Almost 30 years ago, virologists working with RNA tumor viruses (now called retroviruses) were faced with the dilemma of determining the nature of the RNA tumor viruses detected by electron microscopy in the tissues of the New Zealand Black (NZB) mouse. This widely studied murine strain, derived in the 1950s by Marianne Bielschowsky (1), develops autoimmune disorders and B cell lymphomas. Because similar RNA tumor viruses [also known as murine leukemia viruses (MLVs)] were associated with leukemias and lymphomas in many animal species, characterizing the NZB virus offered a clue to the potential causative agent for NZB disease. However, all attempts to grow the NZB virus by standard procedures in cultured mouse cells failed; the virus was considered “replication-defective”.
Working with knowledge gained from pioneers in the RNA tumor virus field (2), the biologic activity of this NZB virus ultimately was demonstrated in 1970 by cultivating NZB mouse embryo cells with hamster or rat tumor cells containing a nonreplicating sequence of the murine sarcoma virus (3). This procedure, using conventional MLV, leads to the rescue of the sarcoma virus genome in the envelope coat of the replication-competent MLV (2). The newly formed transforming virus pseudotype now has the host range of the “helper” MLV. Supernatants from the NZB cell coculture demonstrated the presence of a replicating virus that could induce foci of transformation in rat cells but not mouse embryo cells (3). The NZB MLV host range thus seemed limited to rat cells. Subsequent work demonstrated that the helper MLV visualized in the NZB mouse cells was infectious but was not infectious at all for mouse cells (i.e., complete block). It was infectious only for cells from heterologous species, including those of avian origin (Table 1). Similar viruses were subsequently found in all laboratory strains of Mus musculus domesticus as well as some wild house mice captured in the United States and other parts of the world (4, 5).
Table 1.
Representative cellular host range of xenotropic MLV
| Good | Moderate to low | Resistant |
|---|---|---|
| Bear | Anteater | Mouse |
| Cat | Armadillo | Syrian golden hamster |
| Chimpanzee | Bat | |
| Cow | Chinese hamster | Chicken |
| Dog | Dog | |
| Gorilla | Dolphin | Snake |
| Guinea pig | Deer | Salmon |
| Horse | Gazelle | Goldfish |
| Human | Goat | |
| Lion | Pig | |
| Marmoset | Raccoon | |
| Mink | Rhesus monkey | |
| Orangutan | Sheep | |
| Ring-necked pheasant | Parakeet | |
| Duck | Pigeon | |
| Quail | ||
| Turkey |
Virus replication abilities are summarized from Ref. 5. The differences between good and moderate/low are 10- to 1,000-fold.
The term “xenotropic” (X-tropic) was coined (from the Greek xenos, meaning “foreign,” and tropos, meaning “turning”) for this mouse virus (4, 5). It was distinguished from the already well known MLV that could induce leukemias and lymphomas in mice, termed “ecotropic” (E-tropic; from the Greek oikos, meaning “home”) for their mouse cell tropism (5). A few years later, other MLV were found in wild mice that could infect both mouse cells and cells from heterologous species. They were called “amphotropic” (A-tropic; from the Greek amphos, meaning “both”) to designate this dual-tropism (5–7) (Table 2). Unlike the X-tropic and E-tropic MLV, which are inherited in the mouse genome, A-tropic viruses are acquired by exogenous transmission. The lack of infection of mouse cells by X-tropic MLV was shown to involve an early step in virus entry, most probably the absence of a cell surface molecule required for virus attachment; pseudotype viruses carrying a mouse-tropic envelope protein could permit X-tropic MLV infection of mouse cells with subsequent virus replication (5, 8). Each of these three MLV classes has distinct envelope antigens and appears to use different cellular receptors for entry into cells (5).
Table 2.
Classes of MLV
| X-tropic | (from the Greek xenos, meaning “foreign,” and tropos, meaning “turning”) Viruses that infect and replicate efficiently only in cells from an animal species foreign to the host |
| E-tropic | (from the Greek oikos, meaning “home” or “one’s environment”) Viruses that infect and replicate efficiently in cells from their own host species |
| A-tropic | (from the Greek amphos, meaning “both”) Viruses that infect and replicate efficiently both in cells from their own host species and in cells from heterologous species |
| P-tropic* | Viruses that infect and replicate efficiently both in cells from their own host species and in cells from heterologous species |
Also known as mink cell focus-inducing virus and can be distinguished from A-tropic MLV by sequence and antigenic differences.
To complicate further this burgeoning field of murine retroviruses, mice also were found to release dual-tropic MLV that differed from A-tropic viruses. These polytropic (P-tropic) MLV (9) appeared to result from a recombination of infectious E-tropic virus with endogenous P-tropic MLV-related sequences in mouse cells (10). Many of these P-tropic viruses induced foci in cultured mink lung cells and became known as mink cell focus-forming viruses (11). These latter viruses only arise de novo; like A-tropic MLV, they are not inherited as infectious viruses in the mouse genome (10). The P-tropic MLV can be distinguished with selective neutralizing antibodies, but most genetic studies have suggested that X-tropic and P-tropic MLV share the same cell surface receptor (12–13).
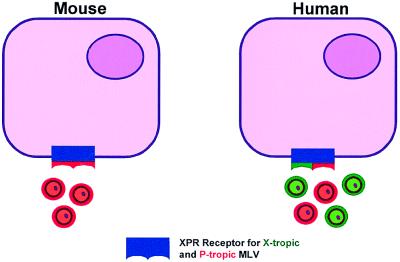
The recognition, by biologic studies, that X-tropic MLV could not infect cells of the host species but these host cells could spontaneously release these viruses supported the virogene hypothesis that viruses could be inherited through the germ cell (14). Similar viruses with an X-tropic host range soon were discovered in cats, baboons, rats, deer, and other animal species (5). The mechanism for resistance of mouse cells to infection by the inherited X-tropic mouse virus remained a long sought-after but unanswered question. The answer now seems provided through the pioneer work of three different groups of investigators in the United States (15–17). One of these groups reports their findings in this issue of the Proceedings. Using present-day molecular biology techniques, Tailor et al. (15) have used human cells to uncover the sequence of the X-tropic virus and P-tropic virus receptor (XPR) previously mapped to the distal arm of mouse chromosome 1 (12, 13, 18). Transfer of specific sequences from a human cDNA library to NIH 3T3 mouse cells allowed the selection of mouse cells susceptible to X-tropic virus entry. This same receptor was found to mediate infection of Chinese hamster ovary cells by both types of viruses. The findings by these investigators indicate that the human cell receptor can be used by both X-tropic and P-tropic viruses but suggest that the receptor counterpart in mice mediates only infection with the P-tropic MLV. The most likely explanation for these observations is that the gene responsible for this receptor is polymorphic. The allele in mouse cells only permits infection by P-tropic-MLV whereas the allele in non-mouse cells is more promiscuous and permits infection by both X-tropic and P-tropic MLV (Fig. 1) (see below).
Figure 1.
The newly recognized MLV receptor (here called XPR) appears to be polymorphic, with alleles in mouse cells that permit infection only with P-tropic MLV; the alleles for this receptor in heterologous cells (e.g., human) permit infection by both X-tropic and P-tropic MLV. In some cells (e.g., M. dunni), the XPR alleles could be heterozygous.
During this past decade, cell surface receptors for several of the murine retroviruses have been characterized. These include the cation amino acid transporter CAT-1, the receptor for E-tropic MLV (19, 20), the sodium-dependent phosphate symporter Pit-2 for both the A-tropic MLV and the 10A1 MLV (21, 22), and the related Pit-1 receptor for another group of murine retroviruses, 10A1 (23, 24) (Table 3). The latter group represents a recombinant virus involving the A-tropic virus genome (25). The nucleotide sequence for the newly discovered MLV receptor (XPR) encodes a protein of 696 amino acids containing several hydrophobic domains, suggesting that XPR transverses the cell membrane multiple times (15–17). Perhaps it is not surprising that preliminary observations suggest that XPR is also a phosphate transporter (15). Moreover, because it shows some sequence similarity to the yeast SYG-1 protein involved in G protein coupled signaling (26), XPR might participate in this type of cellular process (16).
Table 3.
Cell surface receptors for MLV
| MLV Class | Receptor | Function |
|---|---|---|
| E-tropic | CAT-1 | Cationic amino acid transporter |
| A-tropic, 10A-1 | Pit-2 | Sodium-dependent phosphate transporter |
| 10A-1 | Pit-1 | Sodium phosphate symporter |
| X-tropic, P-tropic | XPR* | Phosphate transporter (?) |
Not formally designated.
Another approach used for demonstrating the presence of different cellular receptors for viruses is interference testing. By this method, cells are infected by one virus, and the ability of these infected cells to ward off superinfection with another virus is noted. If a receptor is shared, the first virus infecting the cell will either down-modulate expression of that receptor or will cover it with viral proteins, thus preventing an interaction with the other virus. By these studies, the X-tropic and P-tropic viruses were found to consist of a single receptor group (13); infection by one blocked infection by the other. The inability of mouse cells to be infected by X-tropic viruses then could be explained by small amino acid differences in the murine counterpart to the human receptor for the X-tropic and P-tropic viruses (16). However, in certain wild mouse cells (Mus dunni), nonreciprocal interference has been observed with these two virus classes (27, 28). Thus, although unlikely, it remains possible that a separate X-tropic virus receptor exists and is absent in mouse cells. Conceivably, just as the 10A1 virus can use the A-tropic Pit-2 receptor as well as the Pit-1 receptor, the X-tropic MLV also might have its own cell surface receptor in addition to the newly identified XPR. Alternatively, M. dunni cells could have heterologous alleles for XPR.
Another fascinating aspect linked to the discovery of the X-tropic viruses is the observation that mouse sera can inactivate the ability of these viruses, as well as P-tropic viruses, to infect heterologous cells (5). Although initially believed to be caused by neutralizing antibodies (29), this antiviral activity later was found to be associated with circulating murine lipoproteins (5, 30, 31) and, specifically, with an apolipoprotein (32).
What could be the connection between X-tropic viruses and this neutralizing factor? Because X-tropic viruses were found in normal embryos and placentas as well as in other normal tissues (5), the suggestion was made that these viruses might play a role in normal development (5). This might occur through the interaction of lipoproteins with the X-tropic virus envelope protein expressed on the cell surface (5). Conceivably, the mouse apolipoprotein shares some identity with the X-tropic virus receptor and can use the binding to cell surface-expressed X-tropic MLV for some aspect of lipid metabolism. Alternatively, this lipoprotein:X-tropic virus interaction may have a regulatory role on cell function. It has been proposed that leukemagenesis in the mouse occurs by the interactions of the MLV envelope with a cell surface molecule (33). Similarly, certain physiologic processes may involve lipoproteins, X-tropic or P-tropic MLV, and the XPR receptor. It is perhaps noteworthy that the subgroup A avian sarcoma retroviruses use a low density lipoprotein receptor to infect chicken cells (34). Moreover, human lipoproteins bind to hepatitis C viruses (35).
Decidedly, the identification of the receptor for X-tropic MLV should lead to the discovery of receptors for similar host range viruses isolated from other animals (5). The recognition of this receptor should offer new directions for understanding the role of viruses inherited in mouse cells lacking a functioning receptor. Although carrying the generic term MLV, X-tropic-MLV do not cause leukemia or show any pathogenic role (5). They could, indeed, be involved in some normal physiologic process (5). The achievement of Tailor et al. (15) and the other investigators (16, 17) will now certainly encourage further studies on the biologic and virologic questions of xenotropism and should provide new directions in cell biology not previously recognized. A subject of study for many years, the discovery of the X-tropic MLV receptor greatly enlightens this exciting topic in retrovirology introduced almost three decades ago.
Footnotes
A commentary on this article begins on page 927.
References
- 1.Bielschowsky M, Helyer B J, Howie J B. Proc Univ Otago Med Sch. 1959;37:9–11. [Google Scholar]
- 2.Huebner R J, Hartley J W, Rowe W P, Lane E T, Capps W I. Proc Natl Acad Sci USA. 1966;56:1164–1169. doi: 10.1073/pnas.56.4.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levy J A, Pincus T. Science. 1970;170:326–327. doi: 10.1126/science.170.3955.326. [DOI] [PubMed] [Google Scholar]
- 4.Levy J A. Science. 1973;182:1151–1153. doi: 10.1126/science.182.4117.1151. [DOI] [PubMed] [Google Scholar]
- 5.Levy J A. Curr Top Microbiol Immunol. 1978;79:111–213. doi: 10.1007/978-3-642-66853-1_4. [DOI] [PubMed] [Google Scholar]
- 6.Rasheed S, Gardner M B, Chan E. J Virol. 1976;19:13–18. doi: 10.1128/jvi.19.1.13-18.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hartley J W, Rowe W P. J Virol. 1976;19:19–25. doi: 10.1128/jvi.19.1.19-25.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levy J A. Virology. 1977;77:797–810. doi: 10.1016/0042-6822(77)90500-1. [DOI] [PubMed] [Google Scholar]
- 9.Fischinger P J, Nomura S, Bolognesi D. Proc Natl Acad Sci USA. 1975;72:5150–5155. doi: 10.1073/pnas.72.12.5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kozak C A, Ruscetti S. In: The Retroviridae. Levy J A, editor. Vol. 1. New York: Plenum; 1992. pp. 405–481. [Google Scholar]
- 11.Hartley J W, Wolford N K, Old L J, Rowe W P. Proc Natl Acad Sci USA. 1977;74:789–792. doi: 10.1073/pnas.74.2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kozak C A. J Virol. 1983;48:300–330. doi: 10.1128/jvi.48.1.300-303.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kozak C A. J Virol. 1985;55:690–695. doi: 10.1128/jvi.55.3.690-695.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huebner R J, Todaro G J. Proc Natl Acad Sci USA. 1969;64:1087–1095. doi: 10.1073/pnas.64.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tailor C S, Nouri A, Lee C G, Kozak C, Kabat D. Proc Natl Acad Sci USA. 1999;96:927–932. doi: 10.1073/pnas.96.3.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Battini, J.-L., Rasko, J. E. J. & Miller, A. D. (1999) Proc. Natl. Acad. Sci. USA, in press.
- 17.Yang, Y., Lei, G., Xu, S., Holland, C. A., Kitamura, T., Hunter, K. & Cunningham, J. M. (1999) Nat. Genet., in press. [DOI] [PubMed]
- 18.Hunter K, Housman D, Hopkins N. Somatic Cell Mol Genet. 1991;17:169–183. doi: 10.1007/BF01232974. [DOI] [PubMed] [Google Scholar]
- 19.Wang H, Kavanaugh M P, North R A, Kabat D. Nature (London) 1991;352:729–731. doi: 10.1038/352729a0. [DOI] [PubMed] [Google Scholar]
- 20.Kim J W, Cliss E I, Albritton L M, Cunningham J M. Nature (London) 1991;352:729–731. [Google Scholar]
- 21.Olah Z, Lehel C, Anderson W B, Eiden M V, Wilson C A. J Biol Chem. 1994;269:25426–25431. [PubMed] [Google Scholar]
- 22.Kavanaugh M P, Miller D G, Zhang W, Law W, Kozak S L, Kabat D, Miller A D. Proc Natl Acad Sci USA. 1994;91:7071–7075. doi: 10.1073/pnas.91.15.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller D G, Miller A D. J Virol. 1994;68:8270–8276. doi: 10.1128/jvi.68.12.8270-8276.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson C A, Farrell K B, Eiden M V. J Virol. 1994;68:7697–7703. doi: 10.1128/jvi.68.12.7697-7703.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rein A, Schultz A. Virology. 1984;136:144–152. doi: 10.1016/0042-6822(84)90255-1. [DOI] [PubMed] [Google Scholar]
- 26.Spain B H, Koo D, Ramakrishnan M, Dzudzor B, Colicelli J. J Biol Chem. 1995;270:25435–25444. doi: 10.1074/jbc.270.43.25435. [DOI] [PubMed] [Google Scholar]
- 27.Cloyd M W, Thompson M M, Hartley J W. Virology. 1985;140:1239–1248. doi: 10.1016/0042-6822(85)90362-9. [DOI] [PubMed] [Google Scholar]
- 28.Miller A D, Wolgamot G. J Virol. 1997;71:4531–4535. doi: 10.1128/jvi.71.6.4531-4535.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aaronson S A, Stephenson J R. Proc Natl Acad Sci USA. 1974;71:3941–3945. doi: 10.1073/pnas.71.10.3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levy J A, Ihle J N, Oleszko O, Barnes R D. Proc Natl Acad Sci USA. 1975;72:5071–5075. doi: 10.1073/pnas.72.12.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leong J C, Kane J P, Oleszko O, Levy J A. Proc Natl Acad Sci USA. 1977;74:276–280. doi: 10.1073/pnas.74.1.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kane J P, Hardman D A, Dimpfl J C, Levy J A. Proc Natl Acad Sci USA. 1979;76:5957–5961. doi: 10.1073/pnas.76.11.5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGrath M S, Weissman I L. Cell. 1979;17:65–75. doi: 10.1016/0092-8674(79)90295-2. [DOI] [PubMed] [Google Scholar]
- 34.Bates P, Young J A T, Varmus H E. Cell. 1993;74:1043–1051. doi: 10.1016/0092-8674(93)90726-7. [DOI] [PubMed] [Google Scholar]
- 35.Prince A M, Huima-Byron T, Parker T S, Levine D M. J Viral Hepat. 1996;3:11–17. doi: 10.1111/j.1365-2893.1996.tb00075.x. [DOI] [PubMed] [Google Scholar]