Abstract
Yersinia pestis has a flea-mammal-flea transmission cycle, and is a zoonotic pathogen that causes the systemic diseases bubonic and septicaemic plague in rodents and humans, as well as pneumonic plague in humans and non-human primates. Bubonic and pneumonic plague are quite different diseases that result from different routes of infection. Manganese (Mn) acquisition is critical for the growth and pathogenesis of a number of bacteria. The Yfe/Sit and/or MntH systems are the two prominent Mn transporters in Gram-negative bacteria. Previously we showed that the Y. pestis Yfe system transports Fe and Mn. Here we demonstrate that a mutation in yfe or mntH did not significantly affect in vitro aerobic growth under Mn-deficient conditions. A yfe mntH double mutant did exhibit a moderate growth defect which was alleviated by supplementation with Mn. No short-term energy-dependent uptake of 54Mn was observed in this double mutant. Like the yfeA promoter, the mntH promoter was repressed by both Mn and Fe via Fur. Sequences upstream of the Fur binding sequence in the yfeA promoter converted an iron-repressible promoter to one that is also repressed by Mn and Fe. To our knowledge, this is the first report identifying cis promoter elements needed to alter cation specificities involved in transcriptional repression. Finally, the Y. pestis yfe mntH double mutant had an ~133-fold loss of virulence in a mouse model of bubonic plague but no virulence loss in the pneumonic plague model. This suggests that Mn availability, bacterial Mn requirements or Mn transporters used by Y. pestis are different in the lungs (pneumonic plague) compared with systemic disease.
Introduction
The importance of manganese (Mn) for intermediary metabolism, transcriptional regulation and virulence of pathogens has become apparent in recent years (Anderson et al., 2009; Guedon & Helmann, 2003; Jakubovics & Jenkinson, 2001; Kliegman et al., 2006; Ouyang et al., 2009; Papp-Wallace & Maguire, 2006; Schmitt, 2002; Zaharik & Finlay, 2004). The loss of virulence and/or intracellular survival caused by mutations in Mn transport systems has been documented in a number of different pathogens, including Borrelia burgdorferi, Brucella abortus, Neisseria gonorrhoeae, Porphyromonas gingivalis, Salmonella, various Streptococcus species and Yersinia pseudotuberculosis (Anderson et al., 2009; Arirachakaran et al., 2007; Berry & Paton, 1996; Boyer et al., 2002; Dintilhac et al., 1997; He et al., 2006; Janulczyk et al., 2003; Kehres et al., 2002a; Lim et al., 2008; Marra et al., 2002; Ouyang et al., 2009; Paik et al., 2003; Smith et al., 2003).
In the course of characterizing iron (Fe) transport systems of Yersinia pestis, a zoonotic pathogen that causes the systemic diseases bubonic and septicaemic plague in rodents and humans, as well as pneumonic plague in humans and non-human primates (Inglesby et al., 2000; Perry & Fetherston, 1997), we identified the ABC transporter YfeA-E, which transports Fe and Mn. Deletions or insertions into the yfeABCD operon in a strain which does not make the siderophore yersiniabactin caused significant growth inhibition under Fe-chelated conditions and reduced Fe and Mn uptake. In this same background, a ΔyfeE mutant had only a modest growth delay due to Fe chelation. The yfeE gene is encoded near the yfeABCD operon but transcribed from a separate promoter. The yfeABCD and yfeE promoters both have putative Fur binding sites (FBSs). Although the yfeA–D promoter was repressed by Fe and Mn in a Fur-dependent manner, the yfeE promoter was unaffected by a surplus of either of these cations. In a slightly attenuated background, a ΔyfeAB2031.1 mutation caused an ~75-fold loss of virulence in a mouse model of bubonic plague (Bearden et al., 1998; Bearden & Perry, 1999; Perry et al., 2003).
With two exceptions (FeoB2 of P. gingivalis and BmtA of B. burgdorferi) (Dashper et al., 2005; He et al., 2006; Ouyang et al., 2009), demonstrated Mn transporters fall into two categories: MntH of the NRAMP1 family and the cluster A-1 family of substrate binding proteins (SBPs). Members of the cluster were previously classified as the c9 family of ABC transporters. Some members of the A-1 cluster show specificity for Mn or Zn; others seem to have multiple transition metal substrates (Berntsson et al., 2010; Claverys, 2001; Papp-Wallace & Maguire, 2006). The Yfe/Sit systems, which are members of this cluster, have been shown to acquire Mn and Fe in Y. pestis, Escherichia coli, Salmonella enterica serovar Typhimurium (S. Typhimurium) and Shigella flexneri (Bearden et al., 1998; Bearden & Perry, 1999; Desrosiers et al., 2010; Hazlett et al., 2003; Janakiraman & Slauch, 2000; Janulczyk et al., 1999, 2003; Kehres et al., 2002a; Paik et al., 2003; Runyen-Janecky et al., 2006, 2003; Sabri et al., 2006).
In this study we examine the Mn regulation of the Y. pestis mntH and yfe promoters as well as the role of these systems in Mn uptake and virulence. Our in vitro analyses indicate that Yfe and MntH serve semi-redundant functions in Mn acquisition. Mutation of both systems results in a modest growth inhibition and complete loss of short-term, energy-dependent 54Mn uptake. Like the yfeABCD promoter, the mntH promoter is repressed by both Fe and Mn through Fur. Both promoters show similarity to each other in their FBSs and sequences immediately upstream of the FBS. Transfer of a small region of the yfeA promoter converted the Fur-regulated hmuP′ promoter, which is repressed by Fe but not Mn, to a chimeric promoter that is repressed by both cations. In virulence studies, the yfeAB mntH double mutant had an ~133-fold loss of virulence in a mouse model of bubonic plague compared with its Yfe+ MntH+ parent. This loss of virulence is greater than would be predicted from our in vitro Mn-deficient growth results. Intriguingly, the yfeAB mntH mutant was fully virulent in a mouse model of pneumonic plague.
Methods
Bacterial strains and cultivation.
The bacterial strains used in this study are described in Supplementary Table S1. From glycerol stocks (Beesley et al., 1967), Pgm+ and Pgm− Y. pestis strains were streaked onto Congo red (CR) agar (Surgalla & Beesley, 1969) and incubated at 28–30 °C for 2–3 days prior to transfer of a red or white colony to tryptose blood agar base (TBA) slants. Red colonies on CR plates have retained the chromosomal pgm locus which encodes numerous genes including the yersiniabactin (Ybt) siderophore-dependent Fe transport system, FetMP (an Fe2+ transporter) and the Hms biofilm locus. The pgm locus spans 102 kb and has an in vitro spontaneous deletion rate of 10−5. Strains with a ‘plus’ designation (e.g. KIM6+) have an unmutated pgm locus. Strains without a ‘plus’ designation (e.g. KIM6) either have a mutation within the pgm locus or have deleted the entire locus (Brubaker, 1969; Fetherston et al., 2010; Perry et al., 2012). E. coli DH5α and DH5α (λ pir) were used to propagate recombinant plasmids.
For growth studies of Mn acquisition, Y. pestis cells were harvested from TBA slants after 1–2 days of incubation at 30 °C and grown in a chemically defined medium, PMH2 (pH 7.5), which had been extracted prior to use with Chelex-100 resin (Bio-Rad Laboratories). Correct PIPES and HEPES concentrations should be 50 mM for PMH2 and PMH, respectively, not micromolar concentrations as previously published (Gong et al., 2001; Staggs & Perry, 1991). After Chelex-100 extraction of PMH2, the mean Mn concentration was 0.46 µM (±0.14 µM) (Cornell Nutrient Analysis Laboratory). Y. pestis strains were also cultivated in PMH2 supplemented with MnCl2 to various concentrations. All glassware used for Mn-restricted growth studies was soaked overnight in ScotClean (OWL Scientific) to remove contaminating metals and copiously rinsed in deionized water. Unless indicated otherwise, cultures were aerated (200 r.p.m.) with culture volumes of about 10–20 % of flask volume. Growth through two transfers (about six to eight generations) was used to acclimate cells to PMH2 and varying Mn conditions prior to use in all experimental studies. For growth studies aimed at identifying additional Mn transport systems, PMH2 was treated with three times the normal Chelex-100 concentration [15 g (100 ml)−1 of 2× medium]. For these studies, EDDA [ethylene-di(o-hydroxyphenylacetic acid)] treated to remove contamining iron (Rogers, 1973) was added to a final concentration of 0.5 µM for third-transfer cultures. Growth of all cultures was monitored by determining the OD620 with a Genesys 5 spectrophotometer (Spectronic Instruments). Where appropriate, ampicillin (Ap; 50–100 µg ml−1), chloramphenicol (Cm; 15–30 µg ml−1), kanamycin (Km; 50 µg ml−1), spectinomycin (Spc; 25–100 µg ml−1), streptomycin (Sm; 50 µg ml−1) or tetracycline (Tc; 12.5 µg ml−1) was added to media.
54Mn uptake studies.
Y. pestis cells were grown aerobically in Chelex-100-treated PMH2 for about five generations prior to use in transport assays. Transport was initiated by the addition of 54MnCl2 at a final concentration of 0.4 µCi ml−1 (14.8 kBq ml−1). Parallel cultures preincubated for 10 min with 100 µM carbonyl cyanide m-chlorophenylhydrazone (CCCP) were used to demonstrate energy-independent binding of Mn. Transport assay samples (0.5 ml) were withdrawn at various times after the addition of labelled Mn, filtered through 0.22 µm pore-size nitrocellulose membranes (Millipore) and rinsed twice with PMH2 medium containing 20 µM MnCl2, as previously described. Samples, in the absence of scintillation cocktail, were counted in a Cobra II Auto-Gamma counting system (Packard Instruments) with a 15–2000 keV window. Duplicate, unfiltered samples were used to determine the total amount of radioactivity in each culture. The results are expressed as percentage uptake per 0.4 OD620 units to compensate for increases in cell density during the course of the assay (Bearden et al., 1998; Bearden & Perry, 1999; Perry et al., 2003).
Plasmids and DNA techniques.
Plasmids (see Supplementary Table S1) were purified by alkaline lysis and transformed into E. coli strains by standard calcium chloride transformation or electroporated into Y. pestis cells as previously described (Ausubel et al., 1987; Birnboim & Doly, 1979; Fetherston et al., 1995). DNA restriction endonucleases, T4 DNA ligase, calf intestinal alkaline phosphatase, Klenow and PCR amplifications followed manufacturer’s specifications. Constructed mutations were confirmed by PCR or restriction enzyme digests. Sequences of PCR amplicons used for cloning genes and promoter regions were confirmed by ACGT, Inc. or Elim Pharmaceuticals. Supplementary Table S1 lists the primers (with their sequences) used in PCRs.
Construction of Y. pestis mutants.
Construction of the ΔmntH2122 mutation in Δpgm strains KIM6 and KIM6-2031.1 (ΔyfeAB2031.1), generating strains KIM6-2122 and KIM6-2122.1, has been reported previously (Hazlett et al., 2003). Here we constructed the same mutation in Pgm+ backgrounds using the suicide vector pΔMntH, generating KIM6-2122+ (ΔmntH2122) and KIM6-2122.1+ (ΔmntH2122 ΔyfeAB2031.1). Primers Yp MntH 5′ SalI and Yp MntH 3′ SpeI were used to confirm the ΔmntH2122 mutation in these strains.
For construction of multiple divalent cation transport mutants, we started with Y. pestis strain KIM6-2163.5(pWL204) (ΔyfeABCD2031.4 ΔfeoB2088 ΔfetMP2163.5) and used a combination of red recombinase methods (Datsenko & Wanner, 2000; Lathem et al., 2007) and suicide vectors to introduce new mutations. A ΔefeUOB : : kan2164.1 mutation was introduced into this strain by the red recombinase system using primers YST-1 and YST-2 to amplify the Kmr cassette in pKD4. The PCR product was electroporated into electrocompetent cells and mutants were selected on TBA plates containing Km. The ΔefeUOB : : kan2164.1 mutation was confirmed in several Kmr colonies by PCR with primers YST-3 and YST-4. One mutant was selected and designated KIM6-2163.6(pWL204). The suicide plasmid pΔMntH was introduced into KIM6-2163.6(pWL204) by electroporation and a second cross selected. The introduction of the ΔmntH2122 mutation was verified by PCR using primers mntH-up and mntH-down and the strain was cured of pWL204. The resulting strain was designated KIM6-2163.7 (ΔyfeABCD2031.4 ΔfeoB2088 ΔfetMP2163.5 ΔefeUOB : : kan2164.1 ΔmntH2122). Plasmid pSkippy was introduced into this strain to remove the kan cassette from ΔefeUOB : : kan2164.1, resulting in a ΔefeUOB2164.1 mutation (KIM6-2163.8). Plasmid pWL204 was reintroduced and primers Y2842-KM1 and Y2842-KM2 were used to generate a Δy2482 : : kan2183 strain. The mutation was verified by PCR using primers Y2842-CR and KM-2, and the strain was cured of pWL204 and designated KIM6-2163.11. A znuBC 2077 mutation was introduced into this strain using the suicide plasmid pSUCZnu3.5; the mutation was confirmed by PCR using primers Znu3.2 and Znu del 1 and the strain was designated KIM6-2163.12.
Construction of mntH : : lacZ and chimeric hmu/yfeA : : lacZ reporter plasmids.
A 325 bp region immediately upstream of the start codon for Y. pestis mntH was amplified from KIM6+ DNA using primers MntH-P1 and MntH-P2 (Supplementary Table S1). The PCR product was digested with AscI and Asp718 and cloned into pNEB193. Sequence analysis revealed that the promoter region was intact but that some changes had occurred to the flanking vector sequences during the cloning process. An Asp718/Ecl136II digest liberated the intact mntH promoter (with no errors) from the pNEB193 clone, and this 341 bp fragment was subcloned into the Asp718/AscI sites in pEU730 to yield pEUMntH-P (Supplementary Table S1).
Two hybrid promoters were constructed in which the region upstream of the putative FBS in the hmuP′ promoter was replaced by sequences from the yfeA promoter region. Overlapping primer pairs Hmu1up and Hmu1down as well as Hmu2up and Hmu2down were extended in separate PCRs to generate 6 and 15 bp substitutions in the hmu promoter, respectively. The products of both reactions were then amplified using primers Hmu/Yfe3 and Hmu/Yfe5. The resulting hybrid promoters were digested with Asp718 and AscI, cloned into the corresponding sites of pNEB193 and sequenced. Hybrid promoter regions containing the correct sequence were subsequently cloned into the Asp718 and AscI sites in front of lacZ in pEU730 to generate pEUhmu/yfe6 and pEUhmu/yfe15.
β-Galactosidase assays.
Reporter plasmids with mntH : : lacZ (pEUMnth-P), hmuP′ : : lacZ (pHMU44), yfeA : : lacZ (pEUYfeA), hmu/yfe6 : : lacZ (pEUhmu/yfe6) or hmu/yfe15 : : lacZ (pEUhmu/yfe15) promoter fusions were electroporated into Y. pestis KIM6+ (yfe+ mntH+) and/or KIM6-2030+ (fur : : kan9). For KIM6-2030(pEUMnth-P)+, the fur mutation was complemented with plasmids expressing Y. pestis fur (pFUR1; furYp+) or E. coli fur (pMH15; furEc+). Cells were acclimated to growth at 37 °C in Chelex-100-treated PMH2 as described above, with Mn and Fe supplementation as indicated. Cells were harvested during early exponential phase. β-Galactosidase activities from whole-cell lysates were measured spectrophotometrically with a Genesys 5 spectrophotometer (Spectronic Instruments) following cleavage of ONPG, and the results are expressed in Miller units (Miller, 1992). Assays used two to six replicate samples and the results are means from two or more independent cultures. For assays using hybrid promoter fusions, independent experiments had absolute β-galactosidase values that varied. Consequently, these results are presented as percentage β-galactosidase activity, with the activity in samples with no Mn or Fe supplementation set to 100 %. These results were calculated from four to six replicate samples from six to eight independent cultures.
Virulence testing.
Construction and testing of potentially virulent strains were performed in a CDC-approved BSL3 laboratory following Select Agent regulations using procedures approved by the University of Kentucky Institutional Biosafety Committee. Y. pestis strains were transformed with pCD1Ap by electroporation (Forman et al., 2007; Gong et al., 2001), plasmid profiles analysed, and transformant phenotypes determined on CR agar (Surgalla & Beesley, 1969) and magnesium oxalate plates (Higuchi & Smith, 1961). After growth at 37 °C in PMH2 with and without CaCl2, culture supernatants were tested for LcrV secretion by Western blotting using polyclonal antisera against histidine-tagged LcrV. Growth at 37 °C without CaCl2 causes growth restriction, expression of the pCD1-encoded type III secretion system, and secretion of LcrV and Yops (Fields et al., 1999; Forman et al., 2007).
Subcutaneous (SC) infection and intranasal (IN) instillation of mice have been previously described (Fetherston et al., 2010). Briefly, Y. pestis cells were grown in heart infusion broth (HIB) at 26 °C, resuspended in mouse isotonic PBS (Bearden et al., 1997), and 0.1 ml of 10-fold serially diluted bacterial suspensions was injected SC into groups of four 6- to 8-week-old female Swiss Webster mice (Hsd : : ND4). For IN infections, cells were grown at 37 °C in HIB with 4 mM CaCl2 to prevent full induction of Lcr in vitro and were similarly diluted in mouse isotonic PBS. Twenty microlitres of the bacterial suspension was administered to the nares (~5 µl aliquots alternating between the two nostrils) of mice sedated with ketamine and xylazine. Administered IN and SC bacterial doses were enumerated by plating serial dilutions on TBA plates containing Ap (50 µg ml−1) and colonies were counted after 2 days of incubation at 30 °C (Fetherston et al., 2010). Mice were observed daily for 2 weeks and LD50 values were calculated according to the method of Reed & Muench (1938). All animal care and experimental procedures were conducted in accordance with the Animal Welfare Act, Guide for the Care and Use of Laboratory Animals, PHS Policy and the US Government Principles for the Utilization of and Care for Vertebrate Animals in Teaching, Research, and Training, and approved by the University of Kentucky Institutional Animal Care and Use Committee. The University of Kentucky Animal Care Program is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care, Inc.
Bioinformatics.
The sequenced Y. pestis KIM10+ genome (Deng et al., 2002) was used to search for Mn transport and regulatory systems. KIM10+ is a derivative of KIM6+ cured of two virulence plasmids (pCD1 and pPCP1) (Perry et al., 1990).
Results and Discussion
Bioinformatic analysis of potential Mn transporters in Y. pestis KIM
We had previously implicated the Yfe ABC transporter in Fe and Mn uptake in Y. pestis (Bearden & Perry, 1999; Desrosiers et al., 2010). To identify any additional SBPs in Y. pestis KIM that might be involved in Mn transport, we searched the KIM10+ genome with the sequence of MntC, the periplasmic Mn-binding component of an ABC transporter from N. gonorrhoeae (Lim et al., 2008) but failed to identify any periplasmic binding proteins (i.e. SBPs) with significant similarity. The B. burgdorferi Mn transporter BmtA (Ouyang et al., 2009) does not have an orthologue in the Y. pestis KIM10+ genome. The sequence of P. gingivalis FeoB2, which transports Mn (Dashper et al., 2005; He et al., 2006), identified only one FeoB, which has a demonstrated role in ferrous iron acquisition (J. D. Fetherston and others, unpublished results; Perry et al., 2007). blast analysis did, however, identify Y1500 as an Nramp1-family member with high similarity to E. coli MntH. Y. pestis MntH is a typical family member with a 409 aa ORF, predicted to encode a 43.6 kDa inner-membrane (IM) protein with 11 transmembrane domains (Deng et al., 2002; TMHMM Server v.20). In addition, a recent study in Y. pseudotuberculosis demonstrated that an mntH mutant had reduced Mn but not Fe accumulation (Champion et al., 2011). Thus our bioinformatic analyses identified only Yfe and MntH as proven Mn transporters.
Both Yfe and MntH are involved in Mn acquisition during in vitro growth
Strains with a ΔmntH2122 (KIM6-2122) or a ΔyfeAB2031.1 (KIM6-2031.1) mutation showed no significant difference from their Pgm− parent (KIM6) during growth at 30 or 37 °C in the defined medium, PMH2 treated with Chelex-100 (residual Mn concentration ~0.5 µM; Fig. 1). However, a double ΔyfeAB2031.1 ΔmntH2122 mutant (KIM6-2122.1) exhibited a moderate growth defect compared with the parent strain, which was more pronounced at 30 than at 37 °C (Fig. 1). Thus, under these growth conditions, the Yfe and MntH systems appear to have redundant Mn import functions. The growth defect of the double mutant was alleviated by supplementation with 10 µM MnCl2. However, the growth of the Yfe+ MntH+ parent was only slightly enhanced by Mn supplementation (Fig. 2). This indicates that the Yfe and MntH systems acquire sufficient Mn from the submicromolar concentration in Chelex-100-treated PMH2 to allow full bacterial growth. The growth defect of the double mutant was also alleviated by carriage of recombinant plasmids encoding the yfeABCDE locus (pYFE1.2) or mntH (pMntH-Op) (Fig. 2; data not shown).
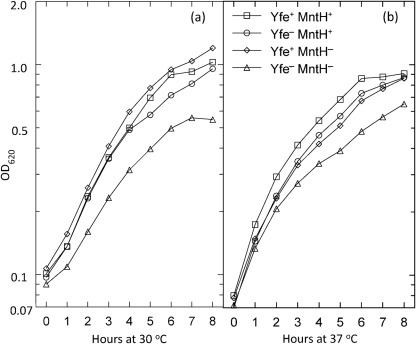
Fig. 1.
Growth of the Y. pestis yfeAB mntH double but not single mutants is reduced in Chelex-100-treated PMH2 at 30 °C (a) and 37 °C (b). Strains: KIM6 (Yfe+ MntH+); KIM6-2031.1 [Yfe− (ΔyfeAB2021.1) MntH+]; KIM6-2122 [Yfe+ MntH− (ΔmntH2122)]; KIM6-2122.1 [Yfe− (ΔyfeAB2031.1) MntH− (ΔmntH2122)]. All strains are Δpgm. The growth curves are from one of two or more independent experiments; all yielded similar results.
Fig. 2.
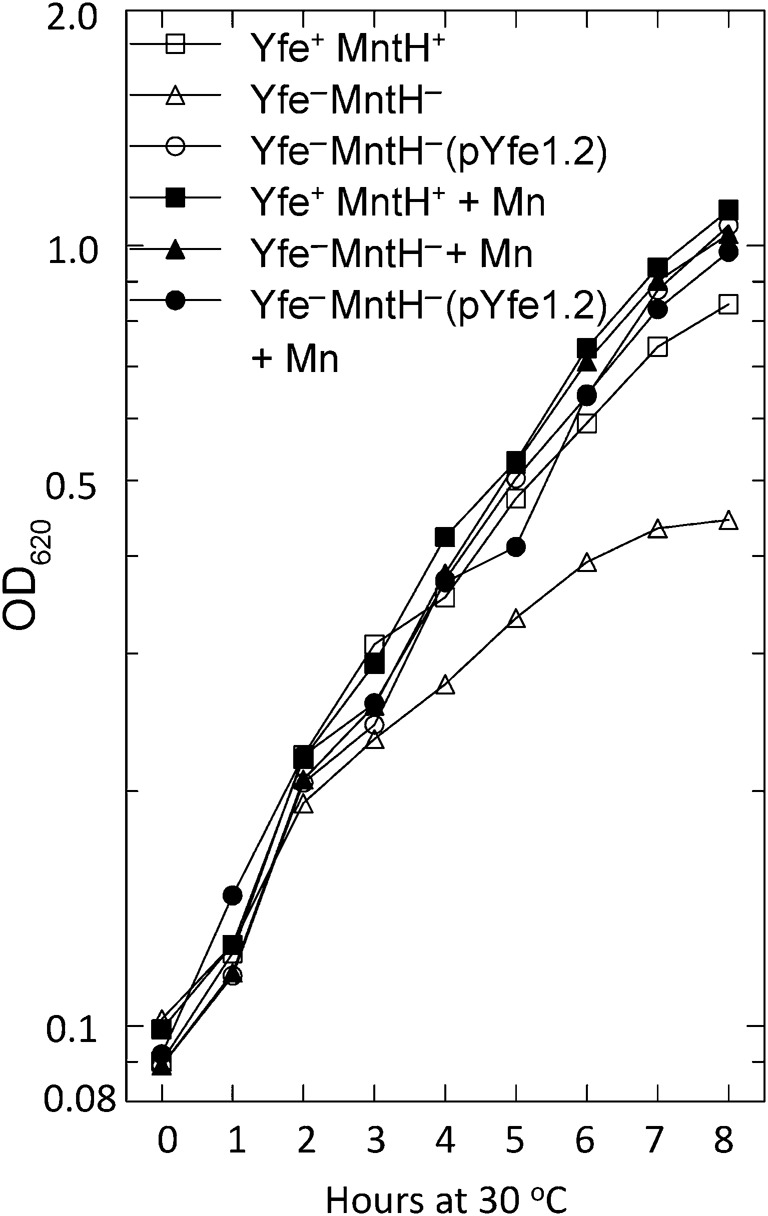
The growth defect of the Y. pestis yfeAB mntH double mutant is alleviated by Mn supplementation or by complementation with the yfeABCDE locus. All strains are Δpgm and were incubated at 30 °C in Chelex-100-treated PMH2. Where indicated, 10 µM Mn was added. Strains and plasmids: KIM6 (Yfe+ MntH+); KIM6-2122.1 [Yfe− (ΔyfeAB2031.1) MntH− (ΔmntH2122)]; pYfe1.2 encodes the yfeABCDE locus. The growth curves are from one of two independent experiments; both yielded similar results.
Since the Yfe system also transports Fe, the growth defect in the yfe mntH mutant could result from a combination of reduced abilities to acquire Fe and Mn. However, our studies with the Yfe and Feo Fe2+ uptake systems have shown that the Y. pestis yfe feo double mutant must be grown under microaerobic conditions for this mutant to exhibit a growth defect. In addition, mutations in Yfe and other inorganic Fe uptake systems do not cause a growth defect under Fe-chelated conditions unless the Ybt system is also mutated or absent (Bearden et al., 1998; Bearden & Perry, 1999; J. D. Fetherston and others, unpublished results; Kirillina et al., 2006; Perry et al., 2003, 2007). In contrast, Supplementary Fig. S1 shows that the Y. pestis yfeAB mntH double mutant exhibited a growth defect even when the Ybt Fe transport system encoded within the pgm locus is present. As a whole, these results indicate that the modest growth defect of the Y. pestis yfeAB mntH mutant is due to decreased Mn uptake when grown in trace concentrations of the metal and is not the result of reduced Fe acquisition.
To confirm the loss of Mn acquisition directly, we performed 54Mn uptake studies. Previously we demonstrated that a yfe mutation reduced energy-dependent 54Mn uptake by ~50 % over a 20 min period (Bearden et al., 1998; Bearden & Perry, 1999; Perry et al., 2003). Fig. 3 shows that the yfe mntH mutant exhibits no energy-dependent uptake over a 10 min period in PMH2. In contrast the Yfe+ MntH+ parent strain accumulated 40–50 % of the extracellular Mn over the same time period (with no additional significant energy-dependent uptake over 40 min). Thus, despite the moderate growth defect in PMH2, the yfe mntH double mutant has no active uptake of Mn at a submicromolar concentration, at least over relatively short time periods. Note that in cells depleted of energy by exposure to CCCP, there is a high level of energy-independent binding of Mn by the parent but not the mutant strain. Previous studies showed that the yfe mutant also exhibits low-level energy-independent Mn binding that is similar to that of the yfe mntH double mutant (Fig. 3) (Bearden et al., 1998; Bearden & Perry, 1999; Perry et al., 2003). Thus, we believe that the cell-associated Mn observed in the CCCP-treated Yfe+ MntH+ parent is due to YfeA binding Mn in the periplasm of cells expressing this SBP.
Fig. 3.
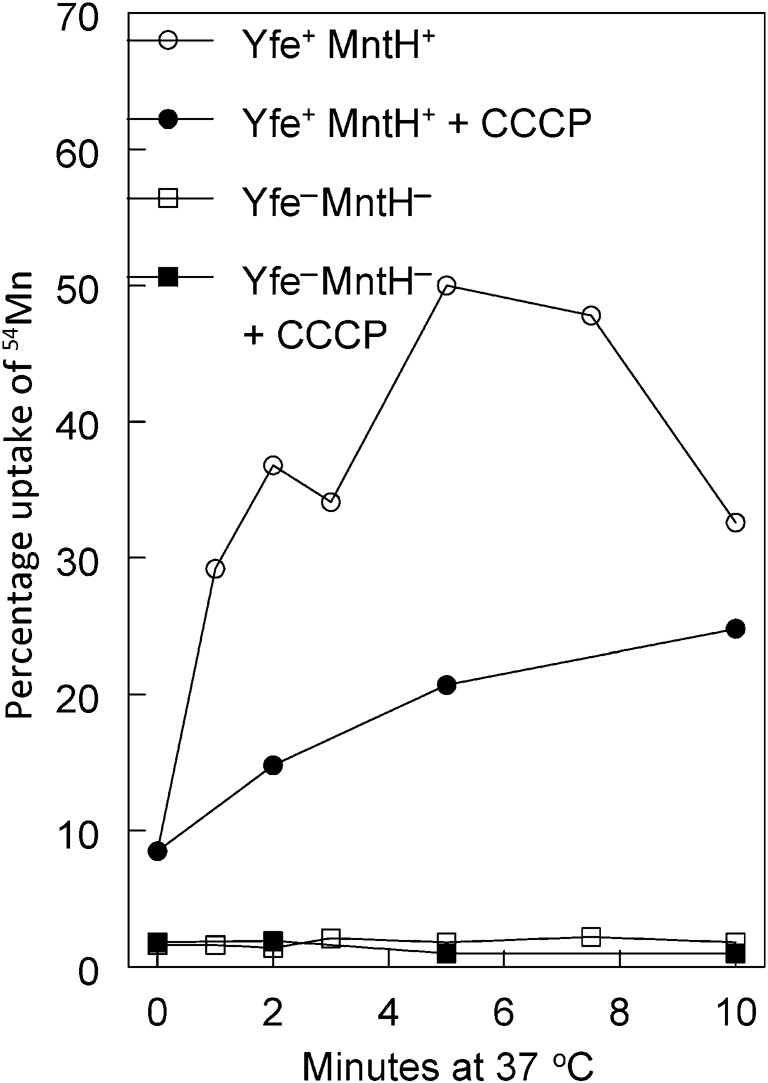
The Y. pestis yfeAB mntH double mutant does not actively transport 54Mn into the cell. Strains were grown at 37 °C in Chelex-100-treated PMH2, and 54MnCl2 was added during early exponential phase to start the Mn uptake assay. For energy-independent binding of 54Mn (closed symbols), CCCP was added 10 min prior to radioisotope addition. Strains used were Pgm+: KIM6+ (Yfe+ MntH+) and KIM6-2122.1+ [Yfe− (ΔyfeAB2031.1) MntH− (ΔmntH2122)]. Results are reported as percentage uptake of 54Mn per 0.4 OD620 unit (cell density). One of two independent experiments with similar results is shown.
The modest in vitro growth defect and intermediate loss of virulence in the bubonic plague model (see below) by the double ΔyfeAB ΔmntH mutant indicate that Y. pestis might have additional transporter(s) capable of Mn acquisition from Mn-deficient environments in vivo and in vitro. Bioinformatic analyses identified only Yfe and MntH as potential Mn transporters. However, sequence similarities of transporters do not always correctly predict their metal specificities (Lim et al., 2008; Rhodes et al., 2005). Since the Yfe system transports both Fe2+ and Mn, we first focused on proven and putative Fe2+ transporters: FeoABC, EfeUOB and FetMP (Cao et al., 2007; J. D. Fetherston and others, unpublished results; Große et al., 2006; Koch et al., 2011; Rajasekaran et al., 2010). The growth of a quintuplet mutant, KIM6-2163.7, containing ΔyfeABCD2031.4 ΔmntH2122 ΔfeoB2088 ΔfetMP2163.5 and ΔefeUOB : : kan2164.1 mutations, in the Chelex-100-treated PMH2 medium showed a defect similar to that of the double ΔyfeABCD ΔmntH mutant (data not shown). Finally, we tested whether the ZnuABC Zn transporter or an SBP that is a member of the TroA-like superfamily (Y2842) might contribute to trace Mn acquisition. Construction and testing of a septuplet mutant (KIM6-2163.12) failed to show a growth defect more severe than that of the yfe mntH mutant. However, this mutant did show a growth response when the Chelex-100-treated PMH2 medium was supplemented with 1 µM Mn (data not shown). This suggests that a high-affinity Mn uptake system is functioning in vitro in Y. pestis, and that the unidentified system is not a Feo, Fet, Efe, Znu or Y2842 transporter. Alternatively, it is possible that Y. pestis has relatively few requirements for Mn and that the modest in vitro growth defect and in vivo phenotypes of the yfe mntH mutant reflect this.
The Aggregatibacter (formerly Actinobacillus) actinomycetemcomitans AfeABCD system is a member of the Yfe/Sit family. An A. actinomycetemcomitans afe mutant is defective for Fe acquisition, and the cloned afe locus restores growth to a Y. pestis yfe feo mutant under Fe-chelated microaerobic conditions and to an E. coli mutant during aerobic growth. However, the yfe mntH double mutant is not complemented by the Afe system (Perry et al., 2012; Rhodes et al., 2005). Thus, it is likely that the A. actinomycetemcomitans AfeABCD system transports Fe2+ but not Mn, currently making it a unique member of the Yfe/Sit ABC transporter family. The reason for the inability of the Afe system to transport Mn remains to be determined.
Transcription from the mntH promoter is repressed by Mn or Fe via Fur
Mn and Fe repression of mntH and sit (yfe) has been demonstrated in E. coli, Shigella flexneri and S. Typhimurium, where these two promoters are primarily repressed by Fe via Fur and by Mn through MntR. In S. Typhimurium, it has been determined that both transcriptional regulators are capable of some repression of both promoters in response to the reciprocal cation. In all three of these organisms, mntH transcription is induced by exposure to H2O2 through OxyR. In contrast, expression of mntH in Brucella abortus is repressed by Mn but not by Fe (Anderson et al., 2009; Ikeda et al., 2005; Kehres et al., 2002b; Patzer & Hantke, 2001; Runyen-Janecky et al., 2006). Previously we demonstrated that Mn and Fe repression of the yfeA promoter is Fur-dependent (Bearden et al., 1998; Perry et al., 2003). A search of the Y. pestis KIM10+ genome failed to identify a homologue of the E. coli Mn transcriptional repressor MntR or Mn-responsive members (Mur) of the Fur superfamily in α-proteobacteria. Thus, Mn regulation in Y. pestis may rely upon Fur alone.
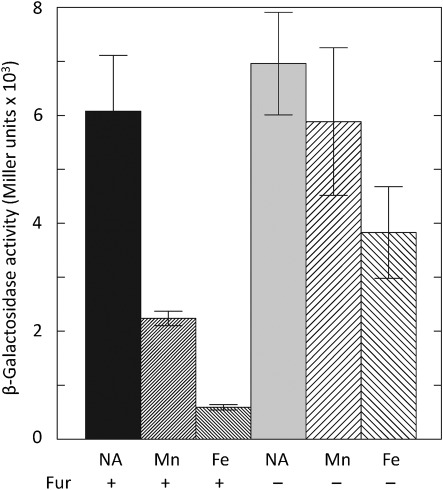
We tested regulation of the mntH promoter using a promoter fusion to lacZ. At 30 °C, transcription from this promoter was repressed by both Fe (10-fold) and Mn (2.7-fold), and this pattern of regulation (Fig. 4) was similar to that previously observed for the yfeA promoter. Similar to the yfeA promoter (Bearden et al., 1998; Perry et al., 2007), repression of the mntH promoter by Mn was Fur-dependent. Curiously, a small degree of Fe repression of the mntH promoter occurred in the Fur mutant (Fig. 4).
Fig. 4.
Fur-dependent transcriptional regulation of the mntH promoter by Fe and Mn. Y. pestis KIM6 (Δpgm Fur+) (bars 1–3 from left) or KIM6-2030 (Δpgm fur9 : : kan) cells (bars 4–6) carrying pEUMntH-P (mntH : : lacZ) were grown in Chelex-100-treated PMH2 at 30 °C with no additions (NA), 10 µM MnCl2 (Mn) or 10 µM FeCl3 (Fe). The values are averages of replicate samples from two or more independent experiments. Error bars, sd.
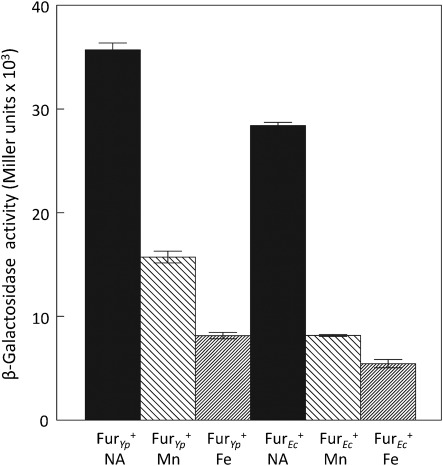
To determine whether Mn repression was specific to Y. pestis Fur, we transformed a Y. pestis fur mutant carrying the mntH reporter with recombinant plasmids expressing FurYp or FurEc. Fig. 5 shows that both Y. pestis Fur and the E. coli Fur restored transcriptional repression by Mn and Fe on the mntH : : lacZ reporter. Thus the ability to repress transcription with excess Mn may be a general property of Fur proteins. When fur is encoded on the Y. pestis chromosome, the mntH reporter is repressed to a greater extent by Fe compared with Mn (Fig. 4). The approximately equivalent repression by both metals when furEc and furYp are encoded on plasmids may be due to the increased number of fur genes. However, the reason for increased expression under Fe- and Mn-deficient conditions with increased copies of fur is unknown (Fig. 5).
Fig. 5.
E. coli Fur causes Mn-dependent repression of transcription from the mntH promoter. Y. pestis KIM6-2030 (Δpgm fur9 : : kan) cells carrying pEUMntH-P (mntH : : lacZ) and pFUR1 (furYp+) or pMH15 (furEc+) were grown in Chelex-100-treated PMH2 at 37 °C with no additions (NA), 10 µM MnCl2 (Mn) or 10 µM FeCl3 (Fe). The values are averages of replicate samples from two or more independent experiments. Error bars, sd.
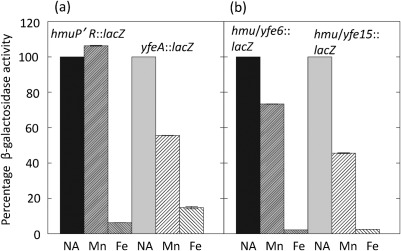
While yfeA and mntH promoters are transcriptionally repressed by excess Mn via Fur, other Y. pestis Fe-repressible, Fur-regulated promoters such as those for fetMP, efeUOB and yiuABC are not affected by Mn supplementation (Perry et al., 2012). The Hmu system, an ABC transporter for haemin uptake, is another example of a Fur-regulated operon repressed by Fe but not by Mn. An hmuP′ : : lacZ promoter fusion construct (pHMU44) in the same vector as the yfeA and mntH reporters (pEU730; Supplementary Table S1) showed transcriptional repression after growth at 37 °C in Chelex-100-treated PMH2 containing 10 µM Fe (~16-fold) but not with 10 µM Mn (Fig. 6a). In contrast, our yfeA : : lacZ reporter displayed approximately seven- and 1.8-fold transcriptional repression by Fe and Mn, respectively. Comparing the yfeA and mntH promoter regions, we noted nucleotide similarities upstream of the FBSs in both promoters (Fig. 7). To determine whether these sequences were involved in Mn-repressible transcription, we constructed two hybrid promoters by replacing 15 or 6 nt of the hmuP′ promoter region with nucleotides from the yfeA promoter region while maintaining the spacing of putative −35, −10 and FBS promoter elements (Fig. 7). Finally, the hybrid promoters were fused to lacZ in pEU730. For unknown reasons the activity of the hms/yfe6 and the native hmuP promoter fusions was about threefold higher than that of the hmu/yfe15 reporter. The hybrid containing 6 nt from yfeA (hmu/yfe6) was repressed ~1.4-fold by Mn and ~45-fold by Fe. In contrast, the hmu/yfeA hybrid promoter with a 15 nt replacement from yfeA (hmu/yfe15) showed a 2.2-fold transcriptional repression by Mn and a similar repression by Fe (~40-fold) (Fig. 6b). Thus, transcriptional repression by Mn in Y. pestis involves Fur and a short nucleotide sequence (>6 and ≤15 nt) upstream of the putative FBS of yfeA. To our knowledge, this study is the first report identifying cis promoter elements needed to alter cation specificities involved in transcriptional repression.
Fig. 6.
Fifteen nucleotides from the yfeA promoter converts hmuP′ to a Mn-repressible promoter. Y. pestis KIM6+ cells carrying pHMU44 (hmuP′R : : lacZ), pEUYfeA (yfeA : : lacZ) (a), pEUHmu/Yfe6 (hmu/yfe6 : : lacZ) or pEUHmu/Yfe15 (hmu/yfe15 : : lacZ) (b) were grown in Chelex-100-treated PMH2 at 37 °C with no additions (NA), 10 µM MnCl2 (Mn) or 10 µM FeCl3 (Fe). The two hybrid promoters have 6 and 15 nt from yfeA replacing nucleotides in the hmuP′R promoter. Due to fluctuations in β-galactosidase activities among eight independent experiments with four to six replicate samples from each experiment, values are presented as means of percentage activity, with the activities of cells grown without Mn or Fe supplementation set at 100 %. Error bars (most not visible), sd.
Fig. 7.
Sequence comparison of the hmuP′, yfeA and two hybrid promoter regions. FBSs are in italic type and boxed, while −35 and −10 regions are underlined. The 15 nt from yfeA used to replace 15 nt from hmuP′R are in bold, lower-case type inside a dashed box in both promoter sequences. For hmu/yfe6, the 6 nt matching those in yfeA are also in bold, lower-case type inside a dashed box. For mntH, the corresponding 15 nt are in bold, lower-case type; nucleotides identical to those in yfeA are underlined.
Mn regulation by Fur is not unique to Y. pestis. Mn repression via Fur has been demonstrated in E. coli for the aerobactin locus and for the fhuF gene using lacZ fusions. The aerobactin locus is fully repressed by 10 µM Mn, while only non-physiological 1 mM Mn was tested for the fhuF gene. In contrast, E. coli sodA (encoding manganese superoxide dismutase; MnSOD) is repressed by Fur in response to Fe but not to Mn (Bagg & Neilands, 1987; Hantke, 1987; Privalle & Fridovich, 1993). The basis for differential cation regulation of these promoters was not explored. Given Mn repression of the E. coli iuc promoter, it is curious that we did not detect Mn repression of the Y. pestis iuc promoter (Perry et al., 2012).
Yfe and MntH are important for the pathogenesis of Y. pestis in bubonic but not pneumonic plague models
Mammalian hosts withhold Mn as a component of innate immunity. Mn levels in human blood and plasma have been measured at 0.2–0.3 and 0.04–0.05 µM, respectively; in rat tissues, Mn concentrations from 0.4 to 1.7 µg (g wet tissue weight)−1 have been reported. More recently, Mn levels of 0.42 and 1.14 µM in the blood and lungs of mice have been reported. Often, these measurements do not differentiate between free and bound forms of the metal, so the bioavailability of Mn in various organs is uncertain. It has been demonstrated that Mn is bound by apoferritin (in vitro) as well as lactoferrin and transferrin (in vitro and in vivo), and that there are different receptors for Mn-transferrin and Zn-transferrin, at least on mouse mammary gland cells. In addition, calprotectin, a protein shown to be produced by neutrophils in tissue abscesses caused by Staphylococcus aureus, chelates Mn and Zn, thereby inhibiting proliferation of the bacterium (Aschner & Aschner, 2005; Aschner & Gannon, 1994; Critchfield & Keen, 1992; Corbin et al., 2008; Davidsson et al., 1989; Kehl-Fie & Skaar, 2010; Lönnerdal et al., 1985; Macara et al., 1973; McDevitt et al., 2011; Moutafchiev et al., 1998; Papavasiliou & Cotzias, 1961; Papp-Wallace & Maguire, 2006; Rehnberg et al., 1980; Zaharik & Finlay, 2004). Finally, mutations in bacterial Mn transporters cause a loss of virulence in a number of pathogens. Indeed, MntH and/or the Yfe/Sit family play a key role in in vivo Mn acquisition in some pathogens, such as Brucella abortus, S. Typhimurium and Y. pseudotuberculosis (Anderson et al., 2009; Arirachakaran et al., 2007; Berry & Paton, 1996; Boyer et al., 2002; Champion et al., 2011; Dintilhac et al., 1997; He et al., 2006; Janulczyk et al., 2003; Kehres et al., 2002a; Lim et al., 2008; Marra et al., 2002; Ouyang et al., 2009; Paik et al., 2003; Sabri et al., 2008; Smith et al., 2003; Zaharik et al., 2004). The Y. pestis strains used in our in vitro growth and transcriptional regulation studies are completely avirulent because they lack virulence plasmid pCD1 (Perry & Fetherston, 1997); therefore, we electroporated recombinant plasmid pCD1Ap into the relevant Pgm+ strains under BSL3 conditions to determine the role of Mn transporters in Y. pestis virulence. As described in Methods, we used these reconstituted strains in SC and IN instillation infections of Swiss Webster mice as bubonic and pneumonic plague models, respectively.
For bubonic plague, a yfeAB mutation caused a reproducible approximately ninefold loss of virulence compared with the parent strain; however, probit analysis indicates that this difference was not significant (P = 0.55) given the number of animals used. An mntH mutant had an LD50 similar to that of the parent strain (Table 1). However, the mntH mutant did display a 3 day delay in reaching the 50 % end point at an infectious dose about six- to eightfold higher than the calculated LD50 (~23 cells) compared with the parent strain. With infectious doses of ~25 cells, more than 60 % of animals survived to day 14; a similar dose (20 cells) of the parent strain was lethal to ~40 % of the mice by day 8 post-infection (Fig. 8). The yfeAB mntH double mutant had an ~133-fold loss of virulence in this model (P = 0.0001) compared with the parent strain (Table 1). This virulence loss is greater than would have been predicted from our in vitro Mn-deficient growth results. Consequently, a defect in Mn acquisition does play a significant role in virulence in this mouse model of bubonic plague.
Table 1. Y. pestis LD50 values and comparative virulence losses in mouse models of pneumonic and bubonic plague.
LD50 values±sd are reported. Virulence loss is compared with the parent/wild-type strain. In the pneumonic plague model, none of the mutations caused a significant loss of virulence as measured by LD50. Strains: KIM5(pCD1Ap)+, wild-type; KIM5-2031.12(pCD1Ap)+, ΔyfeAB2031.1; KIM5-2122(pCD1Ap)+, ΔmntH2122; KIM5-2122.1(pCD1Ap)+, ΔyfeAB2031.1 ΔmntH2122.
| Strain or mutation | Pneumonic plague | Bubonic plague | |
| LD50 | LD50 | Virulence loss | |
| Wild-type | 329±105 | 23±14 | − |
| ΔyfeAB | 139±142 | 205±149 | 8.9-fold |
| ΔmntH | Not tested | 36±33 | Not significant |
| ΔyfeAB ΔmntH | 142±4 | 3068±187 | 133-fold |
Fig. 8.
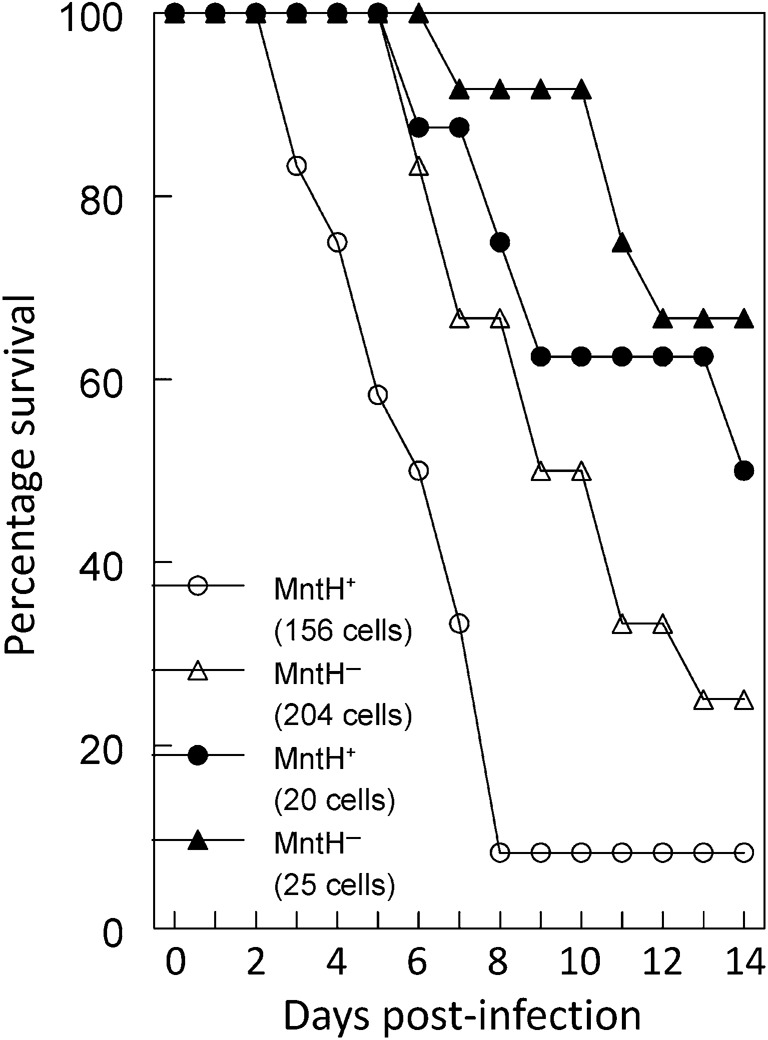
Survival analysis of mice following SC infection with MntH+ or MntH− Y. pestis strains. Low (~1 LD50) and mid-range (~6–8× LD50) doses (cell dose numbers are shown in parentheses in the figure) were used to infect mice. Two or three independent studies with a total of eight to 12 animals were used in the analysis.
In contrast, the yfe single and yfe mntH double mutants, via IN instillation (pneumonic plague model), were fully virulent by LD50 and time-to-death analyses (Table 1; data not shown). It is unlikely that the lung is a Mn-replete environment since Mn levels have been measured at 1 µM to submicromolar concentrations in the lung or the sputum of healthy humans. Indeed, an S. pneumoniae psa Mn transporter mutant was unable to colonize mouse lungs after IN instillation (Gray et al., 2010; McDevitt et al., 2011). Microarray analysis of Y. pestis RNA from the lungs of infected mice showed increased expression of yfe but not mntH compared to in vitro growth. The enhanced expression of yfe in the lung was confirmed by quantitative RT-PCR. Thus, the full virulence of the yfe mntH double mutant in the pneumonic plague model and the intermediate loss of virulence in the bubonic plague model could be due to: (1) the ability of unidentified Mn transporter(s) to provide sufficient Mn in different organ systems; (2) Mn playing a minor role in Y. pestis metabolism and regulation; (3) a shift to metabolic pathways that do not require Mn-dependent enzymes; or (4) a combination of these possibilities.
It is intriguing that a Y. pestis yfe feo Fe2+ uptake mutant showed similar results: significant loss of virulence via SC infection but not by IN infection (J. D. Fetherston and others, unpublished results). These results reinforce our previous conclusion that the importance of Fe transport systems depends upon the organ system in which the bacterium is growing and possibly extends this observation to include Mn transporters.
Divalent cation homeostasis in Y. pestis
Divalent cation homeostasis likely plays a critical role in normal bacterial growth and metabolism, especially in pathogens where the host restricts access to Fe, Mn and Zn. Enzymes with metal cofactors, transport systems, and even transcriptional regulators do not completely discriminate among the relevant divalent transition metal cations. Mn can likely substitute for Fe in some non-redox enzymes, and Zn forms complexes with Mn and Fe metalloproteins (e.g. Fur and MntR appear to have at least limited responses to Mn and Fe, respectively, in some bacteria). However, insertion of an incorrect metal in other proteins may negate their enzymic activity or function (Anjem et al., 2009; Bagg & Neilands, 1987; Fraústo da Silva & Williams, 2001; Hantke, 1987; Ikeda et al., 2005; Privalle & Fridovich, 1993; Tottey et al., 2008). Thus the in vitro and in vivo phenotypes caused by mutations in Y. pestis Fe2+, Mn and Zn transporters could result from a combination of starvation for the relevant cation along with insertion of inappropriate cations into proteins.
Fe, Mn and Zn repression of some metabolic enzymes and various cation transporters, as well as the cation specificities of these transporters and their transcriptional regulators, are all likely important for cation homeostasis in Y. pestis and other bacteria. In Y. pestis, expression of feoABC under aerobic conditions in the presence of Fe, the ability of YfeA to bind Zn in the periplasm but not transport it into the cell, as well as dual Mn and Fe repression of mntH and yfe, may contribute to this homeostasis in different mammalian organ systems and perhaps in the flea (Bearden et al., 1998; Desrosiers et al., 2010; J. D. Fetherston and others, unpublished results; Perry et al., 2003, 2007).
Acknowledgements
We thank P. A. Price and W. E. Goldman, University of North Carolina, for providing plasmids pSkippy and pWL204 for use in red recombinase mutagenesis. This study was partially supported by the National Institutes of Health via Public Health Service grant AI033481. We thank Justin Radolf, University of Connecticut Health Center, for thoughtful discussions of divalent cation transport, regulation and homeostasis.
Abbreviations:
- CCCP
carbonyl cyanide m-chlorophenylhydrazone
- FBS
Fur binding site
- IN
intranasal
- SBP
substrate binding protein
- SC
subcutaneous(ly)
Footnotes
A supplementary figure and a supplementary table are available with the online version of this paper.
References
- Anderson E. S., Paulley J. T., Gaines J. M., Valderas M. W., Martin D. W., Menscher E., Brown T. D., Burns C. S., Roop R. M., II (2009). The manganese transporter MntH is a critical virulence determinant for Brucella abortus 2308 in experimentally infected mice. Infect Immun 77, 3466–3474. 10.1128/IAI.00444-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anjem A., Varghese S., Imlay J. A. (2009). Manganese import is a key element of the OxyR response to hydrogen peroxide in Escherichia coli. Mol Microbiol 72, 844–858. 10.1111/j.1365-2958.2009.06699.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arirachakaran P., Benjavongkulchai E., Luengpailin S., Ajdić D., Banas J. A. (2007). Manganese affects Streptococcus mutans virulence gene expression. Caries Res 41, 503–511. 10.1159/000110883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner J. L., Aschner M. (2005). Nutritional aspects of manganese homeostasis. Mol Aspects Med 26, 353–362. 10.1016/j.mam.2005.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner M., Gannon M. (1994). Manganese (Mn) transport across the rat blood-brain barrier: saturable and transferrin-dependent transport mechanisms. Brain Res Bull 33, 345–349. 10.1016/0361-9230(94)90204-6 [DOI] [PubMed] [Google Scholar]
- Ausubel F. M., Brent R., Kingston R. E., Moore D. D., Seidman J. G., Smith J. A., Struhl K. (editors) (1987). Current Protocols in Molecular Biology. New York: Wiley. [Google Scholar]
- Bagg A., Neilands J. B. (1987). Ferric uptake regulation protein acts as a repressor, employing iron (II) as a cofactor to bind the operator of an iron transport operon in Escherichia coli. Biochemistry 26, 5471–5477. 10.1021/bi00391a039 [DOI] [PubMed] [Google Scholar]
- Bearden S. W., Perry R. D. (1999). The Yfe system of Yersinia pestis transports iron and manganese and is required for full virulence of plague. Mol Microbiol 32, 403–414. 10.1046/j.1365-2958.1999.01360.x [DOI] [PubMed] [Google Scholar]
- Bearden S. W., Fetherston J. D., Perry R. D. (1997). Genetic organization of the yersiniabactin biosynthetic region and construction of avirulent mutants in Yersinia pestis. Infect Immun 65, 1659–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearden S. W., Staggs T. M., Perry R. D. (1998). An ABC transporter system of Yersinia pestis allows utilization of chelated iron by Escherichia coli SAB11. J Bacteriol 180, 1135–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesley E. D., Brubaker R. R., Janssen W. A., Surgalla M. J. (1967). Pesticins. 3. Expression of coagulase and mechanism of fibrinolysis. J Bacteriol 94, 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntsson R. P.-A., Smits S. H. J., Schmitt L., Slotboom D.-J., Poolman B. (2010). A structural classification of substrate-binding proteins. FEBS Lett 584, 2606–2617. 10.1016/j.febslet.2010.04.043 [DOI] [PubMed] [Google Scholar]
- Berry A. M., Paton J. C. (1996). Sequence heterogeneity of PsaA, a 37-kilodalton putative adhesin essential for virulence of Streptococcus pneumoniae. Infect Immun 64, 5255–5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. (1979). A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res 7, 1513–1523. 10.1093/nar/7.6.1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer E., Bergevin I., Malo D., Gros P., Cellier M. F. M. (2002). Acquisition of Mn(II) in addition to Fe(II) is required for full virulence of Salmonella enterica serovar Typhimurium. Infect Immun 70, 6032–6042. 10.1128/IAI.70.11.6032-6042.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brubaker R. R. (1969). Mutation rate to nonpigmentation in Pasteurella pestis. J Bacteriol 98, 1404–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J., Woodhall M. R., Alvarez J., Cartron M. L., Andrews S. C. (2007). EfeUOB (YcdNOB) is a tripartite, acid-induced and CpxAR-regulated, low-pH Fe2+ transporter that is cryptic in Escherichia coli K-12 but functional in E. coli O157 : H7. Mol Microbiol 65, 857–875. 10.1111/j.1365-2958.2007.05802.x [DOI] [PubMed] [Google Scholar]
- Champion O. L., Karlyshev A., Cooper I. A. M., Ford D. C., Wren B. W., Duffield M., Oyston P. C. F., Titball R. W. (2011). Yersinia pseudotuberculosis mntH functions in intracellular manganese accumulation, which is essential for virulence and survival in cells expressing functional Nramp1. Microbiology 157, 1115–1122. 10.1099/mic.0.045807-0 [DOI] [PubMed] [Google Scholar]
- Claverys J. P. (2001). A new family of high-affinity ABC manganese and zinc permeases. Res Microbiol 152, 231–243. 10.1016/S0923-2508(01)01195-0 [DOI] [PubMed] [Google Scholar]
- Corbin B. D., Seeley E. H., Raab A., Feldmann J., Miller M. R., Torres V. J., Anderson K. L., Dattilo B. M., Dunman P. M. & other authors (2008). Metal chelation and inhibition of bacterial growth in tissue abscesses. Science 319, 962–965. 10.1126/science.1152449 [DOI] [PubMed] [Google Scholar]
- Critchfield J. W., Keen C. L. (1992). Manganese+2 exhibits dynamic binding to multiple ligands in human plasma. Metabolism 41, 1087–1092. 10.1016/0026-0495(92)90290-Q [DOI] [PubMed] [Google Scholar]
- Dashper S. G., Butler C. A., Lissel J. P., Paolini R. A., Hoffmann B., Veith P. D., O’Brien-Simpson N. M., Snelgrove S. L., Tsiros J. T., Reynolds E. C. (2005). A novel Porphyromonas gingivalis FeoB plays a role in manganese accumulation. J Biol Chem 280, 28095–28102. 10.1074/jbc.M503896200 [DOI] [PubMed] [Google Scholar]
- Datsenko K. A., Wanner B. L. (2000). One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97, 6640–6645. 10.1073/pnas.120163297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidsson L., Lönnerdal B., Sandström B., Kunz C., Keen C. L. (1989). Identification of transferrin as the major plasma carrier protein for manganese introduced orally or intravenously or after in vitro addition in the rat. J Nutr 119, 1461–1464. [DOI] [PubMed] [Google Scholar]
- Deng W., Burland V., Plunkett G., III, Boutin A., Mayhew G. F., Liss P., Perna N. T., Rose D. J., Mau B. & other authors (2002). Genome sequence of Yersinia pestis KIM. J Bacteriol 184, 4601–4611. 10.1128/JB.184.16.4601-4611.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers D. C., Bearden S. W., Mier I., Jr, Abney J., Paulley J. T., Fetherston J. D., Salazar J. C., Radolf J. D., Perry R. D. (2010). Znu is the predominant zinc importer in Yersinia pestis during in vitro growth but is not essential for virulence. Infect Immun 78, 5163–5177. 10.1128/IAI.00732-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dintilhac A., Alloing G., Granadel C., Claverys J.-P. (1997). Competence and virulence of Streptococcus pneumoniae: Adc and PsaA mutants exhibit a requirement for Zn and Mn resulting from inactivation of putative ABC metal permeases. Mol Microbiol 25, 727–739. 10.1046/j.1365-2958.1997.5111879.x [DOI] [PubMed] [Google Scholar]
- Fetherston J. D., Lillard J. W., Jr, Perry R. D. (1995). Analysis of the pesticin receptor from Yersinia pestis: role in iron-deficient growth and possible regulation by its siderophore. J Bacteriol 177, 1824–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetherston J. D., Kirillina O., Bobrov A. G., Paulley J. T., Perry R. D. (2010). The yersiniabactin transport system is critical for the pathogenesis of bubonic and pneumonic plague. Infect Immun 78, 2045–2052. 10.1128/IAI.01236-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields K. A., Nilles M. L., Cowan C., Straley S. C. (1999). Virulence role of V antigen of Yersinia pestis at the bacterial surface. Infect Immun 67, 5395–5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman S., Nagiec M. J., Abney J., Perry R. D., Fetherston J. D. (2007). Analysis of the aerobactin and ferric hydroxamate uptake systems of Yersinia pestis. Microbiology 153, 2332–2341. 10.1099/mic.0.2006/004275-0 [DOI] [PubMed] [Google Scholar]
- Fraústo da Silva J. J. R., Williams R. J. P. (2001). The Biological Chemistry of the Elements: the Inorganic Chemistry of Life, 2nd edn New York: Oxford University Press. [Google Scholar]
- Gong S., Bearden S. W., Geoffroy V. A., Fetherston J. D., Perry R. D. (2001). Characterization of the Yersinia pestis Yfu ABC inorganic iron transport system. Infect Immun 69, 2829–2837. 10.1128/IAI.67.5.2829-2837.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray R. D., Duncan A., Noble D., Imrie M., O’Reilly D. S. J., Innes J. A., Porteous D. J., Greening A. P., Boyd A. C. (2010). Sputum trace metals are biomarkers of inflammatory and suppurative lung disease. Chest 137, 635–641. 10.1378/chest.09-1047 [DOI] [PubMed] [Google Scholar]
- Große C., Scherer J., Koch D., Otto M., Taudte N., Grass G. (2006). A new ferrous iron-uptake transporter, EfeU (YcdN), from Escherichia coli. Mol Microbiol 62, 120–131. 10.1111/j.1365-2958.2006.05326.x [DOI] [PubMed] [Google Scholar]
- Guedon E., Helmann J. D. (2003). Origins of metal ion selectivity in the DtxR/MntR family of metalloregulators. Mol Microbiol 48, 495–506. 10.1046/j.1365-2958.2003.03445.x [DOI] [PubMed] [Google Scholar]
- Hantke K. (1987). Selection procedure for deregulated iron transport mutants (fur) in Escherichia coli K 12: fur not only affects iron metabolism. Mol Gen Genet 210, 135–139. 10.1007/BF00337769 [DOI] [PubMed] [Google Scholar]
- Hazlett K. R. O., Rusnak F., Kehres D. G., Bearden S. W., La Vake C. J., La Vake M. E., Maguire M. E., Perry R. D., Radolf J. D. (2003). The Treponema pallidum tro operon encodes a multiple metal transporter, a zinc-dependent transcriptional repressor, and a semi-autonomously expressed phosphoglycerate mutase. J Biol Chem 278, 20687–20694. 10.1074/jbc.M300781200 [DOI] [PubMed] [Google Scholar]
- He J., Miyazaki H., Anaya C., Yu F., Yeudall W. A., Lewis J. P. (2006). Role of Porphyromonas gingivalis FeoB2 in metal uptake and oxidative stress protection. Infect Immun 74, 4214–4223. 10.1128/IAI.00014-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi K., Smith J. L. (1961). Studies on the nutrition and physiology of Pasteurella pestis. VI. A differential plating medium for the estimation of the mutation rate to avirulence. J Bacteriol 81, 605–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda J. S., Janakiraman A., Kehres D. G., Maguire M. E., Slauch J. M. (2005). Transcriptional regulation of sitABCD of Salmonella enterica serovar Typhimurium by MntR and Fur. J Bacteriol 187, 912–922. 10.1128/JB.187.3.912-922.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglesby T. V., Dennis D. T., Henderson D. A., Bartlett J. G., Ascher M. S., Eitzen E., Fine A. D., Friedlander A. M., Hauer J. & other authors (2000). Plague as a biological weapon: medical and public health management. JAMA 283, 2281–2290. 10.1001/jama.283.17.2281 [DOI] [PubMed] [Google Scholar]
- Jakubovics N. S., Jenkinson H. F. (2001). Out of the iron age: new insights into the critical role of manganese homeostasis in bacteria. Microbiology 147, 1709–1718. [DOI] [PubMed] [Google Scholar]
- Janakiraman A., Slauch J. M. (2000). The putative iron transport system SitABCD encoded on SPI1 is required for full virulence of Salmonella typhimurium. Mol Microbiol 35, 1146–1155. 10.1046/j.1365-2958.2000.01783.x [DOI] [PubMed] [Google Scholar]
- Janulczyk R., Pallon J., Björck L. (1999). Identification and characterization of a Streptococcus pyogenes ABC transporter with multiple specificity for metal cations. Mol Microbiol 34, 596–606. 10.1046/j.1365-2958.1999.01626.x [DOI] [PubMed] [Google Scholar]
- Janulczyk R., Ricci S., Björck L. (2003). MtsABC is important for manganese and iron transport, oxidative stress resistance, and virulence of Streptococcus pyogenes. Infect Immun 71, 2656–2664. 10.1128/IAI.71.5.2656-2664.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehl-Fie T. E., Skaar E. P. (2010). Nutritional immunity beyond iron: a role for manganese and zinc. Curr Opin Chem Biol 14, 218–224. 10.1016/j.cbpa.2009.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehres D. G., Janakiraman A., Slauch J. M., Maguire M. E. (2002a). SitABCD is the alkaline Mn2+ transporter of Salmonella enterica serovar Typhimurium. J Bacteriol 184, 3159–3166. 10.1128/JB.184.12.3159-3166.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehres D. G., Janakiraman A., Slauch J. M., Maguire M. E. (2002b). Regulation of Salmonella enterica serovar Typhimurium mntH transcription by H2O2, Fe2+, and Mn2+. J Bacteriol 184, 3151–3158. 10.1128/JB.184.12.3151-3158.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirillina O., Bobrov A. G., Fetherston J. D., Perry R. D. (2006). Hierarchy of iron uptake systems: Yfu and Yiu are functional in Yersinia pestis. Infect Immun 74, 6171–6178. 10.1128/IAI.00874-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliegman J. I., Griner S. L., Helmann J. D., Brennan R. G., Glasfeld A. (2006). Structural basis for the metal-selective activation of the manganese transport regulator of Bacillus subtilis. Biochemistry 45, 3493–3505. 10.1021/bi0524215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch D., Chan A. C. K., Murphy M. E. P., Lilie H., Grass G., Nies D. H. (2011). Characterization of a dipartite iron uptake system from uropathogenic Escherichia coli strain F11. J Biol Chem 286, 25317–25330. 10.1074/jbc.M111.222745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathem W. W., Price P. A., Miller V. L., Goldman W. E. (2007). A plasminogen-activating protease specifically controls the development of primary pneumonic plague. Science 315, 509–513. 10.1126/science.1137195 [DOI] [PubMed] [Google Scholar]
- Lim K. H. L., Jones C. E., vanden Hoven R. N., Edwards J. L., Falsetta M. L., Apicella M. A., Jennings M. P., McEwan A. G. (2008). Metal binding specificity of the MntABC permease of Neisseria gonorrhoeae and its influence on bacterial growth and interaction with cervical epithelial cells. Infect Immun 76, 3569–3576. 10.1128/IAI.01725-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lönnerdal B., Keen C. L., Hurley L. S. (1985). Manganese binding proteins in human and cow’s milk. Am J Clin Nutr 41, 550–559. [DOI] [PubMed] [Google Scholar]
- Macara I. G., Hoy T. G., Harrison P. M. (1973). The formation of ferritin from apoferritin. Inhibition and metal ion-binding studies. Biochem J 135, 785–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marra A., Lawson S., Asundi J. S., Brigham D., Hromockyj A. E. (2002). In vivo characterization of the psa genes from Streptococcus pneumoniae in multiple models of infection. Microbiology 148, 1483–1491. [DOI] [PubMed] [Google Scholar]
- McDevitt C. A., Ogunniyi A. D., Valkov E., Lawrence M. C., Kobe B., McEwan A. G., Paton J. C. (2011). A molecular mechanism for bacterial susceptibility to zinc. PLoS Pathog 7, e1002357. 10.1371/journal.ppat.1002357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. H. (1992). A Short Course in Bacterial Genetics. A Laboratory Manual and Handbook for Escherichia coli and Related Bacteria. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory. [Google Scholar]
- Moutafchiev D., Sirakov L., Bontchev P. (1998). The competition between transferrins labeled with 59Fe, 65Zn, and 54Mn for the binding sites on lactating mouse mammary gland cells. Biol Trace Elem Res 61, 181–191. 10.1007/BF02784029 [DOI] [PubMed] [Google Scholar]
- Ouyang Z., He M., Oman T., Yang X. F., Norgard M. V. (2009). A manganese transporter, BB0219 (BmtA), is required for virulence by the Lyme disease spirochete, Borrelia burgdorferi. Proc Natl Acad Sci U S A 106, 3449–3454. 10.1073/pnas.0812999106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik S., Brown A., Munro C. L., Cornelissen C. N., Kitten T. (2003). The sloABCR operon of Streptococcus mutans encodes an Mn and Fe transport system required for endocarditis virulence and its Mn-dependent repressor. J Bacteriol 185, 5967–5975. 10.1128/JB.185.20.5967-5975.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papavasiliou P. S., Cotzias G. C. (1961). Neutron activation analysis: the determination of manganese. J Biol Chem 236, 2365–2369. [PubMed] [Google Scholar]
- Papp-Wallace K. M., Maguire M. E. (2006). Manganese transport and the role of manganese in virulence. Annu Rev Microbiol 60, 187–209. 10.1146/annurev.micro.60.080805.142149 [DOI] [PubMed] [Google Scholar]
- Patzer S. I., Hantke K. (2001). Dual repression by Fe2+-Fur and Mn2+-MntR of the mntH gene, encoding an NRAMP-like Mn2+ transporter in Escherichia coli. J Bacteriol 183, 4806–4813. 10.1128/JB.183.16.4806-4813.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. D., Fetherston J. D. (1997). Yersinia pestis – etiologic agent of plague. Clin Microbiol Rev 10, 35–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. D., Pendrak M. L., Schuetze P. (1990). Identification and cloning of a hemin storage locus involved in the pigmentation phenotype of Yersinia pestis. J Bacteriol 172, 5929–5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. D., Abney J., Mier I., Jr, Lee Y., Bearden S. W., Fetherston J. D. (2003). Regulation of the Yersinia pestis Yfe and Ybt iron transport systems. Adv Exp Med Biol 529, 275–283. 10.1007/0-306-48416-1_53 [DOI] [PubMed] [Google Scholar]
- Perry R. D., Mier I., Jr, Fetherston J. D. (2007). Roles of the Yfe and Feo transporters of Yersinia pestis in iron uptake and intracellular growth. Biometals 20, 699–703. 10.1007/s10534-006-9051-x [DOI] [PubMed] [Google Scholar]
- Perry R. D., Bobrov A. G., Kirillina O., Fetherston J. D. (2012). Yersinia pestis transition metal divalent cation transporters. Adv Exp Med Biol. (in press) [DOI] [PubMed] [Google Scholar]
- Privalle C. T., Fridovich I. (1993). Iron specificity of the Fur-dependent regulation of the biosynthesis of the manganese-containing superoxide dismutase in Escherichia coli. J Biol Chem 268, 5178–5181. [PubMed] [Google Scholar]
- Rajasekaran M. B., Nilapwar S., Andrews S. C., Watson K. A. (2010). EfeO-cupredoxins: major new members of the cupredoxin superfamily with roles in bacterial iron transport. Biometals 23, 1–17. 10.1007/s10534-009-9262-z [DOI] [PubMed] [Google Scholar]
- Reed L. J., Muench H. (1938). A simple method for estimating fifty percent endpoints. Am J Hyg 27, 493–497. [Google Scholar]
- Rehnberg G. L., Hein J. F., Carter S. D., Laskey J. W. (1980). Chronic manganese oxide administration to preweanling rats: manganese accumulation and distribution. J Toxicol Environ Health 6, 217–226. 10.1080/15287398009529844 [DOI] [PubMed] [Google Scholar]
- Rhodes E. R., Tomaras A. P., McGillivary G., Connerly P. L., Actis L. A. (2005). Genetic and functional analyses of the Actinobacillus actinomycetemcomitans AfeABCD siderophore-independent iron acquisition system. Infect Immun 73, 3758–3763. 10.1128/IAI.73.6.3758-3763.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J. (1973). Iron-binding catechols and virulence in Escherichia coli. Infect Immun 7, 445–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runyen-Janecky L. J., Reeves S. A., Gonzales E. G., Payne S. M. (2003). Contribution of the Shigella flexneri Sit, Iuc, and Feo iron acquisition systems to iron acquisition in vitro and in cultured cells. Infect Immun 71, 1919–1928. 10.1128/IAI.71.4.1919-1928.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runyen-Janecky L., Dazenski E., Hawkins S., Warner L. (2006). Role and regulation of the Shigella flexneri Sit and MntH systems. Infect Immun 74, 4666–4672. 10.1128/IAI.00562-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabri M., Léveillé S., Dozois C. M. (2006). A SitABCD homologue from an avian pathogenic Escherichia coli strain mediates transport of iron and manganese and resistance to hydrogen peroxide. Microbiology 152, 745–758. 10.1099/mic.0.28682-0 [DOI] [PubMed] [Google Scholar]
- Sabri M., Caza M., Proulx J., Lymberopoulos M. H., Brée A., Moulin-Schouleur M., Curtiss R., III, Dozois C. M. (2008). Contribution of the SitABCD, MntH, and FeoB metal transporters to the virulence of avian pathogenic Escherichia coli O78 strain χ7122. Infect Immun 76, 601–611. 10.1128/IAI.00789-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt M. P. (2002). Analysis of a DtxR-like metalloregulatory protein, MntR, from Corynebacterium diphtheriae that controls expression of an ABC metal transporter by an Mn2+-dependent mechanism. J Bacteriol 184, 6882–6892. 10.1128/JB.184.24.6882-6892.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. J., Ward P. N., Field T. R., Jones C. L., Lincoln R. A., Leigh J. A. (2003). MtuA, a lipoprotein receptor antigen from Streptococcus uberis, is responsible for acquisition of manganese during growth in milk and is essential for infection of the lactating bovine mammary gland. Infect Immun 71, 4842–4849. 10.1128/IAI.71.9.4842-4849.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staggs T. M., Perry R. D. (1991). Identification and cloning of a fur regulatory gene in Yersinia pestis. J Bacteriol 173, 417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surgalla M. J., Beesley E. D. (1969). Congo red-agar plating medium for detecting pigmentation in Pasteurella pestis. Appl Microbiol 18, 834–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottey S., Waldron K. J., Firbank S. J., Reale B., Bessant C., Sato K., Cheek T. R., Gray J., Banfield M. J. & other authors (2008). Protein-folding location can regulate manganese-binding versus copper- or zinc-binding. Nature 455, 1138–1142. 10.1038/nature07340 [DOI] [PubMed] [Google Scholar]
- Zaharik M. L., Finlay B. B. (2004). Mn2+ and bacterial pathogenesis. Front Biosci 9, 1035–1042. 10.2741/1317 [DOI] [PubMed] [Google Scholar]
- Zaharik M. L., Cullen V. L., Fung A. M., Libby S. J., Kujat Choy S. L., Coburn B., Kehres D. G., Maguire M. E., Fang F. C., Finlay B. B. (2004). The Salmonella enterica serovar Typhimurium divalent cation transport systems MntH and SitABCD are essential for virulence in an Nramp1G169 murine typhoid model. Infect Immun 72, 5522–5525. 10.1128/IAI.72.9.5522-5525.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]