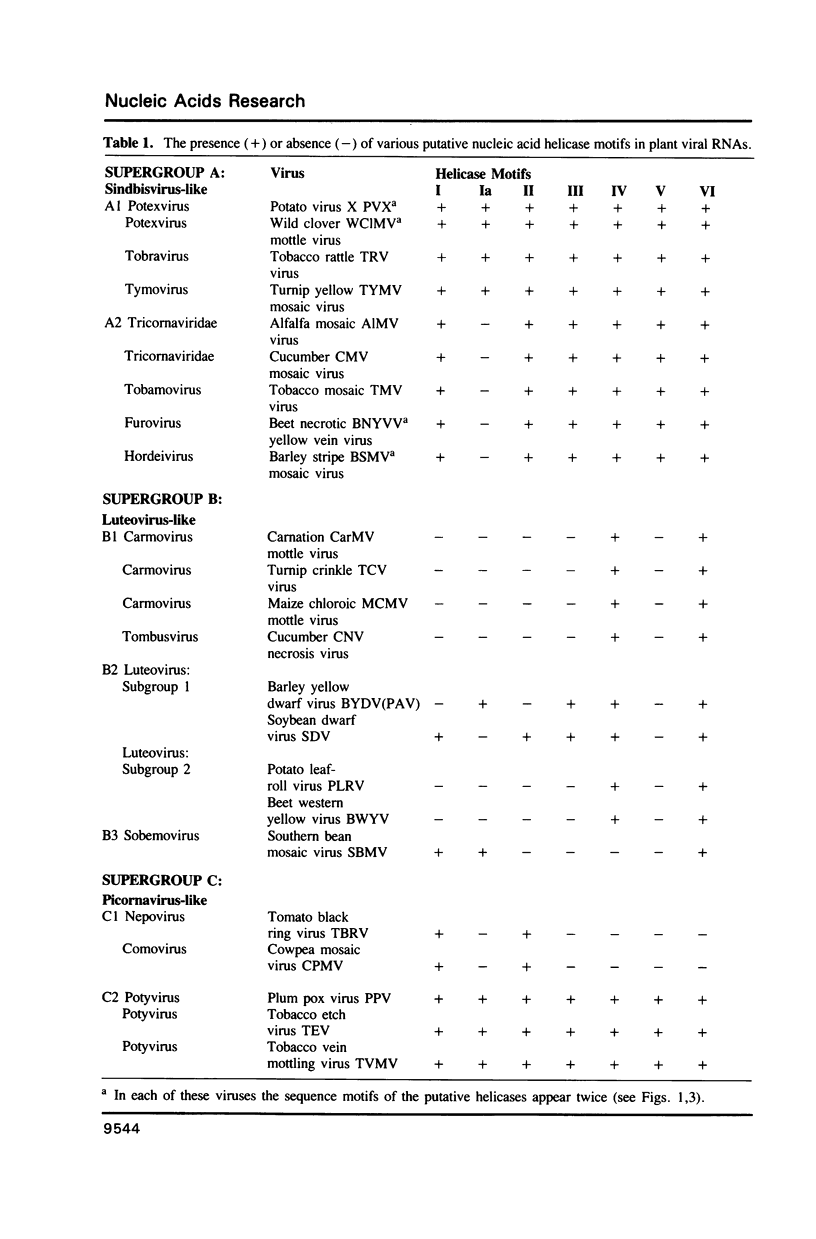
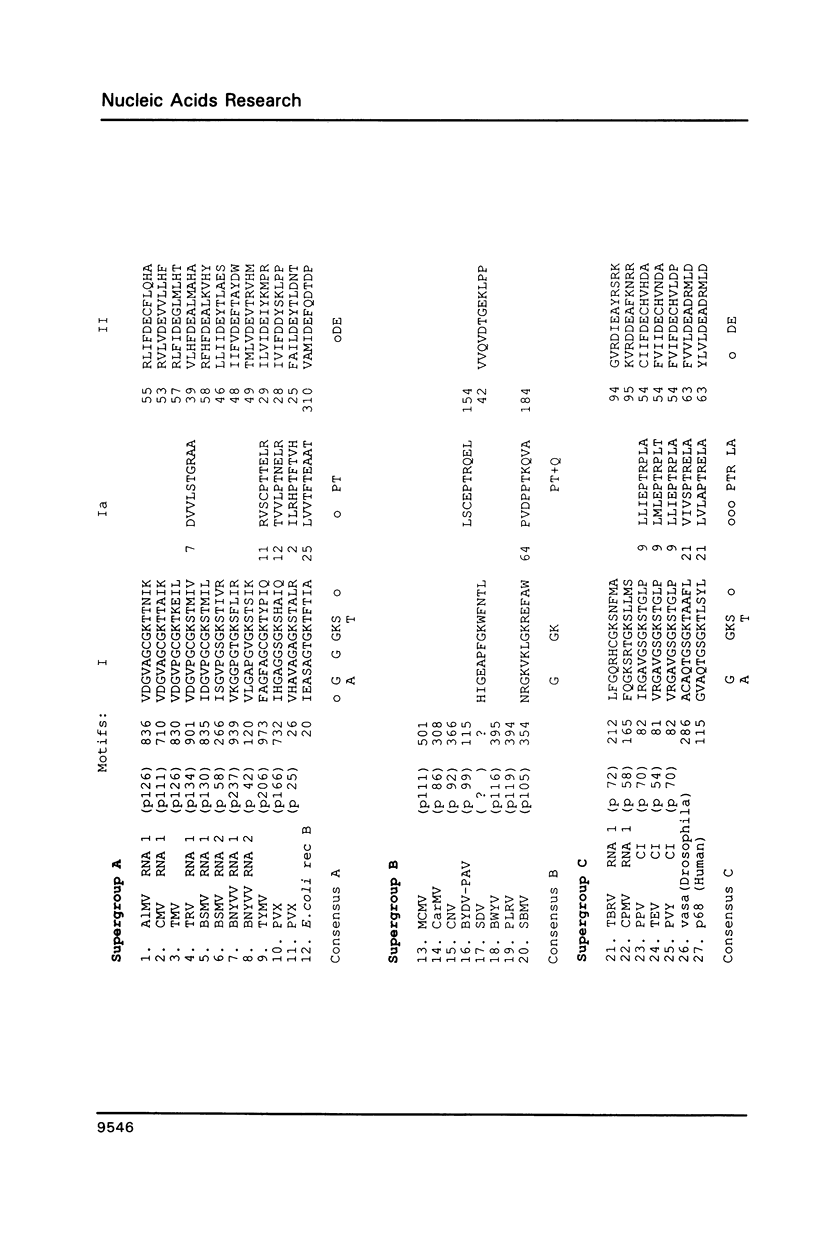
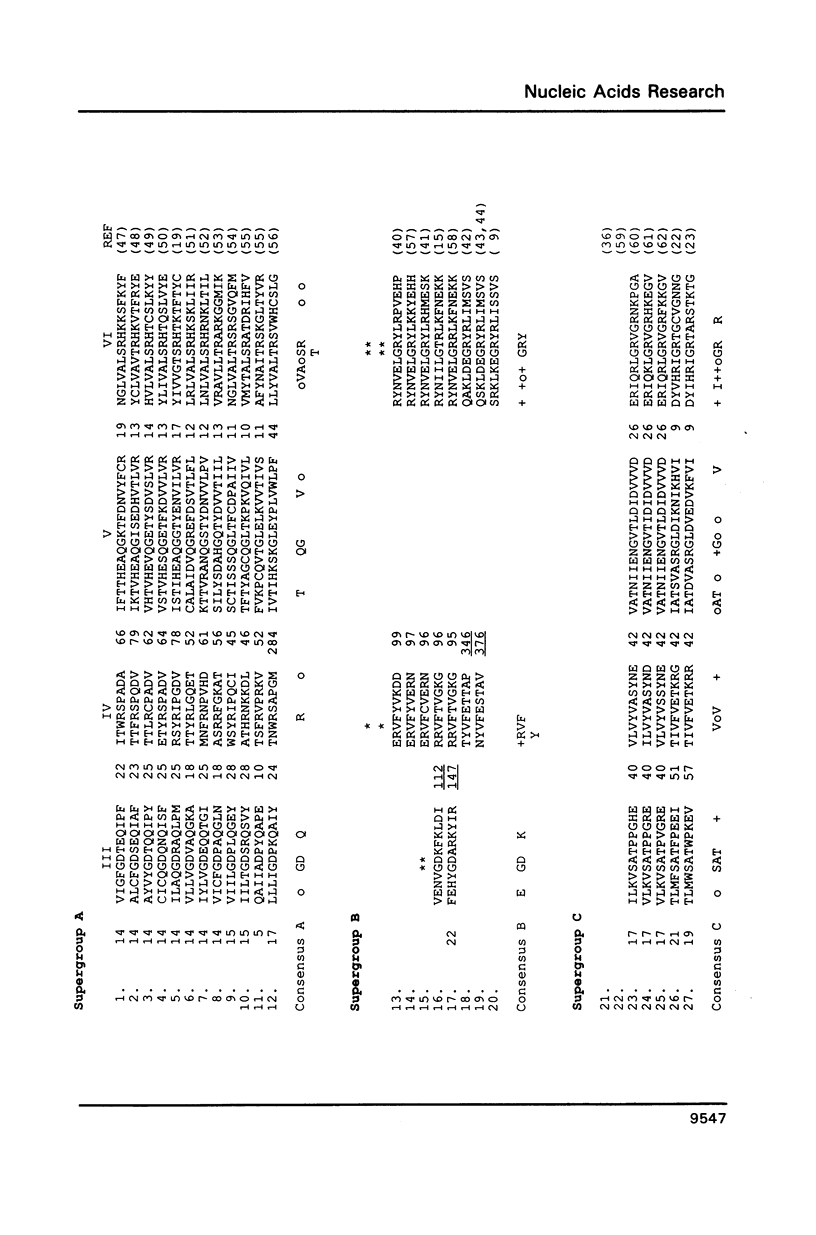
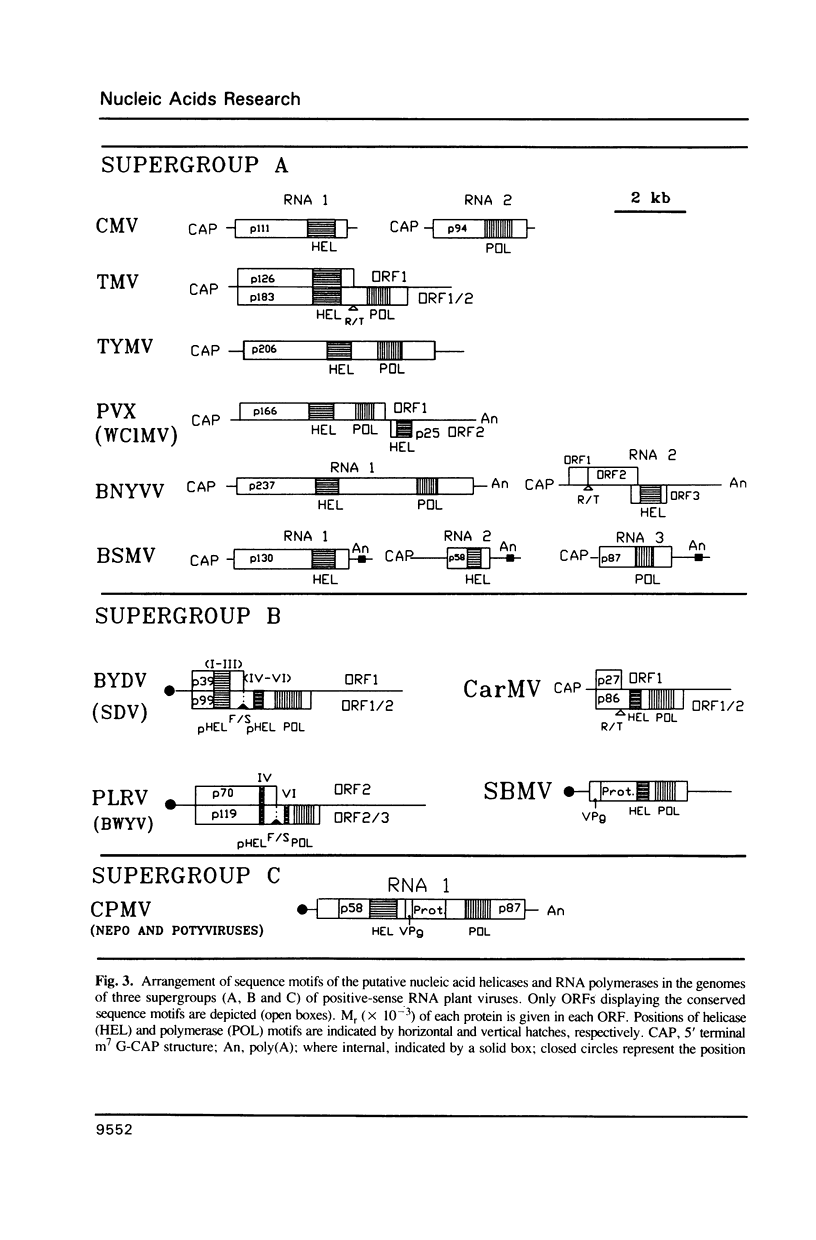
Abstract
Comparative studies of sequence motifs in the RNA polymerases and nucleic acid helicases of positive-sense RNA plant viruses have provided a new scheme for the classification of these pathogens. We propose a new luteovirus supergroup which should be added to the already described Sindbisvirus-like and picornavirus-like supergroups. Sequence motifs of nucleic acid helicases and RNA polymerases which previously were considered to be specific for each of the two supergroups now occur together within this new supergroup. We propose that this new viral supergroup provides an evolutionary link between the other two supergroups.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argos P. A sequence motif in many polymerases. Nucleic Acids Res. 1988 Nov 11;16(21):9909–9916. doi: 10.1093/nar/16.21.9909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal T., Carmichael G. G. RNA replication: function and structure of Qbeta-replicase. Annu Rev Biochem. 1979;48:525–548. doi: 10.1146/annurev.bi.48.070179.002521. [DOI] [PubMed] [Google Scholar]
- Bové J. M., Bové C., Mocquot B. Turnip yellow mosaic virus-RNA synthesis in vitro: evidence for native double-stranded RNA. Biochem Biophys Res Commun. 1968 Aug 13;32(3):480–486. doi: 10.1016/0006-291x(68)90687-6. [DOI] [PubMed] [Google Scholar]
- Bramhill D., Kornberg A. A model for initiation at origins of DNA replication. Cell. 1988 Sep 23;54(7):915–918. doi: 10.1016/0092-8674(88)90102-x. [DOI] [PubMed] [Google Scholar]
- Classification and nomenclature of viruses. Fourth report of the International Committee on Taxonomy of Viruses. Intervirology. 1982;17(1-3):1–199. doi: 10.1159/000149278. [DOI] [PubMed] [Google Scholar]
- Cornelissen B. J., Brederode F. T., Moormann R. J., Bol J. F. Complete nucleotide sequence of alfalfa mosaic virus RNA 1. Nucleic Acids Res. 1983 Mar 11;11(5):1253–1265. doi: 10.1093/nar/11.5.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen B. J., Brederode F. T., Veeneman G. H., van Boom J. H., Bol J. F. Complete nucleotide sequence of alfalfa mosaic virus RNA 2. Nucleic Acids Res. 1983 May 25;11(10):3019–3025. doi: 10.1093/nar/11.10.3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domier L. L., Franklin K. M., Shahabuddin M., Hellmann G. M., Overmeyer J. H., Hiremath S. T., Siaw M. F., Lomonossoff G. P., Shaw J. G., Rhoads R. E. The nucleotide sequence of tobacco vein mottling virus RNA. Nucleic Acids Res. 1986 Jul 11;14(13):5417–5430. doi: 10.1093/nar/14.13.5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. K., Haley B. E., Roth D. A. Photoaffinity labeling of a viral induced protein from tobacco. Characterization of nucleotide-binding properties. J Biol Chem. 1985 Jun 25;260(12):7800–7804. [PubMed] [Google Scholar]
- Finch P. W., Storey A., Chapman K. E., Brown K., Hickson I. D., Emmerson P. T. Complete nucleotide sequence of the Escherichia coli recB gene. Nucleic Acids Res. 1986 Nov 11;14(21):8573–8582. doi: 10.1093/nar/14.21.8573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford M. J., Anton I. A., Lane D. P. Nuclear protein with sequence homology to translation initiation factor eIF-4A. Nature. 1988 Apr 21;332(6166):736–738. doi: 10.1038/332736a0. [DOI] [PubMed] [Google Scholar]
- Forster R. L., Bevan M. W., Harbison S. A., Gardner R. C. The complete nucleotide sequence of the potexvirus white clover mosaic virus. Nucleic Acids Res. 1988 Jan 11;16(1):291–303. doi: 10.1093/nar/16.1.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franssen H., Leunissen J., Goldbach R., Lomonossoff G., Zimmern D. Homologous sequences in non-structural proteins from cowpea mosaic virus and picornaviruses. EMBO J. 1984 Apr;3(4):855–861. doi: 10.1002/j.1460-2075.1984.tb01896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargouri R., Joshi R. L., Bol J. F., Astier-Manifacier S., Haenni A. L. Mechanism of synthesis of turnip yellow mosaic virus coat protein subgenomic RNA in vivo. Virology. 1989 Aug;171(2):386–393. doi: 10.1016/0042-6822(89)90606-5. [DOI] [PubMed] [Google Scholar]
- Goelet P., Lomonossoff G. P., Butler P. J., Akam M. E., Gait M. J., Karn J. Nucleotide sequence of tobacco mosaic virus RNA. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5818–5822. doi: 10.1073/pnas.79.19.5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldbach R., Wellink J. Evolution of plus-strand RNA viruses. Intervirology. 1988;29(5):260–267. doi: 10.1159/000150054. [DOI] [PubMed] [Google Scholar]
- Gorbalenya A. E., Blinov V. M., Donchenko A. P., Koonin E. V. An NTP-binding motif is the most conserved sequence in a highly diverged monophyletic group of proteins involved in positive strand RNA viral replication. J Mol Evol. 1989 Mar;28(3):256–268. doi: 10.1007/BF02102483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A. E., Donchenko A. P., Blinov V. M., Koonin E. V. Cysteine proteases of positive strand RNA viruses and chymotrypsin-like serine proteases. A distinct protein superfamily with a common structural fold. FEBS Lett. 1989 Jan 30;243(2):103–114. doi: 10.1016/0014-5793(89)80109-7. [DOI] [PubMed] [Google Scholar]
- Gorbalenya A. E., Koonin E. V. Birnavirus RNA polymerase is related to polymerases of positive strand RNA viruses. Nucleic Acids Res. 1988 Aug 11;16(15):7735–7735. doi: 10.1093/nar/16.15.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A. E., Koonin E. V., Blinov V. M., Donchenko A. P. Sobemovirus genome appears to encode a serine protease related to cysteine proteases of picornaviruses. FEBS Lett. 1988 Aug 29;236(2):287–290. doi: 10.1016/0014-5793(88)80039-5. [DOI] [PubMed] [Google Scholar]
- Gorbalenya A. E., Koonin E. V., Donchenko A. P., Blinov V. M. A novel superfamily of nucleoside triphosphate-binding motif containing proteins which are probably involved in duplex unwinding in DNA and RNA replication and recombination. FEBS Lett. 1988 Aug 1;235(1-2):16–24. doi: 10.1016/0014-5793(88)81226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A. E., Koonin E. V., Donchenko A. P., Blinov V. M. Two related superfamilies of putative helicases involved in replication, recombination, repair and expression of DNA and RNA genomes. Nucleic Acids Res. 1989 Jun 26;17(12):4713–4730. doi: 10.1093/nar/17.12.4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilley H., Carrington J. C., Balàzs E., Jonard G., Richards K., Morris T. J. Nucleotide sequence and genome organization of carnation mottle virus RNA. Nucleic Acids Res. 1985 Sep 25;13(18):6663–6677. doi: 10.1093/nar/13.18.6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson G., Armour S. L., Gamboa G. C., Burgett S. G., Shepherd J. W. Nucleotide sequence of barley stripe mosaic virus RNA alpha: RNA alpha encodes a single polypeptide with homology to corresponding proteins from other viruses. Virology. 1989 Jun;170(2):370–377. doi: 10.1016/0042-6822(89)90427-3. [DOI] [PubMed] [Google Scholar]
- Gustafson G., Armour S. L. The complete nucleotide sequence of RNA beta from the type strain of barley stripe mosaic virus. Nucleic Acids Res. 1986 May 12;14(9):3895–3909. doi: 10.1093/nar/14.9.3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson G., Hunter B., Hanau R., Armour S. L., Jackson A. O. Nucleotide sequence and genetic organization of barley stripe mosaic virus RNA gamma. Virology. 1987 Jun;158(2):394–406. doi: 10.1016/0042-6822(87)90211-x. [DOI] [PubMed] [Google Scholar]
- Hamilton W. D., Boccara M., Robinson D. J., Baulcombe D. C. The complete nucleotide sequence of tobacco rattle virus RNA-1. J Gen Virol. 1987 Oct;68(Pt 10):2563–2575. doi: 10.1099/0022-1317-68-10-2563. [DOI] [PubMed] [Google Scholar]
- Haseloff J., Goelet P., Zimmern D., Ahlquist P., Dasgupta R., Kaesberg P. Striking similarities in amino acid sequence among nonstructural proteins encoded by RNA viruses that have dissimilar genomic organization. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4358–4362. doi: 10.1073/pnas.81.14.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirling H., Scheffner M., Restle T., Stahl H. RNA helicase activity associated with the human p68 protein. Nature. 1989 Jun 15;339(6225):562–564. doi: 10.1038/339562a0. [DOI] [PubMed] [Google Scholar]
- Hodgman T. C. A new superfamily of replicative proteins. Nature. 1988 May 5;333(6168):22–23. doi: 10.1038/333022b0. [DOI] [PubMed] [Google Scholar]
- Huisman M. J., Linthorst H. J., Bol J. F., Cornelissen J. C. The complete nucleotide sequence of potato virus X and its homologies at the amino acid level with various plus-stranded RNA viruses. J Gen Virol. 1988 Aug;69(Pt 8):1789–1798. doi: 10.1099/0022-1317-69-8-1789. [DOI] [PubMed] [Google Scholar]
- Kamer G., Argos P. Primary structural comparison of RNA-dependent polymerases from plant, animal and bacterial viruses. Nucleic Acids Res. 1984 Sep 25;12(18):7269–7282. doi: 10.1093/nar/12.18.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb R. A., Dreyfuss G. RNA structure. Unwinding with a vengeance. Nature. 1989 Jan 5;337(6202):19–20. doi: 10.1038/337019a0. [DOI] [PubMed] [Google Scholar]
- Lane D. Enlarged family of putative helicases. Nature. 1988 Aug 11;334(6182):478–478. doi: 10.1038/334478a0. [DOI] [PubMed] [Google Scholar]
- Lasko P. F., Ashburner M. The product of the Drosophila gene vasa is very similar to eukaryotic initiation factor-4A. Nature. 1988 Oct 13;335(6191):611–617. doi: 10.1038/335611a0. [DOI] [PubMed] [Google Scholar]
- Linder P., Lasko P. F., Ashburner M., Leroy P., Nielsen P. J., Nishi K., Schnier J., Slonimski P. P. Birth of the D-E-A-D box. Nature. 1989 Jan 12;337(6203):121–122. doi: 10.1038/337121a0. [DOI] [PubMed] [Google Scholar]
- Lomonossoff G. P., Shanks M. The nucleotide sequence of cowpea mosaic virus B RNA. EMBO J. 1983;2(12):2253–2258. doi: 10.1002/j.1460-2075.1983.tb01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiss E., Timpe U., Brisske A., Jelkmann W., Casper R., Himmler G., Mattanovich D., Katinger H. W. The complete nucleotide sequence of plum pox virus RNA. J Gen Virol. 1989 Mar;70(Pt 3):513–524. doi: 10.1099/0022-1317-70-3-513. [DOI] [PubMed] [Google Scholar]
- Mayo M. A., Robinson D. J., Jolly C. A., Hyman L. Nucleotide sequence of potato leafroll luteovirus RNA. J Gen Virol. 1989 May;70(Pt 5):1037–1051. doi: 10.1099/0022-1317-70-5-1037. [DOI] [PubMed] [Google Scholar]
- Miller W. A., Waterhouse P. M., Gerlach W. L. Sequence and organization of barley yellow dwarf virus genomic RNA. Nucleic Acids Res. 1988 Jul 11;16(13):6097–6111. doi: 10.1093/nar/16.13.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morch M. D., Boyer J. C., Haenni A. L. Overlapping open reading frames revealed by complete nucleotide sequencing of turnip yellow mosaic virus genomic RNA. Nucleic Acids Res. 1988 Jul 11;16(13):6157–6173. doi: 10.1093/nar/16.13.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi K., Morel-Deville F., Hershey J. W., Leighton T., Schnier J. An eIF-4A-like protein is a suppressor of an Escherichia coli mutant defective in 50S ribosomal subunit assembly. Nature. 1988 Dec 1;336(6198):496–498. doi: 10.1038/336496a0. [DOI] [PubMed] [Google Scholar]
- Nutter R. C., Scheets K., Panganiban L. C., Lommel S. A. The complete nucleotide sequence of the maize chlorotic mottle virus genome. Nucleic Acids Res. 1989 Apr 25;17(8):3163–3177. doi: 10.1093/nar/17.8.3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaian M. A., Williams R. H., Gordon K. H., Gould A. R., Symons R. H. Nucleotide sequence of cucumber-mosaic-virus RNA 2 reveals a translation product significantly homologous to corresponding proteins of other viruses. Eur J Biochem. 1984 Sep 3;143(2):277–284. doi: 10.1111/j.1432-1033.1984.tb08370.x. [DOI] [PubMed] [Google Scholar]
- Rezaian M. A., Williams R. H., Symons R. H. Nucleotide sequence of cucumber mosaic virus RNA. 1. Presence of a sequence complementary to part of the viral satellite RNA and homologies with other viral RNAs. Eur J Biochem. 1985 Jul 15;150(2):331–339. doi: 10.1111/j.1432-1033.1985.tb09025.x. [DOI] [PubMed] [Google Scholar]
- Robaglia C., Durand-Tardif M., Tronchet M., Boudazin G., Astier-Manifacier S., Casse-Delbart F. Nucleotide sequence of potato virus Y (N Strain) genomic RNA. J Gen Virol. 1989 Apr;70(Pt 4):935–947. doi: 10.1099/0022-1317-70-4-935. [DOI] [PubMed] [Google Scholar]
- Rochon D. M., Tremaine J. H. Complete nucleotide sequence of the cucumber necrosis virus genome. Virology. 1989 Apr;169(2):251–259. doi: 10.1016/0042-6822(89)90150-5. [DOI] [PubMed] [Google Scholar]
- Strauss J. H., Strauss E. G. Evolution of RNA viruses. Annu Rev Microbiol. 1988;42:657–683. doi: 10.1146/annurev.mi.42.100188.003301. [DOI] [PubMed] [Google Scholar]
- Veidt I., Lot H., Leiser M., Scheidecker D., Guilley H., Richards K., Jonard G. Nucleotide sequence of beet western yellows virus RNA. Nucleic Acids Res. 1988 Nov 11;16(21):9917–9932. doi: 10.1093/nar/16.21.9917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. E., Saraste M., Runswick M. J., Gay N. J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1(8):945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S. X., Rinehart C. A., Kaesberg P. Sequence and organization of southern bean mosaic virus genomic RNA. Virology. 1987 Nov;161(1):73–80. doi: 10.1016/0042-6822(87)90172-3. [DOI] [PubMed] [Google Scholar]
- van der Wilk F., Huisman M. J., Cornelissen B. J., Huttinga H., Goldbach R. Nucleotide sequence and organization of potato leafroll virus genomic RNA. FEBS Lett. 1989 Mar 13;245(1-2):51–56. doi: 10.1016/0014-5793(89)80190-5. [DOI] [PubMed] [Google Scholar]



