Be it the B class, or another class of tetracycline (tet) repressor, the utility and specificity of transcriptional regulators based on this family of prokaryotic DNA binding proteins is unparalleled. A method for regulating gene expression at will in mammalian cells has long been the holy grail. Transfections of uncontrolled numbers of plasmids and unregulated gene expression were breakthroughs in the early days of molecular biology when genes encoding abundant proteins first were introduced into cultured cells. Gone are those days and those antiquated and limited methods. Fine tuning is now essential. We need systems in which gene expression can be repressed and then induced at will. Such control is essential for products that are growth inhibitory or toxic, for example, components of the apoptotic cascade. We need to be able to monitor different levels of gene expression during discrete time periods in cultured cells and in animals to understand the regulation of signal transduction that culminates in different cell fates. Cells that stably express deleterious proteins or cytokines may be lost or their phenotype altered during long-term selection. Clearly, for gene therapy, regulation is crucial. Modulating gene expression in cycles that mimic endogenous patterns is highly desirable, and avoiding toxic levels is a must.
Several regulatory systems exist, but the advantages of the tet system are remarkable and many. Because the critical regulatory elements are prokaryotic, this system has no effects on host genes. The absence of pleiotropic effects greatly simplifies the interpretation of the phenotype observed. Moreover, at the doses used, the tet system lacks toxicity in mammalian cells. Because tet is an antibiotic with a long history, extensive documentation exists as to its safety in humans, making it an excellent candidate for gene regulation in gene therapy. The independent and collaborative work of Bujard and Hillen and their colleagues (1, 2) first demonstrated the efficacy of this remarkable regulatable system in mammalian cells. In its initial and simplest rendition, removal of tet causes a bacterial tetracycline repressor (tetR) fused to a viral VP16 transactivator to induce gene expression via seven tandem copies of a tet-operator DNA (tetO) binding site juxtaposed to a minimal promoter. This interaction results in induction of gene expression of up to 5 orders of magnitude. Since then, refinements have greatly improved the versatility and applicability of this system, see for instance refs. 3–5.
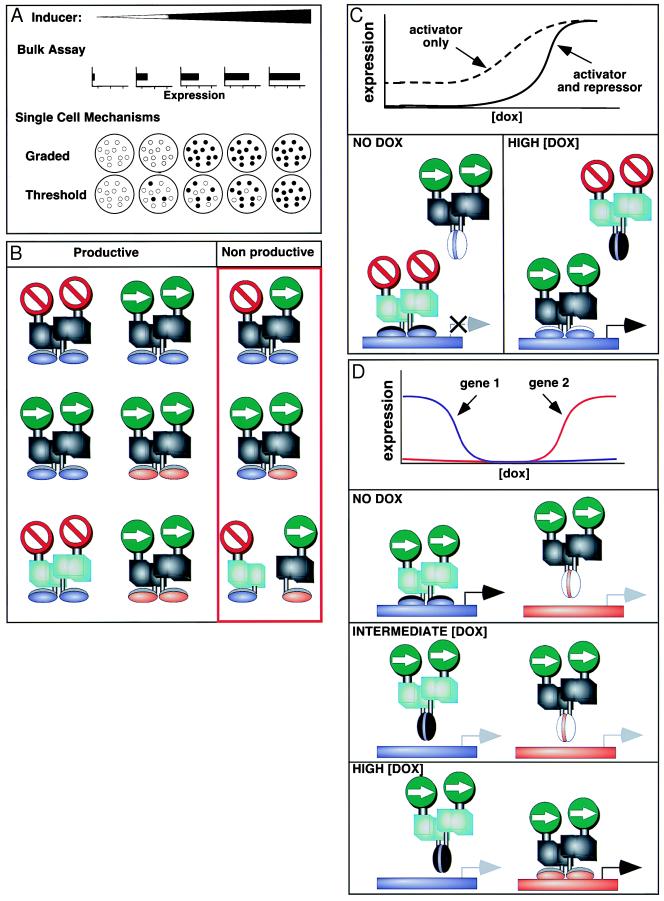
The tet inducer acts as an allosteric effector and becomes an integral part of the transcription factor, and there are no intermediate steps. As a result, the concentration of the inducer directly correlates with the concentration of the transcriptional activator, a feature that differs from all other inducible systems documented to date. As a result, the tet system could be used to test a long-held assumption that transcription is governed by an all-or-none mechanism once transcription factors reach a critical threshold (Fig. 1A). Support for this hypothesis derived from in vitro transcription studies (6, 7). These results also were supported by results obtained in intact live cells in which inducers such as cytokines activated maximal expression of reporter genes on reaching a critical concentration (8–10). These results were not unexpected as the notion that inducers only act once a threshold concentration has been reached was widely accepted and made eminently good sense. How else would the sharp boundaries of gene expression characteristic of early Drosophila development be achieved? How would cells determine when and whether to divide? The consequences of a mechanism that could give a partial effect, for example a range of transcription levels that could vary with the level of the inducer, might well be developmentally devastating.
Figure 1.
(A) Graded transcriptional response to tet at the single cell level. A dose-dependent response to increasing concentrations of an inducer usually can be observed by measuring expression of a reporter gene in bulk assays. Such assays, however, cannot distinguish between two alternative mechanisms operating at the single cell level. In case 1, each cell could respond to intermediate concentrations of inducer by expressing an intermediate amount of gene product (graded response), or in case 2, an intermediate concentration of inducer could result in a fixed maximal level of gene product in a subset of the cells, with the number of cells expressing the gene depending on the concentration of inducer in the medium (threshold response). Case 1 applies to the tet system (3). (B) The need for tetR dimerization specificity. When attempting to coexpress tetR fusion proteins containing different functional domains such as repressor domains (symbolized in the top row by the “do not enter” sign), and activator domains (symbolized by the “go” sign); or DNA binding domains with distinct specificity (symbolized in the middle row by the blue and red “feet”), both productive homodimers and nonproductive heterodimers will form. Nonproductive interactions can be avoided by engineering specific dimerization domains in the tetR portion of the fusion proteins (symbolized by the black and green color midsections in the bottom row). (C) Increasing the dynamic range of the tet system. The basal level of expression of genes under tet control can be reduced without affecting the fully induced level by coexpressing in the same cells a repressor and an activator that respond oppositely to dox and that cannot heterodimerize because of different dimerization domains. The black DNA binding domain symbolizes the wild-type tetR (that binds the tetO in the absence of dox), whereas the white DNA binding domain symbolizes the “reverse” tetR (that binds the tetO in the presence of dox). (D) Independent expression of two different genes. By coexpressing two tetR-based activators that contain DNA binding domains with distinct specificity, respond oppositely to dox, and do not heterodimerize, two independent genes can be regulated by the same inducer. Because of the characteristic dose response of the wild-type and “reverse” tetR, the expression of each gene can be turned off at an intermediate concentration of dox and activated at markedly different dox concentrations.
The simplicity of the tet system allowed a stringent test of the hypothesis that transcription could occur in a graded, rather than a threshold manner (3). The effects of different concentrations of inducer on the expression of a reporter gene were examined at the single cell level by using the fluorescence activated cell sorter. Two retroviruses encoding either the transactivator or a tet-inducible promoter driving expression of a message encoding green fluorescent protein (GFP) were introduced into a polyclonal population of thousands of cells. The results showed a graded response: GFP levels in individual cells containing a single copy of the reporter construct were proportional to the concentration of doxycycline (dox, a tet analog) in the medium. These results showed that a graded transcriptional response is possible (Fig. 1A). However, in nature, as in most of the systems studied above, graded responses are likely to be rare. Instead, complex interactions involving more than one transcriptional regulator (H.M.B. and F.M.V.R., unpublished data) or a series of signal transduction steps from inducer to transcriptional activator, (see for example the mitogen-activated protein kinase cascade; ref. 11), which can serve to convert a given dose of inducer into a fine-tuned all-or-none response are likely to be the rule. Nonetheless, the finding that transcription can be induced to different levels is both of fundamental mechanistic interest and important experimentally, as it provides a means for controlling and studying the effects of precise doses of regulators on growth and differentiation in a manner previously not possible.
A major advance in broadening the utility of the tet system was the use of retroviruses. Although proof of principle was established by using plasmids, this approach definitely involved a labor of love. First, the transactivator plasmid had to be transfected, and then stable clones were selected and tested for their responsiveness to tet by using a second plasmid containing an inducible marker gene. Once the few clones that adequately increased marker gene expression in response to tet were obtained, they were transfected with a third plasmid bearing the inducible gene of interest, and the process of clonal selection and testing was repeated. The yield after several months of cell culture often was a handful of well-regulated clones. If lucky, these did not drift over time, a feature that proved to be highly dependent on cell type, with HeLa cells providing perhaps the most stable tet responders (12).
Because retroviral gene delivery is far more rapid and efficient than stable plasmid transfection and less variable than transient transfection, many investigators turned to tet-regulatable retroviral gene delivery methods. With retroviruses, genes can be introduced with greater than 90% efficiency into tens of thousands of cells, not only into cell lines but also into primary cells, yielding polyclonal populations with diverse integration sites within a week (13). Nonetheless, the inevitable growing pains of any new technology were apparent in the first generation of single tet-regulatable retroviral vectors. In some cases background levels of gene expression were inordinately high because of an autoregulatory feedback loop required to “jump start” the vector (14). In other cases, because the vectors were overly complex with several transcription and translation units (15–19), viral titers were unduly low and few clones could be obtained. These problems now have been largely overcome by the use of two or three different relatively simple retroviral vectors introduced separately, but in close succession into polyclonal populations of cells (3, 4, 20).
One early modification in the tet system was the generation of different tetR transcriptional modulators capable of performing different functions in the same cell. A mutant tetR was generated that differs from the wild type (2) in that the binding of tet causes a conformational change in both tetRs, but the changes have opposite effects. In one case (tT), removal of tet allows interaction of the complex with tetO, inducing gene expression. In the other case, a mutant form (rtT), interacts with tetO and induces gene expression only in the presence of tet. As a result, the same inducer either promotes or blocks transactivation via DNA binding. The dose dependence of these two regulators differs 100-fold. The basis for this difference remains obscure, but is presumably because the mutant binds the inducer with reduced affinity. The kinetics of activation or repression appear similar. Thus, tetR molecules that either function only in the absence or only in the presence of tet can be used with different transcriptional regulatory or DNA binding domains in the same cells. However, a limitation that became apparent was that a mechanism for preventing the formation of nonproductive heterodimers was necessary (Fig. 1B).
Moieties of the tetR transcriptional modulators can be altered and thereby confer different properties on the chimeric proteins, but within each dimer these moieties must be identical or else nonfunctional heterodimeric factors will form. This flexibility allows specificity and a broad range of applications as exemplified in Fig. 1B. For example, the tetR may be produced as a fusion protein with a transactivator that induces transcription (e.g., viral VP16), or as a fusion protein with a transrepressor that inhibits transcription (e.g., the KRAB domain of Kox1) (21). However, a heterodimer of an activator and a repressor would be nonfunctional. A critical advance was the development of a means to avoid the formation of such nonproductive heterodimers between tetR proteins with altered moieties. This advance was achieved by engineering specific dimerization domains. Based on the sequences and the published crystal structures of tetR in several strains of Gram-negative bacteria, as well as mutational analysis (22–24), three such domains from classes B, G, and E now have been identified that are mutually exclusive (4, 5). The discovery of such specific dimerization domains has allowed tet-induced activators and repressors to coexist in the same cells for the first time. As a result, the dynamic range of gene expression has been greatly increased—gene expression now can either be completely repressed or fully induced in a dose-dependent manner (Fig. 1C, see also the RetroTet-ART system in ref. 4). Questions regarding the reversibility of the differentiated state of cells (25, 26) now can be addressed in a systematic manner. For example, cells carrying an inducible gene for toxic proteins or growth arrest proteins, such as p16, can be obtained and induced for a period of time, and then expression can be extinguished (4). This advance will allow a test of plasticity of mammalian cell fate in a manner previously not possible.
Because of the specificity conferred by dimerization domains, independent expression of two genes can be achieved by altering the DNA binding domain of tetR so that it interacts only with a specific modified tetO sequence (5). Two different binding sites were engineered in the tetR forms tTA and rtTA, based on analysis of the tetR sequence and structure. It is remarkable that this protein retains its complex allosteric properties even when mutations are introduced that alter DNA binding specificity, response to dox binding, and dimerizaton specificity. In the paper by Baron et al. (5), DNA binding sites were designed by introducing two distinct point mutations (4C and 6C) in the wild-type tetO. DNA binding domains specific for one or the other site were generated by mutagenesis of the amino terminal region of tetR (27, 28). By using this system it is now possible to either repress the expression of two genes or express either gene alone simply by manipulating the concentration of dox (Fig. 1D). Thus, distinct dimerization domains overcome the problem of forming nonproductive heterodimers and allow at least two types of tetR-based regulators to coexist in the same cells.
For use in gene therapy, two criteria are necessary. The regulators must not be toxic to mammals and must be nonimmunogenic. The analogs dox or anhydrotetracycline appear to have all of the properties of tet yet act at doses 100-fold lower than tet. Tet has been used for decades in humans and animals, and only at high doses have any deleterious effects been detected. In addition, Heard (20) demonstrated that when the tetR-VP16 fusion protein was delivered to mice by using an ex vivo approach, no immune response was observed. These data suggest that these two criteria for gene therapy are met by tet.
Thus, the tet system appears to be advantageous not only for studies of gene expression in cultured cells, but also in controlling the timing and levels of gene expression in transgenic animals and possibly patients. The refinements described in this review are only the tip of the iceberg, and further evolution and applications of this remarkable inducible system undoubtedly will follow.
Footnotes
The companion to this Commentary is published on page 1013.
References
- 1.Gossen M, Bujard H. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gossen M, Freundlieb S, Bender G, Muller G, Hillen W, Bujard H. Science. 1995;268:1766–1769. doi: 10.1126/science.7792603. [DOI] [PubMed] [Google Scholar]
- 3.Kringstein A M, Rossi F M V, Hofmann A, Blau H M. Proc Natl Acad Sci USA. 1998;95:13670–13675. doi: 10.1073/pnas.95.23.13670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rossi F M, Guicherit O M, Spicher A, Kringstein A M, Fatyol K, Blakely B T, Blau H M. Nat Genet. 1998;20:389–393. doi: 10.1038/3871. [DOI] [PubMed] [Google Scholar]
- 5.Baron U, Schnappinger D, Helbl V, Gossen M, Hillen W, Bujard H. Proc Natl Acad Sci USA. 1999;96:1013–1018. doi: 10.1073/pnas.96.3.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carey M, Lin Y S, Green M R, Ptashne M. Nature (London) 1990;345:361–364. doi: 10.1038/345361a0. [DOI] [PubMed] [Google Scholar]
- 7.Laybourn P J, Kadonaga J T. Science. 1992;257:1682–1685. doi: 10.1126/science.1388287. [DOI] [PubMed] [Google Scholar]
- 8.Fiering S, Northrop J P, Nolan G P, Mattila P S, Crabtree G R, Herzenberg L A. Genes Dev. 1990;4:1823–1834. doi: 10.1101/gad.4.10.1823. [DOI] [PubMed] [Google Scholar]
- 9.Karttunen J, Shastri N. Proc Natl Acad Sci USA. 1991;88:3972–3976. doi: 10.1073/pnas.88.9.3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ko M S, Nakauchi H, Takahashi N. EMBO J. 1990;9:2835–2842. doi: 10.1002/j.1460-2075.1990.tb07472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrell J E, Jr, Machleder E M. Science. 1998;280:895–898. doi: 10.1126/science.280.5365.895. [DOI] [PubMed] [Google Scholar]
- 12.Gossen M, Bujard H. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Springer M L, Blau H M. Somatic Cell Mol Genet. 1997;23:203–209. doi: 10.1007/BF02721371. [DOI] [PubMed] [Google Scholar]
- 14.Hofmann A, Nolan G P, Blau H M. Proc Natl Acad Sci USA. 1996;93:5185–5190. doi: 10.1073/pnas.93.11.5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hwang J J, Scuric Z, Anderson W F. J Virol. 1996;70:8138–8141. doi: 10.1128/jvi.70.11.8138-8141.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindemann D, Patriquin E, Feng S, Mulligan R C. Mol Med. 1997;3:466–476. [PMC free article] [PubMed] [Google Scholar]
- 17.Paulus W, Baur I, Boyce F M, Breakefield X O, Reeves S A. J Virol. 1996;70:62–67. doi: 10.1128/jvi.70.1.62-67.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoshimaru M, Ray J, Sah D W, Gage F H. Proc Natl Acad Sci USA. 1996;93:1518–1523. doi: 10.1073/pnas.93.4.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sah D W, Ray J, Gage F H. Nat Biotechnol. 1997;15:574–580. doi: 10.1038/nbt0697-574. [DOI] [PubMed] [Google Scholar]
- 20.Bohl D, Naffakh N, Heard J M. Nat Med. 1997;3:299–305. doi: 10.1038/nm0397-299. [DOI] [PubMed] [Google Scholar]
- 21.Deuschle U, Meyer W K, Thiesen H J. Mol Cell Biol. 1995;15:1907–1914. doi: 10.1128/mcb.15.4.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hillen W, Berens C. Annu Rev Microbiol. 1994;48:345–369. doi: 10.1146/annurev.mi.48.100194.002021. [DOI] [PubMed] [Google Scholar]
- 23.Kisker C, Hinrichs W, Tovar K, Hillen W, Saenger W. J Mol Biol. 1995;247:260–280. doi: 10.1006/jmbi.1994.0138. [DOI] [PubMed] [Google Scholar]
- 24.Schnappinger D, Schubert P, Pfleiderer K, Hillen W. EMBO J. 1998;17:535–543. doi: 10.1093/emboj/17.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blau H M. Annu Rev Biochem. 1992;61:1213–1230. doi: 10.1146/annurev.bi.61.070192.010025. [DOI] [PubMed] [Google Scholar]
- 26.Blau H M, Baltimore D. J Cell Biol. 1991;112:781–783. doi: 10.1083/jcb.112.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Helbl V, Tiebel B, Hillen W. J Mol Biol. 1998;276:319–324. doi: 10.1006/jmbi.1997.1539. [DOI] [PubMed] [Google Scholar]
- 28.Helbl V, Hillen W. J Mol Biol. 1998;276:313–318. doi: 10.1006/jmbi.1997.1540. [DOI] [PubMed] [Google Scholar]