Abstract
Objective
We conducted a single-centre, randomised, double-blinded, placebo-controlled phase II clinical study to test safety and efficacy of a 12-week therapy with low-dose (700 mg/daily) or high-dose (2800 mg/daily) of NAC.
Methods
Twenty-one patients (ΔF508 homo/heterozygous, FEV1 > 40% pred.) were included in the study. After a 3-weeks placebo run-in phase, 11 patients received low-dose NAC, and 10 patients received high-dose NAC. Outcomes included safety and clinical parameters, inflammatory (total leukocyte numbers, cell differentials, TNF-α, IL-8) measures in induced sputum, and concentrations of extracellular glutathione in induced sputum and blood.
Results
High-dose NAC was a well-tolerated and safe medication. High-dose NAC did not alter clinical or inflammatory parameters. However, extracellular glutathione in induced sputum tended to increase on high-dose NAC.
Conclusions
High-dose NAC is a well-tolerated and safe medication for a prolonged therapy of patients with CF with a potential to increase extracellular glutathione in CF airways.
Keywords: Cystic fibrosis, N-acetylcysteine, induced sputum, glutathione, inflammation
Introduction
Cystic fibrosis (CF) lung disease is the main cause of morbidity and mortality in patients with CF. Excessive neutrophil-dominated inflammation in airways is one of the hallmarks of CF lung disease. This uncontrolled inflammation is believed to lead to lung damage and dysfunction. There is a clear need for new anti-inflammatory medications in CF. The use of existing anti-inflammatory therapies, such as oral corticosteroids [1,2] or high-dose ibuprofen [3,4], is limited because of extensive adverse events [5] or concerns thereof [6].
Antioxidant drugs, such as N-acetylcysteine (NAC), have attracted attention recently as potential therapies for CF. The rationale to employ e.g. NAC is based on the premise that CF airways are overexposed to oxidants derived from bacteria [7,8] or activated neutrophils [9]. Overexposure to oxidants, i.e. oxidative stress, is a known amplifier of inflammation. Therefore, antioxidant drugs may be useful to control both oxidative stress and excessive inflammation in CF airways.
While the safety and clinical efficacy of corticosteroids and high-dose ibuprofen have been thoroughly tested in clinical studies, NAC has not been studied as extensively. To date, only one short-term, open, uncontrolled, phase I study on NAC in CF has been published [10]. These authors have tested NAC administered for 4 weeks.
We have designed and conducted a single-centre, randomised, double-blinded, placebo-controlled phase II clinical study. We tested safety of a 12-week therapy with low-dose (700 mg/daily) and high-dose (2800 mg/daily) of NAC, as well as its effects on clinical parameters, concentrations of extracellular glutathione in induced sputum and blood, and inflammatory markers in induced sputum of patients with CF.
Methods
Patients
The study was conducted between January 2000 and September 2001 at the adult out-patient clinic of the Hospital of Johann-Wolfgang-Goethe University, Frankfurt/Main, Germany. Eligible patients were older than 16 yrs and had an established diagnosis of CF (repeatedly positive sweat tests, homo- or heterozygous for ΔF508). Patients had stable disease within the last four weeks before enrolment, and FEV1 > 40% pred. Patients were not to be included if they had recent (i.e. within the last four weeks) exacerbation of CF lung disease, recent use of oral corticosteroids or parenteral antibiotic therapy, history of haemoptysis, known hypersensitivity to NAC or inactive ingredients of the study medication (lactose, sodium cyclamate, saccharin sodium, polyethylene glycol, sodium carbonate, sodium bicarbonate, citric acid), history of severe drug-related allergy, and clinically significant liver impairment (AST/GOT ≥ 50 IU/L). Informed consents were obtained from all patients. The study protocol was approved by the local Human Ethics Board and conducted according to GCP guidelines.
Study design and medications
The study was conducted as a single-centre, randomised, double-blind, placebo-controlled, parallel-group phase II study with a three-week single-blind placebo run-in phase. In total, patients came 5 times to the study centre: visit 1 (screening/wash-out, day 1), visit 2 (randomisation/placebo run-in phase, day 22), visit 3 (begin of therapy with either 700 mg or 2800 mg NAC/daily, day 43), visit 4 (on study medication, day 64), and visit 5 (final visit, day 127). Patients' allocation to either dose of NAC was done according to a randomisation list (1: 1 balanced) generated with Random 1.0 software. Patients were instructed to bring back empty medication containers which were used to assess patients' compliance. A patient was considered as compliant if she or he took at least 80% of the dispensed medication.
Safety measures and adverse events
Safety measures comprised adverse events, changes in vital signs, and safety laboratory parameters. Adverse events were registered during visits 2, 3, 4, and 5. Exacerbations of CF lung disease were counted as adverse events. Vital signs were registered during each visit at the study centre. Safety laboratory comprised analyses of serum (AST/ALT, γ-GT, C-reactive protein), blood, and urine parameters. These were obtained on visit 1 and 5. In addition, an overall tolerability was assessed by both investigator and patient at visit 5.
Efficacy measures
The following parameters were quantified for the subsequent efficacy evaluation: pulmonary functions tests, antioxidant markers (extracellular glutathione in induced sputum and blood), and inflammation markers (total leukocyte counts and cell differentials, Tumour Necrosis Factor (TNF)-α, and Interleukin (IL) -8) in induced sputum. In addition, changes in disease symptoms (clinical symptoms/Quality of Life Questionnaire) were also registered during each visit.
Sputum induction, processing, and outcome measures
Sputum samples were collected during visits 2, 3, 4 and 5. Sputum induction was performed according to the standard protocols as described by us previously [11]. Sputum samples were obtained, and small aliquots were sent for microbiology analysis. The remainders were processed as described in our previous publication [11]. This protocol enabled measures of both extracellular glutathione and cytokines in sputum supernatants, as well as total leukocyte numbers and cell differentials in the sputum's solid phase. In brief, induced sputum was mixed with ice-cold phosphate-buffered saline, gently mixed and vortexed, and centrifuged to separate supernatant (to analyse glutathione and cytokines) and solid phase (to analyse cells) [11]. We did not utilise dithiothreitol to obtain sputum supernatants because this reagent affects glutathione quantification in the sputum [12].
Extracellular glutathione was quantified by the standard methodology as described by us previously [11]. Cytokines (TNF-α and IL-8) were quantified with ELISA kits from R&D Systems.
The solid phase was solubilised with a mixture of dithiothreitol and rhDNAse to obtain cells [11].
Blood samples and outcome measures
Blood samples were obtained during visits 2, 3, 4 and 5. Samples were collected in EDTA tubes and immediately processed for glutathione [13].
Data analyses
Data are presented as median (25 -75%) throughout the text and as box-whisker (min value, 25%, median, 75%, and max value) plots in the figures.
Safety and efficacy measures on treatment with either dose of NAC (visits 4 and 5) were compared to the baseline which was defined as the end of placebo run-in, i.e. visit 3. Categorical data were analysed using Fishers exact test. Ordinal or interval data were analysed using Mann-Whitney (between-group comparisons), Wilcoxon or Friedman tests (within-group comparisons), where appropriate. Two-tailed p values were used, and statistical significance was defined at p < 0.05.
Role of funding source
The sponsor of this study (Hexal AG) participated in the study planning and study performance, but played no role in data collection, data analysis, data interpretation, or summarising the study results and writing this manuscript.
Results
Subjects
A total of twenty-four patients were screened and asked to participate in the study. Twenty-one patients met the inclusion criteria and were included in the intention-to-treat analysis (Table 1). Eleven patients were randomly assigned to receive 700 mg/daily of NAC (low-dose; Table 1), and ten patients were randomly assigned to receive 2800 mg/daily of NAC (high-dose; Table 1). Patients in both groups were comparable in demographic and clinical parameters (Table 1). For instance, both groups were not significantly different in the degree of airway obstruction at visit 1: median (25-75%) FEV1 of 80.60 (55.30-90.00)% pred. in the 700 mg/daily NAC vs. 65.80 (62.90-73.40)% pred. in the 2800 mg/daily NAC (Table 1 and Figure 2, visit 1; p = 0.43).
Table 1.
Patient characteristics.
| 700 mg NAC | 2800 mg NAC | |
|---|---|---|
| Number of patients | 11 | 10 |
| Male/female (n) | 9/2 | 7/3 |
| Age | 27.0 (21.0 - 35.0) | 28.5 (24.0 - 35.0) |
| Height (cm) | 168.0 (163.0 - 178.0) | 174.0 (168.0 - 178.0) |
| Weight (kg) | 65.5 (59.0 - 70.40) | 64.5 (57.4 - 72.0) |
| Pseudomonas aeruginosa | 4 | 3 |
| Staphylococcus aureus | 1 | 1 |
| Various bacteria/saprophytic flora | 6 | 6 |
| FEV1 (% pred) | 80.6 (55.3 - 90.0) | 65.8 (62.9 - 73.4) |
| FVC (% pred) | 82.6 (78.4 - 100.4) | 84.8 (79.6 - 91.2) |
| MEF25-75 (% pred) | 41.1 (12.4 - 68.1) | 21.6 (19.6 - 27.9) |
Data are shown as median (25 - 75%) values
Figure 2.
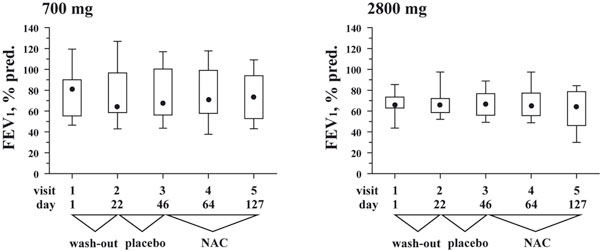
Box and whisker plots for FEV1 (% predicted) at each of the five study visits. Whiskers represent minimum and maximum values, box represents 25 and 75 percentiles, • represents median values. Visit 3 at the end of placebo run-in phase defines the baseline.
Figure 1.
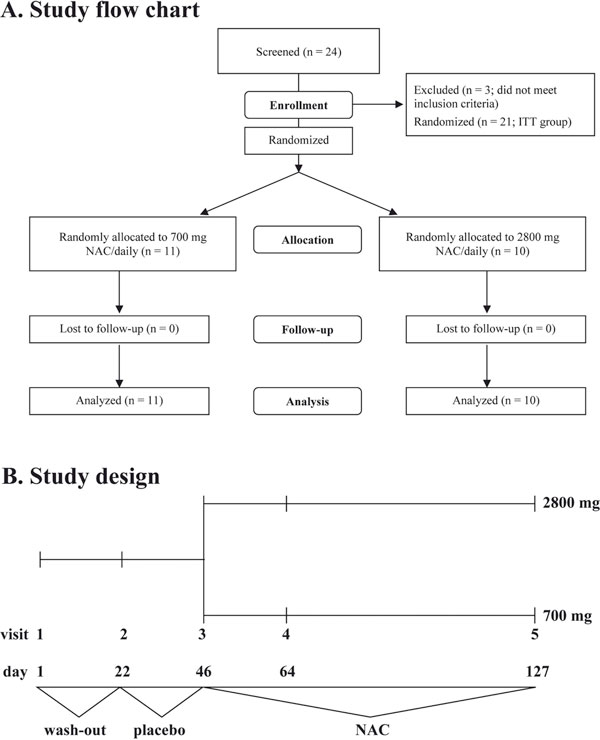
A. A CONSORT study flow chart of patient enrolment, follow-up and analysis. B. Study design. Visit 3 (at the end of placebo run-in phase) was defined as baseline for subsequent analyses.
Compliance
The compliance was found to be good, with the calculated compliance rates of > 80 per cent for both study groups.
Safety
Both study groups exhibited comparable numbers of mild-to-moderate adverse effects (24 on 700 mg/daily of NAC, and 24 on 2800 mg/daily of NAC; p > 0.5). The adverse events were mostly exacerbations of the CF lung disease.
There have been three adverse effects which were rated as "serious". Two adverse effects (polypectomia: the 700 mg/daily NAC group; haemoptysis during a common cold: the 2800 mg NAC/daily group) were rated as non-related to the study medication. With one adverse effect, the causal relationship between the study drug and adverse event was rated as "possible". This adverse event was in one patient in the 700 mg/daily NAC study group who developed gastrointestinal bleeding.
Twenty patients (both groups combined, one report missing) reported the study medication as a "very good" or "good" tolerable.
Efficacy measures
1. Pulmonary function tests
Both study groups demonstrated comparable baseline values of FEV1 before the start of NAC therapy (Figure 2, visit 3; p = 0.65). FEV1 did not change significantly during medication with either doses of NAC (Figure 2; p > 0.3 for both groups).
2. Extracellular total glutathione in induced sputum and blood plasma
Both study groups demonstrated comparable concentrations of extracellular total glutathione in induced sputum at the baseline (i.e. visit 3): 21.2 (7.2 - 34.02) μM in the 700 mg/daily NAC vs. 18.6 (2.8 - 32.4) μM in the 2800 mg/daily NAC (Figure 3A, visit 3; p = 0.71). In the 700 mg/daily NAC, the median sputum concentrations of total glutathione tended to decrease during therapy with NAC, compared to the baseline, i.e. visit 3: 23.2 (7.2 - 34.0) μM vs. 14.0 (2.3 - 24.8) at visit 4 and 16.4 (3.0 - 31.5) μM at visit 5 (Figure 3A). However, these differences did not reach statistical significance (p > 0.3).
Figure 3.
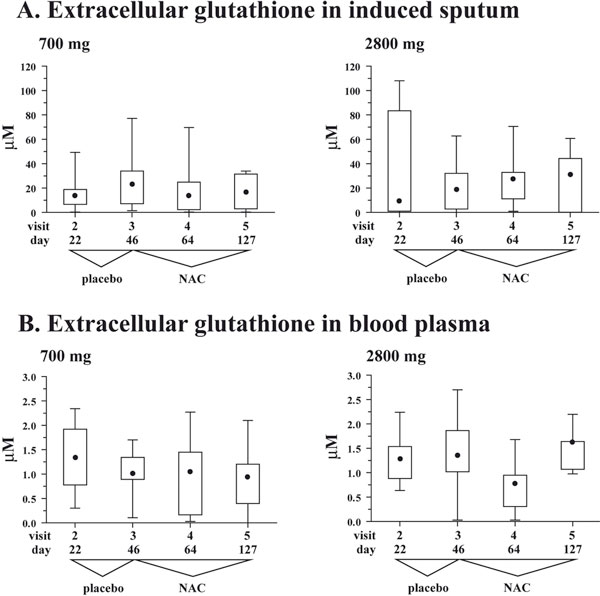
A. Box and whisker plots for extracellular glutathione in induced sputum at the four study visits when induced sputum samples were collected. Whiskers represent minimum and maximum values, box represents 25 and 75 percentiles, • represents median values. Visit 3 at the end of placebo run-in phase defines the baseline. B. Box and whisker plots for extra-cellular glutathione in blood plasma at the four study visits when blood samples were collected. Whiskers represent minimum and maximum values, box represents 25 and 75 percentiles, • represents median values. Visit 3 at the end of placebo run-in phase defines the baseline.
On the contrary, in the 2800 mg/daily NAC, the median values of total glutathione in induced sputum tended to increase on NAC compared to the baseline (visit 3): 18.6 (2.8 - 32.14) μM vs. 27.2 (11.2 - 33.0) μM at visit 4 and 31.3 (0.2 - 44.3) μM at visit 5 (Figure 3A). Similar to the 700 mg/daily NAC group, these differences did not reach statistical significance (Figure 3A, visits 3 - 5; p > 0.8).
This study confirmed our previous report [11] that total glutathione in sputum samples of patients with CF is in reduced form (> 81%; data not shown).
With regard to extracellular total glutathione in blood plasma, both study groups demonstrated comparable concentrations of this antioxidant at the baseline (i.e. visit 3): 1.0 (0.9 - 1.3) μM in the 700 mg/daily NAC vs. 1.4 (1.0 - 1.9) μM in the 2800 mg/daily NAC (Figure 3B, visit 3; p = 0.37). In both study groups, there were no significant changes in concentrations of total glutathione in blood plasma during treatment with NAC (Figure 3B, visits 3 - 5; p > 0.3 for both groups).
3. Inflammatory Markers in Induced Sputum
Both groups were not significantly different in total number of leukocytes in induced sputum at the baseline (visit 3): 56.6 (32.0 - 122.5) × 106 leukocytes in the 700 mg/daily NAC vs. 31.5 (20.0 - 113.7) × 106 leukocytes in the 2800 mg/daily NAC (p = 0.51). In the 700 mg/daily NAC, total number of leukocytes did not change significantly during active therapy (visits 4 and 5: 102 (55.2 - 164.0) × 106 and 56.0 (36.0 - 235.0) × 106 leukocytes, respectively; p > 0.3). The same was true for the 2800 mg/daily NAC study group (visits 4 and 5: 48.6 (42.6 - 186.2) × 106 and 36.8 (19.9 - 110.8) × 106; respectively; p > 0.3).
In both study groups, sputum leukocytes were predominantly neutrophils (data not shown).
Sputum TNF-α. Patients in the 700 mg/daily NAC demonstrated slightly lower concentrations of sputum TNF-α at the baseline (visit 3): 68.4 (30.0 - 140.0) pg/mL vs. 140.0 (105.0 - 541.5) pg/mL; Figure 4A; p = 0.17). Further, in both groups TNF-α exhibited similar kinetics during therapy with NAC: it raised slightly at visit 4 and decreased at visit 5 (Figure 4A). However, these variations did not reach statistical significance in either study group (Figure 4A; p > 0.6 for both groups).
Figure 4.
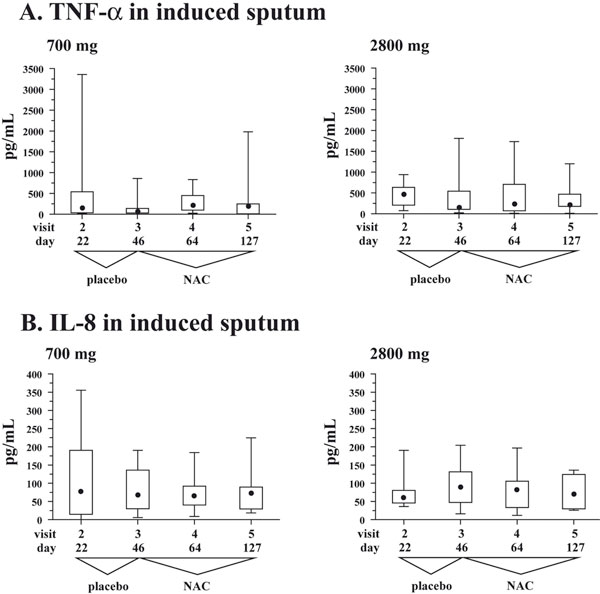
A. Box and whisker plots for TNF-α in induced sputum at the four study visits when induced sputum samples were collected. Whiskers represent minimum and maximum values, box represents 25 and 75 percentiles, • represents median values. Visit 3 at the end of placebo run-in phase defines the baseline. B. Box and whisker plots for IL-8 in induced sputum at the four study visits when induced sputum samples were collected. Whiskers represent minimum and maximum values, box represents 25 and 75 percentiles, • represents median values. Visit 3 at the end of placebo runin phase defines the baseline.
Sputum IL-8. Patients from both study groups demonstrated comparable concentrations of sputum IL-8. In specific, patients in the 700 mg/daily NAC study group had 68.6 (30.0 - 136.1) pg/mL, whereas patients in the 2800 mg/daily NAC study group demonstrated 90.2 (47.4 - 131.2) pg/mL (Figure 4B, visit 3; p = 0.5). In contrast to TNF-α, concentrations of IL-8 in induced sputum demonstrated less variations in both study groups during therapy with NAC (Figure 4B; p > 0.1 for both groups).
Discussion
High-dose oral NAC is a promising new drug to suppress oxidative stress and excessive inflammation in CF airways. We conducted a single-centre, randomised, double-blinded, placebo-controlled phase II study on safety and efficacy of a prolonged (12-weeks) therapy with low- (700 mg/daily) or high-dose (2800 mg/daily) NAC in patients with CF.
Until recently, there was only one report from a phase I study by Tirouvanziam et al [10] on high-dose NAC for therapy of CF. We were unaware of their study when the present study was designed and conducted. Both our and the study Tirouvanziam et al [10] utilised some similar measures, as well as some different ones. Therefore, both studies provide confirmatory as well as non-redundant information about safety and efficacy of high-dose NAC in patients with CF.
The first important observation similar between our and the study by Tirouvanziam et al [10] is that highdose NAC is a safe medication for therapy of CF. In our study, 2800 mg/daily NAC was well tolerated over the course of 12 weeks. Further, neither study revealed any specific risks associated with high-dose NAC in patients with CF.
Second, neither study observed any changes in pulmonary function on high-dose NAC. Yet, it is probably premature to rule out any beneficial effects of high-dose NAC. The timeframes of both studies could have been too short for this outcome measure to show significant changes. The timeframe of our study (12 weeks) was longer, however, it may take months to detect these changes. Indeed, the studies on e.g. high-dose ibuprofen, another anti-inflammatory drug for therapy of CF, went on for 4 or 2 years [3,4] to register a significant impact of the anti-inflammatory drug.
Both studies on high-dose NAC quantified glutathione, important antioxidant in CF, which is expected to be augmented by NAC supplementation. With respect to glutathione, both studies deliver non-redundant information.
Our study focused on extracellular glutathione in induced sputum and blood plasma. Induced sputum samples airway secretions in a non-invasive way, and our research group was the first one to adapt the protocol of induced sputum processing to quantify extracellular glutathione [12], also in patients with CF [11].
The rationale to measure extracellular glutathione comes from the fact that this antioxidant might be in deficiency in CF airways [13]. This deficiency is a direct consequence of the malfunctioning glutathione-exporting function of CF transmembrane conductance regulator (CFTR) protein [14-16]. Decrease in extracellular glutathione is thought to aggravate oxidative stress and promote inflammation in CF airways [17,18]. Therefore, therapeutic interventions capable of increasing extracellular glutathione in CF airways are expected to bring about beneficial clinical effects. Our study demonstrated that high-dose NAC tended to increase concentrations of extracellular glutathione in induced sputum. Further, these trends appeared to be specific to the airways, as no such trends were seen with regard to extracellular glutathione in blood plasma.
By contrast, Tirouvanziam et al [10] focused on studying intracellular glutathione in blood neutrophils and whole blood lysates (predominantly, red blood cells). These authors observed significant changes in intracellular glutathione in neutrophils and in whole blood lysates. It is plausible that blood cells (including neutrophils) increase their intracellular glutathione concentrations on high-dose NAC. Blood cells are likely to be exposed to the much higher concentrations of NAC than the cells in the lung.
The increase of intracellular glutathione in neutrophils could explain the trends towards increased extracellular glutathione in induced sputum samples. Previously, we reported high concentrations of reduced glutathione in sputum samples from patients with CF [11], and the present study confirmed our previous observation. Given the impairment of glutathione-exporting function of mutated CFTR in CF airway epithelium [14-16], high concentrations of reduced glutathione can be explained by its efflux from apoptotic cells, such as neutrophils. Such efflux of reduced glutathione has been shown with respect to many cell types undergoing apoptosis [19-21]. This efflux involves multidrug resistance-associated protein (MRP)1 [22,23], i.e. is CFTR-independent. One could speculate that high-dose NAC provides peripheral neutrophils with higher intracellular glutathione, and that these neutrophils are capable of releasing higher, than normally, concentrations of glutathione in CF airways.
Both studies studied markers of airway inflammation in induced sputum (ours: TNF-α and IL-8, Tirouvanziam et al: neutrophil elastase and IL-8). Both studies should be comparable because of similar methodologies to obtain sputum supernatant. Sputum supernatants were extracted with phosphate-buffered saline, i.e. without dithiothreitol. Dithiothreitol interferes with quantification of glutathione in induced sputum [12] and confounds cell functional outcomes [10].
In neither study were the induced sputum cytokines affected by high-dose NAC. Specifically, the sputum concentrations of TNF-α in the present study were not decreased by high-dose NAC. Further, the sputum IL-8 concentrations also remained unaffected by NAC. Supporting these data, not all patients in the study by Tirouvanziam et al [10] responded to highdose NAC by decreasing sputum IL-8. By contrast, decreases in activity of neutrophil elastase after 4 weeks on high-dose NAC were by far more prominent and significant [10]. Neutrophil elastase is a neutrophil-specific factor. Its suppression caused by high-dose NAC [10] may indicate that neutrophils are the primary target of this drug in patient with CF.
There were some differences between our study and the study by Tirouvanziam et al [10]. For instance, we did not observe any significant changes in total leukocyte numbers in induced sputum, whereas they reported decrease in these numbers on high-dose NAC. These differences can be explained by the differences in lengths between the two studies (12 weeks vs. 4 weeks). The total leukocyte number is sputum varies depending on the phase of CF lung disease (remission vs. exacerbation). A prolonged 12-weeks study is more likely to be affected by clinical exacerbations.
In conclusion, our study demonstrates that high-dose NAC is a well-tolerated and safe medication for a prolonged therapy of patients with CF with a potential capacity to increase extracellular glutathione in CF airways. Long-term and larger studies are necessary to confirm the antioxidant and anti-inflammatory activity of high-dose NAC, and its implications on clinical disease in patients with CF.
Acknowledgements
The authors would like to thank all the patients who participated in this study. The authors further thank Mrs. Rafaela Paxinos, Mrs. Ulrike Müller, Dr. Christian von Mallinckrodt and Dr. Tim Hirche for their invaluable help with the present study.
The study was supported by Hexal AG, Holzkirchen, Germany.
References
- Eigen H, Rosenstein BJ, FitzSimmons S, Schidlow DV. A multicenter study of alternate-day prednisone therapy in patients with cystic fibrosis. Cystic Fibrosis Foundation Prednisone Trial Group. J Pediatr. 1995;126(4):515–23. doi: 10.1016/S0022-3476(95)70343-8. [DOI] [PubMed] [Google Scholar]
- Auerbach HS, Williams M, Kirkpatrick JA, Colten HR. Alternate-day prednisone reduces morbidity and improves pulmonary function in cystic fibrosis. Lancet. 1985;2(8457):686–8. doi: 10.1016/s0140-6736(85)92929-0. [DOI] [PubMed] [Google Scholar]
- Konstan MW, Byard PJ, Hoppel CL, Davis PB. Effect of high-dose ibuprofen in patients with cystic fibrosis. N Engl J Med. 1995;332(13):848–54. doi: 10.1056/NEJM199503303321303. [DOI] [PubMed] [Google Scholar]
- Lands LC, Milner R, Cantin AM, Manson D, Corey M. High-dose ibuprofen in cystic fibrosis: Canadian safety and effectiveness trial. J Pediatr. 2007;151(3):249–54. doi: 10.1016/j.jpeds.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Lai HC, FitzSimmons SC, Allen DB. et al. Risk of persistent growth impairment after alternate-day prednisone treatment in children with cystic fibrosis. N Engl J Med. 2000;342(12):851–9. doi: 10.1056/NEJM200003233421204. [DOI] [PubMed] [Google Scholar]
- Chmiel JF, Konstan MW. Inflammation and anti-inflammatory therapies for cystic fibrosis. Clin Chest Med. 2007;28(2):331–46. doi: 10.1016/j.ccm.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Schwarzer C, Fischer H, Kim EJ. et al. Oxidative stress caused by pyocyanin impairs CFTR Cl(-) transport in human bronchial epithelial cells. Free Radic Biol Med. 2008;45(12):1653–62. doi: 10.1016/j.freeradbiomed.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada B, Lekstrom K, Damian S, Dupuy C, Leto TL. The Pseudomonas toxin pyocyanin inhibits the dual oxidasebased antimicrobial system as it imposes oxidative stress on airway epithelial cells. J Immunol. 2008;181(7):4883–93. doi: 10.4049/jimmunol.181.7.4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witko-Sarsat V, Delacourt C, Rabier D, Bardet J, Nguyen AT, Descamps-Latscha B. Neutrophil-derived long-lived oxidants in cystic fibrosis sputum. Am J Respir Crit Care Med. 1995;152(6 Pt 1):1910–6. doi: 10.1164/ajrccm.152.6.8520754. [DOI] [PubMed] [Google Scholar]
- Tirouvanziam R, Conrad CK, Bottiglieri T, Herzenberg LA, Moss RB, Herzenberg LA. High-dose oral N-acetylcysteine, a glutathione prodrug, modulates inflammation in cystic fibrosis. Proc Natl Acad Sci USA. 2006;103(12):4628–33. doi: 10.1073/pnas.0511304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauletbaev N, Viel K, Buhl R, Wagner TO, Bargon J. Glutathione and glutathione peroxidase in sputum samples of adult patients with cystic fibrosis. J Cyst Fibros. 2004;3(2):119–24. doi: 10.1016/j.jcf.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Dauletbaev N, Rickmann J, Viel K, Buhl R, Wagner TO, Bargon J. Glutathione in induced sputum of healthy individuals and patients with asthma. Thorax. 2001;56(1):13–8. doi: 10.1136/thorax.56.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roum JH, Buhl R, McElvaney NG, Borok Z, Crystal RG. Systemic deficiency of glutathione in cystic fibrosis. J Appl Physiol. 1993;75(6):2419–24. doi: 10.1152/jappl.1993.75.6.2419. [DOI] [PubMed] [Google Scholar]
- Kogan I, Ramjeesingh M, Li C. et al. CFTR directly mediates nucleotide-regulated glutathione flux. EMBO J. 2003;22(9):1981–9. doi: 10.1093/emboj/cdg194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Kim KJ, Yankaskas JR, Forman HJ. Abnormal glutathione transport in cystic fibrosis airway epithelia. Am J Physiol. 1999;277(1 Pt 1):L113–8. doi: 10.1152/ajplung.1999.277.1.L113. [DOI] [PubMed] [Google Scholar]
- Linsdell P, Hanrahan JW. Glutathione permeability of CFTR. Am J Physiol. 1998;275(1 Pt 1):C323–6. doi: 10.1152/ajpcell.1998.275.1.C323. [DOI] [PubMed] [Google Scholar]
- Terheggen-Lagro SW, Rijkers GT, van der Ent CK. The role of airway epithelium and blood neutrophils in the inflammatory response in cystic fibrosis. J Cyst Fibros. 2005;4(Suppl 2):15–23. doi: 10.1016/j.jcf.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Jacquot J, Tabary O, Le RP, Clement A. Airway epithelial cell inflammatory signalling in cystic fibrosis. Int J Biochem Cell Biol. 2008;40(9):1703–15. doi: 10.1016/j.biocel.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Ghibelli L, Coppola S, Rotilio G, Lafavia E, Maresca V, Ciriolo MR. Non-oxidative loss of glutathione in apoptosis via GSH extrusion. Biochem Biophys Res Commun. 1995;216(1):313–20. doi: 10.1006/bbrc.1995.2626. [DOI] [PubMed] [Google Scholar]
- van den Dobbelsteen DJ, Nobel CS, Schlegel J, Cotgreave IA, Orrenius S, Slater AF. Rapid and specific efflux of reduced glutathione during apoptosis induced by antiFas/APO-1 antibody. J Biol Chem. 1996;271(1):15420–7. doi: 10.1074/jbc.271.26.15420. [DOI] [PubMed] [Google Scholar]
- Hammond CL, Madejczyk MS, Ballatori N. Activation of plasma membrane reduced glutathione transport in death receptor apoptosis of HepG2 cells. Toxicol Appl Pharmacol. 2004;195(1):12–22. doi: 10.1016/j.taap.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Hammond CL, Marchan R, Krance SM, Ballatori N. Glutathione export during apoptosis requires functional multidrug resistance-associated proteins. J Biol Chem. 2007;282(19):14337–47. doi: 10.1074/jbc.M611019200. [DOI] [PubMed] [Google Scholar]
- Marchan R, Hammond CL, Ballatori N. Multidrug resistance-associated protein 1 as a major mediator of basal and apoptotic glutathione release. Biochim Biophys Acta. 2008;1778(10):2413–20. doi: 10.1016/j.bbamem.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]


