Abstract
The regional distribution and quantitative frequency of pancreatic endocrine cells were demonstrated in the Korean golden frog (Rana plancyi chosenica Okada), which is known as a Korean endemic species, for the first time by immunohistochemical methods using specific mammalian antisera to insulin, glucagon, somatostatin and human pancreatic polypeptide (PP). In the pancreas of the Korean golden frog, all four endocrine cell types were demonstrated. Insulin- and glucagon-positive cells were located in the pancreas as single cells or islet-like clusters, respectively. Somatostatin-containing cells were also dispersed in the pancreas as single cells or clusters but in the case of clusters, they are exclusively situated in the marginal regions of insulin- or glucagon-positive cell clusters. PP-containing cells were also distributed as single cells or clusters. Clusters consisted of PP-positive cells are distributed as a core type and a marginally distributed type. Overall, there were 40.84±3.81% insulin-, 26.02±1.71% glucagon-, 7.63±2.09% somatostatin- and 25.51±3.26% PP-IR cells.
Key words: Korean golden frog, Rana plancyi chosenica, pancreas, endocrine cells, immunohistochemistry.
Introduction
The Korean golden frog, Rana plancyi chosenica Okada, belonging to the Ranidae in the order of Salientia, is known as a Korean endemic species. They have distinct two golden dorsal bands as peculiar characteristics distinguished from other true frog species. In Korea, numbers and habitats of this frog have dramatically decreased because of pollution and immigration of foreign species of frogs having similar feeding habits, especially bullfrogs. Once Korean golden frogs were widely distributed throughout the Korea, they were listed as a vulnerable species by International Union for Conservation of Nature (IUCN) red list and also listed as an endangered species, grade II in South Korea.
The investigations of gastroenteropancreatic (GEP) endocrine cells have been considered as an important part of phylogenetic studies.1 There has been a surge of interest in the endocrine cells of the GEP system of amphibia in recent years. This is not remarkable considering that so many GEP neuropeptides have been isolated from the amphibian skin.2,3 Although, the regional distribution and frequency of insulin, glucagon, somatostatin and PP have also been reported in the pancreas of some Salientia such as the European common frog,4 the green frog,5 the African clawed toad6–8 and the red-bellied frog9 by immunohistochemical methods, the pancreatic endocrine cells of the endemic Korean golden frog, Rana plancyi chosenica Okada have not been studied yet. And, with the exception of the European common frog4 and red-bellied frog,9 quantitative studies of the Salientia pancreas are scarce. In this study, the regional distribution and quantitative frequency of endocrine cells were demonstrated in the pancreas of the Korean golden frog by immunohistochemistry, for the first time using mammalian antisera specific for insulin, glucagon, somatostatin or human pancreatic polypeptide (PP).
Materials and Methods
Six adult Korean golden frogs (40–60 mm in length) of the Salientia, Rana plancyi chosenica Okada, were captured around Buyeo, Korea. After phlebotomy from the head, samples from the pancreas were fixed in Bouin's solution. After paraffin embedding, serial sections (3–4 µm thick) were prepared. Sections were deparaffinized, rehydrated and stained with hematoxylin and eosin for light microscopic examination of the normal alimentary architecture. Other sections were used for immunostaining using the peroxidase anti-peroxidase (PAP) method.10 Blocking of non-specific peroxidase reactions was performed with normal goat serum prior to incubation with the specific antibodies (Table 1). After rinsing in phosphate buffered saline (PBS; 0.01 M, pH 7.4), sections were incubated with secondary antibodies (goat anti-rabbit IgG, dilution, 1:200; Sigma, St. Louis, MO, USA). Sections were then washed in PBS buffer and finally incubated with PAP complex (dilution, 1:200; Sigma). The peroxidase reaction was carried out using a solution 3,3′-diaminobenzidine tetrahydrochloride containing 0.01% H2O2 in Tris-HCl buffer (0.05 M, pH 7.6). After immunostaining, sections were analyzed with the use of a light microscope.
Table 1. Antisera used in the study.
| Antisera raised* | Code | Source | Dilution |
|---|---|---|---|
| Insulin | 842613 | DiaSorin, Stillwater, USA | 1:2000 |
| Glucagon | 927604 | DiaSorin, Stillwater, USA | 1:2000 |
| Somatostatin | A0566 | DAKO corp., Carpenteria, USA | 1:200 |
| hPP° | A619 | DAKO corp., Carpenteria, USA | 1:600 |
All antisera were raised in rabbits except for insulin, which were raised in a guinea pig;
hPP, humane pancreatic polypeptide.
Specificity of the immunohistochemical staining methods was determined as recommended by Sternberger,10 including preincubation of the antibodies with their corresponding antigens. The frequency of IR cells was calculated as the mean ± standard deviation (SD) of 10 parts of pancreatic parenchyma. Among 1000 cells, including exocrine and endocrine cells, cells stained for each antiserum were counted using an automated image analysis process (DMI, Daegu, Korea) coupled to light microscopy. In addition, the percentage of cells positive to each antiserum was determined among 100 cells of the total IR cell population according to that performed in the red-bellied frog.9
Results and Discussion
In this study, all four types of the IR endocrine cells were detected with the antisera against insulin, glucagon, somatostatin and PP in the pancreas of the Korean golden frog. The frequency of these IR cell types in the pancreas is shown in Table 2. Spherical-to-spindle or occasionally oval- to round- shaped IR cells were present in the pancreas. They were distributed throughout the pancreatic parenchyma between exocrine acinar cells as single cells, and were also observed as clusters. The regional distributional patterns and quantitative frequency of endocrine cells in the pancreas of the Korean golden frog were quite similar to those of other Salientian species but some deviating patterns were also observed, especially on cells stained for somatostatin and PP.
Table 2. Regional distributions and quantitative frequencies of the endocrine cells in the Pancreas of the Korean golden frog.
| Immunoreactive cells |
Number of immunoreactive Cells/1000 cells* |
Percentage of immunoreactive cells against each antiserum* |
|---|---|---|
| Insulin | 85.90±18.28 | 40.84±3.81 |
| Glucagon | 54.30±8.77 | 26.02±1.71 |
| Somatostatin | 15.50±3.10 | 7.63±2.09 |
| hPP° | 53.40±11.96 | 25.51±3.26 |
Quantitative frequencies were calculated using automated image analysis process (DMI, Daegu, Korea) attached to light microscopy;
Hpp, human pancreatic polypeptide.
Cells stained for insulin
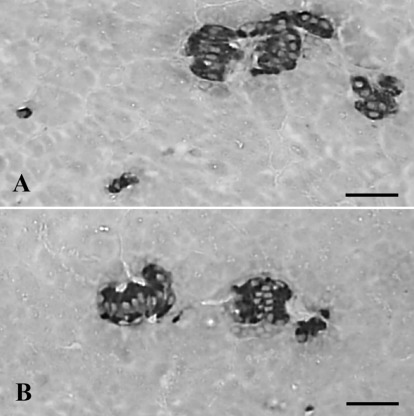
It is well recognized that insulin positive cells are present throughout the Salientia pancreas either in single cells or as clusters based on the previous studied of the European common frog,4,11 African clawed toad8,12,13 and red-bellied frog.9 Our results in the present study concurred with those of the above studies in that the cells stained for insulin were found to be localized in the pancreas of the Korean golden frog as single cells or clusters (Figure 1A,B). Although reports dealing with the abundance of endocrine cells in the Salientian pancreas are scarce, insulin- containing cells were the most predominant type in the pancreas of the Korean golden frog. The insulin-containing cells showed a density of 85.90±18.28/1000 cells and amounted to 40.84±3.81% of the total IR cell population (Table 2). These results are similar to those of the European common frog4 and the red-bellied frog.9
Figure 1.
Cells stained for insulin were distributed as single cells (A) or clusters (A, B) in the pancreas of the Korean golden frog. Scale bars: 80 µm; PAP method.
Cells stained for glucagon
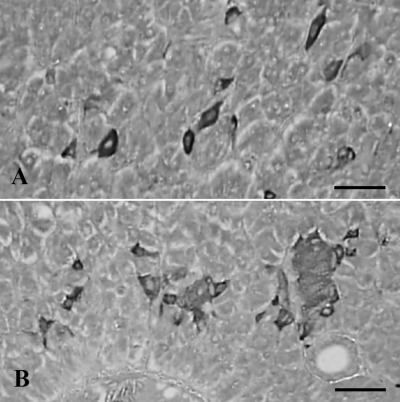
Although most of the glucagon-IR cells were situated in the peripheral regions of pancreatic islets with single cells dispersed between the exocrine acinar cells,14 they were distributed as a single cell or a core clusters in Salientian pancreas.4,9,8,13 In the present study, cells stained for glucagon were found as single cells or clusters in the pancreas of the Korean golden frog (Figure 2A,B), similar to the insulin positive cells and other Salientia.4,9,8,13 In addition, glucagons-containing cells showed the second highest abundance in the present study, 54.30±8.77/1000 cells and 26.02±1.71% of all IR cel1s was positive for glucagon (Table 2). These results are similar to those of European common frog4 and the red-bellied frog.9
Figure 2.
Cells stained for glucagon were located as single cells (A) or clusters (B) in the pancreas of the Korean golden frog. Scale bars: 80 µm; PAP method.
Cells stained for somatostatin
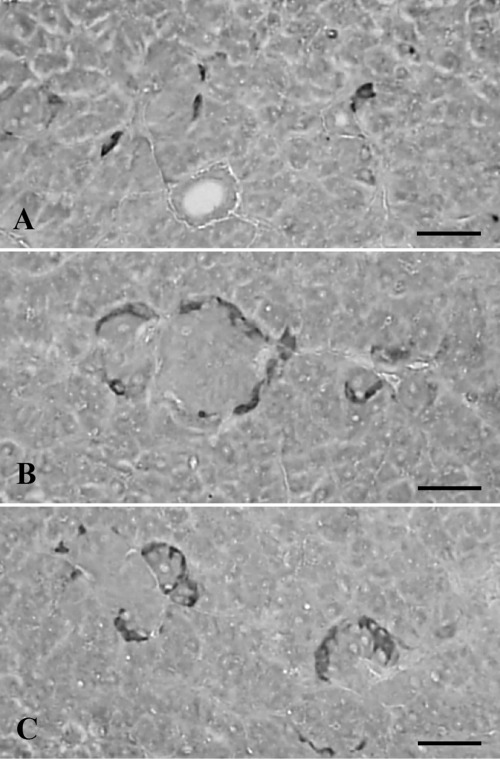
In the Salientian pancreas, cells stained for somatostatin were detected in the European common frog,4 the African clawed toad,6–8,13 the green frog,5 and the red-bellied frog.9 They were present as single cells or clusters in the pancreas of these species, including, in the present study, the Korean golden frog, and in the case of cluster, they occupied marginal regions (Figure 3A−C). Cells stained to somatostatin showed the fourth highest frequency in the present study, 15.50±3.10/1000 cells and approximately 7.63±2.09% of the IR cell population was positive for somatostatin (Table 2). These results indicated that the frequency of somatostatin-positive cells in the pancreas of the Korean golden frog were lowered as compared with the European common frog,4 the African clawed toad6 and the red-bellied frog.9
Figure 3.
Cells stained for somatostatin were dispersed as single cells (A) or clusters (B, C) in the pancreas of the Korean golden frog. In the case of clusters, they are exclusively situated in the marginal regions of insulin- or glucagon-positive cells (B, C). Scale bars: 80 µm; PAP method.
Cells stained for pancreatic polypeptide
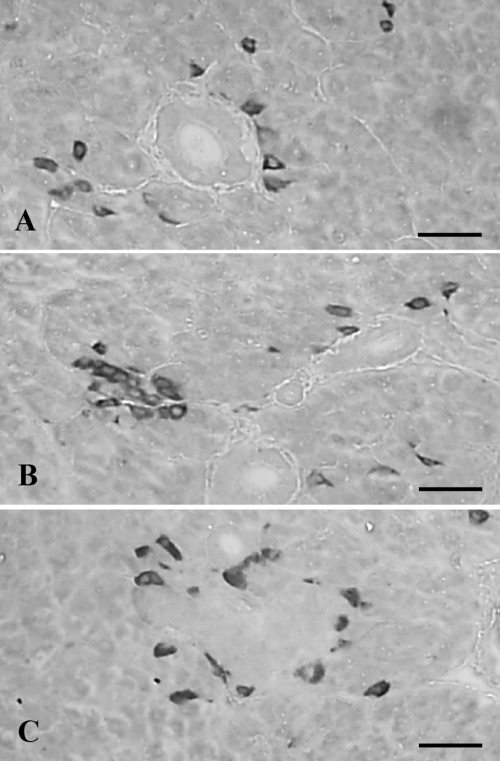
The appearance of PP-positive cells was fairly well identified in various species of Salientian pancreas, and it has been shown that they are conspicuously distributed in the pancreas as single cells, or in peripheral regions of other endocrine cell clusters.12,13 However, in the red-bellied frog,9 they were randomly dispersed in the pancreatic parenchyma only as single cells, between exocrine acinar cells. In the present study, cells stained for PP were also found as single cells or clusters in the pancreas of the Korean golden frog. However, two distributional patterns were detected in the case of clusters as a core type and a marginally distributed type (Figure 4A–C). These differences are considered to be species-dependent characteristics of the Korean golden frog. Cells stained for PP showed quite similar frequencies as compared with glucagon-positive cells as 53.40±11.96/1000 cells in this study, and 25.51±2.26% of the IR cell population was stained for PP (Table 2), more numerous than those of the European common frog4 and the red-bellied frog.9
Figure 4.
Cells stained for PP were dispersed as solitary cells (A, B) or clusters (B, C) in the pancreas of the Korean golden frog. Clusters are distributed as a core type (B) and a marginally distributed type (C). Scale bars: 80 µm; PAP method.
Acknowledgment:
this work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government [MEST] (No. 2011-0030124).
References
- 1.D'Este L, Buffa R, Pelagi M, Siccardi AG, Renda T. Immunohistochemical localization of chromogranin A and B in the endocrine cells of the alimentary tract of the green frog, Rana esculenta. Cell Tissue Res. 1994;277:341–9. doi: 10.1007/BF00327782. [DOI] [PubMed] [Google Scholar]
- 2.Nakajima T, Sasuhara T, Tshikawa O. New frog skin peptides homologous to the ranatensin or bombesin family. In: Miyoshi A, editor. Gut peptides, secretion, function and clinical aspects. Elsevier; Amsterdam, The Netherlands: 1979. pp. 14–18. [Google Scholar]
- 3.Van Noorden S, Polak JM. Hormones of the alimentary tract. In: Barrington EJW, editor. Hormones and evolution. Academic Press; New York, USA: 1979. pp. 791–828. [Google Scholar]
- 4.Etayo JC, Montuenga LM, Sesma P, Diaz de Rada O, Rovira J, Villaro AC. Characterization of pancreatic endocrine cells of the European common frog, Rana temporaria. Gen Comp Endocrinol. 2000;117:366–80. doi: 10.1006/gcen.2000.7427. [DOI] [PubMed] [Google Scholar]
- 5.Trandaburu T, Nurnberger F, Ali SS. Distribution and ultrastructure of somatostatin-immunoreactive cells in the pancreas of Rana esculenta. Anat Anz. 1995;177:213–9. doi: 10.1016/s0940-9602(11)80186-x. [DOI] [PubMed] [Google Scholar]
- 6.Hacker G, Pohlhammer K, Breitfuss A, Adam H. Somatostatin-immunoreactive cells in the gastro-entero-pancreatic endocrine system of Xenopus laevis. Z Mikrosk Anat Forsch. 1983;97:929–40. [PubMed] [Google Scholar]
- 7.Shapiro B, Sheppard M, Kronheim S, Pimstone BL. Tissue distribution of immunoreactive somatostatin in the South African clawed toad (Xenopus laevis) J Endocrinol. 1979;80:407–8. doi: 10.1677/joe.0.0800407. [DOI] [PubMed] [Google Scholar]
- 8.Cowan BJ, Foty RA, Liversage RA. Insulin, glucagon and somatostatin localization in the pancreas of metamorphosed Xenopus laevis. Tissue Cell. 1991;23:777–87. doi: 10.1016/0040-8166(91)90030-w. [DOI] [PubMed] [Google Scholar]
- 9.Lee JH, Ku SK, Lee HS, Kitagawa H. An immunohistochemical study of endocrine cells in the pancreas of the Red-bellied frog (Bombina orientalis) Eur J Histochem. 2003;47:165–72. doi: 10.4081/823. [DOI] [PubMed] [Google Scholar]
- 10.Sternberger LA. 3rd ed. New York: Wiley; 1986. Immunocytochemistry. [Google Scholar]
- 11.Ortiz de Zarate A, Villaro AC, Etayo JC, Diaz de Rada O, Montuenga LM, Sesma P, et al. Development of the endocrine pancreas during larval phases of Rana temporaria. An immunocytochemical and ultrastructural study. Cell Tissue Res. 1991;264:139–50. doi: 10.1007/BF00305732. [DOI] [PubMed] [Google Scholar]
- 12.Maake C, Hanke W, Reinecke M. An immunohistochemical and morphometric analysis of insulin, insulin-like growth factor I, glucagon, somatostatin, and PP in the development of the gastro-entero-pancreatic system of Xenopus laevis. Gen Comp Endocrinol. 1998;110:182–95. doi: 10.1006/gcen.1998.7064. [DOI] [PubMed] [Google Scholar]
- 13.Lozano MT, Hernandez MP, Agulleiro B. Endocrine pancreatic cells from Xenopus laevis: light and electron microscopic studies. Gen Comp Endocrinol. 1999;114:191–205. doi: 10.1006/gcen.1998.7247. [DOI] [PubMed] [Google Scholar]
- 14.Ku SK, Lee HS. An immunohistochemical study of the pancreatic endocrine cells of the nude mouse, Balb/c-nu/nu. Eur J Histochem. 2006;50:61–8. [PubMed] [Google Scholar]