Abstract
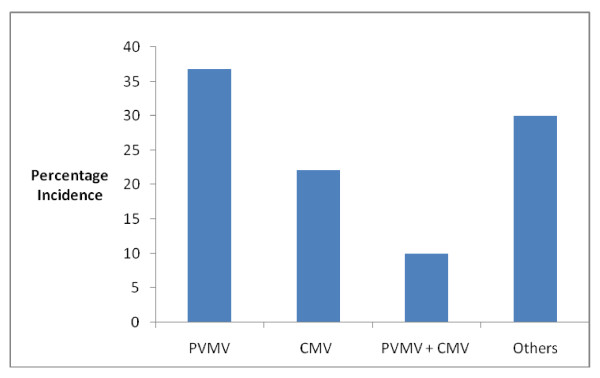
Viral diseases constitute obstacles to pepper production in the world. In Nigeria, pepper plants are primarily affected by pepper veinal mottle virus (PVMV), Cucumber mosaic virus (CMV), Pepper leaf curl Virus (TLCV), Tobacco mosaic virus (TMV), Pepper mottle virus (PMV) and a host of other viruses. The experiment was carried out with a diagnostic survey on the experimental field of the National Horticultural Research Institute, Ibadan, Nigeria and on pepper farms in six local government areas within Ibadan Oyo State, Nigeria, forty samples were collected from each of the farms. Diseased samples were obtained from the field and taken to the laboratory for indexing. In ELISA test some of the samples from the pepper farms showed positive reaction to single infection with PVMV (36.79%), CMV (22.14%) while some others showed positive reaction to mixed infection of the two viruses (10%) but some also negative reaction to PVMV and CMV antisera (31.07).
Keywords: Antisera, CMV, Diagnostic survey, ELISA, PVMV, Nigeria
Introduction
Plant virus diseases cause major losses to agricultural crops around the world. Chemical agents similar to fungicides and bactericides are not effective in controlling virus diseases. Strategies for virus management in plant are mostly aimed at eradicating the source of infection to prevent it from reaching the crop and interfering with the movement of vectors in order to prevent the spread of the disease. However, the most effective means of controlling virus diseases is through cultivating the virus-resistant varieties.
Generally, viral infection causes visible symptoms such as various forms of mosaic and distortions in plants with consequent reductions in crop growth and yield. While reduction in plant size is the most general symptom induced by virus infection, there is probably some stunting of growth even with 'masked' or 'latent' infections, where the systemically infected plant shows no obvious sign of disease [1]. In nature, higher plants are commonly co-infected with multiple viruses and a number of disease syndromes are caused by interaction of two independent viruses. The accumulation dynamics of the interacting viruses in such mixed infection often change drastically [2]. Besides, mixed infections with two unrelated viruses, which are common in field plants, especially in tropical areas, often produce a more severe disease than that caused by either virus alone. For instance, tobacco on infection with potato virus × (PVX) and potato virus Y normally develop a more severe disease than that induced by either virus alone [3].
Pepper veinal mottle virus (PVMV) was first recognized as a distinct member of a group of viruses which was originally designated the Potato virus Y group but was later renamed the Potyvirus group [4]. PVMV occurs mainly in Africa, although it affects Capsicum annuum L. crops in Afghanistan [5] and India [6]. PVMV also occurs in Capsicum spp. in Sierra Leone and Zaire, [7]. PVMV has been reported in several West African countries, and in some parts of Nigeria [8,9]. There was a report that a strain of PVMV occurs naturally in Telfairea occidentalis (Cucurbitaceae) in Nigeria [10]. Strains of the virus are also experimentally transmissible to at least 35 species of the Solanaceae and to nine species of five other families (Aizoaceae, Amaranthaceae, Apocynaceae, Chenopodiaceae and Rutaceae) [11-14]. Symptoms expressed by the leaves of PVMV-infected plants include chlorosis of the veins, followed by systemic interveinal chlorosis, mottle, and small distortion of leaves and at times leaf abscission and fruit distortion occur [11]. There have been reports of one hundred percent losses of marketable pepper fruit due to infection with pepper viruses causing whole field to be abandoned prior to harvest and in some areas making cultivation of pepper to be uneconomical in some parts of Nigeria [8].
Cucumber mosaic virus (CMV) is worldwide in distribution. The virus causing cucumber mosaic has perhaps a wider range of host and attacks a greater variety of vegetables, ornamentals, weeds, and other plants than other viruses [15]. CMV is transmitted mainlyby the green peach aphid, Myzus persicae, and by Aphis gossypii, but it can be transmittedby other species of aphids [16].
In this paper we report the distribution of PVMV and CMV infection in pepper plants within Ibadan, Oyo state, Nigeria.
Materials and methods
Survey for Pepper veinal mottle virus, genus Potyvirus, and Cucumber mosaic virus, genus Cucumovirus was conducted during the 2009 planting season in seven locations in Ibadan, Oyo State in the southwest agro-ecological zone of Nigeria.
The locations were the experimental field of the National Horticultural Research Institute (NIHORT), Lagelu Local government area, Akinyele Local government area, Egbeda Local government area, Ona Ara Local government area, Oluyole Local government area and Ido Local government area.
Collection of diseased leaf samples
Diseased Leaf Samples were randomly collected from the experimental fields containing pepper in NIHORT while in the Local government areas two cultivated pepper farms were randomly surveyed per Local government. On each site 20 plants were randomly sampled from the population of plants on the field. Forty plants were sampled per location from cultivated pepper plants showing symptoms of mosaic, chlorosis, yellowing, stunting and mottle making a total of two hundred and eighty samples in all. The sampled leaves were then stored under Calcium chloride and were placed in the refrigerator prior to indexing.
Virus detection
International Iinstitute of Tropical Agriculture (IITA) Virology laboratory modified protocols for Direct antigen coating (DAC - ELISA) was used for the detection of the presence of PVMV and CMV both from the forty infected pepper leaf samples collected per location. The PVMV and CMV antibodies used were AAB 328 antiserum diluted in the ratio 1:1000 and 1:3000 respectively with Phosphate Buffered Saline (PBS-T) (0.05% Tween 20: pH 7.4: 8.0 g NaCl, 0.2 g KH2 PO4, 1.1 g Na2 HPO4 0.2 g KCl, 0.2 g NaNO3 in 1 l H2O + 0.5 ml Tween 20 (0.05%)) from the Virology Laboratory of the International Institute of Tropical Agriculture (IITA) Ibadan.
Viral indexing protocols
One hundred micro litre of antigen (e.g. sap) ground at 0.1 g of leaf sample in 1 ml of coating buffer was dispensed into each well of ELISA plate. The plate was then incubated at 37°C for 1 h and later washed three times with PBS-T after the incubation period. Cross adsorption was made by grinding 1 g of healthy pepper leaf in 20 mls of conjugate buffer conjugate buffer (1/2 PBS + 0.05% Tween 20 + 0.02% egg albumin + 0.2% PVP + 0.02 g NaN3). 100 μl of PVMV and CMV polyclonal (AAB 328) antisera diluted 1:1000 and 1:3000 in the adsorption solution was added to each of the ELISA plate and then incubated at 37°C for 1 h. After incubation the ELISA plate was washed three times with PBS-T. 100 μl of protein, A- alkaline phosphatase conjugate diluted in the ratio 1:15000 in conjugate buffer (1/2 PBS + 0.05% Tween 20 + 0.02% egg albumin + 0.2% PVP + 0.02 g NaN3) was added per well and the plate incubated at 37°C for 1 h. The plate was washed three times with PBS-T. 100 μl of 0.001 g/ml of p-nitrophenyl phosphate substrate in substrate buffer (97 ml diethanolamine + 800 ml H2O + 0.2 g NaNO3 add HCl to give pH 9.8) was added per well and incubated at room temperature for one hour. For all incubations plates were covered with ELISA cover plates to avoid edge effects and to maintain uniform temperature. Healthy pepper plants (Capsicum sp.) were used as negative control while PVMV and CMV infected Capsicum sp were used as positive control. After one hour the absorbance was measured at 405 nm using multiscan ELISA reader. The samples were considered positive when the ELISA reading exceeded that of the healthy control or was at least twice the reading for the healthy control.
Results and discussion
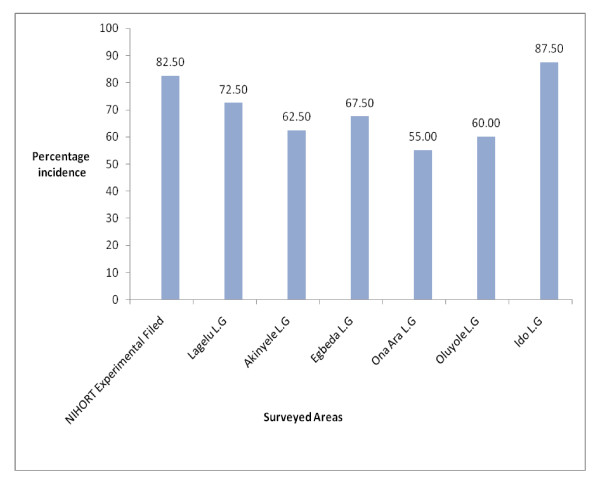
All the farms visited recorded the presence of PVMV and CMV as a single infection as well as mixed infection in some cases. Out of the two hundred and eighty leaf samples suspected to be virus infected collected from the farmers field in Ibadan, Oyo State of Nigeria 36.79% tested positive to PVMV, 22.14% tested positive to CMV, 10% tested positive to both PVMV and CMV through the serological test used, while 31.07% tested negative to the two antisera (Figure 1). However, from Table 1, out of the 40 leaf samples collected from each of the survey point Lagelu have the highest single infection of PVMV [3] while Ido have the highest single infection of CMV [12] while Egbeda have the highest incidence of mixed infection of PVMV and CMV [9].
Figure 1.
Percentage of PVMV, CMV, PVMV + CMV and Other Virus diseases of pepper in Ibada.
Table 1.
Distribution of PVMV and CMV in Ibadan
| Location | Number of samples collected | PVMV | CMV | PVMV + CMV |
|---|---|---|---|---|
| NIHORT Experimental Field | 40 | 17 | 10 | 6 |
| Lagelu Local Government | 40 | 19 | 10 | 0 |
| Akinyele Local Government | 40 | 14 | 7 | 4 |
| Egbeda Local Government | 40 | 11 | 8 | 8 |
| Ona Ara Local Government | 40 | 9 | 6 | 7 |
| Oluyole Local Government | 40 | 15 | 4 | 5 |
| Ido Local Government | 40 | 18 | 13 | 4 |
All the pepper farms surveyed within Ibadan showed differences in PVMV and CMV as observed in the percentage viral incidence (Figure 2). The percentage viral incidence of PVMV and CMV ranges between 55.0% in Ona-ara to 87.50% in Ido.
Figure 2.
Percentage Incidence of PVMV and CMV in NIHORT experimental Station and Six other areas of Oyo State.
Pepper is highly susceptible to virus diseases in Ibadan, Oyo state, Nigeria as all samples picked from all the surveyed locations showed different viral symptoms ranging from different degree of mosaic, mottle, yellowing, stunting, puckering and in some cases reduction in leaf size which was similar to the earlier described symptoms of PVMV and CMV by Atiri [17] and Arogundade et al. [18]. It was however observed that from all the six local governments used as study area in this survey and the experimental field of NIHORT, pepper is mostly infected with PVMV than CMV and the mixed infection of PVMV and CMV, however the combine effect of PVMV and CMV is more on pepper than all other vegetable viruses combine as observed during the survey (Figure 1).
The high percentage incidence of PVMV and CMV in the experimental field of NIHORT could be as a result of numerous alternative host species surrounding the pepper field such as tomato, solanum, cucurbits and a host of other vegetables. This corroborates the findings of Alegbejo [19] who reported that the proximity of pepper plants to certain important weed hosts also has contributed greatly to the spread of viral diseases of pepper. These weeds include Solanum nigrum, S. gracil, Physalis angulata, Vigna rosea, Vigna sinensis, Commelina nudiflora, Petunia hybrida, Physalis floridana, P. micrantha and Solanum incanum.
Contributor Information
Olawale Arogundade, Email: arogundade_olawale@yahoo.co.uk.
Olusegun Samuel Balogun, Email: samcleo1@yahoo.com.
Kehinde Titilope Kareem, Email: rabkareem@yahoo.com.
References
- Matthews REF. Plant Virology. 3. San Diego: Academic; 1991. p. 835. [Google Scholar]
- Otsuki Y, Takebe I. Double infection of isolated tobacco mesophyll protoplasts by unrelated viruses. J Gen Virol. 1976;30:309–316. doi: 10.1099/0022-1317-30-3-309. [DOI] [Google Scholar]
- Vance VB. Replication of potato virus × is altered in co-infections with potato virus Y. Virology. 1991;182:486–489. doi: 10.1016/0042-6822(91)90589-4. [DOI] [PubMed] [Google Scholar]
- Harrison BD, Finch JT, Gibbs AJ, Hollings M, Shepherd RJ, Valenta V, Wetter C. Sixteen groups of plant viruses. Virology. 1971;45:356–363. doi: 10.1016/0042-6822(71)90336-9. [DOI] [PubMed] [Google Scholar]
- Lal SB, Singh S. Identification of some virus diseases of vegetable crops in Afghanistan. Plant Prot Bull (Faridabad) 1988;36(2):83–89. [Google Scholar]
- Nagaraju R, Reddy HR. Occurrence and distribution of bell pepper viruses around Bangalore. 9/10. Vol. 10. Current research, University of Agricultural Sciences, Bangalore; 1980. p. 155; 6. [Google Scholar]
- Huguenot C, Furneaux MT, Clare J, Hamilton RI. Sero-diagnosis of Pepper veinal mottle viru in West Africa using specific monoclonal antibodies in DAS-ELISA. J Phytopathol. 1996;144:29–32. doi: 10.1111/j.1439-0434.1996.tb01484.x. [DOI] [Google Scholar]
- Alegbejo MD, Uvah II. Effect of intercropping pepper with tall companion crops on the incidence of Pepper veinal mottle viru on pepper. Nigerian J Entomology. 1987;7:82–87. [Google Scholar]
- Fajinmi AA. Ph. D thesis. University of Ibadan Oyo State Nigeria; 2006. The incidence, spread and possible control strategies of Pepper veinal mottle Potyvirus (PVMV) disease on pepper (Capsicum annuum.L.) in Nigeria. [Google Scholar]
- Atiri GI. A disease of fluted pumpkin (Telfairia occidentalis Hook.f.) caused by a yellow vein clearing strain of Pepper veinal mottle virus in Nigeria. J Plant Prot Tropics. 1986;3(2):105–110. [Google Scholar]
- Brunt AA, Kenten RH, Phillips S. Symptomatologically distinct strains of Pepper veinal mottle viru from four (4) West African Solanaceous crops. Ann App Biology. 1978;88:115–119. [Google Scholar]
- Igwegbe ECK, Waterworth HE. Properties and serology of the strain of Pepper veinal mottle virus isolated from eggplant (Solanum melongena L.) in Nigeria. Phytopathologische Zeitschrift. 1982;103(1):9–12. doi: 10.1111/j.1439-0434.1982.tb00505.x. [DOI] [Google Scholar]
- Ladipo JL, Roberts IM. Pepper veinal mottle viru associated with a streak disease of tomato in Nigeria. Ann Appl Biol. 1977;87:133–138. [Google Scholar]
- Prasada Rao, RDVJ Yaraguntaiah RC. The occurrence of Pepper veinal mottle virus on chilli in India. Mysore J Agric Sci. 1979;13(4):445–448; 6. [Google Scholar]
- Crescenzy A. Cucumber mosaic cucumovirus populations in Italy under natural epidemic conditions and after a satellite - mediated protection test. Plant Dis. 1993;77:28–33. doi: 10.1094/PD-77-0028. [DOI] [Google Scholar]
- Palukaitis P, Roossinck MJ, Dietzgen RG, Francki RIB. Cucumber mosaicvirus. Adv Virus Res. 1992. pp. 281–349. [DOI] [PubMed]
- Alegbejo MD. Identification of a weed host of Pepper veinal mottle virus in Northern Nigeria. Samaru J Agric Res. 1987;5(1 and 2):65–70. [Google Scholar]
- Atiri GI. Progress of Pepper veinal mottle viru disease in Capsicu peppers. Crop Prot. 1992;11(3):255–259. doi: 10.1016/0261-2194(92)90046-8. [DOI] [Google Scholar]
- Arogundade O, Balogun OS, Shokalu O, Aliyu TH. Influence of Cowpea Mottle Virus and Cucumber Mosaic Virus on the Growth and Yield of six Lines of Soybean (Glycine max L.) J Agric Sci. 2010;2(1):72–78. [Google Scholar]