Abstract
HOXC8 expression is upregulated in diverse cancer types, and a high level of HOXC8 is often associated with the aggressive/metastatic phenotypes. We previously reported that the presence of HOXC8 is essential for breast cancer cell migration and metastasis. However, the underlying molecular mechanism of HOXC8 regulation of cell migration is unclear. Here, we demonstrate that the presence of HOXC8 is required for cadherin 11 (CDH11) expression in breast cancer cells and that HOXC8 regulation of cell migration is mediated by CDH11. To understand the role of HOXC8-CDH11 axis in cell migration, we show that depleting either HOXC8 or CDH11 diminishes the formation of actin-based membrane ruffles, an event essential for cell migration. The loss of membrane ruffles in HOXC8- or CDH11-knockdown cells is apparently caused by reduced Rac activity because ectopically expressing active Rac1 restores cytoskeleton reorganization. CDH11 physically interacts with Trio, a Rac GEF. We show that Trio is responsible for the majority of endogenous Rac activity in migratory breast cancer cells. Because knockdown of CDH11 prevents the plasma membrane localization of Trio, our study indicates that CDH11 may play a role in recruiting Trio to the plasma membrane where Trio activates Rac, leading to cell migration. This study reveals a novel HOXC8-CDH11-Trio-Rac signaling axis that contributes significantly to breast cancer cell migration.
Keywords: cell migration, CDH11, HOXC8, Trio, Rac
Introduction
Metastasis is the overwhelming cause of mortality in cancer patients.1 One of the most significant characteristics of metastatic cancer cells is the aberrant cell migration that is aggravated by the loss of metastasis suppressors, expression of metastasis promoters, and activation of signaling pathways that positively regulate cell migration.2,3 We recently showed that HOXC8 level is significantly elevated in metastatic breast cancer cells.4 Our finding corroborates with 2 early studies in which elevated HOXC8 expression was found to accompany aggressive/metastatic phenotype in prostate and cervical cancers.5,6 These findings indicate that HOXC8 may play a role in cancer metastasis. This possibility is clearly supported by our observation that knockdown of HOXC8 led to the inhibition in breast cancer cell migration and metastasis.4 In murine fibroblasts, HOXC8 has been shown to regulate the expression of several migration-associated genes including cadherin 11 (CDH11) and calponin.7 However, whether these HOXC8-regulated genes are functionally associated with breast cancer cell migration is unclear.
CDH11 is a member of cadherin super-family8 and mediates hemophilic cell-cell adhesion in a calcium-dependent manner.9 CDH11 is predominantly expressed in mesenchyme during early embryogenesis and thus implicated in mediating mesenchymal cell-cell interactions.9,10 Interestingly, CDH11 is also detected in diverse cancer types, especially in metastatic cancer cell lines and aggressive clinical specimens.11,12 As ectopically expressing CDH11 can enhance breast and prostate cancer cell migration and metastasis,13-15 CDH11 has been suggested to play an important role in cancer metastasis. Unfortunately, how CDH11 contributes to cancer cell migration is not fully understood.
Members of Rho GTPase family including Rac, Cdc42, and RhoA are critical in cell migration,16 in that RhoA facilitates actin stress fiber formation/focal adhesion assembly, Rac promotes the formation of membrane ruffles. and Cdc42 props up filopodia.17 Rho GTPases, like other small GTPases, are activated by guanidine exchange factors (GEFs).16-18 In cranial neural crest cells of Xenopus, CDH11 can activate Rac by promoting the enzymatic activity of Trio, a Rac GEF.19 In mammalian system, members of Rho GTPase family have also been shown to act as the key regulators of cadherins.20,21 Since the activities of Rho GTPases are often elevated in metastatic cancer cells,22,23 it is of interest to investigate whether they are functionally linked to the acquired CDH11 expression in these cells.
The objective of this study is to elucidate the molecular mechanism associated with HOXC8 regulation of breast cancer cell migration. We showed that HOXC8 and CDH11 expression were closely correlated in both breast cancer cell lines and clinical breast tumor specimens and that the presence of HOXC8 is required for CDH11 expression. We further showed that HOXC8 regulation of cell migration was mediated through CDH11. These results suggest that HOXC8 regulates breast cancer cell migration by facilitating CDH11 expression. To understand how HOXC8-CDH11 axis regulates breast cancer cell migration, we found that silencing either HOXC8 or CDH11 reduced Rac activity and inhibited actin-based cytoskeleton reorganization. To explore the functional link between CDH11 and Rac activity, we revealed that Trio was in the same immunocomplex of CDH11 and that knockdown of CDH11 decreased the amount of plasma membrane-localized Trio. As silencing Trio decreased Rac activity and forced expression of active Rac1 reversed the inhibitory effect of HOXC8/CDH11-knockdown in cell migration, our study suggests that HOXC8-CDH11 axis regulates cell migration by assisting the plasma membrane localization of Trio and thus elevating Rac activity.
Results
The presence of HOXC8 is required for CDH11 in breast cancer cells
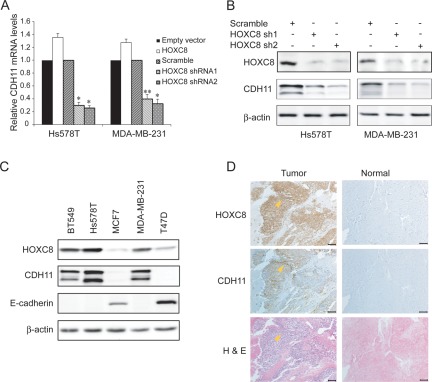
HOXC8 was previously reported to regulate the expression of migration-associated genes including CDH11, embigin, transgelin, and calponin in fibroblasts.7 To determine whether HOXC8 similarly regulated the expression of these genes in breast cancer cells, we either depleted HOXC8 by lentivirally delivering HOXC8 shRNAs or increased HOXC8 level by forcing HOXC8 transgene expression in MDA-MB-231 cells. qRT-PCR showed that HOXC8 positively regulated the expression of CDH11 and embigin while negative regulating the expression of transgelin and calponin (Suppl. Fig. S1), which is in accord with previously observation in fibroblasts.7 We focused on CDH11 because its mRNA was most significantly and consistently regulated by HOXC8 in both Hs578T and MDA-MB-231 cells (Fig. 1A). Immunoblotting with CDH11 mAb also showed that both sequence-distinct HOXC8 shRNAs greatly reduced CDH11 level (Fig. 1B). These results indicate that CDH11 expression is HOXC8-dependent in breast cancer cells.
Figure 1.
HOXC8 and CDH11 expression are well correlated in breast cancer cells. (A) Hs578T and MDA-MB-231 cells were transduced with empty lentiviral vector or vectors encoding scramble sequence, HOXC8 shRNAs, or HOXC8 transgene. Total RNA was isolated from these cells and subjected to qRT-PCR to determine the level of CDH11 mRNA. GAPDH mRNA was used as an internal control for standardization. Data are mean ± SE. n = 4. *P < 0.005 versus empty vector. **P < 0.01 versus empty vector. (B) Hs578T and MDA-MB-231 cells transduced with lentiviral vector encoding scramble sequence or HOXC8 shRNAs were lysed and cell lysates were subjected to immunoblotting to detect HOXC8, CDH11, and β-actin. (C) Overnight cultured cells were lysed and cell lysates were subjected to immunoblotting to detect HOXC8, CDH11, E-cadherin, and β-actin. (D) The continuous sections were prepared from paraffin-embedded normal breast and breast cancer tissues. Sections were subjected to immunohistochemistry with HOXC8 and CDH11 antibodies as well as H&E staining. Scale bar, 100 μm.
We next investigated whether HOXC8 expression and CDH11 expression are correlated by examining their protein levels in a panel of breast cancer cell lines. CDH11 was observed in lines with high HOXC8 level and negative for E-cadherin expression (BT549, Hs578T, and MDA-MB-231) (Fig. 1C). In contrast, lines with low HOXC8 level and positive for E-cadherin displayed no detectable CDH11 (MCF7 and T47D) (Fig. 1C). Subsequently, we performed immunohistochemistry on 25 breast cancer specimens and 12 normal breast samples. None of normal breast tissues were positive for HOXC8 or CDH11 (Fig. 1D, Suppl. Fig. S2, and Suppl. Table S1). In contrast, HOXC8 was positive in 12 and CDH11 was positive in 9 breast cancer specimens (Suppl. Table S1). The positivity of both HOXC8 and CDH11 was significantly correlated with the status of metastasis in these specimens (HOXC8, P < 0.005; CDH11, P < 0.001) but was not associated with the status of ER, PR, or HER2 (Suppl. Table S1). Importantly, all samples positive for CDH11 were positive for HOXC8 (Figv1D, Suppl. Fig. S2, and Suppl. Table S1) and their expression was highly correlated among these samples (R = 0.786, P < 0.001). These results show a strong correlation between CDH11 and HOXC8 expression in breast cancer cells/tissues.
HOXC8 regulates breast cancer cell migration through CDH11
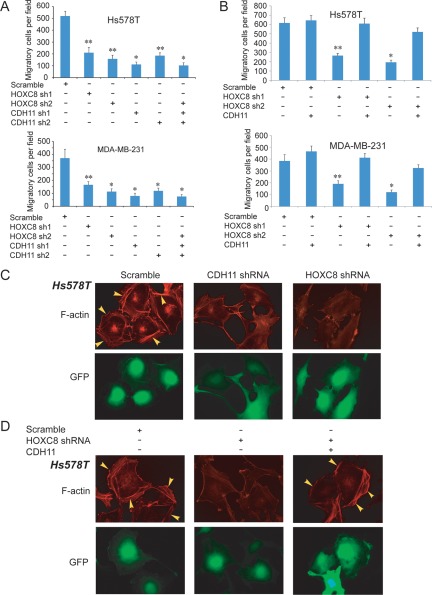
CDH11 has been reported to promote migration of various cancer types including breast cancer cells.13-15 To determine the importance of CDH11 in HOXC8 regulation of breast cancer cell migration, we initially silenced CDH11 expression in Hs578T and MDA-MB-231 cells by lentiviral delivery of sequence distinct CDH11 shRNAs (Suppl. Fig. S3). Transwell migration assay showed that both CDH11 shRNAs were able to decrease cell migration to the extent similar to those observed in HOXC8-knockdown cells (Fig. 2A). Silencing HOXC8 and CDH11 simultaneously did not lead to greater inhibition in cell migration than silencing each one of them individually (Fig. 2A). Importantly, ectopic expression of CDH11 restored more than 80% of cell migration reduced by HOXC8 shRNAs in both lines (Fig. 2B).
Figure 2.
HOXC8 regulation of cell migration is CDH11-dependent. (A) Hs578T or MDA-MB-231 cells were transduced with lentiviral vectors containing scramble sequence, CDH11, or HOXC8 shRNA individually or in combination for 4 days. The population of transduced cells was analyzed for cell migration by Transwell assay. Data are mean ± SE. n = 4. *P < 0.001 versus scramble. **P < 0.005 versus scramble. (B) CDH11 transgene was expressed in Hs578T or MDA-MB-231 cells that lentivirally expressed scramble sequence or HOXC8 shRNAs. Cells were analyzed for cell migration by Transwell assay. Data are mean ± SE. n = 4. *P < 0.001 versus scramble. **P < 0.005 versus scramble. (C) Hs578T cells lentivirally expressing scramble sequence, CDH11, or HOXC8 shRNA were stained with rhodamine-conjugated phalloidin. F-actin was visualized under a fluorescence microscope. Cells expressing GFP were lentivirally transduced. (D) CDH11 transgene was introduced into HOXC8-knockdown Hs578T cells. The cells were stained with rhodamine-conjugated phalloidin, and polymerized actin was visualized under a fluorescence microscope. Cells expressing GFP were lentivirally transduced.
A prerequisite of cell migration is the ability of a cell to undergo cytoskeleton reorganization.24,25 We thus examined how depletion of HOXC8/CDH11 affected actin-based cytoskeleton reorganization. Control, HOXC8-knockdown, and CDH11-knockdown Hs578T cells were fixed, and polymerized actin was visualized by immunofluorescence staining with rhodamine-labeled phalloidin. Although a significant number of membrane ruffles were seen in the control cells, such structures were greatly decreased in HOXC8- and CDH11-knockdown cells (Fig. 2C). When CDH11 transgene was expressed in HOXC8-knockdown cells, a significant number of membrane ruffles were again observed (Fig. 2D). Similar results were obtained with MDA-MB-231 cells (Suppl. Fig. S4). These results show that HOXC8 facilitates cell migration through CDH11 and indicate that HOXC8-CDH11 axis regulates cell migration by affecting actin-based cytoskeleton reorganization.
Knockdown of CDH11 impairs Rac activity in breast cancer cells
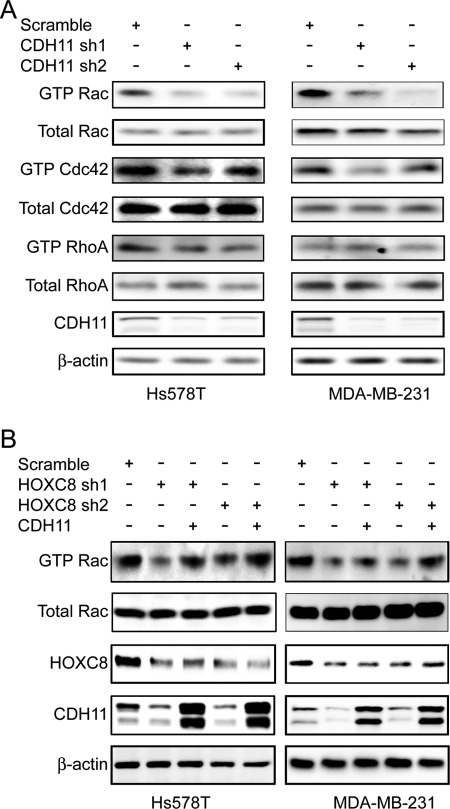
Actin-based cytoskeleton reorganization is tightly regulated by members of Rho GTPase family including Rac, Cdc42, and RhoA.26 To determine the potential functional link between CDH11 and Rho GTPases, we first examined how silencing CDH11 expression affected endogenous Rac, Cdc42, and RhoA activities in Hs578T and MDA-MB-231 cells. Judging by the level of GTP-bound forms, knockdown of CDH11 significantly inhibited Rac activity and also moderately hampered Cdc42 activity (Fig. 3A). In contrast, endogenous RhoA activity was little altered between control and CDH11-knockdown cells (Fig. 3A). Similar results were obtained in HOXC8-knockdown cells (Fig. 3B). However, enforced CDH11 transgene expression rescued Rac activity of HOXC8-knockdown cells (Fig. 3B). Moreover, endogenous Rac activity was significantly higher in CDH11-expressing BT549, Hs578T, and MDA-MB-231 cells compared with CDH11-negative MCF7 and T47D cells (Suppl. Fig. S5). Taken together, these results indicate that HOXC8-CDH11 axis regulates actin-based cytoskeleton reorganization and cell migration by promoting the activities of members of Rho GTPases, especially Rac.
Figure 3.
HOXC8-CDH11 axis promotes endogenous Rac activity in breast cancer cells. (A) Hs578T and MDA-MB-231 cells lentivirally expressing scramble sequence or CDH11 shRNAs were analyzed for Rac, Cdc42, and RhoA activities as described in Materials and Methods. Cell lysates were also collected and subjected to immunoblotting to detect CDH11 and β-actin. (B) HOXC8-knockdown Hs578T or MDA-MB-231 cells were forced to express CDH11 transgene and then analyzed for Rac activities. Cell lysates were also collected and subjected to immunoblotting to detect HOXC8, CDH11, and β-actin.
HOXC8/CDH11 depletion-instigated cell migration reduction is caused by impaired Rac activation
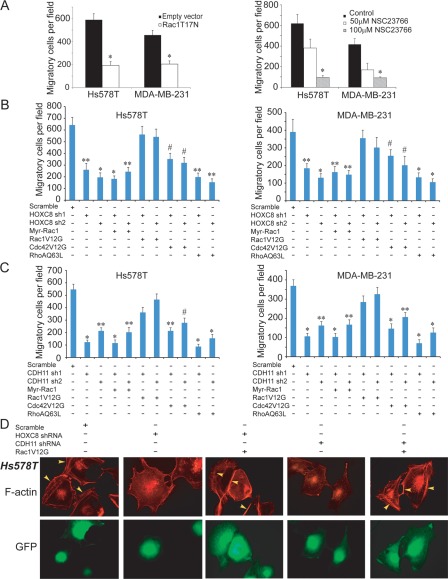
To determine the importance of Rac in breast cancer cell migration, we adenovirally delivered dominant negative Rac1 mutant (Rac1T17N) into Hs578T and MDA-MB-231cells. Transwell migration assay showed that enforced Rac1T17N expression blocked more than 50% of cell migration in both lines (Fig. 4A). In a parallel experiment, we treated Hs578T and MDA-MB-231 cells with Rac inhibitor NSC23766 and observed that NSC23766 inhibited cell migration in a dose-dependent manner (Fig. 4A). As NSC23766 inhibits Rac activity by targeting Rac-GEF Trio and Tiam1,27 these results suggest that Trio- or Tiam1-mediated Rac activation is necessary for breast cancer cell migration.
Figure 4.
Active Rac1 restores cell migration inhibited by HOXC8- or CDH11-knockdown. (A) Left panel: Hs578T and MDA-MB-231 cells were adenovirally transduced with empty vector or vector encoding Rac1T17N for 2 days and then assayed for cell migration by Transwell assay. Right panel: Hs578T and MDA-MB-231 cells were treated with NSC23766 at the indicated concentration for 1 day and then analyzed for cell migration. Data are mean ± SE. n = 4. *P < 0.005 versus empty vector or control. (B) Hs578T and MDA-MB-231 cells with HOXC8-knockdown were adenovirally transduced with vector encoding Myr-Rac1, Rac1V12G, Cdc42V12G, or RhoAQ63L for 2 days and then assayed for cell migration by Transwell assay. Data are mean ± SE. n = 4. *P < 0.001 versus scramble. **P < 0.005 versus scramble. # P < 0.01 versus scramble. (C) Hs578T or MDA-MB-231 cells with CDH11-knockdown were adenovirally transduced with vector encoding Myr-Rac1, Rac1V12G, Cdc42V12G, or RhoAQ63L and then assayed for cell migration by Transwell assay. Data are mean ± SE. n = 4. *P < 0.001 versus scramble. **P < 0.005 versus scramble. # P < 0.01 versus scramble. (D) Hs578T cells lentivirally expressing scramble sequence, HOXC8, or CDH11 shRNA were adenovirally transduced with either empty vector or vector encoding Rac1V12G for 2 days and then subjected immunostaining with rhodamine-conjugated phalloidin. Polymerized actin was visualized under a fluorescence microscope. Cells expressing GFP are lentivirally transduced.
The activation of Rac requires both its initial plasma membrane translocation and subsequent GDP-to-GTP exchange.18 To determine at which step HOXC8-CDH11 axis affects Rac activation, we adenovirally expressed myristoylated membrane-bound Rac1 (Myr-Rac1) or GTP-bound Rac mimic (Rac1V12G) into HOXC8- or CDH11-knockdown Hs578T and MDA-MB-231 cells. Transwell migration assay showed that Rac1V12G, but not Myr-Rac1, was able to restore more than 80% of cell migration caused by HOXC8 or CDH11 depletion (Fig. 4B and 4C). In a parallel experiment, we also examined the potential involvement of Cdc42 and RhoA by individually introducing GTP-bound Cdc42 mimic (Cdc42V12G) and GTP-bound RhoA mimic (RhoAQ63L) into HOXC8- and CDH11-knockdown cells. Cdc42V12G restored cell migration in a lesser degree than RacV12G did whereas RhoAQ63L displayed little effect on cell migration (Fig. 4B and 4C). When polymerized actin was examined by immunofluorescence, we found that enforced Rac1V12G expression largely restored the lost membrane ruffles in both HOXC8- and CDH11-knockdown Hs578T (Fig. 4D) and MDA-MB-231 cells (Suppl. Fig. S6). These results suggest that the HOXC8-CDH11 axis regulates cell migration by promoting Rac activation at the step of GDP-to-GTP exchange.
Trio is involved in high endogenous Rac activity and cell migration
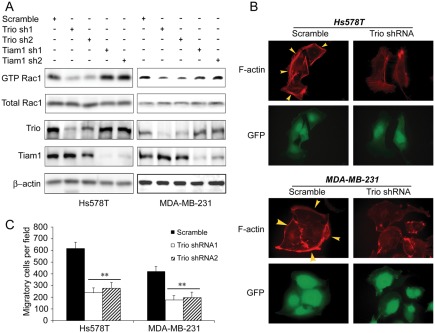
The observation that Trio/Tiam1-targeting NSC23766 inhibited breast cancer cell migration (Fig. 4A) indicated a potential involvement of Trio or Tiam1 in CDH11 regulation of cell migration. To test this, we silenced Trio or Tiam1 in Hs578T and MDA-MB-231 cells with lentivirally delivered shRNAs. Judging Rac activity by measuring the level of GTP-bound Rac, knockdown of Trio, but not Tiam1 substantially reduced Rac activity in both lines (Fig. 5A). The decreased Rac activity in Trio-knockdown cells was also reflected by immunofluorescence staining in which the number of membrane ruffles was much smaller in Trio-knockdown cells than in the control cells (Fig. 5B). Furthermore, Transwell migration assay showed that both Trio shRNAs were capable of abolishing more than 70% of cell migration (Fig. 5C). These results demonstrate that Trio is the main contributor toward high endogenous Rac activity and is required for breast cancer cell migration.
Figure 5.
Trio is required for endogenous Rac activity in breast cancer cells. (A) Hs578T and MDA-MB-231 cells were lentivirally transduced with vector encoding scramble sequence, Trio, or Tiam1 shRNAs for 4 days and then analyzed for Rac activities. Cell lysates were also collected and subjected to immunoblotting to detect Trio, Tiam1, and β-actin. (B) Hs578T and MDA-MB-231 cells lentivirally expressing scramble sequence or Trio shRNA were subjected immunostaining with rhodamine-conjugated phalloidin. Polymerized actin was visualized under a fluorescence microscope. Cells expressing GFP are lentivirally transduced. (C) Hs578T and MDA-MB-231 cells lentivirally expressing scramble sequence or Trio shRNA were assayed for cell migration by Transwell assay. Data are mean ± SE. n = 4. **P < 0.005 versus scramble.
CDH11 is required for the plasma membrane localization of Trio
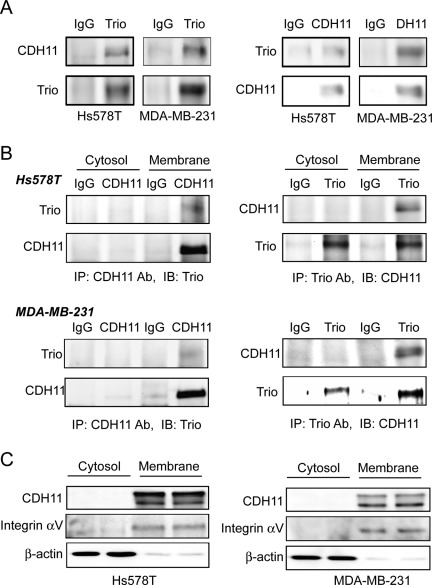
As Rac is mainly activated by Rac GEF on the plasma membrane, the nature of CDH11 as a membrane protein prompted us to test the possibility that CDH11 promotes Rac activity by assisting the plasma membrane localization of Trio through its physical interaction with Trio. To test this possibility, we first examined whether CDH11 was able to interact with Trio in Hs578T and MDA-MB-231 cells. Co-immunoprecipitation with whole cell lysates showed that CDH11 was detected in Trio immunocomplex whereas Trio was seen in CDH11 immunocomplex (Fig. 6A). To further examine the subcellular localization of the CDH11-Trio interaction, we also performed co-immunoprecipitation experiments with either the plasma membrane or cytosol fractions of Hs578T and MDA-MB-231 cells. The interaction between CDH11 and Trio was only detected in the plasma fraction (Fig. 6B), and this may be explained by the observation that CDH11 could only be detected in the plasma fraction of these cells (Fig. 6C). These data thus confirm a physical interaction between CDH11 and Trio in the inner surface of the plasma membrane of breast cancer cells.
Figure 6.
CDH11 interacts with Trio in the plasma membrane regions. (A) Hs578T and MDA-MB-231 cells were lysed and cell lysates were immunoprecipitated with either Trio polyclonal antibody or CDH11 mAb. Immunoblotting was performed to detect CDH11 in Trio immunoprecipitate (left panel) and Trio in CDH11 immunoprecipitate (right panel). IgG was used as negative control. (B) Plasma membrane and cytosolic fractions of Hs578T and MDA-MB-231 cells were immunoprecipitated with either Trio polyclonal antibody or CDH11 mAb. Immunoblotting was performed to detect Trio in CDH11 immunoprecipitate (left panel) and CDH11 in Trio immunoprecipitate (right panel). IgG was used as negative control. (C) Plasma membrane and cytosolic fractions of Hs578T and MDA-MB-231 cells were subjected to immunoblotting to detect CDH11, αv, integrin, and β-actin with the respective antibodies.
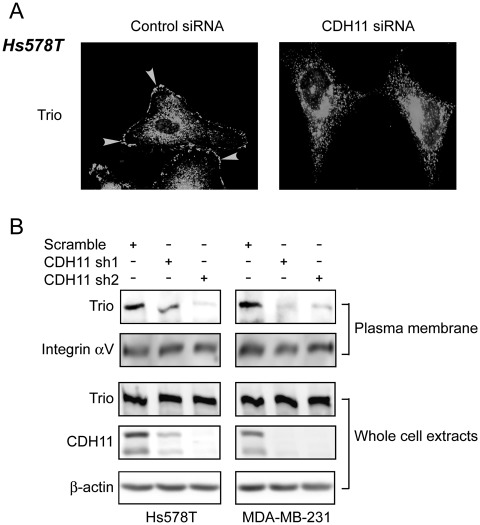
We next investigated how depleting CDH11 affected the subcellular localization of Trio by performing immunofluorescence staining to visualize Trio in both control and CDH11-knockdown Hs578T cells. In control cells, Trio was distributed in both cytoplasm and plasma membrane (Fig. 7A). However, the plasma membrane distribution of Trio was diminished in CDH11-knockdown cells (Fig. 7A). In a parallel experiment, we prepared membrane fractions from both control and CDH11-knockdown cells. Immunoblotting with these membrane fractions showed that the level of Trio, but not αv integrin subunit (a bona fide membrane protein), was significantly lower in CDH11-knockdown cells compared with control (Fig. 7B). Since the level of Trio in total cell lysate was not altered by CDH11-knockdown (Fig. 7B), these results indicate that the plasma membrane localization of Trio depends on the presence CDH11. As Rac is generally activated by its GEF on the plasma membrane, our data suggest that CDH11 elevates Rac activity by facilitating the plasma membrane localization of Trio in breast cancer cells.
Figure 7.
CDH11 facilitates the plasma membrane localization of Trio. (A) Hs578T cells were treated with control or CDH11 siRNA for 3 days and then fixed and stained with anti-Trio polyclonal antibody. The subcellular localization of Trio was visualized under a fluorescence microscope. (B) Plasma membrane fractions were prepared from Hs578T and MDA-MB-231 cells lentivirally expressing scramble sequence or CDH11 shRNAs and then subjected to immunoblotting to detect Trio and αv integrin subunit. Whole cell lysates were also collected and subjected to immunoblotting to detect Trio, CDH11, and β-actin.
Discussion
In this study, we identified a novel signaling axis consisting of HOXC8-CDH11-Trio that promotes Rac activation and cell migration in metastatic breast cancer cells. HOXC8 is a member of homeobox protein, and its aberrant expression is often detected in aggressive/metastatic prostate and breast cancers.4-6 These findings indicate an active role of HOXC8 in cancer metastasis. This notion is supported by our observation that depleting HOXC8 diminishes breast cancer cell migration and metastasis.4 In murine fibroblasts, ectopic expression of HOXC8 alters the expression of several migration-pertinent genes—upregulation of CDH11 and embigin expression and downregulation of transgelin and calponin.7,28,29 In this study, we found that HOXC8 similarly regulated the expression of these genes in metastatic breast cancer cells (Fig. 1 and Suppl. Fig. S1). Especially, we showed that the expression of HOXC8 and CDH11 is well correlated in both breast cancer cell lines and clinical breast tumor specimens (Fig.1, Suppl. Fig. S2, and Suppl. Table S1).
CDH11 belongs to atypical (or type-II) cadherin subfamily,8 and its level is elevated in aggressive breast and prostate cancers.13-15 CDH11 has been suggested to be metastasis-promoting because enforced CDH11 transgene expression enhances cancer cell migration and metatstasis.13-15,30,31 In this study, we showed that knockdown of CDH11 decreased Hs578T and MDA-MB-231 cell migration (Fig. 2). These results indicate that the presence of CDH11 is probably necessary for breast cancer cell migration and metastasis. Clearly, CDH11 is involved is the maintenance of high endogenous Rac activity and effective actin-based cytoskeleton reorganization in migratory breast cancer cells (Fig. 3). Given the critical role Rac plays in cell migration (Fig .4), we conclude that the HOXC8-CDH11 axis acts to promote Rac activation, thus facilitating breast cancer cell migration. This notion is supported by the observation that active Rac1 (RacV12G) restored effective actin-based cytoskeleton reorganization and cell migration of HOXC8- or CDH11-knockdown cells (Fig. 4). We believe that this study provides the first clear evidence on how CDH11 participates in cancer cell migration.
Rac activation requires its plasma membrane translocation and GDP-to-GTP exchange.18 We found that only GTP-bound Rac mimic (Rac1V12G), but not myristoylated membrane-bound Rac1, was able to rescue migration of HOXC8- or CDH11-knockdown cells (Fig. 4). These results indicate that GDP-to-GTP of Rac, rather than the plasma membrane translation of Rac, is defective in the absence of CDH11. A recent study reported that CDH11 triggers Rac activation in a Trio-dependent manner in Xenopus neuronal cells.19 In this study, we found that either Trio/Tiam1-targeting Rac inhibitor NSC2376627 or Trio-specific shRNAs decreased Rac activity and cell migration (Figs. 4 and 5). Moreover, CDH 11 was in the same immunocomplex with Trio in breast cancer cells (Fig. 6), suggesting a physical interaction between them. As knockdown of CDH11 led to the disappearance of Trio in the plasma membrane (Fig. 7) and GDP-to-GTP exchange of Rac usually occurs at inner plasma membrane area, we reason that CDH11 recruits Trio to plasma membrane, leading to robust Rac activation and cell migration.
Metastasis is responsible for the majority of breast cancer mortality, and thus functionally disrupting pathways important for metastasis may improve the survival of breast cancer patients. Therefore, anti-metastasis therapeutic approaches may be developed by targeting HOXC8-CDH11 signaling axis in breast cancer.
Materials and Methods
Cells, shRNAs, qRT-PCR, antibodies, and other reagents
BT-549, Hs578T, MCF-7, MDA-MB-231, and T47D cells were obtained within the last 6 months from ATCC and cultured in the conditions recommended by ATCC. All short hairpin RNAs (shRNAs) were designed using the web-based Invitrogen (Carlsbad, CA) Block-It program and generated by inserting oligonucleotides containing shRNA sequences into pLV-shRNA vector (BioSettia, San Diego, CA). qRT-PCR was performed with gene-specific primer sets using ABI 7500Fast system as previously described.4 Detailed information on antibodies, reagents, shRNAs, and primer sequences is included in the supplementary data.
Transwell migration assays
Cell migration was performed as previously described.4,32 Cell migration was carried out when 10% FBS was present in the lower chambers as the attractant and 5 × 105 cells were added into each chamber. After 4-h migration period, the cells on the undersurface of Transwells were stained with crystal violet solution and counted as migratory cells under a phase-contrast microscope.
Rac, Cdc42, and RhoA activity assays
The activities of Rac, Cdc42, and RhoA were measured by determining the respective levels of GTP-bound Rac, Cdc42, and RhoA using Rac, Cdc42, and RhoA Activity Assay kits (Cell Biolabs, San Diego, CA). The assays were performed according to the manufacturer’s instruction.
Immunohistochemistry
Normal human breast and primary breast cancer tissues were collected in compliance with a protocol approved by the Georgia Health Sciences University Institutional Oversight Advisory Board, and informed consent was received from all subjects. Paraffin-embedded tissues were sectioned and used for immunohistochemistry with HOXC8 and CDH11 mAbs as previously described.33 Sections were also subjected to H&E staining.
Immunofluorescence staining
Cells grown on glass coverslips were fixed with 4% formaldehyde and then permeabilized with 0.2% Triton X-100 and blocked with 3% BSA. To detect actin-based cytoskeleton reorganization, cells were incubated with rhodamine-conjugated phalloidin (Invitrogen). To detect Trio, cells were incubated with anti-Trio polyclonal antibody followed by incubation with AlexaFluor-conjugated secondary antibody (Invitrogen). Polymerized actin and Trio were visualized by a confocal fluorescence microscope.
Plasma membrane fraction preparation
Plasma membrane fractions were prepared as described previously.34 Subconfluent cells were washed twice with PBS and then collected with a cell scraper and suspended in homogenization buffer (250 mM sucrose, 20 mM HEPES pH 7.4, 1 mM EDTA, and protease inhibitors). Cells were homogenized by 4 passages through a tight homogenizer, and debris including nuclei was pelleted by centrifugation at 1,000 × g. The supernatants were further centrifuged at 200,000 × g to obtain plasma membrane fractions.
Statistical analysis of data
Statistical analyses were done by Student t test (2-sided) on data collected from at least 3 independent experiments. Statistical analyses on the pathological parameters of clinical specimens were analyzed by Pearson correlation with SPSS program. The differences were considered statistically significant when P < 0.05.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is supported by NIH grants CA093926 and HL083335.
Supplementary material for this article is available on the Genes & Cancer website at http://ganc.sagepub.com/supplemental.
References
- 1. Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679-95 [DOI] [PubMed] [Google Scholar]
- 2. Yamauchi J, Miyamoto Y, Tanoue A, Shooter EM, Chan JR. Ras activation of a Rac1 exchange factor, Tiam1, mediates neurotrophin-3-induced Schwann cell migration. Proc Natl Acad Sci U S A. 2005;102:14889-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559-64 [DOI] [PubMed] [Google Scholar]
- 4. Li Y, Zhang M, Chen H, et al. Ratio of miR-196s to HOXC8 messenger RNA correlates with breast cancer cell migration and metastasis. Cancer Res. 2010;70:7894-904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alami Y, Castronovo V, Belotti D, Flagiello D, Clausse N. HOXC5 and HOXC8 expression are selectively turned on in human cervical cancer cells compared to normal keratinocytes. Biochem Biophys Res Commun. 1999;257:738-45 [DOI] [PubMed] [Google Scholar]
- 6. Waltregny D, Alami Y, Clausse N, de Leval J, Castronovo V. Overexpression of the homeobox gene HOXC8 in human prostate cancer correlates with loss of tumor differentiation. Prostate. 2002;50:162-9 [DOI] [PubMed] [Google Scholar]
- 7. Lei H, Wang H, Juan AH, Ruddle FH. The identification of Hoxc8 target genes. Proc Natl Acad Sci U S A. 2005;102:2420-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nollet F, Kools P, van Roy F. Phylogenetic analysis of the cadherin superfamily allows intification of six major subfamilies besides several solitary members. J Mol Biol. 2000;299:551-72 [DOI] [PubMed] [Google Scholar]
- 9. Okazaki M, Takeshita S, Kawai S, et al. Molecular cloning and characterization of OB-cadherin, a new member of cadherin family expressed in osteoblasts. J Biol Chem. 1994;269:12092-8 [PubMed] [Google Scholar]
- 10. Hoffmann I, Balling R. Cloning and expression analysis of a novel mesodermally expressed cadherin. Dev Biol. 1995;169:337-46 [DOI] [PubMed] [Google Scholar]
- 11. Sarrio D, Rodriguez-Pinilla SM, Hardisson D, Cano A, Moreno-Bueno G, Palacios J. Epithelial-mesenchymal transition in breast cancer relates to the basal-like phenotype. Cancer Res. 2008;68:989-97 [DOI] [PubMed] [Google Scholar]
- 12. Tomita K, van Bokhoven A, van Leenders GJ, et al. Cadherin switching in human prostate cancer progression. Cancer Res. 2000;60:3650-4 [PubMed] [Google Scholar]
- 13. Huang CF, Lira C, Chu K, et al. Cadherin-11 increases migration and invasion of prostate cancer cells and enhances their interaction with osteoblasts. Cancer Res. 2010;70:4580-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nieman MT, Prudoff RS, Johnson KR, Wheelock MJ. N-cadherin promotes motility in human breast cancer cells regardless of their E-cadherin expression. J Cell Biol. 1999;147:631-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pishvaian MJ, Feltes CM, Thompson P, Bussemakers MJ, Schalken JA, Byers SW. Cadherin-11 is expressed in invasive breast cancer cell lines. Cancer Res. 1999;59:947-52 [PubMed] [Google Scholar]
- 16. Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629-35 [DOI] [PubMed] [Google Scholar]
- 17. Ridley AJ. Rho GTPases and cell migration. J Cell Sci. 2001;114:2713-22 [DOI] [PubMed] [Google Scholar]
- 18. Schmidt A, Hall A. Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev. 2002;16:1587-609 [DOI] [PubMed] [Google Scholar]
- 19. Kashef J, Kohler A, Kuriyama S, Alfandari D, Mayor R, Wedlich D. Cadherin-11 regulates protrusive activity in Xenopus cranial neural crest cells upstream of Trio and the small GTPases. Genes Dev. 2009;23:1393-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Backer S, Hidalgo-Sanchez M, Offner N, et al. Trio controls the mature organization of neuronal clusters in the hindbrain. J Neurosci. 2007;27:10323-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kaibuchi K, Kuroda S, Fukata M, Nakagawa M. Regulation of cadherin-mediated cell-cell adhesion by the Rho family GTPases. Curr Opin Cell Biol. 1999;11:591-6 [DOI] [PubMed] [Google Scholar]
- 22. Vega FM, Ridley AJ. Rho GTPases in cancer cell biology. FEBS Lett. 2008;582:2093-101 [DOI] [PubMed] [Google Scholar]
- 23. Sahai E, Marshall CJ. RHO-GTPases and cancer. Nat Rev Cancer. 2002;2:133-42 [DOI] [PubMed] [Google Scholar]
- 24. Yamaguchi H, Wyckoff J, Condeelis J. Cell migration in tumors. Curr Opin Cell Biol. 2005;17:559-64 [DOI] [PubMed] [Google Scholar]
- 25. Ridley AJ, Schwartz MA, Burridge K, et al. Cell migration: integrating signals from front to back. Science. 2003;302:1704-9 [DOI] [PubMed] [Google Scholar]
- 26. Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247-69 [DOI] [PubMed] [Google Scholar]
- 27. Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc Natl Acad Sci U S A. 2004;101:7618-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lei H, Juan AH, Kim MS, Ruddle FH. Identification of a Hoxc8-regulated transcriptional network in mouse embryo fibroblast cells. Proc Natl Acad Sci U S A. 2006;103:10305-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mueller DW, Bosserhoff AK. MicroRNA miR-196a controls melanoma-associated genes by regulating HOX-C8 expression. Int J Cancer. 2011;129:1064-74 [DOI] [PubMed] [Google Scholar]
- 30. Kiener HP, Stipp CS, Allen PG, Higgins JM, Brenner MB. The cadherin-11 cytoplasmic juxtamembrane domain promotes alpha-catenin turnover at adherens junctions and intercellular motility. Molecular biology of the cell. 2006;17:2366-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Di Benedetto A, Watkins M, Grimston S, et al. N-cadherin and cadherin 11 modulate postnatal bone growth and osteoblast differentiation by distinct mechanisms. J Cell Sci. 2010;123:2640-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bian D, Mahanivong C, Yu J, et al. The G12/13-RhoA signaling pathway contributes to efficient lysophosphatidic acid-stimulated cell migration. Oncogene. 2006;25:2234-44 [DOI] [PubMed] [Google Scholar]
- 33. Su S, Li Y, Luo Y, et al. Proteinase-activated receptor 2 expression in breast cancer and its role in breast cancer cell migration. Oncogene. 2009;28:3047-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Borrow P, Oldstone MB. Characterization of lymphocytic choriomeningitis virus-binding protein(s): a candidate cellular receptor for the virus. J Virol. 1992;66:7270-81 [DOI] [PMC free article] [PubMed] [Google Scholar]