Abstract
Gastrointestinal stromal tumors (GIST) are mesenchymal tumors that in the past were classified as leiomyosarcomas or leiomyomas not responding to standard sarcoma chemotherapy. In several phase I and II trials the efficacy and safety of imatinib was shown before the largest trial ever performed in a single sarcoma entity revealed response rates (CR/PR) of 52%. This multicenter phase II trial presented here was performed to open access to imatinib for patients with unresectable or metastastatic GIST when the EORTC 62005 trial had been closed before imatinib was approved in Germany. It was designed to follow the best clinical response and to assess the efficacy, safety and tolerability of imatinib 400 mg/d in patients with unresectable or metastatic gastrointestinal stromal tumor.
95 patients were treated in this trial with Imatinib 400 mg/d. Four patients (4.6%) attained a complete response and 26 patients (29.9%) a partial response to imatinib treatment. Forty-one patients (47.1%) revealed a stable disease and 16 patients (18.4%) had a progressive disease.
Of the progressive patients 22% showed a partial response and 67% showed stable disease after escalating the dose to 800 mg. According to SWOG tumor response classification, 66 patients (70%) were free of progression within the first year of treatment. Seventy-one patients (74.7%) experienced adverse events or severe adverse events with a suspected relationship to the study drug. Among these, the most common were nausea (n = 27 patients, 28.4%), eyelid edema and peripheral edema in 23 patients each (24.2%), diarrhea in 20 patients (21.1%), muscle cramps in 15 patients (15.8%) and fatigue in 13 patients (13.7%).
Imatinib 400 mg/d led to disease stabilisation in 81,6% of patients with unresectable or metastatic malignant GIST. Thirty-four percent of patients attained a tumor remission (partial or complete response). The safety profile of imatinib based on adverse event assessment is favorable. Imatinib is generally well tolerated in patients with gastrointestinal stromal tumors.
Introduction
Gastrointestinal stromal tumors (GIST) are mesenchymal tumors that in the past were classified as leiomyosarcomas or leiomyomas not responding to standard sarcoma chemotherapy. Recently, GIST were shown to share striking immunohistochemical similarities with interstitial cells of Cajal which are responsible for intestinal motility. In addition, gain of function mutations of the KIT-receptor were found to be an early and pre-eminent oncogenic event in the vast majority of GIST. This has led to therapy with imatinib mesylate, a highly selectively inhibitor of the protein tyrosine kinase family comprising ABL, the chimeric BCR-ABL, the platelet-derived growth factor (PDGF) receptor α and β, and the product of the C-KIT proto-oncogene (KIT) [1-3]. In several phase I and II trials the efficacy and safety of imatinib was shown [4-7] before the largest trial ever performed in a single sarcoma entity revealed response rates (CR/PR) of 52% [8]. This multicenter phase II trial presented here was performed to open access to imatinib for patients with unresectable or metastastatic GIST when the EORTC 62005 trial had been closed before imatinib was approved in Germany.
Patients and Methods
This multicenter open label clinical trial was designed to follow the best clinical response and to assess the efficacy, safety and tolerability of imatinib 400 mg/d in patients with unresectable or metastatic gastrointestinal stromal tumor. Imatinib dose could be increased to 600 mg/d and then 800 mg/d if the patient did not respond. For this purpose, 13 centers were recruited in Germany that actively contributed to the enrollment and assessment of study patients. In each center the trial was approved by the local ethics committee.
Patients had to be 18 years of age with documented histological diagnosis of unresectable or metastatic GIST expressing C-KIT (CD117). Lesions had to be measurable as defined by Southwestern Oncology Group Solid Tumor Response Criteria. Analysis of the efficacy analyzable population was considered primary for this evaluation, using re-calculated tumor surfaces. Patients were allowed to have received previous chemotherapy at least four weeks before study entry. A performance status of 0-3 (ECOG) and adequate total bilirubin < 1.5 × ULN, SGOT and SGPT < 2.5 × UNL (or < 5 × ULN if hepatic metastases were present), creatinine < 1.5 × ULN, absolute neutrophil count > 1.5 × 109/L and platelets > 100 × 109/L were required. the protocol was performed in accordance with the Declaration of Helsinki. All patients gave written informed consent. Baseline assessment of the primary status according to the SWOG response criteria was scheduled within 14 days of first dose of study drug. Patients received physical examinations, evaluation of performance status and measurements of body weight, complete blood count and serum chemistry regularly to monitor potential side effects of imatinib accurately. Tumor response was measured by CT or MRI on days 1, 29, 85, 169, and 351 in relation to baseline.
Statistics
Frequency analyses are provided for the categories of the overall best tumor response according to the definition of the SWOG (primary endpoint). Confidence intervals (two-sided, 95%, Pearson-Copper-limits) were calculated for the rate of responders. In addition to responder analysis, a benefit analysis was performed which included also patients with stable disease defined as overall tumor response. Survival plots for progression free survival and overall survival (including 95% confidence intervals (CI) were calculated according to Kaplan-Meier-method (secondary endpoints).
Results
A total of 95 patients were treated in this multi-center trial between October 2001 (first patient enrolled) and June 2003 (last patient completed). Patients' characteristics are summarized in Table 1. Four patients were judged to be ineligible for evaluation due to major protocol violations, e.g. age < 18 years, no measurable disease as well as two patients who were allocated to surgical resection due to resectable tumor lesions. Recruitment per study site varied between 1 patient and 22 patients. Total study duration was 1.6 years (589 days or 19.4 months). Mean age was 59 years, ranging from 18 to 80 years; 56 patients were male (59%). Mean time from initial diagnosis to start of therapy was 17.4 months and in more than half of the patients (62%), the index disease was recurrent or progressive. The tumor stage diagnosed in most patients (41%) was stage IV. The majority of patients (n = 76, 80%) had an ECOG performance status of grade 0 or 1. Besides gastrointestinal disorders (n = 19 patients, 20%), frequent current medical conditions at time of start of trial were vascular disorders (n = 26 patients, 27%), cardiac disorders (n = 20 patients, 22%), metabolic and nutrition disorders (n = 18 patients, 19%) and psychiatric disorders (n = 16 patients, 17%).
Table 1.
Patients Characteristics (n = 95).
| Age (yrs) | |
|---|---|
| Median | 59 |
| Range | 18-80 |
| Sex (No) | |
| Male | 56 |
| Female | 39 |
| ECOG (No) | |
| Grade 0 | 36 |
| Grade 1 | 40 |
| Grade 2 | 17 |
| Grade 3 | 2 |
| Time since initial diagnosis (days) | |
| Median | 180 |
| Range | 0-6141 |
| Disease Status (%) | |
| Recurrence/Progression | 62 |
| Site of tumor (%) | |
| Stomach | 33 |
| Liver | 28 |
| Small intestine | 23 |
| Peritoneum | 5 |
| Rectum | 5 |
| Colon | 4 |
| Esophagus | 1 |
| Pancreas | 1 |
A dose of 400 mg/day of imatinib was orally applied to 89 patients (94%). Furthermore, 8 patients (8%) were treated with less than 400 mg/d, 9 patients (10%) took more than 400 mg/d, and 1 patient had both dose reduction or interruption and dose escalation, each regimen for at least 4 weeks. No patient took doses higher than 800 mg/d. Treatment was interrupted in 20 patients (22%) for an average duration of 15.1 ± 19.0 days (median 6 days, range 1-77 days). Dose escalations were performed in 9 patients (10%). None of these patients achieved a complete response. But of the patients being progressive 22% showed a partial response and 67% showed stable disease after escalating the dose to 800 mg. According to SWOG tumor response classification, 66 patients (70%) were free of progression within the first year of treatment. Mean time to progression was 302 days (9.9 months).
The primary endpoint of the trial was overall response rate (ORR) based on the SWOG solid tumor response criteria. With respect to overall response, 81.6% (71 patients) had at least a disease stabilisation. Four patients (4.6%) attained a complete and 26 patients (29.9%) a partial response to imatinib treatment. Forty-one patients (47.1%) revealed a stable disease and 16 patients (18.4%) had a progressive disease (Table 2).
Table 2.
Response to imatinib: (n = 87; n = 8 not assessable).
| N pts (%) | ||
|---|---|---|
| Complete Response | 4 | (4.6) |
| Partial Response | 26 | (29.9) |
| Stable disease | 41 | (47.1) |
| Progressive disease | 16 | (18.4) |
Safety Assessment
A total of 84 patients (88.4%) experienced at least a single adverse event (AE), and in 74% of patients the AE was judged as drug-related. The most common adverse events were diarrhea (29% of patients), nausea (27%), eyelid edema (23%), peripheral edema (22%), muscle cramps (15%) and fatigue (13%). The majority of these side effects were mild to moderate. The medications most frequently applied for relief were furosemide (24 patients), metamizole sodium (21 patients), metoclopramide (18 patients), allopurinol (16 patients), omeprazole (14 patients) as well as potassium chloride and actylsalicyclic acid (12 patients each).
Regardless of its relationship to the study drug applied, adverse events were severe in 25 patients (26.3%) and life-threatening in 7 patients (7.4%); in 10 of these patients (10.5%), severe adverse events were considered to be related to the study medication. For none of the life-threatening events such a relationship was reported.
Seventy-one patients (74.7%) experienced adverse events or severe adverse events with a suspected relationship to study drug. Among these, the most common were nausea (n = 27 patients, 28.4%), eyelid edema and peripheral edema in 23 patients each (24.2%), diarrhea in 20 patients (21.1%), muscle cramps in 15 patients (15.8%) and fatigue in 13 patients (13.7%). Of the adverse events with suspected relationship to the study drug, 10 patients (10.5%) experienced severe adverse events but none was life-threatening. Anemia as an adverse event was found in 16 patients (16.8%), in a single case grade III due to bleeding. In 11 patients (11.6%), dose was escalated to 600 or 800 mg/d for at least 4 weeks and in 17 patients (17.9%) for at least 1 day.
Regarding the time since initial diagnosis of GIST, the following events were seen more frequently in patients with relatively longer presence of illness compared to a shorter duration: fatigue (n = 8 vs. n = 5 patients), face edema, conjunctivitis, elevation of lactate dehydrogenase (LDH) (n = 4 vs. n = 2 patients each). It should; however, be noted that subgroups were formed by median split, and the subgroups do not differ considerably.
Suspected unexpected non-serious adverse reactions considered worth mentioning (as recorded in > 3% of patients) were increase in blood urea, decrease in hemoglobin, and leucocytopenia. Neutropenia was not recorded at all. No suspected expected serious adverse reactions (SUSARs) were reported in this trial. Ten patients (10.5%) with normal platelets at baseline shifted outside the normal range at end of treatment, and 16 patients (16.8%) shifted above the leukocyte upper limit of normal. Total protein was decreased in 19 patients (20.0%), and alkaline phosphatase was increased in 11 patients (11.6%). No patient in our trial suffered from clinical cardiotoxicity, as reported elsewere [10]. Our findings support, that imatinib does not induce cardiac toxicity even though we did not monitor routineously cardiac function[11].
NCI/NHI Common Toxicity Criteria (CTC) were applied to all laboratory parameters, and shifts across toxicity grades were evaluated in worst case analysis. Shifts from baseline to end of treatment by at least 2 toxicity grades were seen in the following hematological parameters: from grade 0 to grade 3 in lymphocytes count (n = 3 patients, 3.2%); shifts from grade 1 to grade 3 in hemoglobin (n = 2, 2.1%) and lymphocytes count (n = 1, 1.1%); shifts from grade 0 to grade 2 in lymphocytes count (n = 5, 5.3%) and hemoglobin (n = 3, 3.2%). No increase in hematological toxicity was seen in basophils, eosinophils, and monocytes. (see Table 4)
Table 4.
Number (%) of patients with non-hematological adverse events by severity and relationship to study drug.
| N = 95 | Grade 1/2 | Grade 3/4 | ||
|---|---|---|---|---|
| Preferred term | total | related | Total | related |
| nausea | 34(35.8) | 26(27.4) | 2(2.1) | 1(1.1) |
| oedema | 8(8.4) | 8(8.4) | 2(2.1) | 1(1.1) |
| oedema peripheral | 29(30.5) | 23(24.1) | -- | -- |
| oedema genital | 1(1.1) | 1(1.1) | -- | |
| Eyelid oedema | 23(24.2) | 23(24.2) | -- | -- |
| Face oedema | 6(6.3) | 6(6.3) | -- | -- |
| Periorbital edema | 8(8.4) | 8(8.4) | -- | -- |
| Vomiting | 12(12.6) | 8(8.4) | 5(5.3) | 1(1.1) |
| Muscle cramp | 17(17.9) | 15(15.8) | -- | -- |
| Muscle spasticity | 2(2.1) | 1(1.1) | -- | -- |
| Diarrhea | 25(26.3) | 19(20.0) | 1(1.1) | 1(1.1) |
| Dermatitis allergic | 1(1.1) | 1(1.1) | -- | -- |
| Rash | 2(2.1) | 1(1.1) | -- | -- |
| Headache | 8(8.4) | 2(2.1) | 1(1.1) | 1(1.1) |
| Abdominal pain | 24(25.3) | 4(4.2) | 3(3.2) | -- |
| Arthralgia | 9(9.5) | 2(2.1) | 1(1.1) | -- |
Shifts from baseline to end of treatment by at least 2 toxicity grades were seen in the following non-hematological parameters: from grade 0 to grade 3 in creatinine (n = 1, 1.1%) and bilirubin (n = 1, 1.1%); shifts from grade 1 to grade 3 in albumin (n = 1, 1.1%) and alkaline phosphatase (n = 1, 1.1%); shifts from grade 0 to grade 2 in bilirubin (n = 3, 3.2%) and albumin (n = 2, 2.1%). No increase in non-hematological toxicity was observed in blood urea nitrogen, total protein, or lactate dehydrogenase (Table 3.2).
Table 3.
Number (%) of patients with adverse events, by body system and preferred term, by relation to study drug.
| Table 3.1. Number (%) of patients with adverse events, by body system and preferred term, by relation to study drug. | |||
|---|---|---|---|
| Imatinib mesylate | |||
| N = 95 | not related | related | total |
| Gastrointestinal disorders | 67(70.5) | ||
| Nausea | 9(9.5) | 27(28.4) | 36(37.9) |
| abdominal pain | 23(24.2) | 4 (4.2)27(28.4) | |
| Diarrhea | 6(6.3) | 20(21.1) | 26(27.4) |
| Vomiting | 8(8.4) | 9(9.5) | 17(17.9) |
| constipation | 10(10.5) | 4(4.2) | 14(14.7) |
| Flatulence | 5(5.3) | 6(6.3) | 11(11.6) |
| abdominal discomfort | 8(8.4) | 2(2.1) | 10(10.5) |
| Dyspepsia | 2(2.1) | 3(3.2) | 5(5.3) |
| abdominal pain | 2(2.1) | 2(2.1) | 4(4.2) |
| Subileus | 4(4.2) | -- | 4(4.2) |
| Melaena | 3(3.2) | -- | 3(3.2) |
| Stomatitis | 3(3.2) | -- | 3(3.2) |
| General disorders | 54(56.8) | ||
| oedema peripheral | 6(6.3) | 23(24.2) | 29(30.5) |
| fatigue | 9(9.5) | 13(13.7) | 22(23.2) |
| oedema | 1(1.1) | 9(9.5) | 10(10.5) |
| pain | 7(7.4) | 2(2.1) | 9(9.5) |
| asthenia | 3(3.2) | 6(6.3) | 9(9.5) |
| fever | 6(6.3) | 1(1.1) | 7(7.4) |
| chest pain | 33.2) | -- | 3(3.2) |
| Musculoskeletal and connective tissue disorders | 35(36.8) | ||
| muscle cramp | 2(2.1) | 15(15.8) | 17(17.9) |
| arthralgia | 8(8.4) | 2(2.1) | 10(10.5) |
| pain (extremities) | 6(6.3) | -- | 6(6.2) |
| back pain | 4(4.2) | 1(1.1) | 5(5.3) |
| myalgia | 1(1.1) | 3(3.2) | 4(4.2) |
| Skin and subcutaneous tissue disorders | 34(35.8) | ||
| periorbital oedema | -- | 8(8.4) | 8(8.4) |
| exanthema | 1(1.1) | 6(6.3) | 7(7.4) |
| face oedema | -- | 6(6.3) | 6(6.3) |
| hyperhidrosis | 3(3.2) | 2(2.1) | 5(5.3) |
| alopecia | 1(1.1) | 2(2.1) | 3(3.2) |
| dry skin | -- | 3(3.2) | 3(3.2) |
| erythema | -- | 3(3.2) | 3(3.2) |
| night sweats | 2(2.2) | 1(1.1) | 3(3.2) |
| pruritus | -- | 3(3.2) | 3(3.2) |
| scar pain | 3(3.2) | -- | 3(3.2) |
| Table 3.2. Number (%) of patients with adverse events, by body system and preferred term, by relation to study drug. | |||
| N = 95 | Imatinib mesylate | ||
| not related | related | total | |
| Eye disorders | 27(28.4) | ||
| eyelid oedema | -- | 23(24.2) | 23(24.2) |
| conjunctivitis | -- | 6(6.3) | 6(6.3) |
| lacrimation increased | -- | 3(3.2) | 3(3.2) |
| Nervous system disorders | 23(24.1) | ||
| dizziness | 3(3.2) | 8(8.4) | 11(11.6) |
| headache | 6(6.3) | 3(3.2) | 9(9.5) |
| paraesthesia | -- | 3(3.2) | 3(3.2) |
| Blood and lymphatic system disorders | 21(22.1) | ||
| anaemia | 7(7.4) | 9(9.5) | 16(16.8) |
| leukopenia | -- | 4(4.2) | 4(4.2) |
| Psychiatric disorders | 19(20.0) | ||
| insomnia | 8(8.4) | 1(1.1) | 9(9.5) |
| anxiety | 5(5.3) | -- | 5(5.3) |
| depression | 3(3.2) | 2(2.1) | 5(5.3) |
| sleep disorder | 2(2.1) | 2(2.1) | 4(4.2) |
| Metabolism and nutrition disorders | 18(19.0) | ||
| anorexia | 7(7.4) | 4(4.2) | 11(11.6) |
| hypokalaemia | 6(6.3) | 1(1.1) | 7(7.4) |
| hyperuricaemia | 3(3.2) | -- | 3(3.2) |
| Infections | 18(19.0) | ||
| nasopharyngitis | 6(6.3) | -- | 6(6.3) |
| urinary tract infection | 3(3.2) | -- | 3(3.2) |
| Respiratory, thoracic and mediastinal disorders | 12(12.6) | ||
| cough | 4(4.2) | -- | 4(4.2) |
| pleural effusion | 3(3.2) | -- | 3(3.2) |
| Vascular disorders | 9(9.5) | ||
| hypertension | 4(4.2) | -- | 4(4.2) |
| Laboratory findings | 37(39.0) | ||
| blood lactate dehydrogenase increased | 6(6.3) | 6(6.3) | 12(12.6) |
| blood bililrubin increased | 2(2.1) | 8(8.4) | 10(10.5) |
| blood alkaline phosphatase increased | 5(5.3) | 4(4.2) | 9(9.5) |
| blood creatinine increased | 2(2.1) | 6(6.3) | 8(8.4) |
| blood urea increased | 3(3.2) | 4(4.2) | 7(7.4) |
| alanine aminotransferase (ALT) increased | 2(2.1) | 4(4.2) | 6(6.3) |
| aspartate aminotransferase (AST) increased | 2(2.1) | 4(4.2) | 6(6.3) |
| blood uric acid increased | 6(6.3) | -- | 66.3) |
| haemoglobin decreased | 1(1.1) | 4(4.2) | 5(5.3) |
| hepatic enzyme increased | 1(1.1) | 3(3.2) | 4(4.2) |
Worst case analysis of patients with normal vital signs at baseline and abnormal findings at the most unfavorable post-baseline visit revealed abnormally increased systolic blood pressure and BMI, each in 12 patients (12.6%).
Discussion
The standard treatment of unresectable or metastatic gastrointestinal stromal tumors is imatinib (Glivec) at a dose of 400 mg/d, since several studies have shown a disease control rate (CR, PR, SD) of approximately 75% for this agent [12-14]. Our trial showed a comparable rate of 81% and a comparable CR rate of 4%. CRs have been mainly observed in younger patients not older than 58 years, but these patients did not differ regarding other characteristics e.g. initial tumor stage or time since initial diagnosis. The rate of partial response (29%) was lower in our study than previously reported. One reason might be that the majority (62%) of our patients suffered from recurrent or progressive disease.
Subgroup analysis of patients with partial response including the performance status, time since initial diagnosis and dose regimen (dose stable vs. escalated) showed that none of these variables made a difference concerning disease control rate.
The present data demonstrated that patients with good performance status (ECOG 1 = 39%, ECOG 0 = 38%), were more likely to respond to therapy with imatinib (Glivec). This is in accordance with prognostic data published by the EORTC STBSG [9].
Seventy-five patients (86.2%) survived the first year of treatment. Exploratory analysis suggests that patients with good performance status at baseline were more likely to survive, both within the first year and thereafter. According to tumor response, 66 patients (75.8%) were free of progression throughout the trial, until end of treatment, and 62 of these (71.2%) were progression-free within the first year of treatment. Our analysis suggests, that patients with good performance status at baseline were more likely to attain a sustained response to imatinib, which is translated to a longer period of freedom of progression.
During the present trial side effects were meticulously screened, as experiences with imatinib mesylate were limited. Treatment with an oral drug in patients with abdominal tumors, most of them after major surgery, caused gastrointestinal side effects in 70% of patients. 45% of our patients suffered from gastrointestinal side effects related to imatinib and 25% had gastrointestinal problems likely not to be related to the drug (see Table 3). Nausea, vomiting and diarrhea were among the common gastrointestinal adverse events. Nausea could be improved by taking imatinib during meals and together with 500 ml water. In case of ineffectiveness metoclopramide was prescribed. Loperamide is a reasonable option to treat imatinib-related diarrhea. Commonly observed were periorbital edema, peripheral edema in the lower legs, muscle cramps and fatigue. By monitoring body weight closely and introducing diuretics and fluid retention these side effects could be limited. Muscle cramps responded in some cases to calcium and magnesium supplemention. Overall, drug-related side effects were severe in a small proportion of patients ( < 2%) except for vomiting and abdominal pain both occurring in 5.3% and 3.2% of the study population. No life-threatening events have been observed. In more than 95%, side effects due to imatinib could be managed with standard concomitant drugs, such as furosemide, metoclopamide, metamizole and omeprazole. After one year of treatment, in 50 patients (54.9%) the performance status had been improved or at least maintained compared to baseline assessment. During treatment with imatinib, 81,6% of patients with unresectable or metastatic malignant GIST revealed at least disease stabilisation. Thirty-four percent of patients attained a tumor remission (partial or complete response). The safety profile of imatinib based on adverse event assessment is favorable. Imatinib is generally well tolerated in patients with gastrointestinal stromal tumors. Patients with good performance status tolerate the imatinib even better. The recognition of side effects is mandatory to further develop an appropriate therapeutic management to relief the drug-related symptoms. This is particularly important at doses above 400 mg/d since it became evident that subgroups of patients benefit from a higher initial starting dose of imatinib in the first-line setting and that patients with progressive disease during standard dose should be escalated [15].
Figure 1.
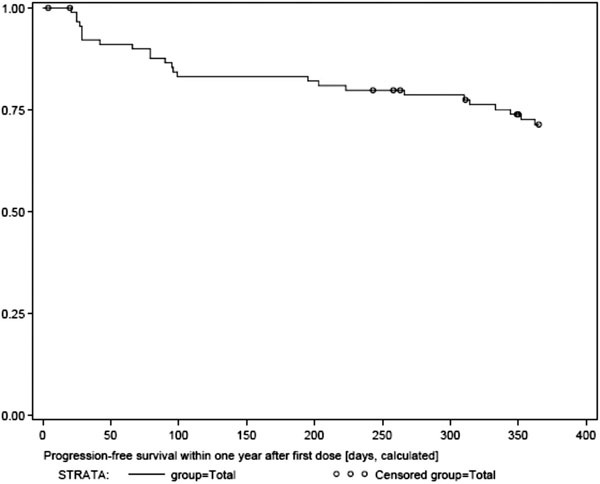
Progression free surival.
Figure 2.
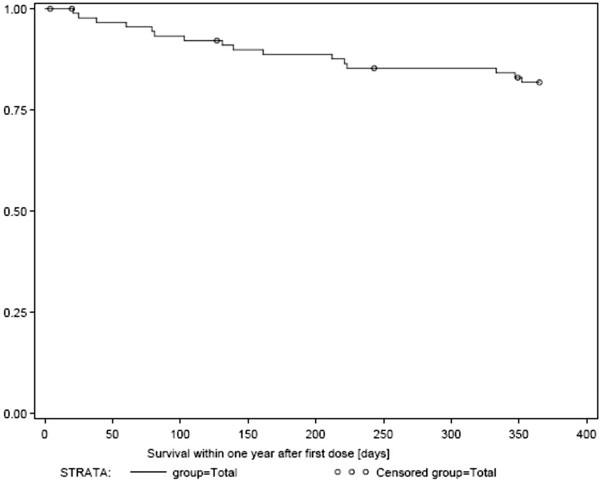
Overall surival.
References
- Druker BJ, Tamura S, Buchdunger E. et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med. 1996;2(5):561–6. doi: 10.1038/nm0596-561. [DOI] [PubMed] [Google Scholar]
- Buchdunger E, Cioffi CL, Law N. et al. Abl protein-tyrosine kinase inhibitor STI571 inhibits in vitro signal transduction mediated by c-kit and platelet-derived growth factor receptors. J Pharmacol Exp Ther. 2000;295(1):139–45. [PubMed] [Google Scholar]
- Heinrich MC, Griffith DJ, Druker BJ. et al. Inhibition of c-kit receptor tyrosine kinase activity by STI 571, a selective tyrosine kinase inhibitor. Blood. 2000;96(3):925–32. [PubMed] [Google Scholar]
- van Oosterom AT, Judson I, Verweij J. et al. Safety and efficacy of imatinib (STI571) in metastatic gastrointestinal stromal tumours: a phase I study. Lancet. 2001;358(9291):1421–3. doi: 10.1016/S0140-6736(01)06535-7. [DOI] [PubMed] [Google Scholar]
- van Oosterom AT, Judson I, Verweij J, Update of phase I study of imatinib (STI571) in advanced soft tissue sarcomas and gastrointestinal stromal tumors: a report of the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer. pp. S83–7. [DOI] [PubMed]
- Verweij J, van Oosterom A, Blay JY. et al. Imatinib mesylate (STI-571 Glivec, Gleevec) is an active agent for gastrointestinal stromal tumours, but does not yield responses in other soft-tissue sarcomas that are unselected for a molecular target. Results from an EORTC Soft Tissue and Bone Sarcoma Group phase II study. Eur J Cancer. 2003;39(14):2006–11. doi: 10.1016/S0959-8049(02)00836-5. [DOI] [PubMed] [Google Scholar]
- Demetri GD, von Mehren M, Blanke CD. et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347(7):472–80. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- Verweij J, Casali PG, Zalcberg J. et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet. 2004;364(9440):1127–34. doi: 10.1016/S0140-6736(04)17098-0. [DOI] [PubMed] [Google Scholar]
- Van Glabbeke M, Verweij J, Casali PG. et al. Initial and late resistance to imatinib in advanced gastrointestinal stromal tumors are predicted by different prognostic factors: a European Organisation for Research and Treatment of Cancer-Italian Sarcoma Group-Australasian Gastrointestinal Trials Group study. J Clin Oncol. 2005;23(24):5795–804. doi: 10.1200/JCO.2005.11.601. [DOI] [PubMed] [Google Scholar]
- Kerkela R, Grazette L, Yacobi R. et al. Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat Med. 2006;12(8):908–61. doi: 10.1038/nm1446. [DOI] [PubMed] [Google Scholar]
- Perik PJ, Rikhof B, de Jong FA. et al. Results of plasma N-terminal pro B-type natriuretic peptide and cardiac troponin monitoring in GIST patients do not support the existence of imatinib-induced cardiotoxicity. Ann Oncol. 2008;19(2):359–61. doi: 10.1093/annonc/mdm468. [DOI] [PubMed] [Google Scholar]
- Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, Heinrich MC, Tuveson DA, Singer S, Janicek M, Fletcher JA, Silverman SG, Silberman SL, Capdeville R, Kiese B, Peng B, Dimitrijevic S, Druker BJ, Corless C, Fletcher CD, Joensuu H. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347(7):472–8. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- Verweij J, Casali PG, Zalcberg J, LeCesne A, Reichardt P, Blay JY, Issels R, van Oosterom A, Hogendoorn PC, Van Glabbeke M, Bertulli R, Judson I. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet. 2004;364(9440):1127–34. doi: 10.1016/S0140-6736(04)17098-0. [DOI] [PubMed] [Google Scholar]
- Blanke CD, Rankin C, Demetri GD, Ryan CW, von Mehren M, Benjamin RS, Raymond AK, Bramwell VH, Baker LH, Maki RG, Tanaka M, Hecht JR, Heinrich MC, Fletcher CD, Crowley JJ, Borden EC. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 2008;26(4):626–32. doi: 10.1200/JCO.2007.13.4452. [DOI] [PubMed] [Google Scholar]
- Debiec-Rychter M, Sciot R, Le Cesne A, Schlemmer M, Hohenberger P, van Oosterom AT, Blay JY, Leyvraz S, Stul M, Casali PG, Zalcberg J, Verweij J, Van Glabbeke M, Hagemeijer A, Judson I. EORTC Soft Tissue and Bone Sarcoma Group; The Italian Sarcoma Group; Australasian Gastrointestinal Trials Group. KIT mutations and dose selection for imatinib in patients with advanced gastrointestinal stromal tumours. Eur J Cancer. 2006;42(8):1093–103. doi: 10.1016/j.ejca.2006.01.030. [DOI] [PubMed] [Google Scholar]


