Abstract
Benzophenone (BP)-type ultraviolet (UV) filters are widely used in a variety of personal care products for the protection of skin and hair from UV irradiation. Despite the estrogenic potencies of BP derivatives, few studies have examined the occurrence of these compounds in human matrices. Furthermore, associations among exposure to these compounds and estrogen-dependent diseases (such as endometriosis) have not been examined previously. In this study, we determined the concentrations of five BP derivatives, 2-hydroxy-4-methoxybenzophenone (2OH-4MeO-BP), 2,4-dihydroxybenzophenone (2,4OH-BP), 2,2’-dihydroxy-4-methoxybenzophenone (2,2’OH-4MeO-BP), 2,2’,4,4’-tetrahydroxybenzophenone (2,2’,4,4’OH-BP) and 4-hydroxybenzophenone (4OH-BP), in urine collected from 625 women in Utah and California, using liquid chromatography (LC)-tandem mass spectrometry (MS/MS). The association of urinary concentrations of BP derivatives with an increase in the odds of a diagnosis of endometriosis was examined in 600 women who underwent laparoscopy/laparotomy (n = 473: operative cohort) or pelvic magnetic resonance imaging (n = 127: population cohort), during 2007-2009. 2OH-4MeO-BP, 2,4OH-BP, and 4OH-BP, respectively, were detected in 99.0%, 93.3%, and 83.8% of the urine samples analyzed, whereas the detection rates for 2,2’,4,4’OH-BP and 2,2’OH-4MeO-BP were below 6.0%. Significant regional differences (higher concentrations in California) and monthly variations (higher concentrations in July and August) were found for urinary concentrations of 2OH-4Me O-BP and 2,4OH-BP. In addition, urinary concentrations of 2OH-4MeO-BP and 2,4OH-BP tended to be higher in more affluent, older, and leaner women. Adjusted odds ratios (ORs) and 95% confidence intervals (CIs) were estimated for the urinary concentrations of BP derivatives and the odds of an endometriosis diagnosis; ORs increased across quartiles of 2OH-4MeO-BP and 2,4OH-BP concentrations, but a significant trend was observed only between 2,4OH-BP and the odds of an endometriosis diagnosis in the operative cohort (OR = 1.19; 95% CI = 1.01, 1.41). When women in the highest quartile of 2,4OH-BP concentrations were compared with women in the first three quartiles, the OR increased considerably (AOR = 1.65; 95% CI = 1.07, 2.53). Given that 2,4OH-BP possesses an estrogenic activity higher than that of 2OH-4MeO-BP, our results invite the speculation that exposure to elevated 2,4OH-BP levels may be associated with endometriosis.
Keywords: Benzophenone derivatives, UV filters, sunscreen agents, estrogenic activity, endometriosis, epidemiology
INTRODUCTION
Ultraviolet (UV) filters that can absorb UV radiation are used in a variety of personal care products to attenuate the negative effects of this harmful radiation on skin and hair. UV filters enter the environment via wash-off from skin or via sewage discharges. UV filters have been detected in environmental matrices such as water, soil, and sediment as well as in human bodily fluids such as urine and blood. 1-4 In vivo and in vitro studies have shown that some organic UV filters possess estrogenic effects. 5, 6 In the United States, 2-hydroxy-4-methoxybenzophenone (2OH-4MeO-BP or BP-3) is widely used in a variety of cosmetics as an UV filter. 7 When a sunscreen lotion that contained 2OH-4MeO-BP was applied on human skin, 1-2% of the amount applied was absorbed into the bloodstream within 10 h. 8 In addition, 2OH-4MeO-BP has been shown to penetrate human skin more readily than do other sunscreen agents. 9, 10 2OH-4MeO-BP is reported to possess weak estrogen-like activity, based on estrogen receptor (ER)-mediated reporter gene and uterotropic assays; 6, 11-14 thus, it is a potential endocrine-disrupting chemical (EDC).
Studies that use experimental animals have demonstrated that 2OH-4MeO-BP is metabolized to 2,4-dihydroxybenzophenone (2,4OH-BP or BP-1) and 2,2’-dihydroxy-4-methoxybenzophenone (2,2’OH-4MeO-BP or BP-8); 15-18 these BP derivatives are also used in cosmetic products as sunscreen agents. 7 It is noteworthy that 2,4OH-BP possesses an estrogenic activity higher than that of 2OH-4MeO-BP. 11, 12, 14, 17, 19 Additive estrogenic effects of 2,4OH-BP and 2OH-4MeO-BP on pS2-gene transcription in MCF-7 cells have been reported. 20 In addition, other BP derivatives, such as 2,2’,4,4’-tetrahydroxybenzophenone (2,2’,4,4’OH-BP or BP-2) and 4-hydroxybenzophenone (4OH-BP), have been shown to have estrogenic activity higher than that of 2OH-4MeO-BP. 11, 12 Despite their potential for endocrine-disrupting activity, information on the occurrence of BP derivatives, except for 2OH-4MeO-BP, in humans is scarce. 2OH-4MeO-BP has been detected in human urine using LC-MS/MS methods. 3, 21-24 Nevertheless, studies that examine BP-type UV filters on human health, including estrogen-mediated outcomes, are not available.
Endometriosis is a gynecological disorder that affects reproductive-age women and is typically defined as an estrogen-dependent disease. 25 Our recent study showed that at least 11% of reproductive-age women in the United States may have undiagnosed endometriosis. 26 Although it is likely that various factors such as genital tract abnormalities, genetic predisposition, hormonal imbalance, and altered immune surveillance are involved in the pathogenesis of endometriosis, its etiology still remains elusive. 27 Exposure to environmental EDCs may be associated with development of endometriosis. 27 However, epidemiological studies that assess the association between endometriosis and exposure to EDCs such as dioxins, 28 polychlorinated biphenyls (PCBs), 29-31 polybrominated biphenyls (PBBs), 30 and organochlorine pesticides (OCPs) 32 have yielded mixed results. To our knowledge, the association between endometriosis and exposure to BP-type UV filters has not been studied to date, despite the relatively high prevalence of exposure to these estrogenic chemicals by prepubescent and reproductive-aged women.
In this study, we determined the concentrations of five BP derivatives, 2OH-4MeO-BP, 2,4OH-BP, 2,2’OH-4MeO-BP, 2,2’,4,4’OH-BP, and 4OH-BP, in urine collected from 625 women who resided within 50 miles of two cities, Salt Lake City (Utah) and San Francisco (California) during 2007-2009, and explored the association between BP derivatives and endometriosis, using the operative reports for 600 women who underwent laparoscopy/laparotomy or pelvic magnetic resonance imaging (MRI).
MATERIALS AND METHODS
Study Cohorts and Data Collection
Urine samples (N = 625) collected in 2007-2009 for the ENDO (Endometriosis, Natural history, Diagnosis, and Outcomes) Study were utilized. The study design and demographic information have been previously described. 26 Briefly, urine samples (approximately 120 mL per person) were collected from 431 and 63 currently-menstruating women, aged 18-44 years, in Salt Lake City, Utah, and San Francisco, California, respectively, who were scheduled to undergo a diagnostic and/or therapeutic laparoscopy or laparotomy at participating surgical centers (referred to as “operative or surgical cohort”). While 495 women were enrolled for the surgeries, one women did not provide a urine sample. In addition, urine samples were collected from 131 (95 from Utah and 36 from California) currently-menstruating women who were matched to the operative cohort on age and residence within the 50-mile catchment area of clinical centers (referred to as “population or unexposed cohort”). The intent of the population cohort was to identify women at risk for endometriosis (i.e., currently menstruating) who did not seek medical care; this group served as a comparison cohort for the operative cohort and for the assessment of consistency of findings across cohorts.
All women met the following three criteria: (1) no breastfeeding for >6 months, (2) no injectable hormone treatment within the past two years, and (3) no cancer history other than non-melanoma skin cancer. Race/ethnicity, marital status, household income, age, hair color (at age 18 years), and smoking habits were obtained by interview prior to surgery for the operative cohort and at their earliest convenience for the population cohort, respectively. However, use of personal care products including sunscreens was not captured during the interview, given that the benzophenone-type UV filters did not become a part of the target compounds for the ENDO Study until recruitment was complete and it became evident that residual urines were available for their analysis. Height and weight of women were measured according to standardized anthropometric methods. 33 Information on age, marital status, smoking habit, hair color, household income, and height and weight were missing for <1.5% of the women. Missing data were minimal, with no systematic difference by cohort or endometriosis status. Institutional Review Board approval was obtained from all participating institutions, and all women consented for participation before the sample collection. All urine samples were stored in a freezer at -20°C until chemical analysis.
Among the 494 women in the operative cohort, 473 underwent laparoscopy or laparotomy, the “gold standard” for the diagnosis of endometriosis, 34 of whom 190 were diagnosed with endometriosis (40% incidence). Endometriosis diagnoses were categorized into four stages, Stage I (minimal [n = 95; 50%]), Stage II (mild [ n = 39; 21%]), Stage III (moderate [ n = 35; 18%]), and Stage IV (severe [n = 21; 11%]), according to the Revised American Society for Reproductive Medicine’s (rASRM) classification. 35 Among the 131 women in the population cohort, 127 (97%) underwent pelvic MRI, of whom 14 (11%) were diagnosed with endometriosis. No staging was performed for the population cohort, given the absence of such an algorithm for MRI diagnosed disease. The diagnostic data obtained from 600 women in the operative and population cohorts were used for the assessment of the association between BP derivatives and the odds of an endometriosis diagnosis.
Chemical Analysis
2OH-4MeO-BP, 2,4OH-BP, 2,2’OH-4MeO-BP, 2,2’,4,4’OH-BP, and 4OH-BP were analyzed in urine by following the procedure reported recently. 3 Briefly, urine samples were removed from the freezer and thawed overnight at 4°C. After a 500-μL aliquot of urine was transferred into a 15-mL polypropylene (PP) tube, 10 ng of deuterated bisphenol-A (d16-BPA) and 100 ng of 4-methylumbelliferyl β-glucuronide were spiked as internal and deconjugation standards, respectively. After gentle mixing, 300 μL of β-glucuronidase/sulfatase (Type HP-2, from Helix pomatia, ≥100,000 units/mL glucuronidase and ≤7,500 units/mL sulfatase; Sigma-Aldrich, St. Louis, MO) buffer, which contained 2 μL enzyme and 1 mL of 1.0 M ammonium acetate, was added. After vortex mixing, the sample was incubated at 37°C for 12 h. Then, 3 mL of 50% MTBE/ethyl acetate were added, shaken for 30 min, using a reciprocating shaker, and centrifuged at 3,000 g for 2 min. The organic phase, containing BP derivatives, was transferred into a new 15-mL PP tube, and the aqueous phase was extracted again with 3 mL of 50% MTBE/ethyl acetate. The organic phases were combined, evaporated to near-dryness using N2, and redissolved in 1 mL of methanol (MeOH). After sonication for 10 sec and centrifugation at 3,000 g for 2 min, the solution was transferred into a 1.5-mL amber glass vial for LC-MS/MS analysis.
An API 2000 electrospray triple quadrupole mass spectrometer (ESI-MS/MS; Applied Biosystems, Foster City, CA), equipped with an Agilent 1100 Series HPLC system (Agilent Technologies Inc., Santa Clara, CA), was used for the measurement of BP derivatives. Ten microliters of urine extract were injected onto a Thermo Betasil C18 (100 mm length × 2.1 mm internal diameter, 5 μm particle diameter) chromatographic column serially connected with a guard column (20 × 2.1 mm, 5 μm; Thermo Electron Co., Bellefonte, PA), at a flow rate of 300 μL/min. The mobile phases were MeOH (solvent A) and deionized water (solvent B); the gradient started at 15% A, ramped to 90% A in 7 min, and held for 4.5 min before reverting back to 15% A. The negative ion multiple reaction monitoring (MRM) mode was used, and the MRM transitions monitored were 227>211 for 2OH-4MeO-BP, 213>91 for 2,4OH-BP, 243>93 for 2,2’OH-4MeO-BP, 245>91 for 2,2’,4,4’ OH-BP, 197>92 for 4OH-BP, 241>142 for d16-BPA, and 175>119 for 4-methylumbelliferone. Nitrogen was used as both curtain and collision gas. MS/MS parameters were optimized for each BP derivative, by infusion of 1 μg/mL standard solution, with a flow injection analysis system; the optimized MS/MS parameters for BP derivatives have been reported previously. 3
The analytes were quantified from an external calibration curve prepared at concentrations ranging from 0.05 to 100 ng/mL. The calibration standards were injected with every batch of samples and the linearity was confirmed to be r > 0.999. If concentrations of BP derivatives in urine were above 100 ng/mL, the sample was re-analyzed using a smaller volume. The limit of detection (LOD) was determined to be 0.082 ng/mL for both 2,4OH-BP and 4OH-BP; 0.13 ng/mL for 2,2’OH-4MeO-BP; and 0.28 ng/mL for both 2OH-4MeO-BP and 2,2’,4,4’OH-BP. The recoveries of five BP derivatives from a triplicate recovery test, through spiking of two concentrations (1.0 and 10 ppb) of each of five BP derivatives into the urine matrix, were between 85.2 and 99.6%, and the coefficients of variation (CVs) were between 0.53 and 5.9%. When variations in the responses of BP derivatives within a day and among 5 consecutive days were compared by replicate analyses of a urine sample that contained 2,4OH-BP, 2OH-4MeO-BP, and 4OH-BP, the respective CVs of five replicate analyses within a day and among 5 days were 1.4 and 3.7% for 2,4OH-BP, 1.7 and 3.0% for 2OH-4MeO-BP, and 2.8 and 4.5% for 4OH-BP. Data processing was performed with the Analyst 1.4.1 software package.
Statistical Analysis
Data were analyzed in two phases, including a descriptive phase for assessing the distribution of BP derivatives and completeness of data for relevant study covariates. Although the LOD for each BP derivative was consistent through batches, all machine observed concentrations irrespective of the LOD were analyzed without substitution, to minimize introducing bias. 36 Differences in concentrations of BP derivatives by the demographic features of women were assessed using the non-parametric Wilcoxon or Kruskal-Wallis test. A p value of < 0.05 denoted significance. The strength of correlation between BP derivative concentrations was evaluated by simple regression analysis. The relation between BP derivative concentrations and odds of an incident endometriosis diagnosis was explored using multivariable logistic regression. Given the uncertain timing of endometriosis onset, we estimated the odds ratios (ORs) for diagnosis along with corresponding 95% confidence intervals (CIs) for each BP derivative, rather than estimating incident disease, per se. Concentrations were log-transformed (x + 1) for all analyses, and first assessed in their continuous form and, subsequently, categorized into quartiles that were defined according to the distributions for unaffected women in both cohorts. The trend test was used to empirically assess linear trends of BP derivative concentrations across the four intervals defined by the 25th, 50th, and 75th percentiles. Odds ratios also were adjusted for women’s natural hair color at age 18 years (categorized as red, blond, or brown/black) and study site (California or Utah), given their observed association with both exposure and endometriosis. 26, 37 We further explored the operative cohort, which permitted sensitive analyses. In particular, we restricted endometriosis to only moderate/severe disease to assess the consistency of our results. We also conducted sensitive analysis by restricting unaffected women to those with a primary postoperative diagnosis of a normal pelvis. Significant ORs are those whose CIs exclude one. Each BP derivative was modeled separately and for each cohort. All analyses were conducted using SAS 9.2 (SAS Institute Inc., Cary, NC).
RESULTS and DISCUSSION
Concentrations of BP Derivatives and Relationship with Demographic Characteristics
2OH-4MeO-BP, 2,4OH-BP, and 4OH-BP were respectively detected in 99.0%, 93.3%, and 83.8% of the 625 urine samples analyzed. The detection rates for 2,2’,4,4’OH-BP and 2,2’OH-4MeO-BP were only 5.6% and 2.6%, respectively, possibly reflecting their limited use relative to 2OH-4MeO-BP and 2,4OH-BP,7 and were not considered in further analytic models.
Urinary concentrations of 2OH-4MeO-BP, 2,4OH-BP, and 4OH-BP and characteristics of the study cohort are summarized in Table 1. The median (range) concentrations in all samples were 6.1 (<0.28-5900) ng/mL for 2OH-4MeO-BP, 6.1 (<0.082-3200) ng/mL for 2,4OH-BP, and 0.36 (<0.082-22) ng/mL for 4OH-BP. An earlier survey in the United States reported a median concentration of 26.0 ng/mL of 2OH-4MeO-BP in urine from 1288 females; 21 however, that study did not measure either 2,4OH-BP or 4OH-BP. The median value for 2OH-4MeO-BP measured in our study (ENDO Study) was lower than that reported previously. 21 This could be partly due to the difference in sampling locations between the studies. In our ENDO Study, we found significantly higher concentrations of 2OH-4MeO-BP in urine samples from women who resided in California than from women who resided in Utah (Table 1). In fact, the median concentration (24.2 ng/mL) of 2OH-4MeO-BP measured in urine samples from California (in our study) was comparable to the value reported previously for the United States. 21 In addition, urinary concentrations of 2OH-4MeO-BP and 2,4OH-BP tended to be higher for more affluent (household income), older (age), and leaner (body mass index) women (Table 1). These findings may suggest that the U.S. women with such characteristics might use personal care products (containing sunscreens) more frequently than do the others. Further analyses with use frequency of personal care products will shed light on the differences in BP derivative concentrations.
Table 1.
Urinary concentrations (ng/mL) of BP derivatives by selected demographic characteristics (ENDO Study)
| 2OH-4MeO-BP | 2,4OH-BP | 4OH-BP | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | Min | 25%a | Median | 75%a | Max | Min | 25%a | Median | 75%a | Max | Min | 25%a | Median | 75%a | Max |
| Total (n =625) | <0.28 | 2.3 | 6.1 | 37 | 5900 | <0.082 | 1.7 | 6.1 | 31 | 3200 | <0.082 | 0.17 | 0.36 | 0.69 | 22 |
| Sampling site | |||||||||||||||
| Utah (n =526) | <0.28 | 1.9 | 5.3 | 28 | 5900 ** | <0.082 | 1.5 | 5.1 | 25 | 3200 ** | <0.082 | 0.17 | 0.35 | 0.68 | 22 |
| California (n =99) | 1.0 | 3.4 | 24 | 100 | 2200 | 0.54 | 3.2 | 15 | 59 | 1800 | <0.082 | 0.23 | 0.42 | 0.73 | 13 |
| Race/ethnicity | |||||||||||||||
| Hispanic (n =82) | <0.28 | 3.3 | 6.9 | 46 | 5900 | <0.082 | 1.7 | 3.9 | 34 | 3200 | <0.082 | 0.19 | 0.36 | 0.70 | 2.6 |
| Non-hispanic while (n =475) | <0.28 | 2.1 | 5.7 | 33 | 2900 | <0.082 | 1.6 | 6.3 | 27 | 2800 | <0.082 | 0.17 | 0.37 | 0.70 | 22 |
| Non-hispanic black (n=10) | 1.0 | 1.1 | 6.6 | 75 | 190 | 0.54 | 1.5 | 6.2 | 24 | 95 | <0.082 | 0.28 | 0.41 | 0.73 | 1.7 |
| Asian/Hawaiian/Pacific Islander/Alaska Native/American Indian (n =34) | <0.28 | 2.2 | 20 | 270 | 2200 | <0.082 | 1.6 | 19 | 120 | 640 | <0.082 | 0.20 | 0.32 | 0.62 | 5.5 |
| Other (n=11) | <0.28 | 1.2 | 3.2 | 110 | 240 | 0.45 | 1.1 | 2.3 | 41 | 130 | <0.082 | <0.082 | 0.37 | 2.7 | 13 |
| Multi-racial (n =13) | <0.28 | 2.6 | 3.1 | 17 | 100 | 1.6 | 2.0 | 2.5 | 20 | 37 | <0.082 | 0.16 | 0.29 | 0.33 | 1.1 |
| Marital status | |||||||||||||||
| Married (n =448) | <0.28 | 2.4 | 6.0 | 41 | 2200 | <0.082 | 1.7 | 6.1 | 31 | 1800 | <0.082 | 0.16 | 0.37 | 0.70 | 22 |
| Other (n =172) | <0.28 | 2.1 | 7.2 | 35 | 5900 | <0.082 | 1.6 | 6.8 | 33 | 3200 | <0.082 | 0.21 | 0.36 | 0.70 | 7.9 |
| Household incomeb | |||||||||||||||
| Below poverty (n =71) | <0.28 | 1.7 | 4.0 | 17 | 2900 ** | <0.082 | 1.2 | 3.8 | 22 | 2800 ** | <0.082 | 0.20 | 0.37 | 0.62 | 2.7 |
| Within 180% poverty (n =75) | <0.28 | 1.6 | 5.5 | 17 | 130 | <0.082 | 1.4 | 4.3 | 16 | 51 | <0.082 | 0.17 | 0.33 | 0.66 | 11 |
| Above 180% poverty (n =470) | <0.28 | 2.6 | 7.3 | 49 | 5900 | <0.082 | 1.8 | 7.5 | 37 | 3200 | <0.082 | 0.17 | 0.38 | 0.73 | 22 |
| Age (in years) | |||||||||||||||
| 20-29(n =221) | <0.28 | 1.7 | 5.1 | 18 | 2900 ** | <0.082 | 1.6 | 5.3 | 21 | 2800 | <0.082 | 0.18 | 0.35 | 0.69 | 22 |
| 30-39(n =264) | <0.28 | 2.6 | 6.9 | 49 | 1700 | <0.082 | 1.6 | 7.5 | 36 | 1800 | <0.082 | 0.14 | 0.33 | 0.64 | 21 |
| ≥40(n =139) | <0.28 | 2.9 | 7.7 | 64 | 5900 | <0.082 | 2.0 | 6.4 | 38 | 3200 | <0.082 | 0.19 | 0.41 | 0.73 | 20 |
| Body mass index | |||||||||||||||
| <25.0 - thin (n =269) | <0.28 | 2.6 | 7.9 | 41 | 2900 * | <0.082 | 2.2 | 9.7 | 40 | 2800 ** | <0.082 | 0.13 | 0.34 | 0.65 | 8.0 |
| 25.0 - 29.9-normal (n =153) | <0.28 | 2.6 | 7.1 | 50 | 5900 | <0.082 | 2.0 | 8.0 | 32 | 3200 | <0.082 | 0.17 | 0.38 | 0.73 | 22 |
| ≥30.0 - overweight (n =198) | <0.28 | 1.8 | 4.5 | 18 | 2200 | <0.082 | 1.3 | 3.1 | 19 | 580 | <0.082 | 0.21 | 0.38 | 0.70 | 21 |
| Natural hair color (at age 18 years) | |||||||||||||||
| Red (n =67) | <0.28 | 3.3 | 7.8 | 53 | 1600 | 0.33 | 2.0 | 6.8 | 57 | 690 | <0.082 | 0.13 | 0.32 | 0.83 | 8.0 |
| Blonde (n =208) | <0.28 | 2.4 | 7.2 | 32 | 2900 | <0.082 | 2.5 | 8.6 | 28 | 2800 | <0.082 | 0.17 | 0.40 | 0.70 | 22 |
| Brown & Black (n =346) | <0.28 | 2.0 | 5.7 | 41 | 5900 | <0.082 | 1.4 | 5.0 | 31 | 3200 | <0.082 | 0.18 | 0.35 | 0.65 | 21 |
| Current smoke | |||||||||||||||
| No (n =538) | <0.28 | 2.5 | 6.8 | 43 | 5900 ** | <0.082 | 1.6 | 6.9 | 32 | 3200 | <0.082 | 0.18 | 0.38 | 0.72 | 22* |
| Yes (n=84) | <0.28 | 1.4 | 4.7 | 14 | 1300 | <0.082 | 2.0 | 4.2 | 19 | 380 | <0.082 | 0.11 | 0.27 | 0.51 | 7.1 |
25 or 75 percentile
Based on the 2007 HHS Poverty Guidlines accounting for the numbers of persons in the household for the 48 contiguous states and District of Columbia.
p <0.05,
p <0.01
Urinary concentrations of 2OH-4MeO-BP and 4OH-BP were significantly lower in smokers than in non-smokers (Table 1). It is known that tobacco smoke contains inducers of cytochrome P450 (CYP) drug-metabolizing enzymes, leading to the increased risk for human health by enhancing the metabolic activation of endogenous and xenobiotic chemicals. 38 As a result, BP derivatives might be efficiently metabolized in active smokers relative to nonsmokers, implying the formation of hydroxylated BP metabolites and their risk for human health. It has been reported that 2OH-4MeO-BP is metabolized principally to 2,4OH-BP, via metabolic activities of CYP enzymes, in animals 15-18. 2,4OH-BP is formed from the metabolism of 2OH-4MeO-BP and possibly 4OH-BP, via catalysis of CYP enzymes. 2,4OH-BP is further metabolized in the body, and that was the reason for the lack of significant difference in urinary concentrations of this BP derivative between smokers and non-smokers.
When the monthly variation in urinary BP derivative concentrations was examined based on the urine collection date, significant (p < 0.01) differences were found for 2OH-4MeO-BP and 2,4OH-BP concentrations (Figure 1). The highest median concentrations of 2OH-4MeO-BP (18 ng/mL) and 2,4OH-BP (23 ng/mL) were observed in July, and the highest values of 2OH-4MeO-BP (5900 ng/mL) and 2,4OH-BP (3200 ng/mL) were found in a urine sample collected in August (Figure 1). These results indicate that women in the ENDO Study were exposed to high concentrations of BP derivatives during the summer months, probably through the application of sunscreens to protect their skin from UV radiation. A previous study that measured 2OH-4MeO-BP concentrations in urine collected from 90 girls in New York City, Cincinnati, and northern California also reported significantly higher concentrations of this BP derivative in urine samples collected in summer than in other seasons. 22 In fact, we previously found notably high concentrations of 2OH-4MeO-BP and 2,4OH-BP in urine from a sunscreen user, compared to those in non-sunscreen users, which suggested that sunscreen products are one of the major sources of exposure to these two BP derivatives in humans. 3 Nevertheless, there was no significant monthly variation in 4OH-BP concentrations in urine (Figure 1), indicating that this compound originates from a different sources.
Fig. 1.
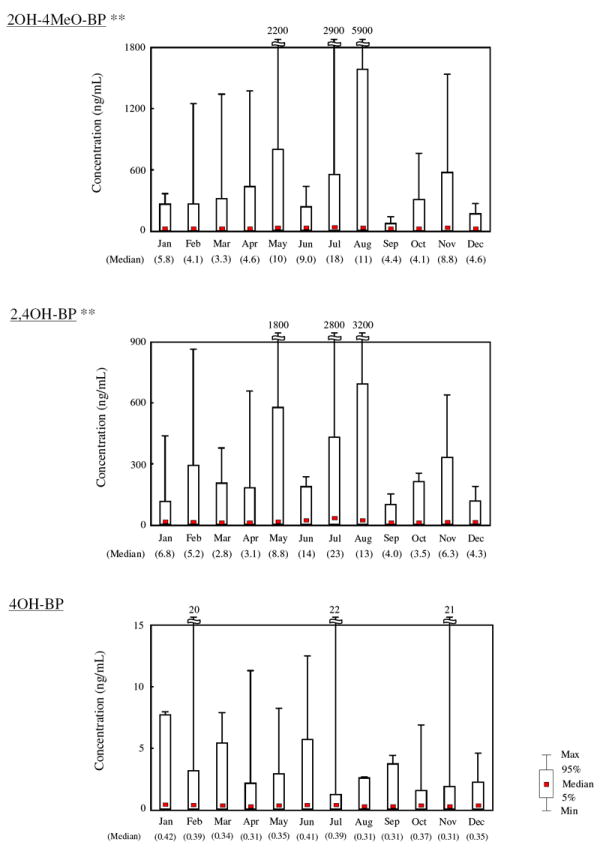
Monthly variation in urinary BP derivative concentrations in women (ENDO Study). **p < 0.01
When the correlations among the three urinary BP derivative concentrations were examined, a significantly higher correlation coefficient (r = 0.92) was found between 2OH-4MeO-BP and 2,4OH-BP concentrations than between 2OH-4MeO-BP and 4OH-BP (r = 0.13) (Figure 2); the correlation coefficient between 2,4OH-BP and 4OH-BP was r = 0.083 (data not shown). These results support the above explanation that the sources of human exposure to 4OH-BP are different from those of 2OH-4MeO-BP and 2,4OH-BP. The positive correlation between 2OH-4MeO-BP and 2,4OH-BP can be attributed to the formation of 2,4OH-BP from 2OH-4MeO-BP via metabolic activities of cytochrome P450 enzymes, as described earlier. In addition, women also can be exposed to 2,4OH-BP directly, through the use of personal care products. 2,4OH-BP is present in some U.S. cosmetics, although the frequency of use of this BP derivative is much lower than that of 2OH-4MeO-BP. 7 A skin absorption study, in which sunscreen lotion containing 2OH-4MeO-BP was applied to the skin of three female volunteers, demonstrated that the major metabolite found in the urine was 2,4OH-BP. 39 4OH-BP detected in our study could have been formed through the oxidative metabolism of BP because the use of 4OH-BP in personal care products and formation of this compound from other BP derivatives are not known. BP has been reported to be metabolized to 4OH-BP, following incubation with rat hepatocytes 40 and in an in vivo study. 15
Fig. 2.
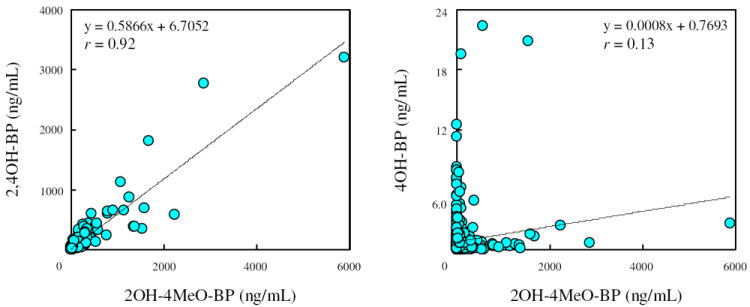
Correlation between urinary concentrations of 2OH-4MeO-BP and 2,4OH-BP or 4OH-BP (ENDO Study).
Benzophenone Derivatives in Urine and Their Association with Endometriosis
The (un)adjusted ORs and corresponding 95% CIs for each BP derivative (in quartiles) are presented for both cohorts in Table 2. The unadjusted and adjusted ORs were elevated for 2OH-4MeO-BP and 2,4OH-BP, particularly at the higher quartiles, but not for 4OH-BP. A significant trend was observed between 2,4OH-BP and the odds of an endometriosis diagnosis, but only in the operative cohort (OR = 1.19; 95% CI = 1.01, 1.41). Given the modest sample size, we also compared the highest quartile to the lower ones. When women in the highest quartile were compared with women in the first three quartiles of 2,4OH-BP concentrations, the OR remained elevated (OR = 1.65; 95% CI = 1.07, 2.53), which denoted an approximate 65% increase in the odds of an endometriosis diagnosis in women with the highest 2,4OH-BP concentration compared to women with lower concentrations. A similar pattern was observed in the population cohort, but the CIs for all BP derivatives included one, denoting the absence of significance, possibly indicative of the limited number (n = 14) of women in the population cohort with endometriosis. Of note is the consistency of the direction of the OR for 2,4OH-BP in sensitivity analyses that restricted the choice of affected individuals to those whose endometriosis was moderate (stage III)/severe (stage IV) (OR = 1.66; 95% CI = 0.89, 3.12). However, a loss of statistical power was observed, which reflected the preponderance of women with minimal (stage I)/mild (stage II) endometriosis in the ENDO study. Similar results were observed when we restrict the choice of unaffected individuals to those with a primary postoperative diagnosis of a normal pelvis (OR = 1.49; 95% CI = 0.91, 2.46).
Table 2.
Odds of an endometriosis diagnosis by urinary concentrations of BP derivatives and cohort (ENDO Study)
| BP analyte | Operative cohort (n=473)
|
Population cohort (n=127)
|
||
|---|---|---|---|---|
| (quarter ng/mL) | OR c (95% CI) | OR d (95% CI) | OR c (95% CI) | OR d (95% CI) |
| 2OH-4MeO-BP | ||||
| 1st quartile (<0.28-2.43) | reference | reference | reference | reference |
| 2nd quartile (2.44-5.99) | 0.80 (0.47, 1.34) | 0.82 (0.48, 1.38) | 0.96 (0.12, 7.38) | 0.89 (0.11, 7.25) |
| 3rd quartile (6.00-36.69) | 0.96 (0.58, 1.61) | 0.99 (0.59, 1.67) | 1.92 (0.34, 10.8) | 2.36 (0.39, 14.2) |
| 4th quartile (36.70-5890) | 1.12 (0.67, 1.88) | 1.24 (0.73, 2.10) | 1.64 (0.29, 9.19) | 1.67 (0.28, 10.1) |
| Trend test a | 1.05 (0.89, 1.24) | 1.08 (0.92, 1.28) | 1.22 (0.73, 2.06) | 1.25 (0.73, 2.15) |
| >Q3 versus <Q3 b | 1.22 (0.79, 1.87) | 1.33 (0.85, 2.06) | 1.22 (0.38, 3.91) | 1.17 (0.34, 3.94) |
| 2,4OH-BP | ||||
| 1st quartile (<0.082-1.68) | reference | reference | reference | reference |
| 2nd quartile (1.69-5.65) | 0.77 (0.45, 1.31) | 0.76 (0.44, 1.31) | 0.44 (0.07, 2.89) | 0.48 (0.07, 3.40) |
| 3rd quartile (5.66-26.59) | 1.14 (0.68, 1.92) | 1.16 (0.68, 1.97) | 0.63 (0.13, 3.13) | 0.73 (0.14, 3.97) |
| 4th quartile (26.60-3200) | 1.43 (0.87, 2.35) | 1.59 (0.94, 2.66) | 0.86 (0.18, 4.03) | 0.89 (0.17, 4.61) |
| Trend test a | 1.15 (0.98, 1.35) | 1.19 (1.01, 1.41) | 1.02 (0.60, 1.74) | 1.03 (0.60, 1.79) |
| >Q3 versus <Q3 b | 1.48 (0.98, 2.25) | 1.65 (1.07, 2.53) | 1.33 (0.41, 4.27) | 1.25 (0.37, 4.24) |
| 4OH-BP | ||||
| 1st quartile (<0.082-0.17) | reference | reference | reference | reference |
| 2nd quartile (0.18-0.35) | 0.87 (0.52, 1.45) | 0.92 (0.55, 1.54) | 1.08 (0.20, 5.85) | 1.51 (0.25, 9.20) |
| 3rd quartile (0.36-0.71) | 1.02 (0.61, 1.71) | 1.03 (0.62, 1.73) | 1.24 (0.25, 6.06) | 2.20 (0.38, 12.7) |
| 4th quartile (0.71-22.40) | 0.84 (0.49, 1.42) | 0.87 (0.51, 1.48) | 1.16 (0.24, 5.66) | 1.69 (0.31, 9.21) |
| Trend test a | 0.97 (0.82, 1.14) | 0.97 (0.82, 1.15) | 1.06 (0.65, 1.73) | 1.19 (0.71, 1.98) |
| >Q3 versus <Q3 b | 0.87 (0.56, 1.36) | 0.89 (0.57, 1.38) | 1.05 (0.31, 3.58) | 1.12 (0.31, 4.01) |
NOTE: Logistic regression models for the operative cohort include 190 women with and 283 without endometriosis, and 14 and 113 women with and without endometriosis, respectively, in the population cohort.
Trend test assessed linear trends of BP derivatives across the four intervals defined by the 25th, 50th, and 75th percentiles.
Women in the highest quartile for each BP derivative were compared with women in the combined first three quartiles.
Odds ratios from unadjusted logistic regressions
Odds ratios from multivariable logistic regressions adjusting for site (Utah, California) and hair color (red, blonde, brown/black)
Our findings suggest an increase in the odds of an endometriosis diagnosis for 2,4OH-BP, which suggests that this BP derivative may be associated with endometriosis diagnosis. To our knowledge, no earlier laboratory or human studies have examined the association between 2,4OH-BP and endometriosis. Nevertheless several studies have reported estrogenic potencies of 2,4OH-BP, 11, 12, 14, 17, 19 which suggests that this compound is a potent xenoestrogen. In vitro studies using recombinant yeast cells that express human ERα (hERα) with the β-galactosidase reporter gene demonstrated an approximate 5-fold higher estrogenic activity of 2,4OH-BP than bisphenol A (BPA), which is a well-known EDC. 11, 12 A recent study using hERα- or hERβ-mediated reporter gene assays displayed a stronger activation of hERβ, in comparison to hERα, by 2,4OH-BP, unlike the activation pattern reported for estradiol (hERα > hERβ). 41 In addition, an in vivo study using ovariectomized female rats showed an increase in uterine weight in rats dosed with 2,4OH-BP. 14 These observations emphasize the need for further studies that examine endometriosis following 2,4OH-BP exposure. In addition, further research should measure BP derivatives and reproductive hormones including endogenous estrogens in blood to help delineate the relation between exposure, hormones, and disease status and, eventually, in the context of other EDCs. A recent laboratory exposure study on 2OH-4MeO-BP showed that the O-dealkylation metabolite, 2,4OH-BP, in rat blood decreased much more slowly over time than did the parent compound. 15 Further, in vitro assays have shown additive estrogenic effects of 2,4OH-BP and other weak estrogen-like EDCs, including UV filters, at relatively low levels. 20, 42, 43
To our knowledge, this is the first epidemiologic study to investigate the occurrence of BP-type UV filters other than 2OH-4MeO-BP in U.S. women and its association with incident endometriosis. Our results suggest that exposure to elevated 2,4OH-BP levels may be associated with increased odds of an endometriosis diagnosis. These exploratory findings await epidemiologic corroboration and support the need for mechanistic studies aimed at delineating the potential endocrine disruption properties of 2,4OH-BP.
Acknowledgments
This study was funded by the Intramural Research Program, Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institutes of Health (contracts NO1-DK-6-3428; NO1-DK-6-3427; 10001406-02).
LITERATURE CITED
- 1.Fent K, Zenker A, Rapp M. Widespread occurrence of estrogenic UV-filters in aquatic ecosystems in Switzerland. Environ Pollut. 2010;158:1817–1824. doi: 10.1016/j.envpol.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Giokas DL, Salvador A, Chisvert A. UV filters: from sunscreen to human body and the environment. Trends Anal Chem. 2007;26:360–374. [Google Scholar]
- 3.Kunisue T, Wu Q, Tanabe S, Aldous KM, Kannan K. Analysis of five benzophenone-type UV filters in human urine by liquid chromatography-tandem mass spectrometry. Anal Methods. 2010;2:707–713. [Google Scholar]
- 4.Zhang Z, Ren N, Li Y-F, Kunisue T, Gao D, Kannan K. Determination of benzotriazole and benzophenone UV filters in sediment and sewage sludge. Environ Sci Technol. 2011;45:3909–3916. doi: 10.1021/es2004057. [DOI] [PubMed] [Google Scholar]
- 5.Fent K, Kunz PY, Gomez E. UV filters in the aquatic environment induce hormonal effects and affect fertility and reproduction in fish. Chimia. 2008;62:368–375. [Google Scholar]
- 6.Schlumpf M, Cotton B, Conscience M, Haller V, Steinmann B, Lichtensteiger W. In vitro and in vivo estrogenicity of UV screens. Environ Health Perspect. 2001;109:239–244. doi: 10.1289/ehp.01109239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Environmental Working Group. EWG’s Skin Deep Cosmetic Database. http://www.ewg.org/skindeep/:available on December 2011.
- 8.Hayden CGJ, Roberts MS, Benson HAE. Systemic absorption of sunscreen after topical application. Lancet. 1997;350:863–864. doi: 10.1016/S0140-6736(05)62032-6. [DOI] [PubMed] [Google Scholar]
- 9.Janjua NR, Mogensen B, Andersson A-M, Petersen JH, Henriksen M, Skakkebæk NE, Wulf HC. Systemic absorption of the sunscreens benzophenone-3, octyl-methoxycinnamate, and 3-(4-methyl-benzylidene) camphor after whole-body topical application and reproductive hormone levels in humans. J Invest Dematol. 2004;123:57–61. doi: 10.1111/j.0022-202X.2004.22725.x. [DOI] [PubMed] [Google Scholar]
- 10.Jiang R, Roberts MS, Collins DM, Benson HAE. Absorption of sunscreens across human skin: an evaluation of commercial products for children and adults. Br J Clin Pharmacol. 1999;48:635–637. doi: 10.1046/j.1365-2125.1999.00056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawamura Y, Ogawa Y, Nishimura T, Kikuchi Y, Nishikawa J, Nishihara T, Tanamoto K. Estrogenic activities of UV stabilizers used in food contact plastics and benzophenone derivatives tested by the yeast two-hybrid assay. J Health Sci. 2003;49:205–212. [Google Scholar]
- 12.Morohoshi K, Yamamoto H, Kamata R, Shiraishi F, Koda T, Morita M. Estrogenic activity of 37 components of commercial sunscreen lotions evaluated by in vitro assays. Toxicol in Vitro. 2005;19:457–469. doi: 10.1016/j.tiv.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Schreurs RHMM, Sonneveld E, Jansen JHJ, Seinen W, Van der Burg B. Interaction of polycyclic musks and UV filters with the estrogen receptor (ER), androgen receptor (AR), and progesterone receptor (PR) in reporter gene bioassays. Toxicol Sci. 2005;83:264–272. doi: 10.1093/toxsci/kfi035. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki T, Kitamura S, Khota R, Sugihara K, Fujimoto N, Ohta S. Estrogenic and antiandrogenic activities of 17 benzophenone derivatives used as UV stabilizers and sunscreens. Toxicol Appl Pharmacol. 2005;203:9–17. doi: 10.1016/j.taap.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Jeon H, Sarma SN, Kim Y, Ryu J. Toxicokinetics and metabolisms of benzophenone-type UV filters in rats. Toxicol. 2008;248:89–95. doi: 10.1016/j.tox.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 16.Kasichayanula S, House JD, Wang T, Gu X. Simultaneous analysis of insect repellent DEET, sunscreen oxybenzone and five relevant metabolites by reversed-phase HPLC with UV detection: application to an in vivo study in a piglet model. J Chromatogr B. 2005;822:271–277. doi: 10.1016/j.jchromb.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 17.Nakagawa Y, Suzuki T. Metabolism of 2-hydroxy-4methoxybenzophenone in isolated rat hepatocytes and xenoestrogenic effects of its metabolites on MCF-7 human breast cancer cells. Chem-Biol Interact. 2002;139:115–128. doi: 10.1016/s0009-2797(01)00293-9. [DOI] [PubMed] [Google Scholar]
- 18.Okereke CS, Abdel-Rhaman MS, Friedman MA. Disposition of benzophenone-3 after dermal administration in male rats. Toxicol Lett. 1994;73:113–122. doi: 10.1016/0378-4274(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 19.Takatori S, Kitagawa Y, Oda H, Miwa G, Nishikawa J, Nishihara T, Nakazawa H, Hori S. Estrogenicity of metabolites of benzophenone derivatives examined by a yeast two-hybrid assay. J Health Sci. 2003;49:91–98. [Google Scholar]
- 20.Heneweer M, Muusse M, Van den Burg M, Sanderson JT. Additive estrogenic effects of mixtures of frequently used UV filters on pS2-gene transcription in MCF-7 cells. Toxicol Appl Pharmacol. 2005;208:170–177. doi: 10.1016/j.taap.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Calafat AM, Wong LY, Ye X, Reidy JA, Needham LL. Concentrations of the sunscreen agent benzophenone-3 in residents of the United States: National Health and Nutrition Examination Survey 2003-2004. Environ Health Perspect. 2008;116:893–897. doi: 10.1289/ehp.11269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolff MS, Teitelbaum SL, Windham G, Pinney SM, Britton JA, Chelimo C, Godbold J, Biro F, Kushi LH, Pfeiffer CM, Calafat AM. Pilot study of urinary biomakers of phytoestrogens, phthalates, and phenols in girls. Environ Health Perspect. 2007;115:116–121. doi: 10.1289/ehp.9488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ye X, Kuklenyik Z, Needham LL, Calafat AM. Automated on-line column-switching HPLC-MS/MS method with peak focusing for the determination of nine environmental phenols in urine. Anal Chem. 2005;77:5407–5413. doi: 10.1021/ac050390d. [DOI] [PubMed] [Google Scholar]
- 24.Ye X, Kuklenyik Z, Needham LL, Calafat AM. Quantification of urinary conjugates of bisphenol A, 2,5-dichlorophenol, and 2-hydroxy-4-methoxybenzophenone in humans by online solid phase extraction-high performance liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2005;383:638–644. doi: 10.1007/s00216-005-0019-4. [DOI] [PubMed] [Google Scholar]
- 25.Giudice LC, Kao L. Endometriosis. Lancet. 2004;364:1789–1799. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- 26.Buck Louis GM, Hediger ML, Peterson CM, Croughan M, Sundaram R, Stanford J, Chen Z, Fujimoto VY, Varner MW, Trumble A, Giudice LC. Incidence of endometriosis by study population and diagnostic method: the ENDO Study. Fertil Steril. 2011;96:360–365. doi: 10.1016/j.fertnstert.2011.05.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garai J, Molnar V, Varga T, Koppan M, Torok A, Bodis J. Endometriosis: harmful survival of an ectopic tissue. Frontiers Biosci. 2006;11:595–619. doi: 10.2741/1821. [DOI] [PubMed] [Google Scholar]
- 28.Niskar AS, Needham LL, Rubin C, Turner WE, Martin CA, Patterson DG, Jr, Hasty L, Wong L-Y, Marcus M. Serum dioxins, polychlorinated biphenyls, and endometriosis: a case-control study in Atlanta. Chemosphere. 2009;74:944–949. doi: 10.1016/j.chemosphere.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Buck Louis GM, Weiner JM, Whitcomb BW, Sperrazza R, Schisterman EF, Lobdell DT, Crickard K, Greizerstein H, Kostyniak PJ. Environmental PCB exposure and risk of endometriosis. Hum Reprod. 2005;20:279–285. doi: 10.1093/humrep/deh575. [DOI] [PubMed] [Google Scholar]
- 30.Hoffman CS, Small CM, Blanck HM, Tolbert P, Rubin C, Marcus M. Endometriosis among women exposed to polybrominated biphenyls. Ann Epidemiol. 2007;17:503–510. doi: 10.1016/j.annepidem.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trabert B, De Roos AJ, Schwartz SM, Peters U, Scholes D, Barr DB, Holt VL. Non-dioxin-like polychlorinated biphenyls and risk of endometriosis. Environ Health Perspect. 2010;118:1280–1285. doi: 10.1289/ehp.0901444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cooney MA, Buck Louis GM, Hediger ML, Vexler A, Kostyniak PJ. Organochlorine pesticides and endometriosis. Reprod Toxicol. 2010;30:365–369. doi: 10.1016/j.reprotox.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lohman TG, Roche AF, Martorell R, editors. Anthropometric standardization reference manual. Champaign, IL: Human Kinetics Books; 1988. [Google Scholar]
- 34.Kennedy S, Bergqvist A, Chapron C, D’Hooghe T, Dunselmans G, Greb R, Hummelshoj L, Prentice A, Saridogan E. ESHRE guideline for the diagnosis and treatmen t of endometriosis. Hum Reprod. 2005;20:2698–2704. doi: 10.1093/humrep/dei135. [DOI] [PubMed] [Google Scholar]
- 35.American Society for Reproductive Medicine. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67:817–821. doi: 10.1016/s0015-0282(97)81391-x. [DOI] [PubMed] [Google Scholar]
- 36.Guo Y, Harel O, Little RJ. How well quantified is the limit of quantification? Epidemiology. 2010;21:S10–S16. doi: 10.1097/EDE.0b013e3181d60e56. [DOI] [PubMed] [Google Scholar]
- 37.Somigliana E, Viganò P, Abbiati A, Gentilini D, Parazzini F, Benaglia L, Vercellini P, fedele L. ‘Here comes the sun’: pigmentary traits and sun habits in women with endometriosis. Hum Reprod. 2010;25:728–733. doi: 10.1093/humrep/dep453. [DOI] [PubMed] [Google Scholar]
- 38.Zevin S, Benowitz NL. Drug interactions with tobacco smoking – An update. Clin Pharmacokinet. 1999;36:425–438. doi: 10.2165/00003088-199936060-00004. [DOI] [PubMed] [Google Scholar]
- 39.Sarveiya V, Risk S, Benson HAE. Liquid chromatographic assay for common sunscreen agents : application to in vivo assessment of skin penetration and systemic absorption in human volunteers. J Chromatogr B. 2004;803:225–231. doi: 10.1016/j.jchromb.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 40.Nakagawa Y, Suzuki T, Tayama S. Metabolism and toxicity of benzophenone in isolated rat hepatocytes and estrogenic activity of its metabolites in MCF-7 cells. Toxicol. 2000;156:27–36. doi: 10.1016/s0300-483x(00)00329-2. [DOI] [PubMed] [Google Scholar]
- 41.Molina-Molina J, Escande A, Pillon A, Gomez E, Pakdel F, Cavaillès V, Olea N, Aït-Aïssa S, Balaguer P. Profiling of benzophenone derivatives using fish and human estrogen receptor-specific in vitro bioassays. Toxicol Appl Pharmacol. 2008;232:384–395. doi: 10.1016/j.taap.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 42.Kunz PY, Fent K. Estrogenic activity of UV filter mixtures. Toxicol Appl Pharmacol. 2006;217:86–99. doi: 10.1016/j.taap.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 43.Silva E, Rajapakse N, Kortenkamp A. Something from “nothing” – eight weak estrogenic chemicals combined at concentrations below NOECs produce significant mixture effects. Environ Sci Technol. 2002;36:1751–1756. doi: 10.1021/es0101227. [DOI] [PubMed] [Google Scholar]


