Abstract
Familial hypophosphatemic rickets is transmitted in most cases as an X-linked dominant trait and results from the mutation of the PHEX gene predominantly expressed in osteoblast and odontoblast. Patients with rickets have been reported to display important dentin defects. Our purpose was to explore the structure, composition and distribution of noncollagenous proteins (NCPs) of hypophosphatemic dentin. We collected teeth from 10 hypophosphatemic patients whose mineralization occurred either in a hypophosphatemic environment or in a corrected phosphate and vitamin environment. Teeth were examined by scanning electron microscopy, immunohistochemistry and Western blot analysis. An abnormal distribution (accumulation in interglobular spaces) and cleavage of the NCPs and particularly of matrix extracellular phosphoglycoprotein were observed in deciduous dentin. In contrast, it was close to normal in permanent dentin mineralized under corrected conditions. In conclusion, dentin mineralization in a corrected phosphate and vitamin D environment compensates the adverse effect of PHEX mutation.
Key Words: Hypophosphatemic rickets, Dentin, Mineralization, Noncollagenous proteins, Matrix extracellular phosphoglycoprotein
Introduction
Hypophosphatemia is in most cases transmitted as an X-linked dominant trait and results from the mutation of the PHEX gene [Hyp Consortium, 1995; Rowe et al., 1997]. This gene encodes an endopeptidase, predominantly expressed in osteoblast and odontoblast, whose only known natural substrate is parathyroid hormone-related peptide [Boileau et al., 2001]. As suggested by recent publications, PHEX may also protect matrix extracellular phosphoglycoprotein (MEPE) from proteolysis by a nonproteolytic interaction, therefore controlling the inhibiting effect of the aspartate serine-rich motif (ASARM) peptide (cleaved C terminal of MEPE) on matrix mineralization [Guo et al., 2002; Rowe et al., 2005]. Hypophosphatemic patients have been reported to display large interglobular spaces in the circumpulpal dentin [Boukpessi et al., 2006], whereas the mantle dentin is unaffected [Goldberg et al., 2002]. Human dentin mineralization is a continuous process that occurs by growth and fusion of calcospherites at the mineralization front [Boyde and Sela, 1978]. This process appears to be controlled by noncollagenous proteins (NCPs), particularly by a family of phosphorylated proteins designated as small integrin-binding ligand N-linked glycoproteins (SIBLINGs) [Fisher and Fedarko, 2003]. Some nonphosphorylated proteins such as osteocalcin, osteonectin and proteoglycans are also involved in this process [Goldberg and Smith, 2004].
Within our specialized outpatient departments for the clinical and odontological survey of hypophosphatemic rickets, we collected teeth from hypophosphatemic patients. The purpose of our work was to explore the structure, composition and distribution of NCPs of hypophosphatemic dentin.
Material and Methods
Sample
Teeth collected from 10 hypophosphatemic patients (aged 3–27 years) and age-matched controls were prepared for scanning electron microscopy (SEM), immunochemistry and Western blot analysis [Boukpessi et al., 2006; Chaussain-Miller et al., 2007]. In order to differentiate the gene mutation effect from the effect of hypophosphatemia, we collected deciduous teeth from children whose dentin mineralization occurred mainly before the onset of the treatment as well as permanent teeth from young adults whose dentin had mineralized in a corrected phosphate and vitamin environment. All teeth were obtained with the parents’ and patients’ informed consent and with approval of our local ethics committee. Immediately after extraction, teeth were either fixed in a 4% paraformaldehyde solution buffered at pH 7.3 by a phosphate-buffered saline and processed for SEM or, after demineralization, immunohistochemistry (IHC) examination, or were gently cleaned with tap water and kept at −20°C prior to protein extraction.
SEM Analysis
Sectioned teeth were prepared for SEM analysis. Carbon or gold sputter-coated surfaces were observed with a scanning electron microscope (JEOL 30B SEM), equipped with an electron microprobe for X-ray microanalysis (EDAX).
Immunohistochemistry
As previously reported, teeth were prepared for IHC [Boukpessi et al., 2006; Chaussain-Miller et al., 2007]. They were demineralized with acetic acid (0.5 M) in a solution of 0.85% NaCl and 4% paraformaldehyde. Five polyclonal antibodies raised against SIBLINGs were used. The anti-dentin sialoprotein (DSP, LF 153), anti-dentin matrix protein 1 (DMP1, LF 143), anti-bone sialoprotein (BSP, LF 100) and anti-osteopontin (OPN, LF 123) antibodies were generous gifts from Larry Fisher, NIDCR. We also used an anti-osteocalcin (OC) antibody (Abcam) and 2 rabbit polyclonal antibodies raised against 2 different regions of human MEPE. One was raised against the midregion of MEPE (NH2-G238SGYTDLQERGDNDISPFSGDGQPF262-COOH) and the other recognized the ASARM motif located in the C-terminal region of MEPE (NH2-R507FSSRRRDDSSESSDSGSSSESDGD525-COOH) [Rowe et al., 2000].
Dentin Protein Extraction and Analysis
Hypophosphatemic and control teeth were prepared for dentin protein extraction [Boukpessi et al., 2006]. Dentin blocks were immersed individually at 4°C for 8 days in 4.13% EDTA (pH 7.2) supplemented with 1/100 Proteinase Inhibitor Cocktail Set V EDTA free (Calbiochem) and the extracts were analyzed by Western blotting.
Results
NCPs Accumulate in Interglobular Spaces of Deciduous Dentin
Hypophosphatemic dentin displayed severe alterations in deciduous teeth. Large interglobular spaces were observed between unmerged calcospherites (fig. 1b), while continuous dentin tubules regularly crossed a homogeneous dentin in control teeth (fig. 1a). Furthermore, fissures were observed in most deciduous incisors, extending from the pulp horn up to the dentinoenamel junction [Chaussain-Miller et al., 2007]. Such fissures may have facilitated bacterial penetration and pulp infection despite the lack of carious decay or trauma, a phenomenon that has been previously reported [Chaussain-Miller et al., 2003]. Using X-ray microanalysis, no mineralization was detected in the interglobular spaces of the primary dentin, whereas it was close to controls in calcospherites [Chaussain-Miller et al., 2007]. IHC revealed accumulation in the interglobular spaces of labeling with antibodies against DSP, DMP1, MEPE (midregion and ASARM, fig. 2b, OC and BSP (fig. 1e). Furthermore, Western blot analysis of dentin extracts demonstrated the abnormal presence of low-molecular-weight protein complexes recognized by antibodies against DSP and OPN [Boukpessi et al., 2006].
Fig. 1.
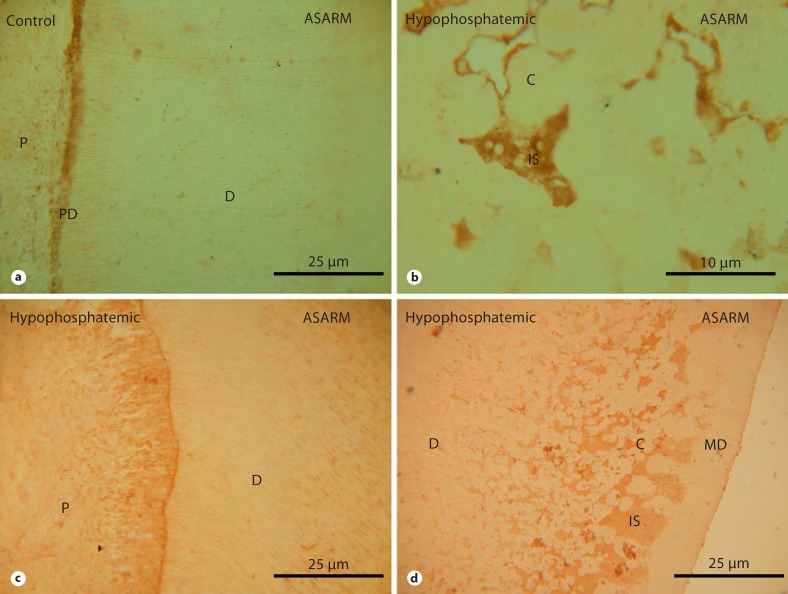
Dentin examination by SEM and IHC (anti-BSP) of a control molar (a, d), a hypophosphatemic primary molar (b, e) and a hypophosphatemic permanent molar (c, f). The inner part of dentin appears normal in the permanent hypophosphatemic molar, whereas discrete calcospherites are observed close to the mantle dentin. P = Pulp; PD = predentin; D = dentin; MD = mantle dentin; C = calcospherite; IS = interglobular space.
Fig. 2.
Dentin examination by IHC (anti-MEPE/ASARM) of a control molar (a), a hypophosphatemic primary molar (b) and a hypophosphatemic permanent molar (c, d). Labeling for ASARM is observed in the interglobular spaces of both hypophosphatemic teeth (b, d); however, for the permanent tooth, the labeling is limited to the outer part of dentin. P = Pulp; PD = predentin; D = dentin; MD = mantle dentin; C = calcospherite; IS = interglobular space.
Permanent Dentin Displays Close to Normal Mineralization
The results were different in permanent teeth from patients whose dentin had mineralized in a corrected phosphate and vitamin environment. Discrete calcospherites were detected in the outer part of dentin, while the inner part appeared normal (fig. 1c) and X-ray microanalysis showed normal mineralization [Chaussain-Miller et al., 2007]. A normal distribution of NCPs was observed in dentin (fig. 1c and f) except for 1 male patient whose outer part of dentin showed faint labeling for DMP1, DSP, OPN, BSP, OC [Chaussain-Miller et al., 2007] and MEPE (fig. 2c and d) in the interglobular spaces.
Discussion
NCPs of bone and dentin matrix have been recently involved in the defects associated with familial X-linked hypophosphatemic rickets [Qin et al., 2004; Liu et al., 2005; Lorenz-Depiereux et al., 2006]. We previously reported severe alterations of the dentin in deciduous teeth from untreated hypophosphatemic patients [Boukpessi et al., 2006; Chaussain-Miller et al., 2007]. IHC analysis of hypophosphatemic dentin showed an accumulation of extracellular matrix molecules in the nonmineralized interglobular spaces. Interestingly, we observed MEPE accumulation in the interglobular spaces using 2 antibodies raised against this protein, one against MEPE midregion and the other against the C-terminal ASARM motif. Protein extraction from hypophosphatemic dentin revealed the unexpected presence of low-molecular-weight proteins recognizing anti-DSP and anti-OPN antibodies, in addition to the immunolabeled proteins and protein complexes found in normal dentin [Boukpessi et al., 2006]. Our data strongly suggested an accumulation of MEPE in the interglobular spaces of deciduous dentin, the major part of which could be possibly cleaved as both antibodies raised against MEPE labeled these spaces. Free ASARM peptides may therefore locally inhibit mineralization in dentin matrix as it has been reported in bone [Rowe et al., 2004].
In addition to the adverse effect of ASARM peptides on mineralization, several hypotheses can be raised concerning the inability of NCPs to regulate mineralization in hypophosphatemic dentin. Western blot analyses suggest that part of the proteins present may have an abnormal structure, which may alter their function and ability to bind other factors in the mineralization process. Also, permanent hypophosphatemia may lead to defective phosphorylation of extracellular matrix proteins like DSP, DMP1, BSP, MEPE and OPN, thus modifying their capacity to promote mineralization. Instead, in the permanent hypophosphatemic dentin, mineralization was close to normal. It is interesting to note that these patients had been treated since early childhood with phosphates and 1-hydroxyvitamin D3, underlying the fact that dentin mineralized in a corrected phosphate and vitamin D environment, which compensates the adverse effect of the PHEX mutation.
In conclusion, abnormal expression and distribution of NCP in hypophosphatemic dentin and liberation of ASARM peptides may partially explain the impaired dentin mineralization associated with the disease. However, correction of phosphatemia and level of vitamin D allows a mineralization that is close to normal, despite PHEX loss of function.
Acknowledgements
This work was supported by the Université Paris Descartes and AP-HP.
Abbreviations used in this paper
- ASARM
aspartate serine-rich motif
- BSP
bone sialoprotein
- DMP1
dentin matrix protein 1
- DSP
dentin sialoprotein
- IHC
immunohistochemistry
- MEPE
matrix extracellular phosphoglycoprotein
- NCPs
noncollagenous proteins
- OC
osteocalcin
- OPN
osteopontin
- SEM
scanning electron microscopy
- SIBLING
small integrin-binding ligand N-linked glycoprotein
References
- Boileau G., Tenenhouse H.S., Desgroseillers Crine L.P. Characterization of PHEX endopeptidase catalytic activity identification of parathyroid-hormone-related peptide107–139 as a substrate and osteocalcin, PPi and phosphate as inhibitors. Biochem J. 2001;355:355–707. doi: 10.1042/bj3550707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukpessi T., Septier D., Bagga S., Garabedian M., Goldberg M., Chaussain-Miller C. Dentin alteration of deciduous teeth in human hypophosphatemic rickets. Calcif Tissue Int. 2006;79:79–294. doi: 10.1007/s00223-006-0182-4. [DOI] [PubMed] [Google Scholar]
- Boyde A., Sela J. Scanning electron microscope study of separated calcospherites from the matrices of different mineralizing systems. Calcif Tissue Res. 1978;26:26–47. doi: 10.1007/BF02013233. [DOI] [PubMed] [Google Scholar]
- Chaussain-Miller C., Septier Wolikow D., Goldberg M., Garabedian M. Dentin structure in familial hypophosphatemic rickets benefits of vitamin D and phosphate treatment. Oral Dis. 2007;13:13–482. doi: 10.1111/j.1601-0825.2006.01326.x. [DOI] [PubMed] [Google Scholar]
- Chaussain-Miller C., Sinding C., Wolikow M., Lasfargues J.J., Godeau G., Garabedian M. Dental abnormalities in patients with familial hypophosphatemic vitamin D-resistant rickets prevention by early treatment with 1-hydroxyvitamin D. J Pedriatr. 2003;142:324–331. doi: 10.1067/mpd.2003.119. [DOI] [PubMed] [Google Scholar]
- Fisher L.W., Fedarko N.S. Six genes expressed in bones and teeth encode the current members of the SIBLING family of proteins. Connect Tissue Res. 2003;44(suppl 1):33–40. [PubMed] [Google Scholar]
- Goldberg M., Septier D., Bourd K., Hall R., Jeanny J.C., Jouet L., et al. The dentino-enamel junction revisited. Connect Tissue Res. 2002;43:43–482. doi: 10.1080/03008200290000817. [DOI] [PubMed] [Google Scholar]
- Goldberg M., Smith A.J. Cells and extracellular matrices of dentin and pulp a biological basis for repair and tissue engineering. Crit Rev Oral Biol Med. 2004;15:15–13. doi: 10.1177/154411130401500103. [DOI] [PubMed] [Google Scholar]
- Guo R., Rowe P.S., Liu S., Simpson L.G., Xiao Z.S., Quarles L.D. Inhibition of MEPE cleavage by Phex. Biochem Biophys Res Commun. 2002;297:297–38. doi: 10.1016/s0006-291x(02)02125-3. [DOI] [PubMed] [Google Scholar]
- Consortium HYP. A gene (PHEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. Nat Genet. 1995;11:11–130. doi: 10.1038/ng1095-130. [DOI] [PubMed] [Google Scholar]
- Liu S., Brown T.A., Zhou J., Xiao Z.S., Awad H., Guilak F., Quarles L.D. Role of matrix extracellular phosphoglycoprotein in the pathogenesis of X-linked hypophosphatemia. J Am Soc Nephrol. 2005;16:16–1645. doi: 10.1681/ASN.2004121060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz-Depiereux B., Bastepe M., Benet-Pages A., Amyere M., Wagenstaller J., Muller-Barth U., et al. DMP1 mutations in autosomal recessive hypophosphatemia implicate a bone matrix protein in the regulation of phosphate homeostasis. Nat Genet. 2006;38:38–1248. doi: 10.1038/ng1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C., Baba O., Butler W.T. Post-translational modifications of sibling proteins and their roles in osteogenesis and dentinogenesis. Crit Rev Oral Biol Med. 2004;15:15–126. doi: 10.1177/154411130401500302. [DOI] [PubMed] [Google Scholar]
- Rowe P.S., Garrett I.R., Schwarz P.M., Carnes D.L., Lafer E.M., Mundy G.R., Gutierrez G.E. Surface plasmon resonance (SPR) confirms that MEPE binds to PHEX via the MEPE-ASARM motif a model for impaired mineralization in X-linked rickets (HYP) Bone. 2005;36:36–33. doi: 10.1016/j.bone.2004.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe P.S., Kumagai Y., Gutierrez G., Garrett I.R., Blacher R., Rosen D., Cundy J., Navvab S., Chen D., Drezner M.K., Quarles L.D., Mundy G.R. MEPE has the properties of an osteoblastic phosphatonin and minhibin. Bone. 2004;34:34–303. doi: 10.1016/j.bone.2003.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe P.S., de Zoysa P.A., Dong R., Wang H.R., White K.E., Econs M.J., Oudet C.L. MEPE, a new gene expressed in bone marrow and tumors causing osteomalacia. Genomics. 2000;67:67–54. doi: 10.1006/geno.2000.6235. [DOI] [PubMed] [Google Scholar]
- Rowe P.S.N., Oudet C.L., Francis F., Sinding C., Pannetier S., Econs J., et al. Distribution of mutations in the Phex gene in families with X-linked hypophosphatemic rickets (HYP) Hum Mol Genet. 1997;6:6–539. doi: 10.1093/hmg/6.4.539. [DOI] [PubMed] [Google Scholar]