Summary
MR elastography is one of the only non-invasive techniques that can accurately and reliably identify and stage liver fibrosis. Importantly, it has been shown to more effectively stage liver fibrosis in adults than other non-invasive assessments and thus can be used to follow treatment response or disease progression. The mechanical properties of liver tissue appear to be the same for adults and children suggesting MRE will prove to be an accurate non-invasive test for identifying, staging and tracking liver fibrosis. In our experience it is technically feasible for pediatric patients, even in young infants. MRE findings appear to correlate well with liver biopsy results in the small number of patients where we have pathologic correlation but larger, studies will be needed to confirm the reliability and accuracy of this technique to establish it as an alternative to pediatric liver biopsy.
Introduction
Children suffer from a wide variety of liver diseases that include congenital, infectious and inflammatory conditions which, when severe, can result in end-stage liver disease and hepatic fibrosis. Traditionally, liver biopsy has been the only accurate method to assess for the presence and severity of fibrosis. With the advent of effective treatments to arrest or hepatic fibrogenesis (1), non-invasive techniques for the identification and grading of hepatic fibrosis have become increasingly sought after. MR elastography (MRE) is one of several novel, non-invasive techniques that assess liver stiffness for the assessment of the presence and severity of liver fibrosis (2, 3). It has recently been shown to accurately identify and stage hepatic fibrosis in adults (4). The use of MRE in pediatric patients has not been reported previously. This review will discuss the physical principles and technical issues related to its performance in children. Additionally, potential future clinical applications will be presented with case examples. Lastly, current limitations and potential areas for future research will be addressed.
Principles and Techniques of MRE
Infiltrative processes or tumors alter the mechanical properties of soft tissues, typically resulting in increased firmness and decreased elasticity. These changes can be qualitatively assessed by physicians with palpation and percussion. In mechanical engineering terms, the pressure applied during palpation or percussion is termed stress and the resulting tissue movement is termed strain. Stresses and strains are related through a number of tissue-specific mechanical parameters (e.g., the shear and Young’s moduli) that characterize the mechanical properties and deformation behavior of the tissue, including its stiffness. In particular, tissues with high shear/Young’s moduli (e.g., cirrhotic liver tissue) are stiff and tissues with low moduli (e.g., normal liver) are soft. Given the same amount of applied pressure (stress) such as that applied during palpation, stiff tissues experience less deformation (strain) than soft tissues. With vibrational stresses, such as percussion, stiffer tissues will transmit the vibrational energy in the form of a shear wave deeper into the tissue whereas more elastic tissues will dissipate the energy and not transmit the shear wave effectively.
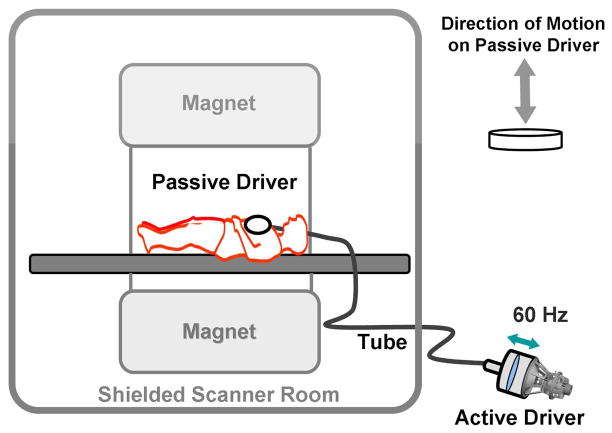
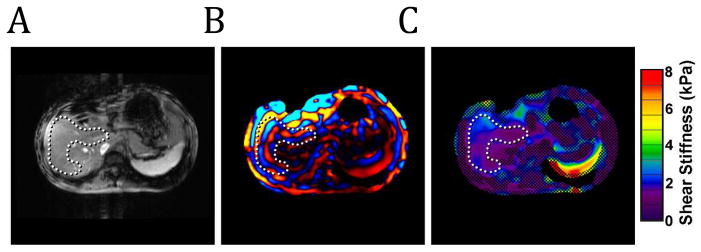
MRE assesses tissue stiffness by measuring the speed of shear waves propagating within it. As with other elasticity imaging techniques (5), this assessment involves three basic steps. First, an external source of tissue stress is applied. In the case of liver MRE this can be performed with an audio sub-woofer. The speaker’s magnet must be located away from the MR imaging magnet, thus a connecting tube is used to transmit the vibrational energy to a passive driver. This driver is placed on the right anterior lower chest/upper abdominal wall and delivers the vibrations transcostally/transabdominally into the liver (fig. 1). The frequency of the vibration is typically 60 Hz. Second, the response of the tissue to the mechanical stress introduced by this vibration is measured using standard MR phase-contrast imaging sequences with the addition of motion-encoding gradients (MEG) synchronized with the vibrational input (fig. 2). These sequences allow for visualization of the propagating shear waves within the target tissue in what are often called wave images. The peaks and troughs of the waves can be identified as concentric rings similar to the effect a pebble thrown into a pond (fig. 3a and b). Regions of interest (ROI) are selected avoiding large blood vessels and areas of low wave amplitude to provide an overall estimate of parenchymal stiffness with units of kiloPascals, kPa. The acquired wave images are used to generate quantitative maps of tissue stiffness referred to as elastograms (fig. 3c).
Figure 1.
Schematic drawing of an acoustic speaker source with connecting tubing and a driver for MRE. The active driver is shielded from the imaging magnet and delivers vibrational energy to the passive driver at 60Hz through the connecting tube. The passive driver is placed across the right anterior chest wall to deliver vibrations transcostally into the liver.
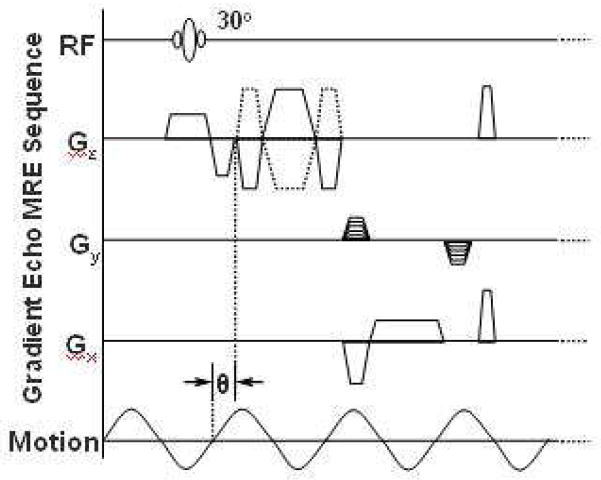
Figure 2.
MRE pulse sequence diagram illustrating the timing of the imaging and motion-encoding gradients in relation to the applied vibration. Theta indicates an adjustable phase delay between the motion and the motion-encoding gradients to capture the tissue motion at different time points during the wave propagation.
Figure 3.
A) MR magnitude image. The C-shaped ROI (dotted line) within the hepatic parenchyma is defined to avoid the central portal vessels. B) Wave image showing the propagation of the shear waves through the hepatic parenchyma. The wave pattern within the liver can be visualized by the alternating colored bands. C) Color-coded elastogram. Post-processing of the MRE wave data identified poor wave propagation in the hatched areas. These areas are not included in the elasticity assessment. The ROI shows blue and purple areas corresponding to normal elasticity values <2.9kPa.
In our practice, MRE is typically incorporated with a diagnostic MRI liver or MR enterography exam, depending on the clinical indication. The MR elastography study requires approximately 5–10 minutes of additional setup and table time. The acquisition parameters have been reported previously (3): axial FOV = 30–44 cm, acquisition matrix = 256×64, TR/TE = 50/20 ms, flip angle = 25°, slice thickness = 10 mm, ±32 kHz receiver bandwidth, 1 pair of 1st moment nulled MEG with motion sensitivity of 6.2 μm/radians, mechanical frequency = 60 Hz, 4 slices, parallel imaging acceleration factor of 2, and 4 time points evenly sampled over 1 period of the motion. The acquisition is typically performed in four 15-second breath holds performed at end expiration.
Technical Issues Unique to Pediatric MRE
In very young children (<1 year old), the standard driver power level is reduced by 50% from what is used in adult patients. A folded towel is placed between the driver and the child to (1) improve the mechanical coupling between the chest and abdomen wall and the comparatively large passive driver, (2) maintain image quality as less driver power is required in a small child to achieve the same displacement amplitude obtained in adults, and (3) to further minimize any potential risk of mechanical or thermal injury to the child. As with other pediatric body MRI examinations, the systemic absorption rate is maintained within acceptable limits based upon patient weight with automated adjustments incorporated by the MRI manufacturer into the imaging protocol. The relatively small surface in infants and children has not been a technical limitation for MRE as the driver can be placed over the lower chest and upper abdomen and still be effective in generating hepatic vibrations. ROI selection in small children, as in adult MRE, must be done with care to stay within the liver but avoid large blood vessels and extra-parenchymal structures such as the gall bladder. Because the increased portal venous flow present after eating can result in transiently increased hepatic stiffness in patients with liver disease (but not in normal subjects), children should be fasting for at least 4 hours prior to MRE (6).
Clinical Applications
In infants, the liver response to injury includes parenchymal cellular injury as well as cholestasis injury as the metabolism of bile formation and excretion are immature. The resulting insult may lead to rapidly progressive liver fibrosis brought about by chronic inflammation. For example, early cirrhosis is common in patients with extra-hepatic biliary atresia. Likewise, several metabolic liver diseases occur at an early age leading to progressive fibrosis such as familial intrahepatic cholestasis syndromes and α-1-antitrypsin deficiency. Liver fibrosis leading to portal hypertension may be the presenting feature in a variety of conditions such as autoimmune hepatitis, sclerosing cholangitis and congenital hepatic fibrosis. At present specific therapeutic tools to treat and reverse fibrosis are lacking but many conditions are amenable to palliative or curative treatment. Examples include the use of replacement bile salt therapy for cholestasis and immune suppression for autoimmune liver disease. With early interventions, hepatic fibrosis can be minimized. Traditionally, percutaneous liver biopsy has been required to monitor the fibrotic changes associated with these conditions.
The need for a noninvasive assessment of hepatic parenchymal inflammation and fibrosis arises in several patient groups: patients with elevated liver function tests of unknown cause; patients with hepatitis; patients with a known diagnosis that is associated with liver fibrosis such as cystic fibrosis, polycystic kidney disease or biliary atresia; and for treatment response assessment. Disadvantages of percutaneous liver biopsy, including expense, need for sedation, potential complications and insufficient sampling, have become more relevant in clinical decision making as potential alternatives to biopsy have been developed. Liver biopsy is an invasive procedure, often requiring deep sedation or general anesthesia in pediatric patients. Though image-guided percutaneous liver biopsy is an extremely safe procedure when performed by an experienced physician (7), the risks of hemorrhage, infection, injury to the liver or adjacent structures and death, are not inconsequential. Additionally, the costs of the biopsy procedure and pathologic analysis of the liver sample exceed those of MRE. Lastly, because of the heterogeneity of liver fibrosis, the accuracy of liver biopsy, the traditional gold standard for parenchymal assessment, is subject to sampling error. Percutaneous liver biopsy obtains a small parenchymal sample, approximately 1/50,000th of the liver mass, and this may not reflect the overall degree of liver damage. MRE samples large fraction of the liver, as much as 20%, and likely gives a more robust assessment of liver fibrosis.
Clinical Cases
Potential clinical applications of MRE include noninvasive assessment of the liver in patients with elevated liver function tests of unknown etiology, screening for liver fibrosis in patients with inflammatory bowel disease and at risk for primary sclerosing cholangitis, PSC, (fig. 4), patients with known congenital fibrogenic liver diseases such as primary familial intrahepatic cholestasis or α-1-antitrypsin deficiency (fig. 5), in patients with auto-immune or infectious conditions associated with liver fibrosis (fig. 6a), and in patients with cystic fibrosis or surgically treated biliary atresia. Additionally, if pediatric MRE proves to accurately and reliably grade hepatic fibrosis, it may be able to replace liver biopsy in the assessment of treatment response (Fig 6b).
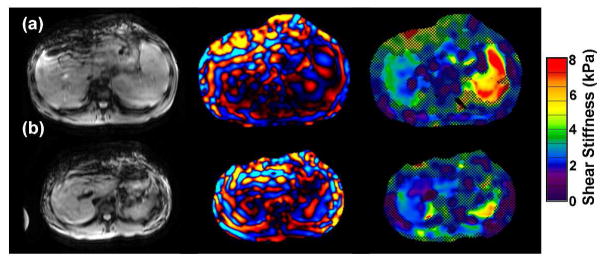
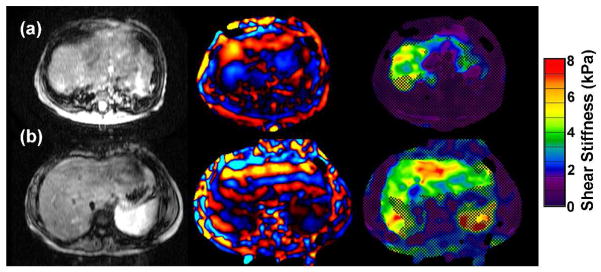
Figure 4.
MRE of 2 patients with inflammatory bowel disease.
A) 16 year old with chronic ulcerative colitis and PSC. MRE demonstrates mildly increased liver elasticity: mean = 3.2 kPa, range = 3.0–3.4 kPa, consistent with mild hepatic fibrosis. Liver biopsy on the same day demonstrated low-grade bridging fibrosis (grade 1–2 of 4).
B) 14 year old with Crohns colitis and PSC. MRE is normal (mean = 2.3 kPa). Biopsy from the same day showed no hepatic fibrosis.
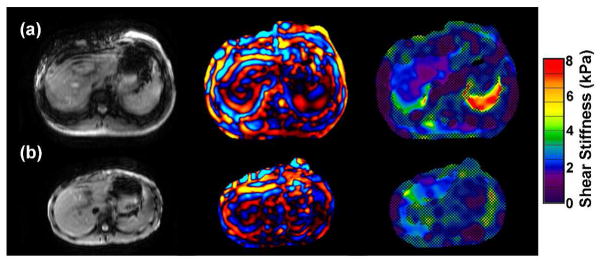
Figure 5.
MRE of two patients with congenital fibrogenic liver disease and marked liver fibrosis on biopsy (grade 3 of 4). Yellow, green and red areas indicate elevated liver stiffness.
A) 10 month old with familial cholestasis type 3. MRE demonstrates markedly elevated liver stiffness, (mean = 3.9 kPa, range = 3.2–6.1 kPa).
B) 10 year old with alpha-1 anti-trypsin deficiency. MRE demonstrates elevated liver stiffness (mean = 4.1 kPa, range = 3.9–4.3 kPa).
Figure 6.
A) 16 year old with chronic hepatitis B. Elastogram was normal (mean = 2.4 kPa) though biopsy showed minimal fibrosis (grade 1–2 of 4).
B) 17 year old with autoimmune hepatitis. Pre-treatment liver biopsy showed marked hepatic fibrosis (grade 3 of 4). Following one month of treatment with the resolution of the clinical and laboratory findings of hepatitis, an MRE demonstrated normal elastogram (mean = 2.5 kPa, range = 2.3–2.9 kPa). Follow up liver biopsy was not performed after the MRE.
Discussion
MRE has been shown to be accurate in the staging of liver fibrosis in adults when compared to liver biopsy and pathologic grading (4, 8). The presence or absence of liver fibrosis is demonstrated with MRE with a sensitivity of 98% and specificity of 99% when using a shear stiffness normal cutoff value of <2.93 kPa. MRE-derived shear stiffness values increased with increasing liver fibrosis grade. Specifically, low grade fibrosis was found in livers with elasticity measurements of > 2.93 and < 5.5 whereas high grade fibrosis was found in livers with values > 5.5 (4). Importantly, shear stiffness values were found to be independent of fatty changes in the liver and could be accurately assessed in the presence of ascites (4). When compared to other non-invasive assessments of liver fibrosis, MRE was found to be superior to ultrasound-based transient elastography, UTE, with higher technical success (94% vs. 84%) and to be more highly correlated with hepatic fibrosis (9). This is felt to be due to several advantages of MRE over UTE. MRE assesses liver strain in 2 or more dimensions and has a much larger sample volume than UTE reducing the potential of sampling error. The use of continuous vibratory compression waves with MRE allows for deeper penetration into the liver (10). MRE is not degraded by iron deposition in the liver except when extensive as in hemochromatosis (11). The incorporation of spin-echo and short-TE gradient-echo sequences has reduced this potential source of technical failure. Additionally, MRE can be performed in the presence of ascites and obesity, both of which are limitations of standard UTE which samples tissues at a set depth from the skin surface, typically 6 cm, which may be too shallow in the presence of obesity or ascites. However, recent developments in UTE will likely permit sampling of deeper tissues overcoming this limitation (12)
Current Limitations and Future Directions of Pediatric Liver MRE
Despite increasing acceptance of MRE for the assessment of liver fibrosis in adults, there are several important considerations to be addressed before it can become an established clinical tool for hepatic fibrosis assessment replacing liver biopsy in children. There is no normal pediatric liver MRE database to date nor have MRE cutoff values been established for mild, moderate or severe fibrosis specific for children. However pediatric liver tissue, normal and diseased, appears to have similar mechanical properties as that of adults. UTE studies performed on normal children and in patients with a wide variety of liver diseases support the use of adult normative data and cutoff values for children. UTE values were found to be independent of age in normal children and in patients with cystic fibrosis (13). Normal pediatric controls have been found to have UTE measurements similar to normal adult patients. Additionally, pediatric UTE appears to identify children with pathologically-proven mild, moderate and severe fibrosis using adult cutoff values (14). Though our experience is limited, our preliminary results have been encouraging with MRE results compared to liver biopsy pathological findings. Thus, it appears that MRE will prove to be an accurate and reliable non-invasive tool for assessment of pediatric liver disease. Prospective studies comparing liver MRE with percutaneous biopsy will be necessary to confirm this as well as to firmly establish the role of MRE in tracking progression and regression of hepatic fibrosis in children.
MRE offers a unique opportunity to examine the spatial patterns of hepatic fibrosis in various diseases. Because MRE visually quantifies and localizes the extent of fibrosis throughout the liver, it provides the opportunity to create a visual map of the extent of fibrosis in the whole liver. This may provide unique diagnostic and prognostic information in various disease states. The range and distribution of elasticity within the liver may also give unique insights into the nature of fibrogenesis. For example, livers with mean stiffness of 3.0 kPa and range of 2.9–3.1 kPa may have different rates of fibrosis progression or treatment response than livers with the same mean stiffness but a broader range with areas of very high stiffness. Additionally, increased liver stiffness in the absence of fibrosis may reflect “pre-fibrotic” changes due to increased extracellular matrix early in the course of fibrotic liver disease (15) and allow for earlier interventions. An excellent review of the stages of fibrogenesis in the liver has recently been published (5). The effect of portal hypertension on splenic stiffness may permit MRE of the spleen to provide an opportunity for non-invasive assessment of portal venous hypertension(16), (Fig 7). Pediatric-specific applications might include distinguishing biliary atresia from neonatal hepatitis, identifying patients with CF or biliary atresia at risk for varices, early recognition of TPN-induced liver disease in short gut patients, and early recognition of hepatic transplant dysfunction.
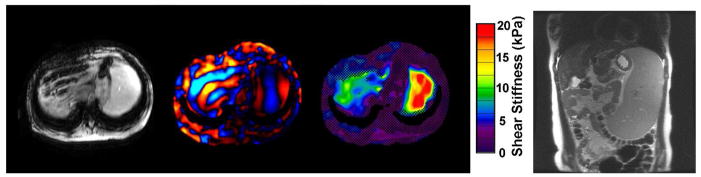
Figure 7.
MRE in a 15 year old with EBV-related liver disease resulting in cirrhosis, marked portal hypertension with varices and severe splenomegaly. Elastogram demonstrates elevated mean liver stiffness of 5kPa. Note elevated splenic stiffness up to 20kPa, (red). Coronal MRI shows severe splenomegaly.
| Figure | Age/gender | Diagnosis | Duration | MRE kPa | Fibrosis grade | Laboratory values |
|---|---|---|---|---|---|---|
| 4a | 16 y/M | Ulcerative colitis Primary sclerosing cholangitis |
2 months | 3.2 kPa | 1–2 of 4 | Elevated LFTs and bilirubin Esophageal varices |
| 4b | 14 y/F | Chrohn colitis | 3 months | 2.3 | Normal | Elevated LFTs Normal bilirubin |
| 5a | 10 mo/F | Primary familial cholestasis, type 3 | congenital | 3.9 | 3 of 4 | Elevatated LFTs and bilirubin |
| 5b | 10 y/F | α-1 antitrypsin, MZ type, Joubert variant, nephronophthisis | congenital | 4.1 | 3 of 4 | Elevated LFTs Normal bilirubin |
| 6a | 16 y/M | Hepatitis B | >12 years | 2.4 | 1–2 of 4 | +HBs antigen, − antibody Elevated HBV DNA |
| 6b | 17 y/F | Autoimmune hepatitis, type 2 | 2 years | 2.5 | 3 of 4 | −ANA, ASMA Elevated IgG1 and 3 |
| 7 | 15 y/F | Hepatoportal sclerosis with idiopathic portal hypertension | Unknown | 5.0 | 3 of 4 | Esophageal varices |
Acknowledgments
This work was supported in part by NIH grant EB001981.
References
- 1.Bonekamp S, Kamel I, Solga S, Clark J. Can imaging modalities diagnose and stage hepatic fibrosis and cirrhosis accurately? JHepatol. 2009 Jan;50(1):17–35. doi: 10.1016/j.jhep.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 2.Yin M, Chen J, Glaser K, et al. Abdominal magnetic resonance elastography. Top Magn Reson Imaging. 2009;20:79–87. doi: 10.1097/RMR.0b013e3181c4737e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mariappan YK, Glaser KJ, Ehman RL. Magnetic resonance elastography: a review. Clin Anat. 2010;23:497–511. doi: 10.1002/ca.21006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yin M, Talwalkar JA, Glaser KJ, et al. Assessment of hepatic fibrosis with magnetic resonance elastography. Clin Gastroenterol Hepatol. 2007 Oct;5(10):1207–1213.e2. doi: 10.1016/j.cgh.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nobili V, Monti L, Alisi A, et al. Transient elastography for assessment of fibrosis in paediatric liver disease. Pediatr Radiol. 2011 Jun 16; doi: 10.1007/s00247-011-2143-y. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Yin M, Talwalkar JA, Venkatesh SK, et al. MR elastography of dynamic postprandial hepatic stiffness augmentation in chronic liver disease. Paper presented at: Proceedings of the International Society for Magnetic Resonance in Medicine; April 18–24 2009; Honolulu HI. [Google Scholar]
- 7.Potter C, Hogan MJ, Henry-Kendjorsky K, et al. Safety of pediatric liver biopsy performed by interventional radiologists. J Pediatr Gastroenterol and Nutr. 2011 Aug;53(2):202–206. doi: 10.1097/MPG.0b013e3182183012. [DOI] [PubMed] [Google Scholar]
- 8.Asbach P, Klatt D, Schlosser B, et al. Viscoelasticity-based staging of hepatic fibrosis with multifrequency mr elastography. Radiology. 2010 Oct;257(5):80–86. doi: 10.1148/radiol.10092489. [DOI] [PubMed] [Google Scholar]
- 9.Huwart L, Sempoux C, Vicaut E, et al. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Clin Gastroenterol Hepatol. 2008 Jul;135(1):32–40. doi: 10.1053/j.gastro.2008.03.076. [DOI] [PubMed] [Google Scholar]
- 10.Huwart L, Sempoux C, Salmeh N, et al. Liver fibrosis: noninvasive assessment with mr elastography versus aspartate aminotransferase-to-platelet ratio index. Radiology. 2007 Nov;245(2):458–466. doi: 10.1148/radiol.2452061673. [DOI] [PubMed] [Google Scholar]
- 11.Faria SC, Ganesan K, Mwangi I, et al. MR imaging of liver fibrosis: current state of the art. Radiographics. 2009;29:1615–1635. doi: 10.1148/rg.296095512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palmeri ML, Wang MH, Rouze NC, et al. Noninvaisve evaluationof hepatic fibrosis using acoustic radiation force-based shear stiffness in patients with nonalcoholic fatty liver disease. J Hepatol. 2011 doi: 10.1016/j.jhep.2010.12.019. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menten R, Leonard A, Clapuyt P, et al. Transient elastography in patients with cystic fibrosis. Pediatr Radiol. 2010;40:1231–1235. doi: 10.1007/s00247-009-1531-z. [DOI] [PubMed] [Google Scholar]
- 14.Nobili V, Vizzutti F, Arena U, et al. Accuracy and reproducibility or transient elastography for the diagnosis of fibrosis in pediatric nonalcoholic steatohepatitis. Hepatology. 2008;48:442–448. doi: 10.1002/hep.22376. [DOI] [PubMed] [Google Scholar]
- 15.Chen J, Talwalkar JA, Meng Y, et al. Early detection of nonalcoholic steatohepatitis inpatients with nonalcoholic fatty liver disease by using mr elastography. Radiology. 2011 Jun;259(3):749–756. doi: 10.1148/radiol.11101942. Epub 2011 Apr 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Talwalkar JA, Meng Y, Venkatesh, et al. Feasibility of in vivo mr elastographic splenic stiffness measurements in the assessment of portal hypertension. AJR. 2009;193:122–127. doi: 10.2214/AJR.07.3504. [DOI] [PMC free article] [PubMed] [Google Scholar]