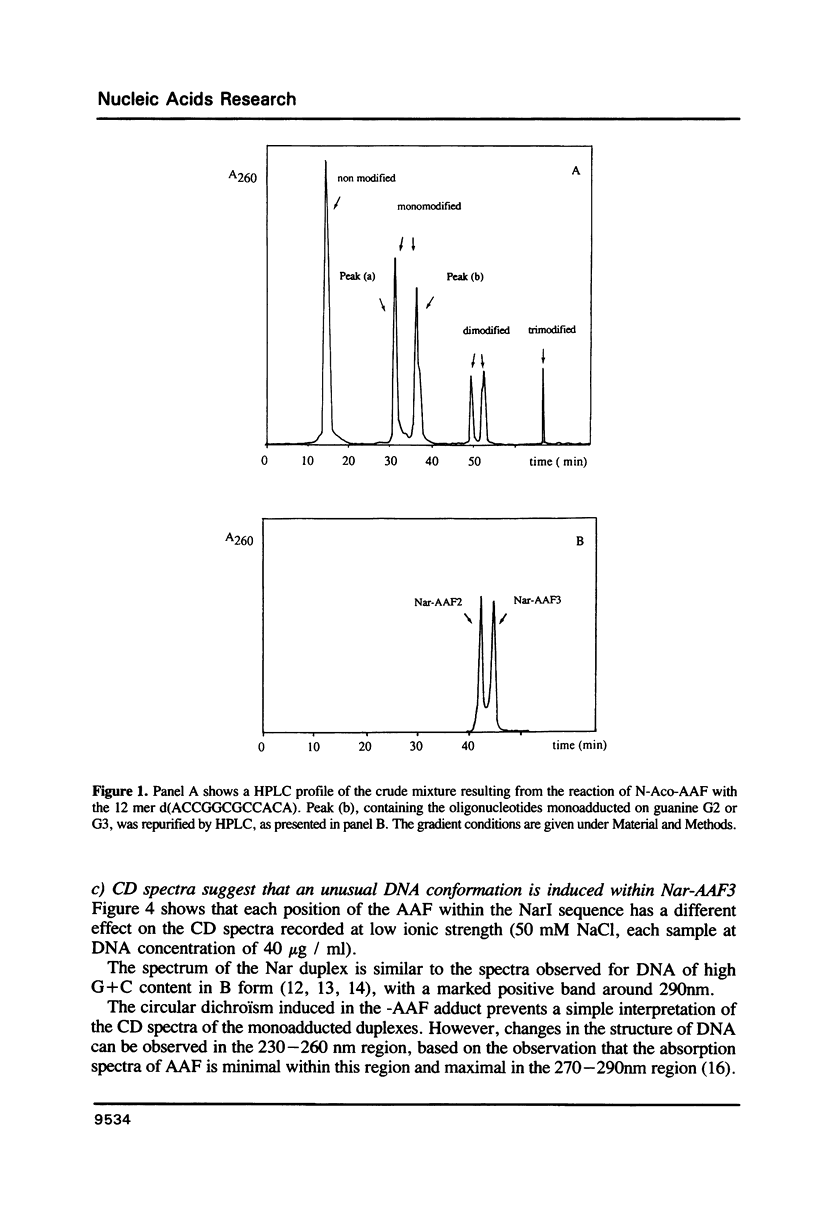
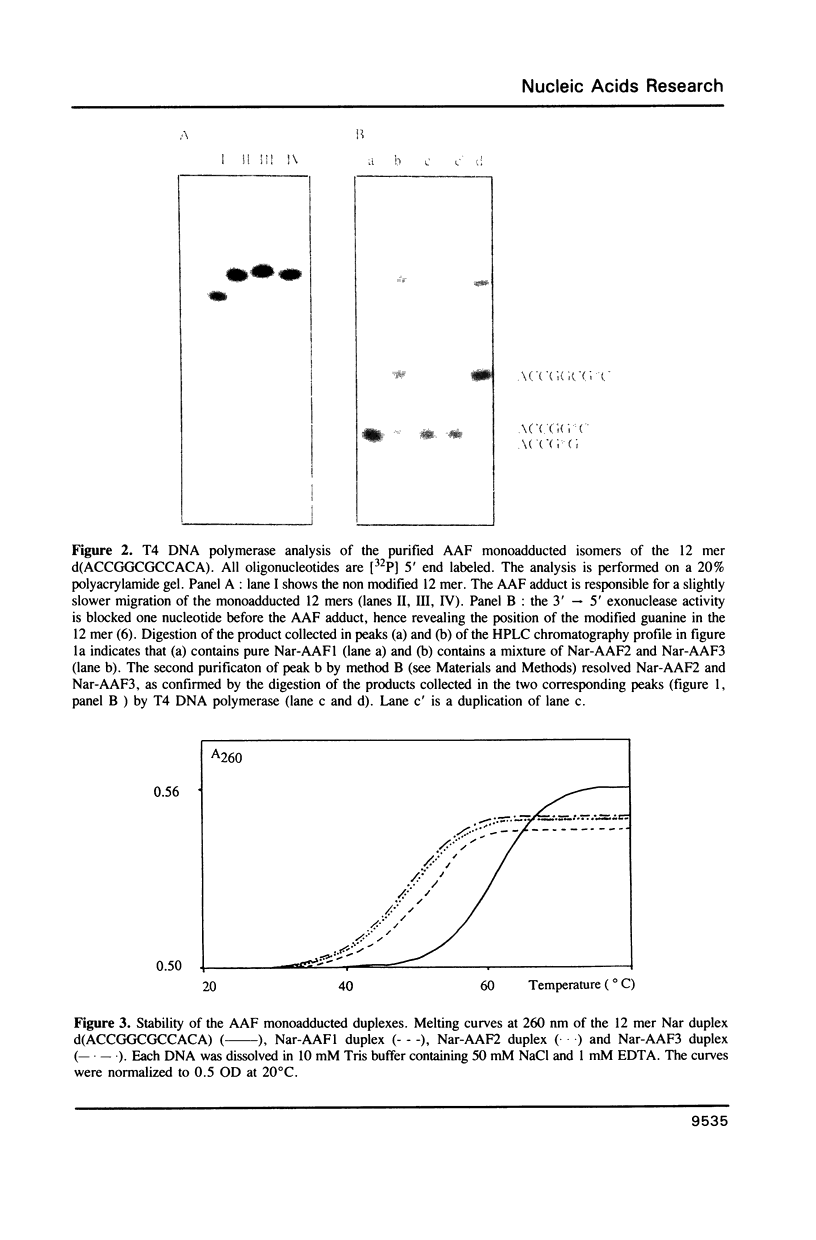
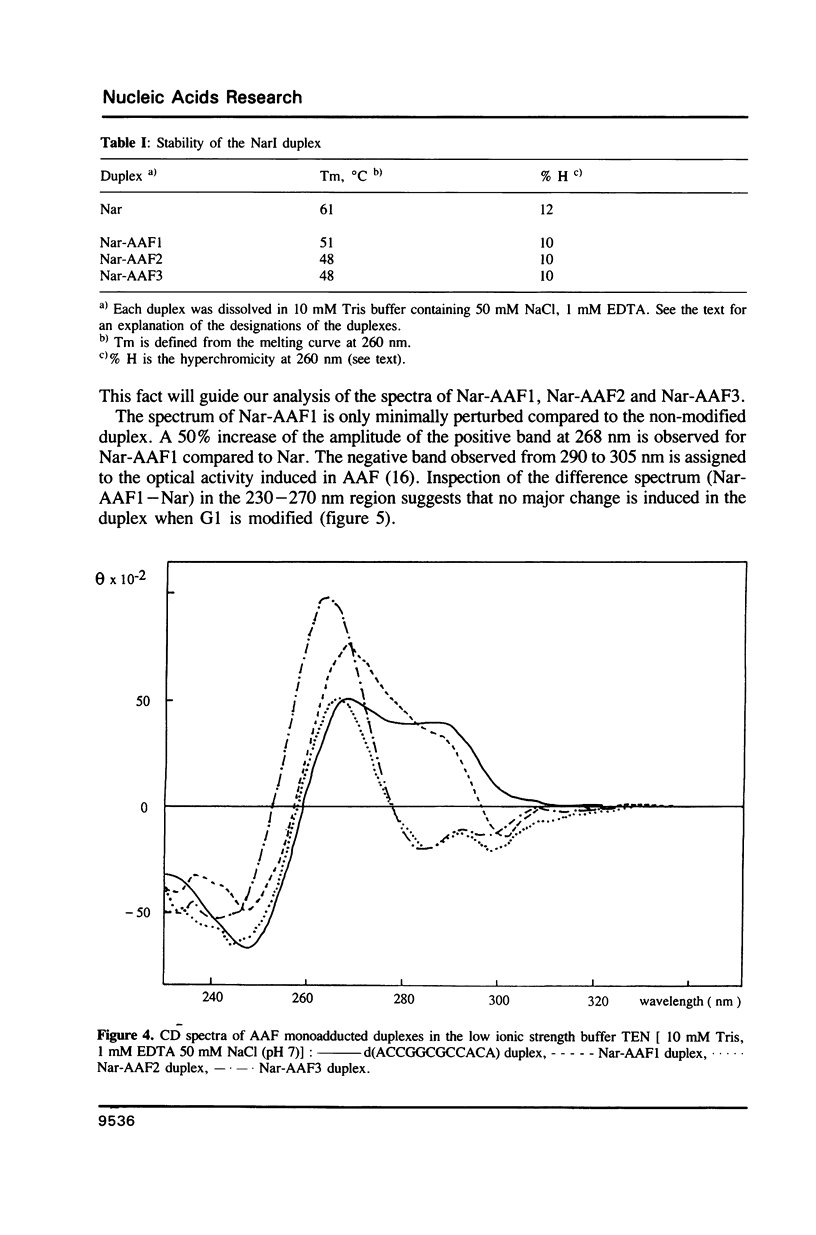
Abstract
The NarI restriction enzyme recognition site, G1G2CG3CC, has been identified as a hotspot for -2 frameshift mutations induced by N-2-acetylaminofluorene (AAF) on the basis of a forward mutation assay in plasmid pBR322 in the bacterium Escherichia coli. AAF binds primarily to the C-8 position of guanine residues, and the three guanines of the NarI site are similarly reactive. Despite this similar chemical reactivity, only binding of AAF to the G3 residue causes the -2 frameshift mutations. To study the mechanisms underlying the specificity of the mutagenic processing further, we monitored the structural changes induced by a single AAF adduct within the NarI site by means of CD spectroscopy and thermal denaturation. The NarI sequence was studied as part of the 12-mer ACCGGCGCCACA. The purification and characterization of the three isomers having a single AAF adduct covalently bound to one of the three guanines of this 12 mer are described. The analysis of the melting profiles of the duplexes formed when these three isomers are annealed with the oligonucleotide of complementary sequence shows the same destabilizing effect of the AAF adduct on the three DNA helices. It is also shown, from the CD spectra, that modification of guanine G1 or G2 by AAF does not induce major changes in the helical structure of DNA. On the other hand, modification of guanine G3 induces a change in the CD signal that suggests the formation of a local left handed structure within the 12-mer duplex. These results show the polymorphic nature of the DNA structure in the vicinity of an AAF adduct.
Full text
PDF


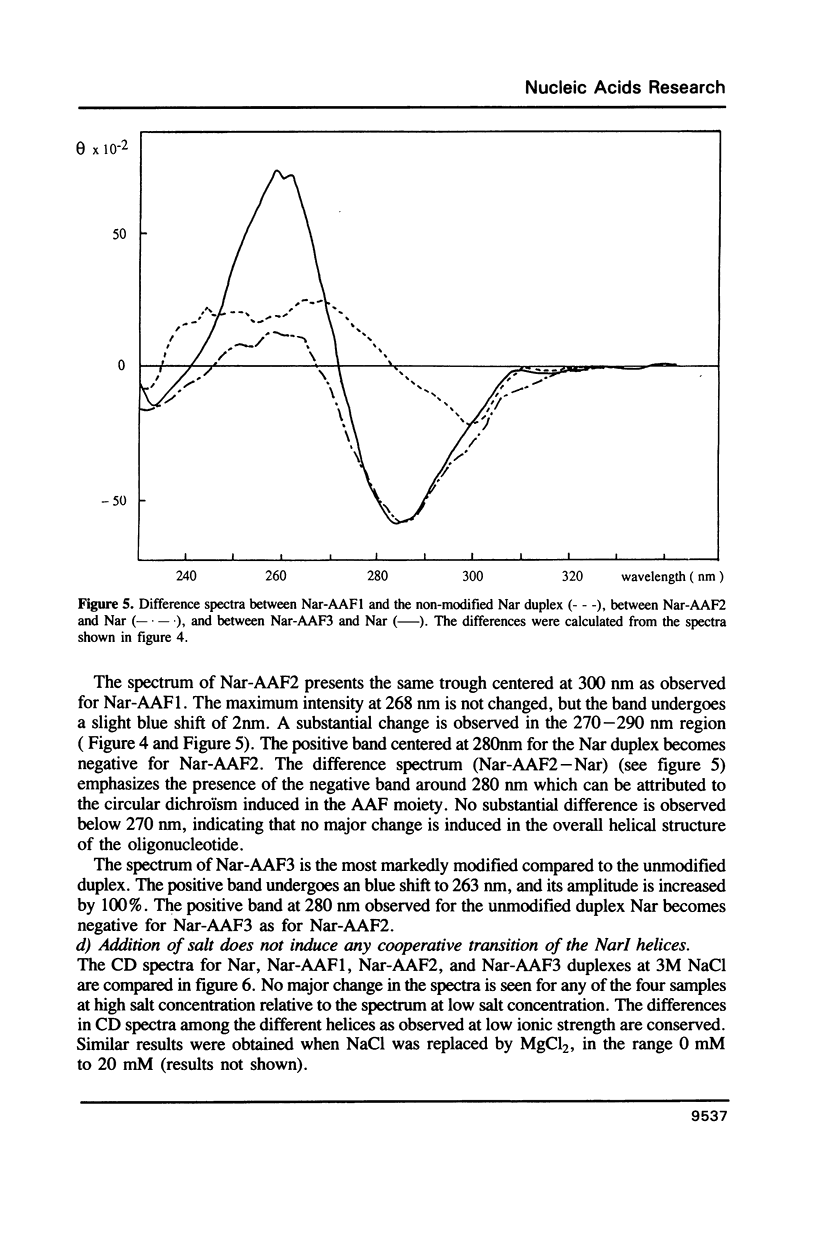
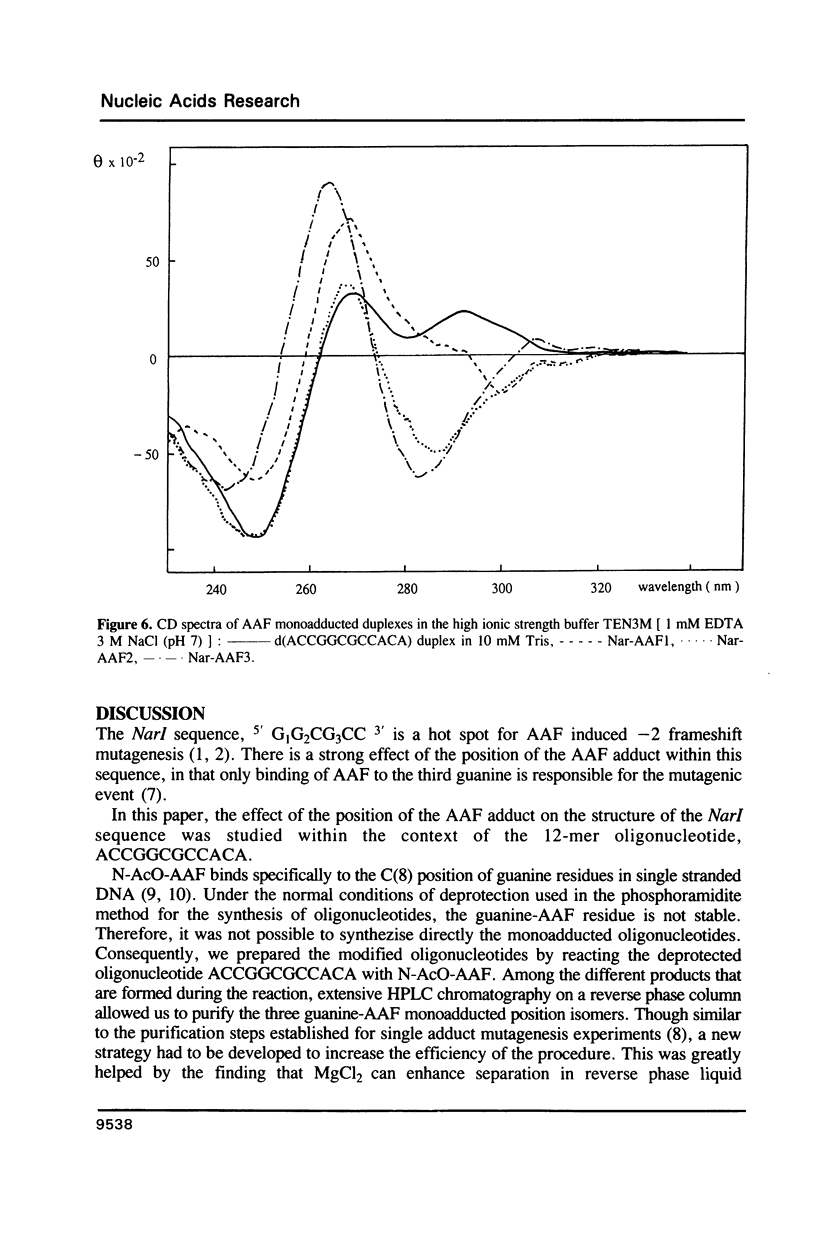



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnott S., Chandrasekaran R., Hall I. H., Puigjaner L. C., Walker J. K., Wang M. DNA secondary structures: helices, wrinkles, and junctions. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):53–65. doi: 10.1101/sqb.1983.047.01.008. [DOI] [PubMed] [Google Scholar]
- Burnouf D., Koehl P., Fuchs R. P. Single adduct mutagenesis: strong effect of the position of a single acetylaminofluorene adduct within a mutation hot spot. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4147–4151. doi: 10.1073/pnas.86.11.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans F. E., Miller D. W., Beland F. A. Sensitivity of the conformation of deoxyguanosine to binding at the C-8 position by N-acetylated and unacetylated 2-aminofluorene. Carcinogenesis. 1980;1(11):955–959. doi: 10.1093/carcin/1.11.955. [DOI] [PubMed] [Google Scholar]
- Fuchs R. P. Arylamidation and arylation by the carcinogen N-2-fluorenylacetamide: a sensitive and rapid radiochemical assay. Anal Biochem. 1978 Dec;91(2):663–673. doi: 10.1016/0003-2697(78)90553-5. [DOI] [PubMed] [Google Scholar]
- Fuchs R. P. DNA binding spectrum of the carcinogen N-acetoxy-N-2-acetylaminofluorene significantly differs from the mutation spectrum. J Mol Biol. 1984 Jul 25;177(1):173–180. doi: 10.1016/0022-2836(84)90063-9. [DOI] [PubMed] [Google Scholar]
- Fuchs R. P., Schwartz N., Daune M. P. Hot spots of frameshift mutations induced by the ultimate carcinogen N-acetoxy-N-2-acetylaminofluorene. Nature. 1981 Dec 17;294(5842):657–659. doi: 10.1038/294657a0. [DOI] [PubMed] [Google Scholar]
- Fuchs R., Daune M. Changes of stability and conformation of DNA following the covalent binding of a carcinogen. FEBS Lett. 1971 May 10;14(4):206–208. doi: 10.1016/0014-5793(71)80618-x. [DOI] [PubMed] [Google Scholar]
- Fuchs R., Daune M. Physical studies on deoxyribonucleic acid after covalent binding of a carcinogen. Biochemistry. 1972 Jul 4;11(14):2659–2666. doi: 10.1021/bi00764a017. [DOI] [PubMed] [Google Scholar]
- Koehl P., Burnouf D., Fuchs R. P. Construction of plasmids containing a unique acetylaminofluorene adduct located within a mutation hot spot. A new probe for frameshift mutagenesis. J Mol Biol. 1989 May 20;207(2):355–364. doi: 10.1016/0022-2836(89)90259-3. [DOI] [PubMed] [Google Scholar]
- Koffel-Schwartz N., Fuchs R. P. Genetic control of AAF-induced mutagenesis at alternating GC sequences: an additional role for RecA. Mol Gen Genet. 1989 Jan;215(2):306–311. doi: 10.1007/BF00339733. [DOI] [PubMed] [Google Scholar]
- Koffel-Schwartz N., Verdier J. M., Bichara M., Freund A. M., Daune M. P., Fuchs R. P. Carcinogen-induced mutation spectrum in wild-type, uvrA and umuC strains of Escherichia coli. Strain specificity and mutation-prone sequences. J Mol Biol. 1984 Jul 25;177(1):33–51. doi: 10.1016/0022-2836(84)90056-1. [DOI] [PubMed] [Google Scholar]
- Kriek E., Miller J. A., Juhl U., Miller E. C. 8-(N-2-fluorenylacetamido)guanosine, an arylamidation reaction product of guanosine and the carcinogen N-acetoxy-N-2-fluorenylacetamide in neutral solution. Biochemistry. 1967 Jan;6(1):177–182. doi: 10.1021/bi00853a029. [DOI] [PubMed] [Google Scholar]
- Kriek E. Persistent binding of a new reaction product of the carcinogen N-hydroxy-N-2-acetylaminofluorene with guanine in rat liver DNA in vivo. Cancer Res. 1972 Oct;32(10):2042–2048. [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M. Salt-induced co-operative conformational change of a synthetic DNA: equilibrium and kinetic studies with poly (dG-dC). J Mol Biol. 1972 Jun 28;67(3):375–396. doi: 10.1016/0022-2836(72)90457-3. [DOI] [PubMed] [Google Scholar]
- Pohl F. M. Polymorphism of a synthetic DNA in solution. Nature. 1976 Mar 25;260(5549):365–366. doi: 10.1038/260365a0. [DOI] [PubMed] [Google Scholar]
- Sage E., Leng M. Conformation of poly(dG-dC) . poly(dG-dC) modified by the carcinogens N-acetoxy-N-acetyl-2-aminofluorene and N-hydroxy-N-2-aminofluorene. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4597–4601. doi: 10.1073/pnas.77.8.4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage E., Leng M. Conformational changes of poly(dG-dC) . poly(dG-dC) modified by the carcinogen N-acetoxy-N-acetyl-2-aminofluorene. Nucleic Acids Res. 1981 Mar 11;9(5):1241–1250. doi: 10.1093/nar/9.5.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santella R. M., Grunberger D., Weinstein I. B., Rich A. Induction of the Z conformation in poly(dG-dC).poly(dG-dC) by binding of N-2-acetylaminofluorene to guanine residues. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1451–1455. doi: 10.1073/pnas.78.3.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheardy R. D. Preliminary spectroscopic characterization of a synthetic DNA oligomer containing a B-Z junction at high salt. Nucleic Acids Res. 1988 Feb 11;16(3):1153–1167. doi: 10.1093/nar/16.3.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheardy R. D., Winkle S. A. Temperature-dependent CD and NMR studies on a synthetic oligonucleotide containing a B-Z junction at high salt. Biochemistry. 1989 Jan 24;28(2):720–725. doi: 10.1021/bi00428a046. [DOI] [PubMed] [Google Scholar]
- Streisinger G., Okada Y., Emrich J., Newton J., Tsugita A., Terzaghi E., Inouye M. Frameshift mutations and the genetic code. This paper is dedicated to Professor Theodosius Dobzhansky on the occasion of his 66th birthday. Cold Spring Harb Symp Quant Biol. 1966;31:77–84. doi: 10.1101/sqb.1966.031.01.014. [DOI] [PubMed] [Google Scholar]
- Streisinger G., Owen J. Mechanisms of spontaneous and induced frameshift mutation in bacteriophage T4. Genetics. 1985 Apr;109(4):633–659. doi: 10.1093/genetics/109.4.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. J., Quigley G. J., Kolpak F. J., van der Marel G., van Boom J. H., Rich A. Left-handed double helical DNA: variations in the backbone conformation. Science. 1981 Jan 9;211(4478):171–176. doi: 10.1126/science.7444458. [DOI] [PubMed] [Google Scholar]
- Wells R. D., Miglietta J. J., Kłysik J., Larson J. E., Stirdivant S. M., Zacharias W. Spectroscopic studies on acetylaminofluorene-modified (dT-dG)n . (dC-dA)n suggest a left-handed conformation. J Biol Chem. 1982 Sep 10;257(17):10166–10171. [PubMed] [Google Scholar]