell.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm MO, Grimm HS, Patzold AJ, et al. Regulation of cholesterol and sphingomyelin metabolism by amyloid-beta and presenilin. Nat Cell Biol. 2005;7:1118–1123. doi: 10.1038/ncb1313. [DOI] [PubMed] [Google Scholar]
- Needham BE, Wlodek ME, Ciccotosto GD, et al. Identification of the Alzheimer's disease amyloid precursor protein (APP) and its homologue APLP2 as essential modulators of glucose and insulin homeostasis and growth. J Pathol. 2008;215:155–163. doi: 10.1002/path.2343. [DOI] [PubMed] [Google Scholar]
- Dawson GR, Seabrook GR, Zheng H, et al. Age-related cognitive deficits, impaired long-term potentiation and reduction in synaptic marker density in mice lacking the beta-amyloid precursor protein. Neuroscience. 1999;90:1–13. doi: 10.1016/s0306-4522(98)00410-2. [DOI] [PubMed] [Google Scholar]
- Phinney AL, Calhoun ME, Wolfer DP, Lipp HP, Zheng H, Jucker M. No hippocampal neuron or synaptic bouton loss in learning-impaired aged beta-amyloid precursor protein-null mice. Neuroscience. 1999;90:1207–1216. doi: 10.1016/s0306-4522(98)00645-9. [DOI] [PubMed] [Google Scholar]
- Senechal Y, Kelly PH, Dev KK. Amyloid precursor protein knockout mice show age-dependent deficits in passive avoidance learning. Behav Brain Res. 2008;186:126–132. doi: 10.1016/j.bbr.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Seabrook GR, Smith DW, Bowery BJ, et al. Mechanisms contributing to the deficits in hippocampal synaptic plasticity in mice lacking amyloid precursor protein. Neuropharmacology. 1999;38:349–359. doi: 10.1016/s0028-3908(98)00204-4. [DOI] [PubMed] [Google Scholar]
- Yang L, Wang Z, Wang B, Justice NJ, Zheng H. Amyloid precursor protein regulates Cav1.2 L-type calcium channel levels and function to influence GABAergic short-term plasticity. J Neurosci. 2009;29:15660–15668. doi: 10.1523/JNEUROSCI.4104-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner T, Fuhrmann M, Burgold S, et al. Gamma-secretase inhibition reduces spine density in vivo via an amyloid precursor protein-dependent pathway. J Neurosci. 2009;29:10405–10409. doi: 10.1523/JNEUROSCI.2288-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KJ, Moussa CE, Lee Y, et al. Beta amyloid-independent role of amyloid precursor protein in generation and maintenance of dendritic spines. Neuroscience. 2010;169:344–356. doi: 10.1016/j.neuroscience.2010.04.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Yang G, Mosier DR, et al. Defective neuromuscular synapses in mice lacking amyloid precursor protein (APP) and APP-Like protein 2. J Neurosci. 2005;25:1219–1225. doi: 10.1523/JNEUROSCI.4660-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Gong YD, Gong K, et al. Reduced synaptic vesicle density and active zone size in mice lacking amyloid precursor protein (APP) and APP-like protein 2. Neurosci Lett. 2005;384:66–71. doi: 10.1016/j.neulet.2005.04.040. [DOI] [PubMed] [Google Scholar]
- Herms J, Anliker B, Heber S, et al. Cortical dysplasia resembling human type 2 lissencephaly in mice lacking all three APP family members. EMBO J. 2004;23:4106–4115. doi: 10.1038/sj.emboj.7600390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenette S, Chang Y, Hiesberger T, et al. Essential roles for the FE65 amyloid precursor protein-interacting proteins in brain development. EMBO J. 2006;25:420–431. doi: 10.1038/sj.emboj.7600926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young-Pearse TL, Bai J, Chang R, Zheng JB, Loturco JJ, Selkoe DJ. A critical function for amyloid precursor protein in neuronal migration revealed by in utero RNA interference. J Neurosci. 2007;27:14459–14469. doi: 10.1523/JNEUROSCI.4701-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norstrom EM, Zhang C, Tanzi R, Sisodia SS. Identification of NEEP21 as a ss-amyloid precursor protein-interacting protein in vivo that modulates amyloidogenic processing in vitro. J Neurosci. 2010;30:15677–15685. doi: 10.1523/JNEUROSCI.4464-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo EH, Squazzo SL, Selkoe DJ, Koo CH. Trafficking of cell-surface amyloid beta-protein precursor. I. Secretion, endocytosis and recycling as detected by labeled monoclonal antibody. J Cell Sci. 1996;109:991–998. doi: 10.1242/jcs.109.5.991. [DOI] [PubMed] [Google Scholar]
- Perez RG, Soriano S, Hayes JD, et al. Mutagenesis identifies new signals for beta-amyloid precursor protein endocytosis, turnover, and the generation of secreted fragments, including Abeta42. J Biol Chem. 1999;274:18851–18856. doi: 10.1074/jbc.274.27.18851. [DOI] [PubMed] [Google Scholar]
- Schwarzman AL, Gregori L, Vitek MP, et al. Transthyretin sequesters amyloid beta protein and prevents amyloid formation. Proc Natl Acad Sci USA. 1994;91:8368–8372. doi: 10.1073/pnas.91.18.8368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Leight SN, Lee VM, et al. Accelerated Abeta deposition in APPswe/PS1deltaE9 mice with hemizygous deletions of TTR (transthyretin) J Neurosci. 2007;27:7006–7010. doi: 10.1523/JNEUROSCI.1919-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum JN, Ye Z, Reixach N, et al. Transthyretin protects Alzheimer's mice from the behavioral and biochemical effects of Abeta toxicity. Proc Natl Acad Sci USA. 2008;105:2681–2686. doi: 10.1073/pnas.0712197105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- Kurosu H, Yamamoto M, Clark JD, et al. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imura A, Tsuji Y, Murata M, et al. alpha-Klotho as a regulator of calcium homeostasis. Science. 2007;316:1615–1618. doi: 10.1126/science.1135901. [DOI] [PubMed] [Google Scholar]
- Barbagallo AP, Wang Z, Zheng H, D'Adamio L. A single tyrosine residue in the amyloid precursor protein intracellular domain is essential for developmental function. J Biol Chem. 2011;286:8717–8721. doi: 10.1074/jbc.C111.219873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King GD, Turner SR. Adaptor protein interactions: modulators of amyloid precursor protein metabolism and Alzheimer's disease risk. Exp Neurol. 2004;185:208–219. doi: 10.1016/j.expneurol.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Zhou D, Noviello C, D'Ambrosio C, Scaloni A, D'Adamio L. Growth factor receptor-bound protein 2 interaction with the tyrosine-phosphorylated tail of amyloid beta precursor protein is mediated by its Src homology 2 domain. J Biol Chem. 2004;279:25374–25380. doi: 10.1074/jbc.M400488200. [DOI] [PubMed] [Google Scholar]
- Russo C, Dolcini V, Salis S, et al. Signal transduction through tyrosine-phosphorylated C-terminal fragments of amyloid precursor protein via an enhanced interaction with Shc/Grb2 adaptor proteins in reactive astrocytes of Alzheimer's disease brain. J Biol Chem. 2002;277:35282–35288. doi: 10.1074/jbc.M110785200. [DOI] [PubMed] [Google Scholar]
- Tarr PE, Roncarati R, Pelicci G, Pelicci PG, D'Adamio L. Tyrosine phosphorylation of the beta-amyloid precursor protein cytoplasmic tail promotes interaction with Shc. J Biol Chem. 2002;277:16798–16804. doi: 10.1074/jbc.M110286200. [DOI] [PubMed] [Google Scholar]
- Tamayev R, Zhou D, D'Adamio L. The interactome of the amyloid beta precursor protein family members is shaped by phosphorylation of their intracellular domains. Mol Neurodegener. 2009;4:28. doi: 10.1186/1750-1326-4-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Zambrano N, Russo T, D'Adamio L. Phosphorylation of a tyrosine in the amyloid-beta protein precursor intracellular domain inhibits Fe65 binding and signaling. J Alzheimers Dis. 2009;16:301–307. doi: 10.3233/JAD-2009-0970. [DOI] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, et al. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Reaume AG, Howland DS, Trusko SP, et al. Enhanced amyloidogenic processing of the beta-amyloid precursor protein in gene-targeted mice bearing the Swedish familial Alzheimer's disease mutations and a 'humanized' Abeta sequence. J Biol Chem. 1996;271:23380–23388. doi: 10.1074/jbc.271.38.23380. [DOI] [PubMed] [Google Scholar]
- Flood DG, Reaume AG, Dorfman KS, et al. FAD mutant PS-1 gene-targeted mice: increased A beta 42 and A beta deposition without APP overproduction. Neurobiol Aging. 2002;23:335–348. doi: 10.1016/s0197-4580(01)00330-x. [DOI] [PubMed] [Google Scholar]
- Wu ZL, Ciallella JR, Flood DG, O'Kane TM, Bozyczko-Coyne D, Savage MJ. Comparative analysis of cortical gene expression in mouse models of Alzheimer's disease. Neurobiol Aging. 2006;27:377–386. doi: 10.1016/j.neurobiolaging.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Chang EH, Savage MJ, Flood DG, et al. AMPA receptor downscaling at the onset of Alzheimer's disease pathology in double knockin mice. Proc Natl Acad Sci USA. 2006;103:3410–3415. doi: 10.1073/pnas.0507313103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, McNeil E, Dressler L, Siman R. Long-lasting impairment in hippocampal neurogenesis associated with amyloid deposition in a knock-in mouse model of familial Alzheimer's disease. Exp Neurol. 2007;204:77–87. doi: 10.1016/j.expneurol.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler C, Ebert U, Baumann K, Schroder H. Alzheimer's disease-like neuropathology of gene-targeted APP-SLxPS1mut mice expressing the amyloid precursor protein at endogenous levels. Neurobiol Dis. 2005;20:528–540. doi: 10.1016/j.nbd.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Hartmann J, Erb C, Ebert U, et al. Central cholinergic functions in human amyloid precursor protein knock-in/presenilin-1 transgenic mice. Neuroscience. 2004;125:1009–1017. doi: 10.1016/j.neuroscience.2004.02.038. [DOI] [PubMed] [Google Scholar]
- Kawasumi M, Chiba T, Yamada M, et al. Targeted introduction of V642I mutation in amyloid precursor protein gene causes functional abnormality resembling early stage of Alzheimer's disease in aged mice. Eur J Neurosci. 2004;19:2826–2838. doi: 10.1111/j.0953-816X.2004.03397.x. [DOI] [PubMed] [Google Scholar]